Abstract
Objective
Hostility is associated with incident cardiovascular disease (CVD) events, independent of traditional risk factors. Increased platelet reactivity and thrombus formation over a disrupted coronary plaque is fundamental for CVD event onset. We examined the association between hostility and platelet reactivity in individuals without a prior history of CVD events.
Methods
Hypertensive patients (n=42) without concomitant CVD event history completed the 50-item Cook-Medley Hostility Scale, and a subset score of 27-items (Barefoot Ho) was derived. We examined the relation between Barefoot Ho scores and platelet aggregation. We also examined individual components of Barefoot Ho (aggressive responding, cynicism, and hostile affect) and their associations with platelet aggregation. Platelet reactivity, induced by ADP, was assessed by standard light transmission aggregometry, the current gold standard method of platelet aggregation assessment.
Results
Barefoot Ho scores were significantly related to increased rate of platelet aggregation in response to ADP. Further, of the three Barefoot Ho components, only aggressive responding was independently associated with increased platelet aggregation rate. The strength of these relationships did not diminish after adjusting for several standard CVD risk factors.
Conclusions
These data demonstrate that hostility, particularly the aggressive responding subtype, is associated with platelet reactivity, a key pathophysiological pathway in the onset of CVD events.
Keywords: platelets, hostility, coronary heart disease
Introduction
Atherosclerosis-related cardiovascular disease (CVD) events including coronary heart disease (CHD), peripheral vascular disease, and stroke remain the leading causes of morbidity and mortality in industrialized nations (1, 2). Multiple risk factors for these events have been identified, including age, male gender, hypertension, hypercholesterolemia, low high-density lipoprotein cholesterol, cigarette smoking, and diabetes (3). These established risk factors do not account for many patients with CVD events, prompting a search for “non-classic” risk factors. This search has identified several psychosocial factors including hostility, that are related to an increased CVD event risk, independent of traditional risk factors (4).
Hostility is generally seen as a persistent personality trait with attitudinal features such as cynicism and suspiciousness of others that predispose hostile individuals to respond to interpersonal stress with anger. Numerous methods have been used previously to characterize hostility including structured interviews and self-report measures (5). The weight of the evidence suggests that hostility, regardless of how it is assessed, is a relatively stable characteristic that prospectively predicts CVD-related morbidity events and mortality (6–11).
Atherosclerosis is the result of a complex interaction between the arterial wall and circulating elements (2). The endothelium plays a pivotal role in regulating hemostatic, inflammatory, and reparative responses to local injury (12). Endothelial dysfunction may promote inflammation, oxidation of lipoproteins and lipid accumulation, smooth muscle proliferation, extracellular matrix deposition or lysis, and thrombogenesis. These processes contribute to plaque development and progression, and subsequent vulnerability and rupture. Platelet-thrombus formation over a disrupted or eroded atherosclerotic plaque plays a major role in CVD event onset (13, 14). The severity of a CVD event is related to the magnitude and stability of the formed thrombus, modulated by a number of local and systemic thrombogenic factors including platelet reactivity (15).
Several mechanisms have been proposed to explain the relation between hostility and incident CVD events including behavioral pathways (i.e. unhealthy lifestyle), co-existance of other psychosocial factors, autonomic imbalance, an abnormal hypothalamic-pituitary-adrenal axis, and elevated inflammatory status (4, 16). Given the central role of platelets in CVD event onset, exaggerated platelet reactivity is a plausible biological mechansim that explains the hostility-CVD incidence link. There are however, only a few studies concerning the relationship between hostility and platelet reactivity. These few studies are limited by the inclusion of individuals with pre-existing CVD event history, some of whom were taking antiplatelet agents at the time of the study (17, 18). In addition, these studies did not examine either the relation between hostility and basal levels of platelet reactivity, or specific receptor pathways of platelet activation. The latter would clarify the molecular basis of hostility’s impact on platelet reactivity. Finally, these studies did not use light transmission aggregometry (LTA), the gold standard methodology for measuring platelet activation that has been used in prior studies linking platelet activity to subsequent CVD events (19–21).
The current study addresses these shortcomings. Specifically, we used LTA to determine whether trait hostility, assessed by the Cook-Medley Hostility Scale, is associated with higher levels of platelet reactivity in a healthy sample without prior CVD event history, none of whom were taking antiplatelet agents. We also explored individual hostility subscales including aggressive responding, cynicism and hostile affect, in order to determine the relative contributions of these components to the reactivity of platelets.
