Oct-3/4 regulates stem cell identity and cell fate decisions by modulating Wnt/β-catenin signalling
The Oct-3/4 transcription factor is well known as a critical regulator ES cell pluripotency. Here, Bergman et al reveal a novel function for Oct-3/4 in modulating Wnt pathway activity, by promoting the degradation of nuclear β-catenin.
Keywords: β-catenin, ES cells, Oct-3/4, Tcf3
Abstract
Although the transcriptional regulatory events triggered by Oct-3/4 are well documented, understanding the proteomic networks that mediate the diverse functions of this POU domain homeobox protein remains a major challenge. Here, we present genetic and biochemical studies that suggest an unexpected novel strategy for Oct-3/4-dependent regulation of embryogenesis and cell lineage determination. Our data suggest that Oct-3/4 specifically interacts with nuclear β-catenin and facilitates its proteasomal degradation, resulting in the maintenance of an undifferentiated, early embryonic phenotype both in Xenopus embryos and embryonic stem (ES) cells. Our data also show that Oct-3/4-mediated control of β-catenin stability has an important function in regulating ES cell motility. Down-regulation of Oct-3/4 increases β-catenin protein levels, enhancing Wnt signalling and initiating invasive cellular activity characteristic of epithelial-mesenchymal transition. Our data suggest a novel mode of regulation by which a delicate balance between β-catenin, Tcf3 and Oct-3/4 regulates maintenance of stem cell identity. Altering the balance between these proteins can direct cell fate decisions and differentiation.
Introduction
Oct-3/4, encoded by the Pou5f1 gene, belongs to the POU-homeodomain transcription factor family. It is an important regulator of pluripotency during the earliest stages of vertebrate development (Brehm et al, 1998; Morrison and Brickman, 2006). Oct-3/4 expression is normally confined to pluripotent cells of the developing embryo, including epiblast and primordial germ cells, as well as their in vitro counterparts, embryonic stem (ES) and embryonic germ cells (Pesce and Scholer, 2001). It is expressed exclusively in embryonic cells during early embryogenesis and its expression is down-regulated during gastrulation, when somatic lineages are first defined. In mature animals, Oct-3/4 expression is confined to the germ cell lineage. The expression pattern of Oct-3/4 in embryonic and postnatal development suggests that it acts as a ‘stem cell survival or maintenance' factor (Boiani and Scholer, 2005). Consistent with this, suppression of Oct-3/4 expression causes complete loss of pluripotent stem cells in early embryonic life, showing that it is involved in maintaining the pluripotent state of ES cells (Nichols et al, 1998). Retinoic acid (RA) treatment induces ES cell differentiation and rapidly down-regulates Oct-3/4 expression. In addition, it has been shown that a critical amount of Oct-3/4 is required to sustain ES cell self-renewal (Niwa et al, 2000). Furthermore, reactivation of Oct-3/4 has been correlated with efficient reprogramming of somatic cells after the transfer of nuclei into oocytes (Boiani et al, 2002; Bortvin et al, 2003).
The Wnt signalling pathway is involved in virtually every aspect of embryonic development. It is one of the earliest signalling pathways necessary for the establishment of the early embryonic axes (Harland and Gerhart, 1997; Marikawa, 2006). The Wnt/β-catenin signalling pathway has multiple functions in stem cell biology, normal development and disease (Logan and Nusse, 2004; Reya and Clevers, 2005; Clevers, 2006). Several studies have shown that activation of Wnt/β-catenin can cause ES cells to remain pluripotent under conditions that would normally induce differentiation (Kielman et al, 2002; Sato et al, 2004a; Hao et al, 2006; Ogawa et al, 2006; Singla et al, 2006; Miyabayashi et al, 2007; Takao et al, 2007), whereas other studies have shown that the Wnt pathway controls differentiation of ES cells and terminal differentiation of post-mitotic cells (Otero et al, 2004; Lindsley et al, 2006).
Oct-3/4 is a potent transcription factor that was found to govern pluripotency by activating or repressing transcription of hundreds of target genes (Boyer et al, 2005). Here, we report a novel mechanism, whereby Oct-3/4 regulates pluripotency by promoting nuclear β-catenin degradation, thereby antagonizing Wnt/β-catenin signalling. We investigated the possible role of this functional interaction in maintaining ES cell pluripotency and regulating differentiation. Our results provide evidence that, in ES cells and Xenopus embryos, cell fate decisions are controlled by a delicate cross-talk between Oct-3/4 and the Wnt/β-catenin signalling pathway.
Results
Oct-3/4 interferes with Wnt/β-catenin signalling and induces a decrease in β-catenin protein levels
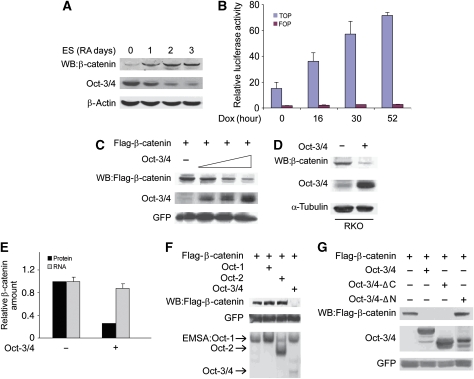
Both Oct-3/4 and the Wnt signalling pathway have been linked to the maintenance of pluripotency and the differentiation of ES cells. Therefore, we wanted to characterize possible regulatory interactions between Wnt signalling and Oct-3/4. We measured β-catenin and Oct-3/4 protein levels during RA-induced ES cell differentiation. Upon induction of differentiation, Oct-3/4 protein levels decreased, whereas β-catenin protein levels increased progressively (Figure 1A). These results are consistent with the previously published data showing that the canonical Wnt signalling pathway is up-regulated during early ES cell differentiation (Lindsley et al, 2006). We used ZHBTc4 ES cells, which harbour a tetracycline-regulated Oct-3/4 transgene, to assess whether the increase in β-catenin levels occurs in response to the decrease in Oct-3/4 expression. In these cells, both endogenous Oct-3/4 alleles are disrupted and the Oct-3/4 transgene is expressed at about 50% of wild-type (wt) expression levels. Treatment with doxycycline (dox) completely abolishes Oct-3/4 protein expression (Niwa et al, 2000). We treated ZHBTc4 with dox for times ranging from 16 to 52 h and examined β-catenin-dependent transcription of a Wnt-responsive reporter construct, TOPFlash. TOPFlash contains a multimeric Tcf/LEF1-binding site upstream of the c-fos promoter. We compared TOPFlash activity with the negative control, FOPFlash, which harbours mutated Tcf-binding sites (Korinek et al, 1997). TOPFlash activity in undifferentiated ZHBTc4 cells was low because of low levels of Wnt/β-catenin signalling. Upon addition of dox, TOPFlash (but not FOPFlash) reporter activity increased progressively throughout 52 h of dox treatment (Figure 1B). As the increase in TOPFlash activity was observed as early as 16 h after Oct-3/4 down-regulation, we suggest that Oct-3/4 inhibits (either directly or indirectly) the transcriptional activity of β-catenin in ES cells in a dose-dependent manner.
Figure 1.
Oct-3/4 promotes β-catenin degradation through its N-terminal domain. (A) Western blot (WB) analysis of Oct-3/4 and β-catenin levels during retinoic acid (RA)-induced ES cell differentiation. Cells were treated with RA for 0–3 days. β-actin served as a loading control. (B) ZHBTc4 ES cells were transfected with TOPFlash or FOPFlash expression vectors. Luciferase activity was assayed in untreated cells and cells treated with dox for the indicated time points. (C) 293T cells were transfected with Flag-β-catenin and GFP expression vectors, together with increasing amounts of Oct-3/4 expression plasmid (1, 5 and 10 μg). β-catenin and Oct-3/4 protein levels were detected using anti-Flag and anti-Oct-3/4, antibodies, respectively. GFP was used as a transfection efficiency control. (D) RKO cells were infected with lentiviral Oct-3/4 expression vector or empty control vector. WB analyses of Oct-3/4 and endogenous β-catenin protein levels were performed using antibodies directed against Oct-3/4 and β-catenin. The α-tubulin served as a loading control. (E) A relative quantification of endogenous β-catenin protein (black bars) and β-catenin mRNA (grey bars) levels in Oct-3/4 transfected (+) or untransfected (−) RKO cells. Ubiquitin C (UBC) and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) were used as loading controls for real-time quantitative PCR. (F) The 293T cells were transfected with Oct-1, Oct-2 and Oct-3/4 expression vectors. β-catenin degradation was shown by WB analysis. GFP served as a loading control. Cell extracts were incubated with P32-end-labelled Octa oligonucleotide, and Oct-1, Oct-2 and Oct-3/4 binding was detected using EMSA. (G) The 293T cells were transfected with the indicated expression vectors. Both Oct-3/4 and β-catenin levels were determined using WB analysis. GFP was used as a transfection efficiency control.
