β-arrestin Kurtz inhibits MAPK and Toll signalling in Drosophila development
β-Arrestins are best known as adaptor proteins involved in GPCR signal transduction, although they are known to regulate multiple signalling cascades. In this study, Tipping et al identify a new function for the Drosophila β-arrestin as an inhibitor of both Toll and RTK pathways during development.
Keywords: β-arrestin, ERK, Kurtz, Toll, Torso.
Abstract
β-Arrestins have been implicated in the regulation of multiple signalling pathways. However, their role in organism development is not well understood. In this study, we report a new in vivo function of the Drosophila β-arrestin Kurtz (Krz) in the regulation of two distinct developmental signalling modules: MAPK ERK and NF-κB, which transmit signals from the activated receptor tyrosine kinases (RTKs) and the Toll receptor, respectively. Analysis of the expression of effectors and target genes of Toll and the RTK Torso in krz maternal mutants reveals that Krz limits the activity of both pathways in the early embryo. Protein interaction studies suggest a previously uncharacterized mechanism for ERK inhibition: Krz can directly bind and sequester an inactive form of ERK, thus preventing its activation by the upstream kinase, MEK. A simultaneous dysregulation of different signalling systems in krz mutants results in an abnormal patterning of the embryo and severe developmental defects. Our findings uncover a new in vivo function of β-arrestins and present a new mechanism of ERK inhibition by the Drosophila β-arrestin Krz.
Introduction
Mammalian β-arrestins were initially characterized as adaptors involved in the desensitization, endocytosis and downregulation of G protein-coupled receptors (GPCRs; Pierce and Lefkowitz, 2001). More recently, it was shown that β-arrestins can initiate a new wave of signalling from the activated GPCR to the MAPK modules, including ERK and JNK cascades (for review, see DeWire et al, 2007; Defea, 2008). Further studies have dramatically expanded our understanding of β-arrestins' signalling functions, and it is now thought that they are involved in the regulation of multiple signalling pathways (Lefkowitz and Shenoy, 2005; Lefkowitz et al, 2006; DeWire et al, 2007; Kovacs et al, 2009).
Despite extensive characterization of the cellular signalling activities of β-arrestins, their role in organism development is only starting to be explored (Wilbanks et al, 2004; Palmitessa et al, 2005; Bryja et al, 2007; Kim and Han, 2007; for review, see Kovacs et al, 2009). Functional studies in mammals have been complicated by the fact that the two mammalian homologues, β-arrestin1 and β-arrestin2, have partially redundant activities. In the mouse, knockout of a single gene for either β-arrestin gives viable and fertile animals (Bohn et al, 1999; Conner et al, 1997), whereas a double-mutant combination results in neonatal lethality attributed to defects in lung development (Zhang et al, 2010).
Drosophila represents an attractive system to study the developmental functions of β-arrestins because the fly genome encodes a single β-arrestin, Kurtz (Krz), which is essential for viability (Roman et al, 2000). We have previously shown that Krz forms a ternary complex with the Notch receptor and a putative ubiquitin ligase Deltex, and that the formation of this complex promotes ubiquitination and proteasomal degradation of Notch (Mukherjee et al, 2005). In addition to its role in Notch downregulation, Krz functions as a bona fide GPCR regulator, as it controls Drosophila olfaction, behaviour, sensitivity to osmotic stress, and has recently been shown to promote internalization of GPCRs on receptor activation (Ge et al, 2006; Liu et al, 2007; Johnson et al, 2008).
All of the previous studies of Krz have focussed on its zygotic functions. However, krz transcript is maternally supplied and is present at high levels from the onset of embryogenesis (Roman et al, 2000). In this study, we analysed the effects of maternal loss of krz on Drosophila development and observed that Krz regulates two maternal patterning systems in the early embryo. Loss of maternal krz expression results in a disruption of signalling through the Toll/NF-κB pathway that specifies the development of the ventral region in the embryo (Gerttula et al, 1988), and the receptor tyrosine kinase (RTK) Torso pathway, which is responsible for the formation of the terminal structures (Sprenger et al, 1989). Using quantitative analysis of expression of the effectors and target genes of the RTK Torso, we observed that Krz prevents inappropriate activation of this pathway in the developing embryo. Protein interaction studies between Krz and the Torso effector ERK have suggested a new mechanism by which Krz can inhibit RTK signalling. Specifically, we observed that Krz preferentially associates with an inactive form of ERK and prevents its activation by the upstream kinase, MEK. Analysis of Krz activity at later developmental stages showed that inhibition of ERK activation by Krz affects not just the Torso pathway but other RTKs that function in diverse tissue contexts. Krz therefore seems to be a general inhibitor of RTK signalling in Drosophila development.
Analysis of the effectors and target genes of Toll showed that the role of Krz in Toll signalling is similarly inhibitory in nature. Krz limits the spread of the nuclear gradient of Dorsal in the embryo and helps establish proper expression domains of Dorsal target genes. In addition to its effects on targets that are specific for Torso and Toll, loss of krz upsets the cross-regulatory balance between these two pathways, which is manifested in an aberrant expression of their common target gene, zerknüllt (zen).
In sum, this study uncovers a key in vivo function of the β-arrestin Krz during Drosophila development, which consists in limiting the activity of the Toll and RTK signalling pathways and preventing inappropriate activation of the patterning regulators under their control. Our study also suggests a new general mechanism of RTK inhibition by Krz. These results represent an important advance in our understanding of the biological functions of β-arrestins.
Results
Maternal loss of krz results in gastrulation defects and embryonic lethality
The krz gene is maternally expressed and its transcript is present ubiquitously throughout early development (Roman et al, 2000). Using a polyclonal antibody against Krz (see Materials and methods section), we observed that the Krz protein is expressed throughout the blastoderm embryo and is predominantly cytoplasmic (Supplementary Figure S1A). To study the effects of loss of krz during early embryonic development, we generated embryos maternally mutant for krz. The krz1 mutation is caused by a P-element insertion in the intron of the krz gene (Roman et al, 2000). Maternal mutant embryos obtained using the FRT krz1 chromosomes lacked Krz protein when stained with our polyclonal anti-Krz antibody (Supplementary Figure S1B). Absence of Krz in krz1 maternal mutants was also observed in extracts from 0- to 4-h embryos on western blots (Figure 1I). These results indicate that krz1 is a strong loss-of-function allele that eliminates all detectable Krz protein.
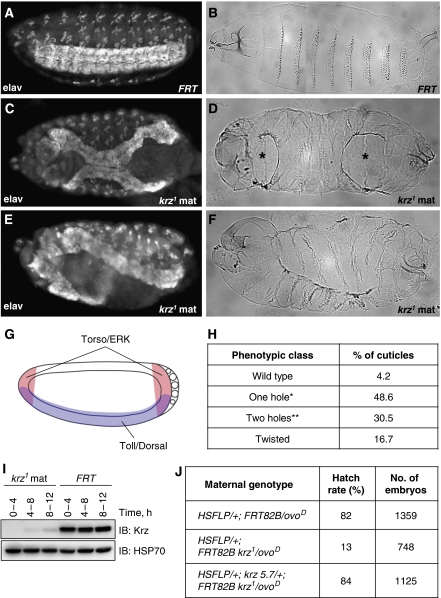
Figure 1.
Maternal krz1 loss of function phenotypes. (A, C, E) General morphology of stage 15 embryos visualized using anti-Elav antibody which stains all neurons. In all embryo panels, anterior is to the left, dorsal is up. (A) FRT control, (C, E) krz1 maternal mutant embryos. (B, D, F) Cuticular preparations of (B) FRT control and (D, F) krz1 maternal mutants. Anterior-ventral and posterior-ventral holes in the mutants (D) are indicated with asterisks. (G) The Torso (pink) and Toll (blue) signalling pathways intersect at the blastoderm stage. (H) Quantification of the phenotypic classes observed in the cuticles of krz1 maternal mutant embryos (72 cuticles scored). *One hole either in the anterior or posterior; **two holes at both anterior and posterior ends. (I) A western blot on extracts from staged FRT and krz1 maternal mutant embryos. IB: immunoblot. HSP70 antibody was used as a loading control. (J) Hatch rates of FRT control embryos, krz1 maternal mutant embryos, and krz1 maternal mutant embryos obtained from females carrying one copy of the genomic rescue construct, krz 5.7.
