Abstract
Breast cancer resistance protein (Bcrp) is a member of the ATP-binding cassette membrane transporter family, which is expressed apically in liver, kidney, and intestine epithelium. Recent reports suggest that in addition to xenobiotics, porphyrins, and food toxins, Bcrp can also transport bile acids and, therefore, may participate in the adaptive response to cholestasis. Bile duct ligation (BDL), an experimental model of obstructive cholestasis, was performed in male wild-type (WT) and Bcrp knockout (KO) mice. An initial time course of 3, 7, and 14 days of BDL in WT mice revealed that Bcrp expression was significantly reduced in liver but increased in ileum by 7 days. Subsequent experiments using 7-day BDL in WT and Bcrp KO mice demonstrated that there was no difference in liver necrosis, serum glutamic pyruvate aminotransferase, bilirubin, or bile acid levels in serum, hepatic tissue, bile, urine, or feces between the two groups. Protein expression levels for liver organic solute transporter (Ost) α and multidrug resistance protein 1 and kidney multidrug resistance-associated protein (Mrp) 2, Mrp3, and Mrp4 were significantly greater in the sham Bcrp KO versus sham WT mice. The expression of Mrp2 and Mrp4 in KO kidneys was further increased after BDL. In contrast, the adaptive response of transporters to BDL in the liver was similar in KO and WT BDL mice, including Ostα and Ostβ expression, which increased in liver and kidney but decreased in the ileum. These findings suggest that Bcrp does not have a significant role in the adaptive response to cholestasis in the liver but may be more important for solute export in the kidney and intestine.
Introduction
The breast cancer resistance protein (BCRP/Bcrp; ABCG2/Abcg2) is a member of the ABC family of membrane transporters with broad substrate specificity (Krishnamurthy and Schuetz, 2006; Polgar et al., 2008). Bcrp was first described in a drug-resistant cancer cell line and has been shown to transport a large number of drugs, environmental agents, and the endogenous solute, protoporphyrin (Krishnamurthy and Schuetz, 2006; Koshiba et al., 2008; Polgar et al., 2008; Robey et al., 2009). The apical membrane localization of BCRP/Bcrp on many cell types, including the canalicular membrane of the hepatocyte, the proximal tubule of the kidney, and the enterocyte of the intestine, permits this transporter to function in a coordinated manner to extrude and prevent absorption of xenobiotics (Maliepaard et al., 2001; Hilgendorf et al., 2007; Huls et al., 2008). In this respect, BCRP/Bcrp shares many substrates with Mdr1 and Mrp2 (Allen et al., 1999; Schinkel and Jonker, 2003). Diverse functional roles for BCRP have also been described. BCRP expression in stem cells leads to the side population phenotype and seems to function to protect these sensitive cells from the accumulation of toxic drugs (Zhou et al., 2001; Abbott, 2006). The localization of BCRP to placental syncytiotrophoblasts may serve as a protective mechanism for the developing fetus (Maliepaard et al., 2001; Robey et al., 2009). BCRP is also expressed in mammary glands where, in apparent contradiction, it transports the same drugs into breast milk (Zarba et al., 1992; DeBruin et al., 2001). For example, the dietary carcinogens aflatoxin B1, 2-amino-3-methylimidazo[4,5-f]quinoline, and 3-amino-1,4-dimethyl-5H-pyrido[4, 3-b]indole are restricted from systemic exposure and the fetus by BCRP, yet are secreted directly into breast milk, thus facilitating exposure to the newborn (van Herwaarden et al., 2006).
The recent availability of the Bcrp1 knockout (KO) mouse has made it possible to study the role of this transporter in greater detail. Such studies have led to the finding that Bcrp1 is responsible for the elimination of the toxic chlorophyll breakdown product pheophorbide a (Jonker et al., 2002). The resemblance of this compound to the heme molecule has shed light on the role of Bcrp in the transport of many porphyrins. The regulation of porphyrins is important in providing a protective mechanism, specifically during hypoxic conditions. Other endogenous substrates for Bcrp include dehydroepiandrosterone sulfate, estrogens, and folic acid (Chen et al., 2003; Grube et al., 2007).
Whether bile acids are endogenous substrates for BCRP/Bcrp is uncertain. Suzuki et al. (2003) were unable to demonstrate transport activity for taurocholic acid or taurolithocholate sulfate in Bcrp-transfected P388 vesicles (from a mouse lymphoma cell line). However, Imai et al. (2003) reported inhibition of [3H]estrone sulfate uptake in the presence of taurolithocholate or taurolithocholate sulfate and to a lesser degree, taurocholate, in transfected K562 cells (human erythroleukemia cells). A third study, using Bcrp-expressing bacteria (Lactococcus lactis), demonstrated that primary bile acids could be transported by this system (Janvilisri et al., 2005).
