Abstract
Loss or dysfunction of podocytes is a major cause of glomerular kidney disease. Several genetic forms of glomerular disease are caused by mutations in genes that encode structural elements of the slit diaphragm or the underlying cytoskeleton of podocyte foot processes. The recent discovery that gain-of-function mutations in Ca2+-permeable canonical transient receptor potential-6 channels (TRPC6) underlie a subset of familial forms of focal segmental glomerulosclerosis (FSGS) has focused attention on the basic cellular physiology of podocytes. Several recent studies have examined the role of Ca2+ dynamics in normal podocyte function and their possible contributions to glomerular disease. This review summarizes the properties of TRPC6 and related channels, focusing on their permeation and gating properties, the nature of mutations associated with familial FSGS, and the role of TRPC channels in podocyte cell biology as well as in glomerular pathophysiology. TRPC6 interacts with several proteins in podocytes, including essential slit diaphragm proteins and mechanosensitive large-conductance Ca2+-activated K+ channels. The signaling dynamics controlling ion channel function and localization in podocytes appear to be quite complex.
Keywords: cytoskeleton, glomerular disease, renal function, FSGS
the discovery in 2005 that mutations in canonical transient receptor potential-6 (TRPC6) channels can cause familial focal segmental glomerulosclerosis (FSGS) (92, 128) brought the analysis of ion channels in podocytes to the forefront of experimental nephrology. Ion channels rarely if ever operate in isolation; in most systems, a number of different channels operate in positive and negative feedback loops to regulate various aspects of cell physiology. TRPC6 channels in podocytes interact with a number of different proteins, including scaffolding molecules, signaling proteins, cytoskeletal elements, and other types of ion channels. Here, we review literature on TRPC6 channels and their binding partners, which include mechanosensitive large-conductance Ca2+-activated K+ channels (BKCa channels), as well as a number of other proteins essential for the normal function of the glomerular slit diaphragm. We also review studies showing that TRPC6 channels in podocytes may affect the cytoskeleton, gene expression, and ultimately glomerular filtration. We begin with a brief overview of podocyte biology.
Role of Podocytes in the Regulation of Glomerular Filtration
The initial steps in renal function entail ultrafiltration of blood in the glomerulus, which functions as a sieve that is permeable to water and small solutes, but that largely excludes macromolecules on the basis of size and charge from the urinary spaces in the lumen of Bowman's capsule. The glomerular filtration barrier is composed of several elements, including the highly fenestrated capillary endothelium, the glomerular basement membrane (GBM) that covers the outer surface of the capillary, and a population of specialized cells called podocytes. In addition, mesangial cells play a role in the process of glomerular filtration by regulating the overall mechanical responses of glomeruli to changes in hydrostatic pressure (23, 30).
Podocytes are highly differentiated cells that extend primary processes that ramify and terminate in specialized structures called foot processes. The podocyte foot processes attach to the GBM in a regularly spaced interdigitating pattern such that a series of filtration slits are formed between adjacent foot processes. These slits are bridged by specialized cell junctions known as slit diaphragms (SDs), whose precise structure and location are essential for normal glomerular filtration (92, 96, 98, 124). Additional specialized contacts between podocyte foot processes and the GBM are also crucial for normal glomerular function, as changes in the pattern of these contacts and in the structure of the foot processes, the most common form of which is known as podocyte effacement, lead to altered glomerular function and proteinuria (107). Podocyte cell bodies and primary processes also contact the GBM by a series of structures called anchoring processes that are structurally similar to foot processes (20, 81) and which give rise to a distinct subpodocyte space (SPS). The SPS in turn maintains contact with the rest of the urinary space in Bowman's capsule through small pores that are few in number (82).
The discovery of glomerular SDs suggested an intuitively simple model of glomerular function in which filtration occurs through a series of increasingly refined sieves. In this model, capillary fenestrations and the GBM prevent permeation of cells and large macromolecules, thereby acting as a type of “prefilter,” whereas the SDs represent the final size-selective barrier to the permeation of macromolecules by excluding proteins with Stokes-Einstein radii comparable to that of albumin (96). In its most basic form, this model implies a static and passive role for podocytes. The underlying glomerular capillary has unusually high pressure compared with other capillary beds, and it has been proposed that podocytes generate force to dynamically counteract distension of the capillary wall that would otherwise lead to protein leakage (64–66). Podocytes were originally predicted to be contractile based on the arrangement of F-actin, myosin, and α-actinin in foot processes (16, 45, 65, 66). There is now direct evidence that podocytes contract in response to a variety of mechanical stimuli and fluid stresses in vitro (21, 24).
Recent ultrastructural observations on the SPS have required some revision to the simple picture of a three-element glomerular filtration barrier. The SPS is defined as the space formed between the GBM and the cell body and primary processes of the podocyte. It covers ∼60% of the glomerular filtration surface, and its boundaries define a highly convoluted pathway that eventually feeds, via a marked constriction called the SPS exit pore, into larger compartments that feed the primary filtrate into the tubules (81, 82, 99). In effect, the SPS adds additional layers with their own resistances and compliances to the overall process of glomerular filtration. Measurements of the dimensions of the SPS have suggested that the SPS is highly restrictive to fluid flow (82), a prediction that has been confirmed by in vivo imaging (99). The hydrodynamic properties of the SPS provide a mechanism for the podocyte to rapidly regulate the overall resistance of the glomerular filtration barrier by changing the volume, pressure, and compliance of this space and by controlling the resistance of the SPS exit pore (82). An important feature of this model is that the highly restrictive SPS could allow for transient flow reversals across the glomerular filtration barrier, which could serve to clean the filtration barrier of clogging proteins (82, 99). The SPS also provides a mechanism for the podocyte to sense and respond to changes in pressure within the urinary space, or changes in glomerular filtration rate, and to make these parameters highly sensitive to any dynamic behavior of the podocyte itself, i.e., the entire podocyte, not just the foot processes (99). By analogy to other cell types, such as neuronal dendritic spines, it is reasonable to suppose that dynamic responses in podocytes and their foot processes are mediated by cytoskeletal elements and Ca2+-dependent processes (21, 44, 78, 93). The discovery that mutations in TRPC6 channels can cause familial FSGS (94, 128) underscored the potential importance of Ca2+ dynamics for podocyte function and the dynamics of glomerular filtration and opened an entirely new line of investigation.
TRPC Channel Family
TRPC channels comprise a family of nonselective Ca2+-permeable cation channels that are widely expressed in vertebrate tissues. There is a general consensus that members of the TRPC family become active in response to signal transduction cascades associated with stimulation of phospholipase C (PLC). These types of cascades lead to hydrolysis and local membrane depletion of phosphatidylinositol-4,5-bisphosphate (PIP2), thereby liberating diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) (116). While all members of the TRPC family are thought to play a role in PLC-mediated signaling cascades, different members of the TRPC family may contribute to these cascades in different ways that are not well understood. TRPC channels are nonselective cation channels, and therefore their activation results in cell depolarization. The fact that TRPC channels are finitely permeable to Ca2+ means that their activation may be sufficient, at least under certain conditions, to modulate a host of secondary cascades, resulting in integration or amplification of cell signals.
There are seven functional members of the TRPC channel family in mice (TRPC1–7), and six in humans, in which TRPC2 is a pseudogene (119). The TRPC channels are members of a larger channel family known as TRP channels that have a broad role in chemo- and mechanosensation and in allowing cells to sense changes in their local environment (19). Based on amino acid sequence homologies, functional human TRPC channels fall into three groups: 1) TRPC1; 2) TRPC4 and TRPC5; and 3) TRPC3, TRPC6, and TRPC7. TRPC channels can form heteromers and homomers and thereby have the potential to form a very large number of unique ion channels (70).
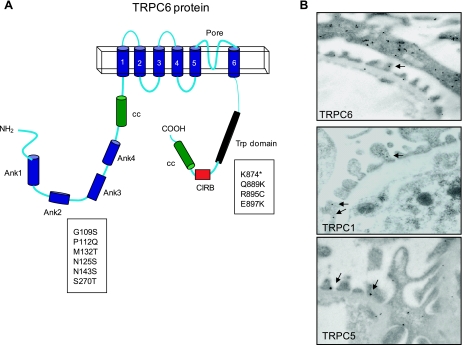
Minimally functional TRPC channels are tetrameric proteins composed of subunits that contain a membrane domain with six transmembrane segments, a pore-forming region between the membrane segments 5 and 6, and with NH2 and COOH termini facing the cytosol. This arrangement is shown schematically in Fig. 1. A variable number of ankyrin repeats, four in the case of TRPC6, are present near the NH2 terminus and are followed by an NH2-terminal coiled-coil domain. All of the TRPC channels have a highly conserved 25-residue TRP domain immediately distal to the transmembrane segments, which includes a motif known as TRP box 1, followed by a proline-rich sequence known as TRP box 2. The TRP domain is followed by a calmodulin and IP3 receptor-binding region (CIRB region) and a COOH-terminal coiled-coil domain (119). Additional calmodulin-binding domains have been identified in both the NH2 and COOH termini of some members of this family, including TRPC6 (134). Ankyrin repeats, coiled-coil domains, and proline-rich domains often play a role in protein-protein interactions, and the domain structure of TRPC channels raises the possibility of extensive associations with other proteins and cytoplasmic factors. Interactions between members of the TRPC family, including formation of homomeric complexes, are known to involve both the NH2 and COOH termini (70).
