Abstract
Uptake of drugs and other xenobiotics from the nasal cavity and into either the brain or systemic circulation can occur through several different mechanisms, including paracellular transport and movement along primary olfactory nerve axons, which extend from the nasal cavity to the olfactory bulb of the brain. The present study was conducted to expand knowledge on a third means of uptake, namely the expression of drug transporters in the rat nasal epithelium. We used branched DNA technology to compare the level of expression of nine transporters [(equilibrative nucleoside transporters (ENT)1 and ENT2; organic cation transporter (OCT)1, 2, and 3; OCTN1; organic anion-transporting polypeptide (OATP)3; and multidrug resistance (MRP)1 and MRP4] in nasal respiratory mucosa, olfactory mucosa, and olfactory bulb to the level of expression of these transporters in the liver and kidney. Transporters with high expression in the nasal respiratory mucosa or olfactory tissues were immunolocalized by immunohistochemistry. ENT1 and ENT2 expression was relatively high in nasal epithelia and olfactory bulb, which may explain the uptake of intranasally administered nucleoside derivatives observed by other investigators. OATP3 immunoreactivity was high in olfactory epithelium and olfactory nerve bundles, which suggests that substrates transported by OATP3 may be candidates for intranasal administration.
Introduction
Interest is increasing in the use of the intranasal route for drug delivery. Although intranasal delivery avoids issues such as first-pass biotransformation by the liver after oral administration, there are significant hurdles such as bioavailability, degradation by nasal proteases, and an apparent size limitation for materials that can be delivered into the systemic circulation or the brain after intranasal administration. Delivery of intranasally administered drugs into either the brain or systemic circulation can occur by means of several processes. Expression of several metal transporters (DMT1, ZIP14) has been shown in rodent olfactory mucosa (Thompson et al., 2007; Genter et al., 2009), and transection of the olfactory nerve stopped the transport of manganese and thallium from the nasal cavity into the brain (Kinoshita et al., 2008). Furthermore, ipsilateral transsynaptic movement of manganese has been shown (Dorman et al., 2002). Movement of molecules between epithelial cells, i.e., paracellular transport, can be promoted by adjuvants such as poly-l-arginine that “loosen” the intercellular junctions that comprise the nasal blood-brain barrier. An unfortunate side effect of some adjuvants is serious and potentially permanent damage to nasal tissues (Natsume et al., 1999). Finally, the nasal mucosa is highly vascularized, allowing drugs applied to the epithelial surface to be rapidly absorbed into the bloodstream (Türker et al., 2004).
The delivery of nucleoside analogs such as zidovudine and 2′,3′-didehydro-3′-deoxythymidine to the cerebral spinal fluid (CSF) has been demonstrated after intranasal administration in rodents (Seki et al., 1994; Yajima et al., 1996). Transport of nucleoside analogs is mediated by the equilibrative nucleoside transporters (ENTs) (Govindarajan et al., 2009). Although ENTs have been localized to various brain regions in rodents and humans (Jennings et al., 2001; Alanko et al., 2006), the presence of ENTs in nasal epithelia has not been shown to date. Nucleoside drugs penetrate the blood-brain barrier poorly (Varatharajan and Thomas 2009), so we hypothesize that the presence of ENTs in nasal epithelia and/or the olfactory bulb (OB) may explain their uptake from the nasal cavity and into the CSF.
Therefore, the goals of the present studies were to examine the expression of some representative drug transporters, including ENTs, in nasal epithelia and olfactory bulb, as a potential basis for predicting which drugs may be candidates for intranasal administration and uptake into the brain. Expression levels were compared to those in liver and kidney. After identifying transporters with significant expression in nasal epithelia and/or OB, we performed immunohistochemistry to localize the cell type(s) expressing the highly expressed transporters.
Materials and Methods
Animals.
Male adult Sprague-Dawley rats (8–9 weeks of age) were obtained from Harlan (Indianapolis, IN). Rats were maintained in 12-h light/dark cycles in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities at the University of Cincinnati and the University of Arizona. Protocols were approved by each Institutional Animal Care and Use Committee. Rats (n = 4) were fed standard rodent chow ad libitum (Harlan Teklad, Indianapolis, IN) and allowed free access to tap water.
RNA Isolation.
