Abstract
We transformed mouse podocytes by ectopic expression of cyclin-dependent kinase 4 (CDK4). Compared with podocytes transformed with a thermo-sensitive SV40 large T antigen mutant tsA58U19 (tsT podocytes), podocytes transformed with CDK4 (CDK4 podocytes) exhibited significantly higher expression of nephrin mRNA. Synaptopodin mRNA expression was significantly lower in CDK4 podocytes and in tsT podocytes under growth-permissive conditions (33°C) compared with tsT podocytes under growth-restricted conditions (37°C), which suggests a role for cell cycle arrest in synaptopodin mRNA expression. Confluent CDK4 podocytes showed significantly higher mRNA expression levels for nephrin, synaptopodin, Wilms tumor 1, podocalyxin, and P-cadherin compared with subconfluent cultures. We carried out experiments to clarify roles of various factors in the confluent podocyte cultures; our findings indicate that cell-cell contact promotes expression of five podocyte marker genes studied, that cellular quiescence increases synaptopodin and podocalyxin mRNA expression, and that soluble factors play a role in nephrin mRNA expression. Our findings suggest that CDK4 podocytes are useful tools to study podocyte biology. Furthermore, the role of cell-cell contact in podocyte gene expression may have relevance for podocyte function in vivo.
Keywords: cyclin-dependent kinase 4, SV40 large T antigen, nephrin, synaptopodin, Wilms tumor-1, P-cadherin
podocyte cultures, especially podocyte cell lines transformed by thermosensitive SV40 large T antigen (SV40 T), have been widely used to study podocyte biology (19, 25). However, as we recently reported in studies on a generation of mouse and human podocyte cell lines, podocytes transformed by thermosensitive SV40 T (tsT) exhibit phenotypic differences compared with podocytes in vivo, with reduced expression of certain differentiation marker genes (13, 24).
Cyclin-dependent kinase 4 (CDK4), a catalytic subunit of the cyclin D-CDK4 serine/threonine kinase complex, is a critical regulator of the cell cycle. CDK4 is the first kinase activated by mitogenic signals. The kinase activity of CDK4 is specifically involved in progression through G1, via phosphorylation of retinoblastoma (Rb) family members (4). Recently, Ramirez et al. (22) immortalized human bronchial epithelial cells by ectopic expression of CDK4 and human telomerase (hTERT). Microarray analysis showed that CDK4/hTERT-immortalized cells showed significantly different gene expression profiles compared with cells immortalized with human papiloma viral oncoproteins E6/E7 or SV40T and that CDK4-transformed cells clustered with primary bronchial epithelial cells. In addition, recent studies showed that cells immortalized with CDK4 are useful tools to study physiology and pathophysiology (8, 23, 30).
We set out to develop an alternative approach to transforming podocytes by comparing the phenotype among primary cells, CDK4-transformed podocytes (CDK4-podocytes) and thermosensitive SV40 T mutant tsA58U19 (tsT-podocytes). We found that CDK4-podocytes and tsT-podocytes showed distinct gene expression patterns. Furthermore, we found that confluent CDK4-podocyte cultures showed higher levels of gene expression for multiple podocyte differentiation genes compared with subconfluent or lower density culture. We defined the role of cellular quiescence (reversible cell cycle arrest) and soluble factors in the confluence effects and conclude that cell-cell contact has a distinct effect in driving expression of particular podocyte differentiation genes.
METHODS
Glomerular isolation.
Isolation of glomeruli from mouse kidneys was performed as previously reported (29). Briefly, a 5-wk-old female CD1 mouse was anesthetized with peritoneal injection of tribromethanol (Avertin, Sigma, St. Louis, MO) and perfused through the heart with Dynabeads M-450 tosylactivated (Invitrogen, Carlsbad, CA) prepared in 50 ml of Hank's buffered salt solution (HBSS) with calcium and magnesium. Kidneys were minced with a razor blade, digested with collagenase and DNAse for 30 min, and passed through 100-μm cell strainer twice. Gomeruli were collected with a magnet and washed with HBSS four times, and placed in culture. Animal experiments were approved by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Animal Care and Use Committee and carried out under the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Primary culture of podocytes.
