Abstract
Background and Purpose
This study investigated the effects of intravenous recombinant Fv-Hsp70 protein on infarction volume and behavior following experimental ischemic stroke.
Methods
Focal cerebral ischemia was produced by occluding the middle cerebral artery (MCA) using the intraluminal suture technique. Rats subjected to 2 hours of focal ischemia were allowed to survive 24 h. At 2 ¼ h and 3 h after onset of ischemia, Fv-Hsp70 recombinant protein (0.5 mg / kg) or saline was injected via the tail vein. Sensory-motor function and infarction volume were assessed at 24 h following ischemia.
Results
Administration of Fv-Hsp70 following focal cerebral ischemia significantly decreased infarct volume by 68% and significantly improved sensory-motor function compared to the saline-treated control group. Western blots showed Fv-Hsp70 in ischemic but not in control brain; and Fv-Hsp70 suppressed endogenous Hsp70.
Conclusion
Fv-Hsp70 protects ischemic brain in this experimental stroke model.
Keywords: Hsp70, antibody, Fv, ischemic stroke, recombinant protein, behavior, rat
Introduction
Tissue plasminogen activator (tPA) is the only medication approved by the FDA for treatment of acute ischemic stroke. Though effective, the limited time window for treatment and its complications limit its use 1. Thus, there is an unmet need for developing effective therapies for stroke.
Hsp70 is a molecular chaperone that protects cells against many types of stress. Neuroprotection by exogenously delivered Hsp70 has been demonstrated by many independent research groups using a variety of approaches including viral transfection of brain; intrathecal delivery of protein; delivery of naked RNA; transgenic and other approaches using both in vitro and in vivo models 2-6. However, none of these methods are likely to be useful or successful in the clinical setting of acute stroke. In order to test the potential of Hsp70 for treating stroke, we searched for a reliable method for in vivo delivery of protein.
The plasma membrane and blood brain barrier present formidable obstacles to delivery of therapeutic molecules to intracellular compartments. Effective molecular therapy depends upon efficient intracellular delivery vehicles. The cell-penetrating single chain fragment of the anti-DNA antibody mAb 3E10 (Fv) is a molecular delivery vehicle that we have shown produces Hsp70 protein-mediated neuroprotection in vitro 7. Thus, this study examined the potential of intravenous administration of Fv-Hsp70 for treating stroke in vivo when given at clinically relevant times following experimental strokes in rats. We show that Fv-Hsp70 given at 2¼ and 3 hours following stroke enters ischemic but not control brain, markedly decreases infarct volume and improves sensory-motor function.
Materials and Methods
Expression of Fv-Hsp70 recombinant protein in P. pastoris
Single chain Fv antibody was derived from mAb 3E10. cDNA for the Fv fragment was ligated into the plasmid pPICZαA, as previously described 7. Briefly, human Hsp70 cDNA was ligated into the Fv containing pPICZαA separated by Myc and His6 tags. The subsequent construct was electroporated into the X-33 methyolotropic yeast strain Pichia pastoris (Invitrogen, Carlsbad, CA). Recombinant protein was purified from the medium using Ni-NTA agarose beads (Qiagen, Valencia, CA) under sterile conditions. Protein was eluted from the column with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 500 mM imidazole, pH 8.0) in five 1.5 ml aliquots. Aliquots were then exchange dialyzed with Dulbecco's PBS (Mediatech, Manassas, VA) to remove the imidazole (with a final concentration of imidazole less than 2 mM). Final sample volume was 1-3 ml with a Fv-Hsp70 concentration of about 0.5 mg/ml. Fv-Hsp70 protein was stored at 4°C and used within 24h.
Animals
Thirty seven male Sprague-Dawley rats weighing 298-346 g (Charles River Labs, USA) were used in this study. The Institutional Animal Care and Use Committee (IACUC) at the University of California at Davis reviewed and approved the animal protocols in accordance with NIH guidelines.
Middle Cerebral Artery Occlusion
Middle cerebral artery occlusion (MCAO) was produced using the intraluminal suture technique 8. Briefly, rats were anesthetized with 3% isoflurane and maintained on 1.5% isoflurane with 100% oxygen. The right common carotid artery was exposed via a ventral midline incision. To occlude the MCA, a 3-0 monofilament nylon suture with the tip rounded by heat was inserted into the external carotid artery (ECA) and advanced into the internal carotid artery approximately 20-23 mm beyond the carotid bifurcation until mild resistance was felt. Rectal temperature was maintained at 36.6°C to 37.5°C with a heating blanket throughout the procedure.
