Abstract
Insects encode multiple ILPs but only one homolog of the vertebrate IR that activates the insulin signaling pathway. However, it remains unclear whether all insect ILPs are high affinity ligands for the IR or have similar biological functions. The yellow fever mosquito, Aedes aegypti, encodes eight ILPs with prior studies strongly implicating ILPs from the brain in regulating metabolism and the maturation of eggs following blood feeding. Here we addressed whether two ILP family members expressed in the brain, ILP4 and ILP3, have overlapping functional and receptor binding activities. Our results indicated that ILP3 exhibits strong insulin-like activity by elevating carbohydrate and lipid storage in sugar-fed adult females, whereas ILP4 does not. In contrast, both ILPs exhibited dose-dependent gonadotropic activity in blood-fed females as measured by the stimulation of ovaries to produce ecdysteroids and the uptake of yolk by primary oocytes. Binding studies using ovary membranes indicated that ILP4 and ILP3 do not cross compete; a finding further corroborated by cross-linking and immunoblotting experiments showing that ILP3 binds the MIR while ILP4 binds an unknown 55 kDa membrane protein. In contrast, each ILP activated the insulin signaling pathway in ovaries as measured by enhanced phosphorylation of Akt. RNAi and inhibitor studies further indicated that the gonadotropic activity of ILP4 and ILP3 requires the MIR and a functional insulin signaling pathway. Taken together, our results indicate that two members of the Ae. aegypti ILP family exhibit partially overlapping biological activity and different binding interactions with the MIR.
Keywords: insect, insulin, metabolism, reproduction, mosquito
1. Introduction
In vertebrates, insulin, IGFs, and relaxins are members of the ILP superfamily that regulate complex physiological processes including metabolism, growth, longevity, and fertility (Halls et al., 2007; Taguchi and White, 2008; Belfiore et al., 2009). Insulin and IGFs are differentially processed from prepropeptides, and preferentially bind structurally related but distinct receptor tyrosine kinase receptors (Taniguchi et al., 2006). Binding of insulin to the IR activates the phosphoinositol 3 kinase/Akt pathway (the canonical insulin signaling pathway) while binding of IGFs to the IGFR activates the MAPK pathway, although it is also recognized extensive cross talk occurs between these ligands, receptors, and their downstream signaling pathways. Relaxins in contrast bind GPCRs, which activate multiple signaling pathways.
Phylogenetically advanced insects like the fruit fly, Drosophila melanogaster, silkmoth, Bombyx mori, honeybee, Apis melifera, and mosquitoes (Aedes aegypti and Anopheles gambiae) also encode multiple, structurally distinct ILPs, but only one homolog of the vertebrate IR that activates the insulin signaling pathway (Wu and Brown, 2006; Ament et al., 2008; Toivonen and Partridge, 2009; Teleman, 2010). Overexpression of any of the seven ILPs encoded by Drosophila during larval development results in increased body size (Ikeya et al., 2002), whereas ablation of the brain neurosecretory cells that produce four of the ILPs (1, 2, 3 and 5) or genetic deletion of ILP1–5 reduces metabolic activity and size (Rulifson et al., 2002; Broughton et al., 2005; Zhang et al., 2009). A recent comprehensive study of gene-knockouts for all seven ILPs in Drosophila further indicates the concurrent loss of dilp1–6 adversely affects development, stress resistance, and lifespan more than deletion of individual dilps (Grönke et al., 2010): a finding which lends additional evidence for the redundant and synergic activation of the insulin signaling pathway. Because mammalian insulins also activate this pathway in insects (Fernandez et al., 1995; Yamaguchi et al., 1995; Riehle and Brown, 1999; Wu and Brown, 2006; Gu et al., 2009), they are often viewed as structurally and functionally equivalent homologs of insect ILPs (Teleman, 2010). On the other hand, insect ILPs exhibit differences in expression patterns and secretion (summarized by Wu and Brown, 2006; Yang et al., 2008; Geminard et al., 2009; Okamoto et al., 2009; Veenstra, 2009; Grönke et al., 2010; Teleman, 2010), suggesting the function of individual ILP members is not fully overlapping. In addition, little is known about the actual binding affinities of insect IRs for different ILPs. Thus, it remains unclear whether all insect ILPs are functionally redundant insulin homologs or high affinity ligands for insect IRs.
Most mosquitoes must feed on blood from a human or other vertebrate host to mature eggs. This aspect of mosquito biology is of great interest, because many species transmit disease-causing pathogens when acquiring a blood meal. Recent studies also indicate that insulin signaling impacts several processes following blood feeding including the cascade of events required for the production of yolk and its uptake into primary oocytes (Roy et al., 2007; Luckhart and Riehle, 2007; Brown et al., 2008; Arik et al., 2009; Sim and Denlinger 2008). The yellow fever mosquito, Aedes aegypti, encodes eight ILP genes of which five (ILP1, 3, 4, 7 and 8) are expressed in the brain (Riehle et al., 2006). Ingestion of a blood meal triggers the release of these ILPs and other neuropeptides, which stimulate the ovaries to produce ecdysteroid hormones (Brown et al, 1998; Brown et al., 2008). These ecdysteroids in turn stimulate the fat body, which is the primary metabolic organ of insects, to synthesize and release yolk proteins produced from the nutrients acquired in the blood meal. Yolk proteins are then packaged into primary oocytes (Roy et al., 2007).
We previously determined that synthetic ILP3 elevates carbohydrate and lipid storage by the fat body in sugar-fed females, and exhibits potent gonadotropic activity as measured by stimulation of ecdysteroid production by the ovaries and yolk uptake by oocytes of blood-fed females (Brown et al., 2008). We also showed that ILP3 binds the MIR with high affinity (IC50= 5.9 nM), while knockdown of the MIR by RNAi greatly reduces ILP3 gonadotropic activity. Earlier studies showed that bovine insulin also stimulates ecdysteroid production by ovaries through insulin signaling (Riehle and Brown, 1999). ILP3, however, exhibits gonadotropic activity at much lower concentrations, and bovine insulin poorly competes ILP3 binding to the MIR (Brown et al., 2008). Taken together, these data indicate that ILP3 exhibits insulin-like activity and suggest bovine insulin is comparatively a weak ligand for the MIR. In the current study, we addressed whether other ILP family members are functionally similar or distinct from ILP3 by producing and characterizing the activity of ILP4. Our results indicate that ILP4 and ILP3 exhibit partially overlapping biological activities and distinctly different binding affinities for the MIR.
2. Experimental procedures
2.1. Mosquitoes
The UGAL strain of A. aegypti was used in all experiments. Mosquitoes were reared as previously described (Riehle et al., 2006). Adult females were blood fed on anesthetized rats.
2.2. ILP4 synthesis
The A and B chains of ILP4 were synthesized using standard Fmoc chemistry with the exception that norleucine was substituted for the single methionine in the B chain to negate the possible effects of oxidation on bioactivity (Supplementary Figure 1). Cross-linking of the chains to form mature ILP4 followed by purification to homogeneity was accomplished as previously described for the biosynthesis of ILP3 (Brown et al., 2008). The structure of ILP4 was then confirmed by MALDI-TOF mass spectroscopy followed by lyophilization and storage at −20° C. ILP3 used in the study was produced as previously described (Brown et al., 2008). Both ILPs were rehydrated in water followed by dilution in Aedes saline (7.5 g NaCl, 0.35 g KCl, and 0.21 g CaCl2 to 1 L, pH 6.5) for use in bioassays.
2.3. Metabolic assays
Newly emerged adult females were provided water only for five days followed on day 6 by access to a 10% sucrose solution for 30 min. Immediately thereafter, females with swollen abdomens indicative of feeding were decapitated and injected with different concentrations of ILP4 or ILP3. Normal, intact mosquitoes (non-decapitated) served as a positive control while mosquitoes decapitated and injected with Aedes saline alone served as the negative control. After 24 h at 27 °C, females were frozen (−80°C), and later processed to measure stored glycogen and lipid as described (Brown et al., 2008).
