Abstract
A positive association between renin-angiotensin system, especially AT1 receptor, and oxidative stress in the pathogenesis of hypertension and cardiovascular/renal diseases has been suggested. However, the role of oxidative stress, especially superoxide radicals in renal sodium handling in response to AT1 and AT2 receptors, is not known. Therefore, the present study was designed to investigate the role of NAD(P)H oxidase (NOX), a major superoxide radical producing enzyme, in AT1 and AT2 receptor function on natriuresis/diuresis in Sprague-Dawley rats. The rats under anesthesia were intravenously infused with NOX inhibitor apocynin (3.5 μg·kg−1·min−1), the AT1 receptor antagonist candesartan (100 μg/kg; bolus), and the AT2 receptor agonist CGP-42112A (1 μg·kg−1·min−1) alone and in combinations. Candesartan alone significantly increased urinary flow (UF; μl/30 min) by 53 and urinary Na excretion (UNaV; μmol/min) by 0.4 over basal. Preinfusion of apocynin had no effect on the net increase in UF or UNaV in response to candesartan. On the other hand, apocynin preinfusion caused profound increases in CGP-42112A-induced UF by 72, UNaV by 1.14, and fractional excretion of Na by 7.8. Apocynin and CGP-42112A alone did not cause significant increase in UF or UNaV over the basal. CGP-42112A infusion in the presence of apocynin increased urinary nitrite/nitrates and cGMP over basal. The infusion of candesartan, apocynin, and CGP-42112A alone or in combinations had no effect on the blood pressure or the glomerular filtration rate, suggesting tubular effects on natriuresis/diuresis. The data suggest that NOX may have an antagonistic role in AT2 receptor-mediated natriuresis/diuresis possibly via neutralizing nitric oxide and thereby influence fluid-Na homeostasis.
Keywords: angiotensin II receptors, apocynin, nitric oxide
increased oxidative stress is one of the contributing factors in the development of hypertension and cardiovascular/renal diseases (1, 6). However, several studies suggest that under physiological conditions, basal level of reactive oxygen species (ROS) plays an important role in normal functioning of organs and cells such as maintaining vascular tone, cell growth, and renal Na homeostasis (4, 8, 18). NAD(P)H oxidase (NOX) is a component of cellular oxidative stress machinery, which produces superoxide radicals (7). Studies suggest that prolonged low-dose angiotensin II (ANG II) infusion possibly via stimulation of ANG II type 1 (AT1) receptors causes increase in the NOX expression and activity in kidney cortex (3, 10, 25). However, the functional end point due to the cross talk between NOX and ANG II receptors, AT1 and AT2 receptors, in normal physiological condition is yet to be determined.
It is well-documented that renal AT1 receptors via activation of renal Na transporters produce antinatriuretic effects and thus play a major role in maintaining renal Na/fluid homeostasis (12, 13). On the other hand, considerable evidence suggests that AT2 receptors exert natriuretic effect and thus influence the renal Na/fluid homeostasis (2). Both the AT1 and AT2 receptors are expressed in various nephron segments with maximal density in the proximal tubules, the site of highest Na reabsorption. Inasmuch as oxidative stress is implicated in renal Na homeostasis (18, 34), the role/effect of renal AT1 and AT2 receptors on the NOX or vice versa in terms of Na handling is not known. Therefore, the aim of our study was to determine whether NOX activity affects the natriuretic/antinatriuretic functions of ANG II receptors in Sprague-Dawley (SD) rats. We found that while there seems to be no interaction between NOX and AT1 receptor, NOX serves a potent inhibitor of AT2 receptor function on natriuresis and diuresis.
METHODS
Animal model.
Male SD rats (Harlan, Indianapolis, IN) weighing 250–310 g were used in the present study. The animals were housed in the University of Houston animal care facility and were maintained on standard rat chow and water ad libitum. The Institutional Animal Use and Care Committee approved the experimental protocols.
Experimental protocols.