The inhibition of the β-catenin-dependent transcriptional activity by Oct-3/4 and the increase in β-catenin protein levels as a function of RA-induced differentiation raise the possibility that Oct-3/4 could modulate β-catenin protein levels. To test this hypothesis, we transfected 293T cells with the following expression vectors: Oct-3/4, β-catenin (Flag tagged) and GFP (as an expression reference). The results from these experiments clearly showed that β-catenin protein levels were reduced by Oct-3/4 in a dose-dependent manner in a gain-of-function setting (Figure 1C), corroborating the loss-of-function results (Figure 1B). In addition to reducing expression of transgenic β-catenin protein in 293T cells, introduction of Oct-3/4 by transfection into the carcinoma cell line RKO reduced endogenous β-catenin protein levels (Figure 1D and E). Importantly, this reduction in β-catenin protein levels took place without a concomitant reduction in the level of β-catenin transcript (Figure 1E), suggesting that the decrease is due to changes in translation or instability of the β-catenin protein.
We investigated whether other members of the POU-homeodomain family also reduce β-catenin protein levels. The 293T cells were co-transfected with Oct-1 or -2 expression vectors along with β-catenin and GFP expression vectors, and the levels of β-catenin and Oct-1, -2, -3/4 in these cells were assessed by western blot and the electromobility shift assay, respectively. Neither Oct-1, which is ubiquitously expressed in vivo, nor Oct-2, which is lymphoid specific, was able to reduce β-catenin protein levels (Figure 1F). We also tested two Xenopus POU-V family members, only one of these—Oct-25 (Pou5f1.1), a functional homologue of Oct-3/4—reduced β-catenin levels. Although transfection of Oct-25 into 293T cells did not affect transgenic β-catenin levels on its own, when Oct-25 was co-transfected with an amount of Oct-3/4 plasmid that was ineffective alone, it induced a dose-dependent reduction in β-catenin levels (Supplementary Figure 1). These data indicate that Oct-3/4 can regulate β-catenin protein levels, raising the possibility that Oct-3/4 participates in the down-regulation of β-catenin that occurs during normal embryonic development. The effect we observed with the Xenopus homologue Oct-25 suggests that this activity may be conserved across species.
We mapped the Oct-3/4 domain(s) necessary for this activity using a series of Oct-3/4 mutants. This series included Oct-3/4-ΔN, which lacks the first 35 amino acids, and Oct-3/4-ΔC, which lacks 75 amino acids constituting almost the entire carboxy-terminal domain. Interestingly, the C-terminal Oct-3/4 mutant reduced β-catenin protein levels to the same extent as wt Oct-3/4, whereas deletion of the N-terminal region abolished this activity completely (Figure 1G).
Oct-3/4 promotes degradation of β-catenin by a proteasomal complex that is blocked by canonical Wnt signalling
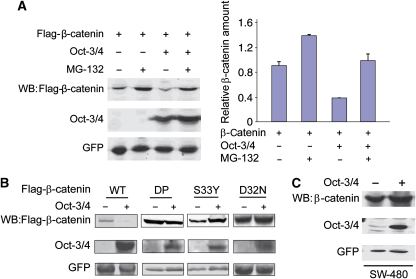
The canonical Wnt pathway functions in part by blocking proteasomal degradation of β-catenin, allowing β-catenin to accumulate and translocate to the nucleus, in which it binds Tcf3 and activates transcription. The proteasome destruction complex includes Axin, GSK3β and the adenomatous polyposis coli (APC) tumour-suppressor gene. On the basis of this, we used three approaches to investigate whether proteasomal degradation is responsible for the Oct-3/4-induced decrease in β-catenin. In the first set of experiments, 293T cells were transfected with β-catenin and Oct-3/4 expression vectors, and then treated for 5 h with and without 20 μM of the proteasome inhibitor MG-132 (Figure 2A). In the absence of MG-132, β-catenin protein levels were significantly reduced in Oct-3/4-expressing cells compared with cells transfected with β-catenin alone. MG-132 treatment of Oct-3/4-expressing cells prevented this decrease (Figure 2A). In the second set of experiments, we tested whether Oct-3/4 can decrease the protein levels of β-catenin mutants lacking phosphorylation residues crucial for proteasomal-mediated degradation. Oct-3/4 was co-transfected with vectors expressing wt or phosphorylation-mutant β-catenin into 293T cells. As described above, wt β-catenin levels decreased when Oct-3/4 was expressed; however, the three β-catenin mutants (DP, dominant-positive mutant in which S33, S37, T40, S45 and S47 were substituted with alanines, and the single-point mutants: D32N and S33Y; Amit et al, 2002) were unaffected by expression of Oct-3/4 (Figure 2B). In the third approach, we investigated whether APC is required for Oct-3/4-induced reduction in β-catenin levels. We infected SW-480 adenocarcinoma cells, which carry a mutated APC gene, with lentivirus-expressing Oct-3/4. In these cells, over-expression of Oct-3/4 had no effect on endogenous β-catenin protein levels (Figure 2C), indicating a requirement for APC. Together, these results suggest that the reduction in β-catenin induced by Oct-3/4 is due to proteasomal degradation of β-catenin mediated by the destruction complex that is blocked by Wnt signalling.
Figure 2.
Oct-3/4 facilitates β-catenin proteasomal degradation. (A) The 293T cells were transfected with the indicated expression vectors. Cells were treated with 20 μM of MG-132 for 5 h. Levels of β-catenin and Oct-3/4 were measured by WB analysis using anti-Flag and anti-Oct-3/4, respectively. A relative quantification of β-catenin levels is shown. GFP served as a loading control. (B) The 293T cells were transfected with wt Flag-β-catenin, or the indicated β-catenin mutants, with or without Oct-3/4 expression vector. DP is a dominant-positive mutant in which S33, S37, T40, S45 and S47 were substituted with alanines; D32N and S33Y are single-point mutants (Amit et al, 2002). Protein levels were detected through WB using anti-Flag and anti-Oct-3/4. GFP served as a loading control. (C) SW480 cells were infected with a lentivirus expressing both Oct-3/4 and GFP. Control infections were carried out with a lentivirus expressing only GFP. Oct-3/4 and endogenous β-catenin were analysed by WB. GFP served as a loading control.
Oct-3/4 forms a complex with β-catenin and Axin
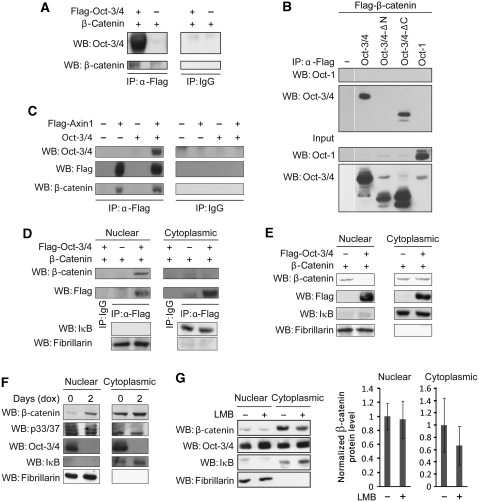
To assess whether Oct-3/4 physically associates with β-catenin, we used co-immunoprecipitation experiments. The 293T cells were transfected with expression vectors for Flag-tagged Oct-3/4 and β-catenin. The analysis was performed in the presence of MG-132 to block β-catenin degradation. Immunoprecipitation was performed with an antibody directed against the Flag-epitope of Oct-3/4 or a control antibody, and the precipitated material was subjected to western blot analysis with anti-Oct-3/4 and anti-β-catenin antibodies. As shown in Figure 3A, β-catenin was only co-precipitated when antibodies directed against the Flag-tagged Oct-3/4 were used, indicating that Oct-3/4 and β-catenin do indeed form a physical complex. Our results corroborate previously published pull-down experiments showing that bacterially expressed GST-β-catenin interacts with myc-tagged Oct-3/4 (Takao et al, 2007).
