Abstract
Activation of V2 receptors (V2R) during antidiuresis increases the permeability of the inner medullary collecting duct to urea and water. Extracellular osmolality is elevated as the concentrating capacity of the kidney increases. Osmolality is known to contribute to the regulation of collecting duct water (aquaporin-2; AQP2) and urea transporter (UT-A1, UT-A3) regulation. AQP1KO mice are a concentrating mechanism knockout, a defect attributed to the loss of high interstitial osmolality. A V2R-specific agonist, deamino-8-d-arginine vasopressin (dDAVP), was infused into wild-type and AQP1KO mice for 7 days. UT-A1 mRNA and protein abundance were significantly increased in the medullas of wild-type and AQP1KO mice following dDAVP infusion. The mRNA and protein abundance of UT-A3, the basolateral urea transporter, was significantly increased by dDAVP in both wild-type and AQP1KO mice. Semiquantitative immunoblots revealed that dDAVP infusion induced a significant increase in the medullary expression of the endoplasmic reticulum (ER) chaperone GRP78. Immunofluorescence studies demonstrated that GRP78 expression colocalized with AQP2 in principal cells of the papillary tip of the renal medulla. Using immunohistochemistry and immunogold electron microscopy, we demonstrate that vasopressin induced a marked apical targeting of GRP78 in medullary principal cells. Urea-sensitive genes, GADD153 and ATF4 (components of the ER stress pathway), were significantly increased in AQP1KO mice by dDAVP infusion. These findings strongly support an important role of vasopressin in the activation of an ER stress response in renal collecting duct cells, in addition to its role in activating an increase in UT-A1 and UT-A3 abundance.
Keywords: urea transporter
in mammalian kidneys, an osmotic gradient exists along the corticomedullary axis with the highest osmolality reached in the papillary tip of the renal inner medulla. Sodium chloride and urea are the major solutes of the interstitial fluid which contribute to the hyperosmolality in the inner medulla. During diuresis and antidiuresis, inner medullary cells are exposed to varying osmolalities; during antidiuresis, local osmolality in the medulla can double to reach 4,000 mosmol/kgH2O in mice, and be reduced to approximately blood osmolality (290 mosmol/kgH2O) with use of diuretics.
During antidiuresis, circulating vasopressin is increased. In the renal collecting duct, vasopressin increases the water permeability of the apical and basolateral membrane by increasing the expression of aquaporins (AQP2, -3, and -4). Vasopressin also increases the urea permeability of the collecting duct by regulating apical membrane accumulation and activity of the medullary urea transporter UT-A1 (3, 16, 18). UT-A3 is also present in the medullary collecting ducts and is located on the basolateral membrane. In vitro studies have demonstrated that vasopressin increases the membrane accumulation and activity (urea flux) of UT-A3 (32). An increase in circulating vasopressin leads to an elevation of the urea concentration in the papillary tip, contributing to the efficiency of the urinary concentrating mechanism (10, 26).
AQP1 knockout mice (AQP1KO) have a defect in the urinary concentrating mechanism, which has been attributed to the loss of AQP1 protein in the descending thin limb of Henle's loop (20). Initial studies suggested that an increase in vasopressin did not increase the urine osmolality of AQP1KO mice; neither a 36-h water restriction protocol nor short-term deamino-8-d-arginine vasopressin [dDAVP; a vasopressin receptor type 2 (V2R)-specific agonist] was successful in increasing AQP1KO urine osmolality (20). However, we recently demonstrated that long-term infusion of dDAVP (7 days) significantly increased the urine osmolality of AQP1KO mice by ∼300 mosmol/kgH2O (6). Yet, this increase in urine osmolality occurred without a vasopressin-mediated increase in the medullary expression of AQP2 (6). Vasopressin regulates both water and urea permeability of the medullary collecting ducts by altering the membrane expression of UT-A1/A3 and AQP2. Both UT-A1 and AQP2 expression are also under the control of the osmotic response element (ORE). However, despite the defect in the urinary concentrating mechanism, only basal UT-A1 expression was decreased in the medulla of AQP1KO mice; AQP2 expression was unaffected (22). As the addition of urea to the interstitial fluid is a key factor in raising inner medullary osmolality (27), this study will determine whether vasopressin increased the expression of UT-A1 and UT-A3 in the medulla of AQP1KO mice.
Urea is also known to alter an array of stress genes in renal medullary cells. Studies in vitro have demonstrated that hyperosmolality can directly activate heat shock proteins and transcription factors (23, 36). Our previous gene array studies identified the endoplasmic reticulum (ER) stress protein GRP78 as significantly increased in the renal medulla following vasopressin-receptor activation (5, 6). ER stress responses can be stimulated by changes in the external environment of a cell, including, potentially, the changes in interstitial osmolality experienced by cells in the renal medulla. An increase in circulating vasopressin precedes the increase in local osmolality during antidiuresis; therefore, we examined the expression of genes involved in the ER stress pathway in response to dDAVP infusion. We used confocal microscopy to localize the increase in GRP78 to AQP2-expressing principal cells of the mouse renal medulla and immunogold labeling to demonstrate that GRP78 is localized to the apical membrane of medullary collecting ducts following dDAVP infusion.
MATERIALS AND METHODS
Experimental animals.
AQP1KO breeder mice on a CD1 background were kindly supplied by Dr. Alan Verkman (University of California, San Francisco, CA). The mice were bred and maintained in the animal facility of the University of Arizona Health Sciences Center under National Institutes of Health guidelines. Genotypes designated (+) for the wild-type allele and (−) for the targeted allele were determined by PCR analysis of genomic DNA isolated from tail biopsies. The mice used in present study were 8-wk-old male mice and received regular food and water ad libitum. Urine osmolality was measured in both wild-type and AQP1KO mice as previously described (6). All protocols were approved by the Institutional Animal Care and Use Committee at the University of Arizona.
