Abstract
The stage at which follicle-enclosed cumulus-oocyte complexes achieve developmental competence in primates is unknown. Therefore, studies were designed to characterize the ability of oocytes in small antral follicles present during the menstrual cycle to spontaneously resume meiosis, fertilize, and support early embryo development. Ovaries were removed from adult rhesus monkeys (n = 12) during the early follicular phase (Days 3–4) of spontaneous cycles. Small antral follicles were divided into five groups according to their diameter; group I: <0.5 mm; group II: 0.5–0.99 mm; group III: 1.0–1.49 mm; group IV: 1.5–1.99 mm; and group V: 2.0–2.5 mm. The cumulus-oocyte complex from healthy small antral follicles (devoid of dark oocytes or granulosa cells) were extracted (n = 199) and cultured for 48 h under different conditions: in TALP (tyrode, albumin, lactate, pyruvate) medium alone, SAGE medium alone, or plus gonadotropins. At 48 h, oocyte meiotic status and diameter were measured after treatment of cumulus-oocyte complexes with hyaluronidase. Cumulus-oocyte complexes derived from follicles of 0.5- to 2-mm diameter contain oocytes that typically reinitiate meiosis in the absence or presence of gonadotropins and fertilize via in vitro fertilization or intracytoplasmic sperm injection. Moreover, the inseminated oocytes can reach the morula stage but arrest. Thus, the ability of these oocytes to complete maturation, as monitored from subsequent embryonic development after fertilization, is suboptimal. Further studies on primate IVM of oocytes from SAFs are warranted in order for them to be considered as an additional, novel source of gametes for fertility preservation in cancer patients.
Keywords: cumulus-oocyte complex, follicular phase, oocyte maturation, rhesus monkeys
Cumulus-oocyte complexes derived from macaque small antral follicles (0.5–2 mm) contain oocytes that can reinitiate meiosis in the absence or presence of gonadotropins and fertilize in vitro.
INTRODUCTION
There has been a remarkable increase in the number of young cancer survivors as a consequence of improvements in therapies [1]. As of 2007, nearly 12 million people were estimated to be living with a history of cancer in the United States [2]. Unfortunately, cancer therapies usually lead to impaired fertility and premature ovarian failure [3]. Approaches for fertility preservation in women have been proposed and are under investigation [4]. The most successful approach involves the traditional reproductive technologies of in vitro fertilization (IVF) and cryopreservation of embryos prior to cancer therapy. However, for girls and many young women, this is not an option. Successful human oocyte cryopreservation for fertility preservation has also been reported [5, 6]. Moreover, cryopreservation of the ovarian cortex is one experimental option for restoring fertility in cancer survivors [7]. So far, two approaches have been considered to support the maturation of cryopreserved immature follicles from the cortex: ovarian tissue transplantation or in vitro follicle maturation (IFM [8]). Transplantation of cryopreserved ovarian cortical strips has been successfully performed in several patients [9, 10]. However, this approach has the risk of introducing cancer cells back into the patient [11, 12]. In contrast, in vitro ovarian follicle culture to achieve mature oocytes for IVF is an alternative without this risk. To date, live offspring in mice have been produced using IFM [13], but limited studies have been performed in primates.
Xu et al. [14] recently reported that an encapsulated three-dimensional (3D) culture system utilizing biomaterials to maintain cell-cell communication [15–17] permitted follicle growth to the small antral stage and supported steroidogenesis of individual secondary macaque follicles. Furthermore, the encapsulated 3D culture system supported the in vitro development of human follicles [18]. However, since mature preovulatory follicles achieve diameters in vivo up to 6 mm [19] in macaques and 20 mm in women [20], the technical challenges of follicle culture in primates relative to mice are obvious. It is estimated that it takes approximately 90 days for a preantral follicle that has entered the growing pool to become a preovulatory follicle in women [21]. But whether this time interval or a preovulatory size follicle is required in vitro to produce an antral follicle that contains a competent oocyte remains unknown. In our laboratory, encapsulated 3D culture of secondary follicles produced antral follicles of ≤1 mm in diameter [14], but whether the oocytes are developmentally competent is under investigation. Therefore, by determining the smallest diameter of an antral follicle that yields a developmentally competent oocyte, this option for fertility preservation can be advanced.
The key process required for growing follicles to yield healthy mature oocytes is the acquisition of developmental competence that includes the ability of the oocyte to spontaneously resume and complete meiosis (on removal from follicles) as well as to support embryonic development after fertilization. In rodents, development competence of oocytes is associated with antrum formation, maximum oocyte size, and nuclear encapsulation or rimming [22–24]. However, in Old World monkeys such as the rhesus macaque, meiotic competence increases with follicle size but has not correlated with antrum formation or maximum oocyte diameter [25, 26]. Moreover, nuclear rimming in primates does not coincide with the maximum oocyte size [25, 26]. In primates, the stage at which the antral follicle-enclosed oocyte achieves developmental competence, such that removal from the follicle permits the spontaneous resumption of meiosis, is unknown. Therefore, studies were designed to characterize the pool of small antral follicles (SAFs) present during the natural menstrual cycle in the monkey ovary for development competence of the oocyte.
The clinical use of in vitro maturation (IVM) of the oocyte as an assisted reproductive technology is increasing, and there are successful clinical IVM protocols even though its efficiency remains low [27–33]. We used similar culture conditions in a nonhuman primate model to assess the maturation of oocytes from SAFs during the natural menstrual cycle. In rhesus monkeys, the highest yield of healthy oocytes and highest ratio of healthy to degenerating oocytes were obtained from SAFs during the early follicular phase compared to those from late follicular or luteal phase of the menstrual cycle [34]. Moreover, the cohort of antral follicles in ovaries from the early follicular phase (Days 1–5 of menstrual cycle) represents those from which the dominant follicle will eventually be selected [35]. Therefore, this study determined the minimal diameter of an antral follicle from the early follicular phase of spontaneous menstrual cycles in macaques that contained a cumulus-oocyte complex (COC) capable of oocyte maturation in vitro, plus the ability of meiotically mature oocytes to fertilize and support preimplantation embryonic development.
MATERIALS AND METHODS
Animals and COC Recovery
The general care and housing of monkeys (Macaca mulatta) at the Oregon National Primate Research Center (ONPRC) was previously described [36]. The studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the ONPRC Animal Care and Use Committee.