Rats subjected to 2 h of focal ischemia followed by 22 h reperfusion were allowed to survive 24h. Behavior was assessed immediately following surgeries and only rats demonstrating circling contralateral to the MCA occlusion were included in the study.
Administration of Fv-Hsp70 Recombinant Protein
Fv-Hsp70 recombinant protein or saline was injected twice via the tail vein. The first injection was performed at 15 min after reperfusion (2¼ hours after onset of ischemia), and the second injection was performed at 1h after reperfusion (3 hours after the onset of ischemia). Each dose of Fv-Hsp70 protein was 0.5mg/kg. Two separate protein injections were performed in order to increase the amount of protein in blood and prolong its action within the 2¼ to 3 hour therapeutic window. The control group was injected with the same volume of 0.9 % sodium chloride at the same time points.
Infarction Volume Measurement
Twenty rats were used for the measurement of infarction volume. Brains were removed from euthanized rats and were cut into 2 mm thick coronal slices with the aid of an acrylic brain matrix (Zivic Instruments, Pittsburgh, PA). Slices were placed into a 2% solution of 2, 3, 5 - triphenyltetrazolium chloride (TTC) and then warmed in a 37°C chamber for 10 minutes. Slices treated with paraformaldehyde (4%) were refrigerated at 4°C for 48 hours prior to digitization on a flatbed scanner at 1200 dpi.
Infarct area of each section was measured by a blinded investigator using NIH ImageJ software 9 and total infarct volumes were calculated. Ipsilateral and contralateral hemisphere volumes were also determined in order to correct for edema, which can artificially affect the infarct volume 10. The formula used to calculate the edema corrected infarct volume was: corrected infarct volume = contralateral volume/ipsilateral volume × infarct volume.
Behavior Testing
Behavior was assessed by a blinded investigator at 24 hours following onset of ischemia in the same animals that were used for the measurement of infarction volume. The vibrissae-induced forelimb placement test was used to assess sensory-motor function 11. This test was specifically chosen since it requires intact vibrissae sensory and forelimb motor cortical function11, and both vibrissae sensory and forelimb motor cortex lie squarely within the MCA vascular territory. Briefly, the rat was held parallel to the edge of a bench and one set of whiskers were brushed against its edge. Movement of the front forelimb, ipsilateral to the whisker stimulation, was monitored as a response and recorded as successful if the paw was placed on the bench surface, with or without pad contact. The percentage of successful forelimb placements over 10 trials was calculated for forelimbs ipsilateral and contralateral to the side of stroke.
Immunohistochemistry
After the measurement of infarction volume, the brain slices were used for immunohistochemistry. Coronal sections 50 μm thick were cut in a cryostat (−20°C). Primary antibody for Hsp70 (monoclonal, 1:200 dilution, BioVision, USA) was incubated with the sections overnight at 4°C. A biotinylated horse anti-mouse IgG (1:200 dilution, Vector Labs, USA) secondary antibody was used The antibody complex was detected using ABC reagent and a substrate solution of H2O2 and diaminobenzidine (DAB) according to the manufacturer's instructions (Vector Labs). Non-specific labeling was assessed by omitting the primary antibody.
Western Blot Analysis
Brains from nine rats were treated as previously described for Western blot analysis12. Fifty μg of protein isolated from the hemisphere ipsilateral to MCAO was loaded into each lane. Membranes were probed overnight at 4°C with rabbit polyclonal anti-Hsp70 (1:4000, Stressgen) antibody. Primary antibody was detected using horseradish peroxidase-conjugated anti-rabbit IgG (Bio-Rad). The signal was detected using the Pierce ECL chemi-luminescent detection system (Thermo Scientific).
Physiological Parameters
Eight rats were used for the measurement of physiologic variables. Physiological variables were measured at 4 time points: 1) Before injection; 2) the first injection; 3) the second injection; and 4) 24h following focal cerebral ischemia. Either saline or Fv-Hsp70 recombinant protein was injected. Heart rate, breathing rate and SpO2 were measured using the MouseOx ® Murine Pulse Oximeter System (Starr Life Sciences Corp, USA). Cerebral blood flow (CBF) was measured using a Laser Doppler Monitor (Perimed Inc, Sweden) 13.