Minipump implantation.
Osmotic minipumps (no. 1007D, Durect, Cupertino, CA) were loaded with dDAVP and subcutaneously implanted into mice under anesthesia with isoflurane. After surgery, mice had free access to food and water. The infusion rate of dDAVP was 5 ng/h for 7 days.
RNA isolation and real-time quantitative PCR.
RNA was isolated from mouse inner medullas using an RNeasy Mini Kit (no. 74104, Qiagen, Valencia, CA) according to the manufacturer's protocol for isolation from tissue, including a genomic DNase digestion step. The RNA was quantified using a spectrophotometer. Real-time quantitative PCR was carried out using the RotorGene RG3000 sequence detection system (Corbett Research, Phoenix, AZ) and a Quantitect Sybr Green PCR Kit (no. 600581, Stratagene, La Jolla, CA) as previously described (21). Primers were designed using Primer3 software, and are listed in Table 1 along with the gene accession numbers for the target genes. Two micrograms of total RNA were reverse transcribed into cDNA. The cDNA was diluted to 8 ng/μl and the PCR mixture contained 5 μl of Sybr master mix, 1.0 μl of RNAse-free water, 2.5 pmol of forward and reverse primers, and 16 ng of cDNA in a volume of 10 μl. Each reaction was performed in triplicate at 95°C, 15 s and 60°C, 30 s for 40 cycles. This was followed by a melt cycle that consisted of a stepwise increase in temperature from 60 to 99°C.
Table 1.
Listed primer pairs were used in real-time PCR
| Gene Name | Accession No. | Primer Sequence | Size of Amplicon, bp |
|---|---|---|---|
| ATF4 | NM_009716 | F: 5′-TCCTGAACAGCGAAGTGTTG-3′ | 90 |
| R: 5′-TTGGCCACCTCCAGATAGTC-3′ | |||
| GADD153 | NM_007837 | F: 5′-CTGGAAGCCTGGTATGAGGAT-3′ | 121 |
| R: 5′-CAGGGTCAAGAGTAGTGAAGGT-3′ | |||
| UT-A1 | AF366052 | F: 5′-GACAGTGAGACGCAGTGAAG-3′ | 193 |
| R: 5′-ACGGTCTCAGAGCTCTCTTC-3′ | |||
| UT-A3 | NM_030683 | F: 5′-AGCAGAAATGCTCTCCTTGC-3′ | 121 |
| R: 5′-CCTCAGGGTTAGGGAGGAAC-3′ | |||
| Dynactin | NM_027151 | F: 5′-GGCTCTTATTGGATGAGGT-3′ | 166 |
| R: 5′-ACTCCACGGTACAACAG-3′ |
ATF4, activating transcription factor 4; GADD153, growth arrest and DNA damage-inducible gene; UT-A1, urea transporter A1; UT-A3, urea transporter A3; F, forward primer; R, reverse primer.
A single predominant peak was observed in the dissociation curve of each gene, supporting the specificity of the PCR product. Ct numbers (threshold values) were set within the exponential phase of the PCR and were used to calculate the expression levels of the genes of interest. Ct values for genes of interest were normalized to endogenous cellular dynactin mRNA measured in parallel samples using dynactin-specific primers.
Protein sample preparation, SDS-PAGE electrophoresis, and Western blotting.
Kidneys were dissected into inner medullas and cortexes, homogenized in ice-cold isolation solution (250 mM sucrose, 10 mM triethanolamine, pH 7.6, containing 1 mg/ml leupeptin, 0.1 mg/ml phenylmethylsulfonyl fluoride) using a tissue homogenizer (Omni 1000 fitted with a microsawtooth generator) at maximum speed at three 15-s intervals. Total protein concentrations were measured using a BCA kit (no. 23227, Pierce, Rockford, IL), and the samples were solubilized in Laemmli sample buffer at 60°C for 15 min. Semiquantitative immunoblotting was carried out, as described previously (4), to assess the relative abundances of the proteins of interest between samples from control and dDAVP-infused mice (in both AQP1KO and wild-type mice). To confirm that protein loading of gels was equal, before immunoblotting was performed 12% polyacrylamide gels were stained with Coomassie blue. Densitometry (BioImaging System, UVP, Upland, CA) was performed on several selected bands to ensure equal loading (<5% variation relative to the mean). Proteins were then separated on SDS-PAGE gels and transferred to polyvinylidene difluoride membrane (no. IPVH00010, Millipore, Billerica, MA). In wild-type mice, 5 μg/lane was sufficient to detect signals with UT-A1 and UT-A3 antibodies. In AQP1KO mice, 15 μg/lane was needed to detect a signal for UT-A1 and UT-A3. For GRP78, 15 μg of total protein was run in wild-type mice and 5 μg of total protein in AQP1KO mice. The membranes were blocked for 1 h at room temperature in 5% nonfat dry milk and then incubated overnight at 4°C with the GRP78 antibody (no. SPA-826, Stressgen Bioreagents, Victoria, BC), or with UT-A1 and UT-A3 antibodies (gifts of Dr. M. A. Knepper, National Institutes of Health), followed by incubation of horseradish peroxidase-conjugated goat anti-rabbit IgG (no. 7074, Cell Signaling Technology) for 1 h at room temperature (1:2,000 dilution). Horseradish peroxidase was visualized using the ECL plus Western Blotting Detection System (no. RPN2132, Amersham Biosciences, Piscataway, NJ). Band densities were determined by BioImaging System (UVP).
Immunohistochemistry.