Primary podocyte cultures were established as previously reported (1, 14). Briefly, isolated glomeruli were plated on type I collagen-coated culture ware (BD Biosciences, San Jose, CA) in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 U/ml of penicillin G sodium (penicillin G), and 100 μg/ml of streptomycin sulfate (streptomycin). Decapsulation of Bowman's capsule was confirmed under phase contrast microscopy. After incubation for 72 h, glomeruli and out-migrating cells were harvested by trypsin digestion. Glomeruli were removed by a 50-μm cell strainer and magnet, and cells were subcultured.
Retroviral infection and culture.
Primary podocytes, after two passages, were infected with retrovirus carrying CDK4 (22) or SV40 large T antigen mutant tsA58U19 (11, 27) with 8 μg/ml polybrene. Beginning after 72 h, cells were treated with 0.2 mg/ml G418 at 37°C (CDK4) or 33°C (tsA58U19) for 14 days. Transformed cells were maintained in RPMI 1640 medium supplemented with 10% FBS, penicillin G, and streptomycin on type I collagen-coated culture ware (BD Biosciences). The SV40-transformed mouse mesangial cell line MES13 (ATCC, Manassas, VA) was used as a negative control for podocyte marker gene expression. The cells were grown in DMEM supplemented with 10% FBS, penicillin G, and streptomycin.
In a preliminary study, nephrin mRNA expression was compared among CDK4-podocytes at passages 10, 15, and 20. Cells at passage 10 showed significantly higher nephrin mRNA expression than passage 15 or 20 while no difference was observed between passages 15 and 20. Thus, CDK4-podocytes from passage 15 to 20 were used to minimize an effect of serial passage on gene expression analysis. tsT-podocytes at passage 10 were used for indicated experiments.
For the cell density study of CDK4-podocytes, cells were plated at 2.13 × 104/cm2 in a six-well dish, 0.71 × 104/cm2 in 60-mm dishes, 0.24 × 104/cm2 on 100-mm dishes, or 0.08 × 104/cm2 in 100-mm dishes and incubated for 72 h. For the cell density study of tsT-podocytes, cells grown at 33°C were suspended with trypsin digestion, plated at 2.13 × 104/cm2 in a six-well dish or 0.24 × 104/cm2 on 100-mm dishes, and cultured for 5 days at 37°C. For the time course study, confluent CDK4-podocytes were suspended by trypsin digestion, seeded at 0.71 × 104/cm2 on 60-mm dishes, and RNA samples were collected daily until day 5. Half the volume of growth medium was replaced with fresh growth medium at day 3. For the serum deprivation study, CDK4-podocytes were plated at 0.71 × 104/cm2 on 60-mm dishes and the growth medium was replaced with medium supplemented with 0.1% FBS at 48 h. The cells were analyzed at 48, 96, and 144 h after start of the culture. For the conditioned medium study, confluent CDK4-podocytes were fed with fresh growth medium; 24 h later, the conditioned medium was collected, centrifuged to remove cellular debris, and frozen. CDK4-podocytes were plated at 0.71 × 104/cm2 on 60-mm dishes and 48 h later, growth medium was replaced with the conditioned medium or as a control, fresh growth medium; after 24 h of further culture, cells were harvested for RNA.
Quantitative RT-PCR.
Total RNA was purified by miRNeasy Mini Kit (Qiagen, Valencia, CA) and cDNA was synthesized using High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA). Quantitative (q) RT-PCR was carried out in ABI Prism 7900HT Sequence Detection System (Applied Biosystems) with Power-SYBR green PCR Master Mix (Applied Biosystems). Relative amounts of indicated genes were calculated by the ΔΔCt method and normalized to β-actin. The primers were as follows: mouse nephrin forward (F) GCCACCACCTTCACACTGAC, reverse (R) AGACCACCAACCGCAAAGAG; mouse synaptopodin (F) CATCGGACCTTCTTCCTGTG, (R) TCGGAGTCTGTGGGTGAG; mouse WT1 (F) CCAGCCTACCATCCGCAAC, (R) GGGTCCTCGTGTTTGAAGG; mouse podocalyxin (F) GAGCACAGCGAGCCATCC, (R) GTGGAGACGGGCAATGTAG; mouse P-cadherin (F) CCTGGAAGGGTGGTTTCATC, (R) TCTCGCGTGTCATCTTCTGG; mouse MYH9 (nonmuscle myosin heavy chain IIA, NMHC-IIA) (F) GAAGAAGGTGAAGGTGAACAAGG, (R) TCTGTGATGGCGTAGATGTGG; mouse podocin (F) GGGCATCAAAGTGGAGAGAACTG, (R) TGGACAGCGACTGAAGAGTGTG; mouse histone cluster 2, H4 (Hist2h4) (F) CACCGAGCACGCCAAGCGCA, (R) TTGAAGCGGCGGCGTCTAGC; mouse GAPDH (F) TGCAGTGGCAAAGTGGAGATT, (R) TTGAATTTGCCGTGAGTGGA; mouse β-actin (F) GTCCACACCCGCCACCAG, (R) TGACCCATTCCCACCATCAC.