Statistical Analysis
Data are reported as mean ± standard error of the mean. T-tests were performed when comparing two groups. One-way ANOVA was performed with a Fisher LSD post hoc test when comparing variables for multiple groups/time points. A p value of less than 0.05 was considered statistically significant.
Results
Physiological Parameters
Compared to baseline, heat rate, breathing rate, CBF, SpO2, and temperature did not significantly differ following either Fv-Hsp70 or saline injection at any time point. CBF was significantly decreased (p < 0.05) at 24 h following ischemia compared to 15 min after reperfusion (first injection of either Fv-Hsp70 or saline). Compared to the control group, heart rate, breathing rate, CBF, SpO2, and temperature did not change significantly (p > 0.05) following Fv-Hsp70 protein injection at any time point (Table 1).
Table 1. Physiological parameters in animals injected with Fv-Hsp70 protein or saline following focal cerebral ischemia (n=4 per group).
| Parameter | Group | Before Injection |
1st Injection |
2nd Injection |
24h after Ischemia |
|---|---|---|---|---|---|
| Breathing Rate (breaths/min) | Control | 64.8 ± 1.7 | 67.0 ± 1.9 | 64.3 ± 2.2 | 68.7 ± 4.4 |
| Fv-Hsp70 | 68.8 ± 3.1 | 73.8 ± 3.9 | 74.8 ± 4.4 | 70.7 ± 4.9 | |
| Heart Rate (beats/min) | Control | 398.0 ± 9.4 | 428.0 ± 11.8 | 430.0 ± 14.4 | 380.3 ± 11.0 |
| Fv-Hsp70 | 396.0 ± 18.6 | 399.8 ± 5.8 | 423.8 ± 13.7 | 403.3 ± 6.2 | |
| SpO2 (%) | Control | 99.0 ± 0.1 | 98.9 ± 0.2 | 99.1 ± 0.1 | 98.7 ± 0.1 |
| Fv-Hsp70 | 99.0 ± 0.1 | 98.7 ± 0.1 | 97.9 ± 0.5 | 98.7 ± 0.1 | |
| Temperature (°C) | Control | 37.1 ± 0.2 | 36.9 ± 0.1 | 36.9 ± 0.2 | 37.3 ± 0.3 |
| Fv-Hsp70 | 37.1 ± 0.2 | 37.0 ± 0.2 | 37.2 ± 0.1 | 37.4 ± 0.1 | |
| CBF (perfusion unit) | Control | 151.0 ± 5.5 | 173.4 ± 8.8* | 153.4 ± 5.7 | 116.3 ± 10.6* |
| Fv-Hsp70 | 125.8 ± 16.5 | 148.5 ± 22.1† | 124.2 ± 10.9 | 102.3 ± 13.1† | |
SpO2: pulse oxygen saturation; CBF: cerebral blood flow.
p < 0.05 compared with each other in the control group.
p < 0.05 compared with each other in the Fv-Hsp70 group using ANOVA following by Fisher LSD Method.
There is no significant difference of any parameters between the two groups at the same time points.
Fv-Hsp70 Reduced Infarct Volume
Fv-Hsp70 protein injection significantly decreased infarct volumes. Average infarct volume, as determined by TTC staining (Figure 1A), was 65.91 ± 27.31 mm3 in the Fv-Hsp70 treated rats, which was significantly (p < 0.05) less than the saline treated control rats, which had an average infarct volume of 208.62 ± 42.59 mm3 (Figure 1B). Thus, the infarct volume was markedly decreased by ∼68% following Fv-Hsp70 injection.
Figure 1.