Adult female rhesus monkeys (n = 12; 5–13 yr of age) exhibiting normal menstrual cycles of approximately 28 days based on serum levels of estradiol and progesterone [14] were used in this study. The first day of menses was considered as Day 1 of the cycle. Ovaries were removed from anesthetized monkeys during the early follicular phase (Days 2–4) of spontaneous cycles by laparoscopy, as previously described [37]. The excised ovaries were transported immediately to the laboratory in holding media (SAGE; CooperSurgical, Inc., Trumbull, CT) supplemented with 0.1% serum protein substitute plus gentamycin. After a gentle treatment with collagenase (0.1 mg/ml collagenase-DNAase-PBS) at 37°C for 20 min, the SAFs were dissected from the ovarian medulla under a stereoscopic microscope using 30-gauge needles. The diameter of healthy SAFs (devoid of dark oocytes or granulosa cells) were measured, and their COCs were extracted (n = 199).
In Vitro Culture of COCs
Individual COCs from each group were cultured for 48 h under different conditions: in tyrode, albumin, lactate, and pyruvate (TALP) alone (n = 45 COCs); TALP + follicle-stimulating hormone (rhFSH; NV Organon, Oss, Netherlands; 9.2 ng/ml) + luteinizing hormone (rhLH; Ares Serono, Randolph, MA; 0.9 ng/ml) (n = 56 COCs); SAGE alone (n = 39 COCs); or SAGE + rhFSH + rhLH (0.75 IU each/ml, as suggested by the manufacturer) (n = 59 COCs). Images of the individual COCs were acquired 0, 24, and 48 h posttreatment using a digital camera attached to an Olympus CK40 microscope. At 48 h, the COCs were treated with hyaluronidase (2 mg/ml) in TALP Hepes-BSA (0.3%) for 30 sec to dissociate the cumulus and obtain denuded oocytes for analysis of the stage of nuclear maturation (germinal vesicle [GV]-intact; metaphase I [MI], and metaphase II [MII]) and diameter. Image Tool Software V3.0 was employed to measure oocyte diameter.
Sample Preparation for Microscopy
A cohort of denuded MII-stage oocytes from each of the four treatment groups was randomly selected and fixed in 4% paraformaldehyde for indirect immunofluorescence to ascertain nuclear maturation, spindle and polar body (PB) organization, and transzonal projections (tzps) after 48 h of oocyte maturation [38]. Oocytes were incubated in primary antibody overnight at 4°C with gentle agitation, followed by three 10-min washes in wash buffer and then a 1-h incubation of secondary antibody at room temperature with agitation. Spindle microtubules were labeled with a-tubulin clone DM1A (1:100; Sigma, St. Louis, MO) followed by Alexa 633 goat anti-mouse IgG (1:500; Invitrogen, Carlsbad, CA); F-actin was probed with Alexa 488-phalloidin (1:50; Invitrogen); chromosomes were labeled by 5 μM ethidium homodimer (Invitrogen). Oocytes were mounted on slides with 2–5 μl of glycerol/PBS solution (1:1) containing 25 mg/ml sodium azide. Samples were analyzed on a Leica SP5 confocal-equipped DM6000 CFS microscope with resonance scanner and argon, HeNe 543, and HeNe 633 lasers. Full Z-stack data sets were collected with a HCX PL Apo CS 40× oil objective (1.25 na) for each oocyte, with images taken every 0.3 μm.
Semen Preparation and IVF/ICSI
Semen was collected by the assisted reproductive technology (ART) core from fertile male rhesus monkeys as previously described [39, 40]. Samples were allowed to liquefy for 15 min and then washed twice in Hepes-buffered TALP medium containing 0.3% bovine serum albumin (BSA; Sigma, St. Louis, MO) by centrifugation (7 min, 1400 rpm). The washed sperm were resuspended in TALP containing 0.3% BSA. Sperm motility and concentration were examined in each sample.
Sperm samples were either used for in IVF of oocytes or intracytoplasmic sperm injection (ICSI), as previously described [40, 41]. Sperm used for IVF was previously activated by a sperm activator containing caffeine and db-cAMP. A cohort of MII oocytes (n = 26) was transferred individually to 100-μl droplets of TALP (serum free) under oil for insemination IVF at 37°C in 5% CO2. Oocytes were examined 16 h postinsemination for the presence of two PB and two pronuclei to confirm fertilization. Fertilized oocytes were transferred to four-well dishes (Nalge Nunc International Co., Naperville, IL) containing 500 μl HECM-9 (serum free) equilibrated at 37°C in 6% CO2, 5% O2, and 89% N2 and covered with tissue culture oil (SAGE) [42, 43]. Embryos were cultured under these conditions for 48 h and thereafter in HECM-9 with 5% FBS. Media was changed every other day, and pictures were taken daily to document embryonic development. Reagents and protocols for embryo culture are routinely used by the ART core and quality control tested every week [44].
An additional cohort of IVM mature oocytes (n = 16) was fertilized within the ART core by ICSI [45]. The micromanipulation chamber (Falcon 1009; Becton-Dickinson, Franklin Lakes, NJ) contained oil and two drops: a sperm drop consisting of 4 μl of 10% polyvinylpyrrolidone (Irvine Scientific, Santa Ana, CA) in TALP-hepes and 1 μl of spermatozoa (3 million/ml) and an oocyte drop, 20 μl of TALP-Hepes, into which mature oocytes were placed. Only progressively motile spermatozoa with normal morphology were selected for ICSI. Individual spermatozoa were immobilized by striking the tail and then injected into oocytes away from the PB using a 7-μm-outer-diameter micropipette (Humagen, Charlottesville, VA). Injected oocytes were transferred and cultured as described after IVF above.
Statistical Analysis
Statistical calculations were performed using Sigma Stat software package (Systat Software, Inc., Richmond, CA). Chi-square or Fisher exact tests were used to analyze differences in proportions among different treatments. Differences in oocyte diameters (MI–MII) among groups were analyzed using a one-factor analysis of variance followed by comparison among means using Kruskal-Wallis tests. Differences were considered significant at P < 0.05.