These later findings led us to examine the possibility that Bcrp might play a protective role in the liver during cholestatic liver disease. In this condition, the liver is faced with high levels of toxic bile acids, a challenge it attempts to overcome in part by altering the expression of bile acid transporters on the basolateral sinusoidal membrane of the hepatocytes (Boyer, 2007). In the present study, we used the Bcrp-null mouse and common bile duct ligation, a model of cholestatic liver disease, to ascertain the importance of Bcrp in this disease model. Our results demonstrate a limited role of Bcrp in the liver during obstructive cholestasis; however, other ABC transporters, which probably compensate for the loss of Bcrp and presumably aid in the excretion of toxic compounds, are induced in the kidney.
Materials and Methods
Animals.
Male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and 9- to 12-week-old Bcrp wild-type [(WT) FVB] and knockout mice (FVB/129 background; model 002767-m) were purchased from Taconic Farms (Germantown, NY). All animals received treatment according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) as adopted and promulgated by the U.S. National Institutes of Health. Animals were housed in a 12-h light/dark cycle environment and allowed free access to food and water. C57BL/6 mice underwent common bile duct ligation (BDL) or a sham operation as described previously (Mennone et al., 2006) for 3, 7, or 14 days. Liver, kidney, and ileum were harvested for mRNA and protein determination. Wild-type and Bcrp KO mice underwent BDL or sham operation for 7 days. Before harvesting, animals were fasted for 10 h. Livers and kidneys were flushed with normal saline to remove blood and then were either quick-frozen for protein and mRNA determination or fixed in 10% formalin for histological staining. Serum, bile, urine, and feces were also collected.
Histology.
Formalin-fixed liver segments were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Slides were coded and graded blindly (by J.L.B.) for necrosis on a scale of 0 to 4.
Bile Acid and Liver Enzyme Assay.
Bile acid quantitation was performed on extracted liver, serum, bile, urine, and feces as described previously using a kit from Trinity Biotech (Wicklow, Ireland) (Mennone et al., 2006). Kits from Thermo Fisher Scientific (Waltham, MA) were used to measure serum bilirubin and glutamic pyruvate aminotransferase.
Quantitative Real-Time Polymerase Chain Reaction.
Total RNA was extracted from tissue using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Five micrograms of RNA was reverse-transcribed into cDNA using a first-strand kit (Stratagene, La Jolla, CA). Real-time polymerase chain reaction was performed on a 7500 sequence detection system (Applied Biosystems, Foster City, CA). Twenty microliters of reaction mixture was used per well containing Master Mix (Applied Biosystems), 1 μl of cDNA, and specific primers and probes at concentrations of 450 to 500 and 125 to 250 nM, respectively. In some cases, TaqMan Gene Expression Assays (Applied Biosystems) were used at a dilution of 1:40. Reactions were run in triplicate in 96-well plates by heating to 50°C for 2 min and 95°C for 10 min and then were cycled at 95°C for 15 s and 60°C for 1 min for 40 cycles. Glyceraldehyde-3-phosphate dehydrogenase was used for all samples to normalize expression.
TaqMan Gene Expression Assays were used for the following: Bcrp (NM_011920), Mm01181556_m1; Mrp2 (NM_013806), Mm00496899_m1; Mrp3 (BC046560), Mm00551550_m1; Ntcp (NM_011387), Mm00441421_m1; Mdr1a (NM_011076), Mm00440761; and Bsep (NM_021022), Mm00445168_m1. The following primers and probes were purchased from Integrated DNA Technologies, Inc. (Coralville, IA): Mrp4 (AK052778) forward, CATCAAGTCCAGGGAAAAGGTTG, reverse GAGGGCCGAGATGAGGGAG, and probe TGGGCAGAACCGGAGCTGGGAAA; glyceraldehyde-3-phosphate dehydrogenase (NM_001001978) forward GCCCAGAACATCATCCCTGC, reverse CCGTTCAGCTCTGGGATGACC, and probe TCCACTGGTGCTGCCAAGGCTGTG; Ostα (NM_145932) forward TGTTCCAGGTGCTTGTCATCC, reverse CCACTGTTAGCCAAGATGGAGAA, and probe CCGCCCTGCAGCCTGCCAT; and Ostβ (NM_178933) forward ATGCGGCTCCTTGGAATTA, reverse GGAGGAACATGCTTGTCATGAC, and probe TCCATCCTGGTCCTGGCAGTCCTG.
Protein Analysis (Western Blotting).
Membrane proteins were isolated, electrophoresed, blotted, and imaged as described previously (Mennone et al., 2006). Densitometry was performed using TotalLab software (FOTODYNE Inc., Hartland, WI). Polyclonal antibodies to Mrp2, Ntcp, and Oatp1 were provided by Bruno Stieger, to Mrp4 by Dietrich Keppler, to Oct1 by Hermann Koepsell, and to Bsep by Victor Ling. Antibodies to Mdr were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and Ostα and Ostβ polyclonals were a gift from Ned Ballatori. Polyclonal Mrp3 antibodies were developed in this laboratory (Wang et al., 2006b).
Statistical Analysis.
All data were compared using unpaired t tests with Instat software (GraphPad Software Inc., San Diego, CA) and are expressed as mean ± S.D. p < 0.05 was considered significant.
Results
BDL Results in a Decrease in Bcrp in Liver and Kidney but an Increase in the Ileum.
