Abstract
The plasma membrane monoamine transporter (PMAT) belongs to the equilibrative nucleoside transporter family (solute carrier 29) and was alternatively named equilibrative nucleoside transporter 4. Previous studies from our laboratory characterized PMAT as a polyspecific organic cation transporter that minimally interacts with nucleosides. Recently, PMAT-mediated uptake of adenosine (a purine nucleoside) was reported, and the transporter was proposed to function as a dual nucleoside/organic cation transporter. To clarify the substrate specificity of PMAT, we comprehensively analyzed the transport activity of human PMAT toward nucleosides, nucleobases, and organic cations in heterologous expression systems under well controlled conditions. Among 12 naturally occurring nucleosides and nucleobases, only adenosine was significantly transported by PMAT. PMAT-mediated adenosine transport is saturable, pH-dependent, and membrane-potential sensitive. Under both neutral (pH 7.4) and acidic (pH 6.6) conditions, adenosine is transported by PMAT at an efficiency (Vmax/Km) at least 10-fold lower than that of the organic cation substrates 1-methyl-4-phenylpyridinium and serotonin. PMAT-mediated adenosine uptake rate was significantly enhanced by an acidic extracellular pH. However, the effect of acidic pH was not adenosine-specific but was common to organic cation substrates as well. Our results demonstrated that although PMAT transports adenosine, the transporter kinetically prefers organic cation substrates. Functionally, PMAT should be viewed as a polyspecific organic cation transporter rather than an archetypical nucleoside transporter.
Introduction
Endogenous nucleosides and nucleobases are important precursors of nucleic acid synthesis in mammalian cells. The purine nucleoside adenosine is also an important signaling molecule that regulates a variety of physiological processes via binding to cell surface adenosine receptors (Olah and Stiles, 1992; Sebastiao and Ribeiro, 2009). Uptake of adenosine and other nucleosides into mammalian cells is mediated by specific membrane transporters. These transporters play an important role in regulating adenosine signaling by controlling its extracellular concentrations at the receptor sites (Griffith and Jarvis, 1996; Kong et al., 2004; Young et al., 2008). Two distinct transporter families are involved in nucleoside uptake. The concentrative nucleoside transporters are encoded by genes in the solute carrier 28 (SLC28) family and mediate Na+-dependent nucleoside transport. Concentrative nucleoside transporters are predominantly found in epithelial cells such as those in kidney, liver, and intestine (Gray et al., 2004; Kong et al., 2004). The equilibrative nucleoside transporters (ENTs) are encoded by genes in the SLC29 family, which contains four isoforms, ENT1 to 4. ENT1 (SLC29A1) and ENT2 (SLC29A2) broadly transport purine and pyrimidine nucleosides via Na+-independent facilitated diffusion. ENT2, but not ENT1, is also able to transport nucleobases. ENT1 and ENT2 can be functionally differentiated by their sensitivity to classic inhibitors such as nitrobenzylmercaptopurine ribonucleoside (NBMPR), dipyridamole, and dilazep. ENT1 is 2 to 4 orders of magnitude more sensitive to these inhibitors than ENT2 (Baldwin et al., 2004; Kong et al., 2004). The third isoform, ENT3 (SLC29A3) also has broad substrate selectivity for nucleosides and nucleobases and may function as an intracellular membrane transporter (Baldwin et al., 2005; Govindarajan et al., 2009). Among the nucleoside transporters, ENT1 is ubiquitously expressed in mammalian cells and represents the most important transporter in regulating adenosine concentrations at its receptor sites (Baldwin et al., 2004; Kong et al., 2004).
The fourth isoform in the ENT family, PMAT (or ENT4, SLC29A4), was first cloned and characterized in our laboratory in 2004 (Engel et al., 2004). In humans, PMAT is broadly expressed in several tissues but is most strongly expressed in the brain (Engel et al., 2004). Using heterologous expression systems, we first demonstrated that PMAT is a plasma membrane transporter and transports monoamine neurotransmitters (e.g., serotonin, dopamine, and norepinephrine) with minimal interactions with nucleosides. We thus named the transporter plasma membrane monoamine transporter (PMAT). Subsequent studies in our laboratory further demonstrated that PMAT shares a large substrate overlap with the organic cation transporters (Wright and Dantzler, 2004; Fujita et al., 2006; Koepsell et al., 2007), transporting a wide array of structurally diversified organic cations including biogenic amines, 1-methyl-4-phenylpyridinium (MPP+), tetraethylammonium, and metformin (Engel and Wang, 2005; Zhou et al., 2007c). Therefore, we have proposed that PMAT functions as a polyspecific organic cation transporter, which may play a role in monoamine clearance and transport of cationic drugs and toxins in vivo (Engel et al., 2004; Engel and Wang, 2005; Zhou et al., 2007c).
Using Xenopus oocytes expressing PMAT, Barnes et al. (2006) reported that PMAT was a pH-activated adenosine transporter, which avidly transports adenosine under acidic pH. These investigators thus proposed that PMAT (ENT4) was a dual nucleoside/organic cation transporter (Barnes et al., 2006; Young et al., 2008). Because the study of Barnes et al. (2006) was mostly conducted at an extremely acidic pH (pH 5.5), the relevance of PMAT to nucleoside uptake versus organic cation uptake at physiological conditions remains unclear. To clarify the substrate specificity of PMAT, we comprehensively analyzed the transport activity of PMAT toward nucleosides, nucleobases, and organic cations in heterologous expression systems under well controlled conditions. In particular, we investigated the interaction of PMAT with adenosine under physiologically relevant conditions. The efficiency of PMAT-mediated adenosine transport was compared with that of organic cations side by side. The in vivo significance of PMAT in adenosine transport was also explored by comparing its expression with ENT1 and ENT2 in physiological sites of relevance.
Materials and Methods
Materials.
[3H]Adenosine (30 Ci/mmol), [3H]uridine (30 Ci/mmol), and [3H]MPP+ (80 Ci/mmol) were purchased from American Radiolabeled Chemicals (St. Louis, MO). [3H]Adenine (43 Ci/mmol), [3H]uracil (43 Ci/mmol), [14C]guanosine (40Ci/mmol), [14C]inosine (34 Ci/mmol), [3H]cytidine (25 Ci/mmol), [3H]thymidine (30 Ci/mmol), [3H]guanine (50 Ci/mmol), [3H]thymine (106 Ci/mmol), [3H]hypoxanthine (27 Ci/mmol), and [3H]cytosine (17 Ci/mmol) were obtained from Moravek Biochemicals (Brea, CA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Stable Expression of Human PMAT in MDCK Cells.