RESULTS
Size Distribution of SAF
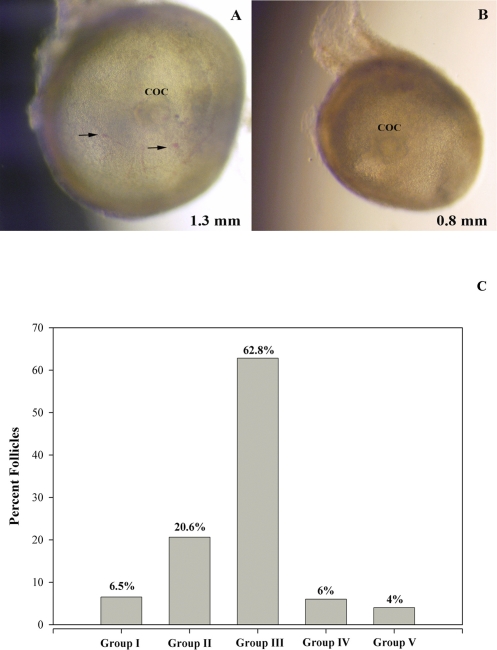
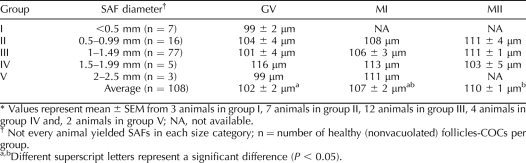
The isolated healthy SAFs (Fig. 1, A and B) were measured and divided into five groups according to their diameter (Fig. 1 C); group I: < 0.5 mm; group II: 0.5–0.99 mm; group III: 1.0–1.49 mm; group IV: 1.5–1.99 mm; and group V: 2.0–2.5 mm. Of the total SAFs collected, the majority distributed into group III (1.0–1.49 mm; 62.8%), with fewer (P < 0.05) in groups II (0.5–0.99 mm; 20.6%), I (<5 mm; 6.5%), IV (1.5–1.99 mm; 6%), and V (2–2.5 mm; 4%). The number of SAFs per animal varied from 3 to 31, with an average of 17 ± 3. Not every animal yielded SAFs in each size category.
FIG. 1.
A and B) Representative pictures of healthy small antral follicles (SAFs) dissected from the ovaries of adult monkeys during the early follicular phase of the menstrual cycle. COCs are easily observed through SAFs of different diameters. Arrows denote the presence of blood vessels containing red blood cells. Size distribution of healthy SAFs dissected from all animals is summarized in C. SAFs were divided into five groups according to their diameter; group I: <0.5 mm; group II: 0.5–0.99 mm; group III: 1.0–1.49 mm; group IV: 1.5–1.99 mm; and group V: 2.0–2.5 mm. Not every animal yielded SAFs in each size group.
Oocyte Maturation After 48 h of Culture
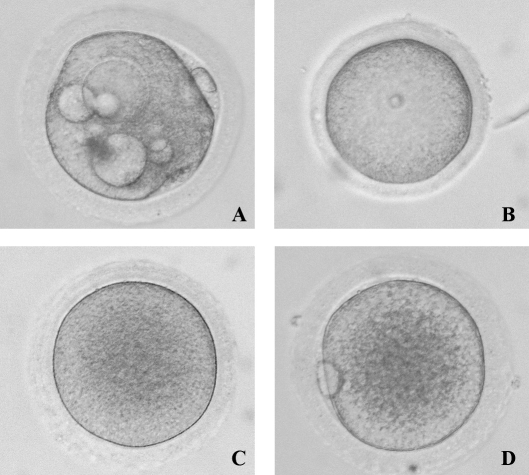
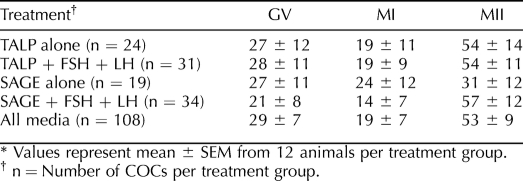
Although we carefully dissected what appeared to be healthy SAFs avoiding those with dark oocytes or granulosa cells, 46% of oocytes within the total number of COCs collected contained vacuoles (Fig. 2A) at 48 h postculture. COCs from group III provided the fewest vacuolated oocytes among the groups. Vacuolated oocytes were considered degenerate and discarded from the statistical analysis. Figure 2 also shows representative pictures of healthy (54%), nonvacuolated oocytes at different stages of nuclear maturation after culture (GV-intact, Fig. 2B; MI, Fig. 2C; and MII, Fig. 2D). The percentage of healthy oocytes resuming maturation to MI and continuing meiosis to MII did not significantly differ between media, nor with or without gonadotropins (Table 1).
FIG. 2.
Representative pictures of monkey oocytes at different stages of nuclear maturation after isolation from SAFs during the early follicular phase of the menstrual cycle and 48 h of culture (GV: B; MI: C; MII: D) as well as degenerating (A). The surrounding cumulus cells were removed by hyaluronidase treatment. Original magnification ×20.
TABLE 1.
Percentage of oocytes from healthy COCs at given stages of nuclear maturation after 48 h in the different culture media.*
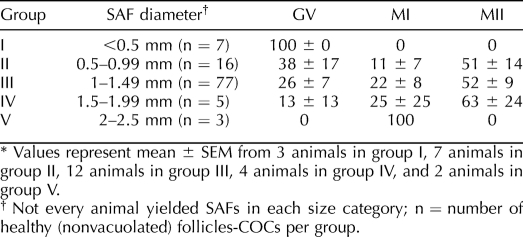
Oocyte nuclear maturation as a function of SAF diameter was also examined (Table 2). Since there were no differences in oocyte maturation between treatments, the data are pooled. Also, not every animal (n = 12) yielded SAFs in each size category. The few oocytes collected in group I did not resume meiosis. In contrast, oocytes from groups II, III, and IV resumed meiosis to the MI stage (Table 2). Moreover, half the oocytes from groups II, III, and IV matured to MII relative to group I. The very few oocytes collected from group V resumed meiosis but precluded statistical analysis.
TABLE 2.
