Abstract
Tetrahydroxy bile acids become major biliary bile acids in Bsep(−/−) mice and Fxr(−/−) mice fed cholic acid; we characterized disposition of these novel bile acids that also occur in patients with cholestasis. We investigated mouse Mrp2 (mMrp2) and P-glycoprotein [(P-gp) mMdr1a]-mediated transport of a tetrahydroxy bile acid, 6α-OH-taurocholic acid (6α-OH-TC), and its biliary excretion in wild-type and Mrp2(−/−) mice in the presence or absence of N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918), a P-gp and breast cancer resistance protein inhibitor. 6α-OH-TC was rapidly excreted into bile of wild-type mice (78% recovery); coinfusion of GF120918 had no significant effect. In Mrp2(−/−) mice, biliary excretion was decreased (52% recovery) and coinfusion of GF120918 further decreased these values (34% recovery). In wild-type, but not Mrp2(−/−), mice, 6α-OH-TC increased bile flow 2.5-fold. Membrane vesicle transport studies of 6α-OH-TC (0.05–0.75 mM) yielded saturation kinetics with a higher apparent affinity for mMrp2 (Km = 0.13 mM) than for mMdr1a (Km = 0.33 mM); mBsep transported 6α-OH-TC with positive cooperativity (Hill slope = 2.1). Human multidrug resistance-associated protein (MRP) 2 and P-gp also transported 6α-OH-TC but with positive cooperativity (Hill slope = 3.6 and 1.6, respectively). After intraileal administration, the time course of 6α-OH-TC biliary recovery was similar to that of coinfused taurocholate, implying that 6α-OH-TC can undergo enterohepatic cycling. Thus, Mrp2 plays a key role in 6α-OH-TC biliary excretion, whereas P-glycoprotein plays a secondary role; Bsep likely mediates excretion of 6α-OH-TC in the absence of Mrp2 and P-gp. In Bsep(−/−) mice, efficient synthesis of tetrahydroxy bile acids that are Mrp2 and P-gp substrates can explain the noncholestatic phenotype.
Introduction
Biliary secretion is a complex process requiring transport of osmotically active solutes, primarily conjugated bile salts and glutathione, into the canalicular space (Blitzer and Boyer, 1982). Canalicular anion transport is mediated mostly by two unidirectional ATP-binding cassette (ABC) transport proteins: bile salt export pump (Bsep; Abcb11) and multidrug resistance-associated protein 2 (Mrp2; Abcc2). P-Glycoprotein [(P-gp) Mdr1a/b; Abcb1a/b] and Bcrp (Abcg2) are two additional ABC transporters that mediate canalicular efflux of drugs and their metabolites. Among the various canalicular transporters, Bsep plays a dominant role in biliary excretion of bile salts (Green et al., 2000; Wang et al., 2001), and it has a much greater transport capacity than any of the other canalicular transporters (Hofmann, 1994).
In humans, mutations in the BSEP gene that impair its function cause a fatal condition called type 2 progressive familial intrahepatic cholestasis (PFIC2) (Strautnieks et al., 1998; Jansen et al., 1999). PFIC2 is characterized by defective biliary bile salt secretion and extensive accumulation of bile salts in the liver, resulting in hepatic failure and death.
In contrast to the severe cholestasis occurring in man when BSEP is deficient, deletion of Bsep in the mouse causes only mild cholestasis. A major biliary bile acid in the Bsep(−/−) mouse was found to be the taurine-conjugated 12α-hydroxy derivative of β-muricholic acid [3α,6β,7β,12α-(OH)4-5β-cholyl taurine; 12α-OH-TβMC], indicating that this tetrahydroxy bile acid was formed in the mouse and could be excreted by one or more canalicular transporters other than Bsep (Wang et al., 2001). Another tetrahydroxy bile acid is formed in the Fxr(−/−) mouse when cholic acid is added to the diet (Cho et al., 2009); this bile acid was shown to be 3α,6α- or 6β,7α,12α-tetrahydroxy bile acid (as the taurine conjugate), and it is likely to be formed by 6-hydroxylation of cholic acid as such and its taurine conjugate. In patients with severe cholestasis, cholic acid is hydroxylated at C-6 to form 3α,6α,7α,12α-(OH)4-tetrahydroxy-5β-cholanoic acid, which is excreted in part in urine (Bremmelgaard and Sjövall, 1979, 1980).
We have shown that human MRP2 transports tauroursodeoxycholate (TUDC), a hydrophilic taurine-conjugated dihydroxy bile acid (Gerk et al., 2007). Lam et al. (2005) showed that the expression of Mdr1a, Mdr2, Mrp2, and Mrp3 was markedly induced in Bsep(−/−) mice. Taken together, these observations suggested that Mrp2 might mediate the Bsep-independent biliary efflux of hydrophilic tetrahydroxy bile acids. In this study, we determined the role of both mouse Mrp2 (mMrp2) and P-glycoprotein (mMdr1a) in the hepatobiliary excretion of the tetrahydroxy bile salt, 6α-OH-taurocholic acid (6α-OH-TC). Single-pass liver perfusion studies (in wild)-type and Mrp2(−/−) mice in the presence and absence of N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918), a P-gp and Bcrp inhibitor, were used to identify the contributions of mMdr1a and mMrp2 to the biliary transport of 6α-OH-TC. To characterize the kinetics of 6α-OH-TC transport, transport studies were performed in plasma membrane vesicles overexpressing mMrp2, mMdr1, or mBsep. We also characterized its transport by human MRP2- and MDR1-expressing membrane vesicles. To determine whether 6α-OH-TC was likely to undergo enterohepatic cycling in the mouse, its absorption from the terminal ileum and subsequent biliary excretion were assessed. Finally, we assessed the ability of 12α-OH-TβMC to inhibit mMrp2-mediated transport of 6α-OH-TC to determine whether it might also be an Mrp2 substrate.
Materials and Methods
Biochemicals and Radiochemicals.
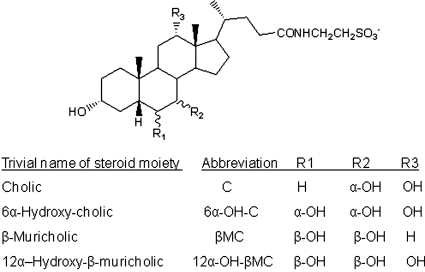
Sodium taurocholate was purchased from Sigma-Aldrich (St. Louis, MO). [14C]Taurocholic acid (55 mCi/mmol) was obtained from American Radiolabeled Chemicals, Inc. (St. Louis, MO). 3α,6α,7α,12α-Tetrahydroxy-5β-cholan-24-oic acid and 3α,6β,7β,12α-tetrahydroxy-5β-cholan-24-oic acid were synthesized from cholic acid as reported previously (Iida et al., 1989; Iida et al., 1990). These compounds can be considered to be the 6α-hydroxy derivative of cholic acid and the 12α-hydroxy derivative of β-muricholic acid (12α-OH-βMC), respectively. The glycine and taurine N-acylamidates of these compounds were prepared using diethylphosphorocyanide as a coupling reagent (Momose et al., 1997). The structures of the bile acids were confirmed by nuclear magnetic resonance (Iida et al., 1993) and are shown in Fig. 1.