PMAT cDNA was previously cloned from a human kidney cDNA library, subcloned into the pcDNA3 vector (Invitrogen, Carlsbad, CA), and transfected into MDCK II cells by liposome-mediated transfection (Lipofectamine; Invitrogen) (Engel et al., 2004). A stable cell line of a single colony origin was obtained by G418 selection and used in this study. PMAT- and vector-transfected MDCK cells were cultured in minimal essential medium containing 10% fetal bovine serum and 200 μg of G418/ml medium.
Uptake Assay in MDCK Cells.
Cells were plated in 24-well plates and allowed to grow at 37°C for 3 days. Growth medium was aspirated, and each well was rinsed with Krebs-Ringer-Henseleit (KRH) buffer (5.6 mM glucose, 125 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgSO4, and 25 mM HEPES, pH 7.4) and then preincubated in KRH buffer for 15 min at 37°C. Transport assays were performed at 37°C by incubating cells in KRH buffer containing an 3H-labeled ligand. For transport studies using 14C- or 3H-labeled nucleosides, 0.5 μM NBMPR was added to the transport buffer to suppress endogenous nucleoside uptake activities. After incubation, uptake was terminated by aspirating the reaction mixture and washing the cells three times with ice-cold KRH buffer. Cells were then solubilized with 0.5 ml of 1 N NaOH and neutralized with 0.5 ml of 1 N HCl. A portion of the lysate (0.5 ml) was quantified by liquid scintillation counting, and 25 μl was used for the protein assay. For nucleoside inhibition studies, cells were preincubated with KRH with no inhibitors and then incubated with [3H]MPP+ in the presence of various nucleosides. Cells were then rinsed three times with ice-cold KRH buffer, and samples were assayed as described above.
PMAT Expression in Xenopus laevis Oocytes.
PMAT cRNA was synthesized in vitro using a method described previously (Zhou et al., 2007a). The purity and integrity of the cRNA were verified by RNase-free agarose gel electrophoresis. Oocytes were harvested from X. laevis (Nasco, Fort Atkinson, WI) and defolliculated with collagenase D. Healthy stage V and VI oocytes were injected with either 50 nl of cRNA (0.8 μg/μl) or water (control) using an automatic nanoliter injector Nanoject II (Drummond, Broomall, PA). Injected oocytes were maintained in a modified Barth's medium [88 mM NaCl, 1 mM KCl, 0.82 mM MgSO4, 0.4 mM CaCl2, 0.33 mM Ca(NO3)2, 2.4 mM NaHCO3 and 10 mM HEPES/Tris, pH 7.4] at 18°C for 2 to 3 days. Uptake assays were performed at 25°C in transport buffer (100 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES, pH 7.4). Oocytes were washed with 2 ml of room temperature transport buffer and incubated in 250 μl of transport buffer containing an 3H-labeled ligand for 20 to 60 min. At the end of the incubation, uptake was terminated by removing the incubation medium. Oocytes were then rapidly washed five times with 3 ml of ice-cold transport buffer. Individual oocytes were solubilized in 10% SDS, and radioactivity was quantified by liquid scintillation counting.
TaqMan Real-Time RT-PCR Quantification of mRNA Transcripts.
Total RNA were extracted from PMAT-expressing MDCK cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Human brain and skeletal muscle total RNA were purchased from Clontech (Mountain View, CA). Total RNA (4 μg) were reverse-transcribed to first-strand cDNA using SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's protocol. TaqMan real-time PCR reagents, supplies, assay primers, and probes for human PMAT (hENT4, SLC29A4), hENT1 (SLC29A1), hENT2 (SLC29A2), and hGUSB (β-glucuronidase) were purchased from Applied Biosystems (Foster City, CA). The primers are designed to span adjacent exons so that genomic DNA will not be amplified. TaqMan real-time PCR reactions were set up and run according to the manufacturer's protocols on an Applied Biosystems 7900HT Fast Real-Time PCR System. Fifty nanograms of cDNA were used per well in a total volume of 25 μl on a 96-well clear-top PCR plate. All samples were run in triplicate. For absolute quantification of PMAT mRNA transcript numbers, the standard curve was generated using serial dilutions of expression vector with known copy numbers. To compare the relative mRNA expression levels of PMAT, hENT1, and hENT2 in human brain and skeletal muscles, hGUSB was selected as the reference gene because previous reports suggested that its expression level is more stable than that of other commonly used housekeeping genes such as β-actin and glyceraldehyde-3-phosphate dehydrogenase (Fink et al., 2008; Romanowski et al., 2008). Absolute or relative amounts for each cDNA were calculated by plotting log(amount) against Ct (threshold cycle) values on a semilogarithmic plot. A linear relationship between log(amount) and Ct was indicated by each standard curve.
Data Analysis.
Uptake experiments were performed in triplicate and repeated two to four times. Studies in Xenopus oocytes were performed on a group of 8 to 10 oocytes for each data point. Data are expressed as means ± S.D. Statistical significance was determined by Student's t test, and kinetic parameters were determined by nonlinear least-squares regression fitting as described previously (Engel and Wang, 2005).
Results
PMAT Sensitivity to Classic Nucleoside Transport Inhibitors.