The kidneys of 7-day dDAVP-infused AQP1KO mice were fixed in 4% paraformaldehyde overnight at 4°C. Four-micrometer sections prepared by the Pathology Laboratory at the University of Arizona were deparaffinized in xylene and rehydrated in graded ethanol. Endogenous peroxidase activity was quenched in absolute methanol plus 0.3% (vol/vol) hydrogen peroxide for 30 min. Antigen was retrieved by heating the sections in citrate buffer (pH 6.0) in a microwave oven for 10 min. Nonspecific binding was blocked in 2% BSA. Sections were then incubated overnight at 4°C with rabbit anti-GRP78, followed by biotin-conjugated goat anti-rabbit IgG (1:200 dilution, no. 81-6140, Zymed Laboratories, South San Francisco, CA) for 30 min at 37°C and horseradish peroxidase-streptavidin conjugate (1:200 dilution, no. 43-4323, Zymed Laboratories) for 30 min at 37°C. Labeling was visualized with chromogen diaminobenzidine (no. 00-2014, Zymed Laboratories). Finally, the slides were counterstained with hematoxylin. For immunofluorescent staining, the protocol used was the same as that for immunohistochemistry except for the secondary antibodies, which were FITC-conjugated donkey anti-rabbit (1:200 dilution, no. 711-095-12, Jackson ImmunoResearch, West Grove, PA) and Texas Red conjugated donkey anti-goat (1:200 dilution, no. 705-075-147, Jackson ImmunoResearch,). The slides were mounted with antifade medium (Vectashield, no. H-1000, Vector Laboratories, Burlingame, CA).
Immunogold labeling.
At death, kidneys were perfused with PBS, and renal medullas were dissected out and fixed in 4% paraformaldehyde overnight at 4°C. Samples were processed by the Research Microscopy Core Facility in the Department of Anatomy at the University of Arizona. For labeling, sections were incubated with 2% fetal calf serum with 10 mM glycine in PBS at room temperature for 1 h to block nonspecific staining. Samples were incubated with rabbit anti-GRP78 overnight at 4°C, followed by 6 nm gold-conjugated anti-rabbit secondary antibody (1:100 dilution, Electron Microscopy Sciences, Hartfield, PA) for 1 h at room temperature. Finally, samples were stained in 2% aqueous uranyl acetate for 5 min at room temperature. Samples were analyzed and photographed under an electron microscope (FEI, Hillsboro, OR).
Statistical analysis.
Statistical analysis was performed using Student's t-test. To facilitate comparisons, the data for the Western blot analysis were normalized such that the mean for the control group was defined as 100%. Results are presented as means ± SE (n = number of individual animals). P < 0.05 was considered statistically significant.
RESULTS
dDAVP increases expression of UT-A1 and UT-A3 in the renal inner medulla of wild-type and AQP1KO mice.
Our previous study demonstrated that dDAVP infusion did not increase the abundance of AQP2 protein or mRNA in the medulla of AQP1KO mice (6). In that same study, dDAVP infusion for 7 days significantly increased the urine osmolality of AQP1KO mice from 581 ± 26 to 893 ± 62 mosmol/kgH2O (6). These data suggest that the renal concentrating mechanism is not entirely lost in the AQP1KO mice; however, vasopressin-mediated regulation of AQP2 did not occur. In this study, dDAVP infusion for 7 days significantly increased urine osmolality in wild-type mice (3,165 ± 279 mosmol/kgH2O in dDAVP-infused wild-type mice compared with 2,173 ± 160 mosmol/kgH2O in control mice, P < 0.05). In AQP1KO mice, there was a small but significant increase in urine osmolality (832 ± 28 mosmol/kgH2O in dDAVP-infused AQP1KO mice compared with 606 ± 8 mosmol/kgH2O in control AQP1KO mice, P < 0.05, n = 3).
Here, we investigated whether the abundance of the apical urea transporter UT-A1 was increased by vasopressin receptor activation (via dDAVP) in the medulla of wild-type and AQP1KO mice. Despite numerous studies on the regulation of urea transporter abundance in rat models, no previous studies have reported on whether vasopressin regulates urea transporter protein abundance in mice.
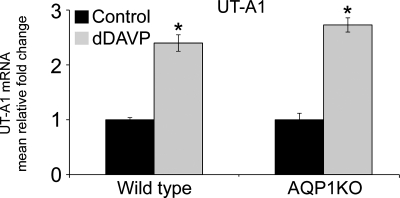
As shown in Fig. 1, dDAVP infusion significantly increased the mRNA abundance of UT-A1 in the renal medulla of wild-type mice (2.40 ± 0.15-fold in dDAVP-infused compared with 1.00 ± 0.04-fold in control mice). Also shown in Fig. 1, dDAVP infusion significantly increased the mRNA abundance of UT-A1 in the renal medulla of AQP1KO mice (2.73 ± 0.13-fold in dDAVP-infused compared with 1.00 ± 0.12-fold in control AQP1KO mice).
Fig. 1.
dDAVP infusion increases mRNA expression of UT-A1 in renal medullas of wild-type and AQP1KO mice. SYBR Green I real-time PCR assay validation for UT-A1 gene was performed. The data shown are the mean relative fold-changes in the renal medullas of control and dDAVP-infused wild-type (WT) mice and control and dDAVP-infused AQP1KO mice. Assays for dynactin were run in parallel on each sample for subsequent normalization of the data. *Significant difference between control and dDAVP-infused mice (t-test; P < 0.05); n = 4.