Cuticular preparations made from krz1 maternal mutant embryos displayed a range of phenotypes. Approximately 80% of the cuticles contained large gaps either in the anterior or posterior regions, or both (Figure 1C, D and H). A more severe defect was observed in 16.7% of the cuticles that displayed twisting along the anterior–posterior axis (Figure 1E, F and H). Using neuron-specific anti-Elav staining as a general marker of embryo morphology (O'Neill et al, 1994), we observed the corresponding developmental abnormalities at earlier stages of embryogenesis (Figure 1A, C and E). Introduction of a genomic rescue construct (krz 5.7, see Materials and methods section) fully rescued all of these defects (Supplementary Figure S1C and E), increased Krz protein to detectable levels (Supplementary Figure S1D), as well as rescued the lethality associated with maternal loss of krz (Figure 1J). Therefore, the developmental defects observed in krz1 maternal mutants are caused specifically by the loss of krz.
The observed cuticular abnormalities reflect the cumulative effect of loss of krz function in the embryo, and are characteristic of phenotypes associated with gastrulation defects (Leptin, 1995). The most affected areas are the anterior-ventral and posterior-ventral regions of the embryo. Patterning of the embryo poles is specified by the RTK Torso pathway, whereas dorsal/ventral differentiation is under the control of the Toll/NF-κB pathway (Nusslein-Volhard, 1991). The activities of these two pathways intersect in the embryo in the anterior-ventral and posterior-ventral regions (Figure 1G). Given that the observed defects in the krz maternal mutants affect these areas, we asked whether Krz had a role in the regulation of Torso and Toll signalling.
Loss of krz affects the expression of Torso target genes
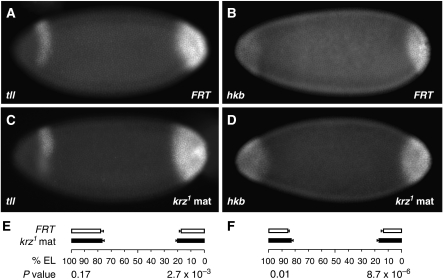
Activation of the Torso signalling pathway turns on the expression of two transcription factors, tailless (tll) and huckebein (hkb), at the embryonic poles (Liaw et al, 1995; Paroush et al, 1997). These patterning regulators are involved in the specification of the embryonic terminal structures. We asked whether loss of maternal krz would affect the expression pattern of these two Torso target genes. Fluorescent RNA in situ hybridization using tll and hkb probes was used as a measure of Torso pathway activity (Figure 2A–D). For both probes, we quantified (1) the distance between the extreme posterior end of the embryo and the anterior boundary of the posterior expression domain, and (2) the distance between the extreme anterior end of the embryo and the posterior boundary of the anterior expression domain (see Materials and methods section for the description of quantification procedure). These distances were normalized by the total embryo length (% EL, Figure 2E and F). On the basis of these measurements, we detected a significant expansion of the posterior domains of expression of both tll and hkb in krz1 maternal mutant embryos, compared with controls (P=8.7 × 10−6 and 2.7 × 10−3, respectively, Figure 2E and F). We also observed a significant expansion of the anterior domain of hkb expression (P=0.01) in krz1 maternal mutants (Figure 2F). The difference in the tll anterior domain was also measurable but not significantly different in krz1 mutants, compared with controls (P=0.17; Figure 2E). These data suggest that loss of maternal krz results in elevated signalling activity of the Torso pathway, manifested as an expansion of expression domains of Torso target genes.
Figure 2.
Loss of krz results in an expansion of expression domains of Torso target genes. (A–D) In situ hybridization with antisense (A, C) tll and (B, D) hkb probes in (A, B) stage-4 FRT control and (C, D) krz1 maternal mutant embryos. (E, F) Quantification of anterior and posterior tll and hkb expression domains. Mean values for the widths of the expression domains are plotted as percent embryo length (% EL, posterior is 0%). Error bars represent one standard deviation. Numbers of embryos quantified: tll in FRT, 19; tll in krz, 18; hkb in FRT, 17; hkb in krz, 19. The P-values for the comparisons between the FRT and mutant embryos were calculated separately for the anterior and posterior domains, and are shown under the graphs.
Loss of krz results in an upregulation of the Torso effector dpERK
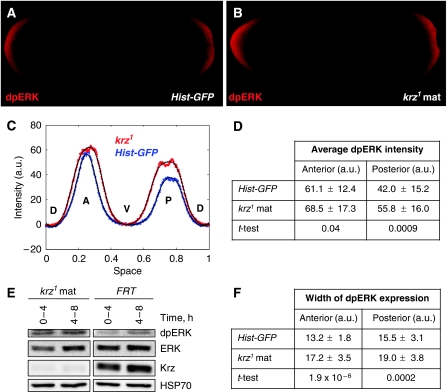
The expression of tll and hkb depends on localized activation of the MAPK ERK (encoded by the rolled locus, rl) downstream of the activated Torso receptor (Gabay et al, 1997b; Sprenger et al, 1989). Given the observed changes in the expression of Torso targets in krz maternal mutants, we asked whether Krz regulates Torso signalling by affecting the activity of ERK. We analysed ERK activation in krz1 maternal mutants using an antibody that specifically recognizes activated doubly phosphorylated ERK (dpERK; Gabay et al, 1997a). The spatial pattern of dpERK distribution was quantified using a previously reported approach (Coppey et al, 2008; see Materials and methods section and Supplementary Figure S2). krz1 maternal mutant embryos showed an overall increase in dpERK intensity as compared with control Hist-GFP embryos (Figure 3A–C). The intensity of dpERK was significantly increased at the posterior termini in krz1 maternal mutants (P=9.0 × 10−4; Figure 3C and D). There was also an increase in intensity in the anterior, but with lower significance (P=0.04; Figure 3C and D). The width of the dpERK gradient at both the anterior (P=1.9 × 10−6) and posterior (P=2.0 × 10−4) termini was also significantly expanded along the length of the embryo in krz1 maternal mutants (Figure 3C and F). Therefore, the most significant differences in dpERK pattern in krz1 maternal mutants can clearly be identified as an expansion of the width of the dpERK domain extending from both the posterior and anterior termini, as well as an increase in dpERK intensity at the posterior end (Figure 3C).
Figure 3.
Loss of krz results in increased levels of dpERK. (A, B) Expression of doubly phosphorylated activated ERK (dpERK) in (A) stage-4 control and (B) krz1 maternal mutant embryos. Embryos were stained with an antibody specific for the activated form of ERK. (C) Average intensity of dpERK expression plotted around the sagittal circumference of the embryos (D, dorsal; A, anterior; V, ventral; P, posterior). (D, F) Quantification of the average dpERK intensity (D) and average width of dpERK expression (F) in 28 Hist-GFP control and 28 krz1 maternal mutant embryos (au, arbitrary signal intensity units). (E) Western blot of protein expression in staged krz1 maternal mutant embryos and FRT controls. HSP70 antibody was used as loading control.
In addition to studying the spatial distribution of dpERK by immunohistochemistry, we analysed dpERK and total ERK levels in the control and krz1 maternal mutant embryos by western blotting. dpERK levels in krz1 maternal mutants were significantly increased in 0–4 and 4–8-h embryos as compared with controls, whereas total ERK levels remained unchanged or were even lower (Figure 3E). Collectively, these data demonstrate that loss of krz function results in an overall increase in ERK activity, with the highest levels present at the embryonic poles. This in turn suggests that the normal function of Krz is to limit the activity of ERK, and hence the Torso pathway, in the early embryo.