The absence of genomic DNA contamination was confirmed by demonstration that there was no amplification following cDNA synthesis and PCR in the absence of reverse transcriptase (RT). qRT-PCR products were subjected to agarose gel electrophoresis and a single band of the expected size was confirmed. RT-PCR products using mouse kidney tissue were used as a positive control.
Immunofluorescent staining and phalloidin staining.
Cells cultured on type I collagen-coated coverslips (BD Biosciences) in six-well dishes were fixed by ethanol acetone (1:1) for 20 min at −20°C or 4% paraformaldehyde in PBS for 30 min at room temperature followed by permeabilization with 0.2% Triton X-100 in PBS for 10 min. After incubation with a blocking solution (2% FBS, 2% bovine serum albumin, 0.2% gelatin in PBS) for 30 min, cells were probed with mouse monoclonal anti-synaptopodin antibody, Clone G1D4 (Fitzgerald Industries International, Concord, MA) ready to use, mouse monoclonal anti-nestin antibody, clone Rat-401, (Millipore) at a dilution of 1:200, mouse monoclonal anti-WT1 antibody, clone 6F-H2 (Millipore) at 1:100 dilution, mouse monoclonal anti-pan cytokeratin (mixture; Sigma) at 1:100 dilution, rabbit polyclonal anti-NMHC-IIA (Covance, Vienna, VA) at 1:500 dilution, rabbit polyclonal anti-nonmuscle myosin heavy chain IIB (NMHC-IIB; Covance) at 1:500 dilution, rabbit polyclonal anti-Ki67 (Abcam, Cambridge, MA) at 1:500 dilution, rabbit polyclonal anti-desmin antibody (Sigma) at 1:25 dilution, or guinea pig anti-nephrin antibody (Fitzgerald Industries International) at 1:100 as a primary antibody for 1 h at room temperature. Signals were visualized by incubating cells with Alexa Fluora 488-conjugated anti-mouse, anti-rabbit, or anti-guinea pig secondary antibody (Invitrogen). To stain F-actin, cells were incubated with Alexa Fluora 594-phalloidin (Invitrogen) at 1:200 dilution for 1 h. Normal mouse IgG or normal rabbit IgG was used as a negative control to determine the specificity of each antibody.
Western blotting.
Cultured cells were lysed and a mouse kidney tissue was homogenized in RIPA buffer with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Thirty micrograms protein extracts were electrophoresed on NuPAGE 4–12% bis-tris gel (Invitrogen) under reducing conditions and transferred to nitrocellulose membrane. The membrane was incubated with polyclonal guinea pig anti-nephrin antibody (Fitzgerald Industries International) at 1:500 dilution or mouse monoclonal anti-β actin antibody (Sigma) at 1:5,000 at 4°C overnight, followed by secondary antibody to guinea pig IgG conjugated with horseradish peroxidase. The blots were developed with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL).
Cell proliferation assay.
Cells cultured in six-well plates, 60-mm or 100-mm dishes at various densities were incubated with 10 μM 5-bromo-2′-deoxyuridine (BrdU; Sigma) for 2 h. The cells were then suspended in growth medium by trypsin digestion, plated in 96-well plates at 1.0 × 104/well, and centrifuged for 10 min at 1,000 rpm at 208 g. Supernatants were discarded and the cells were subjected to cell proliferation assay using BrdU Cell Proliferation Assay (EMD Chemicals, Gibbstown, NJ) according to the manufacturer's instruction.
Statistics.
Statistical analyses were carried out using Prism 5 software (GraphPad Software, San Diego, CA). Significance was evaluated by one-way ANOVA, using Bonferroni testing for intergroup comparisons. A P value of <0.05 was taken as significant.
