Abstract
This paper reviews some of the potential benefits of preserving low-frequency residual hearing using a short-electrode cochlear implant. Both the status of the inner ear and acoustic characteristics of speech cues are important factors. How does the magnitude of the potential benefits depend upon the candidacy criteria for implantation with a hearing-preservation electrode?
Background
Previous research has demonstrated that preserving residual hearing in cochlear implantation can provide significant advantages for the understanding of speech in background noise as well as for the aesthetic qualities of music and other sounds. Developing optimal candidacy guidelines for these devices is a current goal.
Methods
In a large group of Hybrid (acoustic + electric) patients, performance in the recognition of speech in background of other talkers is measured and compared with traditional long-electrode patients. In addition, a number of patient characteristics are compared to success with the short-electrode implant.
Results
Age and duration of hearing loss are found to be predictive factors for the success of the short-electrode approach.
Conclusion
Optimal criterion for candidacy for the use of the short-electrode versus a traditional long electrode can improve the outlook for patients with severe-to-profound high-frequency hearing loss.
Keywords: Cochlear implant, Hybrid, sensorineural hearing loss
Introduction
Until recently, the treatments for sensorineural hearing loss, for the most part, fell into two general categories; 1) hearing aids, which amplify sound to be received by remaining hair cells in the cochlea and 2) cochlear implantation, which bypasses the problem of missing hair cells and stimulates the auditory nerve directly. Each of these approaches has its disadvantages and tends to be appropriate for different patient groups. Hearing aids can only work when there are some surviving hair cells, whereas the cochlear implant is recommended for severe or total hair cell loss. For severe to profoundly deaf patients, the restoration of even moderate speech understanding with a cochlear implant represents a tremendous improvement in their quality of life. For mild to moderate hearing loss patients, the improvements in speech understanding provided by a hearing aid are often quite helpful. However, for patients who have more severe losses, but whose speech recognition is not poor enough to recommend traditional implantation, there has not been a good treatment option. Typically, these patients have relatively sensitive low-frequency thresholds (sometimes in the mild hearing loss range), with a sharply- sloping, severe-to-profound high-frequency hearing loss. They can “hear” speech but without a sufficient level of understanding. To understand the dilemma associated with these patients, it is helpful to consider the nature of the various acoustic cues for speech and also the range of effects of sensorineural hearing loss on the recognition of these speech cues.
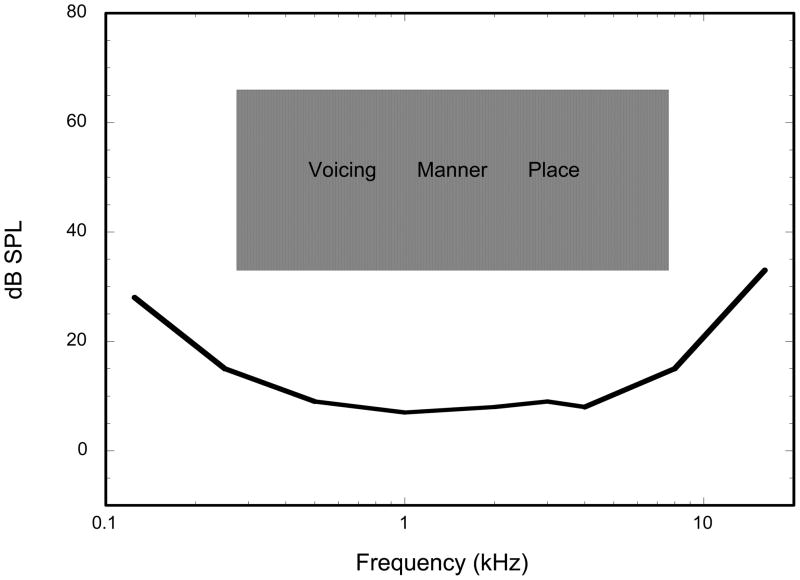
In Figure 1, we present a rough approximation of the location of speech cues in relation to the human audio range. Three types of speech cues are depicted within this shaded area, Voicing, Manner and Place. Strong voicing cues are present in the lower frequencies, and represent the presence or absence of vocal fold vibration in the production of speech (for example; distinguishing /s/ versus /z/). Acoustically, voicing cues are characterized by a broad low-frequency area of increased amplitude accompanied by periodic vibrations ranging from about 100–400 Hz. In contrast, place cues are indications of resonances in the vocal tract created by positions (places) of the articulators (tongue, lips, teeth etc.). They are characterized by the frequency locations of spectral peaks (generally above 1000 Hz) which differ for each type of speech sound (for example; /b/ versus /g/). Manner speech cues distinguish between different classes of speech, such as distinguishing between fricatives and stop consonants. Manner cues tend to lie somewhere between and overlapping the voicing and place cues in terms of frequency and involve timing, loudness and gross spectral shape. In summary, voicing cues are present in the lower frequency regions and only require that the listener be able to detect changes in loudness and temporal patterns, whereas place cues are in the higher frequency regions and require that the listener be able to discriminate between various spectral patterns.