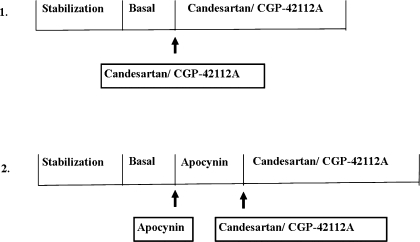
The surgical procedure was performed as described earlier (12, 30). Briefly, rats were anesthetized using Inactin (100 mg/kg body wt ip). Tracheotomy was performed to facilitate breathing. The left carotid artery and jugular vein were catheterized for measurement of blood pressure and saline/drug infusion, respectively. The left ureter was cannulated for urine collection. After 1 h of stabilization period, urine was collected at 30-min intervals. The first two collections were considered as basal. Thereafter, the AT1 receptor antagonist candesartan (100 μg/kg, bolus dose), NOX inhibitor apocynin (3.5 μg·kg−1·min−1), and AT2 receptor agonist CGP-42112 (1 μg·kg−1·min−1) alone or in combinations were intravenously infused. After the drug delivery, two more collections of urine were obtained at 30-min intervals each. The different protocols used in the experiment are shown in Fig. 1. Blood samples were collected at the end of each urine collection and stored in EDTA-coated tubes. Blood pressure and heart rate were recorded throughout the experimental period.
Fig. 1.
Schematic presentation of experimental protocols.
Renal function.
Urine collected for each collection period was measured to determine the urine flow rate (UF). Urinary Na concentration (mmol/l) was calculated using Flame photometer 480 (Ciba Corning Diagnostics, Norwood, MA). The UF and urinary Na concentration were used to compute the urinary Na volume (UNaV). Glomerular filtration rate (GFR; ml/min) was computed from creatinine clearance (mg/dl), which was measured by Creatinine Assay Kit (BioVision, Mountain View, CA). Fractional excretion of Na (FENa%) was computed using UNaV, plasma Na volume, and GFR as reported (12).
Total urinary nitrite/nitrates.
Total nitrite/nitrate in the urine samples was determined as a measure of nitric oxide (NO) production at baseline and during infusion of apocynin, CGP-42112A alone, or in combination. The values are represented as nmoles per minute. We followed the kit-described protocol. Briefly, urine was diluted 10-fold and a volume of 50 μl/well was used for the assay. Reconstituted NADH reagent and nitrate reductase (25 μl of each) were added to all wells. Plate was incubated for 30 min at 37°C followed by addition of 50 μl each of Griess reagent I and II. Plate was incubated again for 10 min at room temperature. Optical density was determined using a microplate reader at 540 nm.
Urinary cGMP levels.
Total cGMP level in urine was determined by using an ELISA kit. Briefly, 100 μl of standard/sample were added to appropriate wells. Fifty microliters of cGMP conjugate and 50 μl of primary antibody were added to each well and the plate was incubated at room temperature for 3 h. After incubation, content of each well was aspirated and washed four times with the wash buffer. Substrate solution (200 μl) was added and the plate was incubated for 30 min. Fifty microliters of stop solution were added and the optical density was determined using a microplate reader at wavelength 450 nm.
Chemicals.
Candesartan was a generous gift from AstraZeneca (Wilmington, DE). CGP-42112A was synthesized by 21st Century Biochemicals (Marlboro, MA). Apocynin and all other chemicals were purchased from Sigma. Creatinine assay kit was purchased from BioVision. The NO/nitrite/nitrate and cGMP assay kit were obtained from R&D Systems (Minneapolis, MN).
Statistical analysis.
Data are represented as means ± SE. One-way ANOVA with Newman-Keuls multiple comparison post hoc test was used to compare variations within groups. Value of P < 0.05 was considered statistically significant.
RESULTS
Effect of apocynin on candesartan-induced diuresis/natriuresis in SD rats.
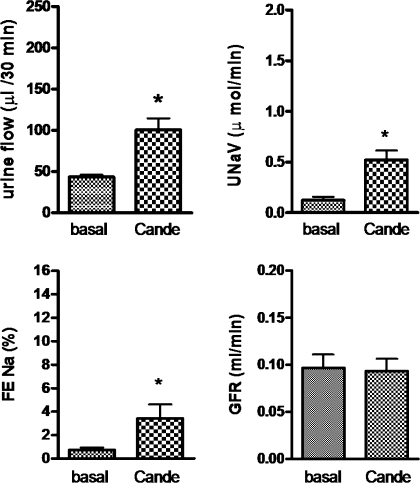
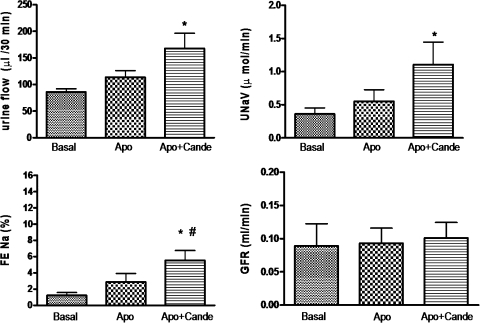
As shown in Fig. 2, a bolus dose of the AT1 receptor antagonist candesartan (100 μg/kg) produced a significant increase in UF (53.08 μl/30 min), UNaV (0.3985 μmol/min), and FENa% (2.673) over the basal. Infusion of apocynin (3.5 μg·kg−1·min−1), a NOX inhibitor, only modestly increased UF (27.77 μl/30 min), UNaV (0.1916 μmol/min), and FENa% (1.648); however, the increases are not significant over the basal (Fig. 3). Preinfusion of apocynin did not change the net natriuretic/diuretic response to candesartan. Apocynin and candesartan together had an additive effect on UF (82.07 μl/30 min), UNaV (0.7443 μmol/min), and FENa% (4.313) over the basal (Fig. 3). Similar results were observed when apocynin was infused after candesartan (data not shown).