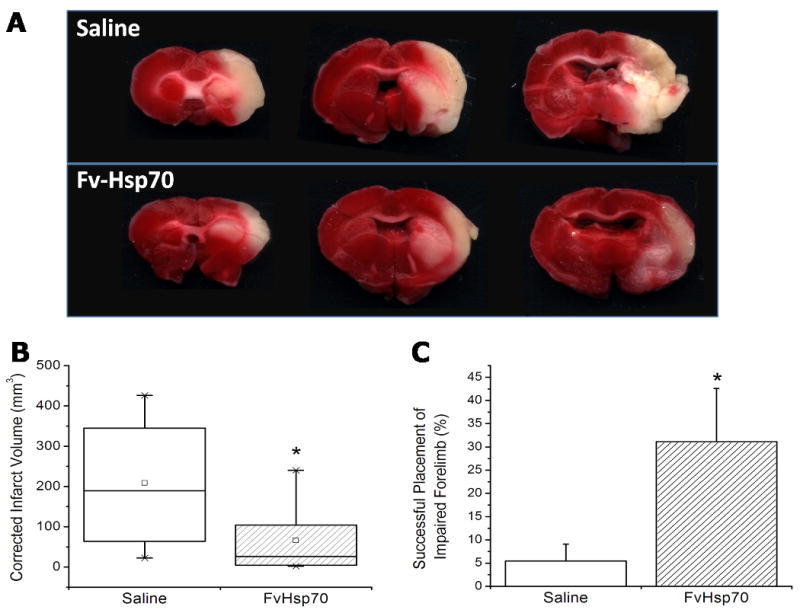
Effect of Fv-Hsp70 protein on the infarct volume and neurological behavior in adult male rats following focal cerebral ischemia. The infarct was produced by occluding the middle cerebral artery (MCA) for 2.0 hours using a suture. Either saline or Fv-Hsp70 recombinant protein (0.5mg/kg) was infused intravenously at 15 min and 1h after reperfusion – that is at 2 ¼ hours and 3.0 hours after the onset of focal ischemia. A. TTC staining in rat brain 24 hours after focal ischemia in saline injected and Fv-Hsp70 injected animals. B. Infarct volumes in rat brain 24 hours after focal ischemia. Data for each treatment group is represented as a separate box. The large box represents the 25th and 75th percentiles. The whiskers demonstrate the 5th and 95th percentiles. Means are indicated by the small squares. * p = 0.015. C. Percentage of successful forelimb placements of rats following stimulation of ipsilateral vibrissae over ten trials. Placement was unsuccessful for nearly all trials in the saline treated rats, whereas Fv-Hsp70 treated rats successfully responded with correct placement at a significantly higher rate. Placement scores for the right forelimb were near perfect for both groups. * p = 0.038.
Fv-Hsp70 Improved Neurological Behavior
Fv-Hsp70 protein injection significantly improved behavioral function on the forelimb placement test (Figure 1C). Forelimb placement in response to whisker stimulation was used to measure the sensory-motor deficit following MCAO. Forelimb placement of the unimpaired side was near perfect for both the Fv-Hsp70 and control groups with 97.8 ± 1.5 % and 100.0 ± 0.0 % successful placements, respectively (data not shown). However, forelimb placement of the impaired side was much lower for the saline treated rats (5.5 ± 3.7 %). The Fv-Hsp70 rats had a statistically significant improvement in the success rate for placement of their impaired forelimb at 31.1 ± 11.5 % (p < 0.05) (Figure 1C).
Fv-Hsp70 Inhibited Endogenous Hsp70 Protein Expression
Western blot analyses showed that the endogenous 70kD Hsp70 was induced at 24h in the brains of rats subjected to MCAO and injected with saline (Figure 2, lane 1). Unexpectedly, there was suppression of the endogenous 70kD Hsp70 protein normally induced by MCAO in the brains of animals subjected to MCAO and who received the Fv-Hsp70 protein (Figure 2, lane 2). Endogenous 70kD Hsp70 was not induced in the brains of sham operated rats (Figure 2, lane 3). Though the 110kD Fv-Hsp70 recombinant protein was not detected, a protein of about 210kD (Figure 2, lane 2) was detected in one third of the brains from the rats subjected to MCAO and who received Fv-Hsp70 protein. This protein is probably a dimer of 110kD Fv-Hsp70 recombinant protein. Immunohistochemistry of brains of animals subjected to MCAO and who received Fv-Hsp70 demonstrated many Hsp70 positive cells (Figure 3A). These cells stained with an anti-Hsp70 antibody probably represent Fv-Hsp70 immunostained cells (Figure 3A) since the endogenous Hsp70 was suppressed by Fv-Hsp70 (Figure 2, lane 2). The Fv-Hsp70 immunostained cells included neurons and glia (Figure 3A) found in the “penumbral cortex” adjacent to the infarction (Figure 3B).
Figure 2.