Methods
Study Population
The sample was comprised of 42 participants, enrolled between May 2003 to January 2006, from a larger intervention study investigating the effects of stress reduction, using the Lifeskills Workshop, on blood pressure and hostility. In the parent study, employees with hypertension (140–179 mmHg/90–109 mmHg) were enrolled from Mount Sinai Medical Center and Columbia University Medical Center. People were excluded for the presence of a DSM-IV mood/thought disorder, chronic renal disease, hypertension related to pregnancy, and congestive heart failure. For this substudy, participants with a history of CHD events, stroke/transient ischemic attack, and/or symptomatic peripheral vascular disease were excluded. Participants on antiplatelet or anticoagulant medications were also excluded, as these agents could affect platelet function. Written informed consent was obtained from all subjects, and the study was approved by the Institutional Review Boards of both Medical Centers.
Procedures
Prior to randomization, subjects reported to the research lab at the Center for Behavioral Cardiovascular Health in the morning after an overnight fast of at least 8 hours. Questionnaires were completed, after which venous blood was collected into 3.2% sodium citrate tubes using a 19-gauge butterfly needle. The citrate tubes were immediately spun at 180 g for 10 minutes to obtain platelet rich plasma (PRP). The remaining plasma was centrifuged at 1800 g to obtain platelet poor plasma (PPP).
Assessment of Hostility
Although there are a number of methods used to assess hostility, the Cook-Medley Hostility Scale was chosen as it is one of the most widely used hostility questionnaires in cardiovascular research (7). It consists of 50 self-report, true-false items drawn from the Minnesota Multiphasic Personality Inventory, and it has acceptable convergent and discriminant validity (22). As in a recently published multi-center study that found a relation between hostility and CVD events in women (11), the primary measure of hostility in our study is a sum of 27 items (Barefoot Ho) from the larger 50-item questionnaire. The Barefoot Ho score is a summary score of three constructs - aggressive responding, cynicism, and hostile affect - relevant to the experience and expression of hostility (11, 23).
Aggressive responding is the tendency to use anger and aggression as primary responses to problems or to endorse these behaviors as justified (23, 24). Cynicism is the inclination to have misanthropic beliefs, including the attribution of selfish motives to other people's acts (5). Hostile affect measures the negative emotions including anger, impatience, and loathing when dealing with others in social relationships (10, 23). Barefoot et al. (23) among others (11) have demonstrated that these three hostility components are key factors in the prediction of all-cause mortality and non-fatal CVD events.
Assessment of Platelet Reactivity
Platelet reactivity was assessed within 2 hours of the blood draw, using standard light transmission aggregometry (LTA), the de facto gold standard and most widely used methodology for the assessment of platelet activation (25). The platelet count of the PRP was assessed, and adjusted to 250,000/mm3 using PPP. LTA was assessed with a Four Channel Optical Aggregometer (Model 470VS, Chronolog Corp, Havertown, PA) with an Aggro/Link computer interface and software, which automatically analyzed and saved each experimental run. With PPP as a reference, aggregation was expressed as the aggregation rate (initial slope) after induction with a platelet agonist, as previously described (26–28). The aggregation rate is an index of the velocity of platelet aggregation (26–28). Platelet aggregation was induced using two doses of adenosine diphosphate (ADP, 5 uM and 10 uM). As described below, the two ADP doses were combined into a single ADP-induced platelet aggregation measure. A multitude of studies that have demonstrated a link between ADP-induced platelet aggregation and CVD events (29–32).
Statistical Analyses
Prior to analysis, the two measures of ADP-induced platelet aggregation were evaluated for outliers and other distributional problems. Each measure of ADP-induced platelet aggregation had one substantial outlier and the measures were winsorized to reduce the effect of these extreme scores on the analyses. Descriptive statistics are presented as mean ± SD. A simple linear regression model was estimated with the Barefoot Ho total score as the primary predictor. Subsequently, a multiple linear regression using the 3 hostility components of the total score (aggressive responding, cynicism, and hostile affect) was estimated. The dependent measure in these analyses was the aggregated measure of the two ADP-induced platelet reactivity indices. Specifically, this measure was formed by standard (z) scoring the two ADP measures and then averaging them. This procedure insures that each measure contributes equally to the aggregate score. We chose to aggregate the two ADP measures because they were substantially correlated (r = .62). This approach created a more reliable index of platelet aggregation, and more efficient analyses.
Both regression models were re-examined after adjusting for age, sex, body mass index, pulse pressure, hyperlipidemia, diabetes, and active smoking, to determine contributions of hostility and its components on platelet reactivity, independent of traditional CVD risk factors. These CVD risk factors were chosen a priori as covariates, because previous studies have shown a strong and consistent relation of each to platelet reactivity (33). Statistical analyses were performed using SPSS v.16 (SPSS Inc, Chicago, IL).