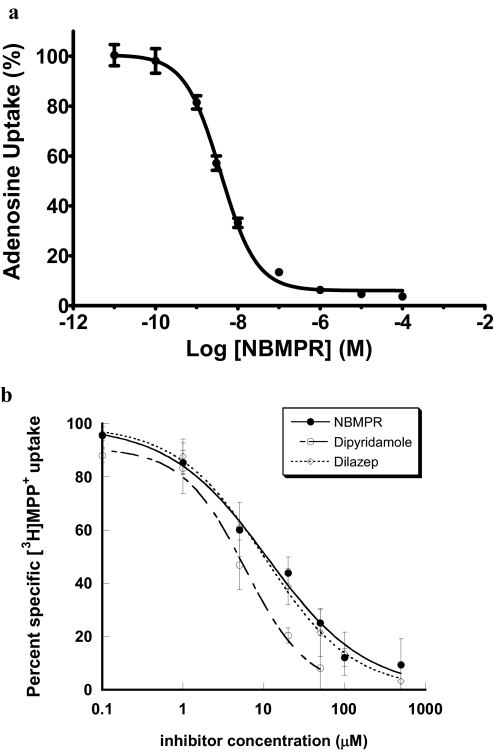
In our original study, we did not observe significant transport of nucleosides by PMAT (Engel et al., 2004). Because equilibrative nucleoside transporters, especially ENT1, are ubiquitously expressed in mammalian cells, it is possible that PMAT-mediated nucleoside transport activity may be masked by high endogenous uptake activity. Hammond et al. (2004) previously showed that MDCK cells contain a single class of high-affinity NBMPR binding sites and only express the canine ENT1 isoform. To confirm these results, we first examined concentration-dependent inhibition of NBMPR on endogenous adenosine uptake in vector-transfected MDCK cells. As shown in Fig. 1a, a monophasic inhibition pattern was observed across a wide NBMPR concentration range (0–100 μM). The fitted IC50 value was 3.4 ± 0.6 nM, which is similar to the IC50 value (2.9 ± 0.6 nM) reported previously (Hammond et al., 2004). The inhibition started to reach the maximum at approximately 0.1 μM, and further increasing the NBMPR concentration had little added effect on total adenosine uptake. At the maximal inhibition, the NBMPR noninhibitable baseline was only approximately 6% of the total adenosine uptake and probably represents nonspecific binding. These data further confirmed that MDCK cells predominantly express ENT1 activity, and it might be feasible to use classic ENT1 inhibitors to suppress endogenous nucleoside uptake and unmask PMAT-mediated nucleoside transport activity in the MDCK expression system. To identify a safe concentration window, we then examined the interaction of PMAT with classic nucleoside transport inhibitors NBMPR, dipyridamole, and dilazep using MDCK cells stably expressing human PMAT. MPP+, a prototype organic cation substrate of PMAT, was used as the probe substrate because it is not metabolized and has low background uptake in MDCK cells (Engel and Wang, 2005). As shown in Fig. 1b, NBMPR, dipyridamole, and dilazep inhibited PMAT-mediated MPP+ uptake with Ki values of 11.1, 5.9, and 10.2 μM, respectively. These Ki values are 3 to 4 orders of magnitude greater than those reported for ENT1 (Ward et al., 2000; Visser et al., 2002; Baldwin et al., 2005) (Table 1). Among the three ENT1 inhibitors, NBMPR demonstrated the highest differential sensitivity toward ENT1 and PMAT; we therefore chose to use 0.5 μM NBMPR to suppress endogenous nucleoside uptake activities.
Fig. 1.
a, concentration-dependent inhibition of endogenous adenosine uptake by NBMPR in vector-transfected MDCK cells. Cells were incubated at 37°C with [3H]adenosine (1 μM) for 2 min in the presence of NBMPR at the indicated concentrations. b, concentration-dependent inhibition of PMAT-mediated MPP+ uptake by classic nucleoside transport inhibitors. PMAT-expressing and vector-transfected MDCK cells were incubated at 37°C with 0.1 μM [3H]MPP+ for 1 min in the presence of inhibitors at indicated concentrations. Each data point represents PMAT-specific uptake, calculated by subtracting the uptake in vector-transfected cells at various inhibitor concentrations from the corresponding uptake in PMAT-expressing cells. Each value represents the mean ± S.D. (n = 3).
TABLE 1.
Potencies of nucleoside transport inhibitors on human ENTs and PMAT
Values are given as means ± S.D. (n = 3). IC50 values are shown in italics.
| Protein |
Ki or IC50 |
||
|---|---|---|---|
| NBMPR | Dipyridamole | Dilazep | |
| nM | |||
| hENT1 | 0.4 ± 0.1a | 5.0 ± 0.9a | 18.7 ± 2.0b |
| hENT2 | 2800 ± 300a | 356 ± 13a | 134,000 ± 40,000b |
| hENT3 | >10,000c | >1000c | >1000c |
| PMAT | 11,074 ± 2800 | 5901 ± 900 | 10,236 ± 1100 |
IC50 values from Ward et al. (2000).
Ki values from Visser et al. (2002).
Data from Baldwin et al. (2005).
Nucleoside and Nucleobase Uptake by PMAT.
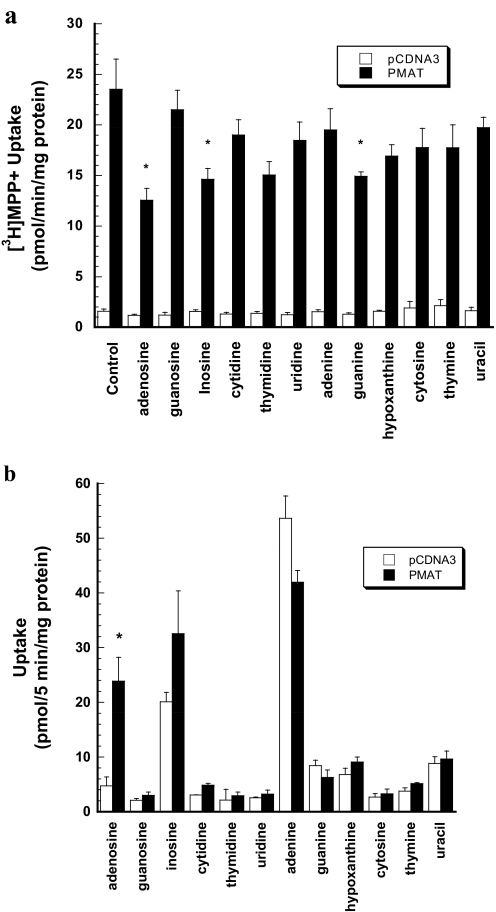
As a first step to analyze PMAT interaction with nucleosides and nucleobases, we examined the effect of 1 mM concentrations of naturally occurring nucleosides (adenosine, guanosine, inosine, cytidine, thymidine, and uridine) and nucleobases (adenine, guanine, hypoxanthine, cytosine, thymine, and uracil) on PMAT-mediated MPP+ (1 μM) uptake. Only adenosine, inosine, and guanine significantly inhibited PMAT-mediated MPP+ uptake (Fig. 2a). To determine whether nucleosides and nucleobases are substrates of PMAT, uptakes of 3H- or 14C-labeled nucleosides and nucleobases were determined at pH 7.4 in the presence of 0.5 μM NBMPR. Compared with vector-transfected cells, a 5-fold increase in adenosine uptake was observed in PMAT-expressing cells during a 5-min incubation time (Fig. 2b). There was no significant uptake for other nucleosides. None of the naturally occurring nucleobases showed PMAT-specific uptake. Background uptake for inosine and adenine was relatively high; however, there was no statistical difference between PMAT-expressing and vector-transfected control cells.