The Krz and ERK proteins directly interact
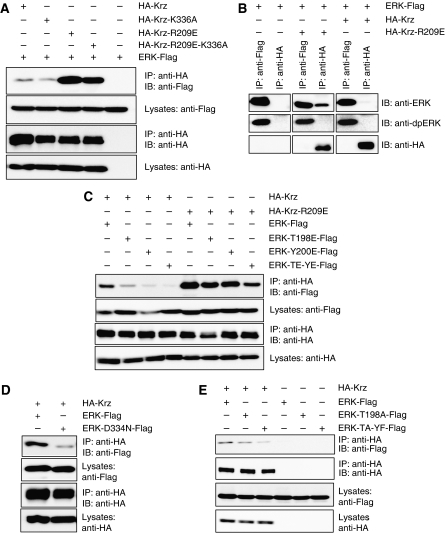
Expansion of expression domains of the Torso effector (dpERK) and target genes (tll and hkb) in krz mutants indicates that the normal function of Krz is to inhibit the activity of the Torso pathway in the embryo. Such an inhibitory action of β-arrestin on RTK signalling in vivo has not been previously reported, and we set out to investigate a possible mechanism for this downregulating effect. As several reports have shown a physical interaction between ERK and mammalian β-arrestins (Luttrell et al, 2001; Tohgo et al, 2002; Xu et al, 2008; Song et al, 2009), we hypothesized that Krz may control ERK activity through a direct protein–protein interaction. In co-immunoprecipitations from Drosophila S2 cells ERK–Flag bound to wild-type HA–Krz (Figure 4A). Substitution of the lysine residue 336 in the Krz protein with alanine (Krz-K336A), corresponding to a mutation that was shown to reduce the binding of β-arrestin2 to ERK in mammalian cells (Xu et al, 2008), had only a slight effect on the binding between Krz and Drosophila ERK in S2 cells (Figure 4A). We also tested an interaction between ERK–Flag and a Krz ‘pre-activated' mutant, in which arginine residue 209 is replaced with glutamic acid (Krz-R209E). A corresponding mutation in mammalian β-arrestins makes their GPCR binding independent of the phosphorylation state of the receptor (Kovoor et al, 1999). We observed that the ‘pre-activated' Krz mutant showed significantly increased binding to ERK (Figure 4A), and that this stronger binding was also observed for the equivalent mutant of human β-arrestin2, β-arrestin2-R170E (Supplementary Figure S3). Our results are generally in agreement with a previous study showing that an interaction between the basal conformation of β-arrestins and ERK is relatively weak (Song et al, 2009). However, a stronger affinity between Krz-R209E and ERK allowed us to observe their interaction by co-immunoprecipitation from in vitro translation reactions, confirming that the binding is direct (Supplementary Figure S4). An increased binding affinity of the Krz-R209E isoform to ERK suggests that the conformation of β-arrestins may influence their association with ERK.
Figure 4.
Krz preferentially interacts with inactive ERK. (A) S2 cells were transfected with HA–Krz, HA–Krz-K336A, HA–Krz-R209E or HA–Krz-R209E-K336A together with Drosophila ERK–Flag. Samples were immunoprecipitated with anti-HA beads and analysed by western blotting. (B) S2 cells were transfected with Drosophila ERK–Flag alone, or together with HA–Krz-R209E or HA–Krz. Cells were treated with insulin at a final concentration of 20 μM. Samples were immunoprecipitated with anti-Flag or anti-HA beads. Immunoprecipitates were analysed by western blot with anti-HA antibody, anti-total ERK antibody and anti-dpERK antibody. (C) S2 cells were transfected with HA–Krz or HA–Krz-R209E together with the indicated Drosophila ERK–Flag versions. Samples were immunoprecipitated with anti-HA beads and analysed by western blotting. (D) S2 cells were transfected with HA–Krz and either Drosophila wild-type ERK–Flag or ERK-D334N–Flag. Lysates were immunoprecipitated with anti-HA beads and immunoblotted. (E) S2 cells were transfected with HA–Krz together with the indicated Drosophila ERK–Flag versions. Samples were immunoprecipitated with anti-HA beads and analysed by western blotting. IP, immunoprecipitated samples; IB, immunoblots.
Krz preferentially associates with an inactive form of ERK
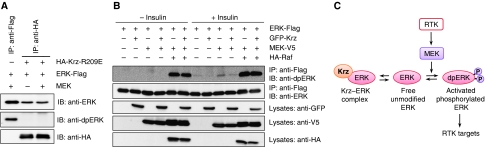
Overexpression of wild-type Krz did not affect ERK stability or ubiquitination (Supplementary Figure S5), suggesting that Krz binding may regulate ERK activity rather than turnover. To address this possibility, we first tested whether activation status of ERK affects its ability to bind Krz. Krz and ERK were transfected into S2 cells and ERK was activated after serum starvation by addition of complete media with insulin (see Materials and methods section). This treatment results in ERK activation through the Drosophila insulin receptor (InR) and the PVR pathway, which are both receptor tyrosine kinases (Friedman and Perrimon, 2006). Under these experimental conditions, ERK could be efficiently immunoprecipitated directly by anti-Flag antibody (Figure 4B, left). A significant proportion of ERK precipitated in this way was the activated form, dpERK. However, when immunoprecipitation was done through the HA–Krz-R209E protein using anti-HA antibody, we observed that ERK could be detected exclusively with anti-total ERK antibody, and there was no signal in the anti-dpERK blots (Figure 4B, middle). The same effect was observed using HA–Krz, though the extent of ERK binding was less pronounced (Figure 4B, right). This result suggests that Krz preferentially associates with inactive ERK.
To further investigate the influence of ERK phosphorylation status on its affinity for Krz, we created versions of ERK mimicking its phosphorylated forms. ERK is phosphorylated by MEK on both threonine and tyrosine in a conserved TEY motif (Canagarajah et al, 1997), and the corresponding sequence is found in residues 198–200 in Drosophila ERK. To test for possible effects of phosphorylation of these residues on ERK–Krz interactions, we changed threonine and tyrosine in the TEY phosphorylation motif to glutamic acid singly and in combination (ERK-T198E–Flag, ERK-Y200E–Flag and ERK-T198E-Y200E–Flag). Immunoprecipitations using these ERK forms showed that single T-to-E or Y-to-E mutations reduced ERK affinity for Krz, and a double mutation reduced it even further (Figure 4C). These effects were most readily observed with HA–Krz but were also clearly seen with HA–Krz-R209E (Figure 4C). We observed a similar effect of the phosphorylation-mimicking mutations on the binding of ERK to human β-arrestin2 (Supplementary Figure S3). Another activating mutation in Drosophila ERK, D334N, is the cause of the dominant gain-of-function properties of the Sevenmaker (rlSem) allele (Brunner et al, 1994). Interestingly, ERK-D334N–Flag exhibited lower binding affinity for HA–Krz, compared with ERK–Flag (Figure 4D). The results of these binding experiments suggest that ERK activation lowers its affinity for Krz.
In addition, we created versions of ERK that are unable to be phosphorylated and tested their ability to bind to Krz. We changed threonine in the TEY phosphorylation motif to alanine singly and in combination with tyrosine substituted to phenylalanine (ERK-T198A–Flag and ERK-T198A-Y200F–Flag). Immunoprecipitations using these ERK forms showed that a single T-to-A mutation reduced ERK affinity for Krz, and a double mutation with Y-to-F led to a further reduction in binding (Figure 4E). The conformation of ERK is dramatically changed on phosphorylation, particularly in the activation loop region (Zhang et al, 1995), which may account for the lack of Krz binding to the active form of ERK. At the same time, mutations rendering ERK inactive also alter the conformation of ERK, in the same region (Zhang et al, 1995). Collectively, the results of binding experiments between Krz and mutant isoforms of ERK are consistent with a model in which Krz preferentially associates with an unmodified form of ERK. Mutations that alter this basal ERK conformation reduce the affinity of ERK for Krz.
Krz-bound ERK is not phosphorylated by MEK
We then asked whether the Krz-bound ERK protein can be efficiently phosphorylated by the upstream kinase, MEK. Interference with ERK phosphorylation represents a simple mechanism that would account for the observed inhibition of ERK activity by Krz in the embryo. To test this hypothesis, we conducted an in vitro kinase assay using purified human MEK2 protein and ERK that was immobilized on beads with and without Krz (see Materials and methods section). ERK–Flag immobilized on anti-Flag beads was readily phosphorylated by MEK (Figure 5A). In contrast, ERK–Flag that was bound to HA–Krz-R209E and treated with MEK was unable to be phosphorylated (Figure 5A). This result shows that not only does Krz preferentially associate with unphosphorylated ERK, but also that this association prevents further phosphorylation of ERK by MEK.
Figure 5.
Krz inhibits ERK phosphorylation by MEK. (A) S2 cells were transfected with Drosophila ERK–Flag alone or in combination with HA–Krz-R209E, and immunoprecipitated with either anti-Flag or anti-HA beads. 320 ng of purified human MEK2 was added to samples as indicated, and extent of phosphorylation of Drosophila ERK–Flag was then assayed with anti-dpERK antibody by western blot analysis. (B) S2 cells were transfected with Drosophila ERK–Flag alone or in the combinations indicated with GFP–Krz, Drosophila MEK–V5 and/or Drosophila HA–Raf. Cells were treated with insulin at a final concentration of 20 μM as indicated. Samples were immunoprecipitated with anti-Flag beads, and extent of phosphorylation of Drosophila ERK–Flag was then assayed with anti-dpERK antibody by western blot analysis. IP, immunoprecipitated samples; IB, immunoblots. (C) A model of Krz regulation of RTK signalling. Krz limits the activity of receptor tyrosine kinases (RTKs) by preferentially binding and sequestering an inactive form of ERK. This sequestration prevents ERK from being phosphorylated by the upstream kinase, MEK, and therefore reduces an overall output of signalling downstream of RTKs.