Results
Characteristics of the Participants
Table 1 shows the characteristics of the study participants. Overall, participants were middle aged, mostly female, and mildly hypertensive.
Table 1.
Sample characteristics*
| Characteristics | Total Sample (N=42) |
|---|---|
| Age, y | 48.5 ± 9.7 |
| Sex, % female | 81.0 |
| Body Mass Index, kg/m2 | 33.7 ± 7.7 |
| Blood Pressure | |
| Systolic blood pressure, mmHg | 145 ± 12 |
| Diastolic blood pressure, mmHg | 88 ± 8 |
| Pulse pressure, mmHg | 56 ± 10 |
| Diabetes Mellitus, %† | 11.9 |
| Diet controlled, % | 4.8 |
| Oral hypoglycemic, % | 7.1 |
| Insulin, % | 4.8 |
| Hyperlipidemia, % | 28.6 |
| Active Smoking, % | 4.8 |
| Medications | |
| Beta-blocker, % | 23.8 |
| Calcium channel blocker, % | 19.0 |
| Angiotensin converting enzyme inhibitor/angiotensin II receptor blocker, % |
35.7 |
| Diuretic, % | 33.3 |
| HMG-CoA reductase inhibitor, % | 4.8 |
| Barefoot Ho score | 13 ± 5 |
| Aggressive responding score | 5 ± 2 |
| Cynicism score | 7 ± 3 |
| Hostile affect score | 2 ± 1 |
| Platelet Aggregation Measures | |
| Aggregation Rate to 5 uM ADP, %/min | 42.3 ± 9.3 |
| Aggregation Rate to 10 uM ADP, %/min | 46.3 ± 9.4 |
Data are expressed as percentage or mean ± SD.
Of the 5 diabetic participants, 2 were diet-controlled, 1 was on metformin alone, and 2 were on both metformin and insulin.
Hostility and Platelet Reactivity
Table 2 shows the relationship between Barefoot Ho score and ADP-induced platelet reactivity. Barefoot Ho score was significantly positively associated with rate of platelet aggregation in response to ADP. This strength of this relation did not diminish after adjustment for several covariates (Table 3).
Table 2.
Coefficients From the Regression of ADP-Induced Platelet Reactivity with Hostility Scores
| Unstandardized | Standardized | 95% CI for B | ||||
|---|---|---|---|---|---|---|
| B | SE | β | p-value | Lower | Upper | |
| Total Barefoot Ho score | .089 | .026 | .472 | .002 | .036 | .142 |
| Aggressive responding | .246 | .090 | .464 | .01 | .063 | .428 |
| Cynicism | .009 | .062 | .026 | .89 | −.117 | .135 |
| Hostile affect | .069 | .117 | .101 | .56 | −.168 | .307 |
Predictors with significant p-values are in bold type.
Table 3.
Coefficients From the Regression of ADP-Induced Platelet Reactivity with Hostility Scores After Adjusting for Covariates*
| Unstandardized | Standardized | 95% CI for B | ||||
|---|---|---|---|---|---|---|
| B | SE | β | p-value | Lower | Upper | |
| Total Barefoot Ho score | .086 | .029 | .459 | .005 | .028 | .145 |
| Aggressive responding | .262 | .103 | .496 | .02 | .052 | .473 |
| Cynicism | .021 | .065 | .062 | .75 | −.112 | .154 |
| Hostile affect | .026 | .132 | .039 | .84 | −.242 | .295 |
Age, sex, body mass index, pulse pressure, hyperlipidemia, diabetes, and active smoking. Predictors with significant p-values are in bold type.