Fig. 1.
Structure of cholic acid, 3α,6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid (6α-OH-C), β-muricholic acid, and 12α-OH-β-muricholic acid as their corresponding taurine conjugates.
The Δ22 derivative of 3α,6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid was prepared as described previously for β-muricholic acid (Iida et al., 1990; Kakiyama et al., 2004). The unconjugated 3α,6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid and its glycine and taurine amidate were then reduced with carrier-free tritium gas (Moravek Biochemicals, Brea, CA) to yield 22,23-3H-3α,6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid, together with its glycine and taurine amidate. These compounds were separated by preparative thin-layer chromatography as described previously (Hofmann, 1961) and eluted using the solvent 15 propionic acid; 20 iso-amylacetate; 5 water; 10 n-propanol. The retention factor for the unconjugated, glycine-conjugated, and taurine-conjugated 22,23-3H-3α,6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acids were 0.66, 0.33, and 0.16, respectively. The taurine-conjugated 22, 23-3H-3α, 6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid ([3H]6α-OH-TC) was extracted with methanol from the silica at the appropriate retention factor value, and further purified by reverse-phase high-performance liquid chromatography (HPLC) using a Symmetry C18, 4.6 × 250-mm column (Waters Corporation, Milford, MA). The flow rate was 0.75 ml/min, and the mobile phase was a mixture of methanol and 0.01 M KH2PO4 (67.4% v/v), adjusted to pH 5.6 with H3PO4 (Hagey et al., 1998). The column effluent was monitored at 200 nm. Nonradiolabeled 6α-OH-TC was used as a standard to obtain the retention time that corresponded to peak fractions between 5 and 6 min. The radioactive fractions were collected every minute for 15 min, and radioactivity in each fraction was assessed by liquid scintillation counting. A single radioactive peak was obtained corresponding to the retention time for 6α-OH-TC. The fraction was collected and dried under a stream of N2, and the residue was resuspended in a small volume of methanol. The final product was 98% radiochemically pure. The 22,23-3H-labeled bile acids have been shown to be stable during enterohepatic cycling (Duane et al., 1996). This same HPLC system was used to analyze bile from wild-type mice infused with 6α-OH-TC for the presence of any potential 6α-OH-TC metabolites.
GF120918 was a generous gift from Dr. Patrick McNamara (University of Kentucky). HPLC grade solvents (methanol, water) were purchased from Thermo Fisher Scientific (Waltham, MA). All other chemicals and reagents were of analytical grade and were available from commercial sources.
Membrane Vesicles.
Sf9 plasma membrane vesicles overexpressing mMrp2 or mouse P-gp (mMdr1a, mP-gp) as well as empty vector (EV) vesicles lacking transporter expression were purchased from CellzDirect (Durham, NC). Sf9 plasma membrane vesicles overexpressing mouse Bsep (mBsep) were obtained from XenoTech, LLC (Lenexa, KS). Mouse Bcrp plasma membrane vesicles were obtained from BD Biosciences (Woburn, MA). Human MRP2 (hMRP2) overexpressing plasma membrane vesicles were prepared as described previously (Gerk et al., 2007). Human MDR1 (hMDR1) recombinant baculovirus was a generous gift from Dr. Suresh Ambudkar (National Cancer Institute, Bethesda, MD). The recombinant baculovirus was amplified and titered by a viral plaque assay. Sf9 cells (5 × 108 cells) in suspension culture were infected in the presence of 5% fetal bovine serum using a multiplicity of infection of 10, and 48 h later, plasma membrane vesicles were isolated and stored as described previously (Ito et al., 2001; Gerk et al., 2004).
Animals.
Male C57BL/6 wild-type mice were purchased from (Harlan Industries, Indianapolis, IN). Abcc2(−/−) [Mrp2(−/−)] mice (25–30 g) in the C57BL/6 background were generously provided by Deltagen (San Mateo, CA) and Eli Lilly and Co. (Indianapolis, IN). Animals had free access to food and water and were maintained in a temperature-controlled environment on a 12-h light/dark cycle. All protocols involving animals were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Single-Pass Liver Perfusion and Enterohepatic Recirculation Studies.
Mice were anesthetized with urethane (2 mg/g b.wt., i.p.). The common bile duct was ligated above the duodenum, and the gallbladder was cannulated with PE-10 tubing. For liver perfusion studies, the portal vein was cannulated with a 20-gauge needle and the liver was perfused via the portal vein with Krebs-Henseleit buffer (118.5 mM NaCl, 24.9 mM NaHCO3, 1.2 mM KH2PO4, 1.19 mM MgSO4, 4.74 mM KCl, 1.27 mM CaCl2, 5 mM glucose, pH 7.4) at a flow rate of 5 ml/min in a single-pass perfusion system. Taurocholate (5 μM) was added to perfusion medium to maintain stable bile flow. Perfusate was saturated with 95% O2/5% CO2, and the liver was maintained at 36 ± 1.0°C. Immediately after the start of perfusion, the abdominal vena cava below the liver was severed and the inferior vena cava above the liver was cannulated with PE-60 tubing. After an ∼10-min period of equilibration of liver temperature and bile flow, [3H]6α-OH-TC (21 nCi/nmol) was infused into the portal vein at a rate of 24 nmol/min over 4 min. For P-gp inhibition studies, GF120918 (10 μM, 0.3 ml of dimethyl sulfoxide) or vehicle was added to the perfusion medium 5 min before [3H]6α-OH-TC infusion. Bile and perfusate outflow were collected in 5- (0–30 min) and 10-min intervals (30–90 min). The bile volume was determined gravimetrically, assuming a density of 1.0. At the end of the perfusion, livers were isolated and snap-frozen.
For the enterohepatic recirculation studies, intraileal administration of bile salts was performed as described previously, with slight modifications (Ballatori et al., 2008). In brief, the ileum was ligated at the ileocaecal junction and 5 cm proximal to the ileocecal junction. Saline (150 μl) containing [3H]6α-OH-TC (2μCi, 150 nmol) and a tracer dose of [14C]taurocholate (1 μCi, 15 nmol, a recovery standard) were injected with a 26-gauge needle directly into the lumen of the ileum between the sutures. Bile was collected in 5- (0–30 min) and 10-min intervals (30–120 min). [3H]6α-OH-TC and [14C]taurocholate in the bile were quantitated by liquid scintillation spectroscopy.