Percentage of oocytes from healthy COCs at given stages of nuclear maturation after 48 h in culture, as a function of SAF size.*
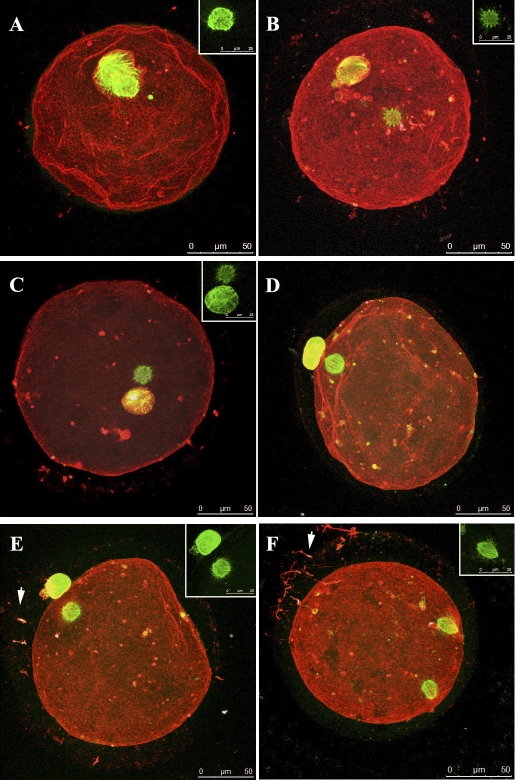
Representative MII oocytes derived from SAFs after 48 h under different culture conditions (TALP + FSH + LH, SAGE + FSH + LH, TALP alone, SAGE alone) were analyzed using immunofluorescence to visualize chromatin, spindles, and actin (Fig. 3). The majority of the MII oocytes showed normal spindle and PB positions regardless of the culture conditions (Fig. 3, A–E). However, some of the spindles and/or PBs were smaller than the expected size (Fig. 3, C–E), and a larger gap between spindle and PB was detected in one of the MII oocytes (Fig. 3B). Abnormal maturation was observed in only one oocyte (Fig. 3F), in which the second spindle was not extruded into a PB showing an incomplete cytokinesis. In several oocytes, tzps were seen (arrows), with F-actin staining along the oolemma (Fig. 3, E and F).
FIG. 3.
Confocal microscopy images of representative oocytes from SAFs after 48 h of culture in media with or without gonadotropins. A) Oocyte matured in SAGE media without supplementation. Oocyte shows first PB adjacent to MII spindles. Spindle shown separately in inset stained with TUBA (green). B) Oocyte matured in TALP without supplementation. The oocyte shows a MII spindle that is somewhat adjacent to the first extruded PB; tzp fragments remain along the oolemma (red). TUBA staining alone (green) is shown in the inset. C and D) Oocytes matured in SAGE media with LH and FSH. MII oocytes (C and D) showing adjacent spindle to PB placement. The oocytes have small MII spindles and small, extruded first PBs. Bright red staining (F-actin) shows remnants of tzp after IVM. Tubulin staining alone (green) is shown in the inset. E and F) oocytes cultured in TALP with LH and FSH. MII oocyte (E) with spindle placement adjacent to extruded polar body. An oocyte (F) with two spindles (green) from incomplete cytokinesis. Numerous transzonal projections (tzps, arrows) seen with F-actin staining (red). Numerous tzps are also seen around the oocyte (red). TUBA staining alone (green) is shown in the inset. Bars = 50 μm and 25 μm (insets).
Oocyte Diameter After 48 h of Culture
Oocyte diameters were analyzed as a function of SAF diameter and meiotic status (Table 3). The minimum and maximum diameters for a GV oocyte were 74 and 119 μm, for an MI oocyte 78.5 and 118 μm, and for an MII oocyte 94.1 and 121.9 μm, respectively. The diameter of MII oocytes was similar regardless of SAF diameter and did not differ between media. When data for all SAFs are pooled, the average diameter of GV oocytes (102 ± 2 μm) was less (P < 0.05) than that of MII oocytes (110 ± 1 μm). Only SAF of <0.5-mm diameter tended to enclose smaller oocytes, none which were capable of resuming meiosis.
TABLE 3.
Diameter of oocytes obtained from SAF of different sizes, as a function of nuclear maturation.*
IVF or ICSI of MII Oocytes
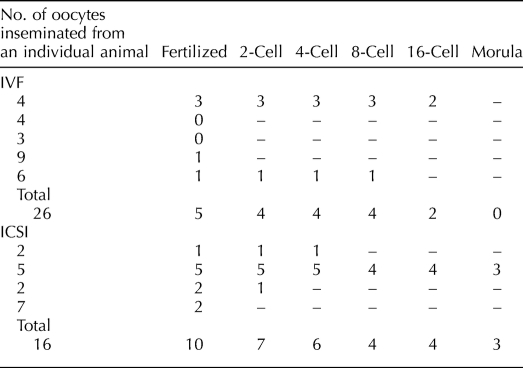
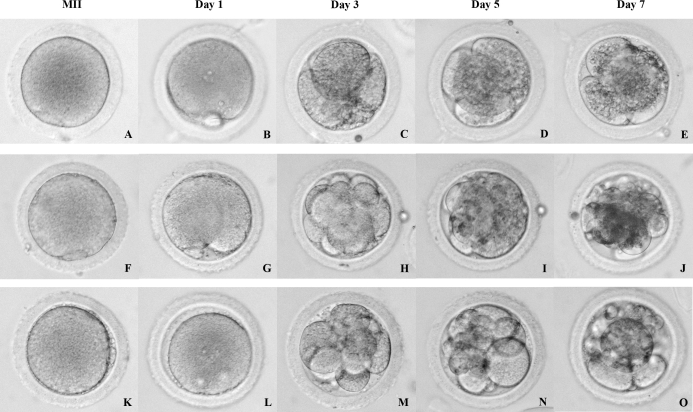
Table 4 depicts fertilization and embryonic development following IVF and ICSI of MII oocytes derived from five and four animals, respectively. Five of 26 oocytes from SAFs fertilized by IVF, and four zygotes cleaved but arrested at the 8- to 16-cell stage. Ten of 16 oocytes that underwent ICSI also fertilized, and zygotes underwent cleavage. In addition, three oocytes from one animal continued cleavage to the morula stage before arrest. In contrast, the current blastocyst rate for rhesus macaque embryos at ONPRC cultured in HCEM-9 media is 53% (Data not shown). Figure 4 shows embryonic development from representative MII oocytes inseminated by ICSI.
TABLE 4.
Fertilization and embryonic development in a cohort of MII oocytes after 48 h of culture and insemination using IVF or ICSI.
FIG. 4.
Representative pictures of MII oocytes prior to (A, F, and K) and after insemination using ICSI (Day 1: B, G, and L; Day 3: C, H, and M; Day 5: D, I, and N; Day 7: E, J, and O). After insemination, the presence of two pronuclei (PN) and two polar bodies (PB) was checked to confirm fertilization (B, G, and L). Some embryos arrested at very early stages (e.g., four cell; D and E), whereas others continued cleavaging until the morula stage (J and O), when they arrested. Original magnification ×20.