Rats were sacrificed by decapitation. The ethmoid turbinates and caudal one-third of the nasal septum were used as the source of olfactory RNA; the nasoturbinates, maxilloturbinates, and the anterior two-thirds of the nasal septum were used for isolation of respiratory RNA (see Wetmore et al., 1999 for a diagram of these regions of the rodent nasal cavity). Tissues were immediately frozen on dry ice and stored at −80°C until RNA isolation. RNA was isolated using Tri Reagent (Molecular Research Center, Cincinnati, OH) or RNAzol B reagent (Tel-Test, Inc., Friendswood, TX) according to the manufacturers' protocols. Sufficient liver, kidney, olfactory, and respiratory tissues were obtained from each of the four rats to isolate RNA from individual rats, whereas olfactory bulbs were pooled from two rats for preparation of two olfactory bulb RNA samples. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm. RNA integrity (relative ratio of 28S and 18S rRNA bands) was analyzed by formaldehyde-agarose gel electrophoresis with ethidium bromide staining.
Gene Expression Analysis.
Specific oligonucleotide probes for organic cation transporter (OCT)1, 2, and 3; multidrug resistance protein (MRP)1 and 4; OCTN1; organic anion-transporting polypeptide (OATP)3; and ENT1 and ENT2 were diluted in lysis buffer supplied in the Quantigene HV Signal Amplification Kit (Panomics, Inc., Freemont, CA) as previously reported (Cherrington et al., 2004). Total RNA (0.5 μg/μl; 10 μl) was added to each well of a 96-well plate containing capture hybridization buffer and 50 μl of each diluted probe set. Total RNA was hybridized to each probe set overnight at 53°C. Subsequent hybridizations were carried out per the manufacturer's protocol, and luminescence was measured with a Quantiplex 320 bDNA luminometer interfaced with Quantiplex Data Management Software version 5.02.
Immunohistochemistry.
Selected proteins were chosen for immunolocalization using these antibodies: ENT1 (Santa Cruz K-18; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); ENT2 (Santa Cruz K-14); OCT2 (Santa Cruz C-13); OCTN1/2/3 (Santa Cruz H-130); MRP1 (Santa Cruz H-70); and OATP3 (Dr. Paul Dawson, Wake Forest University, Winston-Salem, NC). Antibodies were applied to either deparaffinized-archived control rat nasal cavity sections (decalcified in 10% formic acid) or to freshly cut cryosections of control rat nasal cavity (decalcified in 0.1 M EDTA, pH 7.4). Immunolocalization was detected using species-appropriate horseradish peroxidase-conjugated secondary antibodies (Dako, Carpinteria, CA), followed by tyramide signal amplification (PerkinElmer Life and Analytical Sciences, Waltham, MA), and application of aminoethylcarbazole as chromagen (Genter et al., 2002). Selected sections were subjected to antigen retrieval [sections were microwaved in 0.01 M citric acid (pH 5.2) for 16 min, cooled to room temperature, and rinsed in 10 mM phosphate-buffered saline before antibody application]. Immunostained sections were counterstained with hematoxylin and photographed with a Nikon D80 camera (Nikon, Tokyo, Japan). Images were opened and optimized using Irfanview software (http://www.irfanview.com/).
In Silico Evaluation of Subcellular Localization.
Because of the pattern of immunolocalization observed for OCT2 and MRP1 in nasal respiratory mucosa, in silico analysis was undertaken to ask whether OCT2 is likely to be a secreted protein, and whether the MRP1 protein sequence contains a nuclear localization signal. To do this, the respective rat protein sequences were obtained from Uniprot (http://www.uniprot.org) and queried in either SignalP to ascertain the likelihood that OCT2 is secreted (http://www.cbs.dtu.dk/services/SignalP) or the PredictNLS (http://cubic.bioc.columbia.edu/services/predictNLS/) to identify putative nuclear localization sequences.
Results and Discussion
Gene expression studies showed that several of the transporters evaluated were expressed in nasal epithelia and/or OB at levels comparable to or exceeding those in liver or kidney. OCT1, OCT3, and OCTN1 were not appreciably expressed in nasal epithelia or olfactory bulb and therefore were not immunolocalized.