Figure 3.
Oct-3/4 associates with β-catenin and promotes its phosphorylation and destabilization in the nucleus. (A) The 293T cells were transfected with Flag-Oct-3/4 and β-catenin expression vectors. Cell extracts were subjected to immunoprecipitation using either an antibody directed against the Flag-epitope or a control antibody, and the precipitated material was subjected to WB analysis with antibodies directed against Oct-3/4 and β-catenin. (B) The 293T cells were transfected with Flag-β-catenin expression vector together with wt Oct-3/4 or wt Oct-1 or Oct-3/4 mutants with deletion in either the N- or the C-terminal domains. Cell extracts were subjected to immunoprecipitation using anti-Flag, and the precipitated material was subjected to WB analysis with antibodies directed against Oct-1 and Oct-3/4. (C) The 293T cells were co-transfected with expression vector for β-catenin together with Flag-tagged Axin1 and/or Oct-3/4 expression vectors, as indicated. Cell extracts were subjected to immunoprecipitation using either an antibody directed against the Flag-epitope or a control antibody, and the precipitated material was subjected to WB analysis with antibodies directed against Oct-3/4, Flag-epitope and β-catenin. (D) The 293T cells were transfected with expression vectors for Flag-tagged Oct-3/4 and β-catenin, as indicated. Cytoplasmic and nuclear extracts were prepared and subjected to immunoprecipitation using an antibody directed against the Flag-epitope, or a control antibody, and the precipitated material was subjected to WB analysis with antibodies directed against β-catenin and the Flag-epitope. IκB and Fibrillarin were used as cytoplasmic and nuclear control markers, respectively. (E) 293T cells were transfected with expression vectors for β-catenin and Flag-tagged Oct-3/4, as indicated. Cytoplasmic and nuclear extracts were prepared and levels of β-catenin and Oct-3/4 were measured by WB analysis using anti-β-catenin and anti-Flag antibodies. IκB and Fibrillarin were used as cytoplasmic and nuclear control markers, respectively. (F) ZHBTc4 ES cells were either treated or not with 1 μg/ml of dox for 2 days and cytosolic and nuclear extracts were prepared. The phosphorylation levels of β-catenin, as well as total levels of β-catenin and Oct-3/4, were analysed by WB analysis using the indicated antibodies. (G) ZHBTc4 ES cells were either treated or not with 4 ng/ml of LMB for 12 h and cytosolic and nuclear extracts were prepared, and levels of β-catenin and Oct-3/4 were measured by WB analysis using anti-β-catenin and anti-Oct-3/4 antibodies. IκB and Fibrillarin were used as cytoplasmic and nuclear control markers, respectively. Experiments were repeated four times and a relative quantification of β-catenin levels is shown. Arrow bars depict +/− StDV.
To map the domain through which Oct-3/4 interacts with β-catenin, 293T cells were transfected with Flag-tagged β-catenin, together with wt Oct-3/4 (positive control) or wt Oct-1 (specificity control) or Oct-3/4 mutants with deletions in either the N- or C-terminal domains (Figure 3B). Although Oct-3/4 lacking the C-terminus co-precipitated with Flag-β-catenin, Oct-3/4 lacking the N-terminal domain did not. These results corroborate our functional experiments (Figure 1F). We conclude that Oct-3/4 and β-catenin form a physical complex in living cells, for which the N-terminal domain of Oct-3/4 is required. Thus, the N-terminal domain is required both for the interaction between Oct-3/4 and β-catenin (Figure 3B) and for Oct-3/4-induced degradation of β-catenin (Figure 1G).
As discussed above, β-catenin stability is tightly regulated by a multi-subunit destruction complex. We next investigated whether Axin, the concentration-limiting component of this complex, is present in the complex containing Oct-3/4 and β-catenin. The 293T cells were transfected with expression vectors for Flag-tagged Axin1 and Oct-3/4. The analysis was performed in the presence of MG-132. Under these conditions, both β-catenin and Oct-3/4 co-precipitated specifically with Flag-tagged Axin (Figure 3C). In agreement, the reciprocal experiment clearly showed that anti-Flag antibody co-precipitates myc-tagged Axin with Flag-tagged Oct-3/4 (Supplementary Figure 2A). These results indicate that Oct-3/4, β-catenin and Axin1 form a physical complex, further supporting the involvement of the proteasomal destruction complex in Oct-3/4-induced degradation of β-catenin.
Oct-3/4 associates with β-catenin and promotes its phosphorylation and destabilization in the nucleus
As Oct-3/4 is primarily known as a nuclear transcription factor, we investigated whether the interaction between Oct-3/4 and β-catenin takes place in the cytosol or the nucleus. We subjected MG-132-treated 293T cells transfected with expression vectors for β-catenin and Flag-tagged Oct-3/4 to nuclear/cytoplasmic fractionation before co-immunoprecipitation. The purity of the fractions was verified using the nuclear marker Fibrillarin and the cytoplasmic marker IκB, which showed no cross-contamination (Figure 3D). Remarkably, the interaction between Oct-3/4 and β-catenin occurred exclusively in the nucleus (Figure 3D; Supplementary Figure 2B).
Proteasomes are abundant in both the cytosol and the nucleus (Reits et al, 1997; Joseph et al, 2003; Collins and Tansey, 2006; Ben-Aroya et al, 2010). Thus, we next explored whether Oct-3/4-induced β-catenin degradation is observed in the nuclear fraction. We transfected 293T cells with Oct-3/4 (Flag-tagged) and β-catenin expression vectors, and protein levels of β-catenin were measured in nuclear and cytosolic fractions. The results from these experiments clearly showed that β-catenin protein levels were reduced by Oct-3/4 in the nuclear fraction only (Figure 3E).
To analyse whether Oct-3/4-induced degradation occurs in ES cells, which normally express both Oct-3/4 and β-catenin and are a good model for the physiological activity of Oct-3/4, we used ZHBTc4 ES cells, which harbour a tetracycline-regulated Oct-3/4 transgene. Down-modulating endogenous Oct-3/4 expression levels by dox treatment resulted in up-regulation of endogenous β-catenin expression in the nuclear fraction only (Figure 3F), indicating that Oct-3/4 reduces β-catenin protein levels exclusively in the nucleus of ES cells.
To investigate whether Oct-3/4 leads to β-catenin sub-cellular redistribution, similarly to Axin or APC (Henderson, 2000; Rosin-Arbesfeld et al, 2000; Cong and Varmus, 2004), or promotes β-catenin degradation in the nucleus, ZHBTc4 ES cells were treated with LMB, a highly specific inhibitor of the CRAM1-dependent export pathway (Fukuda et al, 1997), and protein levels of β-catenin were measured in nuclear and cytosolic fractions. The results from these experiments clearly showed that LMB treatment did not alter nuclear β-catenin levels, indicating that Oct-3/4 does not reduce nuclear β-catenin through facilitating its export to the cytoplasm (Figure 3G). Lower β-catenin levels were observed in the cytosolic fraction of the LMB-treated versus -untreated ES cells, probably because of the altered β-catenin dynamic balance between the two fractions, a consequence of the LMB-induced block of β-catenin nuclear export.
An alternative model whereby Oct-3/4-induced nuclear β-catenin reduction is due to its ability to interact with Tcf3 (Cao et al, 2007), resulting in β-catenin displacement from the nucleus, was investigated. ZHBTc4 ES cells expressing a dominant-negative (DN) Tcf3 construct lacking the N-terminal region, required for interaction with β-catenin (Molenaar et al, 1996) (designated ZHBTc4/DN, see below), were treated with dox and β-catenin levels were assessed in the nuclear and cytosolic fractions. The results from these experiments clearly show that down-modulating endogenous Oct-3/4 expression levels by dox treatment results in up-regulation of endogenous β-catenin expression in the nuclear fractions of both wt- and DN-expressing ES cells, supporting a Tcf3-independent mechanism (Supplementary Figure 2C). Altogether, these results indicate that Oct-3/4 reduces β-catenin protein levels through its nuclear degradation, rather than shuttling or displacement mechanisms.