Fig. 2.
Candesartan (Cande)-induced diuresis and natriuresis in Sprague-Dawley (SD) rats. The dose of Cande was 100 μg/kg iv bolus. The values in the bar graph are represented as means ± SE. Student's t-test was used to compare the data within the group. The value of P < 0.05 was considered significant (n = 5–10). *Significantly different from basal. FENa%, fractional excretion of Na; UNaV, urinary Na volume; GFR, glomerular filtration rate.
Fig. 3.
Effect of pretreatment of apocynin (Apo) on Cande-induced diuresis and natriuresis in SD rats. Apo was given at a rate of 3.5 μg·kg−1·min−1 iv infusion and the dose of Cande was 100 μg/kg iv bolus. The values in the bar graph are represented as means ± SE. One-way ANOVA with Newman-Keuls multiple comparison post hoc test was used to compare the data within the group. The value of P < 0.05 was considered significant (n = 5–10). *Significantly different from basal. #Significantly different from Apo.
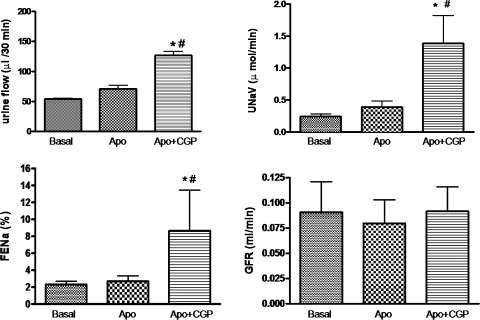
Effect of apocynin on CGP-42112A-induced diuresis/natriuresis in SD rats.
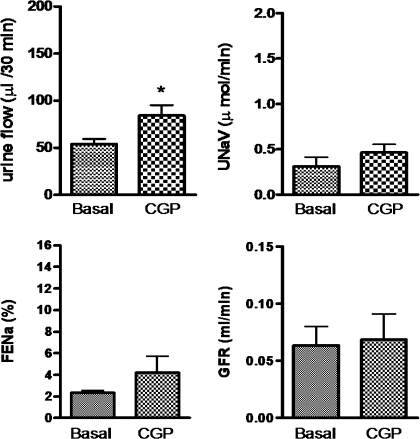
Intravenous infusion of CGP-42112A (1 μg·kg−1·min−1), an AT2 receptor agonist, produced moderate increase in UF (29.97 μl/30 min), UNaV (0.1537 μmol/min), and FENa% (1.449) over the basal; however, it was not statistically significant (Fig. 4). As seen already, infusion of apocynin (3.5 μg·kg−1·min−1) also did not cause significant increase in UF (16.90 μl/30 min), UNaV (0.1475 μmol/min), and FENa% (1.880) over the basal (Fig. 5). However, preinfusion of apocynin significantly increased the net diuretic/natriuretic effect of CGP-42112A. Apocynin and CGP-42112A together had a synergistic effect on UF (72.87 μl/30 min), UNaV (1.142 μmol/min), and FENa% (7.862) over the basal, apocynin alone, and CGP-42112A alone in SD rats (Fig. 5). Similar results were observed when apocynin was infused after CGP-42112A (data not shown).
Fig. 4.
CGP-42112A (CGP)-induced diuresis and natriuresis in SD rats. Intravenous infusion of CGP was given at a rate of 1 μg·kg−1·min−1. The values in the bar graph are represented as means ± SE. Student's t-test was used to compare the data within the group. The value of P < 0.05 was considered significant (n = 5–9). *Significantly different from basal.