Analytical Methods.
Total radioactivity was quantitated in aliquots of bile and perfusate outflow after addition of 5 ml of Bio-Safe II cocktail (Research Products International Corp., Mt. Prospect, IL) by liquid scintillation spectroscopy. Liver radioactivity was counted as described previously (Chong-Rae Lim et al., 1996). Liver (0.2 g) was solubilized with 1 ml of Soluene-35 (PerkinElmer Life and Analytical Sciences, Waltham, MA) at 50°C for 12 h, and then 0.2 ml of isopropyl alcohol and 0.4 ml of hydrogen peroxide (30%) were added to minimize the color quenching. After neutralizing the mixture with 5 N HCl, 10 ml of scintillation cocktail was added and incubated in the dark at 25°C for 24 h; the radioactivity was then measured.
Vesicular Transport Assay.
ATP-dependent transport of [3H]6α-OH-TC into the inside-out Sf9 membrane vesicles overexpressing the mouse and human canalicular transporters and control vesicles were determined as described previously (Gerk et al., 2004). ATP-dependent transport of [3H]6α-OH-TC into membrane vesicles (10 μg/20 μl) was measured in incubations at 37°C for 2 min, transport stopped with 3.5 ml of ice-cold stop buffer, and the mixture was quickly filtered through Durapore 0.4-μm filters (Millipore, Bedford, MA). Radioactivity on the filters was detected by liquid scintillation spectroscopy. Reported transport values were corrected for that occurring in the presence of AMP as well as for any ATP-dependent transport in Sf9 vesicles expressing EV.
Statistical Analysis.
Data are presented as the mean ± S.D. (n = 4–5 animals per group). Statistical significance was assessed by two-way analysis of variance (ANOVA) with Tukey's post hoc test using GraphPad 4.0 (GraphPad Software Inc., San Diego, CA), as indicated in figure legends. For bile flow studies, one-way ANOVA with Bonferroni post hoc test was used. In all cases, P < 0.05 was considered to be statistically significant.
Kinetic Analysis.
Curve fitting was done by nonlinear regression analysis with GraphPad Prism 4. Values are presented as mean ± S.E. To determine allostery, data from concentration-dependent transport assays were analyzed according to the Hill equation:
 |
where v = velocity, Vmax = maximal velocity, [S] = initial substrate concentration, Km = the substrate concentration at half-maximal velocity, and n = Hill coefficient (Hill slope). The data were then compared with a fit to a one-binding site version of eq. 1 (n = 1), the Michaelis-Menten equation. To determine which of the two models best fit the data, the extra sum-of-squares F test was used.
Results
Disposition and Choleretic Effect of 6α-OH-TC in the Single-Pass Perfused Mouse Liver.
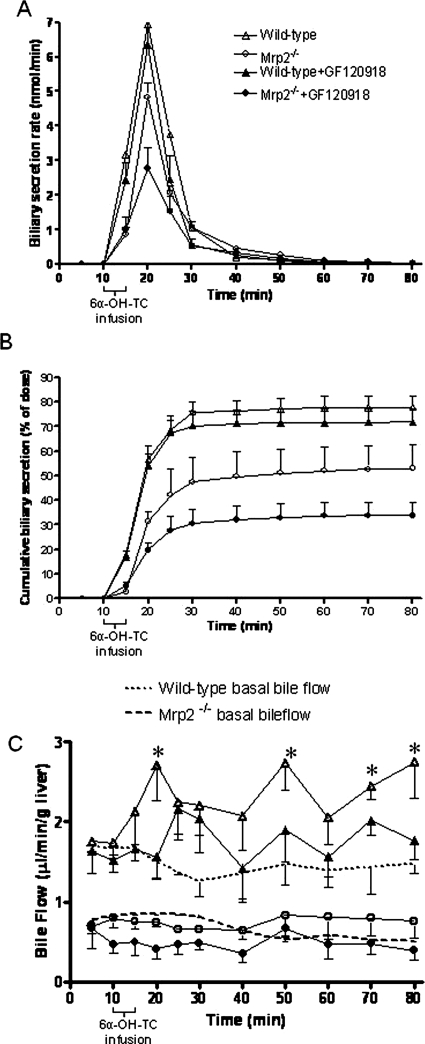
The involvement of Mrp2 and P-gp in the hepatobiliary transport of 6α-OH-TC was investigated by analyzing the uptake and biliary secretion of 6α-OH-TC in the perfused liver of wild-type and Mrp2(−/−) mice in the presence or absence of 10 μM GF120918 (Figs. 2 and 3). 6α-OH-TC was rapidly taken up from the perfusate and secreted in bile of wild-type mice. Sixty percent of the dose was secreted into the bile within 20 min of the start of infusion, and an additional 18% of the dose was secreted into bile over the next 40 min (Fig. 2, A and B; Table 1). Coinfusion of GF120918, a potent inhibitor of Mdr1 and Bcrp, did not significantly alter the biliary secretion of [3H]6α-OH-TC. In Mrp2(−/−) mice, however, the rate of biliary secretion of 6α-OH-TC was significantly decreased (Fig. 2A) compared to that in wild-type mice, both in the presence and absence of GF120918 [Mrp2(−/−), P < 0.001 versus wild-type] [Mrp2(−/−) + GF120918, P < 0.01 versus Mrp2(−/−)]. At each time point, the cumulative biliary secretion of 6α-OH-TC was significantly decreased in Mrp2(−/−) mice (±GF120918) compared with wild-type mice (Fig. 2B). HPLC analysis of radioactivity in bile from wild-type mice infused with 6α-OH-TC showed that all of the radioactivity eluted with the same retention time as the parent compound, indicating the absence of metabolism of 6α-OH-TC under these conditions (data not shown).
Fig. 2.
6α-OH-TC biliary secretion and bile flow in perfused livers from wild-type and Mrp2(−/−) mice in the presence or absence of GF120918, infused with [3H]6α-OH-TC (96 nmol; 2 μCi) from 10 to 14 min. A, 6α-OH-TC biliary secretion versus time. B, cumulative recovery (as percentage of infused dose) of 6α-OH-TC in perfused livers. Mean ± S.D. (n = 4–5 per group). C, bile flow in single-pass perfused livers. Basal bile flow in wild-type and Mrp2(−/−) mice in the absence of [3H]6α-OH-TC infusion is also shown. *, p < 0.05 versus wild-type basal file flow as shown by one-way ANOVA with Bonferroni post hoc test. Data represent the mean ± S.D. of n = 4 to 5 (A and B) or n = 3 to 4 (C) per group.