Cynicism, Hostile Affect, Aggressive Responding and Platelet Reactivity
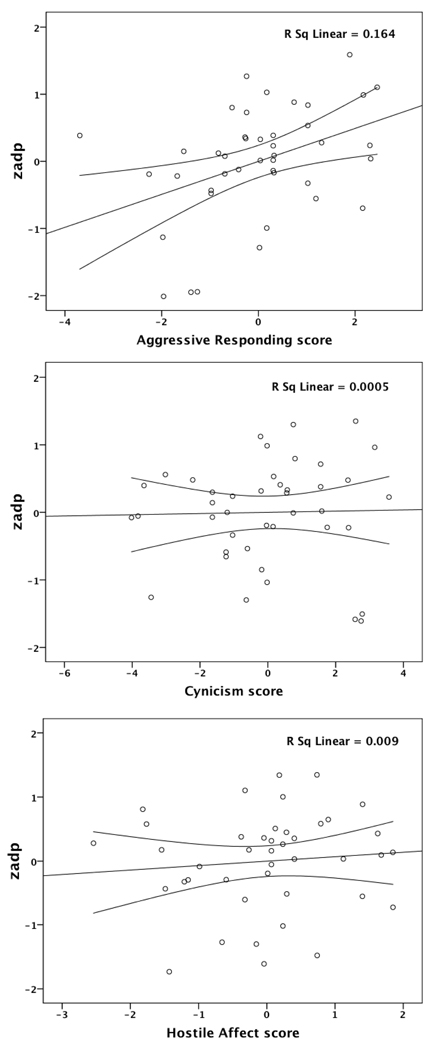
Table 4 shows the correlations among the three hostility components, while Table 2 shows the relationship of these with ADP-induced platelet reactivity. Only aggressive responding independently predicted the rate of platelet aggregation to ADP, while cynicism and hostile affect did not. Figure 1 shows the partial regression plots of the relationship of each hostility component, controlling for the other two subscales, along with mean 95% CI. Finally, as with the results for the total Barefoot Ho score, the strength of the relationship between aggressive responding and ADP-induced platelet reactivity was not reduced after covariate adjustment (Table 3). Cynicism and hostile affect remained unrelated to platelet reactivity.
Table 4.
Correlations Among Hostility Construct Scores*
| Aggressive responding |
Cynicism | Hostile affect | |
|---|---|---|---|
| Aggressive responding |
-- | ||
| Cynicism | .57 | -- | |
| Hostile affect | .47 | .57 | -- |
All ps<.05.
Figure 1.
Partial linear regression scatterplots are shown along with mean 95% CI for each hostility subscale. The three hostility subscale scores (aggressive responding, cynicism, and hostile affect) are included in a regression model. The measure “zadp” was formed by standard (z) scoring the two ADP platelet reactivity measures and then averaging them.
Discussion
Research has shown that hostility is associated with increased risk of incident CVD events, independent of traditional risk factors (6, 10, 23, 34). Given the central role of platelet-thrombosis in initiating CVD events (13, 14), increased platelet reactivity has strong appeal as a viable candidate mechanism linking hostility and incident CVD events. In the present study, we demonstrate for the first time that hostility is significantly associated with increased platelet reactivity in participants without prior CVD event history. Our findings suggest that the higher risk of incident CVD events associated with hostility may in part be explained by exaggerated platelet reactivity.
Few prior studies have examined the relationship between hostility and platelet reactivity, though the methods utilized to assess hostility and platelet function were different than those utilized in the current study. Markovitz et al. (18) found that indices of hostility derived from the Type A Structured Interview (SI) predicted changes in B-thromboglobulin (BTG), a non-specific marker of platelet activation, in response to this interview and additionally a stressful public speaking task. The small sample included both post-myocardial infarction patients (n=14), many of whom were taking anti-platelet agents, and controls (n=15). Within group (post-myocardial infarction patients and controls) relationships between hostility and increases in BTG with psychological stress, and between trait hostility and basal levels of platelet reactivity were not reported. In a subsequent study, Markovitz et al. (17) reported a possible relationship between trait hostility and levels of platelet aggregation in the absence of a psychological stressor. GPIIb/IIIa receptor activation and binding, determined by flow cytometry using either blood from a bleeding time wound or a peripheral vein, was assessed in 55 men and women (32 patients with history of myocardial infarction and 23 healthy controls). SI-rated Hostility (Potential for Hostility) was not related to platelet aggregation in the entire study sample. There was, however, a relationship reported between hostility and wound-induced platelet aggregation in CHD patients but not in healthy controls.
The results of the current study, while demonstrating similarities to these earlier investigations, extend them considerably. Most importantly, the current study utilizes a method to assess platelet reactivity that has been linked to CVD related prognosis (29, 30, 35–40), thereby providing stronger evidence of a link between hostility and CVD events. Furthermore, the study sample, while characterized by the presence of hypertension, was without prior history of CVD events. Yet contrary to the latter study by Markovitz (17), hostility predicted increased platelet reactivity in CVD event-free individuals. This disparity may be explained by the different methods that were used to assess platelet function. With the greater sensitivity in the platelet methods used here, we may have been able to discern aspects of platelet function among at risk, otherwise healthy individuals that the BTG and GP IIb/IIIa receptor flow cytometry methods could not. Furthermore, the ability of these earlier methods to detect an effect for the individuals with CHD event history may have been influenced by that group’s use of anti-platelet agents. Since these agents are known to reduce CVD events (41), it is difficult to fully interpret the earlier findings.