RESULTS
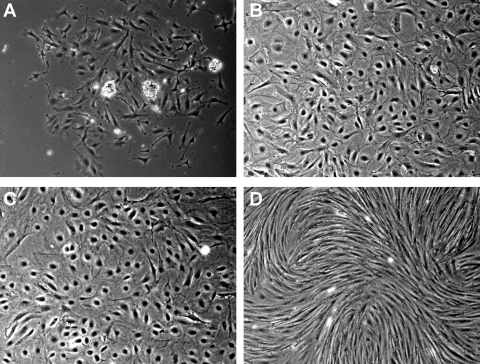
Primary culture of mouse podocytes was established, with representative morphology at 72 h after glomerular isolation shown in Fig. 1A. At this time, irregular shaped cells had migrated from glomeruli. Glomeruli were removed at this time point. Following two subcultures, cells were noted to be larger than before subculture and formed epithelial cell-like monolayers (Fig. 1B). We transformed the primary glomerular epithelial cells after subculturing twice using two different transgenes: CDK4 or tsT, the conventional method. CDK4-podocytes (Fig. 1C) and tsT podocytes, cultured under growth-restricted condition (GR; Fig. 1D), had distinct morpholgies. The CDK4-podocytes preserved an epithelial morphology, while tsT-podocytes exhibited elongated spindle shape.
Fig. 1.
Podocytes transformed by CDK4 display different morphology from podocytes transformed by thermosensitive SV40 large T antigen. A: irregular shaped epithelial cells outgrowing from isolated mouse glomeruli were observed at 72 h after start of the culture. Glomeruli were removed at 72 h and cells were subcultured. B: cells subcultured twice showed irregular or spherical shape and it was larger and more flattened than before subculture. C: cells transformed by CDK4 exhibited similar morphology to the primary cells. D: on the other hand, cells transformed by tsA58U19 and grown under growth-restricted condition showed an elongated spindle shape. Original magnification ×400.
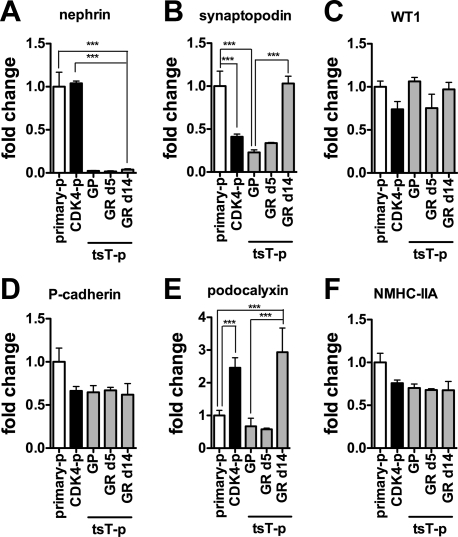
We compared podocyte marker gene expression, assessed by qRT-PCR, between primary podocytes passaged three times, tsT podocytes passaged nine times, and CDK4 podocytes passaged 14 times to define the phenotypic effect of the transformation (Fig. 2). Compared with primary murine podocyte cultures, nephrin mRNA was diminished in tsT-podocytes under both GP and GR conditions, while it was well-preserved in CDK4-podocytes (Fig. 2A). Synaptopodin mRNA expression was significantly lower in CDK4-podocytes than in primary cells. On the other hand, significant upregulation of synaptopodin mRNA after growth restriction was observed in tsT-podocytes. Upregulation after growth restriction in tsT-podocytes was also observed for podocalyxin mRNA expression. WT1, P-cadherin, and MYH9 (NMMHC-IIA) mRNAs were mostly preserved in both CDK4-podocytes and tsT-podocytes and did not change after growth restriction in tsT-podocytes. Podocin mRNA was not detected in primary cells, CDK4-podocytes, or tsT podocytes (data not shown). These findings suggest that CDK4-podocytes and tsT-podocytes have distinct phenotypes in terms of morphology and gene expression. We note that the mRNA expression levels of nephrin, synaptopodin, WT1, P-cadherin, podocalyxin, and MYH9 in mesangial cell line MES13 used as a negative control were significantly lower than those of CDK4-podocytes (Fig. S1A; the online version of this article contains supplemental data).
