Somatic piRNA biogenesis
PIWI-interacting RNAs (piRNAs) play an important role in silencing transposons within the germline; how they are generated and how they exert their silencing function is not well understood. Here Phil Zamore highlights recent work from the Brennecke lab that provides new insight into the factors involved in these small RNA biogenesis pathways.
EMBO J 29 19, 3301–3317 (2010); published online September032010
Piwi-interacting RNAs (piRNAs) protect animal germ cells from transposons and other selfish genetic elements. Of the three types of animal small-silencing RNAs—small-interfering RNAs (siRNAs), microRNAs (miRNAs), and piRNAs—piRNAs are the least well understood, because we lack good tools for studying how they are made and how they function. Brennecke et al have now established a method for triggering RNA interference (RNAi) solely in Drosophila follicle cells, a specialized somatic cell that abuts the developing oocyte and which expresses a simplified version of the piRNA pathway present in animal germ cells. Their initial results already suggest a revision for our model of the piRNA pathway, and promise to accelerate the study of this enigmatic small RNA class.
Transposons and repetitive sequences compose nearly half the human genome and about a third of the genome of flies. Although some transposons are ancient relics that have lost the ability to jump to new locations, others remain active, poised to cause mischief. piRNAs (originally called rasiRNAs) protect animal germ cells from these genetic parasites by preventing transposons from producing the proteins required for them to transpose. Thus, piRNAs ensure the genomic integrity of eggs and sperm, protecting the germ cell DNA from the double-stranded breaks and insertional mutagenesis caused by active transposons.
piRNAs are one of three types of ‘small-silencing RNAs' found in animals (Ghildiyal and Zamore, 2009). Small-silencing RNAs serve as guides for Argonaute proteins, specialized RNA-binding proteins that tightly bind the small RNA guide while allowing it to base pair with and dissociate from its regulatory target RNAs. The most well-studied small-silencing RNAs are siRNAs, which serve as guides for the RNAi pathway. Many readers of this journal routinely use siRNAs to reduce the expression of the genes they study. Most animals (but perhaps not mammals) produce siRNAs to defend against viruses and somatic transposons; many (including mammals) produce endogenous siRNAs to regulate genes. A second class of small-silencing RNAs, miRNAs, selectively reduce the stability and rate of translation of mRNAs with which they can partially base pair (Bartel, 2009). Both siRNAs and miRNAs derive from longer, double-stranded RNA precursors that are diced into 21–23 nt double-stranded small RNAs. Not so for piRNAs.
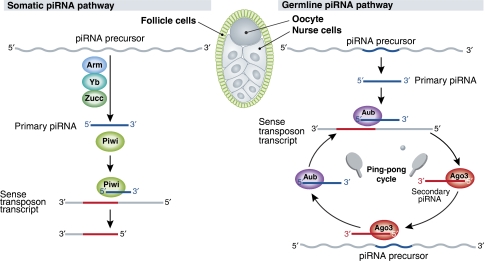
piRNAs derive from long, single-stranded RNAs, nearly all of which are shockingly long and transcribed from genomic ‘clusters'—transposon-rich regions of the genome thought to record the waves of transposon invasions survived by an animal and its evolutionary forebears (Vagin et al, 2006; Brennecke et al, 2007; Carmell et al, 2007; Houwing et al, 2007). Some clusters are convergently transcribed on both genomic strands, whereas others are transcribed in just one direction. Although the first piRNAs were reported in 2001, we still do not understand how these long, single-stranded precursor transcripts are converted into small RNAs. What little we do know about piRNAs comes mainly from the combination of Drosophila genetics and high throughput (or ‘deep') sequencing of piRNAs. These studies have produced a three-part model for piRNA biogenesis (Figure 1) (Brennecke et al, 2007): piRNA precursor transcripts are fragmented and perhaps trimmed to yield primary piRNAs; primary piRNAs initiate an amplification loop (the ‘ping-pong' cycle) that generates secondary piRNAs; and, finally, the resulting amplified piRNAs silence their regulatory targets, such as the mRNA transcripts of transposons, by guiding a specialized sub-class of Argonaute proteins. These specialized Argonaute proteins are called PIWI proteins, after the founding member of the sub-family, the Drosophila protein, P-element-Induced Wimpy Testes or Piwi (Lin and Spradling, 1997).
Figure 1.
Biogenesis of piRNAs in the germline and in somatic follicle cells. In the germline, piRNAs are generated through an Aub- and Ago3-dependent piRNA amplication cycle, whereas in somatic cells, biogenesis occurs through a Piwi-dependent, Aub- and Ago3-independent pathway.