2.4. Yolk uptake and ovary ecdysteroid production assays
Yolk uptake assays were conducted using blood-fed females decapitated within 1 h PBM. Decapitated mosquitoes were then injected with different concentrations of ILP4 or ILP3 in 0.5 μl of saline, followed 24 h later by dissection and measurement of yolk deposition per egg using the long axis of each oocyte (Brown et al., 1998). Decapitated females injected with saline alone served as the negative control, and intact (non-decapitated) blood-fed females injected with saline served as the positive control.
Ecdysteroid production by ovaries was determined using a well-established in vitro bioassay (Sieglaff et al., 2005). Briefly, 2 or 4 pairs of ovaries from non-blood-fed females were incubated with serially diluted ILP3 or ILP4 in modified Aedes saline (140 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 12.5 mM HEPES, 2.5 mM trehalose, 0.5 mM MgCl2, and 0.9 mM NaHCO3, pH 7.0) for 6 h at 27° C followed by quantification of ecdysteroids in the medium by radioimmunoassay (RIA) using an ecdysteroid antiserum (4919) kindly donated by P. Poncheron (Université P. et M. Curie, Paris France) (Sieglaff et al., 2005). To determine the commitment period for ILP activation, ovaries from non-blood fed females were incubated as above with optimal ILP doses (ILP4, 20 pmol or ILP3, 10 pmol/ovary set) for different time periods. At the end of this period, medium was collected (50 μl) for the ecdysteroid RIA, and replaced with medium containing no ILP. After 6 h of total incubation time, medium from the second period was collected and ecdysteroid amounts determined as described above. For the inhibitor experiments, ovaries were set up as above and incubated for 6 h with ILP4 or ILP3 plus different concentrations of the IR inhibitor hydroxyl-2-napthalenylmethyl phosphonic acid – trisacetoxymethyl ester (HNMPA-(AM3), EMD). The concentration of HNMPA that reduced ecdysteroid production by 50% (IC50 value) was calculated by non-linear regression using Sigma Plot (Brown et al., 2008).
2.5. Receptor binding and cross-linking assays
ILP3 and ILP4 were iodinated (125 Iodine, Perkin-Elmer) using the lactoperoxidase-hydrogen peroxide method and purified by HPLC (Crim et al., 2002). Membranes were extracted from 500–600 ovary pairs (4 to 7 day old non-blood fed females) as described previously (Brown et al., 2008). Unlabeled ILP (30 μl at 10X final concentration of 0.1 nM to 20 μM) and 125I-ILP (~200 to 300 pM, ~400K cpm/150 μl) were added to binding buffer (50 mM HEPES (pH 7.6), 1X Hanks balanced salt solution, 3% BSA and 0.1X Roche protease inhibitors) in 1.5 ml polypropylene microtubes (triplicate tubes/concentration) followed by addition of ovary membranes (20 μl) to a final volume of 300 μl. Samples were rotated overnight at 4° C, centrifuged, and the supernatants removed by aspiration. The pelleted membranes were then rinsed with ice-cold binding buffer, re-centrifuged, and the supernatant again removed. Cross-competitive binding experiments were set up similarly with unlabeled ILP3 and 125I-ILP4 and vice versa. Raw counts per minute in the pellet for each sample were converted to percent total binding, and the data from a minimum of three independent assays were analyzed by non-linear regression as described above to generate curves and IC50 values.
Binding assays, set up as described above, were also conducted for cross-linking experiments. After overnight incubation, membranes were pelleted and rinsed 2X with binding buffer minus BSA and protease inhibitors. Membranes were then dispersed in the same buffer (240 μl) followed by addition of 30 μl of Bis[sulfosuccin-imidyl] suberate and Bis[sulfosuccin-imidyl] glutarate (Aculytix, Rockford IL) freshly prepared in water to achieve a 10 μM (ILP4) or 100 μM (ILP3) final concentration of each cross-linking agent. Sample tubes were then rotated for 1 h at 4° C, and the reaction stopped by the addition of 100 mM Tris buffer (pH 7.5). Isolated pellets were then mixed with sample buffer, frozen, and later subjected to SDS-PAGE, immunoblotting and autoradiography as previously described (Brown et al., 2008). The MIR was detected using an anti-MIR primary antibody (377E, 1:6,000), a peroxidase-conjugated goat-anti rabbit secondary antibody (Sigma, 1:15,000) and chemiluminescence substrate (GE Amersham ECL Advance detection kit). Immunoblots were exposed to X-ray film (Kodak XR) for up to three weeks to visualize 125I-ILP-protein conjugates.
2.6. Akt and MAPK immunoblots
Ovaries were pooled from in vitro assays or dissected from adult female mosquitoes and transferred to sample buffer (1 part Novagen PhosphoSafe Extraction Buffer: 1 part Roche protease inhibitor 1X in saline) for extraction. Ovary samples (3–4 ovary pairs per lane) were then electrophoresed on 12.5% Tris-glycine gels (Bio-Rad) followed by immunoblotting using rabbit primary antibodies (Cell Signaling Technologies) for phospho-Drosophila Akt (Ser505) (#9106) 1:1000; phospho-human p44/42 MAP kinase (Thr202/Tyr204) (#9101) 1:2000; and rat P44/42 MAP kinase (#9102) 1:2000. Visualization of MAPK using the above anti-MAPK served as the loading control for immunoblots.
2.7. RNAi assays
Total RNA was extracted from mosquito ovaries, first-strand cDNA synthesized, and MIR cDNA amplified using previously described primers and reaction conditions (Brown et al., 2008). cDNA to the Ae. aegypti relaxin receptor homolog (AaRLGR) was similarly amplified using primers (T7-Fwd: TAATACGACTCACTATAGGG ACATGGTCGGTACCAGAAGC; T7-Rev: TAATACGACTCACTATAGGGTTGAGTGTGTTCCGGGTTT) designed from the annotated AaRLGR sequence (AAEL005095; EAT43473). The resulting PCR products were used as template (1 μg of cDNA) for dsRNA synthesis with the TranscriptAid T7 High Yield Transcription Kit (Fermentas). dsRNA was then treated with DNAse1 and RNAse1, precipitated, and resuspended in nanopure water. dsEGFP was similarly generated for control treatments (Brown et al., 2008). Integrity of the dsRNA was assessed by gel electrophoresis, and its concentration determined by spectrophotometry for dilution to 4 μg/μl, prior to storage at −20°C. dsRNA (2 μg) was injected into intact or decapitated females (3 to 5 days old) at 1 h PBM for the bioassays as described above. We assessed actin and MIR expression using first-strand cDNA synthesized from total RNA, gene specific primers, and reaction conditions as previously described (Sieglaff et al., 2005). We assessed levels of AaRLGR knockdown in dsRLGR and dsEGFP treated samples by rqRT-PCR using a Rotor-Gene 2000 Real-Time PCR Cycler (Corbett Research) using the primers 5′-TCGCTCTACCTGAGGCATCT-3′ (forward) and 5′-GCTCAGGGTGCTGAGAAAAC-3′ (reverse). All experiments were done in triplicate with relative quantitations determined using the ΔΔCT method. Transcript abundance was normalized to internal 18S rRNA levels from the same individual sample.
2.8. Bioassay data analysis
Sample data were statistically analyzed using the JMP 7.0 platform (SAS Inc.). Differences among treatments for the metabolic, yolk and ecdysteroid assays described above were analyzed by one-way ANOVA, and Dunnett’s or the Tukey-Kramer multiple comparison procedure.