Fig. 1.
A: domain structure of transient receptor potential-6 (TRPC6) channels. Boxes indicate human mutations known to cause focal segmental glomerular sclerosis (FSGS). All of the known disease-causing mutations are located in the cytoplasmic NH2 and COOH terminals of TRPC6. B: immunogold localization of TRPC6, TRPC1, and TRPC5 to podocyte foot processes (arrows). The micrograph of TRPC6 expression also shows that it is present along a primary process.
Activation and Modulation of TRPC Channels
The mechanisms underlying activation of TRPC channels are complex and incompletely understood. Moreover, their gating properties depend on whether the channels are part of a heteromeric complex containing other TRPC members, scaffolding proteins, or even members of other ion channel families. For these reasons, studies of TRPC channels carried out in heterologous expression systems need to be interpreted with caution. Under certain conditions, TRPC1, TRPC4, and TRPC5 can become active as a result of Ca2+ mobilization from intracellular stores mediated by IP3 (129). Moreover, knockdown of TRPC4 reduces store-operated Ca2+ current in mouse mesangial cells (122). By contrast, TRPC3, TRPC6, and TRPC7 are not activated by IP3 in heterologous expression systems and instead become active after application of DAG analogs in excised inside-out patches (29, 36). On this basis. it has been suggested that TRPC6 channels are components of signaling cascades that are delimited to the plasma membrane and that the gating of these channels is independent of Ca2+ store depletion (12, 36). There is also evidence that TRPC6 channels are inhibited by PIP2 and that local depletion of this phospholipid may play a role in regulating TRPC6 gating (69).
Recent findings on TRPC-interacting proteins suggest that the gating properties of TRPC family members can vary in different cell types depending on whether certain interacting proteins are expressed. For example, depletion of intracellular calcium stores failed to activate murine TRPC4 and -5 channels expressed in HEK293 cells (100). TRPC1, TRPC2, TRPC4, and TRPC5 are capable of interacting with a protein known as stromal-interacting molecule 1 (STIM1) (129, 131) that can sense the Ca2+ content of intracellular stores (73, 97). STIM1 binding to TRPC1, TRPC4, and TRPC5 can cause them to function as Ca2+ store-operated channels, possibly by conferring Ca2+ sensitivity to their gating, or by simply tethering TRPC channels to the Ca2+ release zone on endosomes (28). STIM1 does not bind to TRPC3 or TRPC6, but it has the potential to alter their behavior indirectly, by promoting their heteromerization with TRPC4 and TRPC5 (131). This raises the possibility that differences in STIM1 expression can underlie markedly different gating behaviors of TRPC channels in different cell types. It is not known whether STIM1 is expressed in podocytes, but such a discovery would be quite significant.
As already noted, the CIRB region of TRPC3 channels can interact physically with IP3 receptors inside cells (62), and it has been suggested that TRPC3 channels can be gated by a conformational coupling mechanism (61). Therefore, whether an endogenously expressed TRPC channel operates as a Ca2+ store-operated or a receptor-operated plasma membrane channel may also depend on its own level of protein expression in a cell. Gating of TRPC3 by mobilization of Ca2+ stores generally occurs only at high expression levels (62, 71, 118) and may also depend on other proteins that regulate their subcellular localization. In podocytes, the role of a TRPC channel may also depend on whether the channels are expressed in foot processes or in the cell body, as endoplasmic reticulum is only found in the later compartment (51).
The potential functional variability that results from formation of TRPC heteromers and from interactions with other proteins complicates interpretation of pharmacological studies of the TRPC channel family. This is because drugs that are selective for a particular TRPC channel overexpressed in a heterologous expression system may have quite different specificities for native TRPC channels expressed in tissues. At this time it is safest to conclude that there is no pharmacological agent that can function as a selective activator or inhibitor of TRPC channels, either collectively or individually. Even experiments with small interference (si) RNA-mediated knockdown and gene knockout have to be interpreted with caution, as there can be compensatory (and even overcompensatory) increases in expression of other members of the family (14, 105).
These considerations are relevant to podocytes because multiple members of the TRPC family are expressed in these cells. TRPC3 has been detected in immortalized mouse podocyte cell lines (53). In addition, TRPC1, TRPC2, and TRPC5 were observed in mouse glomeruli in a pattern consistent with expression in podocytes (94). In Fig. 1 we show immunogold labeling of TRPC6, TRPC5, and TRPC1 in mouse foot processes. It is also possible to see TRPC6 expression in the primary processes. Therefore, it is possible that at least some of the TRPC6 channels in podocytes are components of a heteromeric complex and the TRPC6 subunits may have different functions in different cellular compartments.
There are several other mechanisms for TRPC regulation that may be important in podocytes. In general, these operate over longer time scales. TRPC6 channels normally show very low levels of constitutive activity, a feature that depends on its dual glycosylation at sites on extracellular loops (13). Changes in glycosylation could affect functions regulated by TRPC6 (14). In addition, TRPC6 channels are regulated by direct phosphorylation, for example by Src-family tyrosine kinases, which causes an increase in DAG-evoked TRPC6 activation in excised patches (35). TRPC6 can also be phosphorylated by cAMP-dependent protein kinases (31), although the functional significance of this is as yet unknown. The functional activity of TRPC channels (26), including TRPC6 (7), can be regulated by rapid insertion and removal from the plasma membrane. Given this, it is interesting that TRPC5 and TRPC6 coimmunoprecipitate with dynamin (25), which plays a role in membrane protein trafficking and cytoskeletal regulation in many cell types, including podocytes (106). Finally, there are reports that TRPC6 gating is mechanosensitive owing to effects of membrane curvature on the gating of the channels (17, 112). This mode of gating is not seen in every expression system and may therefore depend on other interacting proteins (27).
TRPC6 Channel Permeation
TRPC channels are neither efficient nor selective Ca2+ channels. The most extensive analyses of TRPC permeation have been carried out on TRPC6 channels. When heterologously expressed in HEK293 cells, TRPC6 forms cation channels that can be activated by DAG analogs. These channels show marked outward and inward rectification with physiological ionic gradients (22, 47, 108). Increasing external Ca2+ concentration causes some reduction in current through TRPC6 channels (22, 47, 108), especially at more depolarized membrane potentials, at least in part because of pore blockade. This blockade can be accurately described with a standard single-site, two-barrier pore model (22). In this model, TRPC6 channels have a single binding site for cations located ∼85% of the distance across the transmembrane electric field. This site has a twofold higher affinity for Ca2+ than it does for monovalent cations, and occupation of this site by a permeant ion results in pore blockade (22). More importantly, as a result of Ca2+ absorption to this site, membrane depolarization markedly reduces the ratio of Ca2+ permeability to that of Na+ (the PCa/PNa ratio), effectively causing TRPC6 to function as a monovalent cation channel. In other words, with membrane depolarization there is a reduction in current, but more importantly, the contribution of Ca2+ influx to the current that remains becomes negligible. This effect can be observed directly when Ca2+ imaging is combined with perforated patch recordings in HEK293 cells (22).
The limited Ca2+ permeability has important implications for TRPC6 channels as regulators of Ca2+ dynamics in different cell types. For example, in excitable cells that express voltage-activated Ca2+ channels (Cav channels), the main role of TRPC6 may be to simply evoke sufficient depolarization to cause activation of Cav, leading to robust Ca2+ influx (111). For this purpose, it is immaterial whether TRPC6 channels have significant permeability to Ca2+. Membrane depolarization by itself is sufficient to trigger another source of Ca2+ influx. However, in nonexcitable cells such as podocytes that lack Cav channels (39), TRPC6 would be unlikely to be a source of significant Ca2+ influx unless there is a mechanism to prevent the depolarization that occurs as a result of TRPC6 activation. As will be discussed further below, BKCa channels, which interact and colocalize with TRPC6 channels, may serve such a function in podocytes (53). It is also worth noting that the permeation model formulated by Estacion and coworkers (22) precisely captures the inward and outward rectification that is observed in TRPC6 channels, including endogenously expressed TRPC6 channels (47).