Fig. 2.
a, effect of naturally occurring nucleosides and nucleobases (1 mM) on PMAT-mediated [3H]MPP+ uptake in MDCK cells. Vector-transfected and PMAT-expressing MDCK cells were incubated with 1 μM [3H]MPP+ for 1 min at 37°C in the presence of various nucleosides and nucleobases at 1 mM. Each bar represents the mean ± S.D. (n = 3). *, p < 0.01, significantly different from the control cells with no inhibitor treatment. b, uptake of 3H- or 14C-labeled nucleosides and nucleobases. Vector-transfected and PMAT-expressing MDCK cells were incubated with 1 μM 3H- or 14C-labeled nucleosides and nucleobases for 5 min at 37°C in the presence of 0.5 μM NBMPR. Each bar represents the mean ± S.D. (n = 3). *, p < 0.01, significantly different from vector-transfected cells.
Effect of MPP+ and Decynium-22 on PMAT-Mediated Adenosine Uptake.
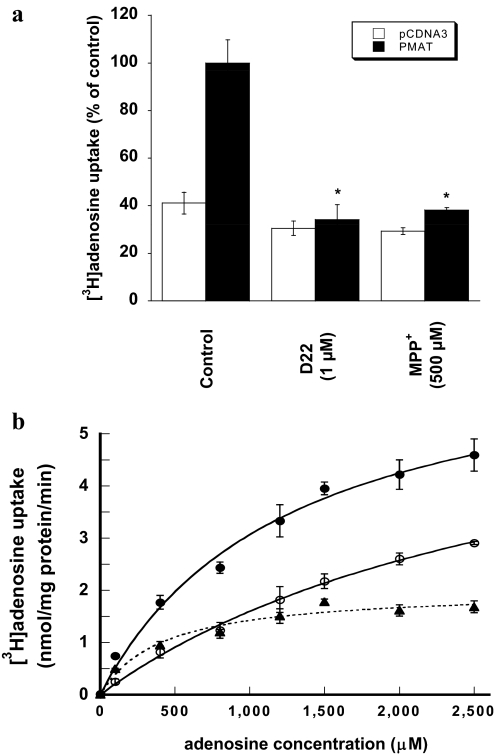
To further confirm that PMAT mediates adenosine transport, we examined the effect of MPP+ and decynium-22 on adenosine uptake in vector- and PMAT-transfected MDCK cells. MPP+ is a prototype organic cation substrate efficiently transported by PMAT, whereas decynium-22 is a well established high-affinity inhibitor for PMAT (Ki = 0.1 μM) (Engel et al., 2004; Engel and Wang, 2005). NBMPR was included in uptake buffer to suppress endogenous nucleoside transporter activity. MPP+ (500 μM) and decynium-22 (1 μM) almost completely abolished adenosine uptake in PMAT-expressing MDCK cells (Fig. 3a) but had no effect on baseline uptake in vector-transfected cells, suggesting that the observed adenosine uptake in PMAT-transfected cells is specifically mediated by the heterologously expressed PMAT transporter.
Fig. 3.
a, effect of decynium-22 (D22) and MPP+ on [3H]adenosine uptake by PMAT-expressing MDCK cells. PMAT- and vector-transfected cells were incubated with 1 μM [3H]adenosine at 37°C in the absence (control) or presence of decynium-22 (1 μM) or MPP+ (500 μM). NBMPR (0.5 μM) was included in the uptake buffer to suppress endogenous nucleoside transporter activity. Each bar represents the mean ± S.D. (n = 3). *, p < 0.001, significantly different from control PMAT-transfected cells. b, concentration-dependent adenosine uptake by PMAT at pH 7.4. PMAT-transfected cells and vector-transfected cells were incubated with various concentrations of adenosine for 1 min at 37°C in the presence of 0.5 μM NBMPR. PMAT-specific uptake (▴– – –▴) was calculated by subtracting the transport activity of vector-transfected cells (○——○) from that of PMAT-transfected cells (●——●).
Adenosine Transport Kinetics.
Concentration-dependent uptake was measured to determine the kinetic property of PMAT toward adenosine at pH 7.4. PMAT-mediated adenosine uptake was saturable with an apparent Km of 413 ± 107 μM and a maximal velocity (Vmax) of 2013 ± 140 pmol/(min · mg) protein (Fig. 3b). The apparent affinity of PMAT toward adenosine is significantly lower than those of hENT1 (Km = 40 μM) and hENT2 (Km = 140 μM) previously determined in PK15 cell expression system (Ward et al., 2000). The apparent efficiency (Vmax/Km) of PMAT-mediated adenosine transport was 4.9 μl/(min · mg) protein, which is 12- and 17-fold lower than the Vmax/Km values for MPP+ and serotonin previously determined in the same cell line (Table 2).
TABLE 2.