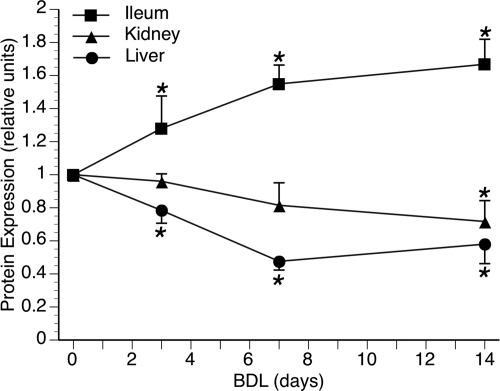
Bcrp protein expression was initially determined over a time course of 3, 7, or 14 days of BDL in WT C57BL/6 mice. Hepatic Bcrp was significantly reduced by 3 days and remained down throughout the 14 days of BDL (Fig. 1). Although renal Bcrp was also significantly reduced by 14 days, it was a more gradual change and did not reach significance at 3 or 7 days (Fig. 1). In contrast, intestinal Bcrp was significantly increased throughout the 14 days of BDL (Fig. 1). Similar results were seen after 7-day BDL in WT FVB mice (data not shown). Therefore, subsequent BDL experiments were conducted on WT FVB and Bcrp KO mice for 7 days.
Fig. 1.
Time course of Bcrp protein expression after BDL in C57BL/6 mice. Densitometry of Western blot analysis of Bcrp expression at 3, 7, and 14 days after BDL showed that liver Bcrp was significantly decreased by 3 days and maintained over 14 days. Kidney levels gradually declined over the time course and were significantly decreased by 14 days. In contrast, Bcrp in the ileum was significantly increased at all time points. *, p < 0.05.
Membrane Transporter Expression in Bcrp KO Mice.
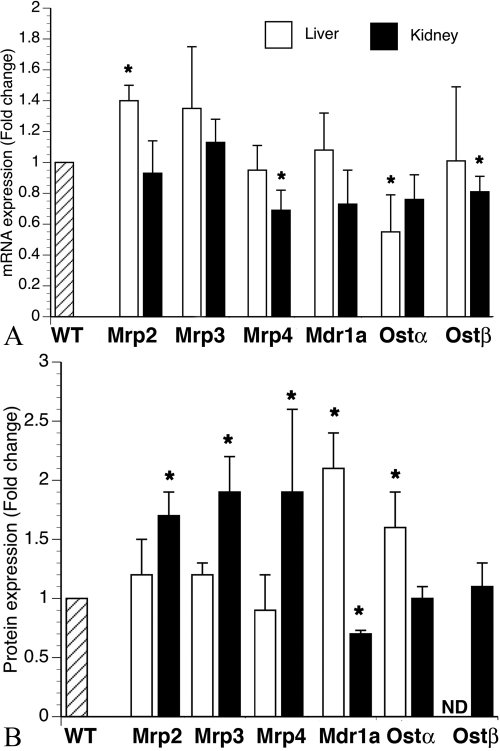
Before performing bile duct ligation in the Bcrp KO mice, we first characterized changes in liver and kidney transporter expression that were present due to the genetic deficiency of Bcrp. Our results show few, slight changes in mRNA levels for hepatic solute transporters. Mrp2 mRNA is up 1.4-fold compared with that in the WT mouse, and Ostα is down by 1.5-fold (Fig. 2A). In the kidney, both Mrp4 and Ostβ mRNA are slightly, although significantly, reduced compared with levels in the WT mouse (Fig. 2A). However, it is interesting to note that protein expression of three members of the multidrug resistance-associated protein family (Mrp2, Mrp3, and Mrp4) is increased in the kidney but not in the liver in Bcrp KO mice (Fig. 2B). Mdr1a is reciprocally increased in the liver and decreased in the kidney (Fig. 2B). This finding suggests that post-translational changes may be important in regulating expression of key ABC export transporters in the kidney in the absence of Bcrp. Background differences between WT (FVB) versus KO (FVB/129), although slight, could account for some of the small differences seen in transporter expression.
Fig. 2.
Transporter expression in liver and kidney of Bcrp-deficient mice. A, in the liver (□) mRNA expression for Mrp2 is increased and that for Ostα is decreased in Bcrp KO mice compared with expression in WT controls. In the kidney (■), both Mrp4 and Ostβ were slightly decreased compared with those in WT controls. n = 6 for all groups. B, in the liver, protein expression of both Mdr1a and Ostα is increased in the Bcrp KO mice compared with that in WT controls. In the kidney, protein expression of Mrp2, Mrp3, and Mrp4 is increased and that of Mdr1a is decreased in Bcrp KO mice compared with expression in WT controls. n = 6 for all groups. ND, non detectable.
Liver Injury in Wild-Type and Bcrp KO Mice 7 Days after BDL.