3. Results
Prior studies firmly establish that the brain is the only source of ILPs in Ae. aegypti that stimulate egg maturation following blood feeding (Lea, 1967; Matsumoto et al., 1989; Brown et al., 2008), but a lack of comparative data makes it unclear whether the five ILP family members (ILP1, 3, 4, 7, and 8) expressed in brain neurosecretory cells have similar or divergent functions in mosquito physiology. ILP3 structurally most resembles mammalian insulin and as previously noted has both metabolic and gonadotropic activity (Brown et al., 2008). Among the four other ILPs, we focused here on ILP4 because it is the only family member expressed in female but not male brains (Riehle et al., 2006), and exhibits structural differences with ILP3 that could affect receptor binding and/or biological activity (Supplementary Fig. 1). ILP1 and 8 in contrast likely form a transcriptionally co-regulated gene complex with ILP3 (Riehle et al., 2006), while an extended B chain makes synthesis of ILP7 much more difficult than ILP4.
3.1. ILP4 and ILP3 exhibit partially overlapping biological activity
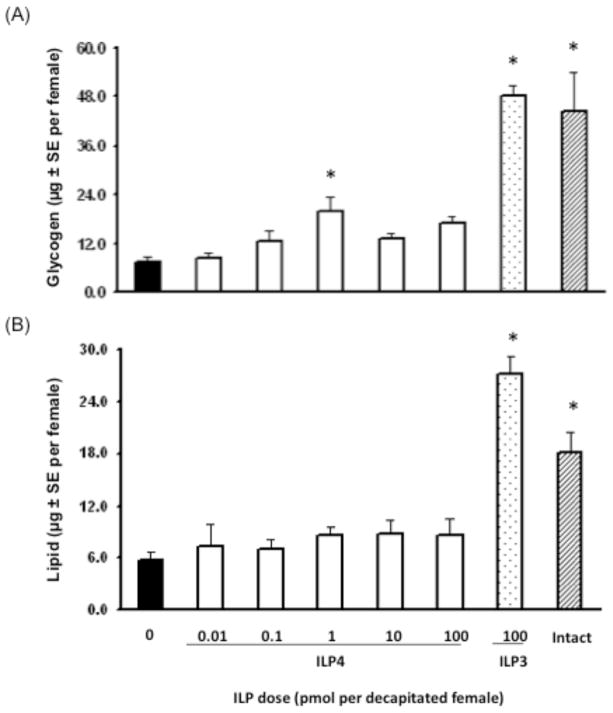
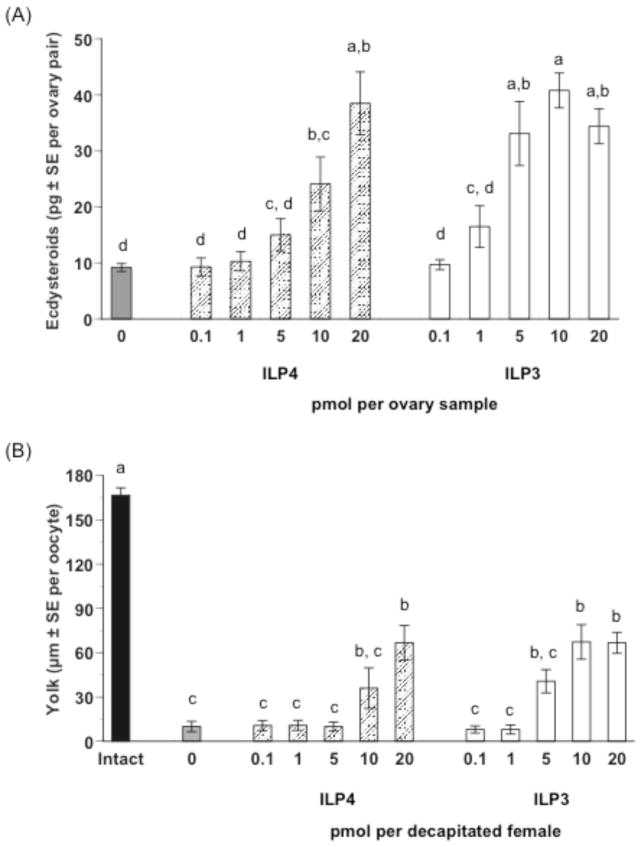
ILP3 exhibits strong, dose-dependent insulin-like activity by elevating carbohydrate and lipid storage in decapitated, sugar-fed adult females to similar levels as control (non-decapitated), sugar-fed females (Brown et al., 2008). We assessed whether ILP4 also exhibits insulin-like activity by conducting the same dose-response experiments using ILP3 as a positive control. Our results indicated that unlike ILP3, ILP4 had almost no effect on glycogen or lipid stores at any concentration tested (Fig. 1A, B). In contrast, ILP4 and ILP3 exhibited similar gonadotropic activity as evidenced by dose-dependent stimulation of ovaries to produce ecdysteroids required for stimulation of yolk protein synthesis and release by the fat body (Fig. 2A). The concentration of ILP4 required to stimulate a significant increase in ecdysteroid production was somewhat higher (20 pmol) than for ILP3 (5 pmol), but the maximum amount of ecdysteroids produced by ovaries (35–45 pg per ovary pair per assay) was very similar for both peptides. ILP4 also dose-dependently stimulated yolk deposition in primary oocytes (Fig. 2B). Again, the dose of ILP4 required to stimulate a significant increase in yolk deposition was higher (20 pmol) than for ILP3 (10 pmol). A single injection of either 20 pmol ILP4 or 10 pmol of ILP3 into decapitated, blood fed females also resulted in very uniform yolk deposition in primary oocytes as evidenced by the very small standard errors for the data. However, as expected, levels of yolk deposition in oocytes from decapitated, ILP treated females were lower than in oocytes from intact (non-decapitated), blood-fed females (Fig. 2B).
Fig. 1.
ILP4 does not stimulate glycogen and lipid storage in sugar-fed, decapitated female Ae. aegypti. Mosquitoes were decapitated 30 min after sugar feeding and injected with ILP4 (0.01–100 pmol). Decapitated mosquitoes injected with 100 pmol of ILP3 or non-decapitated (intact) mosquitoes served as positive controls while decapitated mosquitoes injected with saline only (0) served as a negative control. Glycogen (A) and stored lipid (B) in individual mosquitoes was then measured 24 h later with a minimum of 8 mosquitoes analyzed per treatment. Asterisks indicate treatments that differ significantly from the negative control (Glycogen, F7, 67= 39.0, P<0.0001; Lipid, F7, 62= 18.2, P<0.0001 followed by Dunnett’s multiple comparison with a control procedure, α=0.05). Although marginally statistically significant at the α=0.05 level, the increase in glycogen found with injection of 1 pmol of ILP4 was non-significant at α=0.01.
Fig. 2.
ILP4 and ILP3 stimulate ecdysteroid synthesis by ovaries and yolk uptake by primary oocytes from blood-fed, female Ae. aegypti. (A) Ecdysteroid production (pg ± SE) by ovaries treated with ILP4 or ILP3. Ovaries from sugar-fed females (4 ovary pairs per sample) were incubated 6 h in medium containing a given amount of each ILP (0.1–20 pmol), followed by quantification of ecdysteroid amounts in the medium. Ovaries incubated in medium without ILP (0) served as a negative control. Different letters above a given treatment indicates means that significantly differ from one another (F10, 94= 14.6, P<0.0001; followed by comparison for all pairs using the Tukey-Kramer procedure). (B) Yolk uptake (μm ± SE) by primary oocytes following injection of ILP4 or ILP3. Females were decapitated within 2 h after blooding feeding and injected once with a given ILP (0.1–20 pmol). Yolk deposition was then measured 24 h later. Females injected with saline only served as a negative control while normal, nondecapitated (intact) females served as a positive control. A minimum of 12 mosquitoes was analyzed per treatment. Different letters above a given treatment indicates means that significantly differ from one another (F11, 165= 32.9, P<0.0001 and Tukey-Kramer procedure, α=0.05).