Of note, we found the aggressive responding subscale to be more highly predictive of platelet aggregation than the other two subscales. Only the aggressive responding subtype was independently associated with increased platelet aggregation. The reason for this finding is not known. In our study, there were moderate correlations among the different hostility subscales. These results, which are consistent with other studies (11, 24), suggest that individual hostility components such as aggressive responding may be conceptually distinct. The aggressive responding subtype derived from the Barefoot Ho scale is similar to the “hostile style” component of the SI. Aggressive responding measures the respondent’s tendency to use anger and aggression as instrumental responses, while “hostile style” on the SI assesses the subject’s actual use of these tendencies during the SI. Thus, it may be that individuals with expressive forms of hostility are susceptible to exaggerated platelet aggregation.
Barefoot et al. (23) found aggressive responding, cynicism, and hostile affect were each significantly related to age-adjusted survival. Previously, Kawachi et al. (10) found that aggressive responding independently predicted incident CHD related morbidity/mortality at 7-years among men, whereas cynicism and hostile affect did not. Olson et al. (11) found that aggressive responding and cynicism but not hostile affect predicted 4-year risk of CVD events in women who had undergo diagnostic coronary angiography for suspected CHD. The risk of an adverse cardiovascular event was greater for each point increase in the aggressive responding score (16% excess risk for each point increase) compared to the cynicism score (7% excess risk for each point increase), suggesting that aggressive responding may be a particularly high risk subtype. Lastly, data from the Normative Aging Study (24) showed that aggressive responding was the only one of the three Barefoot Ho hostility components to significantly predict allostatic load score, a cumulative physiological measure of chronic stress. These latter finding suggests that within the trait hostility construct, the aggressive responding component, may be associated not only with increased platelet reactivity, but also with a number of other biological dysregulations.
In our study, hostility was associated with increased platelet reactivity to ADP. ADP stimulates both P2Y12 and P2Y1 platelet receptors (42, 43). The Gq-coupled P2Y1 receptor is responsible for intracellular calcium mobilization, shape change, and initiation of aggregation; the Gi-coupled P2Y12 receptor is responsible for the completion of the aggregation to ADP and potentiation of aggregation and secretion by other platelet agonists (42, 43). It is intriguing to consider that trait hostility may be associated with abnormalities in the P2Y1 and/or P2Y12 receptor pathways. Assuming this hypothesis is correct, however, it is unclear whether the ADP platelet receptors or post-ADP receptor platelet activation pathways are altered among individuals characterized by hostility. Further examination into the relationship between hostility and specific platelet pathways of ADP-induced aggregation may help identify underlying molecular dysregulations of platelet aggregation.
Our study has several limitations. First, although the associations identified are plausible, our cross-sectional study cannot discriminate between the several possible causal directions between trait hostility and platelet reactivity. It is possible that hostility induces a higher level of platelet reactivity. Further, although it is unlikely that platelet reactivity directly induces hostility, exaggerated platelet reactivity could be part of a broader biological dysregulation that renders individuals prone to hostility. Lastly, hostility and increased platelet reactivity may be a consequence of another yet unidentified pathologic process. A second limitation is that history of CVD was determined by self-report. Therefore, we cannot exclude the existence of subclinical CVD in our sample. For instance, as demonstrated by intravascular ultrasound of the coronary arteries in transplanted hearts, subclinical atherosclerotic CHD is relatively prevalent even in donor hearts obtained from young adults and adolescents (44). Thus, from a practical standpoint, we cannot reliably exclude participants with all forms of subclinical CVD, as it would require multi-modality non-invasive and invasive imaging (45–47). Further, it would limit the external validity of our results because most prior studies linking hostility to incident CVD events did not exclude on the basis of subclinical CVD – these studies relied on self-report to define the presence or absence of CVD event history. Therefore, we believe that our inclusion/exclusion criteria are reasonable. Regardless, as prior studies have shown a relationship between hostility and subclinical CVD measures such as carotid atherosclerosis (48, 49), it is possible that the increased platelet reactivity associated with hostility is partially explained by subclinical atherosclerosis. Third, our sample comprised mostly women. We did not examine menopausal status or ovarian function, and therefore cannot comment on how these factors affect the hostility-platelet aggregation link. This is an important research question, given the effects of hormones on cells of the vascular wall (50). Finally, the study is also limited by the size of the cohort. Type I error is a consideration. Overall, although we found a relationship between hostility and levels of platelet reactivity, further research should be conducted to confirm our preliminary findings, to investigate the relations among hostility, subclinical CVD, and platelet aggregation, to examine the role of hormones and menopause in platelet aggregation in hostility, and to examine the generalizability of our findings to other samples including non-employees and participants without hypertension.