Figure 1.
Approximate acoustic region of spoken speech in relaton to the auditory threshold curve.
Sensorineural hearing loss generally affects the sensory hair cells of the cochlea, although some degeneration of afferent nerve fibers can also occur following hair cell loss. Mild to moderate degrees of hearing loss generally involve a loss of outer hair cells, which are not the primary messengers to the brain, but instead are responsible for sensitive cochlear vibration and sharp frequency tuning. Thus patients with lesser degrees of hearing loss can benefit from amplification (to compensate for the reduced cochlear vibration) but also tend to show poorer than normal performance in background noise (due to the poorer frequency resolution). However, once hearing loss exceeds about 60–70 dB, inner hair cells begin to disappear as well (1). These receptors are the primary transmitters to the brain, and even a scattered loss of inner hair cells can presumably affect the brain’s ability to accurately perceive spectral shape characteristics via the frequency-place mapping code along the cochlea.
Thus for patients with nearly any residual low-frequency hearing, amplification at the low frequencies is generally helpful, allowing the perception of voicing (amplitude and timing) and some manner cues. High frequency amplification can be helpful to patients with hearing losses that preserve a reasonable population of inner hair cells in the basal end of the cochlea; however when a more severe loss of inner hair cells occurs, high-frequency amplification is not usually helpful (2–4), and speech recognition errors of place of articulation (and manner) are common. Since lipreading can provide some place cues, these patients usually can understand speech in face-to-face social situations, but accurate communication in many other settings is not possible.
The cochlear implant can address the problem of missing inner hair cells by stimulating the auditory nerve directly. However for the patient with a sloping hearing loss that is severe or profound in the high frequency region, implantation of a traditional long-electrode cochlear implant would generally destroy any residual hearing in the lower frequencies. Why might this be a disadvantage? In other words, what are the disadvantages of losing this low-frequency residual acoustic hearing as compared to hearing via electric-only stimulation? First, acoustic hearing, even when impaired to severe levels, generally provides better frequency resolution than electric stimulation. In one estimate of frequency resolution, the ability to discriminate “rippled noise” spectral shapes was compared between listeners with sensorineural hearing loss listening through hearing aids against patients using traditional cochlear implants (5). Even when the hearing loss was at severe levels, those with acoustic hearing had better resolution than those with electric hearing. Better frequency resolution is an important factor for recognizing speech in noise (6). Even patients with severe hearing loss (listening through hearing aids) can identify easy spondee words at more unfavorable signal-to-noise ratios than could implant patients (7). Additionally, the sound quality for acoustic hearing would be more “natural” as compared to the typical “raspy” or “mechanical” descriptions often given by implant patients. Better frequency resolution would also assist in the appreciation of music, in perceiving intonation cues of spoken speech and even voice recognition. Preserved acoustic hearing could also offer constant sound awareness as opposed to requiring the implant to be activated during such times as sleep or bathing.