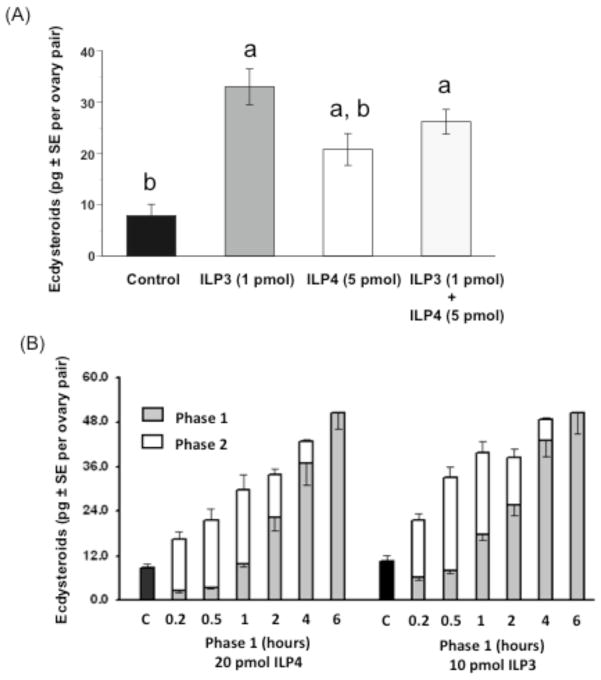
Since brain neurosecretory cells concurrently express and presumably release ILP3 and ILP4 after blood feeding, we considered the possibility that they additively affect ecdysteroid production by ovaries to promote rapid egg maturation. We tested this by incubating ovaries in vitro with a suboptimal dose of ILP4 (5 pmol) or ILP3 (1 pmol) alone or together. Our results, however, revealed no differences between ovaries incubated with each ILP or the two ILPs together, which suggested that ILP4 and ILP3 activity is non-additive (Fig. 3A). We also assessed whether the commitment time required to stimulate a sustained increase in ecdysteroid production by ovaries differed between ILP4 and ILP3. Ovaries were exposed to an optimal dose of ILP4 (20 pmol) or ILP3 (10 pmol) for increasing periods of time (10 min to 6 h). The medium was then removed from each sample (phase 1), and immediately replaced with medium without ILP (phase 2) to complete the 6 h incubation. The amounts of ecdysteroids present in the phase 1 and phase 2 samples were then determined by RIA and summed. Ecdysteroid amounts increased with the amount of time that ovaries were co-incubated with each ILP, although phase 1 samples for ILP3 consistently contained more ecdysteroids than the phase 1 samples for ILP4 (Fig. 3B). Thus, ecdysteroid production positively correlated with exposure time to each ILP, but ILP3, even at a lower concentration, activated ecdysteroid biosynthesis more strongly than ILP4. Taken together, we concluded from these experiments that ILP4 and ILP3 exhibit distinctly different metabolic activity but broadly similar gonadotropic activities in adult female Ae. aegypti.
Fig. 3.
ILP4 and ILP3 non-additively stimulate ecdysteroid production by ovaries. (A) Ovaries (4 pairs per sample) were incubated with suboptimal doses of ILP3 (1 pmol) or ILP4 (5 pmol) for 6 h either alone or together followed by determination of ecdysteroid amounts in the medium by RIA. Ovaries incubated in medium without ILP served as the negative control. Each treatment was replicated 12 times. Asterisks indicate treatments, which differ significantly from the negative control (F3, 44= 8.5, P<0.0002 and the Tukey-Kramer procedure). (B) Effect of ILP4 or ILP3 exposure time on ecdysteroid production by ovaries. Ovaries (4 pairs per sample) were incubated in two phases for a total of 6 h. In phase 1, ovaries were incubated in medium containing 20 pmol of ILP4 or 10 pmol of ILP3 while in phase 2, ovary samples were incubated in fresh medium without ILPs. At the end of each phase, ecdysteroid amounts in the medium were determined by RIA and summed. Nine ovary samples were analyzed per treatment.
3.2. ILP4 weakly competes with ILP3 and does not bind to the MIR
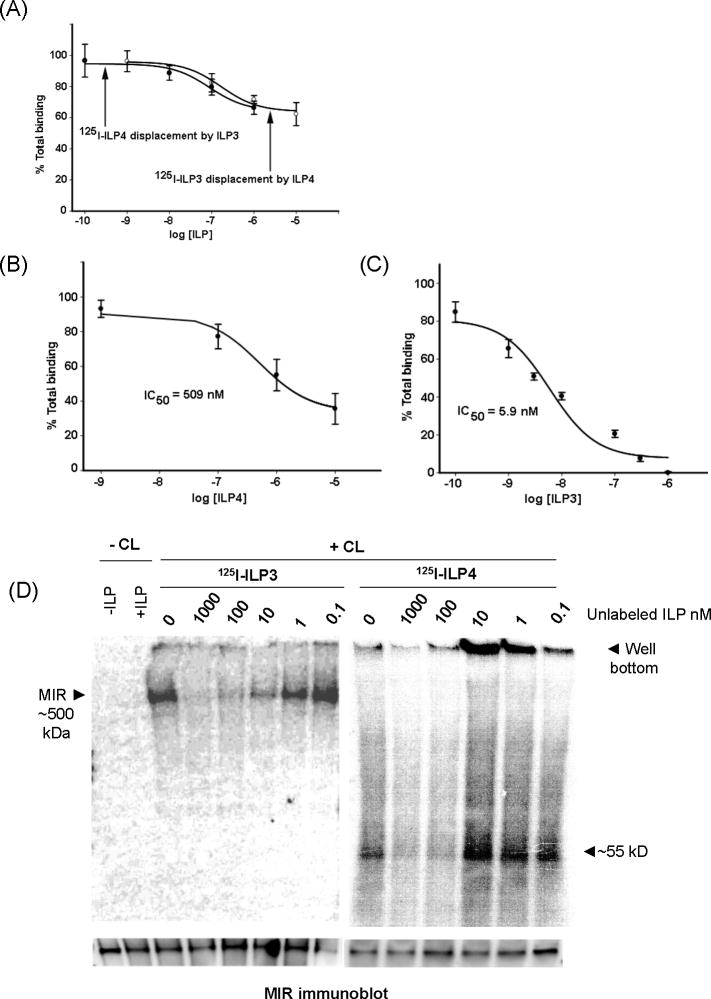
We compared ILP4 and ILP3 binding properties by first conducting competitive ligand-receptor assays using ovary membranes, which are highly enriched with the MIR (Riehle and Brown, 2002; Brown et al., 2008). Cross-competition assays using radiolabeled ILP4 or ILP3 in the presence of increasing concentrations of unlabeled ILP3 or ILP4 respectively showed only a small reduction of total binding (Fig. 4A). Unlabeled ILP3 reduced binding of ILP4 by only 22% when present at 10,000-fold molar excess with a similar trend observed for radiolabeled ILP3 displacement by ILP4 (Fig. 4A). Unlabeled ILP4 displaced radiolabeled ILP4 somewhat more efficiently, although at 10 μM binding was only reduced ca. 40% of total (Fig. 4B). The source of this non-specific binding was not apparent, but the data were nonetheless sufficient to estimate an IC50 value of 509 nM (Fig. 4B). This value, however, was 100X greater than was previously found for radiolabeled ILP3, which is fully competed by unlabeled ILP3 with an IC50 value of 5.9 nM (Fig. 4C).
Fig. 4.