In summary, our findings suggest that trait hostility is associated with increased platelet reactivity in individuals without prior CVD event history. Additionally, these biological dysregulations may be more strongly related to expressive forms of hostility, especially with the aggressive responding subtype. Future studies should delve into the clinical significance of these findings by investigating whether increased platelet aggregation mediates the hostility-CVD event link. Possible underlying mechanisms such as abnormalities of the ADP P2Y1 and/or P2Y12 receptor pathways, which may explain the increased platelet reactivity associated with hostility, should also be examined. Understanding the specific biological mechanisms involved may lead to novel therapeutic strategies to reduce the increased cardiovascular risk associated with hostility.
Acknowledgements
This work was supported by grants HL072866 (D. Shimbo) and HL076857 (D. Shimbo, W. Chaplin, S. Kuruvilla, D. Abraham, M.M. Burg) from the National Institutes of Health.
Abbreviations
- ADP
adenosine diphosphate
- Barefoot Ho
27-item Barefoot Hostility Scale
- BTG
B-thromboglobulin
- CHD
coronary heart disease
- CVD
cardiovascular disease
- LTA
light transmission aggregometry
- PPP
platelet poor plasma
- PRP
platelet rich plasma
- SI
Structured Interview
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Badimon JJ, Zaman A, Helft G, Fayad Z, Fuster V. Acute coronary syndromes: pathophysiology and preventive priorities. Thromb Haemost. 1999;82:997–1004. [PubMed] [Google Scholar]
- 2.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 4.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 5.Miller TQ, Smith TW, Turner CW, Guijarro ML, Hallet AJ. A meta-analytic review of research on hostility and physical health. Psychol Bull. 1996;119:322–348. doi: 10.1037/0033-2909.119.2.322. [DOI] [PubMed] [Google Scholar]
- 6.Barefoot JC, Dahlstrom WG, Williams RB., Jr Hostility, CHD incidence, and total mortality: a 25-year follow-up study of 255 physicians. Psychosom Med. 1983;45:59–63. doi: 10.1097/00006842-198303000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Barefoot JC, Smith TW. The assessment of hostility and anger. In: Siegman AW, Smith TW, editors. Anger, hostility, and the heart. Hillsdale, N.J.: Lawrence Erlbaum Associates, Inc.; 1994. pp. 43–66. [Google Scholar]
- 8.Dembroski TM, MacDougall JM, Costa PT, Jr, Grandits GA. Components of hostility as predictors of sudden death and myocardial infarction in the Multiple Risk Factor Intervention Trial. Psychosom Med. 1989;51:514–522. doi: 10.1097/00006842-198909000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hecker MH, Chesney MA, Black GW, Frautschi N. Coronary-prone behaviors in the Western Collaborative Group Study. Psychosom Med. 1988;50:153–164. doi: 10.1097/00006842-198803000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kawachi I, Sparrow D, Kubzansky LD, Spiro A, 3rd, Vokonas PS, Weiss ST. Prospective study of a self-report type A scale and risk of coronary heart disease: test of the MMPI-2 type A scale. Circulation. 1998;98:405–412. doi: 10.1161/01.cir.98.5.405. [DOI] [PubMed] [Google Scholar]
- 11.Olson MB, Krantz DS, Kelsey SF, Pepine CJ, Sopko G, Handberg E, Rogers WJ, Gierach GL, McClure CK, Merz CN. Hostility scores are associated with increased risk of cardiovascular events in women undergoing coronary angiography: a report from the NHLBI-Sponsored WISE Study. Psychosom Med. 2005;67:546–552. doi: 10.1097/01.psy.0000170830.99263.4e. [DOI] [PubMed] [Google Scholar]
- 12.Corti R, Fuster V, Badimon JJ. Pathogenetic concepts of acute coronary syndromes. J Am Coll Cardiol. 2003;41:7S–14S. doi: 10.1016/s0735-1097(02)02833-4. [DOI] [PubMed] [Google Scholar]
- 13.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1) N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 14.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) N Engl J Med. 1992;326:310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- 15.Viles-Gonzalez JF, Fuster V, Badimon JJ. Links between inflammation and thrombogenicity in atherosclerosis. Current molecular medicine. 2006;6:489–499. doi: 10.2174/156652406778018707. [DOI] [PubMed] [Google Scholar]