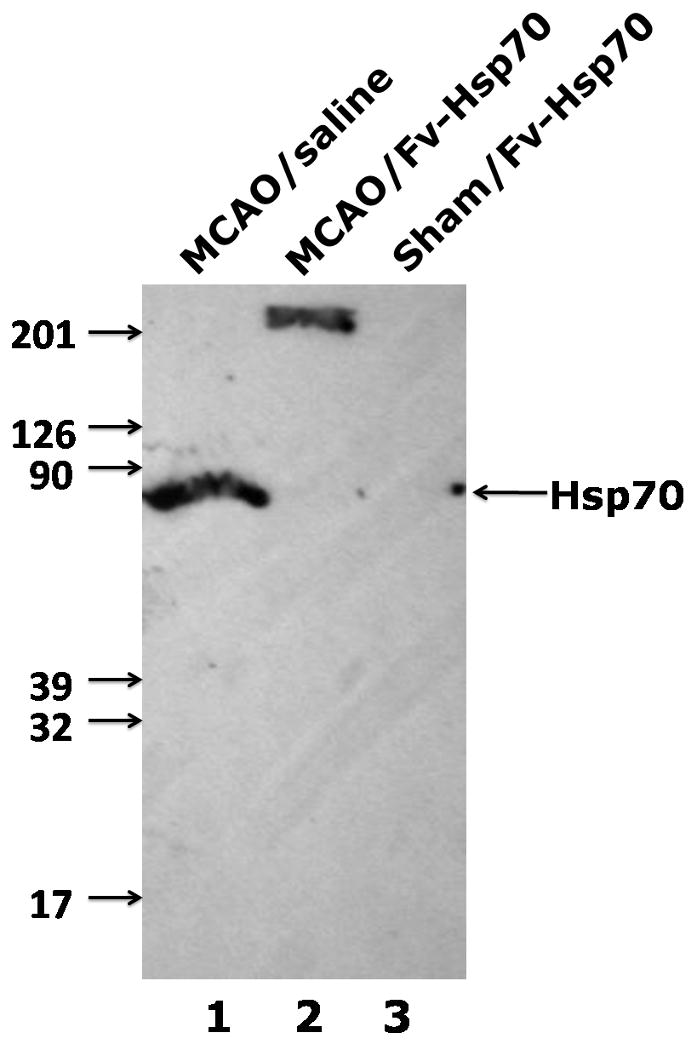
A Western blot is shown for Hsp70 protein in ipsilateral rat cortex after MCAO with Fv-Hsp70 injection (MCAO/Fv-Hsp70) and after MCAO with vehicle-saline injection (MCAO/saline). There was induction of endogenous 70kD Hsp70 protein (lane 1) in the MCAO/saline brain but not in the MCAO/Fv-Hsp70 brain. Instead, a 210kD protein was detected in the MCAO/Fv-Hsp70 brain (lanes 2). Hsp70 protein was not detected in the sham operated rat brain (lane 3).
Figure 3.
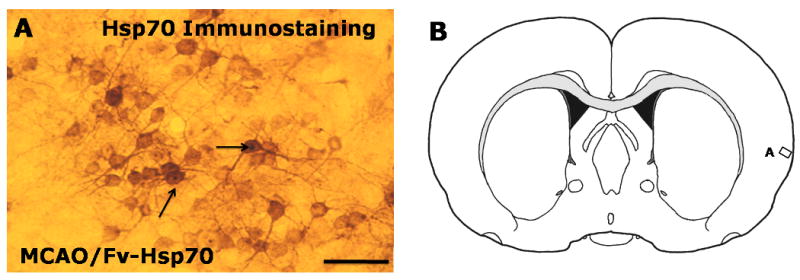
Fv-Hsp70 protein in rat cortex. A. Hsp70 immunostaining in cortex adjacent to an infarction in rat brain at 24h after MCAO and Fv-Hsp70 injections (MCAO/Fv-Hsp70). The Hsp70 stained cells indicate Fv-Hsp70 positive neurons and glial cells. The arrows point to Fv-Hsp70 positive nuclei in neurons. B. Schematic coronal rat brain section with a rectangle labeled A which indicates the location of the image in panel A (after Paxinos and Watson, 1998). Bar =25μm.
Discussion
The results demonstrate that intravenous administration of recombinant Fv-Hsp70 protein protects against focal cerebral ischemia. This is achieved at least in part by reducing cell death as measured by infarct volumes which improves sensory-motor function compared to saline-injected control MCAO animals.
Exogenous Fv-Hsp70 Protects against Focal Ischemic Injury
The mechanism by which Fv-Hsp70 recombinant protein protects against ischemic injury appears to involve exogenous Fv-Hsp70 rather than endogenous Hsp70. Hsp70 has been demonstrated to provide protection against in vivo cerebral ischemia using viral, transgenic, drug and other means of delivery 3, 4, 6, 14.