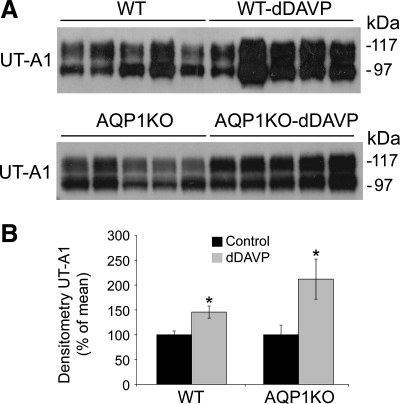
To confirm that these increases in mRNA abundance translated to increases in protein expression, Western blot analyses were performed. Data in Fig. 2 demonstrate that dDAVP infusion significantly increased the protein expression of UT-A1 in the renal medulla of wild-type mice (256 ± 65.8% in dDAVP-infused compared with 100 ± 22.2% in control wild-type mice). Similarly, dDAVP infusion significantly increased the protein expression of UT-A1 in the renal medulla of AQP1KO mice (212 ± 40.4% in dDAVP-infused compared with 100 ± 19.5% in control AQP1KO mice).
Fig. 2.
dDAVP infusion increases the protein expression of UT-A1 in the renal medullas of wild-type and AQP1KO mice. Protein abundance was analyzed by Western blotting. A: representative blots. Each lane represents a homogenate from an individual mouse. B: densitometric analysis of results in A. Values were normalized to control (100%) to facilitate comparison. *Significant difference between control and dDAVP-infused mice (t-test, P < 0.05); n = 5.
Basolateral urea transporter UT-A3 is exclusively expressed in the terminal part of inner medullary collecting ducts (33). Recent in vitro studies have identified a role for vasopressin in the regulation of UT-A3 transcription, and UT-A3-driven urea flux increased with either forskolin or vasopressin treatment in a heterologous system (31, 32).
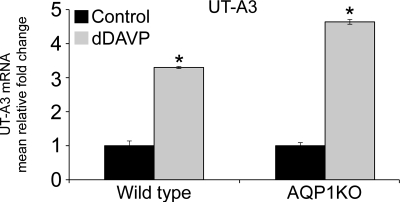
As shown in Fig. 3, 7 days of dDAVP infusion significantly increased the mRNA abundance of UT-A3 in the renal medulla of wild-type mice (3.30 ± 0.03-fold in dDAVP-infused compared with 1.00 ± 0.14-fold in control mice). Also shown in Fig. 3, dDAVP infusion significantly increased the mRNA abundance of UT-A3 in the renal medulla of AQP1KO mice (4.64 ± 0.07-fold in dDAVP-infused compared with 1.00 ± 0.09-fold in control AQP1KO mice).
Fig. 3.
dDAVP infusion increases mRNA expression of UT-A3 in renal medullas of wild-type and AQP1KO mice. SYBR Green I real-time PCR assay validation for UT-A3 gene was performed. The data shown are the mean relative fold-change in the renal medullas of control and dDAVP-infused wild-type (WT) mice and control and dDAVP-infused AQP1KO mice. Assays for dynactin were run in parallel on each sample for subsequent normalization of the data. *Significant difference between control and dDAVP-infused mice (t-test; P < 0.05); n = 4.
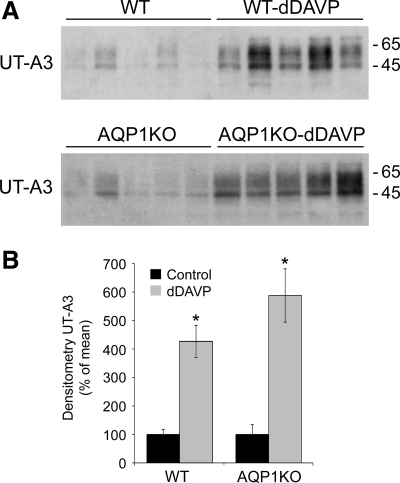
Increases in mRNA abundance were confirmed at the protein level by Western blot analyses. Shown in Fig. 4A, dDAVP infusion significantly increased the protein expression of UT-A3 in the renal medulla of wild-type mice (427 ± 57% in dDAVP-infused compared with 100 ± 17% in wild-type mice) and in the renal medulla of AQP1KO mice (588 ± 93.7% in dDAVP-infused compared with 100 ± 34.4% in control AQP1KO mice).
Fig. 4.
dDAVP infusion increases the protein expression of UT-A3 in the renal medullas of wild-type and AQP1KO mice. Protein abundance was analyzed by Western blotting. A: representative blots. Each lane represents a homogenate from an individual mouse. B: densitometric analysis of results in A. Values were normalized to control (100%) to facilitate comparison. *Significant difference between control and dDAVP-infused mice (t-test, P < 0.05); n = 5.
dDAVP increases expression of ER stress protein GRP78 in principal cells of the mouse renal medulla.
Our previously published gene array studies identified GRP78 as a gene that was upregulated in the renal medullas of AQP1KO mice (6) following long-term dDAVP infusion (7 days) and in wild-type mice following a water-restriction protocol (5).
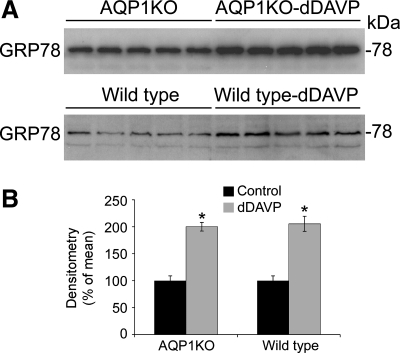
To correlate our previously published changes in mRNA expression with changes in protein expression, quantitative immunoblotting was performed in renal medullas from control and dDAVP-infused wild-type and AQP1KO mice. As demonstrated in Fig. 5, GRP78 protein expression was significantly increased in the renal medulla of AQP1KO mice following 7 days of dDAVP infusion (200 ± 8% in dDAVP-infused compared with 100 ± 9% in control AQP1KO mice, P < 0.05). This response was also seen in wild-type mice following 7 days of dDAVP infusion (205 ± 13.5% in dDAVP-infused compared with 100 ± 9% in control wild-type mice, P < 0.05).