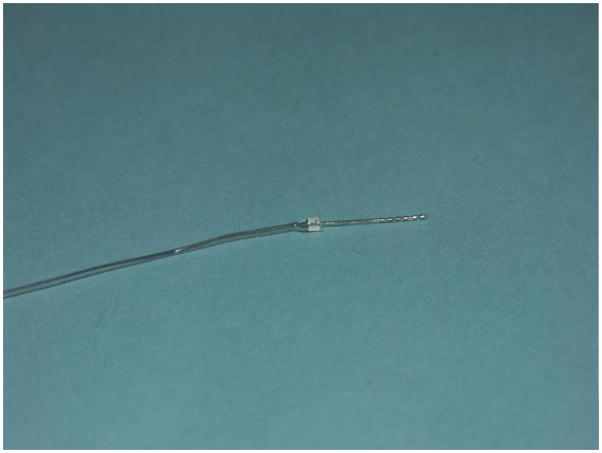
Despite these advantages of residual acoustic hearing, the ineffectiveness of high-frequency amplification for patients with severe-profound hearing loss in the high frequencies means they will still have difficulty in communication, particularly for the speech feature of place, which exhibits itself as errors in consonant recognition. The purpose of the Iowa/Nucleus Hybrid cochlear implant is to provide electric stimulation to the basal end of the cochlea while at the same time attempting to preserve any remaining low-frequency acoustic hearing in the apical end of the cochlea. Figure 2 displays the 10mm Iowa/Nucleus Hybrid electrode. There are 6 channels of electric stimulation located at the apical end of this device. Results of this device in groups of patients have been reported in several previous publications (8–10). In general, mean group recognition of CNC words or consonants in quiet improves by approximately 20–30% when the electric stimulation is added to the low-frequency acoustic hearing., with some patients scoring as high as 80–90% correct on these tests. Much of this improvement occurs as a result of a large increase in the patients’ ability to perceive place cues in the speech materials (11). These patients also out-perform typical long-electrode patients on tasks involving music perception as well (12). One interesting finding is that the typical Hybrid patient keeps improving in their speech recognition for 6–9 months after receiving the device, with some patients showing improvement up to 1 year. Adult patients with traditional long-electrode implants usually reach their maximum performance over a shorter time period. It should also be mentioned that several other approaches to preserving residual hearing in the implanted ear have been reported, with generally comparable results (13–16). This combination of acoustic plus electric hearing (A+E) is a promising solution for many patients who are borderline candidates for a traditional cochlear implant.
Figure 2.
The Iowa/Nucleus Hybrid Electrode
The early reports of the performance of A+E patients were also very encouraging for speech recognition in background noise, where A+E patients showed a considerable advantage over traditional electric-only implant patients (17–19). Many patients in these comparison long-electrode implant groups were implanted years ago with less modern devices and were often patients who, when implanted, had very poor or no pre-operative speech recognition and/or long durations of deafness. Both of these factors have been shown to be strong predictors of traditional implant performance (20), thus possibly biasing these comparison groups toward the lower end of potential performance. On the other hand, the first Hybrid A+E patients were cautiously chosen from populations whose residual hearing was not particularly sensitive, obscuring the true potential of this approach. As the candidacies for both Hybrid and traditional implants naturally have changed over time, along with the development of newer devices, better comparison groups can be assembled to reflect today’s conditions. In this article, we explore some factors that influence candidacy for Hybrid and comparison long-electrode groups.
Two specific questions are addressed:
Does preserving residual acoustic hearing and using a short-electrode implant provide an advantage over traditional long-electrode implants for understanding speech in a background of other talkers?
What patient factors offer predictive value for the probability of success in electrically stimulating an impaired ear with a short electrode?
Methods
These studies were conducted according to the guidelines for the protection of human subjects as set forth by the institutional review board of the University of Iowa, and the methods used were approved by that institutional review board.
Subjects
25 adult Hybrid (short-electrode) implant patients participated in the study. All of these Hybrid users met the criteria for the clinical trials of this device, which consisted of acoustic hearing better than 60 dB HL at frequencies at and below 500 Hz. Hearing levels in the higher frequencies were more severe (poorer than 80 dB HL at 2000 Hz and above). Pre-operative speech recognition for monosyllabic words in the implanted ear was between 10 and 60%-correct. All subjects were using a CIS processing strategy that allocated speech frequencies above approximately 700 Hz to the 6 electrodes of the Hybrid implant.