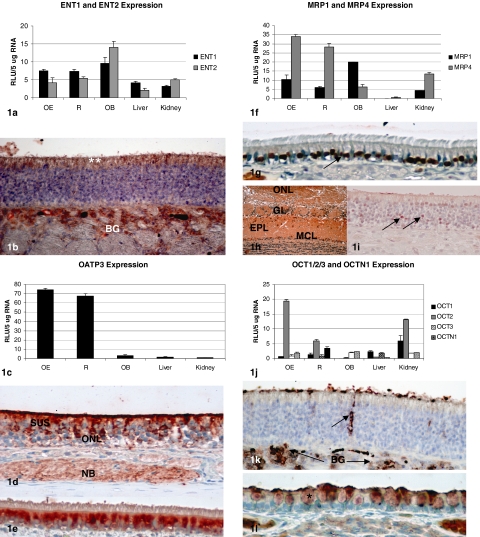
ENT1 and ENT2 expression in nasal epithelia and OB was comparable to the expression in liver and kidney. Immunolocalization with the respective antibodies revealed that both proteins are expressed along the apical surface of the olfactory epithelium (Fig. 1, a and b). Staining in respiratory epithelium was quite weak, but, interestingly, immunolocalization was found in acinar cells of subepithelial mucus glands (data not shown).
Fig. 1.
Gene expression [expressed as relative light units (RLU) per 5 μg of RNA] and immunolocalization (using aminoethylcarbazole as the chromagen) for transporters examined in rat nasal respiratory mucosa (R), olfactory mucosa (OE), OB, liver, and kidney. a, ENT1 and ENT2 expression as assessed by branched DNA analysis. b, immunohistochemical localization of ENT1 (data shown) and ENT2 was similar, with the most prominent at the apical surface of the olfactory epithelium (**) and in the subepithelial BG. Original magnification, 20×. Staining was negligible in respiratory epithelium, with some immunoreactivity noted in the acinar cells of the submucosal mucus glands (data not shown). c, OATP3 expression as assessed by branched DNA analysis. d, OATP3 is localized in the apical cytoplasm of sustentacular cells (SUS), the olfactory nerve layer (ONL), and in subepithelial nerve bundles (NB). Original magnification, 10×. e, immunohistochemistry reveals cytoplasmic localization of OATP3 in ciliated cells of the respiratory epithelium. Original magnification, 10×. f, MRP1 and MRP4 expression as assessed by branched DNA analysis. g, immunohistochemistry localized MRP1 expression to the nuclei in respiratory epithelial cells (arrow). Original magnification, 10×. h, diffuse MRP1 expression was found throughout the olfactory bulb, including the ONL, glomerular layer (GL), external plexiform layer (EPL), and mitral cell layer (MCL). Original magnification, 4×. i, MRP1 immunoreactivity was weakly detected in the olfactory epithelium, with the strongest immunoreactivity in occasional olfactory neuron nuclei (arrows). Original magnification, 20×. j, OCT1, OCT2, OCT3, and OCTN1 expression as assessed by branched DNA analysis. k, immunohistochemistry reveals OCT2 expression primarily in the ducts of BG in the olfactory epithelium (arrow) and in the acinar cells of BG. Original magnification, 10×. l, OCT2 immunohistochemistry reveals expression in the secretory granules (*) of respiratory epithelial cells. Original magnification, 10×.
Expression of OATP3 in respiratory and olfactory tissues were >50 times greater than in liver or kidney. OATP3 was detected by immunohistochemistry in the olfactory nerve layer in the epithelium, as well as in subepithelial nerve bundles, which contain the axons of the olfactory neurons that project into the nasal cavity. Robust staining in the cytoplasm of nasal respiratory epithelium was also observed (Fig. 1, c–e).
MRP1 expression in nasal epithelia was found to be comparable to that in kidney, with even higher expression in OB. MRP4 was likewise found to be expressed at two to three times higher level in nasal epithelia, compared to kidney, with lower but detectable expression in OB (Fig. 1f). MRP1 immunolocalization studies revealed nuclear staining in the nasal respiratory epithelium. Immunoreactivity was weak in olfactory epithelial sections, and MRP protein was found to be diffusely detectable in all layers of the OB (Fig. 1, g–i).
OCT2 localization and activity were previously described in bovine olfactory mucosa (Chemuturi and Donovan, 2007). In our studies, OCT2 expression in olfactory tissue was observed at approximately twice the level detected in kidney (Fig. 1j). Strong immunoreactivity was localized to the apical surface of the olfactory epithelium and in the ducts of Bowman's glands (BG), as well as in subepithelial BG (Fig. 1k). OCT2 was also detected in secretory granules in the respiratory epithelium (Fig. 1l).