Fig. 5.
Effect of pretreatment of Apo on CGP-induced diuresis and natriuresis in SD rats. Intravenous infusion of CGP and Apo was given at a rate of 1 and 3.5 μg·kg−1·min−1, respectively. The values in the bar graph are represented as means ± SE. One-way ANOVA with Newman-Keuls multiple comparison post hoc test was used to compare the data within the group. The value of P < 0.05 was considered significant (n = 5–9). *Significantly different from basal. #Significantly different from Apo.
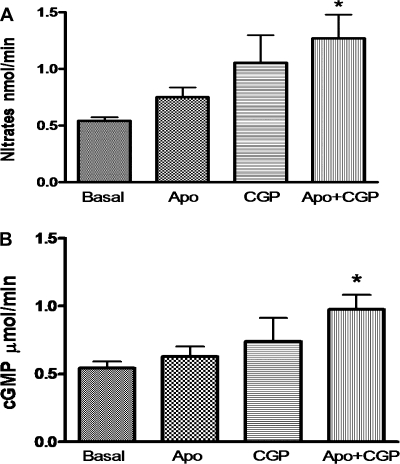
Urinary nitrates and cGMP levels.
Since AT2 receptor has been shown to be linked to NO/cGMP pathway leading to natriuresis/diuresis, we measured urinary nitrates and cGMP levels. The level of nitrates (nmol/min) increased modestly with apocynin (0.21) and CGP-42112A (0.51) alone; however, it was not significant over the basal. A combination of apocynin and CGP-42112A produced a significant increase in nitrate (0.73) levels over the basal (Fig. 6A). Similarly, there was a significant increase (0.4304 μmol/min) in the cGMP level over the basal (0.5442) when apocynin and CGP-42112A were given in combination (Fig. 6B). Apocynin (0.08626) and CGP-42112A (0.1958) alone did not produce a significant increase in urinary cGMP level over the basal.
Fig. 6.
Total nitrates (A) and cGMP (B) measured in urine samples. The values in the bar graph are represented as means ± SE. One-way ANOVA with Newman-Keuls multiple comparison post hoc test was used to compare the data within the group. The value of P < 0.05 was considered significant; n = 4–10. *Significantly different from basal.
Blood pressure and GFR.
There were no significant changes in the GFR with administration of candesartan, CGP-42112A, and/or apocynin over the basal (Figs. 2–5). Also, administration of these drugs had no effect on blood pressure [mean arterial blood pressure (in mmHg): basal = 86.13 ± 4.14, candesartan = 80.20 ± 3.7; basal = 86.83 ± 2.1, apocynin = 78.85 ± 3.9, apocynin + candesartan = 72.33 ± 2.3; basal = 77.5 ± 1.8, CGP-42112A = 72.33 ± 1.5; basal = 80.44 ± 6.3, apocynin = 74.37 ± 2.4, apocynin + CGP-42112A = 69.42 ± 3.6]. These data suggest the tubular effect of the drugs. The tubular effect is further supported by the changes in the FENa% as the results described above. These results are consistent with the earlier reports (11, 12, 18).
DISCUSSION
The major findings of this study are that the natriuretic function of AT2 receptors is masked by NOX by decreasing the bioavailability of NO as a potential mechanism and there is no cross talk between NOX and AT1 receptors function on natriuresis and diuresis in SD rats.
Oxidative stress is mainly defined as an imbalance between rate of generation of ROS (superoxides, hydroxyl ion, peroxynitrite, and H2O2) and the rate at which they are scavenged by the antioxidant enzymes (catalase, glutathione peroxidase, and superoxide dismutases) and other ROS scavengers such as uric acid and ascorbic acid (3, 29, 31). It is agreed on that increased ROS production is deleterious to cells (1, 6); however, their baseline concentration is needed for normal functioning of the cells (4, 8).
Growing bodies of evidence support the role of renin-angiotensin system (RAS) in development of oxidative stress (10, 23, 25). Prolonged ANG II infusion has been shown to increase renal cortical NOX activity (3, 25). ANG II infusion develops a pressor response and mimics the pathological condition of ANG II-mediated hypertension (3, 25). However, in our study, we investigated the role of NOX inhibition on AT1 and AT2 receptor-mediated natriuresis and diuresis in normal SD rats.