Phosphorylation of β-catenin at serines S33 and S37 by GSK-3β marks it for proteasomal degradation. To investigate how Oct-3/4 induces β-catenin nuclear degradation, ES-derived nuclear and cytosolic fractions were analysed with an antibody that recognizes these phosphorylated residues. Interestingly, in the presence of Oct-3/4, endogenous (Figure 3F) as well as transfected (Supplementary Figure 2D) β-catenin phosphorylation levels were enhanced in the nuclear fractions only. Altogether, these results indicate that Oct-3/4-induced β-catenin phosphorylation and degradation are observed in ES cell nuclei.
Oct-3/4 induces β-catenin degradation and interferes with Wnt signalling in Xenopus embryos
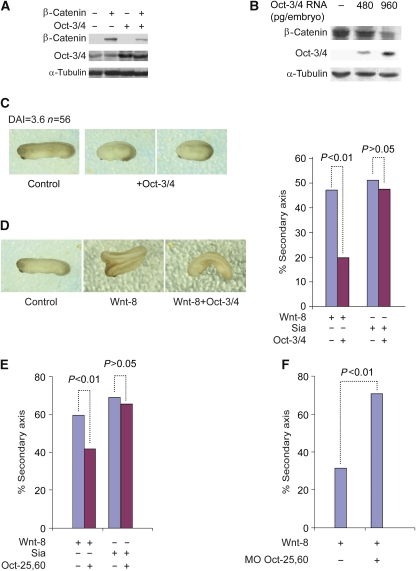
To gain insight into whether the interaction between the Wnt signalling pathway and POU-V domain proteins occurs during normal development, we turned to Xenopus embryos. We injected capped RNA encoding β-catenin and Oct-3/4 into four cell-stage embryos. Immunodetection assays showed that β-catenin protein levels were reduced by Oct-3/4 (Figure 4A). Moreover, injection of Oct-3/4 RNA into Xenopus embryos led to a decrease in the level of endogenous β-catenin in a dose-dependent manner (Figure 4B), indicating that POU-V domain protein-induced β-catenin degradation can occur in developing Xenopus embryos as well, explaining the reduction in Wnt signalling described below.
Figure 4.
Oct-3/4 interferes with Wnt/β-catenin signalling in vivo in Xenopus embryos. (A) Xenopus embryos were radially injected with β-catenin and/or Oct-3/4 mRNA at the 2–4-cell stage. β-catenin and Oct-3/4 protein levels were detected in embryo extracts using WB. The α-tubulin was used as a loading control. (B) Xenopus embryos were injected with increasing amounts of Oct-3/4 mRNA at the 2–4-cell stage. Embryo extracts were analysed using WB for Oct-3/4 and endogenous β-catenin protein levels. The α-tubulin was used as a loading control. (C) Xenopus embryos at the 2–4-cell stage were radially injected with Oct-3/4 mRNA. Examination of the embryos shows a ventralization phenotype. An un-injected embryo is shown as a control. (D) Summary of secondary-axis assays from Xenopus embryos injected at the 2–4 cell stage. Injection of mRNA encoding Wnt8 or Sia (5 pg) resulted in a high (48–55%) frequency of embryos with secondary axes. Co-injection of Oct-3/4 mRNA (30 ng) with Wnt8 mRNA results in inhibition of secondary-axis formation (P⩽0.01). Oct-3/4 does not inhibit Sia-induced secondary-axis formation. Representative embryos injected with the indicated RNAs are shown. (E) Summary of secondary-axis assays from Xenopus embryos injected at the 2–4 cell stage. Injections of mRNAs and analysis were performed as in (D). Oct-25,60 were used instead of Oct-3/4. Co-injection of Oct-25,60 mRNAs (30 ng) with Wnt8 mRNA results in inhibition of secondary-axis formation (P⩽0.01). Oct-25,60 does not inhibit Sia-induced secondary-axis formation. (F) Xenopus embryos were injected at the 2–4 cell stage with mRNA encoding Wnt8 together with antisense morpholinos oligos (MO) directed against Oct-25,60. A full-colour version of this figure is available at The EMBO Journal Online.
The early Wnt signalling pathway is required for formation of the embryonic organizer and promotes dorsal development. The extent of dorsal development can be scored by determining the dorsal-anterior index (DAI) of a group of embryos (Kao and Elinson, 1988). A DAI score of 5 indicates normal dorso-ventral development, whereas a score of 0 indicates fully ventralized embryos. Ectopic over-expression of β-catenin, Wnt8 or Siamois RNAs, all components of early Wnt signalling, results in dorsalized embryos (Christian et al, 1991; Lemaire et al, 1995; Wylie et al, 1996). We found that dorsal over-expression of Oct-3/4 mRNA to target the embryonic organizer yielded a ventralized phenotype (DAI=3.6, n=56; Figure 4C), consistent with down-regulation of endogenous Wnt signalling, probably as a result of β-catenin degradation. These results suggest that Oct-3/4 antagonizes Wnt signalling, leading to reduced transcription of β-catenin/Tcf-activated genes (Cao et al, 2007; Supplementary Figure 3). In agreement with this suggestion, inhibition of two of the three Xenopus POU-V genes, Oct-25 and Oct-60 (Oct-25,60) (Hinkley et al, 1992; Whitfield et al, 1993), resulted in increased expression of Wnt target genes (Supplementary Figure 4A–C). This ability to increase expression of these genes by blocking Oct-25 and -60 occurs at a specific time during embryonic development, and is abrogated by antisense morpholino oligos directed against β-catenin (Supplementary Figure 4D and E). These results show that POU-V genes negatively regulate β-catenin target gene expression in the frog embryo (Cao et al, 2007; Supplementary Figure 4).
To further study the physiological significance of Oct-3/4-induced β-catenin degradation, we tested the ability of Oct-3/4 to inhibit the secondary-axis formation induced by ventral activation of Wnt signalling (Sokol et al, 1991; Sokol, 1999). Secondary-axis formation in Xenopus embryos is a well-established model for assaying activators and inhibitors of the canonical Wnt signalling pathway (Moon et al, 2004). To decipher the epistatic relationship between Oct-3/4 and several components of the Wnt pathway, we examined the ability of Oct-3/4 RNA to inhibit the induction of secondary axis by RNAs encoding components of the Wnt pathway. We induced secondary axis by ectopic expression of Wnt-8 ligand, which promotes β-catenin stabilization, or Siamois, which is a transcriptional target of β-catenin. Injection of Oct-3/4 mRNA effectively blocked the secondary-axis induction by Xwnt-8, but failed to block secondary axis induced by injection of Siamois mRNA, as compared with control injections (Figure 4D). Similar results were obtained when Xenopus Oct-25,60 mRNAs (Figure 4E) were injected instead of Oct-3/4 mRNA, indicating that both Oct-3/4 and Xenopus POU-V proteins inhibit the Wnt signalling pathway downstream of Wnt-8 and upstream of its target gene, Siamois. Furthermore, in the complementary experiment using morpholinos, functional knock-down of Oct-25 and -60 led to an increase in secondary-axis formation (Figure 4F). These gain- and loss-of function assays provide clear evidence that Oct-25 and -60 affect β-catenin signalling in vivo and thereby regulate embryonic development.
Oct-3/4 modulates β-catenin activity in ES cells
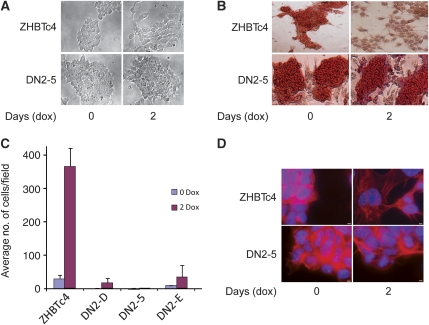
To elucidate whether Wnt signalling has a role in the functional consequences of decreased Oct-3/4 levels, we transfected ZHBTc4 ES cells with a DN Tcf3 construct lacking the N-terminal region required for interaction with β-catenin (Molenaar et al, 1996) (designated ZHBTc4/DN). Tcf3 is the most highly expressed Tcf family member in ES cells (Pereira et al, 2006). Single-cell clones of ZHBTc4/DN were isolated (ZHBTc4/DN2-5, ZHBTc4/DN2-A, ZHBTc4/DN2-D, ZHBTc4/DN2-E) and evaluated for Tcf3 expression (data not shown). Parental and ZHBTc4-DN-expressing clones were treated with dox to decrease Oct-3/4 expression and examined for morphological changes. As previously reported (Niwa et al, 2000), addition of dox resulted in repression of Oct-3/4 mRNA and protein to undetectable levels by 24 h. Although untreated cells had a compact morphology, dox-treated ZHBTc4 cells flattened out over the culture surface. In contrast, dox-treated ZHBTc4-DN cells retained the compact morphology seen in untreated parental cells (Figure 5A). Moreover, although the dox-treated ZHBTc4 cells lost alkaline phosphatase (AP) expression, the Tcf3-DN-expressing cells retained AP expression (Figure 5B). These results indicate that the cellular changes observed upon down-regulation of Oct-3/4 expression occur, in part, as a result of increased Tcf/β-catenin signalling.