Krz does not activate MAPK signalling downstream of RTKs
Several studies in mammalian cells showed that β-arrestins can assemble all of the components of the MAPK cascade into a signalling complex and induce activation of ERK downstream of activated GPCRs (for review, see Defea, 2008; DeWire et al, 2007). One possible explanation for the inhibitory effects of Krz on ERK activation is the absence of Krz binding to the other components of the MAPK cascade in Drosophila. When tested by immunoprecipitation from S2 cells, Krz bound to the Drosophila homologues of Raf (polehole, phl) and MEK (Dsor1; Supplementary Figure S6). We observed that the Krz-R209E mutation increased the affinity of Krz for both Raf and MEK, suggesting that this activating mutation generally increases the binding of β-arrestin to all three MAP kinases. We then created a version of the Drosophila MEK protein that carried two amino-acid substitutions that were shown to activate mammalian MEK (Yan and Templeton, 1994). Unlike what we have seen for ERK (Figure 4C), these mutations increased the association of MEK with Krz (Supplementary Figure S6B). Collectively, these data indicate that the absence of binding to the other MAP kinases cannot explain the inhibitory effects of Krz on ERK activation.
To test the effects of Krz on ligand-dependent activation of the MAPK cascade, we co-expressed ERK with MEK, Raf and GFP–Krz in different combinations in S2 cells, with and without insulin treatment. All constructs were transfected in equal amounts. When all three MAP kinases were co-expressed, we observed a robust activation of ERK (seen as the dpERK signal), which was largely independent from the presence of insulin and was not significantly affected by Krz (Figure 5B). However, when ERK was expressed alone or co-expressed with MEK, and cells were treated with insulin, we observed a reduction in the amount of dpERK in the presence of GFP–Krz (Figure 5B). These data demonstrate that overexpression of Krz can exert a neutral or inhibitory, but not activating, effect on the MAPK cascade downstream of receptor tyrosine kinases.
In sum, the binding and phosphorylation experiments suggest that Krz may limit the extent of Torso activation by preferentially binding and sequestering an inactive form of ERK, making it unavailable for activation by the upstream kinases (Figure 5C). The observed increase in dpERK levels at the embryo poles in krz maternal mutant embryos (Figure 3A and B) may thus be explained at least in part by this previously unknown mechanism. Consistent with this model, we observed that overexpression of Krz reduced the amount of ERK that can be co-precipitated through the MEK protein (Supplementary Figure S6C). Conversely, overexpressed MEK reduced the amount of Krz co-precipitated with ERK (Supplementary Figure S6D). These data support the sequestration model and suggest that the Krz and MEK proteins can compete for ERK when the three proteins are co-expressed.
Krz inhibits other RTKs at later stages of development
Thus far, we have shown genetically a role of Krz in the inhibition of ERK activation in the Torso pathway, and found a likely mechanism for this inhibitory effect. However, ERK is a key signalling component in virtually all RTK pathways that are active throughout Drosophila development (Gabay et al, 1997a). For example, ERK involvement in both wing and eye development is well documented (Biggs et al, 1994; Brunner et al, 1994). We investigated whether negative effects of Krz on ERK were confined to the early embryo, or would also be observed in other tissues.
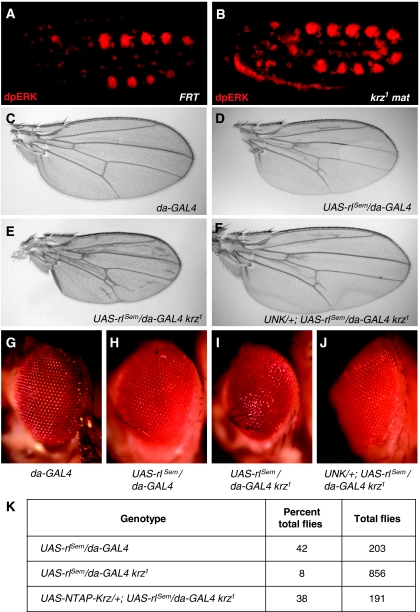
The pattern of ERK activation during embryogenesis is complex and highly dynamic, reflecting the activity of the RTKs EGFR (DER), Heartless (Htl) and Breathless (Btl), in addition to Torso (Gabay et al, 1997a; Gabay et al, 1997b). In krz maternal mutant embryos, we observed a slight expansion of the dpERK pattern in the vicinity of tracheal pits and chordotonal organs (Figure 6A and B). At this stage of development, Torso is no longer active, and dpERK expression is under the control of Btl and EGFR, respectively (Gabay et al, 1997a; Gabay et al, 1997b). An increase in dpERK signal observed in these immunofluorescence staining experiments was consistent with a significant overall dpERK upregulation detected at the same stages of embryogenesis on western blots (see Figure 3E). These data suggest that Krz limits activation of other RTKs that function later in embryogenesis, in addition to its early effects on Torso.
Figure 6.
Krz inhibits RTK signalling in different tissues and at different developmental stages. (A, B) Expression of doubly phosphorylated activated ERK (dpERK) in (A) stage-10.5 FRT and (B) krz1 maternal mutant embryos. An expansion of the dpERK pattern in the mutants is consistent with an overall increase in dpERK levels observed at this stage on western blots (see Figure 3E, 4–8 h). (C–K) Genetic interactions between krz and an activating mutation in ERK, rlSem. (C–F) Wing phenotypes. (G–J) Eye phenotypes. The sizes of the wings can be directly compared because same magnification was used in (C–F). Magnification was also constant for (G–J). The genotypes were, (C, G) da-GAL4, (D, H) UAS-rlSem/da-GAL4, (E, I) UAS-rlSem/da-GAL4 krz1, (F, J) UAS-NTAP-Krz/+; UAS-rlSem/da-GAL4 krz1. UNK is an abbreviation for UAS-NTAP-Krz. (K) Survival to adult reported as percentage of all progeny for the UAS-rlSem/da-GAL4, UAS-rlSem/da-GAL4 krz1, and UAS-NTAP-Krz/+; UAS-rlSem/da-GAL4 krz1 genotypes.
To test for the involvement of Krz in RTK signalling at other developmental stages, we examined phenotypes resulting from hyperactivation of ERK (rl) in combination with loss and gain of function of Krz. Overexpression of a dominant gain-of-function rl isoform, rlSem, using the GAL4–UAS system recapitulates the effects of the original rlSem allele (Brunner et al, 1994). When UAS-rlSem was driven by the ubiquitous da-GAL4 driver (Wodarz et al, 1995), we observed formation of additional vein material in the wing, as well as smaller overall size of the wing (Figure 6C and D). These phenotypes are attributed to elevated EGFR signalling activity (Brunner et al, 1994). Elimination of one copy of krz using the krz1 allele in the UAS-rlSem; da-GAL4 genetic background resulted in the development of even more additional veins in the wing and further reduction in the wing size, which indicates the enhancement of the rl gain-of-function phenotype (Figure 6E). We then asked whether an increase in Krz protein level in this genetic background would cancel the effect of losing one copy of krz. Krz was expressed using the UAS-NTAP-Krz transgene that encodes a fully functional form of Krz (Kyriakakis et al, 2008). In the animals with the UAS-NTAP-Krz/+; UAS-rlSem/da-GAL4 krz1 genotype, the wing venation phenotype was restored to the one in the UAS-rlSem/da-GAL4 flies, despite the fact that one copy of krz was still absent (Figure 6F). It is possible that overexpression of Krz did not fully restore wing venation to a wild-type pattern because the binding affinity between Krz and the rlSem isoform of ERK is weaker than with wild-type ERK (Figure 4D). Therefore, in the background of rlSem overexpression, changes in the level of Krz may more readily affect the wild-type ERK protein, rather than the rlSem form. The wing size, however, increased to that observed in the wild type, when Krz was overexpressed (Figure 6C and F). These genetic interactions are consistent with the view that Krz has an inhibitory effect on ERK activation downstream of EGFR signalling in the wing.
In the eye, expression of rlSem using da-GAL4 resulted in mild irregularities in the ommatidial pattern (Figure 6H), which are likely to be caused by increased activity of the RTKs EGFR and Sev (Biggs et al, 1994; Brunner et al, 1994). This phenotype was strongly enhanced by eliminating one copy of krz, which led to the formation of smaller, rough eyes (Figure 6I). Expression of UAS-NTAP-Krz restored the regular ommatidial pattern and the size of the eye (Figure 6J). The effects of loss of krz on rlSem were also manifested at the level of the whole organism. Thus, UAS-rlSem/da-GAL4 flies survived at 42% (Figure 6K). Survival rate dropped to 8% when one copy of krz was eliminated but restored to 38% by expressing UAS-NTAP-Krz (Figure 6K). Taken together, these results show that the inhibitory effects of Krz on ERK are not limited to early embryogenesis but likely occur throughout Drosophila development. Therefore, Krz seems to be a general inhibitor of RTK signalling in Drosophila development and likely affects the function of different RTKs.