DISCUSSION
This study characterizing the pool of SAFs present in the primate ovaries during the early follicular phase of the natural menstrual cycle, established the ability of the cumulus-enclosed oocytes complexes derived from healthy SAFs to reinitiate meiosis in vitro in culture media with or without gonadotropins. It also demonstrated that the SAFs had to reach at least 0.5 mm in diameter in order for the oocyte to display meiotic competence. Moreover, some inseminated oocytes did fertilize and even reached the morula stage but arrested.
While the number and sizes of presumably healthy SAFs obtained from each animal varied, the majority (63%) measured between 1 and 1.49 mm, followed by 0.5–0.99-mm diameter (21%). The diameters of these macaque SAFs would be equivalent to 4–5 and 2–3 mm, respectively, human follicles [46], which are much smaller than the large, preovulatory follicles from which COCs for human IVF protocols are derived. A recent study from our laboratory using ultrasound imaging identified SAFs (<2 mm) in monkeys throughout the menstrual cycle on the ovary bearing the dominant follicle/corpus luteum and the contralateral ovary [47]. On the first day of the menstrual cycle (first day of menses), these ovaries had between 10 ± 3 (mean ± SEM, larger ovary) and 8 ± 2 (mean ± SEM, smaller ovary) SAFs, respectively. In accordance with these in vivo results, in the present study we obtained an average of 17 ± 3 SAFs (mean ± SEM) per animal. Scheffer et al. [48] reported that during the early follicular phase, the number of SAFs (2–5 mm) in women with regular menstrual cycle was around five to six. The wide range of age (25–46 yr) used to calculate the median for SAFs count in this study may explain the lower number of SAFs reported in women in comparison to rhesus monkey data. It has also been described that the number of SAFs declined with age, whereas the number of larger follicles (7–10 mm) remained nearly constant [46, 48]. However, the number of SAFs obtained per animal was not necessarily related to the ability of oocytes to mature during 48 h in vitro. For example, one animal yielded three SAFs, and all the oocytes resumed meiosis reaching the MII stage. In contrast, several animals yielded many SAFs, but only a few oocytes attained MII in vitro. Thus, this may indicate an inverse correlation between the number and quality of the SAFs. Moreover, perhaps only a small cohort of the SAF population selectively possesses the capacity to continue their development in the early follicular phase of the cycle. Bishop et al. [47] proposed that the dominant follicle in macaques appeared to be selected on the basis of its size differential (>2 mm) from other antral follicles before Day 3 of the follicular phase.
Although we carefully dissected healthy SAFs avoiding dark oocytes or granulosa cells, unexpectedly almost half (46%) of the total COCs contained one or more vacuoles after 48 h of culture. Since the oocytes were not evaluated until removal of the cumulus, it is not clear whether the vacuoles were present at collection or developed during culture. In previous studies on macaque oocytes from COCs, vacuolated oocytes were not subtracted from the MI or MII oocyte categories; thus, higher percentages of maturation were described compared to our results [26, 49]. However, a vacuolated oocyte cannot be considered a healthy oocyte; hence, results from our study may more accurately depict developmental competence of only healthy oocytes.
Notably, we found that oocytes within COCs derived from healthy SAFs of at least 0.5-mm diameter obtained during the early follicular phase can reinitiate meiosis in vitro. However, following fertilization in vitro, embryonic development arrests; therefore, oocyte competence is not fully achieved in these SAFs of ≤2-mm diameter. In our laboratory, the encapsulated 3D culture model permitted isolated secondary follicles from macaques to grow to the small antral stage (≤1 mm) [14]. Whether oocytes derived from the encapsulated 3D culture model are developmentally competent is under investigation.
The current results suggest that the macaque oocyte must achieve a diameter of ≥103–110 μm to reinitiate meiosis. In contrast, it has been reported that human oocytes from early antral follicles may reach a diameter of approximately 120 μm [50], while in rodents the maximum diameter of 70 μm has already been reached at the end of the preantral stage when the oocyte acquires the competence to resume meiosis [50]. The growth phase of the oocyte allows development of the zona pellucida and production of mRNA and proteins required for subsequent fertilization and early embryonic development [50]. However, size cannot be the only requirement for developmental competence in primates since GV oocytes of up to 116 μm were obtained from larger (1.5–1.99 mm) SAFs.
Nuclear events endow oocytes with the competence to resume and complete meiosis whereas, cytoplasmic programs enable the oocyte to be fertilized successfully and support embryo development to term. Failure to complete cytoplasmic maturation can block the development at fertilization, embryonic genome activation, blastocyst formation, or even postimplantation events [51]. To date, the ability of the primate oocyte to complete nuclear and cytoplasmic maturation in vitro is markedly inferior to that of oocytes from other species [52–58]. In addition, the quality of primate oocytes matured in vitro is much less compared with their counterparts matured in vivo [26, 49, 59–62]. Acquisition of oocyte meiotic maturation potential, especially developmental ability, requires mutual interactions between the oocyte and its surrounding somatic cells (cumulus cells plus mural granulosa cells) throughout oogenesis. However, oocyte maturation encompasses both nuclear and cytoplasmic programs, and the full developmental competence is conferred only when these two processes are closely integrated. The intimate associations between these two cell types are established through gap junctions as well as paracrine interactions and persist until ovulation [51, 63, 64]. Therefore, the early interruption that occurs when the COCs are dissected from the follicle for in vitro culture may compromise the meiotic and developmental competence of the oocytes. However, it remains to be determined whether the failure in embryo development in oocytes derived from SAF is due to suboptimal culture conditions or a requirement for the COC to reside for a longer time within the growing antral follicle in primates.
Gonadotropins (FSH and LH) are not required for the cumulus-enclosed oocyte from macaque SAFs to resume meiosis since COCs cultured in either TALP or SAGE media without gonadotropins yielded MI and MII oocytes. These results agree with a previous report in primates wherein no differences were observed between the proportion of MI or MII oocytes obtained from COCs cultured in the absence or presence of gonadotropins [49, 65]. In primates, although follicles that reach the antral stage become dependent on these pituitary hormones for further growth and maturation [66], their involvement in oocyte nuclear and/or cytoplasmic development remains unknown. In mice, low doses of FSH in vitro improve the rates of oocyte fertilization and blastocyst development, whereas high doses reduce oocyte competence to undergo fertilization and preimplantation development but increase granulosa cell differentiation [67, 68]. The use of FSH experimentally, in vitro, avoids the fact that mouse cumulus cells reportedly lack LH receptors and thus cannot respond to LH in vitro [67]. Whether primate cumulus cells are LH insensitive is unknown.