A recent in vivo study has provided evidence that Hsp70 provides protection via its effects on apoptosis since it showed that the C-terminal portion of Hsp70 was as protective as the full length molecule 13. This is an important observation since the N-terminal portion of Hsp70 is the ATP-binding portion that is essential for protein refolding, whereas the C-terminus is the peptide binding, anti-apoptotic portion of the Hsp70 molecule 15. Since the full length Hsp70 was used in the current study, it is possible that the re-folding functions and/or the anti-apoptotic functions accounted for the neuroprotection. Future studies are needed to address the alternative mechanisms.
Since the entire Fv-Hsp70 construct was neuroprotective, it is difficult to know whether the Fv portion of the construct provided any protection. We doubt Fv is protective since administration of Fv to neurons in vitro did not protect against hydrogen peroxide toxicity or against oxygen-glucose deprivation 7. However, future in vivo studies will need to compare Fv-Hsp70 to Fv alone.
Endogenous Hsp70 Induction Is Suppressed Following Fv-Hsp70 Administration
One of the intriguing findings of this study was the inhibition of endogenous Hsp70 expression following Fv-Hsp70 protein administration. Heat shock genes have functional heat shock elements (HSE) in their promoters that are bound and activated by heat shock factor proteins (HSF, a family of transcription factors) to initiate Hsp transcription following heat shock or stress 16-18. HSF is normally bound to Hsp90 in the resting cell. With the presence of denatured proteins, Hsp90 dissociates from HSF and binds the abnormal proteins 19. The HSFs are freed, form trimers, and are activated. The activated trimers act at the HSE of Hsp70 to initiate Hsp70 transcription. Once Hsp70 is induced, it binds to denatured proteins to prevent further protein denaturation in collaboration with other chaperones 20.
Administration of a drug that binds Hsp90, like geldanamycin, stimulates the transcription of heat shock genes. Once Hsp70 protein is produced in large amounts, it binds to HSF1 in the transcriptional domains to suppress endogenous Hsp70 protein production 21. Here we show that exogenous Hsp70 suppresses the induction of endogenous Hsp70. We propose that Fv-Hsp70 binds to HSF1 and prevents HSF1 from binding to HSEs in the Hsp70 promoter, thereby preventing induction of endogenous Hsp70 mRNA and protein following MCAO. Thus, exogenous Fv-Hsp70 protein provides neuroprotection in spite of suppression of endogenous Hsp70 production.
We postulate that the reason for the marked protection afforded by exogenous Fv-Hsp70 relates to timing. The endogenous Hsp70 protein is not induced until around 4-6 hours after brain ischemia and is not maximal until 24 hours after ischemia. In contrast, exogenous Fv-Hsp70 protein was given at 2 ¼ and 3 hours following brain ischemia in this study. The exogenous Fv-Hsp70 likely protects because it peaks in brain shortly after administration at 2 ¼ and 3 hours whereas endogenous Hsp70 protection is probably not maximal until 24 hours after ischemia. It is possible that Fv-Hsp70 protection might be improved by giving it at even earlier times (for example, 1-2 hours). This was not tested here, and would not be practical clinically in most cases at least at the present time.
Using Fv-Fragment to Deliver Hsp70 into Brain
The Fv antibody, mAb 3E10, was developed and used to deliver biologically active recombinant proteins to living cells in vitro and in vivo 22, 23. These studies have demonstrated that the Fv-Hsp70 construct penetrated COS-7 cells and primary rat neurons and protected both cells from oxidative stress. These results provide evidence that Fv is capable of delivering a functional protein to neurons and indicate its potential in the development of Hsp70 protein therapy. Since we did not detect any Fv-Hsp70 in the brains of sham animals, this would suggest that despite the ability of Fv proteins to gain entry into many types of cells, it requires alterations of the blood-brain-barrier for large amounts of Fv-Hsp70 to enter the brain. With an intact blood brain barrier it is unlikely that much intravascular Fv-Hsp70 enters normal brain based upon the data here.