Because we found OCT2 immunolocalization in the secretory granules of the nasal respiratory epithelium, as well as immunolocalization of MRP1 in the nuclei of the respiratory epithelium (and to a lesser extent, in nuclei of the olfactory epithelium), in silico analysis was undertaken to attempt to explain these observations. SignalP analysis of the rat OCT2 protein sequence revealed that OCT2 has a targeting sequence (between positions 33 and 34) that would predict that OCT2 could be secreted; when similarly evaluated, MRP1 (known to be an integral membrane protein), was identified as a nonsecreted protein. A nuclear localization sequence (NLS) was not found in MRP1 when analyzed using PredictNLS; the aryl hydrocarbon nuclear translocator protein sequence was used as a “positive control” for this analysis, and, as is known that NLS were found in the aryl hydrocarbon nuclear translocator sequence.
The transfer of drugs and toxicants from the nasal cavity to the brain and bloodstream can occur by several different mechanisms. The original goal of this work was to determine whether ENT1 and ENT2 (encoded by SLC29A1 and SLC29A2) are expressed in nasal epithelia, because substrates for these transporters, e.g., zidovudine and 2′,3′-didehydro-3′-deoxythymidine, are detected in CSF fluid after intranasal administration to rodents (Seki et al., 1994; Yajima et al., 1996). We found that ENT1 expression was higher in olfactory mucosa, respiratory mucosa, and OB than that in liver and kidney, and that ENT2 expression in nasal epithelia was comparable to that in liver and kidney, and 2- to 3-fold higher in OB. Immunohistochemical results showed that these proteins had essentially the same pattern of immunolocalization, namely at the apical surface of the olfactory epithelium, in the Bowman's glands beneath the olfactory epithelial basement membrane and in mucus glands under the respiratory epithelium.
The expression of OATP3 (encoded by SLC21A7 and involved in thyroid hormone transport) was detected at remarkably high levels in olfactory and nasal respiratory tissues. It is established that propylthiouracil-induced hypothyroidism in humans and rodents is associated with olfactory dysfunction and decreased olfactory neurogenesis (e.g., Paternostro and Meisami, 1996; Tong et al., 2007). Thus, the high OATP3 expression in the olfactory mucosa may be a reflection of the important role that thyroid hormone plays in olfactory function and homeostasis. OATP3 was detected in the apical cytoplasm of sustentacular cells, as well as in the mature olfactory neuron layer and in olfactory nerve bundles. This distribution suggests that OATP3 protein may participate in uptake and translocation of materials from the nasal airway into the brain. Although intestinal transport of thyroid hormone and bile salts has been attributed to OATP3, there is only limited data available on drugs that are transported by OATP3 (e.g., fexofenadine) (Kikuchi et al., 2006).
These studies extend previous observations to show that drug transporters are present in nasal respiratory and olfactory tissues as well as in OB. In contrast to the observations of other investigators, who detected OATP3 by immunohistochemistry in the mouse olfactory bulb (Ohtsuki et al., 2004), we did not detect immunostaining in the rat olfactory bulb, even when antigen retrieval was undertaken. Likewise, human MRP1 was immunolocalized in the cytoplasm in human nasal respiratory mucosa (Wioland et al., 2000), in contrast to the nuclear localization that we observed herein. These observations suggest that there may be significant interspecies differences in the expression and localization of these and other transporters.
Acknowledgments.
OATP3 antibody was provided by Dr. Paul A. Dawson (Wake Forest University, Winston-Salem, NC).
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK068039]; and the University of Cincinnati Dean's Bridge Funding Program and Center for Environmental Genetics.
Parts of this work were presented at the following meeting: Genter MB, Augustine LM, and Cherrington NJ (2010) Transporter expression in rat nasal olfactory and respiratory epithelia. Society of Toxicology Annual Meeting; 2010 Mar 10; Salt Lake City, UT. Society of Toxicology, Reston, VA.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.034611.
- CSF
- cerebral spinal fluid
- ENT
- equilibrative nucleoside transporters
- OB
- olfactory bulb
- OCT
- organic cation transporter
- OATP
- organic anion-transporting polypeptide
- MRP
- multidrug resistance protein
- BG
- Bowman's glands
- NLS
- nuclear localization sequence.