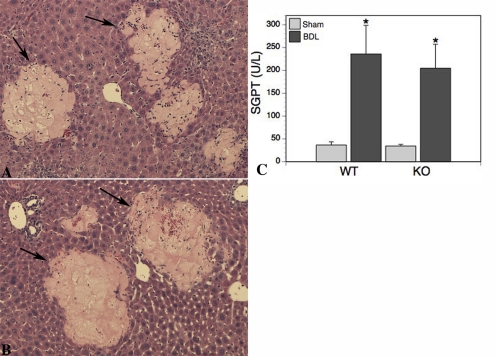
Liver sections stained with hematoxylin and eosin were graded blindly (0–4 scale) for liver cell injury. Sham livers from both groups had complete absence of necrosis, and the histological appearance was normal. In contrast, varying degrees of parenchymal necrosis were noted in both WT (2.0 ± 0.8) and Bcrp KO (2.7 ± 0.6) BDL livers, although there was no significant difference between the two groups (Fig. 3, A and B). Serum glutamic pyruvate aminotransferase values increased approximately 16-fold in both BDL groups, confirming the histological grading (Fig. 3C).
Fig. 3.
A and B, liver necrosis is the same in WT and Bcrp KO mice. Necrotic regions (arrows) were easily distinguished from healthy parenchyma. The extent of necrosis in WT and KO livers was comparable. C, the serum liver enzyme alanine aminotransferase [glutamic pyruvate aminotransferase (SGPT)] level was significantly (p < 0.005) increased in both WT and Bcrp KO animals after BDL. This increase was the same in both (n = 6) groups of animals.
Serum Bilirubin and Bile Acid Levels.
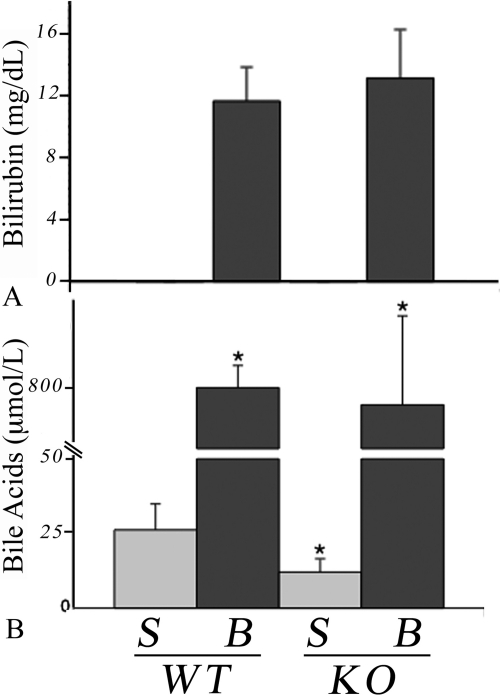
Serum bilirubin levels were undetectable in sham groups and were increased in both BDL groups to an equal extent (Fig. 4A). Serum bile acid levels increased after BDL as expected and reached similar levels in both groups (Fig. 4B). However, the concentration of bile acids in the sham Bcrp KO mice was less than half that of the sham WT group (p < 0.01). Bile acid concentrations in extracted liver were similarly elevated after BDL: WT (34-fold) and KO (29-fold). There were no significant differences between groups for bile acid levels in bile, urine, or feces (data not shown).
Fig. 4.
Serum bilirubin and bile acid levels are increased in both WT and Bcrp KO mice after BDL. A, bilirubin was below detection levels in sham mice (S, n = 6) but rose to similar levels after BDL (B, n = 6). B, there was no difference in the increase in serum bile acids after BDL in WT and KO animals. However, there was a significantly (*, p < 0.05) lower concentration of serum bile acids in the KO sham compared with the WT group. n = 6 for both groups.
Expression of Liver Transporters after BDL in Bcrp KO Mice.
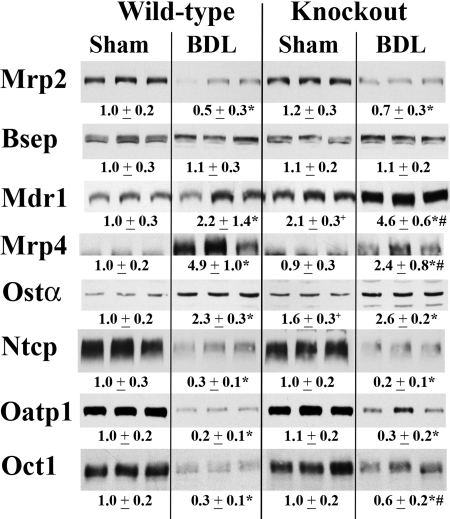
Mrp2 mRNA expression was significantly down-regulated in both WT (1.4-fold) and Bcrp KO (1.2-fold) livers after 7 days of BDL. However, the expression remained significantly higher in Bcrp KO mice compared with that in the WT mice (p < 0.005). There was no significant difference in mRNA expression between the WT and Bcrp KO mice after BDL for any of the other transporters tested (Mrp3, Mrp4, Mdr1a, Ostα, Ostβ, Bsep, and Ntcp) (data not shown). As with mRNA expression, Mrp2 protein expression was significantly down-regulated in both WT and Bcrp KO mice after BDL, although there was no significant difference between the two groups (Fig. 5). Mrp4 was induced after BDL in both groups, although the increase in the KO BDL mice was only half that seen in the WT animals (Fig. 5). The up-regulation of Mdr1a after BDL was twice as great in the KO mice compared with the WT mice (Fig. 5). Bsep protein levels were unchanged after BDL in both groups. Oatp1, Ntcp, and Oct1 expression was significantly decreased after BDL in the livers of both WT and KO animals, although the decrease for Oct1 was significantly less in the Bcrp KO mice than that seen in the WT mice.