In addition to pore blockade, Ca2+ produces complex effects on the gating properties of TRPC channels. For example, Ca2+-calmodulin appears to facilitate or accelerate activation of TRPC5 channels (85) and TRPC6 channels (4, 108). Cytosolic Ca2+ concentrations at the high end of physiological cause inhibition of TRPC6 channels independently of pore blockade, which may entail channel phosphorylation by protein kinase C (108). This provides a potential mechanism for negative feedback. It also raises an interesting possibility for signaling cross talk, as it is possible that Ca2+ influx or mobilization through one type of channel could modulate signaling mediated by TRPC6 channels.
Mutations in TRPC6 Gene and Glomerular Disease
The importance of ion channels for podocyte function was underscored by the discovery that the TRPC6 mutations shown in Fig. 1 can cause inherited glomerular disease such as FSGS, which generally entails effects on podocytes at early stages (94, 128). TRPC6 mutations accounted for 5 of 71 families with FSGS in the study by Reiser and colleagues (94). The ethnic backgrounds and age range at disease presentation of patients in that study were broad, ranging from 16–61 yr. This relatively late onset stands in marked contrast to several other genetic glomerulopathies that manifest in infancy, such as congenital nephrotic syndrome of the Finnish type (52), steroid-resistant nephrotic syndrome of the podocin type (5), or Wilms' tumor protein 1 (WT1)-related diseases (83). The early onset of those diseases can be readily explained by the disruption or loss of essential structural components of the SD. The question remains as to why the onset of kidney disease in patients with TRPC6 mutations occurs at a relatively advanced age. One possible explanation is that mutations in TRPC6 may produce subtle changes in intracellular function that lead to irreversibly altered cell behavior only over time and possibly after the accumulation of other renal insults. These could include elevated glomerular capillary pressure, ischemia, metabolic stresses such as diabetes, or serum factors, or the presence of other mutations. Similar explanations have been offered for the adult onset and dominantly inherited forms of FSGS associated with mutations in α-actinin-4 (49) and CD2AP (42).
Interestingly, patients with TRPC6-related FSGS do not generally present with any other pathological phenotype. This is surprising because TRPC6 is expressed in many other tissues, for example, in smooth muscle, where it is thought to contribute to the regulation of vascular tone (14, 126). It is possible that the glomerular phenotype is the consequence of a unique role of TRPC6 in the organization of the filtration apparatus or an unusual susceptibility of this structure to even subtle changes in Ca2+ dynamics or cytoskeletal regulation. TRPC6 is not unique in this respect, as mutations in the genes encoding α-actinin-4 and CD2AP result primarily in glomerular kidney disease even though they are widely expressed and associated with a variety of cellular functions (42, 49).
All of the TRPC6 mutations associated to date with FSGS map to the intracellular NH2-terminal and COOH-terminal domains of the channel protein, and most are associated with some detectable increase in activity compared with wild-type in heterologous expression systems. These include six NH2-terminal missense mutations (G109S, P112Q, M132T, N125S, N143S, and S270T) that are located in or near the various NH2-terminal ankyrin repeats (94, 128). These mutations may compromise the ability of TRPC6 to oligomerize with other TRPC subunits (101) or alter the location or steady-state level of channel expression at the cell surface (125). Among these, the P112Q mutation described in a large family from New Zealand exhibits increased surface expression as assessed by cell-surface biotinylation assays as well as an increase in angiotensin II-mediated Ca2+ influx in heterologous expression systems (128). NH2-terminal mutations can also affect TRPC6 gating properties, as the N143S and S270T mutations result in channels with an increased mean open-time compared with wild-type (32). The NH2-terminal mutation with the largest effect on channel function observed to date is M132T. This mutation gives rise to maximal currents in heterologous expression systems three- to fivefold larger than those of wild-type channels, as well as delayed inactivation in the presence of continuous agonist stimulation (32). Interestingly, the M132T mutation gives rise to FSGS disease in children as early as 9 yr of age, suggesting that the age of onset may be related to the magnitude of the gain of function with respect to integral Ca2+ influx over time.
At the COOH terminus of TRPC6, the mutation L780P is located near the Trp box 1 motif that is conserved in all TRPC channels, whereas four additional mutations (K874*, Q889K, R895C, and E897K) map to a predicted coiled-coil domain located further downstream. While no study has specifically investigated the importance of the COOH-terminal coiled-coil motif in TRPC channel gating, one study suggests that the COOH-terminal tails of TRPC4 and TRPC6 participate in channel oligomerization (70). Importantly, the R895C and E897K mutations both resulted in TRPC6 channels with higher maximal current amplitudes in heterologous expression systems, effectively resulting in a net gain in function. It is possible that these are caused by increased trafficking to the cell surface.
Role of TRPC6 in Acquired Forms of Proteinuric Kidney Disease
The discovery of TRPC6 mutations provided a molecular basis for understanding certain hereditary forms of proteinuric glomerular disease. A recent study showed that wild-type TRPC6 was upregulated in a subset of acquired human proteinuric kidney diseases as well as in experimental models of acquired glomerular disease (78). Of particular interest was the induction of TRPC6 detected in patients with membranous glomerulonephritis (78). In membranous nephropathy, subepithelial immune deposits form in situ as a result of circulating antibodies against as yet unidentified antigens (46). The formation of immune deposits is associated with complement activation, leading to assembly of C5b-9 membrane attack complexes on podocyte plasma membranes (90). It has also been reported that podocyte damage mediated by the complement C5b-9 complex is associated with an activation of PLC and an increase in intracellular free Ca2+ (11). This raises the interesting question of whether podocyte TRPC6 channels are regulated by complement and whether this contributes to the pathophysiology of membranous disease. Podocyte TRPC6 channels are also upregulated in response to angiotensin II (133) and as a result of increased generation of reactive oxygen species during puromycin aminonucleoside nephrosis (123), as well as in neonatal nephrin−/− mice (94). With respect to angiotensin II, siRNA knockdown of TRPC6 reduces angiotensin II-mediated Ca2+ transients in podocyte cell lines (133), and this neurohormone causes an increase in cytosolic Ca2+ concentrations in primary cultures of podocytes through stimulation of Ca2+ influx and mobilization of intracellular Ca2+ stores (33). Thus sustained excessive Ca2+ influx through TRPC6 channels may be a common pathway underlying a host of glomerulodegenerative conditions that originate in podocytes, although it is possible that TRPC6 dysfunction in other glomerular cell types could contribute to the overall pathology. Some of these changes presumably entail changes in gene expression. In this regard, TRPC6 appears to be a component of a Ca2+-dependent regulatory loop that drives NFAT-dependent transcription in podocytes (102) and that may also cause activation of NF-κB signaling (133). Over time, alterations in intracellular Ca2+ dynamics may result in irreversible damage and loss of podocytes (107) or podocyte dedifferentiation that ultimately leads to glomerulosclerosis (1). It is also possible that sustained disruptions of TRPC6 signaling could lead to podocyte detachment from the GBM owing to changes in cytoskeletal dynamics of the foot processes, altered integrin function, or altered susceptibility to fluid sheer stress (107).
Interaction of TRPC6 with Podocin and Nephrin
TRPC6 is expressed in podocyte foot processes in the vicinity of the SD as well as in the cell body and throughout the major processes (94) (Fig. 1). TRPC6 interacts with nephrin and podocin, at least in cultured podocytes (41, 43, 94), and these interactions may be central to the physiological function of these channels at the SD.
Podocin is a member of the stomatin gene family, and its homolog Mec-2 contributes to mechanosensation in Caenorhabditis elegans (37). Podocin has been shown to regulate the activity of TRPC6 in a cholesterol-dependent manner, and Huber and coworkers (43) have proposed that podocin binds sterols and alters the local lipid environment surrounding the channel molecule, thereby facilitating the ability of TRPC6 to respond directly to deformation of the plasma membrane. In heterologous expression systems, TRPC6 has also been reported to respond to pressure stimuli in membrane patches even when PLC is inhibited, and the authors of that study suggest a common biophysical basis for membrane stretch activation and activation of TRPC6 by DAG based on inhibition of both processes by the spider toxin GsMTx-4 (112). Podocin may cause local depletion of membrane sterols, resulting in local changes in membrane fluidity, thereby lowering energy barriers for conformational changes in the channel molecule caused by membrane curvature.