References
- Alanko L, Porkka-Heiskanen T, Soinila S. (2006) Localization of equilibrative nucleoside transporters in the rat brain. J Chem Neuroanat 31:162–168 [DOI] [PubMed] [Google Scholar]
- Chemuturi NV, Donovan MD. (2007) Role of organic cation transporters in dopamine uptake across olfactory and nasal respiratory tissues. Mol Pharm 4:936–942 [DOI] [PubMed] [Google Scholar]
- Cherrington NJ, Slitt AL, Li N, Klaassen CD. (2004) Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats. Drug Metab Dispos 32:734–741 [DOI] [PubMed] [Google Scholar]
- Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Roberts KC, Wong BA. (2002) Olfactory transport: a direct route of delivery of inhaled manganese phosphate to the rat brain. J Toxicol Environ Health A 65:1493–1511 [DOI] [PubMed] [Google Scholar]
- Govindarajan R, Leung GP, Zhou M, Tse CM, Wang J, Unadkat JD. (2009) Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am J Physiol Gastrointest Liver Physiol 296:G910–G922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genter MB, Burman DM, Vijayakumar S, Ebert CL, Aronow BJ. (2002) Genomic analysis of alachlor-induced oncogenesis in rat olfactory mucosa. Physiol Genomics 12:35–45 [DOI] [PubMed] [Google Scholar]
- Genter MB, Kendig EL, Knutson MD. (2009) Uptake of materials from the nasal cavity into the blood and brain: are we finally beginning to understand these processes at the molecular level? Ann NY Acad Sci 1170:623–628 [DOI] [PubMed] [Google Scholar]
- Jennings LL, Hao C, Cabrita MA, Vickers MF, Baldwin SA, Young JD, Cass CE. (2001) Distinct regional distribution of human equilibrative nucleoside transporter proteins 1 and 2 (hENT1 and hENT2) in the central nervous system. Neuropharmacology 40:722–731 [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Nozawa T, Wakasawa T, Maeda T, Tamai I. (2006) Transporter-mediated intestinal absorption of fexofenadine in rats. Drug Metab Pharmacokinet 21:308–314 [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Shiga H, Washiyama K, Ogawa D, Amano R, Ito M, Tsukatani T, Furukawa M, Miwa T. (2008) Thallium transport and the evaluation of olfactory nerve connectivity between the nasal cavity and olfactory bulb. Chem Senses 33:73–78 [DOI] [PubMed] [Google Scholar]
- Natsume H, Iwata S, Ohtake K, Miyamoto M, Yamaguchi M, Hosoya K, Kobayashi D, Sugibayashi K, Morimoto Y. (1999) Screening of cationic compounds as an absorption enhancer for nasal drug delivery. Int J Pharm 185:1–12 [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Takizawa T, Takanaga H, Hori S, Hosoya K, Terasaki T. (2004) Localization of organic anion transporting polypeptide 3 (oatp3) in mouse brain parenchymal and capillary endothelial cells. J Neurochem 90:743–749 [DOI] [PubMed] [Google Scholar]
- Paternostro MA, Meisami E. (1996) Essential role of thyroid hormones in maturation of olfactory receptor neurons: an immunocytochemical study of number and cytoarchitecture of OMP-positive cells in developing rats. Int J Dev Neurosci 14:867–880 [DOI] [PubMed] [Google Scholar]
- Seki T, Sato N, Hasegawa T, Kawaguchi T, Juni K. (1994) Nasal absorption of zidovudine and its transport to cerebrospinal fluid in rats. Biol Pharm Bull 17:1135–1137 [DOI] [PubMed] [Google Scholar]
- Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. (2007) Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J 21:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Chen GH, Liu RY, Zhou JN. (2007) Age-related learning and memory impairments in adult-onset hypothyroidism in Kunming mice. Physiol Behav 91:290–298 [DOI] [PubMed] [Google Scholar]
- Türker S, Onur E, Ozer Y. (2004) Nasal route and drug delivery systems. Pharm World Sci 26:137–142 [DOI] [PubMed] [Google Scholar]
- Varatharajan L, Thomas SA. (2009) The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res 82:A99–A109; erratum in Antiviral Res 2009 84:203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore BA, Mitchell AD, Meyer SA, Genter MB. (1999) Evidence for site-specific bioactivation of alachlor in the olfactory mucosa of the Long-Evans rat. Toxicol Sci 49:202–212 [DOI] [PubMed] [Google Scholar]
- Wioland MA, Fleury-Feith J, Corlieu P, Commo F, Monceaux G, Lacau-St-Guily J, Bernaudin JF. (2000) CFTR, MDR1, and MRP1 immunolocalization in normal human nasal respiratory mucosa. J Histochem Cytochem 48:1215–1222 [DOI] [PubMed] [Google Scholar]
- Yajima T, Hasegawa T, Juni K, Saneyoshi M, Kawaguchi T. (1996) Nasal absorption of 2′,3′-didehydro-3′-deoxythymidine (D4T) and its esters in rats. Biol Pharm Bull 19:1234–1237 [DOI] [PubMed] [Google Scholar]