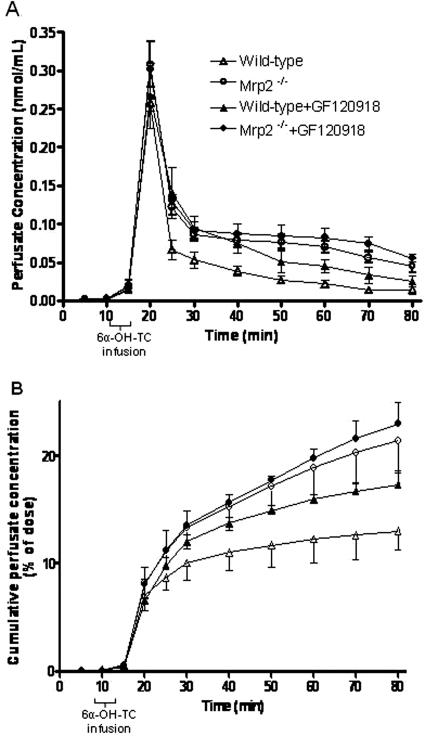
Fig. 3.
6α-OH-TC in the perfusate outflow in single-pass perfused livers from wild-type and Mrp2(−/−) mice in the presence or absence of GF120918. [3H]6α-OH-TC was infused from 10 to 14 min (96 nmol; 2 μCi). A, 6α-OH-TC concentration in perfusate outflow versus time. B, cumulative perfusate (shown as % of dose administered) of 6α-OH-TC. Data represent the mean ± S.D. (n = 4–5 per group) of animals shown in Fig. 2.
TABLE 1.
Percent recovery of 6α-OH-TC in perfused mouse liver
Data analyzed by two-way ANOVA with Tukey's post hoc test. Data represent the mean ± S.D. of 4 to 5 animals per group and are obtained from Figs. 2 and 3.
| −GF120918 |
+GF120918 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bile | Perfusate | Liver | Total | Bile | Perfusate | Liver | Total | |
| Wild-type mice | 78 ± 5 | 14 ± 2 | 0.2 ± 0.03 | 92 ± 4 | 75 ± 6 | 17 ± 3 | 0.23 ± 0.04 | 89 ± 8 |
| Mrp2(−/−) mice | 52 ± 3* | 26 ± 4* | 5.4 ± 2* | 84 ± 2 | 34 ± 5*‡ | 30 ± 2*‡ | 10 ± 2.2*‡ | 74 ± 5 |
p < 0.05 vs. wild-type mice.
p < 0.05 vs. Mrp2(−/−) mice.
The basal bile flow before 6α-OH-TC infusion in wild-type and Mrp2(−/−) mice was 1.22 ± 0.24 and 0.61 ± 0.20 μl/(min · g liver), respectively (Fig. 2C). Incorporation of 10 μM GF120918 into the perfusion medium throughout the perfusion period did not alter basal bile flow (data not shown), and bile flow in each group was stable throughout the perfusion period. In wild-type mice, a single bolus dose of 96 nmol of 6α-OH-TC infused via the portal vein produced an immediate increase in bile flow that was significantly greater compared with basal bile flow at some, but not all, time points (Fig. 2C). 6α-OH-TC administration in wild-type mice in the presence of GF120918 increased bile flow approximately 1.5-fold, but this increase was not statistically significant compared with wild-type basal bile flow (Fig. 2C). In contrast, 6α-OH-TC failed to produce choleresis in Mrp2(−/−) mice, either in the presence or absence of GF120918 (Fig. 2C).
The concentration of 6α-OH-TC in perfusate outflow versus time is shown in Fig. 3. Approximately 8 to 10% of the dose was seen in the perfusate within the first 5 min after infusion of 6α-OH-TC in all treatment groups and indicates the portion of the dose that was not extracted by the liver. However, at later time points, the concentration of 6α-OH-TC in the perfusate outflow in Mrp2(−/−) mice was higher compared with wild-type mice (Fig. 3, A and B), suggesting that the accumulated 6α-OH-TC in the liver, and that which was not secreted into bile in Mrp2(−/−) mice, was in part effluxed across the basolateral membrane into the perfusate. 6α-OH-TC was not retained extensively in the livers of wild-type mice, either in the presence or absence of GF120918. As noted in Table 1, 6α-OH-TC accumulated >20-fold in the livers of Mrp2(−/−) compared with wild-type mice, and infusion of GF120918 further increased the retention of 6α-OH-TC in the liver in Mrp2(−/−) mice.
Kinetics of ATP-Dependent [3H]6α-OH-TC Transport by Mouse and Human Canalicular Transporters in Vesicular Transport Assays.
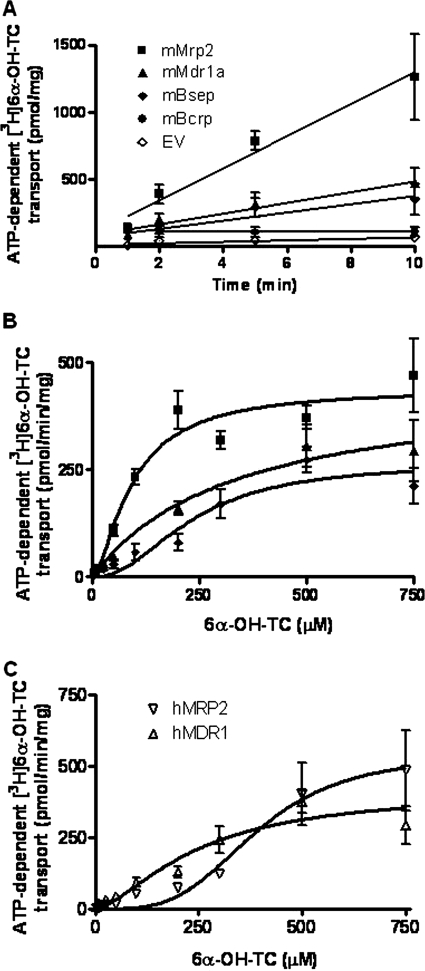
We characterized the transport of a broad concentration range (5–750 μM) of [3H]6α-OH-TC by membrane vesicles overexpressing mMrp2, mMdr1a, or mBsep to obtain the kinetic parameters. The transport of [3H]6α-OH-TC by mMrp2, mP-gp, and mBsep was linear with respect to incubation time (Fig. 4A); furthermore, linearity with respect to membrane protein concentration (5–20 μg) was established (data not shown). Membrane vesicles from Sf9 cells transfected solely with EV did not transport [3H]6α-OH-TC, nor did membrane vesicles expressing mouse Bcrp (Fig. 4A).
Fig. 4.
[3H]6α-OH-TC transport in membrane vesicles overexpressing mouse and human canalicular transporters. A, linearity of ATP-dependent [3H]6α-OH-TC transport with time using 10 μg of membrane protein and 150 μM [3H]6α-OH-TC. Data represent mean ± S.D. of triplicate determinations. B, saturation kinetics of ATP-dependent [3H]6α-OH-TC transport by mouse Mrp2, Mdr1a, and Bsep. Data are the mean ± S.E. of two independent experiments, each determined in triplicate. C, saturation kinetics of ATP-dependent [3H]6α-OH-TC transport by human MRP2 and MDR1. Data are the mean ± S.E. of two independent experiments, each determined in triplicate.