- 16.Suarez EC, Lewis JG, Kuhn C. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain Behav Immun. 2002;16:675–684. doi: 10.1016/s0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- 17.Markovitz JH. Hostility is associated with increased platelet activation in coronary heart disease. Psychosom Med. 1998;60:586–591. doi: 10.1097/00006842-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Markovitz JH, Matthews KA, Kiss J, Smitherman TC. Effects of hostility on platelet reactivity to psychological stress in coronary heart disease patients and in healthy controls. Psychosom Med. 1996;58:143–149. doi: 10.1097/00006842-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505–1516. doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 20.Maree AO, Fitzgerald DJ. Variable platelet response to aspirin and clopidogrel in atherothrombotic disease. Circulation. 2007;115:2196–2207. doi: 10.1161/CIRCULATIONAHA.106.675991. [DOI] [PubMed] [Google Scholar]
- 21.Wang TH, Bhatt DL, Topol EJ. Aspirin and clopidogrel resistance: an emerging clinical entity. European heart journal. 2006;27:647–654. doi: 10.1093/eurheartj/ehi684. [DOI] [PubMed] [Google Scholar]
- 22.Cook W, Medley D. Proposed hostility and pharasaic-virtue scales for the MMPI. J Appl Physiol. 1954;38:414–418. [Google Scholar]
- 23.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB., Jr The Cook-Medley hostility scale: item content and ability to predict survival. Psychosom Med. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Kubzansky LD, Kawachi I, Sparrow D. Socioeconomic status, hostility, and risk factor clustering in the Normative Aging Study: any help from the concept of allostatic load? Ann Behav Med. 1999;21:330–338. doi: 10.1007/BF02895966. [DOI] [PubMed] [Google Scholar]
- 25.Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2007;50:1822–1834. doi: 10.1016/j.jacc.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 26.Vlasova II. The effect of oxidatively modified low-density lipoproteins on platelet aggregability and membrane fluidity. Platelets. 2000;11:406–414. doi: 10.1080/09537100020000157. [DOI] [PubMed] [Google Scholar]
- 27.Williams MS, Coller BS, Vaananen HJ, Scudder LE, Sharma SK, Marmur JD. Activation of platelets in platelet-rich plasma by rotablation is speed-dependent and can be inhibited by abciximab (c7E3 Fab; ReoPro) Circulation. 1998;98:742–748. doi: 10.1161/01.cir.98.8.742. [DOI] [PubMed] [Google Scholar]
- 28.Xu W, Favaloro EJ, Medbury H, Zoellner H. Human endothelial cells maintain anti-aggregatory activity for platelets during apoptosis. Thromb Haemost. 2001;85:915–923. [PubMed] [Google Scholar]
- 29.Geisler T, Langer H, Wydymus M, Gohring K, Zurn C, Bigalke B, Stellos K, May AE, Gawaz M. Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. European heart journal. 2006;27:2420–2425. doi: 10.1093/eurheartj/ehl275. [DOI] [PubMed] [Google Scholar]
- 30.Hochholzer W, Trenk D, Bestehorn HP, Fischer B, Valina CM, Ferenc M, Gick M, Caputo A, Buttner HJ, Neumann FJ. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol. 2006;48:1742–1750. doi: 10.1016/j.jacc.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 31.Kabbani SS, Watkins MW, Ashikaga T, Terrien EF, Holoch PA, Sobel BE, Schneider DJ. Platelet reactivity characterized prospectively: a determinant of outcome 90 days after percutaneous coronary intervention. Circulation. 2001;104:181–186. doi: 10.1161/01.cir.104.2.181. [DOI] [PubMed] [Google Scholar]
- 32.Kabbani SS, Watkins MW, Ashikaga T, Terrien EF, Sobel BE, Schneider DJ. Usefulness of platelet reactivity before percutaneous coronary intervention in determining cardiac risk one year later. The American journal of cardiology. 2003;91:876–878. doi: 10.1016/s0002-9149(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 33.Gresele P, Page CP, Fuster V, Vermylen J, editors. Platelets in thrombotic and non-thrombotic disorders. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 34.Barefoot JC, Larsen S, von der Lieth L, Schroll M. Hostility, incidence of acute myocardial infarction, and mortality in a sample of older Danish men and women. American journal of epidemiology. 1995;142:477–484. doi: 10.1093/oxfordjournals.aje.a117663. [DOI] [PubMed] [Google Scholar]