Comparison of adenosine transport kinetics with organic cation substrates
Values are given as means ± S.D. (n = 3). All values were determined in the same MDCK cell line stably expressing human PMAT. For organic cations, kinetic data were taken from Engel and Wang (2005).
| Substrate | Km | Vmax | Vmax/Km |
|---|---|---|---|
| μM | pmol/(min · mg) protein | μl/(min · mg) protein | |
| MPP+ | 33 ± 7 | 2800 ± 116 | 85 |
| Serotonin | 114 ± 12 | 6524 ± 197 | 57 |
| Dopamine | 329 ± 8 | 18,222 ± 168 | 55 |
| Tyramine | 283 ± 23 | 5055 ± 147 | 18 |
| Histamine | 10,471 ± 2250 | 99,610 ± 17,299 | 9.5 |
| Norepinephrine | 2606 ± 258 | 20,561 ± 902 | 7.9 |
| Adenosine | 413 ± 107 | 2013 ± 140 | 4.9 |
| Epinephrine | 15,323 ± 3947 | 38,442 ± 7705 | 2.5 |
| Tetraethylammonium | 6593 ± 1702 | 5827 ± 918 | 0.9 |
Comparison of Adenosine and Organic Cation Transport by PMAT in MDCK Cells.
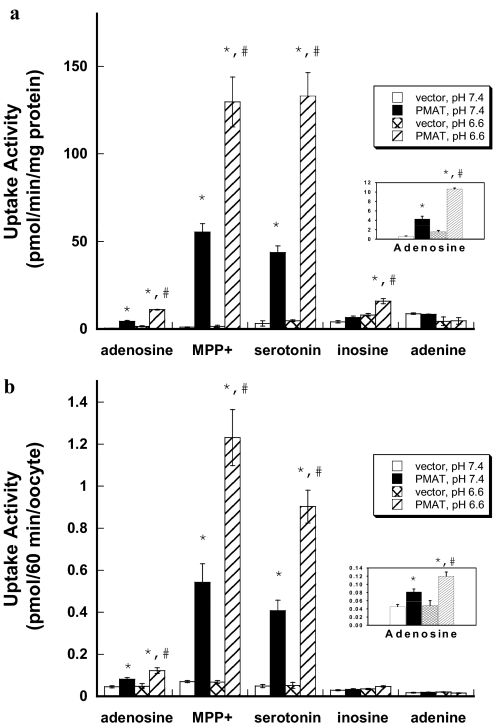
To directly compare PMAT transport activity toward adenosine and organic cation substrates in the same experiment, we conducted uptake studies (1 min) of adenosine, MPP+, and serotonin side by side in PMAT- and vector-transfected MDCK cells in the presence of 0.5 μM NBMPR under both normal (pH 7.4) and acidic (pH 6.6) conditions. All compounds were used at 1 μM, which is much lower than their Km values toward PMAT (Table 2). Under these conditions (i.e., [S] ≪ Km), the rate of uptake, determined by V = Vmax/Km × [S], directly reflects the apparent transport efficiency (Vmax/Km). At pH 7.4, PMAT-expressing cells showed significantly enhanced uptake for adenosine, MPP+, and serotonin (Fig. 4a). Consistent with the 12- and 17- fold differences in the transport efficiency (Table 2), PMAT-mediated MPP+ and serotonin uptake was 11 and 14 times higher than that of adenosine. Under acidic conditions (pH 6.6), PMAT-mediated adenosine uptake is enhanced by 2.5-fold (Fig. 4a). However, a similar magnitude of increase was also observed for the cationic substrates MPP+ (∼2-fold) and serotonin (∼3-fold), resulting in an equally higher (11- and 16-fold) uptake of organic cations at pH 6.6. No statistically significant PMAT-mediated uptake was observed for adenine under either neutral or acidic pH. For inosine, PMAT-mediated uptake was insignificant at pH 7.4 and only reached marginal significance at pH 6.6.
Fig. 4.
a, effect of extracellular pH on PMAT-mediated uptake in MDCK cells. Vector-transfected and PMAT-expressing MDCK cells were incubated with 1 μM concentrations of various 3H-labeled compounds for 1 min at 37°C in the presence of NBMPR (0.5 μM). Each value represents the mean ± S.D. (n = 3). b, effect of extracellular pH on PMAT-mediated uptake in Xenopus oocytes. Water- or PMAT cRNA-injected oocytes were incubated with 1 μM concentrations of various 3H-labeled compounds for 60 min at 25°C in the presence of NBMPR (0.5 μM). Each value represents the mean ± S.E. (n = 8–10). *, p < 0.01, significantly different from vector-transfected cells. #, p < 0.01, significantly different from PMAT activity measured at pH 7.4.
Comparison of Adenosine and Organic Cation Transport by PMAT Expressed in Xenopus Oocytes.
Our study in PMAT-expressing MDCK cells showed that the organic cation substrates MPP+ and serotonin are transported at much higher efficiencies by PMAT at both pH 6.6 and 7.4. Furthermore, the stimulatory effect of acidic pH on PMAT activity is not adenosine-specific as the rates of PMAT-mediated MPP+ and serotonin uptake were also increased at similar magnitudes at acidic pH. These findings are different from those of Barnes et al. (2006), who reported that the pH effect was adenosine-specific. To explore whether the difference was due to the use of different expression systems, we expressed PMAT in Xenopus oocytes, the system used in the study by Barnes et al. (2006). Uptake of nucleosides and organic cations was performed side by side in the same experiment using the same batch of oocytes. All substrates were used at 1 μM and incubated for 60 min with water- or PMAT cRNA-injected oocytes in the presence of 0.5 μM NBMPR. Similar to results obtained in MDCK cells, PMAT-specific uptake was much greater for MPP+ and serotonin than for adenosine at both pH 7.4 and 6.6 (Fig. 4b). Lowering extracellular pH from 7.4 to 6.6 had a similar stimulatory effect on the transport of all three substrates. Endogenous uptake of adenine and inosine was lower in oocytes than in MDCK cells; however, no significant PMAT-mediated uptake was observed at pH 7.4 or 6.6. Taken together, our results suggest that PMAT transports organic cations (e.g., MPP+ and serotonin) at much higher efficiencies than adenosine at both neutral and acidic conditions (pH 6.6). The stimulatory effect of PMAT by acidic pH is not adenosine-specific but rather is characteristic of all substrates tested.
Effect of Membrane Potential on PMAT-Mediated Adenosine Transport.