Mrp2 mediated saturable transport of [3H]6α-OH-TC that was best fitted to classic Michaelis-Menten kinetics (Fig. 4B; Table 2). mP-gp also transported [3H]6α-OH-TC via classic Michaelis-Menten kinetics with a lower apparent affinity compared with mMrp2. mBsep transported [3H]6α-OH-TC with positive cooperativity, as shown in Fig. 4B and Table 2. These data indicate that [3H]6α-OH-TC is a substrate for multiple canalicular transporters, namely mMrp2, mP-gp, and mBsep.
TABLE 2.
Kinetic analysis of [3H]6α-OH-TC transport by mouse and human canalicular transporters
Nonlinear regression results showing best-fit values calculated from the data shown in Fig. 4, B and C. Values in brackets indicate the 95% confidence interval.
| Kinetic Values | mMrp2 | mMdr1a | mBsep | hMRP2 | hMDR1 |
|---|---|---|---|---|---|
| Km (μM) | 133 (80–165) | 334 (257–411) | 232 (170–303) | 391 (362–420) | 245 (160–330) |
| Vmax [pmol/min · mg] | 522 (456–588) | 456 (411–500) | 267 (208–325) | 543 (500–587) | 415 (327–503) |
| Hill slope | 2.1 (1.2–3.2) | 3.6 (3–4) | 1.6 (1.1–2.1) |
In view of the formation of tetrahydroxy bile acids under cholestatic conditions in humans, we also investigated the [3H]6α-OH-TC transport by hMRP2 and hMDR1 overexpressing plasma membrane vesicles under conditions of linearity with respect to time and protein concentration. hMRP2 transported the bile acid with low apparent affinity but with strong evidence of positive cooperativity. hMDR1 showed higher apparent affinity for [3H]6α-OH-TC than mMdr1a and the transport was also positively cooperative, although to a lesser extent than that observed with hMRP2 (Fig. 4C; Table 2).
Biliary Secretion of [3H]6α-OH-TC and [14C]Taurocholate after Its Intraileal Administration in Wild-Type Mice.
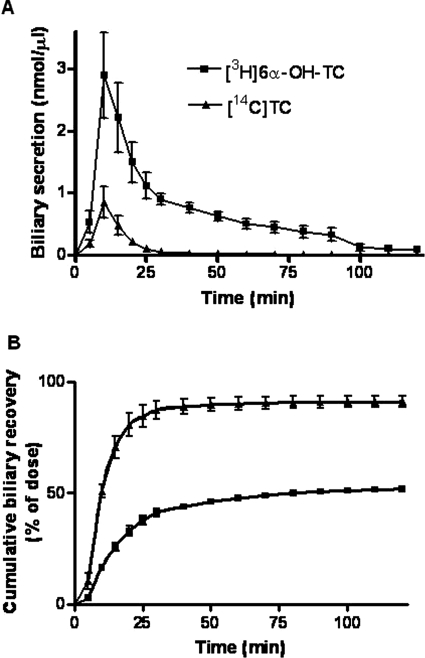
To determine the absorption of [3H]6α-OH-TC by the terminal ileum and therefore its potential for enterohepatic recirculation, [3H]6α-OH-TC was injected into the terminal ileum lumen of wild-type mice and its biliary recovery was measured. [14C]Taurocholate was coadministered as a recovery marker. The biliary secretion of [3H]6α-OH-TC and [14C]taurocholate followed a similar time course, with the majority of the dose excreted in bile within the first 15 min of their intraileal administration (Fig. 5A). However, the total recovery of [3H]6α-OH-TC radioactivity was lower (51%) than that of [14C]taurocholate (91%) at the end of the 120-min collection period (Fig. 5B). Nonetheless, these data indicated that [3H]6α-OH-TC is absorbed from the terminal ileum and can undergo enterohepatic recirculation, as do other major conjugated bile acids.
Fig. 5.
Biliary recovery of [3H]6α-OH-TC and [14C]TC after their intraileal administration in wild-type mice. A, biliary concentration of [3H]6α-OH-TC and [14C]TC versus time. B, cumulative biliary concentration of [3H]6α-OH-TC and [14C]TC acid versus time in wild-type mice. Data represent the mean ± S.D. (n = 4–5 per group).
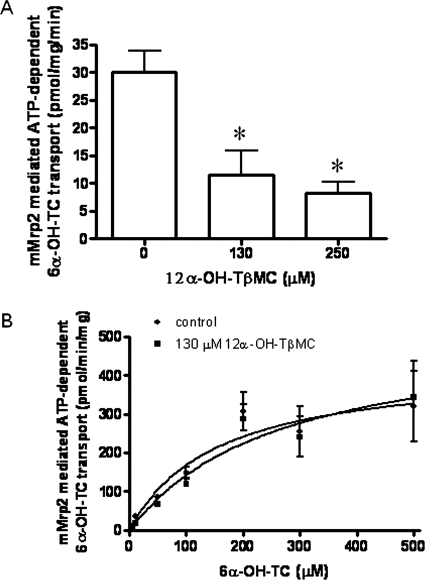
Inhibition of 6α-OH-TC Transport by 12α-OH-TβMC.
12α-OH-TβMC is the predominant tetrahydroxylated bile acid species in Bsep(−/−) mice (Perwaiz et al., 2003). To determine whether it might also be an Mrp2 substrate, we investigated its effect on 6α-OH-TC (13 μM) transport (Fig. 6A); 130 and 250 μM 12α-OH-TβMC significantly inhibited 6α-OH-TC transport by 61 and 72%, respectively (Fig. 6A). In the presence of 130 μM 12α-OH-TβMC, there was an increase in the Km for 6α-OH-TC from 150 to 263 μM, whereas the Vmax was increased from 426 to 536 pmol/(min · mg protein) (Fig. 6B), suggesting competitive inhibition.
Fig. 6.
Effect of 12α-OH-TβMC on 6α-OH-TC transport. A, inhibition of 6α-OH-TC (13 μM) transport by 12α-OH-TβMC in mMrp2-overexpressing plasma membrane vesicles. Each data point represents mean ± S.D. of triplicate determinations. Comparisons were made by Dunnett's test after one-way ANOVA. *, p < 0.05 versus control. B, saturation kinetics of ATP-dependent [3H]6α-OH-TC transport by murine Mrp2 in the absence and presence of 130 μM 12α-OH-TβMC. Data represent mean ± S.D. of triplicate determinations.