Fig. 5.
dDAVP infusion increases the protein expression of GRP78 in the renal medullas of wild-type and AQP1KO mice. Protein abundance was analyzed by Western blotting. A: representative blots. Each lane represents a homogenate from an individual mouse. B: densitometric analysis of results in A. Values were normalized to control (100%) to facilitate comparison. *Significant difference between control and dDAVP-infused mice (t-test, P < 0.05); n = 5.
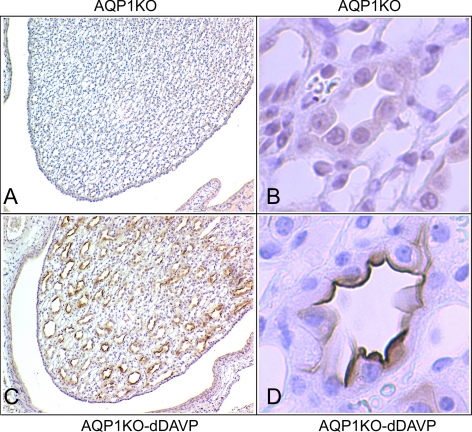
Immunohistochemistry data presented in Fig. 6 demonstrate that the increase in GRP78 protein in vasopressin-infused mice was localized to collecting ducts of the renal medulla. There was no obvious staining in medullary cells from control mice that were not infused with dDAVP (Fig. 6, A and B), but a dramatic increase in GRP78 immunostaining was seen in collecting duct tubules of the inner medulla following dDAVP infusion (Fig. 6, C and D); this expression was localized to the papillary tip of the renal medulla; there was no staining for GRP78 in the initial part of the inner medullary collecting ducts.
Fig. 6.
Localization of dDAVP-induced GRP78 expression to the apical membrane of collecting ducts in the papillary tip of AQP1KO mice. GRP78 immunoperoxidase labeling was performed in paraffin-embedded mouse kidney sections from AQP1KO and dDAVP-infused AQP1KO mice. A and C: renal medullas of AQP1KO and dDAVP-infused AQP1KO mice; dDAVP-induced GRP78 expression is localized to the papilla of the renal medulla in dDAVP-infused AQP1KO mice (magnification ×40). B and D: immunoperoxidase labeling indicates that GRP78 expression is localized to the apical membrane of collecting duct cells in dDAVP-infused AQP1KO mice (magnification ×400).
GRP78 colocalizes with AQP2 to the apical membrane of inner medullary collecting ducts following dDAVP infusion.
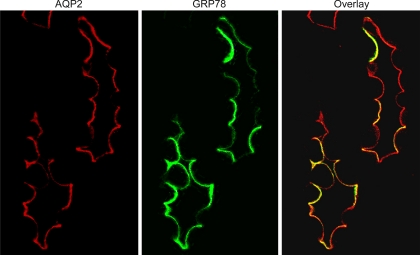
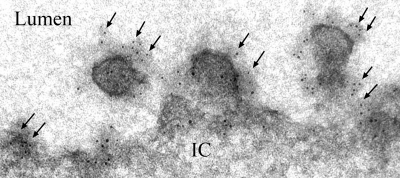
AQP2 is a marker for collecting duct principal cells. When circulating vasopressin is high, AQP2 is trafficked to the membrane of collecting duct cells where it can be visualized in the apical membrane by immunofluorescence microscopy. Using dual-labeling techniques for AQP2 and GRP78, we demonstrated that the increase in GRP78 expression in the renal medulla was localized to AQP2-positive cells, i.e., principal cells of the medullary collecting ducts. In addition, similar to the expression pattern for AQP2 following dDAVP infusion, GRP78 expression was localized to the apical membrane of collecting duct principal cells; this is shown in Fig. 7. There was no GRP78 expression on the apical membrane of collecting duct cells from control AQP1KO mice that had not been infused with dDAVP infusion. Traditionally thought of as an ER protein, localization of GRP78 to the apical membrane of medullary principal cells, following dDAVP, was surprising. To confirm that the location of GRP78 was truly apical, immunogold labeling was performed on medullary sections from dDAVP-infused AQP1KO mice. Electron micrograph data, shown in Fig. 8, clearly demonstrate that GRP78 is localized to the apical membrane of medullary collecting ducts following dDAVP infusion.
Fig. 7.
Immunolocalization of GRP78 to apical membrane of principal cells of renal collecting ducts. Confocal image of immunofluorescently labeled aquaporin-2 (AQP2; red; A) and GRP78 (green; B) localized to the apical membrane of collecting duct principal cells in the mouse medulla of AQP1KO mice. C: merged image (magnification ×600).
Fig. 8.
Immunogold electron microscopy of GRP78 in principal cells of dDAVP-infused AQP1KO mice. Immunogold labeling of GRP78 is prominently observed in the apical membrane of collecting duct principal cells of dDAVP-infused AQP1KO mice (gold particles, 10 nM, arrows). IC, intracellular. Lumen, lumen of tubule (magnification ×53,000).
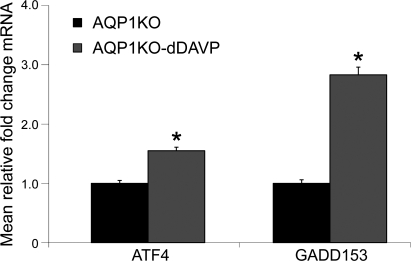
dDAVP upregulates mRNA expression of ATF4 and GADD153 in the renal medulla of AQP1K0 mice.