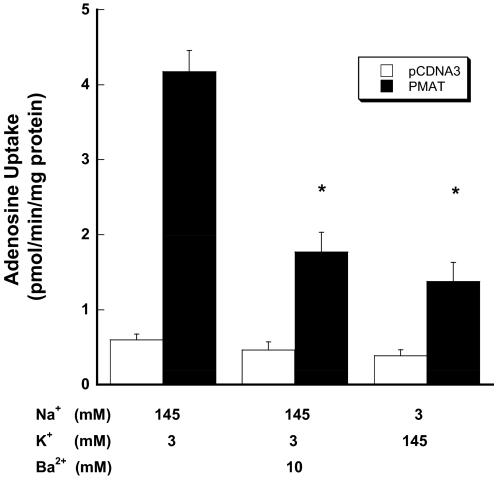
We previously showed that PMAT-mediated organic cation transport is sensitive to changes in membrane potential (Engel et al., 2004). To examine whether PMAT-mediated adenosine transport is affected by membrane potential, uptake was measured under various depolarization conditions at pH 7.4 in the presence of 0.5 μM NBMPR. Depolarization of cells with increased extracellular K+ strongly reduced PMAT-mediated adenosine uptake (Fig. 5). Barium, a potent potassium channel blocker, also substantially reduced PMAT-mediated adenosine uptake. These data suggest that PMAT-mediated adenosine transport is electrogenic and is favored by the physiological inside negative membrane potential.
Fig. 5.
Influence of membrane potential on PMAT-mediated adenosine uptake. Vector-transfected and PMAT-expressing cells were incubated with [3H]adenosine (1 μM) for 1 min at 37°C in the presence of 0.5 μM NBMPR. Ba2+ was added to block the potassium channels. To avoid precipitation of barium by sulfate and phosphate in the KRH buffer, a chloride salt-based buffer (5 mM glucose, 145 mM NaCl, 3 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, and 5 mM HEPES, pH 7.4) with different compositions of potassium and sodium was used. Each value represents mean ± S.D. (n = 3). *, p < 0.01, significantly different from activity tested in PMAT-transfected cells under normal physiological conditions.
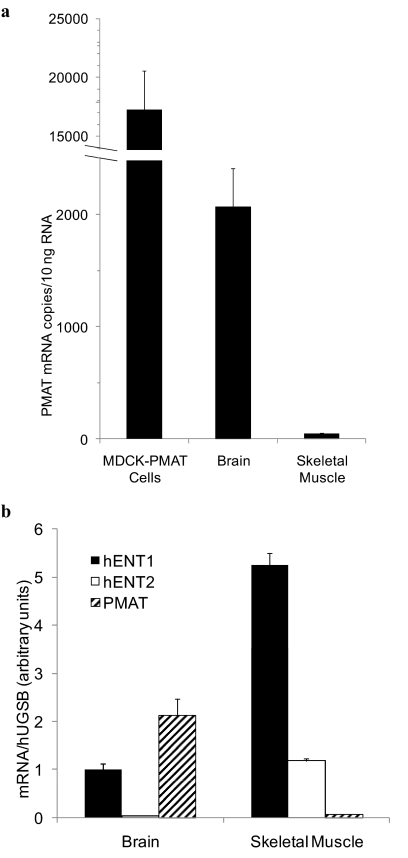
PMAT Expression Levels in Transfected-MDCK Cells and Human Brain and Skeletal Muscle.
The above studies were conducted in MDCK cells overexpressing human PMAT. To explore the significance of PMAT in adenosine transport in a physiologically relevant context, we quantified and compared PMAT mRNA expression levels in the overexpression system with those found in human brain and skeletal muscle by real-time RT-PCR. Compared with the PMAT-transfected MDCK cells, PMAT mRNA copy numbers per 10 ng of total RNA were only approximately 8-fold lower (Fig. 6a), consistent with our previous finding that PMAT is highly expressed in the brain (Engel et al., 2004; Dahlin et al., 2007). PMAT transcripts in the skeletal muscle were 40-fold lower than those in the brain and 340-fold lower than those in the stably transfected MDCK cells (Fig. 6a). To evaluate the relevance of PMAT in adenosine transport in the presence of major nucleoside transporters ENT1 and ENT2, we also determined the relative expression levels of PMAT, hENT1, and hENT2 in human brain and skeletal muscle (Fig. 6b). In the brain, PMAT and hENT1 were expressed at much higher levels than hENT2, with PMAT expression being approximately 2-fold higher than that of hENT1. In the skeletal muscle, hENT1 was highly expressed, and its expression was 4.4-fold higher than that of hENT2 and 66-fold higher than that of PMAT.
Fig. 6.
a, expression levels of PMAT transcripts in transfected MDCK-PMAT cells compared with human brain and skeletal muscle tissues as determined by real-time RT-PCR. b, relative expression levels of hENT1, hENT2, and PMAT transcripts normalized to hGUSB in human brain and skeletal muscle. Total RNA was extracted from PMAT-transfected MDCK cells or purchased from commercial sources (human brain and skeletal muscle). Real-time RT-PCR was used to quantify expression levels of each gene. Data represent the mean ± S.D. (n = 3).
Discussion
Studies in our laboratory previously demonstrated that PMAT functions as an organic cation but not as a nucleoside transporter (Engel et al., 2004). However, recent reports classified this transporter as a dual nucleoside/organic cation transporter (Barnes et al., 2006; Young et al., 2008). To clarify the biological function of PMAT, we reinvestigated its substrate specificity by focusing on its interaction with nucleosides/nucleobases and by comparing the relative transport efficiency of PMAT in transporting organic cations and nucleoside substrates.
We first confirmed that MDCK cells predominantly express ENT1 activity, which can be effectively suppressed by low concentrations of NBMPR (Fig. 1a). We then analyzed the interaction of PMAT with classic ENT1 inhibitors with the goal of identifying a concentration window to effectively suppress endogenous ENT1 without affecting PMAT. Our data revealed that PMAT is 3 to 4 orders of magnitude more resistant to NBMPR, dipyridamole, and dilazep than hENT1 (Table 1; Fig. 1b). For example, the Ki values of NBMPR are 0.4 and 11,074 nM toward hENT1 and PMAT, respectively. Because endogenous adenosine uptake in MDCK cells was almost fully inhibited by NBMPR at concentrations greater than 0.1 μM (Fig. 1a), we chose to use 0.5 μM NBMPR to suppress endogenous uptake in all adenosine transport studies in MDCK cells. At this concentration, NBMPR suppressed 94% of total endogenous adenosine uptake (Fig. 1a) with a negligible effect on PMAT (Fig. 1b). A cross-comparison with other hENT isoforms (Table 1) also revealed that PMAT, like ENT2, has low-affinity interaction with classic ENT inhibitors, although a subtler isoform-dependent difference was observed toward specific inhibitors. PMAT displays similar affinities toward NBMPR, dipyridamole, and dilazep, whereas hENT2 exhibits differential sensitivities toward these inhibitors. The nature of the low-affinity interaction between classic ENT inhibitors and PMAT is unclear. Our recent structure-function analysis studies suggest that PMAT and the ENTs share a similar protein organization, despite their marked differences in substrate specificity (Zhou et al., 2007b). It is possible that the low-affinity NBMPR binding site in PMAT is similar to that in ENT2. Alternatively, these inhibitors may interact with PMAT at a structurally distinct site.