ILP4 and ILP3 are weak cross-competitors and bind different proteins in ovary membranes. (A) Binding of 125I-ILP4 or 125I-ILP3 to ovary membranes in the presence of increasing concentrations of unlabeled ILP3 or ILP4. (B) 125I-ILP4 binding to ovary membranes in the presence of increasing concentrations of unlabeled ILP4 with calculated IC50 value indicated. Bound counts in the presence of increasing concentrations of each unlabeled competitor, minus non-specific binding, are plotted as a decreasing percentage of total binding. (C) 125I-ILP3 binding to ovary membranes in the presence of increasing concentrations of unlabeled ILP3 with calculated IC50 value indicated (from Brown et al., 2008). (D) Autoradiograph of ovary membrane blots showing competitive binding of 125I-ILP3 to the MIR complex (left) and competitive binding of 125I-ILP4 to ~55 kDa and 120 kDa proteins. Ovary membranes (20–30 ovary pair equivalents) were incubated with each labeled ILP (250 pmol) and increasing concentrations of the corresponding unlabeled ILP. Samples were then cross-linked, subjected to SDS-PAGE and blotted for autoradiography. The first two lanes of the blot show samples incubated with (+) or without (-) ILP3. Samples with cross-linker present (Cl+) or absent (Cl-) are indicated at the top of autoradiograph. The bottom of the autoradiograph shows the corresponding immunoblot probed with our anti-MIR antibody, which detects a single band at ~500 kDa in each lane.
These results indicated that ILP3 and ILP4 weakly compete with one another and suggested they either bind different receptors or different domains on the same receptor complex. To distinguish between these possibilities, we conducted cross-linking experiments using a modified approach from Brown et al. (2008) that included two cross linkers of different size. These results showed that ILP3 binds a ~500 kDa complex containing the MIR as shown on immunoblots (Fig. 4D). ILP3 binding to this complex was also fully competed by increasing concentrations of unlabeled ILP3. Radiolabeled ILP4 in contrast did not cross-link to the MIR, yet did cross-link to a 55 kDa protein (Fig. 4D). Competition by unlabeled ILP4 showed near complete loss of label from the 55 kDa protein by 100 nM ILP4. In other replicates of this cross-linking experiment, we also sometimes observed cross-linking of ILP4 to a 120 kDa protein that was likewise competed by unlabeled ILP4 (data not shown). However, this observation was inconsistent. Whether the the 55 kDa protein, 120 kDa protein, or a combination of the two represent the specific binding shown in Fig. 4B remained unclear, but our results do suggest ILP4 binds a novel protein but not the MIR as observed for ILP3 (Fig. 4D).
3.3. ILP4 and ILP3 activate the insulin signaling pathway in ovaries
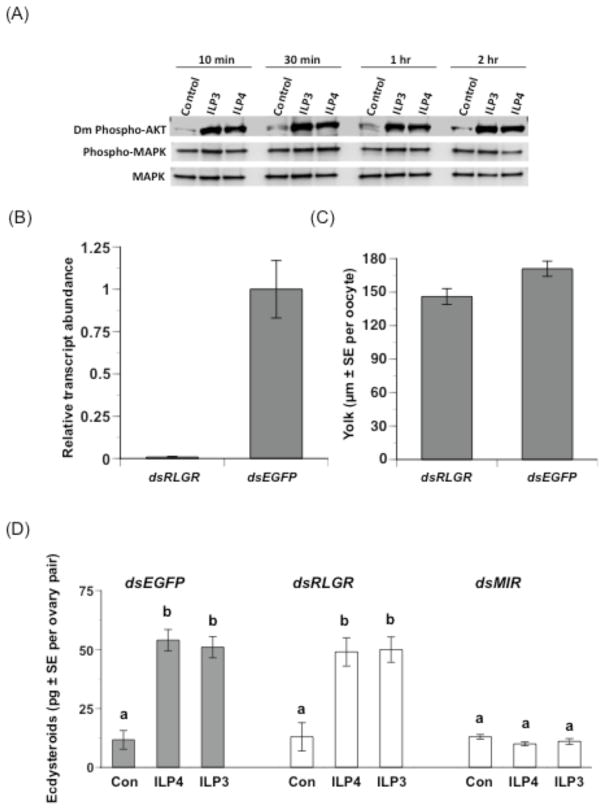
The functional and receptor binding activities of ILP3 clearly suggested this family member should exhibit insulin-like signaling properties such as phosphorylation of the serine-threonine kinase, Akt. In contrast, the lack of metabolic activity by ILP4 combined with a lack of binding to the MIR suggested it might exhibit other signaling properties like activation of the MAPK pathway as seen for vertebrate IGFs. To assess these possibilities, we repeated our in vitro gonadotropic bioassay by treating ovaries with optimal doses of each ILP. We then collected ovary samples at different time points post-treatment for immunoblot analysis using a Drosophila anti-phospho-Akt and two antibodies specific for non-phosphorylated and phosphorylated forms of human MAPK that cross-react with mosquito Akt and MAPK respectively (Kang et al, 2008). Preliminary studies confirmed each antibody recognized Ae. aegypti proteins of expected size for Akt and MAPK (Supplementary Fig. 2). Our experimental results further indicated that ILP3 and ILP4 both increased the level of phosphorylated Akt detected in ovaries at each sample time relative to untreated control ovaries (Fig. 5A). In contrast, we detected no changes in the relative levels of phosphorylated and non-phosphorylated MAPK after ILP3 or ILP4 treatment (Fig. 5A). We also observed the same pattern with ovaries collected from mosquitoes 10 min to 2 h after blood feeding, which further corroborated that ILPs released after blood feeding activate the insulin signaling pathway in ovaries.
Fig. 5.
ILP4 and ILP3 activate the insulin signaling pathway while knockdown of the MIR but not RLGR disables gonadotropic activity. (A) Ovaries (4 pairs per sample) were incubated in vitro with ILP3 (10 pmol) or ILP4 (20 pmol) for 10 min–2 h. Samples were then subjected to SDS-PAGE and immunoblot analysis using primary antibodies to Drosophila-phospho-Akt (ser505), human P44/42 MAPK, and phospho-P44/42 MAPK (Thr202/Tyr204). Three ovary pair equivalents were loaded per lane. Increased phosphorylation of Akt was detected within 10 min of treatment with each ILP and sustained for 2 h, whereas no change was detected in phosphorylation levels of MAPK. (B) rqRT-PCR analysis of RLGR transcript abundance in mosquito ovaries treated with dsRLGR or dsEGFP (negative control). Mosquitoes were injected with each double-stranded RNA 1 h after blood feeding and transcript abundance was determined 24 h later. Transcript levels were standardized to a level of 1 for mosquitoes treated with dsEGFP while transcript levels for mosquitoes treated with dsRLGR are expressed relative to the negative control. Each treatment was replicated three times using samples generated from ovaries collected from four individuals. Error bar =1 SD. (C) dsRLGR and dsEGFP have no effect on yolk uptake by primary oocytes (t-test, P>0.05). Mosquitoes injected with dsRLGR (n=36) or dsEGFP (n=53) as described for (A) were dissected 24 h later and the mean size of oocytes determined. (D) dsMIR treatment significantly reduces ecdysteroid production by ovaries while dsRLGR and dsEGFP does not. Ovaries (2 ovary pairs per sample) were dissected from treated females 24 hours PBM and incubated for 6 hours with ILP3 (10 pmol), ILP4 (20 pmol) or no ILP (Con), followed by ecdysteroid RIA of the sample medium. A minimum of 6 samples was analyzed per treatment. Different letters above a given treatment indicates means that significantly differ from one another (F8, 105= 19.4, P<0.0001; followed by comparison for all pairs using the Tukey-Kramer procedure).
3.4. ILP3 and ILP4 activity requires the MIR
The preceding results fully supported the idea that ILP3 requires the MIR for function. In contrast, our results with ILP4 show binding to a 55 and 120 kDa protein, but whether activity was independent of the MIR remained unclear given this family member also stimulated phosphorylation of Akt. Thus, ILP4 could depend on the MIR for function, albeit indirectly, or the 55 and/or 120 kDa proteins could be receptors that stimulate Akt phosphorylation and ecdysteroid production independently of the MIR. We further noted that the 55 kDa protein is within the molecular mass range of specific leucine-rich repeat-peptide G protein-coupled receptors (LGRs) that bind relaxin and other ILPs in mammals (Hsu et al., 2002). Insects also encode one or more LGRs for which no ligand has been identified (Hsu et al., 2002; Loy et al., 2008).