Loss of krz results in an upregulation of Toll signalling
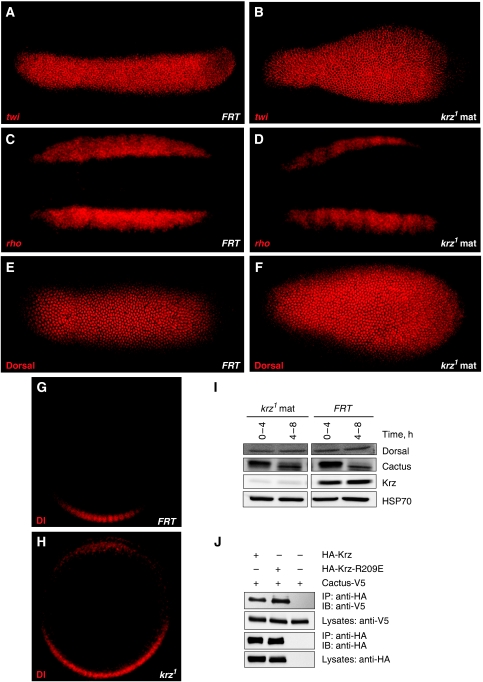
In addition to the defects at the anterior and posterior ends of the embryo, phenotypic analysis of krz1 maternal mutants revealed abnormal development of the ventral structures (see Figure 1C–F). Specification of the ventral embryonic region is under the control of the Toll signalling pathway. Activation of the Toll receptor results in a nuclear gradient of the NF-κB homologue Dorsal protein, with the highest levels present along the ventral midline of the embryo (Roth et al, 1989; Rushlow et al, 1989; Steward, 1989). Nuclear translocation of Dorsal results in a direct transcriptional activation of twist (twi) along the ventral midline (Thisse et al, 1991). To determine the effects of loss of krz on Toll pathway signalling, we studied the pattern of twi expression in krz mutants. In situ hybridization using the twi probe revealed an expansion of the twi expression domain in krz1 maternal mutant embryos, especially in the posterior half of the embryo (Figure 7A and B), suggesting that in krz mutants Toll signalling is abnormally upregulated. Therefore, Krz functions during normal development to limit the activity of Toll.
Figure 7.
Loss of krz results in increased nuclear Dorsal as well as expanded Toll target gene expression domains. (A, B) In situ hybridization with antisense twist (twi) probe in (A) stage-4 FRT control and (B) krz1 maternal mutant embryos. (C, D) In situ hybridization with antisense rhomboid (rho) probe in (C) stage-4 FRT control and (D) krz1 maternal mutant embryos. (E–H) Visualization of Dorsal protein localization with anti-Dorsal antibody viewed (E, F) ventrally and (G, H) in a transverse optical cross section in (E, G) stage-4 control FRT and (F, H) krz1 maternal mutant embryos. (I) Western blot of protein expression in staged krz1 maternal mutant embryos and FRT controls. HSP70 antibody was used as loading control. (J) S2 cells were transfected with HA–Krz or HA–Krz-R209E together with Cactus-V5. Samples were immunoprecipitated with anti-HA beads and analysed by western blotting. IP, immunoprecipitated samples; IB, immunoblots.
Given that twi is directly activated by Dorsal, we asked whether the observed expansion of its expression domains resulted from a dysregulation of Dorsal nuclear localization. We observed that the nuclear gradient of Dorsal protein is significantly expanded in the lateral regions of the embryo, particularly in the posterior (Figure 7E and F). Higher nuclear levels of Dorsal in these cells were even more readily observed in transverse optical sections (Figure 7G and H). These data indicate that the twi expression domain in krz mutants closely follows the expansion of the Dorsal nuclear gradient, suggesting that the abnormal pattern of Dorsal localization is a likely cause for the observed change in the expression of this primary Toll target.
A secondary Toll target gene, rhomboid (rho), is expressed as two longitudinal ventro-lateral stripes in the blastoderm embryo. The dorsal boundary of the rho stripe is determined by a combined activity of both Dorsal and Twist, whereas the ventral boundary is set by a direct repression by Snail, which is in turn activated by both Dorsal and Twist (Ip et al, 1992; Hong et al, 2008). Given our data on the expansion of the Dorsal gradient and the twi expression domain, it would be expected that the two rho stripes would be pushed further apart in krz maternal mutants. Indeed, in situ hybridization using a rho probe revealed this to be the case (Figure 7C and D), which shows that secondary Toll targets are also affected by the loss of krz function in the embryo.
Dorsal levels in extracts from krz1 maternal mutants appeared unchanged compared with control (Figure 7I). At the same time, the level of the Dorsal inhibitor Cactus (IκBα homologue) was lower in the 0–4-h embryos (Figure 7I). These findings suggest that the expansion of the Dorsal expression pattern in krz1 maternal mutants is a result of inappropriate nuclear translocation of Dorsal in cells of the lateral regions of the embryo, rather than increased Dorsal protein levels. This aberrant expansion of the Dorsal nuclear localization may be explained at least in part by the lower levels of Cactus that normally sequesters Dorsal in the cytoplasm.
β-Arrestins have been reported to bind to IκBα in mammalian cells and inhibit its phosphorylation (Gao et al, 2004). As phosphorylation leads to IκBα degradation, its association with β-arrestin stabilizes IκBα and results in downregulation of NF-κB signalling (Gao et al, 2004). We hypothesized that a similar mechanism may be responsible for the effects of Krz on the Toll pathway and tested for an interaction between Krz and Cactus, the Drosophila homologue of IκBα. We detected an interaction using co-immunoprecipitation from S2 cells (Figure 7J), as well as using in vitro translated proteins (Supplementary Figure S7), suggesting a direct binding. Unlike ERK–Flag, Cactus-V5 bound to HA–Krz with about the same affinity as to HA–Krz-R209E (Figure 7J), suggesting that the ‘pre-activated' conformation of Krz does not affect Cactus binding. A direct interaction between the Krz and Cactus proteins, together with an observed decrease in Cactus levels in krz maternal mutant embryos (Figure 7I), suggests that Krz may limit Toll pathway activity by stabilizing Cactus and thus inhibiting an inappropriate spreading of the Dorsal nuclear gradient.
Loss of krz affects the balance of cross-regulatory interactions between Torso and Toll
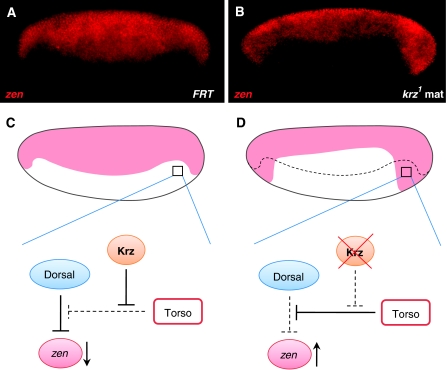
As the activities of the Torso and Toll pathways intersect at the blastoderm stage to jointly control expression of the transcription factor zerknüllt (zen), we asked whether loss of krz would affect the balance of cross-regulation between these pathways in the embryo. High levels of the Dorsal protein in the ventral nuclei repress zen expression in the ventral region, whereas Torso signalling relieves this repression at the embryo poles, allowing zen to be expressed at the embryonic termini (Ip et al, 1991; Rusch and Levine, 1994). In situ hybridization using zen probe in krz1 maternal mutant embryos showed that zen expression contracted in the dorsal region, but at the same time expanded at the embryo poles, especially at the posterior end (Figure 8A and B). This result can be interpreted as an increased repression of zen by Dorsal in the lateral regions, and a simultaneous increase in de-repression of zen by the Torso pathway at the embryo poles (Figure 8C and D). These complex changes in the zen expression pattern, which result from the loss of a single β-arrestin in the embryo, reveal an important developmental role of Krz in the regulation of signalling activities of the Torso and Toll pathways. In this context, Krz is helping Torso and Toll to achieve a precise balance of cross-regulation, which is necessary for the correct spatial expression of their common targets. Dysregulation of this fine balance in krz mutants results in inappropriate expression of developmental regulators such as zen, and contributes to patterning defects in the embryo.