Data shown in Fig. 9 demonstrate that dDAVP infusion significantly increased the mRNA abundance of growth arrest and DNA damage-inducible gene 153 (GADD153) and activating transcription factor 4 (ATF4) in the medulla of AQP1KO mice (ATF4: 1.55 ± 0.06 in dDAVP-infused compared with 1.00 ± 0.05 in control AQP1KO mice; GADD153: 2.83 ± 0.13 in dDAVP-infused AQP1KO mice compared with 1.00 ± 0.06 in control AQP1KO mice, P < 0.05). These data confirm that several components of an ER stress response are upregulated by vasopressin in the renal medulla.
Fig. 9.
dDAVP infusion increases mRNA expression of GADD153 and ATF4 in renal medullas of AQP1KO mice. SYBR Green I real-time PCR assay validation was performed. The data shown are the mean relative fold-changes in the renal medullas of control and dDAVP-infused AQP1KO mice. Assays for dynactin were run in parallel on each sample for subsequent normalization of the data. *Significant difference between AQP1KO mice and dDAVP-infused AQP1KO mice (t-test; P < 0.05); n = 4.
DISCUSSION
In the present study, we demonstrated that 7 days of dDAVP infusion increases the expression of urea transporters UT-A1 and UT-A3 in the medullary collecting duct of both wild-type and AQP1KO mice. In addition, dDAVP infusion increases the expression of ER stress-associated proteins GRP78, ATF4, and GADD153. AQP1KO mice lack a urinary concentrating mechanism (there was little increase in interstitial osmolality in the inner medulla of AQP1KO mice following dDAVP stimulation); thus our data suggest that vasopressin may directly increase the expression of urea transporters, in concert with ER stress-associated proteins, in the mouse renal medulla.
Urea transporters and vasopressin signaling.
Our study demonstrates that long-term infusion of V2R agonist dDAVP increased the mRNA and protein abundance of the basolateral urea transporter UT-A3 and the apical urea transporter UT-A1 in the medullary collecting ducts of both wild-type and AQP1KO mice. These data demonstrate that in vivo, vasopressin upregulates UT-A1 and UT-A3 expression in the mouse renal collecting duct. In isolated rat inner medullary collecting ducts, short-term vasopressin rapidly increases the membrane accumulation and activity of UT-A1 (18). Vasopressin has been shown to increase UT-A3-driven urea flux via an increased accumulation of the transporter in the basolateral membrane of transfected cell lines (32). However, several studies demonstrated that long-term (3–7 days) vasopressin infusion fails to increase the mRNA and protein abundance of UT-A1 in the rat renal medulla. In fact, dDAVP infusion in Brattleboro rats decreased UT-A1 protein and mRNA abundance. Similarly, a water-restriction protocol also decreased UT-A1 mRNA abundance (29). However, when Sprague-Dawley rats were water restricted, there was a small, but not significant, increase in UT-A1 mRNA abundance (1). These data had therefore suggested that vasopressin did not directly regulate the transcription of UT-A1 in the rat renal medulla. However, no studies to date have demonstrated that vasopressin can increase the protein abundance of UT-A1 or UT-A3 in mice in vivo. Our data demonstrate a clear difference between what has been previously shown in rats and what is true in mice.
Osmolality is known to play a role in the regulation of UT-A1 transcription and protein synthesis in rats and mice. In vitro studies have demonstrated that the tonicity-responsive enhancer/osmotic response element (TonE/ORE) is present in the promoter of UT-A1 and UT-A3; UT-A promoter activity was increased when conditions were made hypertonic (25). However, in a mouse model where TonE binding protein (TonEBP) function was disrupted, the basal expression of UT-A1 in the renal medulla was significantly decreased. Yet, when these mice were water restricted (to increase endogenous vasopressin levels), the mRNA expression of UT-A1 increased (19), indicating that an increase in tonicity is not necessary for the vasopressin-mediated upregulation of UT-A1 in vivo. Consistent with this, we demonstrated that in AQP1KO mice, which also have a urinary concentrating defect, dDAVP significantly increased UT-A1 and UT-A3 expression, confirming that an increase in osmolality is not necessary for vasopressin-mediated upregulation of collecting duct urea transporters. Thus, in contrast to data from the rat renal medulla, our data suggest a potential role for cAMP in the transcriptional regulation of UT-A1 and UT-A3 in the mouse renal medulla. This correlates with in vitro studies where cAMP significantly increased the activity of the mouse UT-A promoter (9) but did not increase the activity of the rat UT-A promoter (24).
However, further studies are needed to identify the signaling mechanism by which vasopressin increases the abundance of UT-A1 and UT-A3 in the mouse renal medulla. Vasopressin is known to phosphorylate UT-A1, which leads to an increased accumulation in the apical membrane (18), and phosphorylation of UT-A1 relates to the activity of the transporter. However, little is known about how vasopressin may regulate transcription and translation of these transporters, and our study did not look at whether UT-A1 or UT-A3 was phosphorylated after 7 days of dDAVP infusion.
ER stress proteins and vasopressin signaling.
Cells in the renal medulla are unique as they face and tolerate large changes in local osmolality during diuresis and antidiuresis, due in part to the activity of UT-A1 and UT-A3. Both UT-A1 and UT-A3 are highly expressed in the papillary tip of the medulla, and an increase in activity of both transporters increases the urea concentration in the interstitial fluid of the papillary tip (33). This was clearly demonstrated in the UT-A1/3 knockout mouse, where urea accumulation in the inner medulla was markedly reduced (11). ER stress responses can be stimulated by changes in the external environment of a cell, including, potentially, the changes in interstitial osmolality experienced by cells in the renal medulla. An increase in circulating vasopressin precedes the increase in local osmolality during antidiuresis; therefore, we examined the expression of genes involved in the ER stress pathway in response to dDAVP infusion.