TRPC6 interactions with nephrin near the SD may underlie regulation of gating and trafficking of these channels (38, 120). Functional interactions between these proteins may serve to monitor the integrity of the filtration apparatus in podocytes to sense mechanical stimuli and to trigger Ca2+ signaling cascades that could alter cytoskeletal dynamics (64–66). For example, engagement of the ectodomains of nephrin results in localized activation of Src family tyrosine kinases (120). TRPC6 channels are substrates for Src family kinases such as Fyn, and tyrosine phosphorylation by these types of kinases causes an increase in TRPC6 activity (35), which would in turn cause alterations of cytoskeletal dynamics. Moreover, the absence of nephrin as shown in neonatal nephrin−/− mice led to increased expression of TRPC6 in podocytes compared with podocytes of wild-type littermates (94).
Biochemical and Biophysical Properties of BKCa Channels
BKCa channels interact with TRPC6 channels and other SD proteins and may play an important role in podocyte signaling (53–55, 58, 95). Functional BKCa channels were first reported in human podocyte cell lines (79) and were later described in mouse podocyte cell lines (53–55, 58, 95). Slo1 proteins can also be detected by immunofluorescence and immunoblotting in human (79) and mouse (58) glomeruli. In mice, BKCa channels colocalize with synaptopodin, suggesting a podocyte pattern of expression (58). As we will describe further below, the BKCa channels of podocytes are mechanosensitive (79).
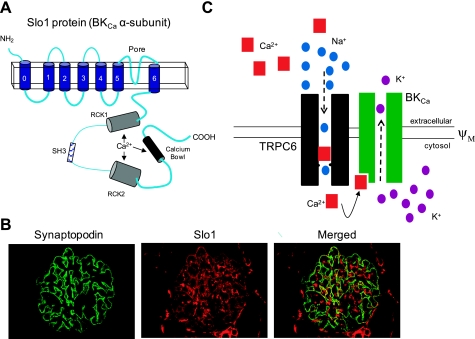
Functional BKCa channels are composed of four pore-forming subunits, often referred to as Slo1 proteins, which are encoded by the KCNMA1 gene (also known as Slo1 and KCa1.1). BKCa channels are expressed in a remarkably wide range of tissues, and in several cell types in kidney, including arteriolar smooth muscle and the distal nephrons, as well as in podocytes (Fig. 2). Open BKCa channels always have a very large unitary conductance (180–220 pS in symmetrical 150 mM KCl and ∼105 pS with physiological ionic gradients). This means that activation of a relatively small number of them can have profound effects on cell physiology, especially in electrically compact cells. Each vertebrate Slo1 protein has seven membrane-spanning domains with an extracellular NH2 terminus and a large globular cytoplasmic COOH-terminal domain that comprises about half of each Slo1 subunit.
Fig. 2.
Large-conductance Ca2+-activated K+ (BKCa) channels in podocytes. A: topography of the pore-forming subunits of BKCa channels (Slo1 proteins) showing Ca2+-binding domains in the large cytoplasmic COOH terminal. Also shown is a noncanonical SH3 domain that mediates stretch-sensitive gating by interactions with actin-binding proteins. B: colocalization of Slo1 (red) and synaptopodin (green) in a mouse glomerulus revealed by double-label immunofluorescence and a merged image. Areas of colocalization indicate expression in podocytes. Slo1 also appears to be expressed in the mesangium, where there is no synaptopodin. C: model for coordinated activation of TRPC6 and BKCa. Although Ca2+ can permeate TRPC6, it also causes pore blockade and with depolarization TRPC6 behaves as a monovalent cation channel. However, as a result of physical interactions and resulting colocalization, Ca2+ influx through TRPC6 can cause activation of BKCa. This may prevent membrane depolarization, thereby providing a driving force that can sustain Ca2+ infux through TRPC6.
The gating of BKCa channels is markedly voltage dependent, and Ca2+ binding to multiple allosterically interacting domains in the cytoplasmic COOH terminus of Slo1 proteins shifts activation into the physiological range of membrane potentials (130, 132). These Ca2+-activation domains include the so-called “calcium bowl” (103) and a pair of “regulator of potassium conductance” (RCK) domains (2) that are separated by a flexible hinge region that allows for their conformational coupling (60, 91). The domain structure is summarized schematically in Fig. 2A.
The mammalian KCNMA1 gene is expressed in a large number of splice variants with markedly different voltage and Ca2+ sensitivities and gating kinetics, although all variants have high unitary conductance. This gene has 35 exons and is subject to alternative splicing at no fewer than 7 different sites (3). More than one BKCa channel splice variant can exist in a single cell type (55, 57), and it is therefore possible that some native BKCa channels are heteromeric. This has important consequences for the regulation of both gating and trafficking to the cell surface (9, 57, 76).
A large number of factors determine the overall activity of BKCa channels in cells. These include 1) gating properties, especially voltage and Ca2+ sensitivities, which can be acutely altered by posttranslational modification and by interactions with other proteins; 2) the extent to which BKCa channels are either trafficked into the plasma membrane or trapped in intracellular compartments; 3) variations in KCNMA1 transcript levels and alternative splicing; and 4) the nature and proximity of Ca2+ sources that are usually needed to drive BKCa gating. A great deal of literature on the biochemical and biophysical factors controlling the gating of BKCa channels has been recently reviewed (10). Here, it is important to note that robust activation of BKCa channels usually requires cell depolarization and micromolar concentrations of free Ca2+ in the cytosolic microenvironment of the channels, far above the concentrations normally found in bulk cytosol. From the laws of diffusion, this implies that BKCa channels must in the majority of cases be closely colocalized to their Ca2+ sources, generally within nanometers of Ca2+-permeable pores, to see concentrations sufficient for substantial activation (80). In addition, reversible phosphorylation of various residues within the large intracellular COOH terminus of BKCa channels can have complex effects on their gating properties. For example, proto-oncogenic tyrosine kinases such as Src and Fyn can enhance the activity of BKCa channels when Ca2+ is present (72), which suggests that they could be modulated coordinately with nephrin and TRPC6 in podocytes (35, 38, 120). Changes in BKCa gating provide a means to integrate several other cellular pathways including cellular REDOX potential (113) and hypoxia (77, 127) that may be important in the context of kidney pathophysiology.
BKCa gating is typically sensitive to mechanical stimuli, a process that in some cell types requires interactions with actin filaments subjacent to the plasma membrane (18, 89). This type of gating has been described in some detail in immortalized human podocyte cell lines (79). Membrane stretch-sensitive gating is mediated at least in part by binding of adaptor proteins such as cortactin and Grb2 (and possibly many others) to a highly conserved noncanonical Src-homology 3 domain that can tether plasma membrane channels to subjacent actin filaments (114, 115). This proline-rich site is located directly between the two RCK domains in the large cytoplasmic COOH terminal of the Slo1 protein. It is possible that mechanical forces cause displacement of this hinge region, leading to perturbation of the allosteric coupling between the two RCK domains, thereby altering BKCa activation. Perturbing these types of interactions with actin may also underlie actions of certain neurohumoral signals on BKCa gating (84). BKCa channels can potentially interact with actin by at least three other mechanisms: these include binding to filamin A (56), interactions with caveolins (6), and by direct interaction with an actin-binding domain present in a more downstream region of the COOH terminus of Slo1 that is present in all vertebrate variants (135). It is not known whether these latter interactions play a role in regulating BKCa gating, but they clearly play a role in BKCa trafficking to the cell surface (56, 135). Thus interactions between BKCa channels and actin filaments are complex and are likely to have multiple effects on the overall activity of BKCa channels.
The properties of BKCa channels are markedly altered by auxiliary β-subunits that do not form functional pores by themselves, but which alter the gating properties of associated Slo1 proteins (75). There are four distinct vertebrate genes that encode BKCa channel β-subunits (β1–4). All share a basic membrane topology consisting of two transmembrane segments connected by an extracellular loop domain and with cytoplasmic NH2 and COOH termini (86). The presence of β-subunits can cause complex effects on the Ca2+ and voltage sensitivity of BKCa gating, as well as the access of the channel pore to scorpion toxin inhibitors such as charybdotoxin and iberiotoxin. Mouse and human podocytes express β4-subunits (55, 79). These subunits cause marked slowing of BKCa activation at all membrane potentials, which is one of the hallmarks of macroscopic BKCa currents in podocytes (55). β4-Subunits also cause reduced channel activation at low cytosolic Ca2+ concentrations, but markedly enhanced activation when cytosolic Ca2+ is elevated (121). In other words, β4-subunits cause BKCa channels to function as a robust on-off switch, rather than to become active in a graded fashion. Furthermore, the slow kinetics conferred by the presence of β4-subunits suggests that these channels would not normally respond to transient changes in membrane potential. The presence of β4 is also reported to cause a reduction or loss of sensitivity to blockade of BKCa channels by charybdotoxin and iberiotoxin (48).