A comparison group of 31 adult long-electrode cochlear implant patients was also recruited. These subjects were selected from a larger group of implant patients in order to match the Hybrid patients in their ability to understand speech in quiet (see below). All were using the Cochlear Freedom speech processor with their own preferred clinical program. All were implanted within the past 5 years at the University of Iowa.
One way to examine the potential advantages of the Hybrid implant and preserving low-frequency acoustic hearing for speech recognition in background noise is to choose the comparison group of long-electrode patients so that their post-operative speech recognition scores in quiet match that of the Hybrid electrode group. Both groups in this comparison listened through the implanted ear only. Thus, some poorer performing long-electrode patients (who presumably may have had very poor pre-operative speech recognition or long durations of deafness) are not included in the comparison. The logic here is that these poorer patients would not be candidates for a Hybrid electrode in any case. The Hybrid patients are all of those tested at Iowa (excluding two who lost all residual hearing). These comparison group patients were first sorted according to their scores for recognizing consonants in a quiet background and starting from the highest performers were added to the comparison group until their group average speech in quiet scores matched the Hybrid group (who listened with A+E). Thus the long-electrode comparison group consisted of the “best performing” (for speech in quiet) of all research patients at Iowa.
Procedures
The task was the recognition of spondee words in a background of other talkers as described more completely elsewhere (17). Briefly, the patient was asked to choose the spoken target spondee word from 12 alternatives while a 2-talker competing background was presented at the same time. An adaptive procedure was used to determine the 50%-correct Speech Recognition Threshold (SRT), expressed as a signal-to-babble ratio in dB; thus a lower number represents better performance. For reference, normal-hearing listeners usually score about −25 dB on this same task (17).
For the second set of measures, the same group of Hybrid patients were tested using electric-only stimulation (direct electric connection) using CNC words.
Results
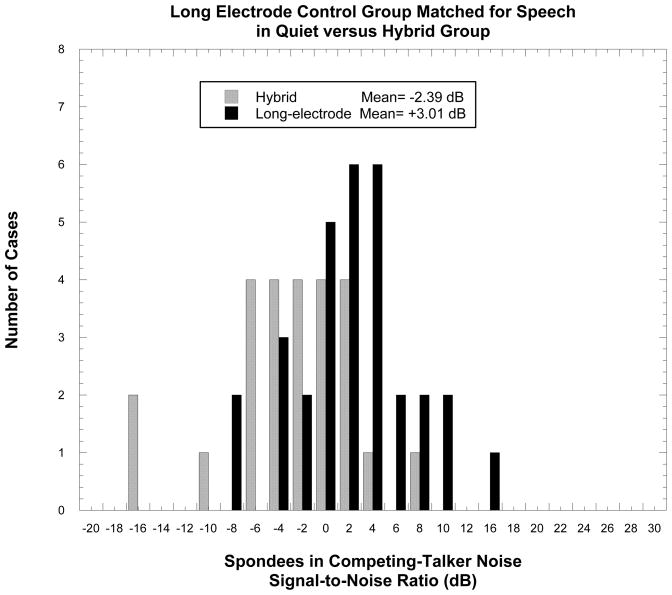
In Figure 3 we present such a comparison in a histogram displaying the recent results of individual patients using either a traditional long-electrode implant or the Hybrid. The Hybrid patients, on average, outperformed the long-electrode group by more than 5 dB, with most of the highest performing patients coming from the Hybrid group. This advantage for preserving acoustic hearing in cochlear implantation is most likely related to the better frequency resolution provided by acoustic hearing versus electrical stimulation.
Figure 3.
The Signal to Noise ratio for 50%-correct recognition of spondee words in a background of competing talkers (SRT) for individual patients using either a traditional long-electrode or the A+E Hybrid device.