Of major interest is the question of how Fv gains entry into brain cells at all. Recent work by Hansen and colleagues has shown that Fv enters cells via a nucleoside salvage transporter, ENT2 24. The fact that the ENT2 transporter is highly expressed in brain makes it possible that Fv might be particularly useful for delivery of proteins into brain in a variety of acute neurological diseases including stroke. In addition, the fact that very little Fv-Hsp70 entered normal brain, but large amounts entered ischemic brain, could suggest that the ENT2 transporter is activated by ischemia, and that this leads to selective up regulation of ENT2 function and transport of nucleosides and Fv-Hsp70 into ischemic brain. Thus, this could provide a disease and stroke-specific uptake of Fv-Hsp70 so that Fv-Hsp70 would not be taken up into normal brain and affect normal brain function but would be taken up into ischemic brain and help it survive.
Summary
Intravenous administration of recombinant Fv-Hsp70 protein decreased infarct volume and improved sensory-motor function following focal cerebral ischemia. Fv-Hsp70 protein enters ischemic but not normal brain and suppresses endogenous Hsp70.
Acknowledgments
This work was supported by a NIH/NINDS grant to FRS (NS054652) and Department of Veterans Affairs Research office Merit Review to RHW.
Footnotes
Disclosure/conflict of interest: The authors have no conflict of interest to declare in this study.
References
- 1.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, Lo EH. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 2.Fink SL, Chang LK, Ho DY, Sapolsky RM. Defective herpes simplex virus vectors expressing the rat brain stress-inducible heat shock protein 72 protect cultured neurons from severe heat shock. J Neurochem. 1997;68:961–969. doi: 10.1046/j.1471-4159.1997.68030961.x. [DOI] [PubMed] [Google Scholar]
- 3.Giffard RG, Xu L, Zhao H, Carrico W, Ouyang Y, Qiao Y, Sapolsky R, Steinberg G, Hu B, Yenari MA. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J Exp Biol. 2004;207:3213–3220. doi: 10.1242/jeb.01034. [DOI] [PubMed] [Google Scholar]
- 4.Lu A, Ran R, Parmentier-Batteur S, Nee A, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002;81:355–364. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 5.Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice over expressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- 6.Yenari MA. Heat shock proteins and neuroprotection. Adv Exp Med Biol. 2002;513:281–299. doi: 10.1007/978-1-4615-0123-7_10. [DOI] [PubMed] [Google Scholar]
- 7.Hansen JE, Sohn W, Kim C, Chang SS, Huang NC, Santos DG, Chan G, Weisbart RH, Nishimura RN. Antibody-mediated Hsp70 protein therapy. Brain Res. 2006;1088:187–196. doi: 10.1016/j.brainres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 9.Rasband WS. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997-2009. Imagej. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 10.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semi-automated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 11.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhan X, Kim C, Sharp FR. Very brief focal ischemia simulating transient ischemic attacks (TIAs) can injure brain and induce Hsp70 protein. Brain Res. 2008;1234:183–197. doi: 10.1016/j.brainres.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nito C, Kamiya T, Ueda M, Arii T, Katayama Y. Mild hypothermia enhances the neuroprotective effects of FK-506 and expands its therapeutic window following transient focal ischemia in rats. Brain Res. 2004;1008:179–185. doi: 10.1016/j.brainres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Plumier JC, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Anti-apoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- 16.Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: Regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- 18.Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol. 2007;594:89–99. doi: 10.1007/978-0-387-39975-1_9. [DOI] [PubMed] [Google Scholar]
- 19.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor hsf1 activation by hsp90 (hsp90 complex) that forms a stress-sensitive complex with hsf1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 20.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as hsf1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisbart RH, Miller CW, Chan G, Wakelin R, Ferreri K, Koeffler HP. Nuclear delivery of p53 c-terminal peptides into cancer cells using scfv fragments of a monoclonal antibody that penetrates living cells. Cancer Lett. 2003;195:211–219. doi: 10.1016/s0304-3835(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 23.Weisbart RH, Wakelin R, Chan G, Miller CW, Koeffler PH. Construction and expression of a bi-specific single-chain antibody that penetrates mutant p53 colon cancer cells and binds p53. Int J Oncol. 2004;25:1113–1118. [PubMed] [Google Scholar]
- 24.Hansen JE, Tse CM, Chan G, Heinze ER, Nishimura RN, Weisbart RH. Intranuclear protein transduction through a nucleoside salvage pathway. J Biol Chem. 2007;282:20790–20793. doi: 10.1074/jbc.C700090200. [DOI] [PubMed] [Google Scholar]