Properties of BKCa Channels in Podocytes
Slo1 immunostaining of mouse glomeruli is consistent with expression of BKCa channels in podocytes and mesangial cells (58) (Fig. 2), and Slo1 subunits and β4-subunits are also expressed in mouse and human podocyte cell lines (55, 79). Functional BKCa channels with slow kinetics are readily detected in single-channel recordings from inside-out patches excised from immortalized podocytes (79). Podocyte BKCa channels with unusually slow activation kinetics can also be detected in whole-cell recordings and are most easily observed by including micromolar Ca2+ in the recording pipettes and evoking currents by application of depolarizing voltage steps (53–55) (Fig. 2B). These outward currents are not seen when recording electrodes contain 10 mM EGTA to buffer the free Ca2+ to much lower concentrations (55). Currents with similar properties can be detected in podocytes dissociated acutely from rat glomeruli. The macroscopic BKCa currents in mouse podocyte cell lines do not inactivate in the face of continued depolarization. Podocyte BKCa channels are only partially inhibited by iberiotoxin (55), but they are completely blocked by the selective BKCa inhibitors paxilline (55) and penetrem A (79) as well as by nonselective K+ channel inhibitors such as tetraethylammonium (79).
The BKCa channels in human podocyte cell lines become active in response to membrane stretch (79). Thus the probability of BKCa opening in cell-attached patches is increased an average of fourfold by applying modest negative pressure (−20 cmH2O) to recording pipettes. A similar effect is observed by placing podocytes in a bath solution with a tonicity that is 70% of control, which would be expected to cause swelling and stretch of the podocyte plasma membrane (79). These effects were seen in patches that were substantially depolarized from the resting membrane potential, and it is not known whether this reflects a direct effect of membrane stretch on BKCa gating or whether it is an indirect effect mediated by a stretch-dependent increase in local Ca2+ concentration. We have observed that acute depolymerization of actin with cytochalasin-D does not affect the amplitude or kinetics of macroscopic BKCa channels when intracellular Ca2+ is clamped at 5 μM (58), which suggests that the stretch sensitivity in these cells may not be mediated by actin interactions. Several alternative mechanisms are available in podocytes. For example, Huber and colleagues (43) demonstrated that coexpression of the cholesterol-binding protein podocin in heterologous systems can increase the activity of TRPC6 channels and that this effect appears to be attenuated by prior cholesterol depletion of cells and requires the cholesterol-binding domain of podocin. Given this, it is interesting to note that cholesterol depletion increases activity of BKCa channels in a variety of systems (8, 68, 110), most likely by changing the local fluidity of the surrounding membrane and by altering association of the channels with lipid rafts. It will be important to determine whether podocin affects BKCa gating and whether it mediates stretch sensitivity of BKCa function in podocytes.
Interactions Between TRPC6 and BKCa Channels in Podocytes
Activation of BKCa channels at physiological membrane potentials usually requires micromolar concentrations of free Ca2+ or even higher. It is rare for bulk cytoplasmic concentrations of Ca2+ to reach these levels except in extreme pathological situations. However, nanodomains immediately surrounding the pores of Ca2+-permeable channels can exceed this concentration (80). For this reason, the functional coupling of Slo1 channels with Ca2+-permeable channels usually requires very close colocalization. There is literature on excitable cells showing BKCa channels bind directly to voltage-activated Ca2+ channel proteins, which ensures the necessary colocalization for functional coupling of their gating (74, 136). However, podocytes are not excitable cells and do not express Cav channels (39). Instead, coimmunoprecipitation and glutathione-S-transferase pull-down assays have shown that Slo1 subunits can bind to TRPC6 channels (53). This occurs with endogenously expressed channels of mouse podocyte cell lines, as well as with channels, and domains of channels, expressed in HEK293T cells. These interactions may allow for sufficient colocalization to allow Ca2+ influx through TRPC6 channels to cause a coordinate activation of BKCa channels. It is also interesting that coexpression of TRPC6 with certain Slo1 splice variants increases surface expression of the latter in heterologous expression systems, whereas siRNA knockdown of TRPC6 reduces surface expression of endogenous BKCa channels in mouse podocyte cell lines (53). Somewhat surprisingly, this does not occur with TRPC3, which is also able to bind to BKCa channels. These results raise the possibility that at least a subset of TRPC6 and BKCa channels traffics as a complex in podocytes.
The available evidence suggests that TRPC6 channels represent a physiologically important source of Ca2+ influx in podocytes (133). For this reason, it is important to consider how podocytes maintain conditions that allow TRPC6 channels to transport Ca2+. As already discussed, depolarization tends to cause TRPC6 to function as a nonselective monovalent cation channel, owing to voltage-dependent pore blockade by Ca2+, which causes large effects on the PNa/PCa ratio (22). Conversely, hyperpolarization allows TRPC6 to transport Ca2+ into the cell more efficiently both by increasing the driving force and by reducing pore blockade (22). Activation of TRPC6 will also cause cell depolarization unless there is an opposing mechanism to prevent this from happening. A physical interaction between BKCa and TRPC6 channels on the cell could provide such a mechanism by keeping BKCa channels within the Ca2+ microdomain that surrounds the pores of active TRPC6 channels. Such colocalization would allow for their coordinated activation in space and time. The resulting Ca2+ activation of BKCa would oppose cell depolarization caused by TRPC6 activation and thereby maintain the conditions needed for efficient Ca2+ influx into the cell. In this model, cell surface BKCa channels colocalized with TRPC6 provide positive feedback to Ca2+ influx in nonexcitable cells such as podocytes. By contrast, they have long been known to provide negative feedback to Ca2+ influx in excitable cells that express Cav channels that colocalize with BKCa (74). The difference is a simple consequence of the fact that TRPC6 and Cav channels have an effectively opposite dependence on membrane potential, through voltage effects on gating in the case of Cav (74) and voltage effects on Ca2+ permeability in the case of TRPC6 (22).
Another possible role of BKCa as part of the TRPC6 complex is to increase the mechanosensitivity of the overall complex. The extent to which native TRPC6 channels in podocytes are stretch sensitive is not known, although their interaction and colocalization with podocin in situ makes this a very real possibility (43). BKCa channels are demonstrably stretch sensitive in podocyte cell lines (79), which could provide a mechanism to fine-tune Ca2+ influx during normal glomerular function. This is because BKCa activation would increase the driving force for Ca2+ influx through TRPC6 channels.
Are there other mechanisms that could contribute to coordinated activation or deactivation of TRPC6 and BKCa? Src family protein kinases have been reported to increase the activity of both TRPC6 (35) and BKCa channels (72). In podocytes, Src activation can occur as a result of engagement of the nephrin ectodomain (120), which raises the possibility that both of these channels are components of a nephrin-dependent outside-in signaling cascade. It is also possible that certain signals have opposing effects on these two channel molecules, PIP2 being one possibility (69, 117). This could provide a mechanism to terminate or fine-tune the Ca2+ signaling mechanism, but to date this has not been investigated in podocytes.
Interaction of BKCa Channels with Other Podocyte Proteins
The COOH-terminal domains of BKCa channels interact with >100 different proteins (50), but the functional significance has only been established for a small subset of proteins. We have already noted that TRPC6 channels interact with the COOH-terminal domains of Slo1 proteins in heterologous expression systems and in podocyte cell lines (53). Other SD proteins that interact with BKCa channels include nephrin (55), Neph1 (54), MAGI-1 (95), and synaptopodin (58). None of these protein interactions directly regulate the gating of BKCa channels in HEK293T cells, but all appear to play a role in regulating the steady-state surface expression of functional BKCa channels. Thus siRNA-mediated knockdown of nephrin, Neph1, or synaptopodin reduced the number of functional surface BKCa channels observed by electrophysiology or immunochemistry in mouse podocyte cell lines (54, 55 58). Conversely, coexpression of either nephrin or synaptopodin with Slo1 increases surface expression of BKCa channels in HEK293T cells compared with what was observed when Slo1 is expressed by itself (55, 58). The situation with Neph1 is interesting because the functional consequences of its interaction with BKCa channels depend on the cell type examined. Thus coexpression of Neph1 with Slo1 proteins in HEK293T cells suppressed expression of functional BKCa channels on the cell surface (54). An effect in the same direction was also seen in parasympathetic neurons, which express Neph1 endogenously. In those cells, siRNA knockdown of Neph1 caused an increase in steady-state surface expression of Slo1 and an increase in macroscopic Ca2+-dependent K+ current (54). It is not known why Neph1 produces different effects on BKCa channels in different cell types. MAGI-1 is a scaffolding protein expressed in podocyte SDs that also interacts with nephrin (34), synaptopodin (87), and α-actinin (87). Coexpression of MAGI-1 suppresses expression of BKCa channels on the surface of HEK293T cells (95). Conversely, siRNA knockdown of MAGI-1 expression in podocytes causes an increase in functional surface expression of BKCa channels (95).