Figure 5.
Oct-3/4 repression induces cellular motility in ES cells through activation of the Wnt signalling pathway. (A) Morphological changes in ZHBTc4 and ZHBTc4/DN-5 ES cells upon dox-induced repression of Oct-3/4. Left—photomicrographs of undifferentiated ES cells cultured in the presence of LIF, and the absence of dox. Right—cells were grown in the presence of LIF and dox for 2 days. All photographs are in the same magnification (× 200). (B) Alkaline phosphatase staining for the indicated ES cells before and after dox treatment in the presence of LIF. All photographs are in the same magnification (× 200). (C) Cellular motility of the indicated ES cells grown in the presence of LIF, treated or untreated with dox. Data represent the average number of migrating cells, counting in magnification of × 100. (D) Actin filaments phalloidin immunofluorescent staining of the indicated ES cells, either treated or untreated with dox. Scale bar, 80 μm.
Down-regulation of Oct-3/4 enhances Wnt signalling and cellular motility
The epithelial to mesenchymal transition (EMT) is a critical part of normal embryogenesis (Cavallaro and Christofori, 2004). Typically, EMT involves increased cellular motility. To further explore the functional effects of Oct-3/4 expression, we analysed whether down-regulating Oct-3/4 in ZHBTc4 ES cells induces cellular motility. We assessed the ability of dox-treated or -untreated cells to migrate through 8 μm pore size Transwell plates (Figure 5C). The motility of dox-treated cells (24 or 48 h treatment) increased ∼13-fold when compared with untreated cells. To determine whether this increase in motility is mediated through the β-catenin signalling pathway, three independent ZHBTc4/DN clones were analysed. Strikingly, expression of Tcf3-DN had a dramatic effect on cell migration. It considerably diminished dox-induced migration of clones ZHBTc4/DN2-D and 2-E, and almost completely blocked migration of ZHBTc4/DN2-5 (Figure 5C). In addition, staining with flourescent phalloidin clearly shows that, while dox induced reorganization of the cytoskeleton in ZHBTc4 cells, no cytoskeletal reorganization occurred in cells expressing the Tcf3-DN (Figure 5D). To verify that the Oct-3/4-induced suppression of motility is mediated by β-catenin, we knocked down β-catenin expression in these cells with shRNA (Supplementary Figure 5A and B). Migration of these clones after dox treatment was almost completely blocked, despite the persistence of low levels of β-catenin (Supplementary Figure 5C), suggesting that fine-tuning of β-catenin signalling has a biological readout. In summary, down-regulation of Oct-3/4 in ZHBTc4 cells induced a migratory phenotype that was blocked by expression of Tcf3-DN or suppression of β-catenin. These results strongly implicate the Wnt pathway in the increased cellular motility resulting from Oct-3/4 repression and suggest that POU-V domain proteins participate in maintaining the undifferentiated state in ES cells.
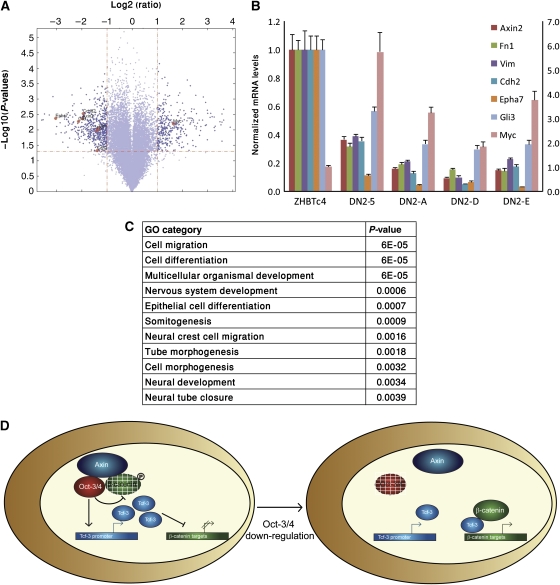
To identify genes that are involved in cell migration and are targets of the Wnt/β-catenin signalling pathway, but not of Oct-3/4, we used microarray analysis with an Affymetrix Mouse Gene 1.0 ST Array to examine differential gene expression in dox-treated ZHBTc4 and ZHBTc4/DN2-5 cells grown in the presence of LIF. Using a Benjamini—Hochberg-adjusted P-value of <0.05 as a cutoff, 878 known genes were significantly different (by at least two-fold) in dox-treated ZHBTc4 and ZHBTc4/DN2-5 cells. A total of 337 of these genes were up-regulated and 541 were down-regulated (Figure 6A; Supplementary Table 1). Quantitative RT–PCR analysis validated the results of the microarray for seven out of nine (77%) representative genes tested (Figure 6B). Moreover, similar expression patterns for these seven genes were detected in three additional independent ZHBTc4/DN single-cell clones (DN2-A, DN2-D and DN2-E) (Figure 6B), suggesting that our microarray results are valid for all Tcf3-DN-expressing ES clones.
Figure 6.
Genome-wide analysis of differentiated ZHBTc4 and ZHBTc4/DN-5 cells. (A) To challenge the statistical significance of the microarray results, we used the data obtained from the four arrays to perform the volcano analysis. Using thresholds of ⩾two-fold decrease or increase in expression and a P-value of ⩽0.05, 878 genes were detected. All dots in the scatter plot above the horizontal line have P-values <0.05 (calculated using the t-test). All points to the left of the left vertical line are down-regulated by at least two-fold in dox-treated ZHBTc4/DN-5 relative to the dox-treated ZHBTc4. The dots to the right of the right vertical line are up-regulated by at least two-fold. (B) Seven indicated genes were measured using quantitative real-time PCR in order to validate the array results. The levels of Myc are shown on the scale to the right. UBC and Gapdh were used as endogenous controls. (C) Gene ontology (GO) analysis of the genes with a differential expression between dox-treated ZHBTc4 and ZHBTc4/DN-5 ES cells. The P-value of this representation is based on a hypergeometric distribution. Lowest P-value GO categories are shown. (D) A model for the functional cross-talk between Oct-3/4 and Wnt/β-catenin signalling, see text.
The spectrum of changes that occur during EMT is diverse. Interestingly, expression of mesenchymal markers such as N-cadherin, fibronectin and vimentin was markedly decreased in all dox-treated ZHBTc4/DN clones in comparison with dox-treated ZHBTc4 parent cells (Figure 6B). To gain insight into biological processes that are regulated by Tcf3/β-catenin in ES cells in the absence of Oct-3/4 expression, we performed a Gene Ontology analysis on the 878 genes identified by our microarray analysis. Interestingly, in agreement with our morphological and functional assays, the most highly represented genes are associated with the following terms: cell migration, cell differentiation and multicellular organismal development (Figure 6C). Together, these results strongly suggest that the ability of Oct-3/4 to down-regulate β-catenin has a critical factor in maintaining undifferentiated ES cell phenotype by preventing premature EMT-like events.
Discussion
In this study, we provide evidence for a novel activity of the POU-homeodomain transcription factor Oct-3/4, revealing an unexpected function for this embryo-specific factor as a gatekeeper of the Wnt/β-catenin signalling pathway. In ES cells and Xenopus embryos, we found a marked increase in β-catenin/Tcf transcriptional activity after repression of either Oct-3/4 or its putative functional homologues in Xenopus, Oct-25 and -60. We showed that the effect of Oct-3/4 is downstream of the Wnt ligand, but upstream of the β-catenin/Tcf target genes. This antagonistic relationship between Oct-3/4 and Wnt signalling was also shown by the ability of Oct-3/4 to ventralize Xenopus embryos and inhibit secondary-axis formation by Wnt-8. Furthermore, gain- and loss-of-function experiments clearly showed that the Oct-25 and -60 proteins modulated secondary-axis formation induced by Wnt/β-catenin signalling in vivo, indicating that the ability of POU-V family proteins to modulate the responsiveness of embryonic cells to canonical Wnt signalling is biologically significant.