In the present study, all the COCs from SAFs of young adult rhesus monkeys were obtained during the early follicular phase (Days 2–4) of natural menstrual cycles, and the oocyte's ability to resume meiosis in vitro was carefully analyzed. This source of the COCs is distinct from those in previous reports using both nonhuman primates and women. Most prior studies evaluating IVM in nonhuman primate models used oocytes from large antral follicles of monkeys primed with FSH for 6–7 days [42, 60, 69, 70]. Both human and macaque oocytes isolated from large antral follicle resume spontaneous meiosis [52, 71–74]. In nonhuman primates [42, 60, 69, 70], as in women [75, 76], gonadotropin priming in vivo has been shown to increase the number of oocytes collected and improve their maturation as well as to enhance the embryonic competence. In contrast, oocytes from small follicles unresponsive to an ovulatory gonadotropin surge or from dissected ovaries collected at unknown stages of the menstrual cycle, presumably from small follicles not exposed to gonadotropins, are also used in human IVM, but their competence is lower [29, 31, 32, 77–81]. Furthermore, the specific gonadotropin regimen to use in vitro (FSH alone, hCG alone, or the combination of both) to obtain mature oocytes remains controversial [28, 29, 31, 32, 78, 82]. Although the efficiency of IVM remains low, over 900 babies have currently been born following this technique (with or without gonadotropin priming) around the world [83]. Further studies on primate IVM of oocytes from SAFs are warranted in order to provide an additional, novel source of gametes for fertility preservation in cancer patients.
In summary, primate COCs derived from SAFs of 0.5- to 2-mm diameter during the early follicular phase contain oocytes that can reinitiate meiosis and be fertilized in vitro. Thus, the cohort of SAF present in the medulla of ovaries from spontaneous menstrual cycles prior to selection of the dominant follicle can provide COCs as an additional source of oocytes for IVM and fertilization. However, the competence of these oocytes to achieve cytoplasmic maturation prior to fertilization with subsequent embryonic development is still under investigation.
Acknowledgments
We are grateful for the expert contributions of the animal care staff and surgical unit of the Division of Animal Resources and the outstanding technical support of the ART core and the Endocrine Services and Technology Laboratory at ONPRC. Recombinant human FSH (Organon) and LH (Merck Serono) were generously donated for this project. A special thanks to Dr. Dick Yeoman, Maralee Lawson, Dr. Jing Xu, Dr. Alison Ting, and Melinda Murphy for their scientific assistance.
Footnotes
Supported by Oncofertility Consortium NIH 1 UL1 RR024926 (R01-HD058294, PL1-EB008542), the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement (U54 HD18185, U54 HD55744) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, NCRR-RR00163 and Lalor Foundation Postdoctoral Basic Research Fellowship (M.C.P.). Also, this work was funded by UL1: UL1DE019587; R01C: RL1HD058295: T32 HD007068-31 (S.L.B.).
REFERENCES
- McVie JG.Cancer treatment: the last 25 years. Cancer Treat Rev 1999; 25: 323–331. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlander N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, et al. SEER cancer statistics review, 1975–2005. National Cancer Institute, Bethesda, MD,http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER Web site,2008. [Google Scholar]
- Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN.Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab 2003; 88: 5307–5314. [DOI] [PubMed] [Google Scholar]
- Jeruss JS, Woodruff TK.Preservation of fertility in patients with cancer. N Engl J Med 2009; 360: 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Kort HI, Vajta G.The efficacy and safety of human oocyte vitrification. Semin Reprod Med 2009; 27: 450–455. [DOI] [PubMed] [Google Scholar]
- Borini A, Coticchio G.The efficacy and safety of human oocyte cryopreservation by slow cooling. Semin Reprod Med 2009; 27: 443–449. [DOI] [PubMed] [Google Scholar]
- Sonmezer M, Oktay K.Assisted reproduction and fertility preservation techniques in cancer patients. Curr Opin Endocrinol Diabetes Obes 2008; 15: 514–522. [DOI] [PubMed] [Google Scholar]
- West ER, Zelinski MB, Kondapalli LA, Gracia C, Chang J, Coutifaris C, Critser J, Stouffer RL, Shea LD, Woodruff TK.Preserving female fertility following cancer treatment: current options and future possibilities. Pediatr Blood Cancer 2009; 53: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J.Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 2005; 353: 318–321. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A.Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 364: 1405–1410. [DOI] [PubMed] [Google Scholar]
- Meirow D, Ben Yehuda D, Prus D, Poliack A, Schenker JG, Rachmilewitz EA, Lewin A.Ovarian tissue banking in patients with Hodgkin's disease: is it safe? Fertil Steril 1998; 69: 996–998. [DOI] [PubMed] [Google Scholar]
- Shaw J, Trounson A.Oncological implications in the replacement of ovarian tissue. Hum Reprod 1997; 12: 403–405. [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK.Tissue-engineered follicles produce live, fertile offspring. Tissue Eng 2006; 12: 2739–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB.Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod 2009; 81: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ER, Shea LD, Woodruff TK.Engineering the follicle microenvironment. Semin Reprod Med 2007; 25: 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ.Cellular basis for paracrine regulation of ovarian follicle development. Reproduction 2001; 121: 647–653. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Shea LD.The role of the extracellular matrix in ovarian follicle development. Reprod Sci 2007; 14: 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK.In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 2009; 24: 2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koering MJ, Baehler EA, Goodman AL, Hodgen GD.Developing morphological asymmetry of ovarian follicular maturation in monkeys. Biol Reprod 1982; 27: 989–997. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Hillier SG, van den Boogaard AM, Trimbos-Kemper TC, Reichert LE, Jr, van Hall EV.Follicular development during the luteal phase of the human menstrual cycle. J Clin Endocrinol Metab 1983; 56: 1022–1031. [DOI] [PubMed] [Google Scholar]