These data indicate that protein interactions in SD domains regulate trafficking of ion channels and possibly other proteins into the plasma membrane. It is possible that signaling cascades differentially alter the stability of certain of these protein complexes, thereby affecting the steady-state surface expression of ion channels.
Conclusion
The discovery that mutations in TRPC6 channels cause familial FSGS has made analysis of ion channels in podocytes an important subject in renal physiology and experimental nephrology. Many questions remain unanswered, including the mechanisms whereby enhanced TRPC6 function leads to podocyte dysfunction and glomerular disease, the signaling cascades that control gating of podocyte ion channels, the role of multichannel complexes in controlling overall Ca2+ flux through TRPC6 channels, and the role of other members of the TRPC family and other types of cation channels in podocyte function.
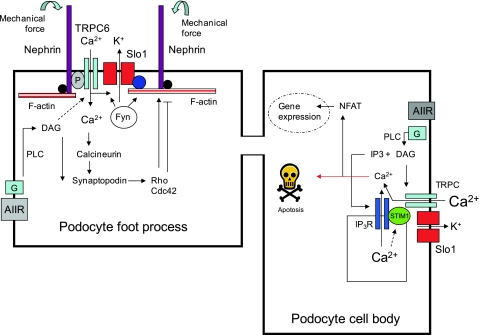
One question that has not been addressed is what terminates Ca2+ signaling in podocytes. It seems likely that podocytes contain a number of systems for sequestering and buffering Ca2+ in podocytes, and it is quite possible that Ca2+-dependent cascades lead to termination of DAG-dependent TRPC6 activation through negative feedback. It is possible that these cascades eventually cause suppression of the gating of TRPC6 and BKCa channels, and possibly, over longer time scales, their expression on the cell surface. Some of the interactions between TRPC6 channels and other proteins are shown schematically in Fig. 3, which also shows a few of the many unresolved questions. The available data already suggest reciprocal interactions between podocyte ion channels and the cytoskeleton. The net effect of Ca2+ influx controlled by TRPC6 appears to include regulation of podocyte actin dynamics, possibly to allow SDs to adapt to mechanically adapt to changes in their immediate environment. Interactions with the cytoskeleton may in turn regulate the gating of podocyte ion channels by contributing to their mechanosensitivity, or by controlling their steady-state expression at the SD and at other locations on the cell surface. Ultimately, it is likely that sustained changes in podocyte ion channel function lead to Ca2+-dependent changes in gene expression, cell metabolism, and alterations in the efficiency of the glomerular filtration barrier that can lead over time to maintenance or loss of the entire nephron.
Fig. 3.
Models of TRPC6 and BKCa function in podocytes. These channels form a complex with each other in the plasma membrane, which ensures that Ca2+ influx through TRPC6 can cause coordinated activation of BKCa. This serves to prevent membrane depolarization and thereby maintain the Ca2+ permeability of TRPC6. In foot processes (left), these channels also interact with nephrin, podocin (P) and with the underlying actin cytoskeleton, which allows them to function as mechanosensors and to allow the resulting Ca2+ influx to modulate nephrin signaling and cytoskeletal dynamics acting in part through small GTPases and proteins such as Nck and CD2AP (shown schematically as a black circle). BKCa channels are also mechanosensitive through interactions with the cytoskeleton mediated by cortactin (blue circle) or other actin-binding proteins. TRPC6 channels can become active in response to G protein-mediated signaling cascades associated with PLC activation, for example, during angiotensin signaling. The entire slit diaphragm complex may be also regulated by tyrosine kinases such as Fyn. Other TRPC family members are expressed in podocytes, and their functions are unknown. In the cell body (right), they may form heteromeric complexes with TRPC6 and in this way function as Ca2+-store operated channels in G protein signaling cascades associated with PLC activation, possibly in complex with other proteins such as STIM1 and the 1,4,5-trisphosphate (IP3) receptor. Influx of Ca2+ in the cell body can regulate gene expression in podocytes. Excessive Ca2+ influx through TRPC6 channels as a result of mutations or other pathological events can ultimately lead to loss of podocytes through apoptosis as well as detachment from the glomerular basement membrane (GBM). Foot process effacement and podocyte hypertrophy may represent an adaptive response of the remaining podocytes to maintain coverage of the GBM.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1 DK82529 (to S. E. Dryer) and RO1 DK73495 (to J. Reiser).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao L, Rapin AM, Holmstrand EC, Cox DH. Elimination of the BKCa channel's high-affinity Ca2+ sensitivity. J Gen Physiol 120: 173–189, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beisel KW, Rocha-Sanchez SM, Ziegenbein SJ, Morris KA, Kai C, Kawai J, Carninci P, Hayashizaki Y, Davis RL. Diversity of Ca2+-activated K+ channel transcripts in inner ear hair cells. Gene 386: 11–23, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Boulay G. Ca2+-calmodulin regulates receptor-operated Ca2+ entry activity of TRPC6 in HEK-293 cells. Cell Calcium 32: 201–207, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Brainard AM, Miller AJ, Martens JR, England SK. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am J Physiol Cell Physiol 289: C49–C57, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem 279: 7241–7246, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Chang HM, Reitstetter R, Mason RP, Gruener R. Attenuation of channel kinetics and conductance by cholesterol: an interpretation using structural stress as a unifying concept. J Membr Biol 143: 51–63, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Chiu YH, Alvarez-Baron C, Kim EY, Dryer SE. Dominant-negative regulation of cell surface expression by a pentapeptide motif at the extreme COOH terminus of a Slo1 calcium-activated potassium channel splice variant. Mol Pharmacol 77: 497–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci 66: 852–875, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cybulsky AV, Bonventre JV, Quigg RJ, Lieberthal W, Salant DJ. Cytosolic calcium and protein kinase C reduce complement-mediated glomerular epithelial injury. Kidney Int 38: 803–811, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Dietrich A, Kalwa H, Rost BR, Gudermann T. The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: functional characterization and physiological relevance. Pflügers Arch 451: 72–80, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Dietrich A, Mederos y Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J Biol Chem 278: 47842–47852, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Dietrich A, Mederos Schnitzler MY, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol 25: 6980–6989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, Singhal PC. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol 283: F173–F180, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 59: 673–682, 1988 [PubMed] [Google Scholar]
- 17.Dyachenko V, Husse B, Rueckschloss U, Isenberg G. Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium 45: 38–54, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Ehrhardt AG, Frankish N, Isenberg G. A large-conductance K+ channel that is inhibited by the cytoskeleton in the smooth muscle cell line DDT1 MF-2. J Physiol 496: 663–676, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eid SR, Cortright DN. Transient receptor potential channels on sensory nerves. Handb Exp Pharmacol 194: 261–281, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Elias H. The renal glomerulus by light and electron microscopy. Res Serv Med 46: 1–28, 1956 [Google Scholar]
- 21.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Estacion M, Sinkins WG, Jones SW, Applegate MA, Schilling WP. Human TRPC6 expressed in HEK 293 cells forms non-selective cation channels with limited Ca2+ permeability. J Physiol 572: 359–377, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Friedrich C, Endlich N, Kriz W, Endlich K. Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol 291: F856–F865, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Goel M, Sinkins W, Keightley A, Kinter M, Schilling WP. Proteomic analysis of TRPC5- and TRPC6-binding partners reveals interaction with the plasmalemmal Na+/K+-ATPase. Pflügers Arch 451: 87–98, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Goel M, Sinkins WG, Zuo CD, Hopfer U, Schilling WP. Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct. Am J Physiol Renal Physiol 293: F1476–F1488, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honoré E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch 455: 1097–1103, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Gross SA, Guzmán GA, Wissenbach U, Philipp SE, Zhu MX, Bruns D, Cavalié A. TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. J Biol Chem 284: 34423–34432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gudermann T, Hofmann T, Mederos y Schnitzler M, Dietrich A. Activation, subunit composition and physiological relevance of DAG-sensitive TRPC proteins. Novartis Found Symp 258: 103–118, 2004 [PubMed] [Google Scholar]