Our genetic and biochemical tests showed that Oct-3/4 promotes a dramatic decrease in β-catenin protein levels. This decrease occurred without a concomitant reduction in β-catenin transcript level. β-catenin stability is known to be regulated by proteosomal degradation, suggesting that the decrease we documented in β-catenin protein levels is due to degradation. Interestingly, we showed that when Oct-3/4 expression is suppressed in ES cells, β-catenin levels increase, leading to increased Wnt signalling and ES cell differentiation. How does Oct-3/4 promote β-catenin degradation? We have shown that Oct-3/4 (but not Oct-1 or -2) interacts with β-catenin to form a physical complex. Although our studies do not reveal whether the interaction is direct or indirect, previous work using bacterially expressed proteins indicates that Oct-3/4 and β-catenin can interact directly (Takao et al, 2007). Oct-3/4 and β-catenin also associate with Axin1, the rate-limiting component of the proteasomal destruction complex. We found that Oct-3/4 interacts with β-catenin exclusively in the nucleus, mediating its phosphorylation and proteasomal degradation. These results are compatible with the previous studies showing that proteasomes are abundant in the nucleus in which their proteolytic activity has a crucial function in double-strand break repair and transcription (Reits et al, 1997; Joseph et al, 2003; Collins and Tansey, 2006; Ben-Aroya et al, 2010). Interestingly, a number of other transcription factors inhibit β-catenin/Tcf activity and promote degradation of β-catenin through the formation of a mutual complex (Zorn et al, 1999; Akiyama et al, 2004; Han et al, 2006). Our data suggest that neither enhanced nuclear cytoplasmic shuttling nor an indirect mechanism involving squelching of Tcf3 have a function in Oct-3/4-induced β-catenin nuclear reduction.
Previous work (Cao et al, 2007) proposed that Oct-3/4 and Xenopus POU-V proteins antagonize β-catenin function by forming a ternary repressive complex with Tcf3. However, in ES cells, many of the repressing complexes contain Tcf3, TLE2 and CtBP, but not POU-V or β-catenin, suggesting that Oct-3/4 acts through another mechanism. In fact, genome-wide analysis did not reveal any colocalization of β-catenin with both Oct-3/4 and Tcf3, although Oct-3/4 and Tcf3 co-occupy many target promoters (Tam et al, 2008). Our suggestion that Oct-3/4 regulates β-catenin by promoting its degradation is consistent with these findings.
Oct-3/4-induced inhibition of Tcf/β-catenin activity prevents ES cell transition to a migratory phenotype
EMT is a dynamic process that is important for the formation of mesoderm and endoderm in the gastrulating embryo. Interestingly, epiblast cells from mutant embryos that express a truncated stabilized form of β-catenin not only express mesenchymal markers prematurely, but also lose E-cadherin expression, resulting in premature EMT (Kemler et al, 2004). Multiple pathways contribute to EMT during mouse gastrulation; however, β-catenin is an important player as the canonical Wnt pathway regulates the formation of the node, which serves as an embryonic organizing centre for further development. EMT events have been described in ES cells from Rhesus monkey, human and mouse (Denker et al, 2007; Spencer et al, 2007; Ullmann et al, 2007), showing that they are a useful model for investigating the mechanisms underlying EMT events. Normally, ES cells in culture are maintained in a pluripotent, non-migratory state by LIF. Here, we showed that down-modulating Oct-3/4 with dox in ZHBTc4 ES cells grown in the presence of LIF results in morphological changes, up-regulation of β-catenin signalling and increased invasive activity, all consistent with transition to a migratory phenotype. These changes were blocked by expressing a DN Tcf3 (Tcf3-DN) deficient in its ability to interact with β-catenin, but capable of DNA binding and repression (Molenaar et al, 1996). Knocking down β-catenin expression with shRNA also blocked the changes induced by Oct-3/4 down-regulation. Thus, preventing formation of a β-catenin–Tcf3 complex maintains the non-migratory phenotype of ES cells after down-regulation of Oct-3/4. These findings strongly suggest that Oct-3/4 has a function in preventing premature EMT during normal development by promoting β-catenin degradation, thereby blocking transcriptional changes induced by β-catenin–Tcf3 complexes.
Three independent studies have reported genome-wide effects on gene expression in mouse ES cells in response to Tcf3 knock-down (shRNA) or knock-out (Tcf3−/−) (Cole et al, 2008; Tam et al, 2008; Yi et al, 2008). These studies showed that in ES cells, Tcf3 functions mainly as a transcriptional repressor of two sets of genes: genes important for maintaining ES cell pluripotency and self-renewal, and genes involved in lineage commitment and stem cell differentiation. To identify a sub-set of Tcf3-regulated genes that are not affected by Oct-3/4, we performed a microarray analysis to highlight changes in gene expression between dox-treated ZHBTc4 and ZHBTc4/DN-Tcf3 cells. Our genomic analysis identified a set of genes, which are repressed in Tcf3 DN-expressing ES clones and are involved in cell migration. These data suggest that the biological significance of Oct-3/4-mediated β-catenin degradation is to protect ES cells from untimely β-catenin accumulation, which, together with Tcf3, up-regulates genes involved in cell migration and differentiation.
Intricate relationships between Oct-3/4 and Tcf/β-catenin pathways regulate embryonal stem cell identity
Tcf3−/− ES cells showed delayed differentiation after LIF withdrawal (Pereira et al, 2006), and ES cells with stable knock-down of Tcf3 were completely resistant to differentiation upon LIF withdrawal (Tam et al, 2008). These phenotypes were thought to be the result of elevated expression of pluripotent genes such as Oct-3/4. Indeed, ablation of Oct-3/4 in Tcf3 knock-down cells induced differentiation, indicating that the undifferentiated phenotype of these cells was dependent on Oct-3/4 expression (Tam et al, 2008). Unlike these cells, our Tcf3-DN-expressing ZHBTc4 ES clones did not undergo morphological changes and invasive capabilities after Oct-3/4 repression. These results suggest that Oct-3/4 expression is less critical for maintaining the pluripotent undifferentiated phenotype if β-catenin signalling through Tcf3 is ablated. This could be because Tcf3-DN can bind and repress genes that are involved in lineage commitment and stem cell differentiation. Thus, the DN Tcf3 most likely maintains the pluripotent phonotype repression of Wnt signalling by preventing Tcf3–β-catenin interaction. These data suggest that limiting the transcriptional activities of β-catenin is important for maintaining pluripotent phenotype.
It is clear that the relationship between Oct-3/4 and Tcf3/β-catenin pathways is complex. Published studies, together with our results, suggest that a balance between the expression of Tcf3/β-catenin and Oct-3/4 dictates the pluripotency and self-renewal properties of ES cells. Tcf3 is an integral component of the core regulatory circuitry of ES as it co-occupies promoters in association with Oct-3/4 and Nanog (Cole et al, 2008). These pathways also regulate each other. Tcf3 regulates Oct-3/4 expression by binding directly to the Oct-3/4 promoter and inhibiting Oct-3/4 expression (Tam et al, 2008). This repression can be relieved by stimulation of Wnt signalling through the addition of Wnt3a (Tam et al, 2008), or by over-expression of a non-degradable β-catenin (Takao et al, 2007), probably by titrating the transcriptional repressor, Tcf3. On the other hand, Oct-3/4 activates Tcf3 expression (Tam et al, 2008), and in somatic cells of the skin and intestine of adult mice, ectopically expressed Oct-3/4 elevates β-catenin levels, possibly through a positive feedback loop mediated by Tcf3, which is positively regulated by Oct-3/4 (Hochedlinger et al, 2005). In addition, the Xenopus POU-V proteins, Oct-25 and -60, were shown to inhibit transcription of β-catenin target genes through the formation of a ternary repressive complex with Tcf3 (Cao et al, 2007). Our results showing Oct-3/4-dependent β-catenin degradation add another level of interaction between the Oct-3/4 and β-catenin/Tcf signalling pathways. Interestingly, the outcome of these feedback loops results in non-overlapping temporal, spatial and cellular distribution of POU-V and β-catenin proteins in the embryo and in ES cells (Cao et al, 2006; Tam et al, 2008).