AT1 and AT2 receptors are reported to be expressed in the various kidney parts, including in the proximal tubules, which is the site of major Na reabsorption (12, 21, 27). In the present study, inhibition of AT1 receptors by candesartan or selective activation of AT2 receptors by CGP-42112A caused natriuresis. However, the natriuresis produced by CGP-42112A was not significant over the basal. The less natriuretic response to AT2 receptor activation compared with AT1 receptor activation can be attributed to either low renal AT2 receptor density (5, 15) or to masking effect of NOX on AT2 receptor function as determined in the present study.
It is worth mentioning that apocynin was used as NOX inhibitor in the study; however, some other studies showed its antioxidant activity (14, 32). The inhibitory role of apocynin is well-documented demonstrating that it prevents the translocation of p47phox, NOX cytosolic subunit, to the membrane reducing its assembly with the membrane-bound NOX subunits, gp91phox and p22phox, and thus inhibits NOX activity (29). Whatever property apocynin may have, NOX inhibitor vs. antioxidant activity, the present study clearly demonstrates that reducing baseline ROS levels significantly impacts AT2 receptor function. The present study also demonstrated that apocynin alone produces a certain degree of natriuresis/diuresis indicating an independent role of ROS on Na and fluid homeostasis. This finding is in agreement with another study supporting the natriuretic role of NOX by showing a dose-dependent increase in Na excretion with apocynin (18). The mechanism for this was attributed to an increase in GFR due to release of NO with apocynin (18). However, in our study, we did not observe any significant increase in NO with apocynin infusion, and also the GFR remained unchanged. The difference between our study and the study by Lopez et al. (18) could possibly be due to the low dose of apocynin, used by us.
The response of candesartan on natriuresis did not change by NOX inhibitor. Studies supported that during pathological conditions, activation of AT1 receptors increases NOX activity (3, 7, 19). Had there been any relationship between AT1 receptors and NOX in these SD rats the natriuretic response of NOX alone should have been comparable to that of candesartan. This suggests that under normal physiological conditions, NOX may not be a part of AT1 receptor signaling leading to antinatriuresis. However, during pathological conditions, it may be the higher AT1 receptor function in response to elevated ANG II concentration which links RAS to oxidative stress. Selective activation of the AT2 receptor by the agonist CGP-42112A while NOX was inhibited, to our surprise, produced a tremendous increase in UF, UNaV, and FENa% over the basal. The mechanism behind this increase in renal function by a combination of CGP-42112A and apocynin at present is not clear. However, it can be speculated that there is a certain interaction between the downstream signaling molcules, which amplifies the net urinary Na out-put.
The superoxide radicals are reported to quickly quench the NO molcules and convert them to peroxynitrite (22, 24, 26). The NO is known to inhibit various Na transporters in the proximal tubules with a potential to regulate urinary Na excretion (16, 17). Several studies including from our laboratory showed that CGP-42112A via AT2 receptors activates NO/cGMP pathway leading to an increase in urinary Na excretion (2, 9, 11, 20, 28, 33). Considering these findings, we measured the total nitrate (as an index of NO levels) and cGMP excreted in urine, in response to apocynin and CGP-41221A infusion alone or in combination, as a potential mechanism of enhanced AT2 receptor-mediated natriuresis in the presence of NOX inhibitor apocynin. The total nitrate and cGMP significantly increased over the basal when apocynin and CGP-42212A were administered together. However, the increase in total nitrate and cGMP levels was not synergistic as we observed with Na. Although reasons are not clear why we did not see a similar response with nitrate/cGMP, a plausible explanation for this could be that systemic administration of these drugs to some extent could have caused NO/cGMP release from the vasculature which might have masked the tubular NO/cGMP release affecting the net urinary nitrate and cGMP excretion pattern. On the other hand, the changes in Na excretion in response to drugs are a tubular effect as suggested by an increase in FENa% without any changes in GFR or blood pressure. Also, certain threshold increase in NO-cGMP could have been enough to synergistically amplify the AT2 receptor signal leading to inhibition in Na transport and promoting natriuresis.
Considering these studies, it may be proposed that in pathological conditions where there is increased oxidative stress, lesser bioavailability of NO will decrease AT2 receptor-mediated natriuresis leaving AT1 receptor-mediated antinatriuresis unopposed. This might increase Na retention-shifting pressure natriuresis contributing to hypertension. On the other hand, inhibition or lowering of NOX activity can lead to beneficial effects of AT2 receptors like cell proliferation, vasorelaxation, and natriuresis.