Fig. 5.
Western blot and densitometric analysis of transporter protein expression in total liver membrane of WT and Bcrp KO mice. Representative blots (n = 3) are shown, and densitometry values for all six mice are shown below the blots. Expression of apical Mrp2 is down equally in WT and KO mice after BDL. There was no difference in Bsep expression levels after BDL in either group. However, Mrp4 and Mdr1a were up-regulated after BDL in WT and KO mice. The increase in Mrp4 in the KO mice was only half that seen in the WT BDL mice, whereas the increase in Mdr1a was twice as great in the KO mice compared with that in the WT mice. Oatp1, Ntcp, and Oct1 protein expression was significantly decreased after BDL in the livers of both WT and KO animals, although the decrease for Oct1 was significantly less in the Bcrp KO mice than that seen in the WT mice. *, p < 0.05, sham versus BDL; #, p < 0.05, WT BDL versus KO BDL; +, p < 0.05, WT sham versus KO sham.
Expression of Kidney Transporters after BDL in Bcrp KO Mice.
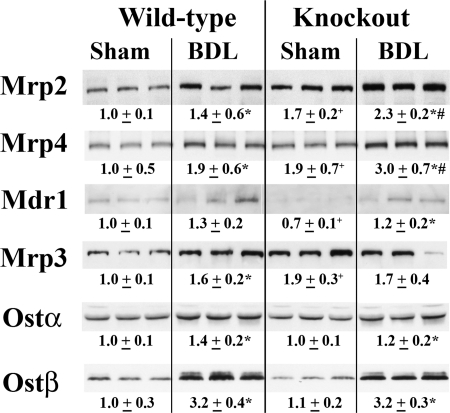
Both Mdr1a and Ostβ mRNA levels were significantly decreased after BDL in the Bcrp KO mice compared with the WT mice after BDL (data not shown). There was no significant difference between the two groups for mRNA for any other transporter tested (data not shown). As stated earlier, the basal levels of Mrp2, Mrp3, and Mrp4 protein were significantly higher than those in the wild-type mice (Fig. 2). After BDL, protein expression for Mrp2 and Mrp4 was further increased in the KO mice (Fig. 6) and was significantly higher than that in the WT BDL mice. All other membrane transporters tested (Mrp3, Mdr1a, Ostα, and Ostβ) were similar to those in the WT mice after BDL (Fig. 6).
Fig. 6.
Western blot and densitometric analysis of transporter protein expression in total kidney membrane of WT and Bcrp KO mice. Representative blots (n = 3) are shown, and densitometry values for all six mice are shown below the blots. As shown in Fig. 2, the basal levels of Mrp2, Mrp3, and Mrp4 protein were significantly higher than those in the WT mice. After BDL, Mrp2 and Mrp4 were further increased in the KO mice and remained significantly higher than those in the WT BDL mice. *, p < 0.05, sham versus BDL; #, p < 0.05, WT BDL versus KO BDL; +, p < 0.05, WT sham versus KO sham.
Ileum Protein Expression.
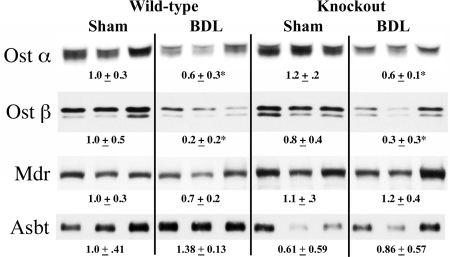
Protein expression in ileum for Ostα, Ostβ, Mdr, and Asbt was compared between WT and Bcrp KO sham and BDL mice (Fig. 7). Both Ostα and Ostβ levels were significantly decreased after BDL in both WT and Bcrp KO mice. The degree of down-regulation was similar between WT and Bcrp KO animals. Mdr and Asbt expression was statistically unchanged for all four groups of animals.
Fig. 7.
Representative Western blots and densitometry values of ileum transporters in Bcrp WT and KO sham and BDL mice. n = 6 for each group. Ostα: WT BDL, *, p < 0.05 versus WT sham; *, p < 0.0005 for KO BDL versus KO sham. Ostβ: * p < 0.05 versus respective sham.
Discussion
During cholestatic liver injury, a number of adaptive responses occur in the liver in an attempt to minimize the toxic consequence of hepatic bile acid accumulation. These adaptations include reduction in bile acid synthesis, enhanced conversion of bile acid to more hydrophobic compounds via phase I and phase II metabolic pathways, reduced hepatic bile acid uptake, and increased expression of alternative pathways of bile acid excretion across the basolateral membrane back into the systemic circulation (Boyer, 2007). Whether Bcrp plays a role in this adaptive response is not known.