Which patients are most appropriate for the Hybrid device? Clearly the patient should have some residual hearing to preserve. The clinical trials of the Hybrid device limited eligibility to patients with 60 dB HL or better thresholds at 500 Hz and below, so outcome data on patients with greater losses are harder to come by. However, several patients’ low-frequency hearing fell to poorer levels in the months following implantation, so a few data points from greater hearing losses are available. If one uses Hybrid patients’ speech recognition in a background of other talkers (as in Figure 3 above) as the outcome measure, when plotted as a function of the low-frequency pure-tone average, the advantage of preserving residual acoustic hearing over a traditional implant occurs for hearing levels up to approximately 90 dB HL (21). This is in general agreement with other findings (18) showing that even severely impaired hearing in the opposite ear could assist in an A+E advantage over electric alone hearing.
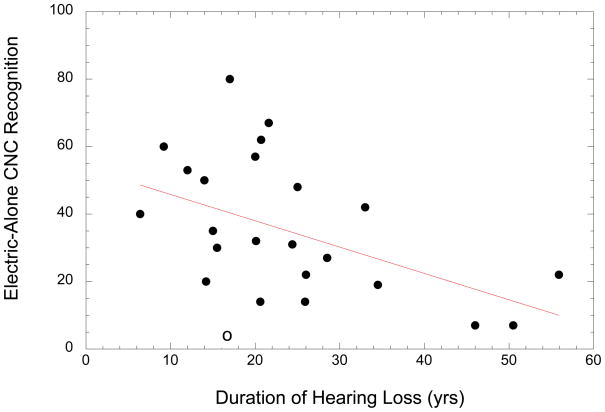
Another consideration for Hybrid candidacy is the effectiveness of a short electrode for providing the missing speech cues via electrical stimulation. Although no clear link has yet been shown between a patient’s nerve survival and their speech recognition (22), it does stand to reason that a longer electrode might have a better chance of stimulating remaining nerve fibers wherever they may be in the cochlea. On the other hand, a shorter electrode might be expected to be less invasive and better at preserving residual hearing (although again, no clear data on this issue is available at this time). Thus our question is: Who can benefit from a short electrode? There are two measures one might use to determine the effectiveness of electrical stimulation in the Hybrid population; 1) the increase in speech recognition when electric stimulation is added to the existing acoustic signal, or 2) the electric-alone speech score. The first option, which is the increase in speech recognition, suffers from potential ceiling effects, as a patient with high acoustic-only speech recognition is limited in how much electrical stimulation can improve their score. For our analysis we therefore used the second measure, electric-alone speech recognition (of CNC word lists). The Hybrid was programmed to present the usual frequency range of speech that the patient used in their everyday life (typically from 687–8000 Hz). We looked at a wide range of possible predictor variables that might offer some guidance in determining candidacy for a Hybrid device in terms of the effectiveness of electrical stimulation. These possible predictor variables included duration of hearing loss prior to implantation, duration of severe/profound high-frequency loss, age of onset of hearing loss (both for overall and just high-frequency loss), age at implantation, duration of hearing aid use, preoperative speech scores, various measures of pre-operative pure tone thresholds, and etiology. The only factors with significant predictive value were 1) duration of hearing loss (r = −.48, p < .05), and 2) age of implantation (r = −.49, p<. 05). Together these two variables account for 36% of the variance in the effectiveness of electric stimulation by the Hybrid electrode. Duration of hearing loss versus electric-alone speech recognition is plotted in Figure 4.
Figure 4.
The relation between the duration of hearing loss for Iowa Hybrid patients and their electric-only recognition of CNC words. The open-circle data point represents the single patient with a hearing loss that occurred at a very young age.
A more detailed analysis of the data in Figure 4 reveals that one patient in particular (open circle data point), with duration of hearing loss of 17 years and electric-alone speech score of 2% was by far the youngest recipient of a Hybrid device (age 18 at implantation), meaning that for this individual the hearing loss occurred prior to language development. This patient’s performance is well below the regression line for the effect of duration of loss, suggesting that another factor may also be playing a role. This implies that providing high-frequency speech cues later in life to individuals who have not had experience with them may not be productive. If this one patient’s data is not included in the analysis, the correlation between duration of hearing loss and speech recognition increases to r = .55. Although this one example is not conclusive, it does suggest that pre-lingual high-frequency hearing loss may possibly be a cautionary factor for A+E candidacy.