Using 0.5 μM NBMPR to control endogenous nucleoside uptake, we observed significant transporter-mediated adenosine uptake in PMAT-expressing MDCK cells and Xenopus oocytes at physiologic pH (Figs. 2 and 4). No uptake activity was detected for any other nucleosides (guanosine, inosine, cytidine, thymidine, and uridine) or nucleobases (adenine, guanine, hypoxanthine, cytosine, thymine, and uracil). Such adenosine specificity of PMAT is in sharp contrast to that of ENT1 and ENT2, which broadly transport all naturally occurring pyrimidine and purine nucleosides and even some nucleobases in the case of ENT2 (Ward et al., 2000; Kong et al., 2004). On the other hand, a large number of organic cations are accepted by PMAT as substrates, including MPP+, serotonin, dopamine, histamine, epinephrine, tetraethylammonium, and metformin (Engel and Wang, 2005; Zhou et al., 2007c). The apparent transport efficiency (Vmax/Km) for adenosine is significantly lower than that of most organic cation substrates (Table 2). Indeed, when transport activities were measured side by side, the transport activity of adenosine was only a fraction of those of MPP+ and serotonin uptake in both MDCK and oocyte expression systems (Fig. 4). These data strongly argue against PMAT as a typical nucleoside transporter but confirmed our original hypothesis that PMAT functions as a polyspecific organic cation transporter.
Using Xenopus oocytes expressing human PMAT, Barnes et al. (2006) reported that PMAT was a pH-activated adenosine transporter, whose activity toward adenosine is activated at acidic pH but absent at pH 7.4. On the other hand, PMAT-mediated serotonin transport was reported to be insensitive to pH changes, and the pH effect on PMAT was described as adenosine-specific (Barnes et al., 2006). In contrast, we previously observed a pronounced pH effect on PMAT-mediated organic cation transport (Xia et al., 2007). In this study, we reinvestigated the effect of extracellular pH on PMAT-mediated adenosine and organic cation uptake side by side in MDCK cells and in Xenopus oocytes. Our data consistently showed that PMAT-mediated uptakes of adenosine, serotonin, and MPP+ were equally sensitive to extracellular pH and were enhanced at similar magnitudes by acidic pH (Fig. 4). These results demonstrated that the stimulatory effect of proton is not substrate-specific but rather is a general characteristic of PMAT-mediated transport.
The mechanism by which acidic pH stimulates PMAT activity is still unclear. Limited data from our laboratory indicate that it may be due to transport coupling with a transmembrane proton gradient (i.e., a proton-substrate cotransport mechanism) (Xia et al., 2007). It is interesting to note that PMAT-mediated adenosine transport is sensitive to membrane potential changes and decreases under depolarizing conditions (Fig. 5). These data suggest that PMAT-mediated adenosine transport is electrogenic, and there is net transfer of positive charges across the membrane during adenosine translocation. Because adenosine itself does not carry a charge at neutral pH, this electrogenic property may result from net transfer of a positive charge of a cotransported proton ion. More direct studies using electrophysiological measurements are necessary to elucidate the precise mechanism of the observed proton effect on PMAT.
Adenosine regulates a variety of physiological processes. In the brain, adenosine exerts an inhibitory tone and serves as an endogenous neuroprotective agent against ischemia- and seizure-induced neuronal injury (Rathbone et al., 1999; Latini and Pedata, 2001). In the skeletal muscle, adenosine functions as a locally produced regulator of muscle blood flow and plays a major protective role during systemic hypoxia (Hellsten et al., 1998; MacLean et al., 1998). Our quantitative PCR results showed that hENT1 is highly expressed in both human brain and skeletal muscle (Fig. 6b), suggesting a major role of this transporter in regulating adenosine levels in these tissues. Consistent with our previous reports (Engel et al., 2004; Dahlin et al., 2007), PMAT is highly expressed in the brain (Fig. 6b). Whereas our previous Northern blot showed significant PMAT expression in human skeletal muscle (Engel et al., 2004), quantitative real-time PCR revealed that its expression in this tissue is much lower than that in the brain. The reported extracellular concentrations of adenosine, determined by in vivo microdialysis, under normal physiological conditions are between 40 and 460 nM in the mammalian brain (Zetterström et al., 1982; Ballarín et al., 1991; Latini and Pedata, 2001) and between 220 and 440 nM in human skeletal muscle (Hellsten et al., 1998; MacLean et al., 1998). Given its low affinity and low activity toward adenosine and in the presence of other highly active nucleoside transporters, especially ENT1, PMAT is not likely to function as a major contributor for adenosine uptake in vivo. However, during ischemia or hypoxia, extracellular adenosine concentrations rise drastically (up to 30- to 46-fold) (Latini and Pedata, 2001), and there is evidence that ENT1 expression and activity may be repressed by hypoxia (Eltzschig et al., 2005). Under such conditions, PMAT may play a backup role in adenosine uptake, especially in the brain where the transporter is abundantly expressed.
In summary, our results demonstrated that PMAT does not function as a typical nucleoside transporter and transports adenosine much less efficiently than organic cations. PMAT is relatively insensitive to classic nucleoside transport inhibitors and is stimulated by acidic pH in a substrate-independent manner. Functionally, PMAT should be viewed as a polyspecific organic cation transporter rather than an archetypical nucleoside transporter.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM066233].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.032987.