We identified that vasopressin receptor activation (via dDAVP) increased the expression of GRP78, an ER chaperone protein, in the renal medulla of both wild-type and AQP1KO mice. GRP78 is an ER chaperone protein, whose expression increases in response to a disturbance to the ER environment, such as a change in calcium homeostasis, redox status, or the accumulation of unfolded proteins. An increase in GRP78 expression has been implicated in the ability of renal cells to survive challenges such as toxic chemicals (15) and oxidative stress (13). Previous proteomic studies in the Brattleboro rat medulla identified GRP78 as a protein whose abundance was increased following the infusion of vasopressin (35). However, in a rat model of vasopressin escape, where urinary osmolality is low despite high vasopressin levels, GRP78 expression was decreased in the renal medulla (12). AQP1KO mice also have a low urinary osmolality, due to a renal concentrating defect. However, dDAVP increased GRP78 expression in these mice, suggesting that vasopressin can increase the abundance of GRP78 in renal collecting duct cells without large changes in medullary osmolality.
We also demonstrate that expression of ATF4, GADD153, and ATF3 (6), all components of the ER stress pathway, are increased in the renal medulla of AQP1KO mice following dDAVP infusion. ATF3 and GADD153 are known to be induced by urea in a collecting duct cell line; an increase in osmolality with urea (300 mosmol/kgH2O) increased the mRNA and protein expression of ATF3 and GADD153 in mIMCD3 cells (34, 36). In contrast, an increase in tonicity via sodium (300 mosmol/kgH2O) did not activate these genes. The activation of these ER stress pathway genes in the medulla of AQP1KO mice is intriguing in light of the vasopressin-mediated increase in UT-A1 and UT-A3 expression and the small increase in urinary osmolality. Our future studies will determine whether vasopressin-mediated urea accumulation contributes to this ER stress response in the renal collecting duct.
It is possible that sustained stimulation of V2R could cause disturbances in collecting duct cell calcium homeostasis sufficient to trigger ER stress. Collecting duct cells express the V2R. Activation of the V2R is known to cause transient increases in intracellular calcium, mediated through calcium release from ryanodine-sensitive stores (2, 7). Further in vitro studies are needed to confirm or negate the possibility that a V2R-mediated calcium release activates an ER stress pathway in collecting duct cells.
Surprisingly, not only did dDAVP increase GRP78 protein abundance, but following dDAVP stimulation, GRP78 protein was located at the apical membrane of medullary collecting duct principal cells. The pattern of expression was similar to the pattern of vasopressin-mediated AQP2 expression. GRP78 is widely known as an ER-resident chaperone protein. However, the first report of GRP78 appearing in a cell surface localization was documented in tumor cells following stimulation with thapsigargin, a well-known ER stress inducer (8). More recent studies have demonstrated that several heat shock proteins, including GRP78, are highly abundant on the cell surface of different cell types (14, 30, 37). The function of cell surface GRP78 localization in the collecting duct is currently unknown, but a functional role clearly exists for GRP78 beyond an ER chaperone. In tumor cells, the cell surface expression of GRP78 is associated with an inhibition of transforming growth factor-β signaling (28); in proximal tubule cells it may play a role in the trafficking of Na-K-ATPAse to the basolateral membrane (17). Further in vitro studies are needed in collecting duct cell lines to determine the signaling mechanism behind the observed vasopressin-mediated increase in GRP78 expression and to identify the functional relevance of GRP78 cell surface expression.
In summary, the present study demonstrates that dDAVP increases the mRNA and protein expression of UT-A1 and UT-A3 in renal medulla of wild-type and AQP1KO mice. The AQP1KO mouse is a renal concentrating mechanism knockout; thus our data suggest that increases in osmolality are not involved in the vasopressin-mediated upregulation of either UT-A1 or UT-A3 abundance in the mouse renal medulla. We also confirm previous observations that vasopressin induces an ER stress-like response in the collecting ducts of the renal medulla. During conditions of antidiuresis, when vasopressin is high, the upregulation of GRP78 expression may play a physiological role in collecting duct cell homeostasis.