Discussion
There are numerous reports of patients with traditional long-electrode implants getting many of these same potential advantages by using the residual acoustic hearing in the contralateral, non-implanted ear (eg., 23, 24, 25). Often, this simply involves encouraging the patient to wear a hearing aid in the non-implanted ear. However, the traditional, long-electrode typically destroys any residual hearing in the implanted ear; this may be a potential disadvantage for some patients, particularly if the patient has sensitive low-frequency thresholds. The candidacy guidelines for the Iowa/Nucleus/Hybrid electrode included patients with normal thresholds up to 1500 Hz; such patients have, in the past, been quite reluctant to undergo implantation with a traditional electrode. However, if a patient is implanted with a short-electrode and does not receive much benefit from the device, then perhaps implantation with a longer electrode might have been a better choice (26). The analysis of the present data can perhaps provide some information to assit in developing appropriate candidacy considerations.
One can speculate as to the underlying reasons for these particular factors to correlate with the success of electric stimulation with a short electrode. Duration of hearing loss prior to implantation is similar to the factor “duration of deafness” which is a strong predictor of success for long-electrode patients (20). This could be related to degeneration of auditory nerve fibers over time following the death of the sensory receptor cells, or more generally to the failure of auditory pathways up to and including the brain to maintain the ability to decode speech cues without proper input signals from the periphery.
The factor of age of implantation exhibited itself primarily as poor performance with the electrical stimulation for elderly patients in particular (especially those over the age of 65–70 years). One hypothesis is that this factor is important due to the severe tonotopic mismatch that the short-electrode and its speech processor programming imposes on the auditory system. Remember that this electrode only extends 10 mm into the cochlea, placing the most apical electrode at approximately the 2800–4000 Hz region of the cochlea. These patients, when listening through acoustic hearing, are typically missing all speech cues above about 750 Hz. Since the bulk of speech information is located in the region from 300–3000 Hz (27), the clinical decision of assigning speech frequencies to these electrodes has been to direct as much of the missing speech spectrum to the Hybrid electrode, regardless of its extreme basal location. Thus the Hybrid patient is expected to 1) adjust to speech frequencies in abnormal absolute locations relative to the normal tonotopic map and 2) adjust to speech frequencies that are in abnormal locations relative to the acoustic hearing in the apex of the cochlea, which still has the essentially normal tonotopic mapping. We know (28) that in long-electrode patients can at least partially adjust over time to an overall shift in absolute tonotopic locations (which basically preserves the relative frequency-place mapping). However, adjusting to the severe discrepancies between the low-frequency acoustic and high-frequency electric signals is most likely an even more difficult task and may account for the longer than normal time course of improvement with the Hybrid device. These more elderly patients may be less able to adapt to these severe mismatch problems. There is related evidence that older users of implants have more difficulty adjusting to a second implant (29) and also that people with poorer cognitive abilities (usually older patients) had more difficulty adjusting to hearing aids with large amounts of amplitude compression (30).
The ability to adapt to new tonotopic maps in the cochlea has been suggested by other work from our research program. Hybrid patients’ perception of pitch has been shown to change over the months following implantation (31). This presumably occurs in order for the patient to reconcile different places of stimulation between the acoustic and electric stimulation in both the implanted and the contralateral ear. For many patients the eventual pitch sensation of each electrode (obtained by matching to the opposite, unimplanted ear) ended up corresponding to the frequency settings that the speech processor assigned to that electrode (32). This suggests that the brain’s interpretation of the tonotopic information coming from the implanted cochlea is plastic and adapts to the new stimulation pattern provided by the Hybrid implant. This ability to adapt would seem to be an important prerequisite for success with this device.