The Ae. aegypti genome encodes one predicted relaxin LGR homolog of 573 amino acids and predicted molecular mass of 63.1 kDa (AaRLGR; DrmLGR3- CG31096 homolog). RT-PCR analysis also indicated the corresponding transcript is expressed in ovaries and other tissues (data not shown). We therefore assessed whether the egg maturation activities of ILP3 and ILP4 depended upon the RLGR or MIR using two complementary approaches. In the first, we knocked down RLGR and MIR expression in ovaries by RNAi followed by functional assays. Prior studies confirmed that dsMIR treatment of blood fed mosquitoes significantly reduces MIR transcript and protein levels in ovaries, which correspondingly results in reduced yolk uptake by oocytes and reduced ecdysteroid production by ovaries (Brown et al., 2008). No antibody is available for the AaRLGR protein, but rqRT-PCR analysis indicated that injection of dsRLGR into blood fed mosquitoes greatly reduced AaRLGR transcript abundance in ovaries (110-fold) relative to mosquitoes injected with dsEGFP (negative control) (Fig. 5B). However, treatment of blood fed mosquitoes with dsRLGR or dsEGFP had no effect on yolk deposition in primary oocytes (Fig. 5C) suggesting AaRLGR is not required for egg maturation. Comparing the effects of dsMIR and dsRLGR on ecdysteroid production by ovaries from blood-fed, decapitated females corroborated this conclusion (Fig. 5D). Ovaries from dsRLGR treated mosquitoes produced near-identical amounts of ecdysteroid after treatment with ILP4 or ILP3 as ovaries from mosquitoes treated with dsEGFP. In contrast, ovaries from females treated with dsMIR produced almost no ecdysteroids after treatment with ILP4 or ILP3 (Fig. 5D).
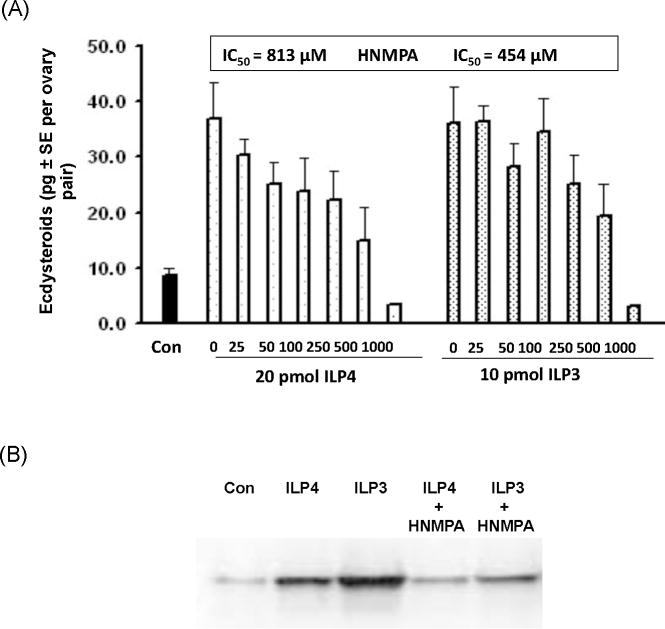
As a second approach, we tested the effects of HNMPA-(AM3), a specific cell-permeable inhibitor of insulin receptor tyrosine kinase activity and tyrosine autophosphorylation of the mammalian IR (IC50=200 μM) (Saperstein et al. 1989) required for insulin-stimulated signaling. Our results indicated that HNMPA-(AM3) dose-dependently reduced ecdysteroid production by ovaries following treatment with ILP4 or ILP3 at IC50 values similar to those previously determined for mammals (Fig. 6A). Immunoblotting experiments further indicated that, similar to mammals, HNMPA-(AM3) disabled MIR signaling as evidenced by the inability of ILP3 and ILP4 to increase levels of phosphorylated Akt in ovaries in the presence of the inhibitor (Fig. 6B).
Fig. 6.
HNMPA-(AM3) dose-dependently reduces ecdysteroid production by ovaries treated with ILP4 and ILP3. (A) Ovaries (4 ovary pairs per sample) were dissected from mosquitoes 24 h PBM and incubated with ILP4 (20 pmol) or ILP3 (10 pmol) and increasing concentrations of HNMPA-(AM3) for 6 h followed by ecdysteroid RIA of the medium. Ovaries incubated in medium without ILP or HNMPA-(AM3) served as a negative control. Nine samples were analyzed per treatment with the IC50 value for inhibiting ILP4 and ILP3 activity indicated above the graph. (B) Ovaries (4 pairs per sample) were incubated in vitro 2 h with ILP3 (10 pmol) or ILP4 (20 pmol) for 2 h alone or with HNMPA-(AM3) (500 μM). Samples were then subjected to SDS-PAGE and immunoblot analysis using the Drosophila-phospho-Akt (ser505) antibody. Three ovary pair equivalents were loaded per lane. Increased phosphorylation of Akt was detected following treatment with each ILP alone, whereas no change in phosphorylation was detected in control ovaries (con) incubated without ILPs or ovaries incubated with ILPs plus HNMPA-(AM3).
Discussion
Our understanding of ILP function in invertebrates derives primarily from characterizing the effects of overexpressing (Ikeya et al., 2002) or deleting multiple family members (Rulifson et al., 2002; Broughton et al., 2005; Belgacem and Martin, 2006; Zhang et al., 2009; Grönke et al., 2010). As a consequence, the direct endocrine function(s) of individual ILP family members and their interactions with the single IR expressed by insects remains unclear. Here we produced two ILPs from Ae. aegypti and present evidence that they exhibit different metabolic but very similar gonadotropic activities in adult females. Our results further show that synthetic ILP3 binds the MIR with high affinity, whereas ILP4 does not. Combined with earlier results showing that ILP3 binding is poorly competed by bovine insulin (Brown et al., 2008), our results suggest that individual ILPs from Ae. aegypti are not functionally equivalent, and that the MIR does not bind endogenous ILPs and mammalian insulins with similar affinity. Because invertebrates encode multiple ILPs but only one IR homolog, the diverse functions associated with insulin signaling in lower metazoans have been attributed to developmental differences in the timing of expression or secretion of ILP family members (Brogiolo et al., 2001; Broughton et al., 2005; Gershman et al., 2007; Geminard et al., 2009; Taguchi and White, 2009; Grönke et al., 2010). Our results, however, suggest differing affinities by the IR for individual ILPs may be a more important control mechanism for function than previously recognized.
Our results fully support the conclusion that ILP3 directly binds the MIR with high affinity. Our functional studies further indicate ILP3 signaling depends upon activation of the MIR since dsMIR and HNMPA-(AM3) treatment both block gonadotropic activity. Prior results showed that agonists of Akt phosphorylation stimulate mosquito ovaries to produce ecdysteroids (Riehle and Brown, 1999, 2003). Results from the current study are in keeping with this finding as they indicate the gonadotropic activity of ILP3 also depends on signaling through a functional Akt. In contrast, our binding and cross-linking experiments indicate ILP4 binds a 55 kDa protein in ovary membranes with much lower affinity than found for ILP3. One explanation for this result is that the synthetic ILP4 we produced improperly refolded, due to incorrect disulfide bond formation between the A and B chains, to yield a structure that does not bind the MIR. We cannot fully discount this possibility, but also emphasize that ILP4 exhibited very similar gonadotropic activity to synthetic ILP3 and much stronger gonadotropic activity than purified bovine insulin (see Brown et al., 2008), which we would not expect if ILP4 was structurally aberrant. It is also possible our binding data with ILP4 reflect improper binding conditions for interaction with the MIR. We think this is unlikely, however, given preliminary studies we conducted in which a variety of conditions were tested yet none resulted in detectable binding of ILP4 to the MIR.