Figure 8.
Krz regulates Torso and Toll shared target gene zen. (A, B) In situ hybridization with antisense zen probe in (A) stage-4 FRT control and (B) krz1 maternal mutant embryos. (C, D) A diagram explaining the effects of loss of krz on Torso and Toll mutual regulation of zen. A boxed area indicates the same position in (C) the wild-type and (D) krz1 maternal mutant embryos. (C) In the wild-type embryo, Dorsal nuclear translocation represses zen transcription in the cells inside the boxed area. By limiting Torso activity in these cells, Krz ensures that zen is repressed. (D) In krz maternal mutant embryos, loss of krz results in an upregulation of Torso activity, which overcomes Dorsal-mediated repression and leads to an abnormal de-repression of zen in the boxed area. Dashed line in the embryo schematic in (D) indicates a boundary of the wild-type zen expression pattern.
Discussion
Despite a large body of evidence on the signalling functions of β-arrestins, few studies address the in vivo roles of these proteins, and still fewer characterize their developmental functions (Kovacs et al, 2009). In this study, we have uncovered a previously uncharacterized function of the Drosophila β-arrestin Kurtz in regulating early development. Our results demonstrate that the Krz protein is necessary for setting a precise level of activation of two maternal signalling pathways, Torso and Toll. This activity of Krz helps to establish the correct domains of expression of developmental patterning regulators that are under the control of these pathways.
Our genetic and protein interaction data suggest a new mechanism by which Krz may limit the activity of Torso (Figure 5C). We have observed that Krz preferentially binds and sequesters an inactive form of ERK, thereby making it unavailable for activation by the upstream kinases such as MEK. Such a mechanism of direct inhibition of ERK activation by β-arrestin binding has not been previously reported. This mechanism is consistent with the observed in vivo effects of loss of krz on ERK activity. In krz maternal mutant embryos, ERK is not sequestered and therefore more ERK is available to transduce Torso signals, resulting in hyperactivation of Torso target genes, tll and hkb. Furthermore, consistent with this model is the observation that Krz and MEK apparently compete for ERK when all three proteins are co-expressed in S2 cells (Supplementary Figure 6C and D).
Interaction assays using mutated forms of Krz and ERK indicate that the conformations of both proteins have an effect on their binding affinity. On binding to an activated GPCR, the arrestin molecule undergoes a dramatic conformational change that can be mimicked by specific mutations (Gurevich and Gurevich, 2004). We have observed in immunoprecipitation experiments that such ‘pre-activated' form of Krz (R209E) has a much greater affinity for ERK, compared with the wild-type Krz protein, and that this higher affinity is also observed for the equivalent mutant of human β-arrestin2. This suggests that the ERK-binding ability of β-arrestin may be affected by its conformation, but it is unknown at present whether any upstream signals convert Krz into an activated form in the embryo. Overexpression of Krz-R209E using the da-GAL4 driver did not result in any observable phenotype and could rescue zygotic loss of krz, suggesting that it retains most of the functions of wild-type Krz (data not shown).
We observed that the conformation of ERK itself has a large effect on its interactions with Krz. In our binding experiments, activated forms of ERK bind Krz (and human β-arrestin2) with lower affinity, compared with wild-type inactive ERK. Moreover, mutations in the TEY motif, which render ERK constitutively inactive, also lower its affinity for Krz, which is at a first glance a surprising result. However, previous studies have shown that both types of mutations in the TEY motif, which is a part of the activation loop, increase disorder in the lip region and cause a conformational change in the ERK molecule that makes it different from the basal state (Zhang et al, 1995). We therefore speculate that the activation loop may be involved in mediating an interaction of ERK with β-arrestin. Any deviation of ERK structure from the basal state would decrease its association with β-arrestin, which is consistent with our results.
Other studies have reported formation of protein complexes containing β-arrestins and an activated form of ERK (DeFea et al, 2000a; DeFea et al, 2000b; Tohgo et al, 2002; Ge et al, 2003). It is possible that in those experimental conditions other binding partners, such as Raf or the activated receptor, assist in stabilizing the complex of MAP kinases with β-arrestin (Luttrell et al, 2001). We have shown that although Krz can bind to the Drosophila homologues of both MEK and Raf, overexpression of Krz does not increase production of dpERK by the MAPK cascade downstream of activated RTKs, but instead appreciably inhibits it in the absence of overexpressed Raf. Our data do not rule out a possibility that Krz may still promote ERK activation in other biological contexts, particularly downstream of activated GPCRs, but this question awaits further investigation.
Interestingly, the sequestration mechanism of ERK inhibition described here is different from the effects of Krz on Notch. We have previously shown that Krz inhibits Notch activity by forming a ternary complex with Deltex and the Notch receptor. Formation of this complex increases Notch turnover and thereby downregulates Notch signalling (Mukherjee et al, 2005). We have not observed a change in ERK turnover in the presence of wild-type overexpressed Krz, suggesting that Krz is unlikely to be involved in the regulation of ERK stability. However, given the versatility of molecular functions displayed by β-arrestins, it is possible that there are other, as yet uncharacterized mechanisms by which Krz controls signalling downstream of RTKs.
The inhibitory effects of Krz on ERK activity are not limited to the Torso pathway and early embryogenesis, but are also observed in other tissues and at later developmental stages. Thus, broadening of the dpERK patterns activated by EGFR and Btl was observed in krz maternal mutant embryos. An increase in the overall levels of dpERK during mid-to-late embryogenesis was also detected on western blots. Later in development, ERK is activated by EGFR in the wing and both EGFR and Sevenless in the eye (Biggs et al, 1994; Brunner et al, 1994). Our genetic data suggest that Krz also inhibits ERK activity in these tissues during larval development. A broad involvement of Krz in inhibiting ERK activity suggests that Krz has a general inhibitory role to limit the activity of different RTKs in Drosophila development.
In addition to its effects on RTK signalling, we observed that Krz has an important role in limiting the activity of the Toll receptor, which specifies the development of the ventral structures. Other studies have reported that mammalian β-arrestins can downregulate NF-κB signalling by binding and stabilizing the NF-κB inhibitor IκBα (Gao et al, 2004; Witherow et al, 2004). The inhibitory effects of Krz on Dorsal may involve a similar mechanism. We observed that Krz can directly bind to the Drosophila orthologue of IκBα, Cactus, suggesting that the mechanism of NF-κB inhibition by β-arrestins at the level of IκBα may be conserved. Consistent with this finding, we detected a decrease in the level of the Cactus protein in krz maternal mutants at 0–4 h of development, which may explain the observed expansion of the nuclear gradient of Dorsal in these mutants. It is still unclear why expansion of Dorsal nuclear localization is more pronounced in the posterior half of the embryo.
In the developing embryo, the Torso and Toll pathways do not work in isolation, but are involved in cross-regulatory interactions on certain common targets, such as zen. zen is repressed by nuclear Dorsal in the ventral part of the embryo, and relieved of this repression (de-repressed) by the signalling activity of Torso emanating from the embryo poles (Rusch and Levine, 1994). The molecular mechanism of this de-repression is still unknown. We have observed that loss of krz shifts the balance of the effects of Torso on Toll, which results in an inappropriate expansion of zen expression at the embryo poles (Figure 8C and D). We speculate that Krz helps Torso to achieve a precise level of de-repression of zen by limiting the activity of ERK. Krz is thus able to control the separate activities of the Torso and Toll pathways (reflected in its effects on tll, hkb, twi, and rho), as well as regulate common Torso and Toll targets such as zen. For such pathways that are engaged in cross-regulatory interactions, Krz ensures that a proper level of signalling activity from one pathway reaches the other. This function adds an important new mechanism to our understanding of the ways in which signalling pathways are coordinately regulated during development (Nagaraj and Banerjee, 2009; Shilo, 2009).
A ubiquitous distribution of Krz in the embryo agrees with the dysregulation of multiple pathways observed in krz mutant animals. As overexpression of Krz does not cause any obvious defects (data not shown), the level of Krz itself is not limiting for the regulation of signalling. Instead, Krz apparently makes other signalling co-factors limiting for their respective pathways, essentially working as a molecular ‘sponge' to prevent pathway hyperactivity. Specificity of Krz function is likely to be determined by its selective interactions with specific pathway co-factors. Maternal loss of krz function thus affects multiple developmental signalling pathways, resulting in an accumulation of defects that ultimately lead to severe morphological abnormalities such as a disruption of gastrulation movements. By analysing the effects of loss of krz on individual pathways in vivo, we were able to show its role in the regulation of RTK and Toll signalling. Future studies will likely reveal other pathways and levels of regulation that are under the control of the Drosophila β-arrestin Krz.