Discussion
Tetrahydroxy bile acids occur very rarely as biliary bile acids in vertebrates (Hofmann et al., 2010). They have recently been identified in substantial amounts in mice under two circumstances. The first is the Bsep(−/−) mouse, in which β-muricholic acid (3α,6β,7β-trihydroxy) undergoes C-12 hydroxylation, presumably by the microsomal hydroxylase Cyp8b1. The second is the Fxr(−/−) mouse ingesting a diet containing cholic acid, in which cholic acid (3α,7α,12α-trihydroxy) undergoes hydroxylation at C-6, preferably by Cyp3A11 (Cho et al., 2009). In humans, genetic defects in the BSEP gene that result in loss of BSEP expression cause the potentially fatal condition PFIC2, a disorder associated with markedly elevated serum bile acid levels and a low content of bile acids in bile (Jansen et al., 1999). In contrast to the fatal condition caused by BSEP deficiency in man, targeted inactivation of Bsep in mice is associated with a much less-severe phenotype. Bsep(−/−) mice have mild intrahepatic cholestasis with biliary bile acid secretion ∼30% of that in wild-type mice (Wang et al., 2001). The identification of tetrahydroxy biliary bile acids in the Bsep(−/−) mouse requires that such bile acids use canalicular transporters other than Bsep. Our data indicate that at least one tetrahydroxy bile acid is transported by at least three canalicular transporters in mice, Mrp2, Mdr1a, and Bsep. We also used vesicular transport studies to show that the human canalicular transporters, hMRP2 and hMDR1 also transport this tetrahydroxy bile acid.
6α-OH-TC was taken up efficiently and secreted rapidly into the bile of wild-type mice. A role of Mrp2 in its biliary secretion was clearly shown by the marked decrease in the cumulative biliary secretion of 6α-OH-TC in Mrp2(−/−) mice from 78 to 52% of the dose. However, the remaining 52% of the dose secreted in bile of Mrp2(−/−) mice implied the role of yet other canalicular transporters in 6α-OH-TC biliary secretion. Wang et al. (2009) recently generated the Bsep, Mdr1a, and Mdr1b triple knockout mice, which had a markedly increased severity of cholestasis, implying that P-gp can function as a compensatory mechanism in Bsep(−/−) mice. Therefore, we investigated the role of P-gp as a mechanism of biliary secretion of 6α-OH-TC using GF120918, a very potent inhibitor of P-gp and Bcrp (Hyafil et al., 1993; Allen et al., 2003). Infusion of 10 μM GF120918 has been shown to effectively inhibit P-gp in mouse liver (Tian et al., 2007). When GF120918 was coinfused with 6α-OH-TC in Mrp2(−/−) mice, cumulative biliary secretion of 6α-OH-TC was even further reduced to 34% of the dose, indicating that P-gp is likely another canalicular transporter for this bile salt. However, it should be noted that coinfusion of GF120918 in the wild-type mice did not alter biliary secretion of 6α-OH-TC. Based on these observations and the fact that P-gp expression in Mrp2(−/−) mice is similar to that in wild-type mice (Chu et al., 2006), we conclude that P-gp plays a secondary role to Mrp2 in 6α-OH-TC biliary secretion. The canalicular transporter Bcrp is also inhibited by GF120918. Thus, decreased biliary secretion of [3H] 6α-OH-TC in the presence of GF120918 could suggest a role for Bcrp in canalicular transport of [3H]6α-OH-TC, although Bcrp expression is not changed in Mrp2(−/−) mice (Chu et al., 2006). Although human BCRP expressed in Lactococcus lactis was shown to transport cholate, deoxycholate, and taurocholate (Janvilisri et al., 2005), we did not detect ATP-dependent transport of 6α-OH-TC in plasma membrane vesicles overexpressing murine Bcrp.
6α-OH-TC was taken up efficiently into hepatocytes, as indicated by its extensive and rapid elimination into bile. The efficiency of uptake may be somewhat less than that observed for taurocholate in the perfused rat liver, which exceeds 90% (Holzinger et al., 1998). The sodium-dependent uptake (Ntcp) and sodium-independent uptake (Oatp) transporters in the basolateral membrane play an important role in the hepatic uptake of physiologic conjugated bile acids. However, identification of the specific carriers responsible for 6α-OH-TC uptake requires further investigation. The concentration of 6α-OH-TC in the perfusate outflow was also measured. The continuing regurgitation of 6α-OH-TC at later time points from the liver of Mrp2(−/−) mice was greater than in wild-type mice, consistent with its decreased biliary clearance, and is likely due to efflux of 6α-OH-TC by basolateral transporters.
The above in vivo findings obtained from perfusion experiments were confirmed by in vitro transport experiments. [3H]6α-OH-TC was transported by membrane vesicles from mMrp2 expressing Sf9 cells in an ATP-dependent manner that was saturable, confirming that Mrp2 was capable of transporting this hydrophilic bile salt. mMdr1a overexpressing Sf9 membrane vesicles also transported 6α-OH-TC but with a lower apparent affinity than mMrp2. Therefore, it is possible that P-gp can play a significant role as an alternative canalicular transporter for tetrahydroxy bile salts in Bsep(−/−) mice, where its expression level is significantly up-regulated (Wang et al., 2009).
Characterization of transport of 3[H]6α-OH-TC by mBsep-overexpressing membrane vesicles showed that Bsep transported 6α-OH-TC with lower apparent affinity but also with significant positive cooperativity. Thus, Bsep could account for 34% of the [3H]6α-OH-TC dose recovered in bile in the absence of Mrp2 and P-gp, as seen in Mrp2(−/−) + GF120918 mice (Table 1). Our studies clearly imply that multiple canalicular transporters can mediate biliary secretion of 6α-OH-TC, as has been shown for substrates such as estradiol-17β-glucuronide (Vore et al., 1996; Morikawa et al., 2000), the opioid peptide [d-Pen2, d-Pen5]-enkephalin (Hoffmaster et al., 2004), and spiramycin (Tian et al., 2007). 12α-OH-TβMC appeared to inhibit mMrp2-mediated 6α-OH-TC transport in a competitive manner. This tetrahydroxy bile acid, which was unavailable to us in radioactive form, is thus also a likely substrate of mMrp2.
The low-affinity transport of 6α-OH-TC by hMRP2 and hMDR1, together with the low formation of tetrahydroxy bile acids in humans deficient in BSEP, provides a compelling rationale for the severity of PFIC2. However, the MRP2-mediated transport of 6α-OH-TC demonstrated marked homotropic cooperativity. It would be of interest to determine whether tetrahydroxy bile acids also exhibit heterotrophic cooperativity, that is, whether they can stimulate MRP2-mediated transport of other substrates or bile acids. Such heterotrophic cooperativity is demonstrated by the hydrophilic bile acid TUDC, which stimulates MRP2-mediated transport of estradiol-3-glucuronide and estradiol-17β-glucuronide (Gerk et al., 2007).