Fig. 2.
mRNA expression of podocyte markers in podocytes transformed by CDK4 is distinct from podocytes transformed by thermosensitive SV40 large T antigen. Expression of nephrin (A), synaptopodin (B), Wilms tumor 1 (WT1; C), P-cadherin (D), podocalyxin (E), or nonmuscle myosin heavy chain IIA (MYH9; F) mRNA in primary podocytes (primary-p), podocytes transformed by CDK4 (CDK4-p), or podocytes transformed by tsA58U19 (tsT-p) under growth-permissive condition (GP), growth-restricted condition (GR) day 5, or GR day 14 grown in confluence was analyzed by quantitative RT-PCR. A: nephrin mRNA was diminished in tsT-p in contrast to CDK4-p in which it was preserved. B: synaptopodin mRNA was suppressed in CDK4-p and tsT-punder GP and GR at day 5, whereas it was retained in tsT-p under GR at day 14. D: podocalyxin mRNA was higher in CDK4-p and tsT-p under GR day 14 compared with primary podocytes. C, D, F: expression of WT1, P-cadherin, and MYH9 mRNA was mostly preserved in both CDK4-p and tsT-p. The data were normalized to β-actin mRNA. Data are presented as means ± SD of triplicate cultures, relative to primary podocytes. ***P < 0.001.
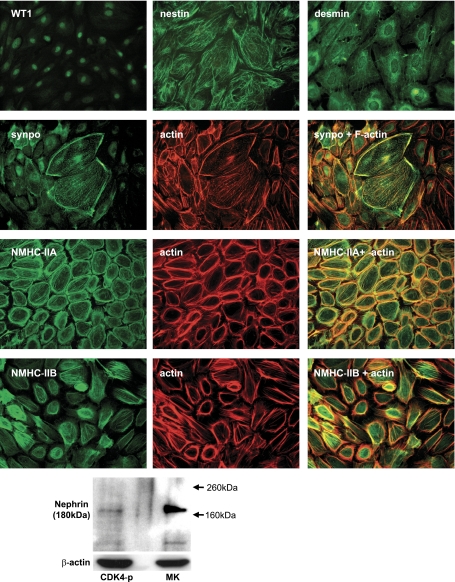
We next characterized CDK4-podocytes for podocyte markers by immunofluorescent staining (Fig. 3). Transcription factor WT1 was identified in nucleus. Intermediate filament proteins nestin and desmin were expressed in a fine filamentous pattern in the cytoplasm. Cortical F-actin and a moderate degree of stress fiber formation were demonstrated by phalloidin staining. The actin-binding protein synaptopodin was expressed in the cytoplasm, closely colocalized with F-actin, and expression was especially in flat, large cells suggestive of either quiescence or senescence. NMHC-IIA (MYH9 gene product) and NMHC-IIB (MYH10 gene product) were stained in the cytoplasm and colocalized with F-actin. Notably, NMHC-IIA was present in cortical actin and stress fibers, while NMHC-IIB was largely localized with stress fibers. Although nephrin was not detected by immunofluorescent staining, low expression of nephrin was observed by Western blotting (Fig. 3). Cytokeratins, a family of intermediate filament protein characteristic of parietal epithelial cells, were not expressed. Specificity of the immunostaining was confirmed by lack of signals in normal mouse or rabbit IgG control (data not shown).
Fig. 3.
Podocyte culture transformed by CDK4 expresses podocyte marker proteins. Immunofluorescent staining of podocytes transformed by CDK4 was carried out for WT1, nestin, desmin, synaptopodin, nonmuscle myosin heavy chain IIA (NMHC-IIA), or nonmuscle heavy chain IIB (NMHC-IIB). Transcription factor WT1 was localized to nuclei. Intermediate filament protein nestin and desmin exhibited a fine filamentous pattern. The actin-binding protein synaptopodin colocalized with actin and was expressed most abundantly in large flattened cells suggestive of quiescent or senescent cells. The nonmuscle myosin heavy chains NMHC-IIA and NMHC-IIB were detected and colocalized with actin. Original magnification: WT1, ×200; and others, ×400. Nephrin expression was detected by Western blotting. The expression level was lower than that of mouse kidney tissue used as a positive control. β-Actin served as a loading control.