GRANTS
This work was funded by National Institutes of Health Grant RO1-DK073611 (H. L. Brooks), Southwest Environmental Health Sciences Center Grant ES006694, and by the American Physiological Society's Postdoctoral Fellowship in Physiological Genomics (QC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Bagnasco SM, Peng T, Nakayama Y, Sands JM. Differential expression of individual UT-A urea transporter isoforms in rat kidney. J Am Soc Nephrol 11: 1980–1986, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanian L, Sham JS, Yip KP. Calcium signaling in vasopressin-induced aquaporin-2 trafficking. Pflügers Arch 456: 747–754, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Blount MA, Mistry AC, Frohlich O, Price SR, Chen G, Sands JM, Klein JD. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol 295: F295–F299, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks HL, Sorensen AM, Terris J, Schultheis PJ, Lorenz JN, Shull GE, Knepper MA. Profiling of renal tubule Na+ transporter abundances in NHE3 and NCC/TSC null mice using targeted proteomics. J Physiol 530: 359–366, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Q, Keck M, McReynolds MR, Klein JD, Greer KA, Sharma K, Hoying JB, Sands JM, Brooks HL. Effects of water restriction on gene expression in mouse renal medulla: identification of 3β-HSD4 as a collecting duct protein. Am J Physiol Renal Physiol 291: F218–F224, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cai Q, McReynolds MR, Keck M, Greer KA, Hoying JB, Brooks HL. Vasopressin receptor subtype 2 activation increases cell proliferation in the renal medulla of AQP1 null mice. Am J Physiol Renal Physiol 293: F1858–F1864, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Delpino A, Piselli P, Vismara D, Vendetti S, Colizzi V. Cell surface localization of the 78 kD glucose regulated protein (GRP 78) induced by thapsigargin. Mol Membr Biol 15: 21–26, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Fenton RA, Cottingham CA, Stewart GS, Howorth A, Hewitt JA, Smith CP. Structure and characterization of the mouse UT-A gene (Slc14a2). Am J Physiol Renal Physiol 282: F630–F638, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA 101: 7469–7474, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoorn EJ, Hoffert JD, Knepper MA. Combined proteomics and pathways analysis of collecting duct reveals a protein regulatory network activated in vasopressin escape. J Am Soc Nephrol 16: 2852–2863, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung CC, Ichimura T, Stevens JL, Bonventre JV. Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem 278: 29317–29326, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Jang JH, Hanash S. Profiling of the cell surface proteome. Proteomics 3: 1947–1954, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Jia Z, Person MD, Dong J, Shen J, Hensley SC, Stevens JL, Monks TJ, Lau SS. Grp78 is essential for 11-deoxy-16,16-dimethyl PGE2-mediated cytoprotection in renal epithelial cells. Am J Physiol Renal Physiol 287: F1113–F1122, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kato A, Naruse M, Knepper MA, Sands JM. Long-term regulation of inner medullary collecting duct urea transport in rat. J Am Soc Nephrol 9: 737–745, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Kesiry R, Liu J. GRP78/BIP is involved in ouabain-induced endocytosis of the Na/K-ATPase in LLC-PK1 cells. Front Biosci 10: 2045–2055, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Klein JD, Frohlich O, Blount MA, Martin CF, Smith TD, Sands JM. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J Am Soc Nephrol 17: 2680–2686, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lam AK, Ko BC, Tam S, Morris R, Yang JY, Chung SK, Chung SS. Osmotic response element-binding protein (OREBP) is an essential regulator of the urine concentrating mechanism. J Biol Chem 279: 48048–48054, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem 273: 4296–4299, 1998 [DOI] [PubMed] [Google Scholar]
- 21.McReynolds MR, Taylor-Garcia KM, Greer KA, Hoying JB, Brooks HL. Renal medullary gene expression in aquaporin-1 null mice. Am J Physiol Renal Physiol 288: F315–F321, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Morris RG, Uchida S, Brooks HL, Knepper MA, Chou CL. Altered expression profile of transporters in the inner medullary collecting duct of aquaporin-1 knockout mice. Am J Physiol Renal Physiol 289: F194–F199, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Nahm O, Woo SK, Handler JS, Kwon HM. Involvement of multiple kinase pathways in stimulation of gene transcription by hypertonicity. Am J Physiol Cell Physiol 282: C49–C58, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Nakayama Y, Naruse M, Karakashian A, Peng T, Sands JM, Bagnasco SM. Cloning of the rat Slc14a2 gene and genomic organization of the UT-A urea transporter. Biochim Biophys Acta 1518: 19–26, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Nakayama Y, Peng T, Sands JM, Bagnasco SM. The TonE/TonEBP pathway mediates tonicity-responsive regulation of UT-A urea transporter expression. J Biol Chem 275: 38275–38280, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Sands JM. Critical role of urea in the urine-concentrating mechanism. J Am Soc Nephrol 18: 670–671, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Nielsen B, Graves B, Roth J. Water removal and solute additions determining increases in renal medullary osmolality. Am J Physiol Renal Fluid Electrolyte Physiol 244: F472–F482, 1983 [DOI] [PubMed] [Google Scholar]
- 28.Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol Cell Biol 28: 666–677, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shayakul C, Smith CP, Mackenzie HS, Lee WS, Brown D, Hediger MA. Long-term regulation of urea transporter expression by vasopressin in Brattleboro rats. Am J Physiol Renal Physiol 278: F620–F627, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Shin BK, Wang H, Yim AM, Le NF, Brichory F, Jang JH, Zhao R, Puravs E, Tra J, Michael CW, Misek DE, Hanash SM. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem 278: 7607–7616, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Stewart GS, King SL, Potter EA, Smith CP. Acute regulation of mUT-A3 urea transporter expressed in a MDCK cell line. Am J Physiol Renal Physiol 292: F1157–F1163, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Stewart GS, Thistlethwaite A, Lees H, Cooper GJ, Smith C. Vasopressin regulation of the renal UT-A3 urea transporter. Am J Physiol Renal Physiol 296: F642–F648, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Terris JM, Knepper MA, Wade JB. UT-A3: localization and characterization of an additional urea transporter isoform in the IMCD. Am J Physiol Renal Physiol 280: F325–F332, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Tian W, Cohen DM. Urea stress is more akin to EGF exposure than to hypertonic stress in renal medullary cells. Am J Physiol Renal Physiol 283: F388–F398, 2002 [DOI] [PubMed] [Google Scholar]
- 35.van Balkom BW, Hoffert JD, Chou CL, Knepper MA. Proteomic analysis of long-term vasopressin action in the inner medullary collecting duct of the Brattleboro rat. Am J Physiol Renal Physiol 286: F216–F224, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Yang XY, Cohen DM. Urea-associated oxidative stress and Gadd153/CHOP induction. Am J Physiol Renal Physiol 276: F786–F793, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Zwang NA, Hoffert JD, Pisitkun T, Moeller HB, Fenton RA, Knepper MA. Identification of phosphorylation-dependent binding partners of aquaporin-2 using protein mass spectrometry. J Proteome Res 8: 1540–1554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]