In the mouse liver perfusion studies, we noticed a mild but prolonged choleresis after administration of 6α-OH-TC in wild-type but not Mrp2(−/−) mice. These results imply that Mrp2-mediated secretion of 6α-OH-TC is responsible for the enhanced bile flow observed after its administration. In Bsep(−/−) mice, the average bile flow is reduced only slightly compared with wild-type controls [6.85 ± 2.58 versus 8.33 ± 2.71 μl/(min · 100 g b.wt.)] (Wang et al., 2001). Because Bsep(−/−) mice secreted unusually large quantities of tetrahydroxy bile salts, our findings that these bile salts possess choleretic effects can explain the nearly normal bile flow seen in Bsep(−/−) mice. The choleretic effect of tetrahydroxy bile acids may be considerably greater than that of trihydroxy bile acids, because they are likely to have a high critical micellization concentration and induce little phospholipid secretion (Gurantz and Hofmann, 1984).
Finally, the present studies showed that a taurine-conjugated tetrahydroxy bile acid is likely to undergo enterohepatic recycling as do other major bile acids. Recovery of 6α-OH-TC was less than that of taurocholate, however, indicating less efficient transport by the ileal enterocyte or decreased hepatic uptake, or both.
In conclusion, this study demonstrated that Mrp2 is the most likely primary canalicular transporter responsible for the biliary secretion of the tetrahydroxy bile acid, 6α-OH-TC, in mice. P-gp and Bsep also contributed to the residual secretion of this bile acid in Mrp2(−/−) mice. The apparent affinity of these transporters for 6α-OH-TC was in the order of Mrp2 > Pgp > Bsep (positive cooperativity). Thus, Mrp2 likely plays a beneficial role in cholestasis in mice due to loss of function of Bsep. The inability of Mrp2 to protect against the liver disease and mortality in the Bsep/mdr1a/mdr1b null mice (Wang et al., 2009) is not explained by our work. It could be due to poor transport of 12α-OH-TβMC by Mrp2, which we were unable to study directly, or possibly to the accumulation of other hepatotoxic metabolic products, including bacterial metabolites that might affect canalicular transport.
Identification of human MRP2 as a transporter of hydrophilic tetrahydroxy bile acids, together with the potential of 6α-OH-TC to increase MRP2 transport by heterotrophic activation, suggests that it might have utility in the treatment of PFIC2. Although tetrahydroxy bile acids are formed and excreted in urine in patients with cholestasis (Bremmelgaard and Sjövall, 1980), tetrahydroxy bile acids have never been shown to become major biliary bile acids in man as they do in the Bsep(−/−) or cholic acid-fed Fxr(−/−) mouse. Thus, in the mouse, formation of tetrahydroxy bile acids and their elimination by multiple canalicular carriers are life-saving adaptations to cholestasis, which do not occur in man.
Acknowledgments.
We gratefully acknowledge Dr. Thomas Raub of Eli Lilly for providing the Mrp2(−/−) mice. We also thank Mrs. Baoxiang Yan for her assistance in mice perfusion studies and Dr. Maciej J. Zamek-Gliszczynski (Eli Lilly) for helpful discussions regarding mice perfusion studies.
This work was supported in part by the United States Public Health Service [Grant HD 58299] (to M.V.); and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan [Grant 19510223] (to T.I.) for 2009–2011 and Research Project of the Institute of Natural Sciences Nihon University for 2009–2010.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.033480.
- ABC
- ATP-binding cassette
- BSEP/Bsep
- bile salt export pump
- MRP/Mrp
- multidrug resistance-associated protein
- P-gp
- P-glycoprotein
- Bcrp
- breast cancer resistance protein
- PFIC2
- type 2 progressive familial intrahepatic cholestasis
- 12α-OH-TβMC
- taurine-conjugated 12α-hydroxy β-muricholic acid
- Fxr
- farnesoid X receptor
- mMrp2
- mouse Mrp2
- 6α-OH-TC
- 6α-OH-taurocholic acid
- MDR1
- multidrug resistance 1
- [3H]6α-OH-TC
- taurine-conjugated 22, 23-3H-3α, 6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid
- HPLC
- high-performance liquid chromatography
- GF120918
- N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide
- mP-gp
- mouse P-gp
- EV
- empty vector
- mBsep
- mouse Bsep
- hMRP
- human MRP
- hMDR1
- human MDR1
- TUDC
- tauroursodeoxycholate
- ANOVA
- analysis of variance.
References
- Allen JD, Van Dort SC, Buitelaar M, van Tellingen O, Schinkel AH. (2003) Mouse breast cancer resistance protein (Bcrp1/Abcg2) mediates etoposide resistance and transport, but etoposide oral availability is limited primarily by P-glycoprotein. Cancer Res 63:1339–1344 [PubMed] [Google Scholar]
- Ballatori N, Fang F, Christian WV, Li N, Hammond CL. (2008) Ostalpha-Ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am J Physiol Gastrointest Liver Physiol 295:G179–G186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer BL, Boyer JL. (1982) Cellular mechanisms of bile formation. Gastroenterology 82:346–357 [PubMed] [Google Scholar]
- Bremmelgaard A, Sjövall J. (1979) Bile acid profiles in urine of patients with liver diseases. Eur J Clin Invest 9:341–348 [DOI] [PubMed] [Google Scholar]
- Bremmelgaard A, Sjövall J. (1980) Hydroxylation of cholic, chenodeoxycholic, and deoxycholic acids in patients with intrahepatic cholestasis. J Lipid Res 21:1072–1081 [PubMed] [Google Scholar]
- Cho JY, Matsubara T, Kang DW, Ahn SH, Krausz KW, Idle JR, Luecke H, Gonzalez FJ. (2010) Urinary metabolomics in Fxr-null mice reveals activated adaptive metabolic pathways upon bile acid challenge. J Lipid Res 51:1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong-Rae Lim K-HO, Kyoung Mi Kim, Soon-Hong Yuk, Hai-Bang Lee, Chong-Kook Kim. (1996) The enhancement of liver targetability of [3H]methotrexate-galactosylated serum albumin conjugate in mice. Int J Pharm 132:175–182 [Google Scholar]
- Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, et al. (2006) Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2). J Pharmacol Exp Ther 317:579–589 [DOI] [PubMed] [Google Scholar]
- Duane WC, Schteingart CD, Ton-Nu HT, Hofmann AF. (1996) Validation of [22,23-3H]cholic acid as a stable tracer through conversion to deoxycholic acid in human subjects. J Lipid Res 37:431–436 [PubMed] [Google Scholar]