Assuming ILP4 is properly folded, then our binding data together with the outcome of our dsMIR and HNMPA-(AM3) experiments suggest interaction with the 55 kDa and possible 120 kDa proteins in some manner activates the MIR and insulin signaling to drive ecdysteroid biosynthesis and yolk uptake. The identity of these proteins, however, and how they might activate the MIR remains unclear. Our results strongly suggest the 55 kDa protein is not AaRLGR, since knockdown of the corresponding transcript has no effect on ILP4 activity. A large number of intracellular adapter proteins have also been identified that bind and positively modulate the activity of mammalian insulin/IGF receptors (APS, CB, DOCK2, GAB1, IRS1–4, SHC, SH2B). Yet, only IRS1 and SH2B have been shown to bind the IR/IGRF of insects, and neither of these intracellular factors are known to directly interact with ILPs which would suggest the 55 and 120 kDa proteins are unrelated to these factors (Bohni et al., 1999; Werz et al., 2009). The 55 kDa and 120 kDa proteins could also be extracellular ILP binding factors such as Imp-L2 and dALS that antagonize ILP function in Drosophila (Honegger et al., 2008; Arquier et al., 2008). We think this too is unlikely though, because highly purified ovary membranes were used for the ILP4 binding and cross-linking assays we performed. In contrast, recent studies in mammals do show that G-protein coupled receptors (GPCRs) can interact with receptor tyrosine kinases, including the IGFR, resulting in their transactivation (Delcourt et al., 2007), and that β-arrestin-2, an intracellular modulator of GPCR activity, scaffolds with the IR and Akt to directly activate signaling (Luan et al, 2009). These data thus suggest the possibility that the 55 kDa protein could be a GPCR capable of interacting with the MIR and activating insulin signaling. Obviously, identification of these proteins is essential to understanding their interaction with ILP4.
As previously noted, ILP3 is expressed in the medial neurosecretory cells of both male and female adult mosquitoes, whereas ILP4 expression is female specific (Riehle and Brown, 2006; Brown et al., 2008). The gonadotropic activity of ILP4 is fully consistent with regulation of a sex-specific function like egg maturation, whereas the broader metabolic and gonadotropic activities of ILP3 are in keeping with functions relevant to both sexes. How ILP3 and ILP4 stimulate insect ovaries to produce ecdysteroids remains undefined, although recent findings indicate another neuropeptide, prothoracicotropic hormone (PTTH), stimulates ecdysteroid production in the prothoracic glands by binding a receptor tyrosine kinase (Torso) that stimulates signal-regulated kinase (ERK) phosphorylation (Rewitz et al., 2009). Interestingly, insulin/ILP treatment also activates Ras signaling and the ERK cascade in both mammals and insects suggesting a potential link between PTTH and ILP-mediated regulation of ecdysteroid production (Kim et al., 2004; Orme et al., 2006; Teleman, 2010). Downstream events leading to increased ecdysteroid production by mosquito ovaries also remain unclear although prior studies do suggest ILPs activate the transcription of genes encoding cytochrome P450 enzymes required for ecdysteroid biosynthesis (Sieglaff et al., 2005). ILP3 likely modulates glycogen and lipid storage in the fat body by activation of insulin signaling, which regulates metabolic control in mammals and insects (summarized by Teleman, 2010). Insulin signaling is also known to stimulate vitellogenin synthesis in the fat body of blood-fed Ae. aegypti females (Roy et al., 2007). In contrast, understanding why ILP4 lacks metabolic activity will require identification of the ILP4 binding proteins.
All known insulin/IGFR receptors are encoded by single genes that are post-translationally processed and linked as homo- or heterodimers composed of two extracellular α subunits, which form the extracellular ligand binding domain, and two transmembrane, tyrosine kinase β-subunits, which activate the insulin signaling pathway (Taguchi and White, 2008). The MIR from Ae. aegypti and the Drosophila insulin receptor (DIR) are similar to mammalian IR/IGFRs with the exception that the DIR includes approximately 300 additional amino acid residues at both the N- and C-termini (Fernandez et al., 1995; Graf et al., 1997). The MIR and overlapping portions of the DIR are only 35% identical to the mammalian IR, yet the literature often suggests contrary to our results that the DIR binds mammalian insulin with similar affinity to mammalian IRs (Taguchi and White, 2008; Telman, 2010). The evidence cited as support for this conclusion, however, derives from studies conducted in mammalian cells expressing a recombinant chimeric receptor comprised of the human extracellular ligand binding domain (α subunit) attached to the Drosophila β subunit (Yamaguchi et al., 1995). This expression system showed that the essential signal transducing capacity of vertebrate and insect IRs is conserved, but it is not surprising that a chimeric receptor comprised of human α subunits has near identical binding affinities for mammalian insulin as the human IR. The only study to our knowledge that examines the affinity of the DIR for mammalian insulins is that of Petruzzelli et al. (1985) who determined a Km of 15 nM for bovine insulin using a partially purified receptor preparation obtained from whole body fly extracts. This result is consistent with the biological activity that mammalian insulins have in Drosophila, mosquitoes and other insects (summarized by Wu and Brown, 2006), but also provide no insights about the relative affinity of the DIR for endogenous ILPs. Our results, therefore, offer the first insight into the selectivity of an insect IR for endogenous ILPs and mammalian insulins, while also providing the first evidence that individual insect ILP family members exhibit differences in biological activity.
Supplementary Material
Acknowledgments
We thank D. Fendley and I. Haider for technical assistance. This work was supported by National Institutes of Health Grant AI031108 and the Georgia Agricultural Experiment Station.