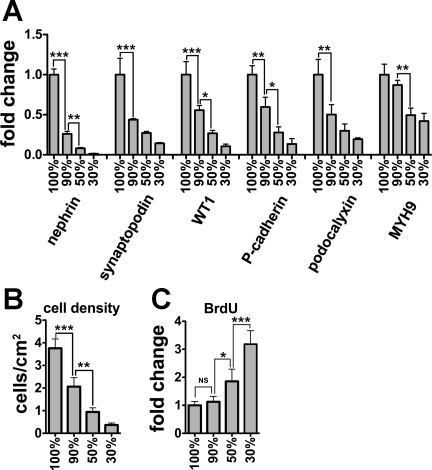
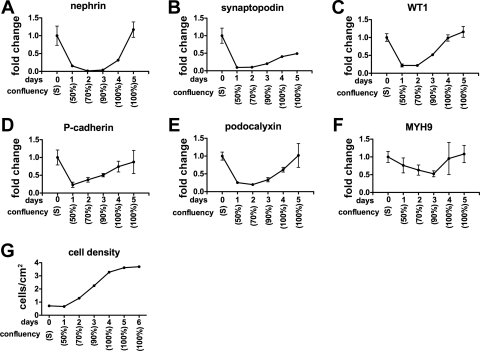
To investigate culture methods that might enhance expression of podocyte marker genes, we compared mRNA expression by qRT-PCR among CDK4-podocytes cultured at several densities (Fig. 4A). Interestingly, mRNA expression of all genes analyzed (nephrin, synaptopodin, WT1, P-cadherin, podocalyxin, and MYH9) was highest in cells in confluent cells and diminished with decreasing cell density. The expression level correlated directly with cellular density, with the weakest effect seen for MYH9 (Fig. 4B). Notably, even subconfluent cells (estimated at 90% confluence) exhibited significantly lower mRNA expression of all genes, with the exception of MYH9, compared with confluent cultures. To exclude the possibility that the high expression of these genes in the confluent culture was due to downregulation of the normalizing gene β-actin, we analyzed mRNA expression of Hist2h4 and GAPDH, alternative internal control genes to β-actin. Hist2h4 or GAPDH expression normalized to β-actin was not significantly different among various densities (Fig. S1B). To demonstrate that the effects of cell density extended to podocytes other than CDK4-podocytes, we cultured tsT-podocytes at high or low density and compared mRNA expression for the six podocyte markers. Expression of all the genes analyzed was significantly higher in tsT-podocytes at high density than low density (Fig. S1C).
Fig. 4.
Culture at high cellular density induces mRNA expression of multiple podocyte markers. A: mRNA expression of nephrin, synaptopodin, WT1, P-cadherin, podocalyxin, and nonmuscle myosin heavy chain IIA (MYH9) in podocytes transformed by CDK4 at diverse cell densities was analyzed by quantitative RT-PCR, normalized to β-actin mRNA, and shown relative to expression at 100% confluence. Compared with confluent cells, subconfluent cells exhibited significantly lower mRNA expression of every gene, except for MYH9 for which the differences were only apparent at 50% confluence. B: corresponding number of cells/cm2 is shown. C: cell proliferation was measured by the rate of 5-bromo-2′-deoxyuridine (BrdU) incorporation. BrdU incorporation levels were similar between confluent and subconfluent cells, but were significantly higher in cells at 50 and 30% confluent cells. Cellular confluency (A, B, C) are estimates. Data are presented as means ± SD of triplicate cultures (A–C). *P < 0.05. **P < 0.001. ***P < 0.001. NS, not significant.
To further evaluate an effect of cellular density on podocyte marker gene expression, we carried out a time course study, using cells harvested at various times after plating (Fig. 5). Confluent cells suspended in medium before plating in dishes (day 0) exhibited high expression of each gene. Expression of each gene was immediately diminished after subculture, reached the lowest levels within 2 days, and then consistently increases as cellular density was increased, with more modest effects for synaptopodin and MYH9 compared with the other genes.
Fig. 5.
Time course study for podocyte marker mRNA expression in podocytes transformed by CDK4. Podocytes transformed by CDK4 were grown in confluence, passaged, and RNA samples were collected every day until day 5 for expression of nephrin (A), synaptopodin (B), WT1 (C), P-cadherin (D), podocalyxin (E), and MYH9 (F) mRNA. Cells suspended in medium before plating exhibited high mRNA expression of each gene. Expression of all genes except MYH9 were decreased at day 1, reached a nadir at day 2, and then increased as cellular density increased (G). Gene expression data were normalized to β-actin mRNA. Data are presented as means ± SD of triplicate cultures, expressed relative to the cells at day 0. S, cells in suspension.