BCRP/Bcrp is expressed at the apical membranes of the bile canaliculus, the intestine, and the renal proximal tubule together with other ABC transporters and might be expected to contribute to this adaptive response. In addition, the possibility that Bcrp might transport bile acids as suggested by some (Imai et al., 2003; Janvilisri et al., 2005) led us to examine the expression and role of Bcrp in these three tissues in cholestasis in wild-type and Bcrp KO mice.
Our findings indicate that both mRNA and Bcrp protein expression in mice decreases over time after BDL in both liver and kidney. The findings in the liver are consistent with previous studies in rat in which Bcrp protein expression was reduced after BDL for 6 weeks (Dietrich et al., 2004b). However, no change in hepatic Bcrp mRNA was seen after lithocholate-induced cholestasis in mice (Beilke et al., 2008). In contrast, we found that expression levels of Bcrp protein in the ileum steadily increased over 14 days of BDL in mice. Conflicting findings exist for obstructive cholestasis in humans, in whom BCRP protein was decreased in duodenal biopsy samples (Zimmermann et al., 2006) in one study but was unchanged in another (Dietrich et al., 2004a). Intestinal Bcrp expression is significantly lower in mouse compared with that in rat and human (Tanaka et al., 2005; Gupta et al., 2006) so that species differences may account for some of these discrepancies.
The mechanism by which Bcrp expression is differentially regulated in the three tissues was not examined in this study; however, estradiol down-regulates Bcrp in several cell lines (Imai et al., 2005; Wang et al., 2006a), whereas testosterone has been shown to have inductive effects on Bcrp expression in vivo (Tanaka et al., 2005). During cholestasis, serum testosterone levels are significantly reduced, whereas estrogen is increased 3-fold in male rats (Chen et al., 1995). Whether such hormone-induced changes observed in vitro account for altered Bcrp expression in the present study remains to be determined.
Unlike earlier studies in Mrp4-null mice (Mennone et al., 2006), we found no evidence that BDL caused more liver injury in Bcrp-null mice. Thus, Bcrp does not seem to play a protective role in cholestatic liver injury. Indeed, histological evidence of necrosis and serum aminotransferase levels was similar in WT and Bcrp KO mice after BDL for 7 days as were elevations in serum bile acids and bilirubin. Liver bile acid levels were also similar between the two groups of bile duct-ligated animals, suggesting that Bcrp plays little or no role in hepatic bile acid transport. However, the basal level of serum bile acids in the Bcrp KO sham animal was less than half of that seen in the WT sham animal. This difference was not explained by increased urinary or fecal excretion, because bile acid levels in the urine and feces were not different between WT and KO animals. Despite these findings, we cannot exclude the possibility that renal or intestinal clearance of bile acids might be increased in the absence of Bcrp, given the changes in expression of renal ABC transporters.
In contrast with minor changes in bile acid transporters in the liver of KO mice (only Mrp2 showed small but significant increases at the mRNA level, and Ostα mRNA was reduced, whereas small increases in Mdr1 and Ostα protein expression were noted), and the absence of changes in expression of Mdr1, Asbt, Ostα, or Ostβ in the intestine, significant differences were found in the expression of renal transporters in the Bcrp-null mice. Mrp4 and Ostβ mRNA were reduced in Bcrp-null mice compared with that in wild-type animals, whereas protein expression of Mrp3 and 4 was up-regulated. These findings suggest that Bcrp may have an important role in transport of endogenous substrates in the kidney requiring compensatory responses in other transporters in its absence. It is interesting to speculate that sulfate conjugates may be one important group because they are known substrates for Bcrp (Imai et al., 2003; Polgar et al., 2008) as well as Mrp4 (Zelcer et al., 2003) and Ostα/β (Seward et al., 2003). Recent studies in Bcrp knockout mice indicate impaired renal excretion of the sulfate metabolite of the drug edaravone (Mizuno et al., 2004) in support of this hypothesis. Impairment of biliary but not intestinal excretion of troglitazone sulfate, the major metabolite of the antidiabetes agent troglitazone, is also impaired in the Bcrp-null mouse (Enokizono et al., 2007). Glucuronide conjugates are also likely Bcrp substrates and could account for compensatory increases in Mrp2 and Mrp3, which are known transporters for these compounds (Krishnamurthy and Schuetz, 2006; Polgar et al., 2008).
After BDL, liver mRNA levels of Mdr1 and Bsep are relatively increased in Bcrp-null mice compared with those in the WT mice, whereas changes in transporter protein expression paralleled those in WT BDL animals although the magnitude of increase in Mrp4 was less and that in Mdr1 was more than in WT BDL animals. These findings again suggest that liver Bcrp plays little or no role in the adaptive response to cholestatic liver injury.
As seen in earlier studies, Ntcp and Oct1, two basolateral transporters for the hepatic uptake of conjugated bile acids and small organic cations, respectively, are down-regulated after BDL in both rats and mice (Denk et al., 2004; Mennone et al., 2006). The degree of decrease in Oct1 expression in the present study was significantly less in the Bcrp-null liver. This may indicate an increased need for this transporter in this disease model and thus may imply a shared, as yet unidentified, substrate(s) with Bcrp.