- Gougeon A.Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996; 17: 121–155. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Sorensen RA.In vitro maturation of mouse oocytes isolated from late, middle, and pre-antral graafian follicles. J Exp Zool 1974; 190: 123–127. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T, Yanagimachi R.Maturation in vitro of ovarian oocytes of prepubertal and adult hamsters. J Reprod Fertil 1975; 45: 83–90. [DOI] [PubMed] [Google Scholar]
- Mattson BA, Albertini DF.Oogenesis: chromatin and microtubule dynamics during meiotic prophase. Mol Reprod Dev 1990; 25: 374–383. [DOI] [PubMed] [Google Scholar]
- Lefevre B, Gougeon A, Nome F, Testart J.In vivo changes in oocyte germinal vesicle related to follicular quality and size at mid-follicular phase during stimulated cycles in the cynomolgus monkey. Reprod Nutr Dev 1989; 29: 523–531. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Tennier MT, Boatman DE, Bavister BD.Chromatin configurations and meiotic competence of oocytes are related to follicular diameter in nonstimulated rhesus monkeys. Biol Reprod 1993; 48: 349–356. [DOI] [PubMed] [Google Scholar]
- Trounson A, Wood C, Kausche A.In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril 1994; 62: 353–362. [DOI] [PubMed] [Google Scholar]
- Mikkelsen AL, Lindenberg S.Benefit of FSH priming of women with PCOS to the in vitro maturation procedure and the outcome: a randomized prospective study. Reproduction 2001; 122: 587–592. [DOI] [PubMed] [Google Scholar]
- Mikkelsen AL.FSH priming in IVM cycles. Tan LS, Chian RC, Buckett WM.In-Vitro Maturation of Human Oocytes: Basic Science to Clinical Application. London:Informa Healthcare;2007: 237–242. [Google Scholar]
- Chian RC, Buckett WM, Too LL, Tan SL.Pregnancies resulting from in vitro matured oocytes retrieved from patients with polycystic ovary syndrome after priming with human chorionic gonadotropin. Fertil Steril 1999; 72: 639–642. [DOI] [PubMed] [Google Scholar]
- Chian RC, Gulekli B, Buckett WM, Tan SL.Priming with human chorionic gonadotropin before retrieval of immature oocytes in women with infertility due to the polycystic ovary syndrome. N Engl J Med 1999; 341: 1624–1626. [DOI] [PubMed] [Google Scholar]
- Chian RC, Buckett WM, Tulandi T, Tan SL.Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum Reprod 2000; 15: 165–170. [DOI] [PubMed] [Google Scholar]
- Bergh C, Loft A, Lundin K, Ziebe S, Nilsson L, Wikland M, Grondahl C, Arce JC.Chromosomal abnormality rate in human pre-embryos derived from in vitro fertilization cycles cultured in the presence of follicular-fluid meiosis activating sterol (FF-MAS). Hum Reprod 2004; 19: 2109–2117. [DOI] [PubMed] [Google Scholar]
- Alak BM, Wolf DP.Rhesus monkey oocyte maturation and fertilization in vitro: roles of the menstrual cycle phase and of exogenous gonadotropins. Biol Reprod 1994; 51: 879–887. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Hodgen GD.The ovarian triad of the primate menstrual cycle. Recent Prog Horm Res 1983; 39: 1–73. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL.In vitro fertilization-embryo transfer in nonhuman primates: the technique and its applications. Mol Reprod Dev 1990; 27: 261–280. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL.Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod 2002; 17: 2825–2831. [DOI] [PubMed] [Google Scholar]
- Barrett SL, Albertini DF.Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod 2007; 76: 949–957. [DOI] [PubMed] [Google Scholar]
- Lanzendorf SE, Gliessman PM, Archibong AE, Alexander M, Wolf DP.Collection and quality of rhesus monkey semen. Mol Reprod Dev 1990; 25: 61–66. [DOI] [PubMed] [Google Scholar]
- Lanzendorf SE, Zelinski-Wooten MB, Stouffer RL, Wolf DP.Maturity at collection and the developmental potential of rhesus monkey oocytes. Biol Reprod 1990; 42: 703–711. [DOI] [PubMed] [Google Scholar]
- Mitalipov SM, Nusser KD, Wolf DP.Parthenogenetic activation of rhesus monkey oocytes and reconstructed embryos. Biol Reprod 2001; 65: 253–259. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Paprocki AM.Birth of rhesus monkey infant after transfer of embryos derived from in-vitro matured oocytes: short communication. Hum Reprod 2000; 15: 2411–2414. [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Leibo SP, Tarantal AF.Improved collection and developmental competence of immature macaque oocytes. Theriogenology 2003; 59: 699–707. [DOI] [PubMed] [Google Scholar]
- Mitalipov SM, Zhou Q, Byrne JA, Ji WZ, Norgren RB, Wolf DP.Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum Reprod 2007; 22: 2232–2242. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Thormahlen S, Ramsey C, Yeoman RR, Fanton J, Mitalipov S.Use of assisted reproductive technologies in the propagation of rhesus macaque offspring. Biol Reprod 2004; 71: 486–493. [DOI] [PubMed] [Google Scholar]
- Haadsma ML, Bukman A, Groen H, Roeloffzen EM, Groenewoud ER, Heineman MJ, Hoek A.The number of small antral follicles (2–6 mm) determines the outcome of endocrine ovarian reserve tests in a subfertile population. Hum Reprod 2007; 22: 1925–1931. [DOI] [PubMed] [Google Scholar]
- Bishop CV, Sparman ML, Stanley JE, Bahar A, Zelinski MB, Stouffer RL.Evaluation of antral follicle growth in the macaque ovary during the menstrual cycle and controlled ovarian stimulation by high-resolution ultrasonography. Am J Primatol 2009; 71: 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER.Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril 1999; 72: 845–851. [DOI] [PubMed] [Google Scholar]
- Morgan PM, Warikoo PK, Bavister BD.In vitro maturation of ovarian oocytes from unstimulated rhesus monkeys: assessment of cytoplasmic maturity by embryonic development after in vitro fertilization. Biol Reprod 1991; 45: 89–93. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Vanderhyden BC.Oocyte growth and developmental competence. Tan LS, Chian RC, Buckett WM.In-Vitro Maturation of Human Oocytes: Basic Science to Clinical Application. London:Informa Healthcare;2007: 1–14. [Google Scholar]