For individuals who do not have a long duration of hearing loss (20 years or less) and who may have the ability to adapt to the new stimulation patterns of A+E hearing, the short, Hybrid electrode would seem to be a promising treatment for patients with good low-frequency hearing and severe-to-profound high frequency hearing loss. This type of hearing loss has long been a particularly difficult hearing loss to treat and, the short-electrode implant, when successful, offers the advantages of preserving the patient’s acoustic hearing along with the benefits of electric stimulation. Additional experience with A+E hearing from both ipsilateral and contralateral preserved hearing patients is still required to determine appropriate guidelines for this new approach. In addition, hearing preservation electrodes will most likely undergo even further technological development in the future.
Acknowledgments
This work was supported by the National Institutes of Health.
References
- 1.Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hearing Research. 1984;16:55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- 2.Hogan C, Turner CW. High-frequency amplification: Benefits for hearing-impaired listeners. J Acoust Soc Am. 1998;104:432–441. doi: 10.1121/1.423247. [DOI] [PubMed] [Google Scholar]
- 3.Ching T, Dillon H, Bryne D. Speech recognition of hearing-impaired listeners: Predictions from audibility and the limited role of high-frequency amplification. J Acoust Soc Am. 1998;103:1128–1140. doi: 10.1121/1.421224. [DOI] [PubMed] [Google Scholar]
- 4.Vickers D, Moore BCJ, Baer T. Effects of low-pass filtering on the intelligibility of speech in quiet for people with and without dead regions at high frequencies. J Acoust Soc Am. 2001;110:1164–1175. doi: 10.1121/1.1381534. [DOI] [PubMed] [Google Scholar]
- 5.Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: Normal hearing, hearing impaired and cochlear implant listeners. J Acoust Soc Am. 2005;118:1111–1121. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- 6.Fu QJ, Shannon RV, Wang X. Effects of noise and spectral resolution on vowel and consonant recognition: Acoustic and electric hearing. J Acoust Soc Am. 1998;104:3586–3596. doi: 10.1121/1.423941. [DOI] [PubMed] [Google Scholar]
- 7.Turner CW. Hearing Loss and the Limits of Amplification. Audiology and Neurotology, 2006. 2006;11(S):2–5. doi: 10.1159/000095606. [DOI] [PubMed] [Google Scholar]
- 8.Gantz BJ, Turner CW. Combining acoustic and electric speech processing:Iowa/Nucelus Hybrid Implant. Acta Otolaryngolica. 2004;124:344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- 9.Gantz BJ, Turner CW, Gfeller K. Acoustic Plus Electric Speech Processing: Results of a Multicenter Clinical Trial of the Iowa/Nucleus Hybrid Implant. Audiology and Neurotology. 2006;11(S1):63–68. doi: 10.1159/000095616. [DOI] [PubMed] [Google Scholar]
- 10.Reiss LA, Gantz BJ, Turner CW. Cochlear Implant Speech Processor Frequency Allocations May Influence Pitch Perception. Otology and Neurology. 2008;29:160–167. doi: 10.1097/mao.0b013e31815aedf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gantz BJ, Turner CW. Combining Acoustic and Electric Hearing. Laryngoscope. 2003 October;113:1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Gfeller K, Turner C, Zhang X, Olszewski C, Gantz B. Accuracy of Cochlear Implant Recipients on Pitch Perception, Melody Recognition and Speech Reception in Noise. Ear and Hearing. 2007;28:412–423. doi: 10.1097/AUD.0b013e3180479318. [DOI] [PubMed] [Google Scholar]
- 13.Von Ilberg C, Kiefer J, Tillein J, Pfenningdorff T, Hartmann R, Sturzebacher E, Klinke R. Electro-Acoustic stimulation of the auditory system. ORL. 1999;61:334–340. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- 14.Skarzynski H, Lorens A, Piotrowska A. A new method of partial deafness treatment. Med Sci Monit. 2003;9:26–30. [PubMed] [Google Scholar]