Most details of our current model for piRNA production and function remain to be confirmed. Now Brennecke et al (Olivieri et al, 2010) find that even the details we thought we knew—such as which proteins act at which steps—are not quite right. These authors studied the piRNA pathway in Drosophila ovarian follicle cells, which use a variant piRNA pathway that is simpler than the three-step pathway found in germ cells (Li et al, 2009; Malone et al, 2009). In flies, somatic follicle cells surround each developing oocyte and its associated 15 nurse cells, providing signals that help establish the anterior–posterior and dorsal–ventral axes of the oocyte, as well as laying down the protective egg shell that surrounds the egg. Although most transposons act in the germline, gypsy evades the germline piRNA pathway by producing virus-like particles within the follicle cells, then infecting the adjacent oocyte. Hence, the follicle cell piRNA pathway, which silences transposons such as gypsy, Tabor, and ZAM. Of the three Drosophila PIWI proteins, only Piwi itself has been detected in follicle cells. Piwi, unlike the two germline PIWI proteins, Aubergine and Argonaute3, resides mainly in the nucleus.
Brennecke et al used an extraordinary public resource—the Vienna Drosophila RNAi collection of fly strains able to express double-stranded RNA in vivo to trigger RNAi against nearly every fly gene (Dietzl et al, 2007)—to reduce the expression of candidate genes specifically in the follicle cells. Their great insight was to use the traffic jam promoter—which they discovered was expressed solely in follicle cells—to produce the double-stranded RNA just in that one cell type. Beyond the specific results reported in their paper, Brennecke et al have set the stage for a whole genome RNAi screen to identify genes required for a variety of follicle cell functions, without disrupting those genes in the adjacent germ cells.
Using this follicle cell-specific RNAi strategy to study the piRNA pathway, the authors discovered that the putative helicase Armitage, the putative nuclease Zucchini, and the putative helicase/tudor domain protein Yb were all required to make or stabilize the piRNAs that guide Piwi to silence transposons such as gypsy in follicle cells. This is surprising, as previous genetic data suggested that Armitage acts only in the germline, leading to the view that the mechanism of piRNA production in the somatic follicle cells was fundamentally different from that in the oocyte and its nurse cells. (At least 10 other genes implicated in the germline piRNA pathway were not required to silence gypsy in follicle cells.) As Armitage acts early in the piRNA pathway—no piRNAs accumulate in an armitage mutant—and follicle cells possess only a primary piRNA pathway, Armitage may assist in the production of piRNA from cluster transcripts or in loading piRNAs into PIWI proteins.
Combining RNAi in follicle cells with immunofluorescent antibody detection of proteins, as well as co-immunoprecipitation experiments, the authors discovered that Armitage and Yb are both localized to cytoplasmic foci. Piwi may transit these foci en route to the nucleus, and without Armi or Yb, Piwi remains in the cytoplasm, rather than accumulating in the nucleus. Without Zucchini, Piwi accumulates on the cytoplasmic face of the nuclear envelope. Brennecke et al suggest that in the absence of piRNAs, which are lost in armitage, Yb, and zucchini mutants, Piwi is not licensed to enter the nucleus. Alternatively, Piwi may cycle between the nucleus and cytoplasm, accumulating in the nucleus only when piRNAs are available to tether it to nuclear regulatory targets, such as nascent transcripts not yet exported to the cytoplasm. Distinguishing between these ideas will no doubt be made easier by the new tools established by the Brennecke group.
Footnotes
The author declares that he has no conflict of interest.
References
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ (2007) Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128: 1089–1103 [DOI] [PubMed] [Google Scholar]
- Carmell MA, Girard A, van de Kant HJ, Bourc'his D, Bestor TH, de Rooij DG, Hannon GJ (2007) MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell 12: 503–514 [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156 [DOI] [PubMed] [Google Scholar]
- Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10: 94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, van den Elst H, Filippov DV, Blaser H, Raz E, Moens CB, Plasterk RH, Hannon GJ, Draper BW, Ketting RF (2007) A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 129: 69–82 [DOI] [PubMed] [Google Scholar]
- Li C, Vagin VV, Lee S, Xu J, Ma S, Xi H, Seitz H, Horwich MD, Syrzycka M, Honda BM, Kittler EL, Zapp ML, Klattenhoff C, Schulz N, Theurkauf WE, Weng Z, Zamore PD (2009) Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell 137: 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Spradling AC (1997) A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 124: 2463–2476 [DOI] [PubMed] [Google Scholar]
- Malone CD, Brennecke J, Dus M, Stark A, McCombie WR, Sachidanandam R, Hannon GJ (2009) Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell 137: 522–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri D, Sykora MM, Sachidanandam R, Mechtler K, Brennecke J (2010) An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila. EMBO J 29: 3301–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD (2006) A distinct small RNA pathway silences selfish genetic elements in the germline. Science 313: 320–324 [DOI] [PubMed] [Google Scholar]