- 30.Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Hassock SR, Zhu MX, Trost C, Flockerzi V, Authi KS. Expression and role of TRPC proteins in human platelets: evidence that TRPC6 forms the store-independent calcium entry channel. Blood 100: 2801–2811, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Heeringa SF, Möller CC, Du J, Yue L, Hinkes B, Chernin G, Vlangos CN, Hoyer PF, Reiser J, Hildebrandt F. A novel TRPC6 mutation that causes childhood FSGS. PLoS One 4: e7771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henger A, Huber T, Fischer KG, Nitschke R, Mundel P, Schollmeyer P, Greger R, Pavenstädt H. Angiotensin II increases the cytosolic calcium activity in rat podocytes in culture. Kidney Int 52: 687–693, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Hirabayashi S, Mori H, Kansaku A, Kurihara H, Sakai T, Shimizu F, Kawachi H, Hata Y. MAGI-1 is a component of the glomerular slit diaphragm that is tightly associated with nephrin. Lab Invest 85: 1528–1543, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem 279: 18887–18894, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Huang M, Gu G, Ferguson EL, Chalfie M. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature 378: 292–295, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 14: 211–216, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Huber TB, Gloy J, Henger A, Schollmeyer P, Greger R, Mundel P, Pavenstädt H. Catecholamines modulate podocyte function. J Am Soc Nephrol 9: 335–345, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, Shaw AS, Walz G, Benzing T. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 23: 4917–4928, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T. Interaction with podocin facilitates nephrin signaling. J Biol Chem 276: 41543–41546, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Huber TB, Kwoh C, Wu H, Asanuma K, Gödel M, Hartleben B, Blumer KJ, Miner JH, Mundel P, Shaw AS. Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin Invest 116: 1337–1345, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber TB, Schermer B, Müller RU, Höhne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, Shirokova E, Krautwurst D, Harteneck C, Simons M, Pavenstädt H, Kerjaschki D, Thiele C, Walz G, Chalfie M, Benzing T. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci USA 103: 17079–17086, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt JL, Pollak MR, Denker BM. Cultured podocytes establish a size-selective barrier regulated by specific signaling pathways and demonstrate synchronized barrier assembly in a calcium switch model of junction formation. J Am Soc Nephrol 16: 1593–1602, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Ichimura K, Kurihara H, Sakai T. Actin filament organization of foot processes in vertebrate glomerular podocytes. Cell Tissue Res 329: 541–557, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int 51: 270–276, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88: 325–332, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Jin P, Weiger TM, Levitan IB. Reciprocal modulation between the alpha and beta 4 subunits of hSlo calcium-dependent potassium channels. J Biol Chem 277: 43724–43729, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodríguez-Pérez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Kathiresan T, Harvey M, Orchard S, Sakai Y, Sokolowski B. A protein interaction network for the large conductance Ca2+-activated K+ channel in the mouse cochlea. Mol Cell Proteomics 8: 1972–1987, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerjaschki D, Miettinen A, Farquhar MG. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med 166: 109–128, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Kim EY, Alvarez-Baron CP, Dryer SE. Canonical transient receptor potential channel (TRPC)3 and TRPC6 associate with large-conductance Ca2+-activated K+ (BKCa) channels: role in BKCa trafficking to the surface of cultured podocytes. Mol Pharmacol 75: 466–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim EY, Chiu YH, Dryer SE. Neph1 regulates steady-state surface expression of Slo1 Ca2+-activated K+ channels: different effects in embryonic neurons and podocytes. Am J Physiol Cell Physiol 297: C1379–C1388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim EY, Choi KJ, Dryer SE. Nephrin binds to the COOH terminus of a large-conductance Ca2+-activated K+ channel isoform and regulates its expression on the cell surface. Am J Physiol Renal Physiol 295: F235–F246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim EY, Ridgway LD, Dryer SE. Interactions with filamin A stimulate surface expression of large-conductance Ca2+-activated K+ channels in the absence of direct actin binding. Mol Pharmacol 72: 622–630, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Kim EY, Ridgway LD, Zou S, Chiu YH, Dryer SE. Alternatively spliced C-terminal domains regulate the surface expression of large conductance calcium-activated potassium channels. Neuroscience 146: 1652–1661, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim EY, Suh JM, Chiu YH, Dryer SE. Regulation of podocyte BKCa channels by synaptopodin, Rho, and actin microfilaments. Am J Physiol Renal Physiol (First published July 14, 2010). doi:10.1152/ajprenal.00206.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim EY, Zou S, Ridgway LD, Dryer SE. β1-Subunits increase surface expression of a large-conductance Ca2+-activated K+ channel isoform. J Neurophysiol 97: 3508–3516, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Kim HJ, Lim HH, Rho SH, Bao L, Lee JH, Cox DH, Kim H, Park CS. Modulation of the conductance-voltage relationship of the BKCa channel by mutations at the putative flexible interface between two RCK domains. Biophys J 94: 446–456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiselyov K, Mignery GA, Zhu MX, Muallem S. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol Cell 4: 423–429, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature 396: 478–482, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Kretzler M, Koeppen-Hagemann I, Kriz W. Podocyte damage is a critical step in the development of glomerulosclerosis in the uninephrectomised-desoxycorticosterone hypertensive rat. Virchows Arch 425: 181–193, 1994 [DOI] [PubMed] [Google Scholar]
- 64.Kriz W, Elger M, Mundel P, Lemley KV. Structure-stabilizing forces in the glomerular tuft. J Am Soc Nephrol 5: 1731–1739, 1995 [DOI] [PubMed] [Google Scholar]
- 65.Kriz W, Hackenthal E, Nobiling R, Sakai T, Elger M, Hähnel B. A role for podocytes to counteract capillary wall distension. Kidney Int 45: 369–376, 1994 [DOI] [PubMed] [Google Scholar]
- 66.Kriz W, Kretzler M, Provoost AP, Shirato I. Stability and leakiness: opposing challenges to the glomerulus. Kidney Int 49: 1570–1574, 1996 [DOI] [PubMed] [Google Scholar]
- 67.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Lam RS, Shaw AR, Duszyk M. Membrane cholesterol content modulates activation of BK channels in colonic epithelia. Biochim Biophys Acta 1667: 241–248, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Large WA, Saleh SN, Albert AP. Role of phosphoinositol 4,5-bisphosphate and diacylglycerol in regulating native TRPC channel proteins in vascular smooth muscle. Cell Calcium 45: 574–582, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Lepage PK, Lussier MP, Barajas-Martinez H, Bousquet SM, Blanchard AP, Francoeur N, Dumaine R, Boulay G. Identification of two domains involved in the assembly of transient receptor potential canonical channels. J Biol Chem 281: 30356–30364, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Lièvremont JP, Bird GS, Putney JW., Jr Canonical transient receptor potential TRPC7 can function as both a receptor- and store-operated channel in HEK-293 cells. Am J Physiol Cell Physiol 287: C1709–C1716, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Ling S, Woronuk G, Sy L, Lev S, Braun AP. Enhanced activity of a large conductance, calcium-sensitive K+ channel in the presence of Src tyrosine kinase. J Biol Chem 275: 30683–30689, 2000 [DOI] [PubMed] [Google Scholar]
- 73.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loane DJ, Lima PA, Marrion NV. Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain. J Cell Sci 120: 985–995, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol 570: 65–72, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S. Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Lett 581: 1000–1008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCartney CE, McClafferty H, Huibant JM, Rowan EG, Shipston MJ, Rowe IC. A cysteine-rich motif confers hypoxia sensitivity to mammalian large conductance voltage- and Ca-activated K (BK) channel alpha-subunits. Proc Natl Acad Sci USA 102: 17870–17876, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 18: 29–36, 2007 [DOI] [PubMed] [Google Scholar]
- 79.Morton MJ, Hutchinson K, Mathieson PW, Witherden IR, Saleem MA, Hunter M. Human podocytes possess a stretch-sensitive, Ca2+-activated K+ channel: potential implications for the control of glomerular filtration. J Am Soc Nephrol 15: 2981–2987, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Müller A, Kukley M, Uebachs M, Beck H, Dietrich D. Nanodomains of single Ca2+ channels contribute to action potential repolarization in cortical neurons. J Neurosci 27: 483–495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neal CR, Crook H, Bell E, Harper SJ, Bates DO. Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J Am Soc Nephrol 16: 1223–1235, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Neal CR, Muston PR, Njegovan D, Verrill R, Harper SJ, Deen WM, Bates DO. Glomerular filtration into the subpodocyte space is highly restricted under physiological perfusion conditions. Am J Physiol Renal Physiol 293: F1787–F1798, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Niaudet P, Gubler MC. WT1 and glomerular diseases. Pediatr Nephrol 21: 1653–1660, 2006 [DOI] [PubMed] [Google Scholar]