Materials and methods
Drosophila stocks and cuticular preparations
The krz1 allele was obtained from the Szeged Stock Center (Hungary), and is synonymous with krzs047819 allele described in FlyBase. yw ;; UAS-rlSem3.1/TM3 was kindly provided by Ernst Hafen. krz 5.7 genomic rescue construct covers the same genomic interval used by Roman et al (2000). It was cloned into the pattB vector and transgenic lines were created using ϕC31-mediated integration into the second chromosome acceptor site 51D (Venken et al, 2006; Bischof et al, 2007). krz1 maternal mutant embryos were generated using the FLP-DFS technique with krz1 recombined onto the FRT82B chromosome (Chou and Perrimon, 1996). To obtain cuticles embryos were dechorionated after being aged for 24 h at 25°C. Dechorionated embryos were shaken in methanol and incubated in glycerol:acetic acid 1:4 mixture for 1 h at 65°C. Embryos were then mounted in 3:1 CMCP:lactic acid (CMCP is from Masters Company) and incubated overnight at 65°C.
Fluorescent in situ hybridization, antibodies and immunostaining
Probe construction and in situ hybridization was performed as described in (Kosman et al, 2004). To detect tll, hkb, twist, rho and zen biotinylated RNA probes were generated and hybridized to krz1 maternal mutant embryos and to FRT controls. Mouse anti-biotin primary (Sigma, St Louis, MO), and goat anti-mouse Alexa555 secondary antibodies (Invitrogen, Eugene, OR) were used to detect probes. Embryos were mounted in Prolong Gold anti-fade mounting reagent (Invitrogen). Embryos were immunostained for protein detection using the same protocol with mouse anti-dpERK (Sigma), or mouse anti-Dorsal (Developmental Studies Hybridoma Bank, IA) primary antibodies and donkey anti-mouse Cy3 secondary antibodies (Jackson Immunoresearch). dpERK staining was performed immediately after fixing. Embryos immunostained with dpERK antibody were then mounted in Aqua-Poly/Mount (Polysciences, Warrington, PA). Anti-Krz antibody was generated against full-length Krz protein in guinea pig, and resulting serum was affinity purified. Primary and secondary antibodies used to detect Krz protein were diluted 1:10 in PBS and pre-absorbed on krz mutant embryos. Images were obtained using the Zeiss LSM510 confocal microscope. For optical transverse cross section images of Dorsal, embryos stained with anti-Dorsal antibody were mounted perpendicular to the coverslip and imaged at ∼75 μm from the posterior pole. Mouse anti-HSP70 antibody was from Sigma, rabbit anti-GFP antibody was from Invitrogen, and rabbit anti-Cactus antibody was a gift from Steven Wasserman.
Quantification of expression patterns
Quantification of dpERK patterns was performed as described previously (Coppey et al, 2008). To minimize staining variability, krz1 maternal mutants and control embryos were stained in the same tube. The Hist-GFP transgene encodes a nuclear form of GFP and allows for a simple discrimination between the marked and non-marked embryos (Coppey et al, 2008). As Hist-GFP and FRT control embryos showed identical dpERK staining patterns (see Supplementary Figure S2C–G), Hist-GFP embryos were used as controls for stainings with krz maternal mutant embryos. The boundaries of tll and hkb expression domains were determined using an automated image processing program in Matlab. The script finds the perimeter of the embryo and then averages staining intensity along the dorsoventral axis. This was done for 1000 points uniformly spaced along the anterior–posterior axis, generating an anterior–posterior expression profile of the gene. The locations at which the signal becomes half of the maximal level were used as boundaries of the expression domains. Student's t-test was calculated in Excel using one-tailed distribution, two-sample unequal variance setting.
Expression constructs
Full-length Drosophila ERK (rl), MEK (Dsor1), Raf (phl), Cactus and Krz open-reading frames were amplified by PCR using epitope tag-containing primers and cloned into the pMT/V5-His series vectors (Invitrogen) to generate amino-terminally tagged HA–Krz, HA–Raf, GFP–Krz, Myc–Krz and carboxy-terminally tagged MEK-V5 and ERK–Flag. All Krz, ERK and MEK mutations were generated using these initial constructs as templates with GeneTailor site-directed mutagenesis system (Invitrogen).
Cell culture and immunoprecipitation
Drosophila S2 cells were maintained at 25°C in Schneider's Drosophila medium (Gibco, Grand Island, NY) supplemented with 10% heat inactivated fetal bovine serum (Gibco) and 5% Pen/Strep antibiotic mixture. All transfections were performed using Effectene transfection reagent (Qiagen, Valencia, CA). Equal amounts of plasmids were transfected, and the total amount of DNA was kept constant by adding empty vector. Proteins were induced with 0.35 mM CuSO4 overnight, cells were lysed in default lysis buffer (50 mM Tris (pH 7.5), 125 mM NaCl, 5% glycerol, 0.2% IGEPAL, 1.5 mM MgCl2, 1 mM DTT, 25 mM NaF, 1 mM Na3VO4, 1 mM EDTA and 2 × Complete protease inhibitor (Roche, Indianapolis, IN)) and lysates were incubated with anti-Flag, anti-V5 or anti-HA affinity beads (Sigma) for 2 h at 4°C, followed by extensive washes. Protein complexes were eluted with SDS sample buffer, separated on SDS protein gels, transferred onto Immun-Blot PVDF membranes (Bio-Rad, Hercules, CA) and probed with rabbit anti-Flag, mouse anti-HA, anti-GFP or anti-V5 antibodies (Sigma).
Insulin treatment
The S2 cells were transfected with ERK–Flag alone, or together with indicated combinations of HA–Krz, HA-R209E, GFP–Krz, MEK–V5 or HA–Raf and induced overnight with 0.35 mM CuSO4 in serum-free media. The following day complete S2 cell-conditioned media containing 20 μM of bovine pancreatic insulin (Sigma) was added, and cells were incubated for 20 min at 25°C. Cells were lysed and the extracts were used for co-immunoprecipitation assays as described above, and probed with mouse anti-ERK, mouse anti-dpERK, rabbit anti-Flag, mouse anti-HA, mouse anti-V5 (Sigma) or guinea pig anti-Krz antibodies for western blot analysis.
In vitro kinase assay
The S2 cells were transfected with ERK–Flag alone or together with HA–Krz-R209E. Extracts were incubated with anti-HA beads, and extracts transfected with only ERK–Flag were incubated with anti-Flag beads. After several washes, 320 ng of human purified MEK2 and 200 μM ATP in kinase buffer (Cell Signaling Technologies, Danvers, MA) were added and samples were incubated at 30°C for 30 min. The ERK–Flag sample was used as a positive control for ERK phosphorylation. Samples were analysed by western blot for ERK phosphorylation status. Proteins were detected by mouse anti-HA (Sigma), mouse anti-dpERK (Sigma) and mouse anti-panERK (BD Biosciences) antibodies.
Supplementary Material
Acknowledgments
We thank Mathieu Coppey for help with dpERK quantifications, and members of the Veraksa lab for fruitful discussions on the paper. Some of the Drosophila stocks described in this study were obtained from the Bloomington Drosophila Stock Center, Bloomington, IN. Anti-Cactus antibody was a gift from Steven Wasserman. HS-HA-Ub construct was a gift from R Fehon. Anti-Elav and anti-Dorsal antibodies were obtained from the Developmental Studies Hybridoma Bank, University of Iowa. This study was supported by the grant from NSF 0640700 to AV, and by a grant from the Nancy Goranson Research Fund to MT.
Footnotes
The authors declare that they have no conflict of interest.