- 15.Gstoettner W, Kiefer J, Baumgartner W-D, Pok S, Peters S, Adunka O. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otol. 2004;124:348–352. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- 16.James C, Albegger Battmer R, Burdo S, et al. Preservation of residual hearing with cochlear implantation: How and why? Acta Otol. 2005;125:481–491. doi: 10.1080/00016480510026197. [DOI] [PubMed] [Google Scholar]
- 17.Turner CW, Gantz BJ, Vidal C, Behrens A, Henry B. Speech recognition in noise for cochlear implant listeners: Benefits of residual acoustic hearing. J Acoust Soc Am. 2004;115:1729–1735. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- 18.Kong Y-L, Stickney G, Zeng FG. Speech and melody recognition in binaurally combined acoustic and electric hearing. J Acoust Soc Am. 2005;117:1351–1361. doi: 10.1121/1.1857526. [DOI] [PubMed] [Google Scholar]
- 19.Wilson BS, Dorman MF. Cochlear implants: Current designs and future possibilities. J Rehab Res Devel. 2008;45:695–730. doi: 10.1682/jrrd.2007.10.0173. [DOI] [PubMed] [Google Scholar]
- 20.Rubinstein JT, Parkinson WS, Tyler RS, Gantz BJ. Residual speech recognition and cochlear implant performance: Effect of implantation criteria. A, J Otol. 1999;20:445–452. [PubMed] [Google Scholar]
- 21.Turner CW, Gantz LR, Reiss LAJ. Integration of acoustic and electric hearing. Journal of Rehabilitation Research and Developments. 2008;45:769–778. doi: 10.1682/jrrd.2007.05.0065. [DOI] [PubMed] [Google Scholar]
- 22.Nadol JB, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos IK, Montandon P, Coker NJ, Roland JT, Shallop JK. Histopathology of cochlear implants in humans. Ann Otol, Rhino Laryng. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- 23.Ching TY. The evidence calls for making binaural-bimodal fitting routine. Hearing Journal. 2005;58:32–41. [Google Scholar]
- 24.Ching TYC, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004;25:9–21. doi: 10.1097/01.AUD.0000111261.84611.C8. [DOI] [PubMed] [Google Scholar]
- 25.Dorman MF, Gifford RH, Spahr AJ, McKarns SA. The benefits of combining acoustic and electric stimulation for the recognition of speech, voice and melodies. Audiol Neurootol. 2008;13(2):105–12. doi: 10.1159/000111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald MB, Sagi E, Jackson M, Shapiro WH, Roland JT, Waltzman SB, Svirsky M. Reimplantation of Hybrid cochlear implant users with a full length electrode after loss of residual hearing. Otol And Neurol. 2007;29(2):168–173. doi: 10.1097/mao.0b013e31815c4875. [DOI] [PubMed] [Google Scholar]
- 27.French NR, Steinberg JC. Factors governing the intelligibility of speech sounds. J Acoust Soc Am. 1947;19:90–119. [Google Scholar]
- 28.Fu QJ, Shannon RV, Galvin JJ. Perceptual learning following changes in the frequency-to-electrode assignment with the Nucleus 22 cochlear implant. J Acoust Soc Am. 2002;112:1664–1674. doi: 10.1121/1.1502901. [DOI] [PubMed] [Google Scholar]
- 29.Noble W, Tyler RS, Dunn C, Bhullar N. Younger and older-age adults with unilateral and bilateral cochlear implants: Speech and spatial hearing self ratings and performance. Otology and Neurology. 2009;30:921–929. doi: 10.1097/MAO.0b013e3181b76b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gatehouse S, Naylor G, Elberling C. Linear and nonlinear hearing aid fittings – 2. Patterns of candiditure. Int J Audiol. 2006;45:153–171. doi: 10.1080/14992020500429484. [DOI] [PubMed] [Google Scholar]
- 31.Reiss LAJ, Turner CW, Erenberg SR, Gantz B. Changes in pitch with a cochlear implant over time. J Assoc Res Otol. 2007 Jun;8(2):241–57. doi: 10.1007/s10162-007-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiss LA, Gantz BJ, Turner CW. Cochlear implant speech processor frequency allocations may influence pitch perception. Otol Neur. 2008;29:160–167. doi: 10.1097/mao.0b013e31815aedf4. [DOI] [PMC free article] [PubMed] [Google Scholar]