BDL also produced no differences in expression in Ostα in the liver of Bcrp KO mice, which was up-regulated at the protein level in both bile duct-ligated groups as noted previously in WT mice and in patients with primary biliary cirrhosis (Boyer et al., 2006). Ostα and Ostβ mRNA and protein were also increased in the kidney and down-regulated in the intestine. These findings are consistent with farnesoid X receptor-mediated feedback regulation of the expression of this transporter because of increases in tissue levels of bile acids in liver and kidney and their absence from the intestine after BDL.
Bcrp is expressed on the apical membrane of the proximal tubule in the kidney where it is involved with the excretion of a variety of compounds including glucuronide and glutathione drug conjugates (Huls et al., 2008; Polgar et al., 2008) similar to Mrp2 and Mrp4. Thus, it is not surprising to see increases in kidney Mrp2 and Mrp4 protein expression in the Bcrp knockout mice. Several studies have also documented increases in renal Mrp2 mRNA in mouse (Slitt et al., 2007) and rat (Lee et al., 2001) after BDL, although not consistently (Mennone et al., 2006). That the expressions of Mrp2 and Mrp4 were augmented further after BDL suggests that these renal ABC transporters are responding further to the effects of cholestasis in addition to the effects of the absence of Bcrp.
In contrast to the lack of significant effects of the loss of liver Bcrp in the adaptive response to cholestasis in hepatocytes, the abundance of Bcrp in the terminal portions of the small intestine is significantly increased after BDL (Fig. 1). One possible explanation is that intestinal Bcrp might play a role in the systemic response to cholestasis and contribute to the efflux of as yet to be identified substrates.
In summary, in the present study we found no evidence for a primary protective role for Bcrp in cholestasis produced by BDL in the mouse. Nevertheless, compensatory changes in renal Mrp2, Mrp3, and Mrp4 in Bcrp-null mice that are augmented after BDL suggest that Bcrp is playing an important role in substrate transport in the kidney both under normal as well as cholestatic conditions. Further studies will be needed to access the significance of these findings in the kidney. Likewise, the significance of the up-regulation of Bcrp in the intestine after BDL, which is in striking contrast to the down-regulation of this transporter in liver and kidney, merits further analysis.
This study was supported by the U.S. Public Health Service [Grants DK R37 25636, DK P30 34989]; and the Yale Liver Center Morphology and Cellular and Molecular Physiology Cores.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.034512.
- BCRP/Bcrp
- breast cancer resistance protein
- WT
- wild-type
- KO
- knockout
- ABC
- ATP-binding cassette
- Mdr
- multidrug resistance protein
- Mrp
- multidrug resistance-associated protein
- BDL
- bile duct ligation
- Ntcp
- sodium taurocholate-cotransporting polypeptide
- Bsep
- bile salt export pump
- Ost
- organic solute transporter
- Oatp
- organic anion-transporting polypeptide
- Oct
- organic cation transporter
- Asbt
- apical sodium-dependent bile acid transporter.
References
- Abbott BL. (2006) ABCG2 (BCRP): a cytoprotectant in normal and malignant stem cells. Clin Adv Hematol Oncol 4:63–72 [PubMed] [Google Scholar]
- Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. (1999) The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res 59:4237–4241 [PubMed] [Google Scholar]
- Beilke LD, Besselsen DG, Cheng Q, Kulkarni S, Slitt AL, Cherrington NJ. (2008) Minimal role of hepatic transporters in the hepatoprotection against LCA-induced intrahepatic cholestasis. Toxicol Sci 102:196–204 [DOI] [PubMed] [Google Scholar]
- Boyer JL. (2007) New perspectives for the treatment of cholestasis: lessons from basic science applied clinically. J Hepatol 46:365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. (2006) Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTα-OSTβ in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol 290:G1124–G1130 [DOI] [PubMed] [Google Scholar]
- Chen J, Murray M, Liddle C, Jiang XM, Farrell GC. (1995) Downregulation of male-specific cytochrome P450s 2C11 and 3A2 in bile duct-ligated male rats: importance to reduced hepatic content of cytochrome P450 in cholestasis. Hepatology 22:580–587 [PubMed] [Google Scholar]
- Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, Ross DD, Bates SE, Kruh GD. (2003) Transport of methotrexate, methotrexate polyglutamates, and 17β-estradiol 17-(β-d-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res 63:4048–4054 [PubMed] [Google Scholar]
- DeBruin LS, Martos PA, Josephy PD. (2001) Detection of PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) in the milk of healthy women. Chem Res Toxicol 14:1523–1528 [DOI] [PubMed] [Google Scholar]
- Denk GU, Soroka CJ, Mennone A, Koepsell H, Beuers U, Boyer JL. (2004) Down-regulation of the organic cation transporter 1 of rat liver in obstructive cholestasis. Hepatology 39:1382–1389 [DOI] [PubMed] [Google Scholar]
- Dietrich CG, Geier A, Salein N, Lammert F, Roeb E, Oude Elferink RP, Matern S, Gartung C. (2004a) Consequences of bile duct obstruction on intestinal expression and function of multidrug resistance-associated protein 2. Gastroenterology 126:1044–1053 [DOI] [PubMed] [Google Scholar]