In summary, we found that NOX inhibitor causes synergistic effect on AT2 receptor-mediated natriuresis/diuresis in SD rats, suggesting a potential interaction between AT2 receptor and NOX. Such interaction appears to be at the level of reactions between NO and superoxide reducing NO bioavailability and thereby regulating renal tubular Na reabsorption. To our knowledge, this is the first report suggesting a potential role of NOX via reducing AT2 receptor function in tubular Na reabsorption/excretion and blood pressure control.
Perspectives
Present studies demonstrate the beneficial role of inhibition of NOX and selective activation of renal AT2 receptors on natriuresis and diuresis. In management of pathological conditions like hypertension and renovascular diseases, AT1 receptor blockers (ARBs) are preferentially used. Considering that AT1 receptors activate ROS during pathological conditions, ARBs will bring the ROS activity down. On the other hand, ARBs can lead to unopposed action of ANG II on AT2 receptors. So, the present study supports the notion that it is not only blocking of AT1 receptors rather it is activation of AT2 receptors which brings about the beneficial changes during pathological conditions.
GRANTS
The present work was supported by National Institutes of Health Grant R01-DK-61578, Grant to Enhance and Advance Research by the University of Houston and Norman Hackerman Advanced Research Program, Texas.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Candesartan was a generous gift from AstraZeneca.
REFERENCES
- 1.Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes 54: 2219–2226, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension 35: 155–163, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117–R124, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chakraborti T, Ghosh SK, Michael JR, Batabyal SK, Chakraborti S. Targets of oxidative stress in cardiovascular system. Mol Cell Biochem 187: 1–10, 1998 [DOI] [PubMed] [Google Scholar]
- 5.De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000 [PubMed] [Google Scholar]
- 6.Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson's disease. Free Radic Biol Med 44: 1873–1886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol 296: F1484–F1493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas JA, Krier JD, Bolterman RJ, Juncos LA, Romero JC. Low-dose angiotensin II increases free isoprostane levels in plasma. Hypertension 34: 983–986, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol 290: F1430–F1436, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Hakam AC, Hussain T. Renal angiotensin II type 2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension 45: 270–275, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hall JE. The kidney, hypertension, and obesity. Hypertension 41: 625–633, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press 12: 70–88, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Liang M, Knox FG. Nitric oxide activates PKCα and inhibits Na+-K+-ATPase in opossum kidney cells. Am J Physiol Renal Physiol 277: F859–F865, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Liang M, Knox FG. Production and functional roles of nitric oxide in the proximal tubule. Am J Physiol Regul Integr Comp Physiol 278: R1117–R1124, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Lopez B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension 42: 1150–1156, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am 93: 569–582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millatt LJ, Abdel-Rahman EM, Siragy HM. Angiotensin II and nitric oxide: a question of balance. Regul Pept 81: 1–10, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol 277: F437–F446, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Nistala R, Whaley-Connell A, Sowers JR. Redox control of renal function and hypertension. Antioxid Redox Signal 10: 2047–2089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz MC, Sanabria E, Manriquez MC, Romero JC, Juncos LA. Role of endothelin and isoprostanes in slow pressor responses to angiotensin II. Hypertension 37: 505–510, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 31, Suppl 2: S170–S180, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18: 2439–2446, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Shah S, Hussain T. Enhanced angiotensin II-induced activation of Na+, K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens 28: 29–40, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest 100: 264–269, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefanska J, Pawliczak R. Apocynin: molcular aptitudes. Mediators Inflamm 2008: 106507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tallam LS, Jandhyala BS. Significance of exaggerated natriuresis after angiotensin AT1 receptor blockade or angiotensin-converting enzyme inhibition in obese Zucker rats. Clin Exp Pharmacol Physiol 28: 433–440, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Titova E, Ostrowski RP, Sowers LC, Zhang JH, Tang J. Effects of apocynin and ethanol on intracerebral hemorrhage-induced brain injury in rats. Clin Exp Pharmacol Physiol 34: 845–850, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Zhuo JL, Li XC. Novel roles of intracrine angiotensin II and signalling mechanisms in kidney cells. J Renin Angiotensin Aldosterone Syst 8: 23–33, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou AP, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001 [DOI] [PubMed] [Google Scholar]