- 35.Bliden KP, DiChiara J, Tantry US, Bassi AK, Chaganti SK, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007;49:657–666. doi: 10.1016/j.jacc.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 36.Cuisset T, Frere C, Quilici J, Barbou F, Morange PE, Hovasse T, Bonnet JL, Alessi MC. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost. 2006;4:542–549. doi: 10.1111/j.1538-7836.2005.01751.x. [DOI] [PubMed] [Google Scholar]
- 37.Cuisset T, Frere C, Quilici J, Morange PE, Nait-Saidi L, Carvajal J, Lehmann A, Lambert M, Bonnet JL, Alessi MC. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol. 2006;48:1339–1345. doi: 10.1016/j.jacc.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 38.Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961–965. doi: 10.1016/s0735-1097(02)03014-0. [DOI] [PubMed] [Google Scholar]
- 39.Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, Bassi AK, Tantry US. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol. 2005;46:1820–1826. doi: 10.1016/j.jacc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 40.Stejskal D, Vaclavik J, Lacnak B, Proskova J. Aspirin resistance measured by cationic propyl gallate platelet aggregometry and recurrent cardiovascular events during 4 years of follow-up. European journal of internal medicine. 2006;17:349–354. doi: 10.1016/j.ejim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Mehta SR, Yusuf S. Short- and long-term oral antiplatelet therapy in acute coronary syndromes and percutaneous coronary intervention. J Am Coll Cardiol. 2003;41:79S–88S. doi: 10.1016/s0735-1097(02)02831-0. [DOI] [PubMed] [Google Scholar]
- 42.Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb Haemost. 2008;99:466–472. doi: 10.1160/TH07-11-0673. [DOI] [PubMed] [Google Scholar]
- 43.Michelson AD. P2Y12 antagonism: promises and challenges. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:s33–s38. doi: 10.1161/ATVBAHA.107.160689. [DOI] [PubMed] [Google Scholar]
- 44.Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, Young JB, Nissen SE. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705–2710. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 45.Greenland P, Abrams J, Aurigemma GP, Bond MG, Clark LT, Criqui MH, Crouse JR, 3rd, Friedman L, Fuster V, Herrington DM, Kuller LH, Ridker PM, Roberts WC, Stanford W, Stone N, Swan HJ, Taubert KA, Wexler L. Prevention Conference V: Beyond secondary prevention: identifying the high-risk patient for primary prevention: noninvasive tests of atherosclerotic burden: Writing Group III. Circulation. 2000;101:E16–E22. doi: 10.1161/01.cir.101.1.e16. [DOI] [PubMed] [Google Scholar]
- 46.Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, Fayad Z, Budoff MJ, Rumberger J, Naqvi TZ, Shaw LJ, Faergeman O, Cohn J, Bahr R, Koenig W, Demirovic J, Arking D, Herrera VL, Badimon J, Goldstein JA, Rudy Y, Airaksinen J, Schwartz RS, Riley WA, Mendes RA, Douglas P, Shah PK. From vulnerable plaque to vulnerable patient--Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. The American journal of cardiology. 2006;98:2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Van Mieghem CA, McFadden EP, de Feyter PJ, Bruining N, Schaar JA, Mollet NR, Cademartiri F, Goedhart D, de Winter S, Granillo GR, Valgimigli M, Mastik F, van der Steen AF, van der Giessen WJ, Sianos G, Backx B, Morel MA, van Es GA, Zalewski A, Serruys PW. Noninvasive detection of subclinical coronary atherosclerosis coupled with assessment of changes in plaque characteristics using novel invasive imaging modalities: the Integrated Biomarker and Imaging Study (IBIS) J Am Coll Cardiol. 2006;47:1134–1142. doi: 10.1016/j.jacc.2005.09.075. [DOI] [PubMed] [Google Scholar]
- 48.Everson-Rose SA, Lewis TT, Karavolos K, Matthews KA, Sutton-Tyrrell K, Powell LH. Cynical hostility and carotid atherosclerosis in African American and white women: the Study of Women's Health Across the Nation (SWAN) Heart Study. Am Heart J. 2006;152(982):e7, e13. doi: 10.1016/j.ahj.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Matthews KA, Owens JF, Kuller LH, Sutton-Tyrrell K, Jansen-McWilliams L. Are hostility and anxiety associated with carotid atherosclerosis in healthy postmenopausal women? Psychosom Med. 1998;60:633–638. doi: 10.1097/00006842-199809000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Miller VM, Jayachandran M, Owen WG. Ageing, oestrogen, platelets and thrombotic risk. Clin Exp Pharmacol Physiol. 2007;34:814–821. doi: 10.1111/j.1440-1681.2007.04685.x. [DOI] [PubMed] [Google Scholar]