- Dietrich CG, Geier A, Wasmuth HE, Matern S, Gartung C, de Waart DR, Elferink RP. (2004b) Influence of biliary cirrhosis on the detoxification and elimination of a food derived carcinogen. Gut 53:1850–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Sugiyama Y. (2007) Involvement of breast cancer resistance protein (BCRP/ABCG2) in the biliary excretion and intestinal efflux of troglitazone sulfate, the major metabolite of troglitazone with a cholestatic effect. Drug Metab Dispos 35:209–214 [DOI] [PubMed] [Google Scholar]
- Grube M, Reuther S, Meyer Zu Schwabedissen H, Köck K, Draber K, Ritter CA, Fusch C, Jedlitschky G, Kroemer HK. (2007) Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab Dispos 35:30–35 [DOI] [PubMed] [Google Scholar]
- Gupta N, Martin PM, Miyauchi S, Ananth S, Herdman AV, Martindale RG, Podolsky R, Ganapathy V. (2006) Down-regulation of BCRP/ABCG2 in colorectal and cervical cancer. Biochem Biophys Res Commun 343:571–577 [DOI] [PubMed] [Google Scholar]
- Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. (2007) Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos 35:1333–1340 [DOI] [PubMed] [Google Scholar]
- Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ, Heemskerk S, Russel FG, Masereeuw R. (2008) The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int 73:220–225 [DOI] [PubMed] [Google Scholar]
- Imai Y, Asada S, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. (2003) Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol Pharmacol 64:610–618 [DOI] [PubMed] [Google Scholar]
- Imai Y, Ishikawa E, Asada S, Sugimoto Y. (2005) Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2. Cancer Res 65:596–604 [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Janvilisri T, Shahi S, Venter H, Balakrishnan L, van Veen HW. (2005) Arginine-482 is not essential for transport of antibiotics, primary bile acids and unconjugated sterols by the human breast cancer resistance protein (ABCG2). Biochem J 385:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, et al. (2002) The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA 99:15649–15654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba S, An R, Saito H, Wakabayashi K, Tamura A, Ishikawa T. (2008) Human ABC transporters ABCG2 (BCRP) and ABCG4. Xenobiotica 38:863–888 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Schuetz JD. (2006) Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol 46:381–410 [DOI] [PubMed] [Google Scholar]
- Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL. (2001) Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology 121:1473–1484 [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. (2001) Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 61:3458–3464 [PubMed] [Google Scholar]
- Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, Boyer JL. (2006) Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology 43:1013–1021 [DOI] [PubMed] [Google Scholar]
- Mizuno N, Suzuki M, Kusuhara H, Suzuki H, Takeuchi K, Niwa T, Jonker JW, Sugiyama Y. (2004) Impaired renal excretion of 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl) benzothiazole (E3040) sulfate in breast cancer resistance protein (BCRP1/ABCG2) knockout mice. Drug Metab Dispos 32:898–901 [PubMed] [Google Scholar]
- Polgar O, Robey RW, Bates SE. (2008) ABCG2: structure, function and role in drug response. Expert Opin Drug Metab Toxicol 4:1–15 [DOI] [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. (2009) ABCG2: a perspective. Adv Drug Deliv Rev 61:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Jonker JW. (2003) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev 55:3–29 [DOI] [PubMed] [Google Scholar]
- Seward DJ, Koh AS, Boyer JL, Ballatori N. (2003) Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTα-OSTβ. J Biol Chem 278:27473–27482 [DOI] [PubMed] [Google Scholar]
- Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. (2007) Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta 1768:637–647 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Suzuki H, Sugimoto Y, Sugiyama Y. (2003) ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem 278:22644–22649 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. (2005) Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun 326:181–187 [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Wagenaar E, Karnekamp B, Merino G, Jonker JW, Schinkel AH. (2006) Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis 27:123–130 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou L, Gupta A, Vethanayagam RR, Zhang Y, Unadkat JD, Mao Q. (2006a) Regulation of BCRP/ABCG2 expression by progesterone and 17β-estradiol in human placental BeWo cells. Am J Physiol Endocrinol Metab 290:E798–E807 [DOI] [PubMed] [Google Scholar]
- Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, Boyer JL. (2006b) Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology 131:878–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarba A, Wild CP, Hall AJ, Montesano R, Hudson GJ, Groopman JD. (1992) Aflatoxin M1 in human breast milk from The Gambia, west Africa, quantified by combined monoclonal antibody immunoaffinity chromatography and HPLC. Carcinogenesis 13:891–894 [DOI] [PubMed] [Google Scholar]
- Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, Borst P. (2003) Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4). Biochem J 371:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7:1028–1034 [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Hruz P, Gutmann H, Terracciano L, Beuers U, Lehmann F, Beglinger C, Drewe J. (2006) Decreased expression of breast cancer resistance protein in the duodenum in patients with obstructive cholestasis. Digestion 74:101–108 [DOI] [PubMed] [Google Scholar]