References
- Biggs WH III, Zavitz KH, Dickson B, van der Straten A, Brunner D, Hafen E, Zipursky SL (1994) The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J 13: 1628–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA 104: 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286: 2495–2498 [DOI] [PubMed] [Google Scholar]
- Brunner D, Oellers N, Szabad J, Biggs WH III, Zipursky SL, Hafen E (1994) A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell 76: 875–888 [DOI] [PubMed] [Google Scholar]
- Bryja V, Gradl D, Schambony A, Arenas E, Schulte G (2007) Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci USA 104: 6690–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ (1997) Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90: 859–869 [DOI] [PubMed] [Google Scholar]
- Chou TB, Perrimon N (1996) The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144: 1673–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG (1997) beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res 81: 1021–1026 [DOI] [PubMed] [Google Scholar]
- Coppey M, Boettiger AN, Berezhkovskii AM, Shvartsman SY (2008) Nuclear trapping shapes the terminal gradient in the Drosophila embryo. Curr Biol 18: 915–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defea K (2008) Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. Br J Pharmacol 153(Suppl 1): S298–S309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW (2000a) The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA 97: 11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW (2000b) beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148: 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK (2007) Beta-arrestins and cell signaling. Annu Rev Physiol 69: 483–510 [DOI] [PubMed] [Google Scholar]
- Friedman A, Perrimon N (2006) A functional RNAi screen for regulators of receptor tyrosine kinase and ERK signalling. Nature 444: 230–234 [DOI] [PubMed] [Google Scholar]
- Gabay L, Seger R, Shilo BZ (1997a) In situ activation pattern of Drosophila EGF receptor pathway during development. Science 277: 1103–1106 [DOI] [PubMed] [Google Scholar]
- Gabay L, Seger R, Shilo BZ (1997b) MAP kinase in situ activation atlas during Drosophila embryogenesis. Development 124: 3535–3541 [DOI] [PubMed] [Google Scholar]
- Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G (2004) Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell 14: 303–317 [DOI] [PubMed] [Google Scholar]
- Ge H, Krishnan P, Liu L, Krishnan B, Davis RL, Hardin PE, Roman G (2006) A Drosophila nonvisual arrestin is required for the maintenance of olfactory sensitivity. Chem Senses 31: 49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Ly Y, Hollenberg M, DeFea K (2003) A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem 278: 34418–34426 [DOI] [PubMed] [Google Scholar]
- Gerttula S, Jin YS, Anderson KV (1988) Zygotic expression and activity of the Drosophila Toll gene, a gene required maternally for embryonic dorsal–ventral pattern formation. Genetics 119: 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2004) The molecular acrobatics of arrestin activation. Trends Pharmacol Sci 25: 105–111 [DOI] [PubMed] [Google Scholar]
- Hong JW, Hendrix DA, Papatsenko D, Levine MS (2008) How the Dorsal gradient works: insights from postgenome technologies. Proc Natl Acad Sci USA 105: 20072–20076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip YT, Kraut R, Levine M, Rushlow CA (1991) The dorsal morphogen is a sequence-specific DNA-binding protein that interacts with a long-range repression element in Drosophila. Cell 64: 439–446 [DOI] [PubMed] [Google Scholar]
- Ip YT, Park RE, Kosman D, Bier E, Levine M (1992) The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Dev 6: 1728–1739 [DOI] [PubMed] [Google Scholar]
- Johnson EC, Tift FW, McCauley A, Liu L, Roman G (2008) Functional characterization of kurtz, a Drosophila non-visual arrestin, reveals conservation of GPCR desensitization mechanisms. Insect Biochem Mol Biol 38: 1016–1022 [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK (2007) Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J 26: 2513–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosman D, Mizutani CM, Lemons D, Cox WG, McGinnis W, Bier E (2004) Multiplex detection of RNA expression in Drosophila embryos. Science 305: 846. [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ (2009) Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell 17: 443–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV (1999) Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J Biol Chem 274: 6831–6834 [DOI] [PubMed] [Google Scholar]
- Kyriakakis P, Tipping M, Abed L, Veraksa A (2008) Tandem affinity purification in Drosophila: the advantages of the GS-TAP system. Fly (Austin) 2: 229–235 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ (2006) New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell 24: 643–652 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK (2005) Transduction of receptor signals by beta-arrestins. Science 308: 512–517 [DOI] [PubMed] [Google Scholar]
- Leptin M (1995) Drosophila gastrulation: from pattern formation to morphogenesis. Annu Rev Cell Dev Biol 11: 189–212 [DOI] [PubMed] [Google Scholar]
- Liaw GJ, Rudolph KM, Huang JD, Dubnicoff T, Courey AJ, Lengyel JA (1995) The torso response element binds GAGA and NTF-1/Elf-1, and regulates tailless by relief of repression. Genes Dev 9: 3163–3176 [DOI] [PubMed] [Google Scholar]
- Liu L, Davis RL, Roman G (2007) Exploratory activity in Drosophila requires the kurtz nonvisual arrestin. Genetics 175: 1197–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ (2001) Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA 98: 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S (2005) Regulation of Notch signalling by non-visual beta-arrestin. Nat Cell Biol 7: 1191–1201 [DOI] [PubMed] [Google Scholar]
- Nagaraj R, Banerjee U (2009) Regulation of Notch and Wingless signalling by phyllopod, a transcriptional target of the EGFR pathway. EMBO J 28: 337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslein-Volhard C (1991) Determination of the embryonic axes of Drosophila. Dev Suppl 1: 1–10 [PubMed] [Google Scholar]
- O'Neill EM, Rebay I, Tjian R, Rubin GM (1994) The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell 78: 137–147 [DOI] [PubMed] [Google Scholar]
- Palmitessa A, Hess HA, Bany IA, Kim YM, Koelle MR, Benovic JL (2005) Caenorhabditus elegans arrestin regulates neural G protein signaling and olfactory adaptation and recovery. J Biol Chem 280: 24649–24662 [DOI] [PubMed] [Google Scholar]
- Paroush Z, Wainwright SM, Ish-Horowicz D (1997) Torso signalling regulates terminal patterning in Drosophila by antagonising Groucho-mediated repression. Development 124: 3827–3834 [DOI] [PubMed] [Google Scholar]
- Pierce KL, Lefkowitz RJ (2001) Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci 2: 727–733 [DOI] [PubMed] [Google Scholar]
- Roman G, He J, Davis RL (2000) kurtz, a novel nonvisual arrestin, is an essential neural gene in Drosophila. Genetics 155: 1281–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Stein D, Nusslein-Volhard C (1989) A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell 59: 1189–1202 [DOI] [PubMed] [Google Scholar]
- Rusch J, Levine M (1994) Regulation of the dorsal morphogen by the Toll and torso signaling pathways: a receptor tyrosine kinase selectively masks transcriptional repression. Genes Dev 8: 1247–1257 [DOI] [PubMed] [Google Scholar]
- Rushlow CA, Han K, Manley JL, Levine M (1989) The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell 59: 1165–1177 [DOI] [PubMed] [Google Scholar]
- Shilo BZ (2009) Phyllopod at the intersection of developmental signalling pathways. EMBO J 28: 311–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Coffa S, Fu H, Gurevich VV (2009) How does arrestin assemble MAPKs into a signaling complex? J Biol Chem 284: 685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger F, Stevens LM, Nusslein-Volhard C (1989) The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature 338: 478–483 [DOI] [PubMed] [Google Scholar]
- Steward R (1989) Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell 59: 1179–1188 [DOI] [PubMed] [Google Scholar]
- Thisse C, Perrin-Schmitt F, Stoetzel C, Thisse B (1991) Sequence-specific transactivation of the Drosophila twist gene by the dorsal gene product. Cell 65: 1191–1201 [DOI] [PubMed] [Google Scholar]
- Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM (2002) beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem 277: 9429–9436 [DOI] [PubMed] [Google Scholar]
- Venken KJ, He Y, Hoskins RA, Bellen HJ (2006) P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314: 1747–1751 [DOI] [PubMed] [Google Scholar]
- Wilbanks AM, Fralish GB, Kirby ML, Barak LS, Li YX, Caron MG (2004) Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science 306: 2264–2267 [DOI] [PubMed] [Google Scholar]
- Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ (2004) beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci USA 101: 8603–8607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Hinz U, Engelbert M, Knust E (1995) Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82: 67–76 [DOI] [PubMed] [Google Scholar]
- Xu TR, Baillie GS, Bhari N, Houslay TM, Pitt AM, Adams DR, Kolch W, Houslay MD, Milligan G (2008) Mutations of beta-arrestin 2 that limit self-association also interfere with interactions with the beta2-adrenoceptor and the ERK1/2 MAPKs: implications for beta2-adrenoceptor signalling via the ERK1/2 MAPKs. Biochem J 413: 51–60 [DOI] [PubMed] [Google Scholar]
- Yan M, Templeton DJ (1994) Identification of 2 serine residues of MEK-1 that are differentially phosphorylated during activation by raf and MEK kinase. J Biol Chem 269: 19067–19073 [PubMed] [Google Scholar]
- Zhang J, Zhang F, Ebert D, Cobb MH, Goldsmith EJ (1995) Activity of the MAP kinase ERK2 is controlled by a flexible surface loop. Structure 3: 299–307 [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu X, Zhang Y, Zhao J (2010) Loss of betaarrestin1 and betaarrestin2 contributes to pulmonary hypoplasia and neonatal lethality in mice. Dev Biol 339: 407–417 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.