- Zheng P.Effects of in vitro maturation of monkey oocytes on their developmental capacity. Anim Reprod Sci 2007; 98: 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RG.Maturation in vitro of human ovarian oocytes. Lancet 1965; 2: 926–929. [DOI] [PubMed] [Google Scholar]
- Galli C, Moor RM.Gonadotrophin requirements for the in vitro maturation of sheep oocytes and their subsequent embryonic development. Theriogenology 1991; 35: 1083–1093. [Google Scholar]
- Pinyopummintr T, Bavister BD.Effects of amino acids on development in vitro of cleavage-stage bovine embryos into blastocysts. Reprod Fertil Dev 1996; 8: 835–841. [DOI] [PubMed] [Google Scholar]
- Pinyopummintr T, Bavister BD.Energy substrate requirements for in vitro development of early cleavage-stage bovine embryos. Mol Reprod Dev 1996; 44: 193–199. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD.Development of in-vitro-fertilized primate embryos into blastocysts in a chemically defined, protein-free culture medium. Hum Reprod 1996; 11: 1690–1697. [DOI] [PubMed] [Google Scholar]
- Sirard MA, Parrish JJ, Ware CB, Leibfried-Rutledge ML, First NL.The culture of bovine oocytes to obtain developmentally competent embryos. Biol Reprod 1988; 39: 546–552. [DOI] [PubMed] [Google Scholar]
- Schroeder AC, Eppig JJ.The developmental capacity of mouse oocytes that matured spontaneously in vitro is normal. Dev Biol 1984; 102: 493–497. [DOI] [PubMed] [Google Scholar]
- Bavister BD, Boatman DE, Leibfried L, Loose M, Vernon MW.Fertilization and cleavage of rhesus monkey oocytes in vitro. Biol Reprod 1983; 28: 983–999. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD.Follicle-stimulating hormone priming of rhesus monkeys enhances meiotic and developmental competence of oocytes matured in vitro. Biol Reprod 1994; 51: 904–912. [DOI] [PubMed] [Google Scholar]
- Wolf DP, VandeVoort CA, Meyer-Haas GR, Zelinski-Wooten MB, Hess DL, Baughman WL, Stouffer RL.In vitro fertilization and embryo transfer in the rhesus monkey. Biol Reprod 1989; 41: 335–346. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Paprocki AM, VandeVoort CA.Causes of developmental failure of in-vitro matured rhesus monkey oocytes: impairments in embryonic genome activation. Hum Reprod 2003; 18: 826–833. [DOI] [PubMed] [Google Scholar]
- Atef A, Francois P, Christian V, Marc-Andre S.The potential role of gap junction communication between cumulus cells and bovine oocytes during in vitro maturation. Mol Reprod Dev 2005; 71: 358–367. [DOI] [PubMed] [Google Scholar]
- Jamnongjit M, Hammes SR.Oocyte maturation: the coming of age of a germ cell. Semin Reprod Med 2005; 23: 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD.Effects of granulosa cells and gonadotrophins on meiotic and developmental competence of oocytes in vitro in non-stimulated rhesus monkeys. Hum Reprod 1995; 10: 887–895. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ.The physiology of follicle selection. Reprod Biol Endocrinol 2004; 2: 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ, Pendola FL, Watanabe S.Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod 1998; 59: 1445–1453. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Albertini DF.Assessment of oocyte quality following repeated gonadotropin stimulation in the mouse. Biol Reprod 2003; 68: 812–821. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD.Granulosa cells from follicle stimulating hormone-primed monkeys enhance the development competence of in-vitro-matured oocytes from non-stimulated rhesus monkeys. Hum Reprod 1996; 11: 1698–1702. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Tennier MT, Boatman DE, Bavister BD.Effects of gonadotropins upon the incidence and kinetics of meiotic maturation of macaque oocytes in vitro. Mol Reprod Dev 1994; 37: 467–472. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Sowa M, Nakano R.Relationship between human oocyte maturation and different follicular sizes. Biol Reprod 1985; 32: 413–417. [DOI] [PubMed] [Google Scholar]
- Thibault C.Hammond Memorial Lecture. Are follicular maturation and oocyte maturation independent processes? J Reprod Fertil 1977; 51: 1–15. [DOI] [PubMed] [Google Scholar]
- Lefevre B, Gougeon A, Testart J.In-vitro oocyte maturation: some questions concerning the initiation and prevention of this process in humans. Hum Reprod 1987; 2: 495–497. [DOI] [PubMed] [Google Scholar]
- Jensen JT, Schwinof KM, Zelinski-Wooten MB, Conti M, DePaolo LV, Stouffer RL.Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Hum Reprod 2002; 17: 2079–2084. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD.A macaque model for studying mechanisms controlling oocyte development and maturation in human and non-human primates. Hum Reprod 1999; 14: 2544–2555. [DOI] [PubMed] [Google Scholar]
- Fadini R, Dal Canto MB, Mignini Renzini M, Brambillasca F, Comi R, Fumagalli D, Lain M, Merola M, Milani R, De Ponti E.Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online 2009; 19: 343–351. [DOI] [PubMed] [Google Scholar]
- Wynn P, Picton HM, Krapez JA, Rutherford AJ, Balen AH, Gosden RG.Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in-vitro maturation. Hum Reprod 1998; 13: 3132–3138. [DOI] [PubMed] [Google Scholar]
- Hwang JL, Lin YH.Combination of FSH priming and hCG priming in IVM cycles. Tan LS, Chian RC, Buckett WM.In-Vitro Maturation of Human Oocytes: Basic Science to Clinical Application. Informa Healthcare;2007: 243–252. [Google Scholar]
- Child TJ, Abdul-Jalil AK, Gulekli B, Tan SL.In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril 2001; 76: 936–942. [DOI] [PubMed] [Google Scholar]
- Liu S, Li Y, Gao X, Yan JH, Chen ZJ.Changes in the distribution of mitochondria before and after in vitro maturation of human oocytes and the effect of in vitro maturation on mitochondria distribution. Fertil Steril 2010; 93: 1550–1555. [DOI] [PubMed] [Google Scholar]
- Liu S, Jiang JJ, Feng HL, Ma SY, Li M, Li Y.Evaluation of the immature human oocytes from unstimulated cycles in polycystic ovary syndrome patients using a novel scoring system. Fertil Steril 2010; 93: 2202–2209. [DOI] [PubMed] [Google Scholar]
- Lin YH, Hwang JL, Huang LW, Mu SC, Seow KM, Chung J, Hsieh BC, Huang SC, Chen CY, Chen PH.Combination of FSH priming and hCG priming for in-vitro maturation of human oocytes. Hum Reprod 2003; 18: 1632–1636. [DOI] [PubMed] [Google Scholar]
- Hashimoto S.Application of in vitro maturation to assisted reproductive technology. J Reprod Dev 2009; 55: 1–10. [DOI] [PubMed] [Google Scholar]