- Gerk PM, Li W, Megaraj V, Vore M. (2007) Human multidrug resistance protein 2 transports the therapeutic bile salt tauroursodeoxycholate. J Pharmacol Exp Ther 320:893–899 [DOI] [PubMed] [Google Scholar]
- Gerk PM, Li W, Vore M. (2004) Estradiol 3-glucuronide is transported by the multidrug resistance-associated protein 2 but does not activate the allosteric site bound by estradiol 17-glucuronide. Drug Metab Dispos 32:1139–1145 [DOI] [PubMed] [Google Scholar]
- Green RM, Hoda F, Ward KL. (2000) Molecular cloning and characterization of the murine bile salt export pump. Gene 241:117–123 [DOI] [PubMed] [Google Scholar]
- Gurantz D, Hofmann AF. (1984) Influence of bile acid structure on bile flow and biliary lipid secretion in the hamster. Am J Physiol 247:G736–G748 [DOI] [PubMed] [Google Scholar]
- Hagey LR, Schteingart CD, Rossi SS, Ton-Nu HT, Hofmann AF. (1998) An N-acyl glycyltaurine conjugate of deoxycholic acid in the biliary bile acids of the rabbit. J Lipid Res 39:2119–2124 [PubMed] [Google Scholar]
- Hofmann AF. (1962) Thin-layer adsorption chromatography of free and conjugated bile acids on silicic acid. J Lipid Res 3:127–128 [Google Scholar]
- Hofmann AF, Hagey LR, Krasowski MD. (2010) Bile salts of vertebrates: structural variation and possible evolutionary significance. J Lipid Res 51:226–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann AF. (1994) Biliary secretion and excretion: the hepatobiliary component of the enterohepatic circulation of bile acids, in Physiology of the Gastrointestinal Tract, 3rd ed, vol 2 (Johnson LR, Alpers DH, Christensen J, Jacobson ED, Walsh JH. eds) pp 1555–1576, Raven Press, New York [Google Scholar]
- Hoffmaster KA, Zamek-Gliszczynski MJ, Pollack GM, Brouwer KL. (2004) Hepatobiliary disposition of the metabolically stable opioid peptide [D-Pen2, D-Pen5]-enkephalin (DPDPE): pharmacokinetic consequences of the interplay between multiple transport systems. J Pharmacol Exp Ther 311:1203–1210 [DOI] [PubMed] [Google Scholar]
- Holzinger F, Schteingart CD, Ton-Nu HT, Cerrè C, Steinbach JH, Yeh HZ, Hofmann AF. (1998) Transport of fluorescent bile acids by the isolated perfused rat liver: kinetics, sequestration, and mobilization. Hepatology 28:510–520 [DOI] [PubMed] [Google Scholar]
- Hyafil F, Vergely C, Du Vignaud P, Grand-Perret T. (1993) In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res 53:4595–4602 [PubMed] [Google Scholar]
- Iida T, Chang FC, Mushiake K, Goto J, Nambara T. (1993) Complete 1H and 13C resonance assignments of stereoisomeric 3α,6α,7α,12α-tetrahydroxycholanoic acids by two-dimensional shift-correlated NMR. Magn Reson Chem 31:645–651 [Google Scholar]
- Iida T, Komatsubara I, Yoda S, Goto J, Nambara T, Chang FC. (1990) Potential bile acid metabolites. 16. Synthesis of stereoisomeric 3 alpha,6,7,12 alpha-tetrahydroxy-5 beta-cholanoic acids. Steroids 55:530–539 [DOI] [PubMed] [Google Scholar]
- Iida T, Momose T, Tamura T, Matsumoto T, Chang FC, Goto J, Nambara T. (1989) Potential bile acid metabolites. 14. Hyocholic and muricholic acid stereoisomers. J Lipid Res 30:1267–1279 [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Ito K, Suzuki H, Sugiyama Y. (2001) Single amino acid substitution of rat MRP2 results in acquired transport activity for taurocholate. Am J Physiol 281:G1034–G1043 [DOI] [PubMed] [Google Scholar]
- Jansen PL, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal EM, Hooiveld GJ, Koning JH, De Jager-Krikken A, Kuipers F, Stellaard F, et al. (1999) Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology 117:1370–1379 [DOI] [PubMed] [Google Scholar]
- Janvilisri T, Shahi S, Venter H, Balakrishnan L, van Veen HW. (2005) Arginine-482 is not essential for transport of antibiotics, primary bile acids and unconjugated sterols by the human breast cancer resistance protein (ABCG2). Biochem J 385:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiyama G, Iida T, Yoshimoto A, Goto T, Mano N, Goto J, Nambara T, Hagey LR, Hofmann AF. (2004) Chemical synthesis of (22E)-3α,6α,7α-trihydroxy-5β-chol-22-en-24-oic acid and its taurine and glycine conjugates: a major bile acid present in the rat. J Lipid Res 45:567–573 [DOI] [PubMed] [Google Scholar]
- Lam P, Wang R, Ling V. (2005) Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry 44:12598–12605 [DOI] [PubMed] [Google Scholar]
- Momose T, Tsubaki T, Iida T, Nambara T. (1997) An improved synthesis of taurine- and glycine-conjugated bile acids. Lipids 32:775–778 [DOI] [PubMed] [Google Scholar]
- Morikawa A, Goto Y, Suzuki H, Hirohashi T, Sugiyama Y. (2000) Biliary excretion of 17beta-estradiol 17beta-D-glucuronide is predominantly mediated by cMOAT/MRP2. Pharm Res 17:546–552 [DOI] [PubMed] [Google Scholar]
- Perwaiz S, Forrest D, Mignault D, Tuchweber B, Phillip MJ, Wang R, Ling V, Yousef IM. (2003) Appearance of atypical 3 alpha,6 beta,7 beta,12 alpha-tetrahydroxy-5 beta-cholan-24-oic acid in spgp knockout mice. J Lipid Res 44:494–502 [DOI] [PubMed] [Google Scholar]
- Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, et al. (1998) A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet 20:233–238 [DOI] [PubMed] [Google Scholar]
- Tian X, Li J, Zamek-Gliszczynski MJ, Bridges AS, Zhang P, Patel NJ, Raub TJ, Pollack GM, Brouwer KL. (2007) Roles of P-glycoprotein, Bcrp, and Mrp2 in biliary excretion of spiramycin in mice. Antimicrob Agents Chemother 51:3230–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vore M, Hoffman T, Gosland M. (1996) ATP-dependent transport of beta-estradiol 17-(beta-D-glucuronide) in rat canalicular membrane vesicles. Am J Physiol 271:G791–G798 [DOI] [PubMed] [Google Scholar]
- Wang R, Chen HL, Liu L, Sheps JA, Phillips MJ, Ling V. (2009) Compensatory role of P-glycoproteins in knockout mice lacking the bile salt export pump. Hepatology 50:948–956 [DOI] [PubMed] [Google Scholar]
- Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, Helgason CD, Ackerley C, Phillips MJ, Ling V. (2001) Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci USA 98:2011–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]