It seems that β-catenin has a dual, context-dependent function in ES cell biology that is maintaining stem cell identity and directing ES cell differentiation. Several studies have shown that activation of the Wnt pathway can cause ES cells to remain pluripotent under conditions that induce differentiation (Aubert et al, 2002; Kielman et al, 2002; Sato et al, 2004b; Hao et al, 2006; Ogawa et al, 2006). However, other studies have shown that the Wnt pathway has an important function in directing differentiation of ES cells (Otero et al, 2004; Dravid et al, 2005; Lindsley et al, 2006). Moreover, immunofluorescence analysis showed that in ES cells, β-catenin was largely restricted to the cytoplasm, and a genome-wide analysis indicated that Oct-3/4 and Tcf3 co-occupy many target promoters, no co-localization of β-catenin with them was observed (Tam et al, 2008). When taken together, these studies suggest that Wnt/β-catenin regulation is an important component of stem cell fate determination (self-renewal or differentiation) that depends on extrinsic signals (such as FGF, BMP, LIF) and intrinsic cell factors (such as Oct-3/4). In ES cells, Oct-3/4 prevents over-accumulation of β-catenin signalling through two distinct molecular mechanisms (Figure 6D). First, it activates expression of Tcf3, which in ES cells functions mainly as a repressor of genes crucial for lineage commitment and stem cell differentiation (Tam et al, 2008). Second, it promotes nuclear β-catenin degradation, thereby decreasing Tcf/β-catenin-mediated transcriptional regulation. This molecular device keeps nuclear β-catenin levels relatively low, which is compatible with proliferation and pluripotency. In this way, Oct-3/4 protects the cells from transient external differentiating signals that may direct the cells prematurely towards an incorrect fate.
Materials and methods
Cell culture and transfection
ES and ZHTc4 ES cells were cultured without a feeder layer on gelatin-coated dishes, in LIF medium supplemented with 15% foetal calf serum. ES differentiation was induced by the addition of 1 μM RA (Sigma) for 0–4 days. Oct-3/4 reduction was induced by the addition of 2 μg/μl dox for 0–52 h. ES cells were treated with 4 ng/ml leptomycin B (LMB, Sigma) for 12 h. The 293T, RKO and SW480 cells were cultured in DMEM supplemented with 10% foetal calf serum. The 293T cells were transiently transfected using the calcium phosphate procedure (Wigler et al, 1979). Transfection of ZHTc4 ES cells was carried out using Lipofectamine 2000 (Invitrogen). RKO and SW480 cells were infected with a lentivirus that expresses Oct-3/4.
Expression plasmids
The pCS2-Oct-3/4 was constructed by cloning the full-length Oct-3/4 cDNA into the ClaI site of pCS2. The other expression plasmids were pEVRF-Oct-3/4, Oct-3/4-ΔN, Oct-3/4-ΔC (Ben-Shushan et al, 1998); pOct1 (Tanaka and Herr, 1990); pOct-2 (Clerc et al, 1988); pOct-6 (Meijer et al, 1992); Flag-β-catenin, Flag-DP, Flag-D32N, Flag-S33, Flag-Axin1, Myc-Axin1 and GFP (Amit et al, 2002); pCS2-xWnt-8 (Kelly et al, 1995); pCS2-xSiamois (Lemaire et al, 1995); pCS2-x β-catenin (Kurth et al, 1999) and TOP-Flash and FOP-Flash (Korinek et al, 1997). The pLKO.1 lentivral vector-expressing shRNA against β-catenin was bought from Openbiosystems (Cat RMM4534). Oligonucleotides encoding shRNA against scrambled (5′CCGGTCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGGTTTTTG 3′) were annealed and inserted downstream of the U6 promoter between AgeI and EcoRI sites of pLKO construct.
Antibodies and morpholino oligonucleotides
Polyclonal rabbit antisera directed against the mouse Oct-3/4 proteins were raised in rabbits after injection of full-length recombinant proteins (Ben-Shushan et al, 1998). Anti-β-catenin (H-102; Santa Cruz Biotechnology), anti-Flag (M2; Sigma), anti-Myc (Ab-1; Oncogene), anti-mouse β-actin (clone AC-15; Sigma), anti-GFP (JL-8; Clontech), anti-phospho β-catenin ser33/37/Thr41 (#9561; Cell Signaling), anti-Fibrillarin (ab-4566; Abcam), anti-IkB (C21; Santa Cruz Biotechnology) and anti-α-tubulin (clone DNIA; Sigma) were used for western blot analysis (Liu et al, 2005). Antibodies against E-cadherin (BD Biosciences) were used for immunostaining. Morpholino oligonucleotides (MO) were purchased from gene tools. Sequences were as follows:
Oct-60MO: 5′ CTGGTCCATCTCCAGCACTTGCTC
Oct-25MO: 5′ TGTTGGCTGTACATGGTGTCCAAGA
Immunoprecipitation and western blotting
The 293T, ES, RKO and SW480 cells were lysed in a solution of 50 mM Tris–HCl (pH 7.6), 150 mM NaCl, 0.5% NP40, 5 mM EDTA and protease inhibitor cocktail for 20 min on ice. Lysates were centrifuged for 5 min at 4°C. The lysates were normalized by protein concentration assays, separated by SDS–PAGE and transferred to nitrocellulose membrane (Protran), (Schleicher and Schuell). The blots were probed with the indicated antibodies and developed with the appropriate HRP-conjugated secondary antibodies (Pierce). For immunoprecipitation, the cells were treated with 20μM MG-132 (Calbiochem) for 3 h, the lysates were rotated with agarose-conjugated anti-Flag (Sigma) overnight at 4°C, and then precipitated at 12K r.p.m. All lanes of each individual western blot are from the same gel.
DNA microarrays
Gene expression profiling was performed using the Affymetrix Mouse Gene 1.0 ST Array (Santa Clara, CA), comprised of ∼770 317 distinct probes representing 28 853 well-annotated genes, according to the manufacturer's instructions. Data was imported and normalized using Partek Genomic v6.4 program and analysed using SpotfireDecisionSite™ software for functional genomics. For analysis of gene expression, RNA from dox-treated ZHBTc4 and ZHBTc4/DN-5 ES cells was extracted and processed according to the manufacture's protocol, and the results of two independent experiments were averaged. Genes that were up- or down-regulated by ⩾two-fold with a P-value of ⩽0.05 were considered statistically significant.
Methods for nuclear/cytoplasmic fractionation, electrophoretic mobility shift assay, RT–PCR and real-time PCR, in vitro-transcribed RNAs, luciferase reporter assay, AP staining, matrigel invasion assay, phalloidin immunoflourescence and confocal microscopy can be found in the online Supplementary data.