- 84.O'Malley D, Irving AJ, Harvey J. Leptin-induced dynamic alterations in the actin cytoskeleton mediate the activation and synaptic clustering of BK channels. FASEB J 19: 1917–1919, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Ordaz B, Tang J, Xiao R, Salgado A, Sampieri A, Zhu MX, Vaca L. Calmodulin and calcium interplay in the modulation of TRPC5 channel activity. Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. J Biol Chem 280: 30788–30796, 2005 [DOI] [PubMed] [Google Scholar]
- 86.Orio P, Torres Y, Rojas P, Carvacho I, Garcia ML, Toro L, Valverde MA, Latorre R. Structural determinants for functional coupling between the beta and alpha subunits in the Ca2+-activated K+ (BK) channel. J Gen Physiol 127: 191–204, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patrie KM, Drescher AJ, Welihinda A, Mundel P, Margolis B. Interaction of two actin-binding proteins, synaptopodin and alpha-actinin-4, with the tight junction protein MAGI-1. J Biol Chem 277: 30183–30190, 2002 [DOI] [PubMed] [Google Scholar]
- 88.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 89.Piao L, Ho WK, Earm YE. Actin filaments regulate the stretch sensitivity of large-conductance, Ca2+-activated K+ channels in coronary artery smooth muscle cells. Pflügers Arch 446: 523–528, 2003 [DOI] [PubMed] [Google Scholar]
- 90.Pippin JW, Durvasula R, Petermann A, Hiromura K, Couser WG, Shankland SJ. DNA damage is a novel response to sublytic complement C5b-9-induced injury in podocytes. J Clin Invest 111: 877–885, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qian X, Niu X, Magleby KL. Intra- and intersubunit cooperativity in activation of BK channels by Ca2+. J Gen Physiol 128: 389–404, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11: 1–8, 2000 [DOI] [PubMed] [Google Scholar]
- 93.Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem 279: 34827–34832, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ridgway LD, Kim EY, Dryer SE. MAGI-1 interacts with Slo1 channel proteins and suppresses Slo1 expression on the cell surface. Am J Physiol Cell Physiol 297: C55–C65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodewald R, Karnovsky MJ. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 60: 423–433, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 96: 7962–7967, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salmon AH, Toma I, Sipos A, Muston PR, Harper SJ, Bates DO, Neal CR, Peti-Peterdi J. Evidence for restriction of fluid and solute movement across the glomerular capillary wall by the subpodocyte space. Am J Physiol Renal Physiol 293: F1777–F1786, 2007 [DOI] [PubMed] [Google Scholar]
- 100.Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem 275: 17517–17526, 2000 [DOI] [PubMed] [Google Scholar]
- 101.Schindl R, Frischauf I, Kahr H, Fritsch R, Krenn M, Derndl A, Vales E, Muik M, Derler I, Groschner K, Romanin C. The first ankyrin-like repeat is the minimum indispensable key structure for functional assembly of homo- and heteromeric TRPC4/TRPC5 channels. Cell Calcium 43: 260–269, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Schlöndorff J, Del Camino D, Carrasquillo R, Lacey V, Pollak MR. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol 296: C558–C569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys J 73: 1355–1363, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci 22: 505–512, 2001 [DOI] [PubMed] [Google Scholar]
- 105.Selli C, Erac Y, Kosova B, Tosun M. Post-transcriptional silencing of TRPC1 ion channel gene by RNA interference upregulates TRPC6 expression and store-operated Ca2+ entry in A7r5 vascular smooth muscle cells. Vascul Pharmacol 51: 96–100, 2009 [DOI] [PubMed] [Google Scholar]
- 106.Sever S, Altintas MM, Nankoe SR, Möller CC, Ko D, Wei C, Henderson J, del Re EC, Hsing L, Erickson A, Cohen CD, Kretzler M, Kerjaschki D, Rudensky A, Nikolic B, Reiser J. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest 117: 2095–2104, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol 561: 415–432, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shirato I, Sakai T, Kimura K, Tomino Y, Kriz W. Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. Am J Pathol 148: 1283–1296, 1996 [PMC free article] [PubMed] [Google Scholar]
- 110.Shmygol A, Noble K, Wray S. Depletion of membrane cholesterol eliminates the Ca2+-activated component of outward potassium current and decreases membrane capacitance in rat uterine myocytes. J Physiol 581: 445–456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem 280: 39786–39794, 2005 [DOI] [PubMed] [Google Scholar]
- 112.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586–16591, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang XD, Daggett H, Hanner M, Garcia ML, McManus OB, Brot N, Weissbach H, Heinemann SH, Hoshi T. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol 117: 253–274, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tian L, Chen L, McClafferty H, Sailer CA, Ruth P, Knaus HG, Shipston MJ. A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. FASEB J 20: 2588–2590, 2006 [DOI] [PubMed] [Google Scholar]
- 115.Tian L, McClafferty H, Chen L, Shipston MJ. Reversible tyrosine protein phosphorylation regulates large conductance voltage- and calcium-activated potassium channels via cortactin. J Biol Chem 283: 3067–3076, 2008 [DOI] [PubMed] [Google Scholar]
- 116.Trebak M, Lemonnier L, Smyth JT, Vazquez G, Putney JW., Jr Phospholipase C-coupled receptors and activation of TRPC channels. Handb Exp Pharmacol 179: 593–614, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Vaithianathan T, Bukiya A, Liu J, Liu P, Asuncion-Chin M, Fan Z, Dopico A. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J Gen Physiol 132: 13–28, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vazquez G, Wedel BJ, Trebak M, St. John Bird G, Putney JW., Jr Expression level of the canonical transient receptor potential 3 (TRPC3) channel determines its mechanism of activation. J Biol Chem 278: 21649–21654, 2003 [DOI] [PubMed] [Google Scholar]
- 119.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 76: 387–417, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang B, Rothberg BS, Brenner R. Mechanism of β4 subunit modulation of BK channels. J Gen Physiol 127: 449–465, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang X, Pluznick JL, Wei P, Padanilam BJ, Sansom SC. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol 287: C357–C364, 2004 [DOI] [PubMed] [Google Scholar]
- 123.Wang Z, Wei X, Zhang Y, Ma X, Li B, Zhang S, Du P, Zhang X, Yi F. NADPH oxidase-derived ROS contributes to upregulation of TRPC6 expression in puromycin aminonucleoside-induced podocyte injury. Cell Physiol Biochem 24: 619–626, 2009 [DOI] [PubMed] [Google Scholar]
- 124.Wartiovaara J, Ofverstedt LG, Khoshnoodi J, Zhang J, Mäkelä E, Sandin S, Ruotsalainen V, Cheng RH, Jalanko H, Skoglund U, Tryggvason K. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest 114: 1475–1483, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wedel BJ, Vazquez G, McKay RR, St. Bird G J, Putney JW., Jr A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process. J Biol Chem 278: 25758–25765, 2003 [DOI] [PubMed] [Google Scholar]
- 126.Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M, Ghofrani HA, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA 103: 19093–19098, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science 306: 2093–2097, 2004 [DOI] [PubMed] [Google Scholar]
- 128.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 129.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium 42: 205–211, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature 418: 880–884, 2002 [DOI] [PubMed] [Google Scholar]
- 131.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9: 636–645, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zeng XH, Xia XM, Lingle CJ. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J Gen Physiol 125: 273–286, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang H, Ding J, Fan Q, Liu S. TRPC6 up-regulation in Ang II-induced podocyte apoptosis might result from ERK activation and NF-kappaB translocation. Exp Biol Med 234: 1029–1036, 2009 [DOI] [PubMed] [Google Scholar]
- 134.Zhu MX. Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflügers Arch 451: 105–115, 2005 [DOI] [PubMed] [Google Scholar]
- 135.Zou S, Jha S, Kim EY, Dryer SE. A novel actin-binding domain on Slo1 calcium-activated potassium channels is necessary for their expression in the plasma membrane. Mol Pharmacol 73: 359–368, 2008 [DOI] [PubMed] [Google Scholar]
- 136.Zou S, Jha S, Kim EY, Dryer SE. The β1 subunit of L-type voltage-gated Ca2+ channels independently binds to and inhibits the gating of large-conductance Ca2+-activated K+ channels. Mol Pharmacol 73: 369–378, 2008 [DOI] [PubMed] [Google Scholar]