- SLC
- solute carrier
- ENT
- equilibrative nucleoside transporter
- NBMPR
- nitrobenzylmercaptopurine ribonucleoside
- PMAT
- plasma membrane monoamine transporter
- MPP+
- 1-methyl-4-phenylpyridinium
- MDCK
- Madin-Darby canine kidney
- KRH
- Krebs-Ringer-Henseleit
- RT
- reverse transcriptase
- PCR
- polymerase chain reaction
- h
- human.
References
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. (2004) The equilibrative nucleoside transporter family, SLC29. Pflugers Arch 447:735–743 [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, Ritzel MW, Cass CE, Young JD. (2005) Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem 280:15880–15887 [DOI] [PubMed] [Google Scholar]
- Ballarín M, Fredholm BB, Ambrosio S, Mahy N. (1991) Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol Scand 142:97–103 [DOI] [PubMed] [Google Scholar]
- Barnes K, Dobrzynski H, Foppolo S, Beal PR, Ismat F, Scullion ER, Sun L, Tellez J, Ritzel MW, Claycomb WC, et al. (2006) Distribution and functional characterization of equilibrative nucleoside transporter-4, a novel cardiac adenosine transporter activated at acidic pH. Circ Res 99:510–519 [DOI] [PubMed] [Google Scholar]
- Dahlin A, Xia L, Kong W, Hevner R, Wang J. (2007) Expression and immunolocalization of the plasma membrane monoamine transporter (PMAT) in the brain. Neuroscience 146:1192–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schönfeld C, Löffler M, Reyes G, Duszenko M, Karhausen J, et al. (2005) HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med 202:1493–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K, Wang J. (2005) Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol Pharmacol 68:1397–1407 [DOI] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J. (2004) Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem 279:50042–50049 [DOI] [PubMed] [Google Scholar]
- Fink T, Lund P, Pilgaard L, Rasmussen JG, Duroux M, Zachar V. (2008) Instability of standard PCR reference genes in adipose-derived stem cells during propagation, differentiation and hypoxic exposure. BMC Mol Biol 9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Urban TJ, Leabman MK, Fujita K, Giacomini KM. (2006) Transport of drugs in the kidney by the human organic cation transporter, OCT2 and its genetic variants. J Pharm Sci 95:25–36 [DOI] [PubMed] [Google Scholar]
- Govindarajan R, Leung GP, Zhou M, Tse CM, Wang J, Unadkat JD. (2009) Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am J Physiol Gastrointest Liver Physiol 296:G910–G922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JH, Owen RP, Giacomini KM. (2004) The concentrative nucleoside transporter family, SLC28. Pflugers Arch 447:728–734 [DOI] [PubMed] [Google Scholar]
- Griffith DA, Jarvis SM. (1996) Nucleoside and nucleobase transport systems of mammalian cells. Biochim Biophys Acta 1286:153–181 [DOI] [PubMed] [Google Scholar]
- Hammond JR, Stolk M, Archer RG, McConnell K. (2004) Pharmacological analysis and molecular cloning of the canine equilibrative nucleoside transporter 1. Eur J Pharmacol 491:9–19 [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Maclean D, Rådegran G, Saltin B, Bangsbo J. (1998) Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation 98:6–8 [DOI] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. (2007) Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24:1227–1251 [DOI] [PubMed] [Google Scholar]
- Kong W, Engel K, Wang J. (2004) Mammalian nucleoside transporters. Curr Drug Metab 5:63–84 [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. (2001) Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem 79:463–484 [DOI] [PubMed] [Google Scholar]
- MacLean DA, Sinoway LI, Leuenberger U. (1998) Systemic hypoxia elevates skeletal muscle interstitial adenosine levels in humans. Circulation 98:1990–1992 [DOI] [PubMed] [Google Scholar]
- Olah ME, Stiles GL. (1992) Adenosine receptors. Annu Rev Physiol 54:211–225 [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. (1999) Trophic effects of purines in neurons and glial cells. Prog Neurobiol 59:663–690 [DOI] [PubMed] [Google Scholar]
- Romanowski T, Sikorska K, Bielawski KP. (2008) GUS and PMM1 as suitable reference genes for gene expression analysis in the liver tissue of patients with chronic hepatitis. Med Sci Monit 14:BR147–152 [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA. (2009) Adenosine receptors and the central nervous system. Handb Exp Pharmacol 471–534 [DOI] [PubMed] [Google Scholar]
- Visser F, Vickers MF, Ng AM, Baldwin SA, Young JD, Cass CE. (2002) Mutation of residue 33 of human equilibrative nucleoside transporters 1 and 2 alters sensitivity to inhibition of transport by dilazep and dipyridamole. J Biol Chem 277:395–401 [DOI] [PubMed] [Google Scholar]
- Ward JL, Sherali A, Mo ZP, Tse CM. (2000) Kinetic and pharmacological properties of cloned human equilibrative nucleoside transporters, ENT1 and ENT2, stably expressed in nucleoside transporter-deficient PK15 cells. Ent2 exhibits a low affinity for guanosine and cytidine but a high affinity for inosine. J Biol Chem 275:8375–8381 [DOI] [PubMed] [Google Scholar]
- Wright SH, Dantzler WH. (2004) Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev 84:987–1049 [DOI] [PubMed] [Google Scholar]
- Xia L, Engel K, Zhou M, Wang J. (2007) Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells. Am J Physiol Renal Physiol 292:F682–F690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD, Yao SY, Sun L, Cass CE, Baldwin SA. (2008) Human equilibrative nucleoside transporter (ENT) family of nucleoside and nucleobase transporter proteins. Xenobiotica 38:995–1021 [DOI] [PubMed] [Google Scholar]
- Zetterström T, Vernet L, Ungerstedt U, Tossman U, Jonzon B, Fredholm BB. (1982) Purine levels in the intact rat brain. Studies with an implanted perfused hollow fibre. Neurosci Lett 29:111–115 [DOI] [PubMed] [Google Scholar]
- Zhou M, Engel K, Wang J. (2007a) Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain. Biochem Pharmacol 73:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Xia L, Engel K, Wang J. (2007b) Molecular determinants of substrate selectivity of a novel organic cation transporter (PMAT) in the SLC29 family. J Biol Chem 282:3188–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Xia L, Wang J. (2007c) Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos 35:1956–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]