Supplementary Material
Acknowledgments
We are grateful to Nirit Feldman, Ada Hatzubai, Yaara Birman and Rachel Schyr for their helpful suggestions and cooperation. This work was supported by grants from the Israel Academy of Sciences, Philip Morris USA Inc. and Philip Morris International, and the National Institutes of Health to YB, and a grant from the Israel Science Foundation and the Wolfson Family Chair in Genetics to AF. MA-R is supported by the Adams Fellowship Program of the Israel Academy of Sciences and Humanities.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B (2004) Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev 18: 1072–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I (2002) Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 16: 1066–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert J, Dunstan H, Chambers I, Smith A (2002) Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol 20: 1240–1245 [DOI] [PubMed] [Google Scholar]
- Ben-Aroya S, Agmon N, Yuen K, Kwok T, McManus K, Kupiec M, Hieter P (2010) Proteasome nuclear activity affects chromosome stability by controlling the turnover of mms22 a protein important for DNA repair. PLoS Genet 6: e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shushan E, Thompson JR, Gudas LJ, Bergman Y (1998) Rex-1, a gene encoding a transcription factor expressed in the early embryo, is regulated via Oct-3/4 and Oct-6 binding to an octamer site and a novel protein, Rox-1, binding to an adjacent site. Mol Cell Biol 18: 1866–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiani M, Eckardt S, Scholer HR, McLaughlin KJ (2002) Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev 16: 1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiani M, Scholer HR (2005) Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol 6: 872–884 [DOI] [PubMed] [Google Scholar]
- Bortvin A, Eggan K, Skaletsky H, Akutsu H, Berry DL, Yanagimachi R, Page DC, Jaenisch R (2003) Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development 130: 1673–1680 [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122: 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T (1998) Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 391: 597–601 [DOI] [PubMed] [Google Scholar]
- Cao Y, Siegel D, Donow C, Knochel S, Yuan L, Knochel W (2007) POU-V factors antagonize maternal VegT activity and beta-Catenin signaling in Xenopus embryos. EMBO J 26: 2942–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Siegel D, Knochel W (2006) Xenopus POU factors of subclass V inhibit activin/nodal signaling during gastrulation. Mech Dev 123: 614–625 [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G (2004) Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 4: 118–132 [DOI] [PubMed] [Google Scholar]
- Christian JL, McMahon JA, McMahon AP, Moon RT (1991) Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development 111: 1045–1055 [DOI] [PubMed] [Google Scholar]
- Clerc RG, Corcoran LM, LeBowitz JH, Baltimore D, Sharp PA (1988) The B-cell-specific Oct-2 protein contains POU box- and homeo box-type domains. Genes Dev 2: 1570–1581 [DOI] [PubMed] [Google Scholar]
- Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA (2008) Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev 22: 746–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GA, Tansey WP (2006) The proteasome: a utility tool for transcription? Curr Opin Genet Dev 16: 197–202 [DOI] [PubMed] [Google Scholar]
- Cong F, Varmus H (2004) Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin. Proc Natl Acad Sci USA 101: 2882–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker HW, Behr R, Heneweer C, Viebahn C, Thie M (2007) Epithelial-mesenchymal transition in Rhesus monkey embryonic stem cell colonies: a model for processes involved in gastrulation? Cells Tissues Organs 185: 48–50 [DOI] [PubMed] [Google Scholar]
- Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L (2005) Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells 23: 1489–1501 [DOI] [PubMed] [Google Scholar]
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E (1997) CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390: 308–311 [DOI] [PubMed] [Google Scholar]
- Han G, Li AG, Liang YY, Owens P, He W, Lu S, Yoshimatsu Y, Wang D, Ten Dijke P, Lin X, Wang XJ (2006) Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell 11: 301–312 [DOI] [PubMed] [Google Scholar]
- Hao J, Li TG, Qi X, Zhao DF, Zhao GQ (2006) WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol 290: 81–91 [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J (1997) Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol 13: 611–667 [DOI] [PubMed] [Google Scholar]
- Henderson BR (2000) Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat Cell Biol 2: 653–660 [DOI] [PubMed] [Google Scholar]
- Hinkley CS, Martin JF, Leibham D, Perry M (1992) Sequential expression of multiple POU proteins during amphibian early development. Mol Cell Biol 12: 638–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R (2005) Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121: 465–477 [DOI] [PubMed] [Google Scholar]
- Joseph TW, Zaika A, Moll UM (2003) Nuclear and cytoplasmic degradation of endogenous p53 and HDM2 occurs during down-regulation of the p53 response after multiple types of DNA damage. FASEB J 17: 1622–1630 [DOI] [PubMed] [Google Scholar]
- Kao KR, Elinson RP (1988) The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol 127: 64–77 [DOI] [PubMed] [Google Scholar]
- Kelly RE, DeRose ML, Draper BW, Wahl GM (1995) Identification of an origin of bidirectional DNA replication in the ubiquitously expressed mammalian CAD gene. Mol Cell Biol 15: 4136–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo MM, de Vries WN, Knowles BB, Solter D (2004) Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development 131: 5817–5824 [DOI] [PubMed] [Google Scholar]
- Kielman MF, Rindapaa M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, Taketo MM, Roberts S, Smits R, Fodde R (2002) Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet 32: 594–605 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Kurth T, Fesenko IV, Schneider S, Munchberg FE, Joos TO, Spieker TP, Hausen P (1999) Immunocytochemical studies of the interactions of cadherins and catenins in the early Xenopus embryo. Dev Dyn 215: 155–169 [DOI] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB (1995) Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell 81: 85–94 [DOI] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM (2006) Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development 133: 3787–3796 [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR (2005) Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol 15: 1989–1997 [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Marikawa Y (2006) Wnt/beta-catenin signaling and body plan formation in mouse embryos. Semin Cell Dev Biol 17: 175–184 [DOI] [PubMed] [Google Scholar]
- Meijer D, Graus A, Grosveld G (1992) Mapping the transactivation domain of the Oct-6 POU transcription factor. Nucleic Acids Res 20: 2241–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M (2007) Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci USA 104: 5668–5673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H (1996) XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86: 391–399 [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A (2004) WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 5: 691–701 [DOI] [PubMed] [Google Scholar]
- Morrison GM, Brickman JM (2006) Conserved roles for Oct4 homologues in maintaining multipotency during early vertebrate development. Development 133: 2011–2022 [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95: 379–391 [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24: 372–376 [DOI] [PubMed] [Google Scholar]
- Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H (2006) Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun 343: 159–166 [DOI] [PubMed] [Google Scholar]
- Otero JJ, Fu W, Kan L, Cuadra AE, Kessler JA (2004) Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development 131: 3545–3557 [DOI] [PubMed] [Google Scholar]
- Pereira L, Yi F, Merrill BJ (2006) Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol 26: 7479–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce M, Scholer HR (2001) Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells 19: 271–278 [DOI] [PubMed] [Google Scholar]
- Reits EA, Benham AM, Plougastel B, Neefjes J, Trowsdale J (1997) Dynamics of proteasome distribution in living cells. EMBO J 16: 6087–6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H (2005) Wnt signalling in stem cells and cancer. Nature 434: 843–850 [DOI] [PubMed] [Google Scholar]
- Rosin-Arbesfeld R, Townsley F, Bienz M (2000) The APC tumour suppressor has a nuclear export function. Nature 406: 1009–1012 [DOI] [PubMed] [Google Scholar]
- Sato K, Saito-Ohara F, Inazawa J, Kudo A (2004a) Pax-5 is essential for k sterile transcription during Igk chain gene rearrangement. J Immunol 172: 4858–4865 [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH (2004b) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 10: 55–63 [DOI] [PubMed] [Google Scholar]
- Singla DK, Schneider DJ, LeWinter MM, Sobel BE (2006) wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun 345: 789–795 [DOI] [PubMed] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA (1991) Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67: 741–752 [DOI] [PubMed] [Google Scholar]
- Sokol SY (1999) Wnt signaling and dorso-ventral axis specification in vertebrates. Curr Opin Genet Dev 9: 405–410 [DOI] [PubMed] [Google Scholar]
- Spencer HL, Eastham AM, Merry CL, Southgate TD, Perez-Campo F, Soncin F, Ritson S, Kemler R, Stern PL, Ward CM (2007) E-cadherin inhibits cell surface localization of the pro-migratory 5T4 oncofetal antigen in mouse embryonic stem cells. Mol Biol Cell 18: 2838–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao Y, Yokota T, Koide H (2007) Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun 353: 699–705 [DOI] [PubMed] [Google Scholar]
- Tam WL, Lim CY, Han J, Zhang J, Ang YS, Ng HH, Yang H, Lim B (2008) T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells 26: 2019–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Herr W (1990) Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell 60: 375–386 [DOI] [PubMed] [Google Scholar]
- Ullmann U, In't Veld P, Gilles C, Sermon K, De Rycke M, Van de Velde H, Van Steirteghem A, Liebaers I (2007) Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Mol Hum Reprod 13: 21–32 [DOI] [PubMed] [Google Scholar]
- Whitfield T, Heasman J, Wylie C (1993) XLPOU-60, a Xenopus POU-domain mRNA, is oocyte-specific from very early stages of oogenesis, and localised to presumptive mesoderm and ectoderm in the blastula. Dev Biol 155: 361–370 [DOI] [PubMed] [Google Scholar]
- Wigler M, Pellicer A, Silverstein S, Axel R, Urlaub G, Chasin L (1979) DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci USA 76: 1373–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie C, Kofron M, Payne C, Anderson R, Hosobuchi M, Joseph E, Heasman J (1996) Maternal beta-catenin establishes a ‘dorsal signal' in early Xenopus embryos. Development 122: 2987–2996 [DOI] [PubMed] [Google Scholar]
- Yi F, Pereira L, Merrill BJ (2008) Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells 26: 1951–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE (1999) Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell 4: 487–498 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.