Abbreviations
- BSA
bovine serum albumen
- ILP
insulin-like peptides
- DIR
Drosophila insulin receptor
- GPCR
G-protein coupled receptor
- IGFs
insulin-like growth factors
- IGFR
insulin growth factor-like receptor
- IR
insulin receptor
- MAPK
mitogen-activated protein kinase
- MIR
mosquito (Aedes aegypti) insulin receptor
- PBM
post-blood meal
- RNAi
RNA interference
- RT-PCR
reverse transcriptase polymerase chain reaction
- rqRT-PCR
relative quantitative RT-PCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ament SA, Corona M, Pollock HS, Robinson GE. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc Natl Acad Sci USA. 2008;105:4226–31. doi: 10.1073/pnas.0800630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arik AJ, Rasgon JL, Quicke KM, Riehle MA. Manipulating insulin signaling to enhance mosquito reproduction. BMC Physiol. 2009;9:15, 1–11. doi: 10.1186/1472-6793-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arquier N, Geminard C, Bourouis M, Jarretou G, Honegger B, Paix A, Leopold P. Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab. 2008;7:333–338. doi: 10.1016/j.cmet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Barve V, Ahmed F, Adsule S, Banerjee S, Kulkarni S, Katiyar P, Anson CE, Powell AK, Padhye S, Sarkar FH. Synthesis, molecular characterization, and biological activity of novel synthetic derivatives of chromen-4-one in human cancer cells. J Med Chem. 2006;49:3800–3808. doi: 10.1021/jm051068y. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- Belgacem YH, Martin JR. Disruption of insulin pathways alters trehalose level and abolishes sexual dimorphism in locomotor activity in Drosophila. J Neurobiol. 2006;66:19–32. doi: 10.1002/neu.20193. [DOI] [PubMed] [Google Scholar]
- Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell. 1999;97:865–75. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–21. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MDW, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistence in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005;102:3105–10. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Graf R, Swiderek KM, Fendley D, Stracker TH, Champagne DE, Lea AO. Identification of a steroidogenic neurohormone in female mosquitoes. J Biol Chem. 1998;273:3967–3971. doi: 10.1074/jbc.273.7.3967. [DOI] [PubMed] [Google Scholar]
- Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2008;105:5716–21. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crim JW, Garczynski SF, Brown MR. Approaches to radioiodination of insect peptides. Peptides. 2002;23:2045–2051. doi: 10.1016/s0196-9781(02)00192-4. [DOI] [PubMed] [Google Scholar]
- Delcourt N, Bockaert J, Marin P. GPCR-jacking: from a new in RTK signaling to a new concept in GPCR activation. Trends Pharmacol Sci. 2007;28:603–607. doi: 10.1016/j.tips.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Fernandez R, Tabarini D, Azpiazu N, Frasch M, Schlessinger J. The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO J. 1995;14:3373–84. doi: 10.1002/j.1460-2075.1995.tb07343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géminard C, Rulifson EJ, Léopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metabol. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Gershman B, Puig O, Hang L, Peitzsch RM, Tatar M, Garofalo RS. High-resolution dynamics of the transcriptional response to nutrition in Drosophila: a key role for dFOXO. Physiol Genomics. 2007;29:24–34. doi: 10.1152/physiolgenomics.00061.2006. [DOI] [PubMed] [Google Scholar]
- Graf R, Neuenschwander S, Brown MR, Ackermann U. Insulin-mediated secretion of ecdysteroids from mosquito ovaries and molecular cloning of the insulin receptor homologue from ovaries of bloodfed Aedes aegypti. Insect Molec Biol. 1997;6:151–63. doi: 10.1111/j.1365-2583.1997.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Grönke S, Clark DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLOS Genetics. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SH, Lin JL, Lin PL, Chen CH. Insulin stimulates ecdysteroidogenesis by prothoracic glands in the silkworm, Bombyx mori. Insect Biochem Molec Biol. 2009;39:171–9. doi: 10.1016/j.ibmb.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Halls ML, van der Westhuizen ET, Bathgate RAD, Summers RJ. Relaxin family pepide receptors: former orphans reunite with their parent ligands to activate multiple signaling pathways. Br J Pharacol. 2007;150:677–691. doi: 10.1038/sj.bjp.0707140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger B, Galic M, Köhler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–4. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Kang MA, Mott TM, Tapley EC, Lewis EE, Luckhart S. Insulin regulates aging and oxidative stress in Anopheles stephensi. J Exp Biol. 2008;211:741–8. doi: 10.1242/jeb.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Cho JY, Kim KS, Lee SJ, Lee KH, Choi KY. Drosophila PI3 kinase and Akt involved in insulin-stimulated proliferation and ERK pathway activation in Schneider cells. Cell Signaling. 2004;16:1309–1317. doi: 10.1016/j.cellsig.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Lea AO. The medial neurosecretory cells and egg maturation in mosquitoes. J Insect Physiol. 1967;13:419–429. doi: 10.1016/0022-1910(67)90082-0. [DOI] [PubMed] [Google Scholar]
- Loy TV, Vandersmissen HP, Van Hiel MB, Poels J, Verlinden H, Badisco L, Vassart G, Vanden Broeck JV. Comparative genomics of leucine-rich repeats containing G protein-coupled receptors and their ligands. Gen Comp Endocrinol. 2008;155:14–21. doi: 10.1016/j.ygcen.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Luan B, Zhao J, Wu H, Duan B, Shu G, Wang X, Li D, Jia W, Kang J, Pei G. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457:1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- Luckhart S, Riehle MA. The insulin signaling cascade from nematodes to mammals: insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev Comp Immunol. 2007;31:647–56. doi: 10.1016/j.dci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Brown MR, Suzuki A, Lea AO. Isolation and characterization of ovarian ecdysteroidogenic hormones from the mosquito, Aedes aegypti. Insect Biochem. 1989;19:651–656. [Google Scholar]
- Okamoto N, Yamanaka N, Satake H, Saegusa H, Kataoka H, Mizoguchi A. An ecdysteroid-inducible insulin-like growth factor-like peptide regulates adult development of the silkmoth Bombyx mori. FEBS J. 2009;276:1221–1232. doi: 10.1111/j.1742-4658.2008.06859.x. [DOI] [PubMed] [Google Scholar]
- Orme MH, Alrubaie S, Bradley GL, Walker CD, Leevers SJ. Input from RAS is required for maximal PI3K signaling in Drosophila. Nature Cell Biol. 2006;8:1298–1302. doi: 10.1038/ncb1493. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L, Herrera R, Garcia R, Rosen OM. The insulin receptor of Drosophila melanogaster. In: Feramisco J, Ozanne B, Stiles C, editors. Cancer Cells 3: Growth Factors and Transformation. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1985. pp. 115–121. [Google Scholar]
- Rewitz KF, Yamanaka N, Gilbert LI, O’Connor MB. The insect neuropeptide PTTH activates receptor tyrosine kinase Torso to initiate metamorphosis. Science. 2009;326:1403–1405. doi: 10.1126/science.1176450. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Brown MR. Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti. Insect Biochem Molec Biol. 1999;29:855–860. doi: 10.1016/s0965-1748(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Brown MR. Insulin receptor expression during development and a reproductive cycle in the ovary of the mosquito Aedes aegypti. Cell Tis Res. 2002;308:409–420. doi: 10.1007/s00441-002-0561-8. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Brown MR. Molecular analysis of the serine/threonine kinase Akt and its expression in the mosquito, Aedes aegypti. Insect Molec Biol. 2003;12:225–232. doi: 10.1046/j.1365-2583.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Fan Y, Cao C, Brown MR. Molecular characterization and developmental expression of insulin-like peptides in the yellow fever mosquito, Aedes aegypti. Peptides. 2006;27:2535–3028. doi: 10.1016/j.peptides.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Roy SG, Hansen IA, Raikhel AS. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Molec Biol. 2007;37:1317–1326. doi: 10.1016/j.ibmb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Saperstein R, Vicario PP, Strout HV, Brady E, Slater EE, Greenlee WJ, Ondeyka DL, Patchett AA, Hangauer DG. Design of a selective insulin receptor tyrosine kinase inhibitor and its effect on glucose uptake and metabolism in intact cells. Biochemistry. 1989;28:5694–5701. doi: 10.1021/bi00439a053. [DOI] [PubMed] [Google Scholar]
- Sieglaff DH, Duncan KA, Brown MR. Expression of genes encoding proteins involved in ecdysteroidogenesis in the female mosquito, Aedes aegypti. Insect Biochem Molec Biol. 2005;35:369–514. doi: 10.1016/j.ibmb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci USA. 2008;105:6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nature: Molec Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Teleman AA. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J. 2010;425:13–26. doi: 10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- Toivonen JM, Partridge L. Endocrine regulation of aging and reproduction in Drosophila. Molec Cell Endocrinol. 2009;299:39–50. doi: 10.1016/j.mce.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 2009;336:309–23. doi: 10.1007/s00441-009-0769-y. [DOI] [PubMed] [Google Scholar]
- Werz C, Köhler K, Hafen E, Stocker H. The Drosophila SH2B family adaptor Lnk acts in parallel to chico in the insulin signaling pathway. PLoS Genet. 2009;5:e1000596. doi: 10.1371/journal.pgen.1000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Ann Rev Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Fernandez R, Roth RA. Comparison of the signaling abilities of the Drosophila and human insulin receptors in mammalian cells. Biochemistry. 1995;34:4962–4968. doi: 10.1021/bi00015a007. [DOI] [PubMed] [Google Scholar]
- Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science. 2008;319:1679–1683. doi: 10.1126/science.1151842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lui J, Li CR, Kohanski RA, Pick L. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc Natl Acad Sci USA. 2009;106:19617–19622. doi: 10.1073/pnas.0905083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.