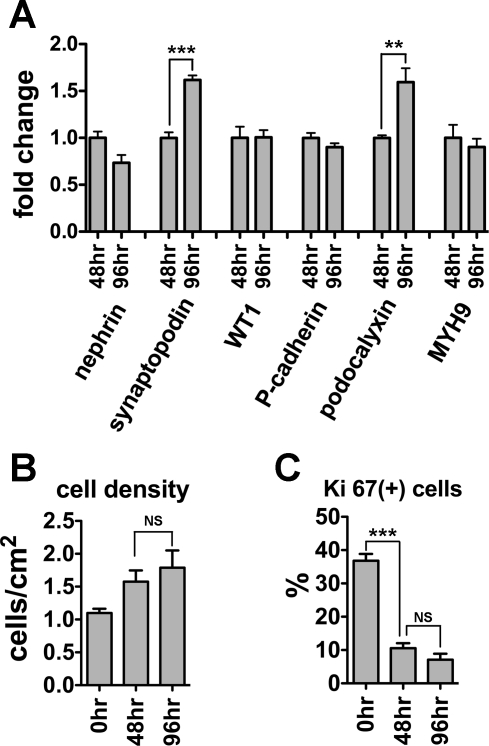
We next addressed possible mechanisms by which cell density might regulate podocyte gene expression; specifically, we considered possible roles for cell-cell contact, cellular quiescence, and soluble autocrine/paracrine factors released from cells. Initially, we considered whether cell cycling contributed. First, while BrdU incorporation was much higher at 50 and 30% confluence, there was no difference in BrdU incorporation between 90 and 100% confluent cultures, despite substantial differences in gene expression (Fig. 4C). Second, following cellular quiescence induced by serum deprivation, growth arrest at 48 and 96 h was confirmed by similar cell density (Fig. 6B) and similar (low) levels of Ki67 expression (Fig. 6C). Nevertheless, after 96 h of serum deprivation compared with 48 h, synaptopodin and podocalyxin mRNA levels were significantly increased, whereas expression of other genes were unchanged (Fig. 6A). These findings suggest the distinct pattern of synaptopodin and podocalyxin gene expression is either due to very modest (nonsignificant) changes in cell density or that cell cycle arrest induces synaptopodin and podocalyxin mRNA. This latter possibility is supported by the observation that synaptopodin and podocalyxin mRNAs were also the two genes upregulated in tsT-podocytes after growth arrest (Fig. 2, B and E).
Fig. 6.
Cellular quiescence promotes mRNA expression of synaptopodin and podocalyxin. A: mRNA expression of nephrin, synaptopodin, WT1, P-cadherin, podocalyxin, and MYH9 in podocytes transformed by CDK4 at 48 or 96 h after serum starvation was measured by quantitative RT-PCR. At 96 h, compared with 48 h, synaptopodin and podocalyxin mRNA expression was significantly increased. Cellular quiescence at 48 and 96 h was confirmed by similar cell densities (B) and reduction of Ki67-positive cells compared with 0 h (C). Data were normalized to β-actin mRNA and relative expressions to 48 h are shown as means ± SD of triplicate samples (A). ***P < 0.001, **P < 0.01.
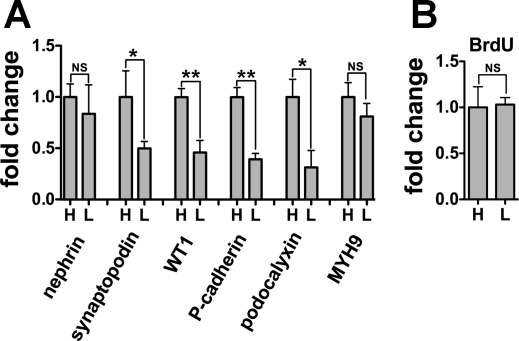
We next carried out an experiment to put cells in growth arrest to see whether gene expression was still affected by cell density. Cells were cultured at high or low density and then subjected to serum starvation to suppress cell proliferation (Fig. 7). After 96 h of serum deprivation, BrdU incorporation was identical between the cultures (Fig. 7B). However, synaptopodin, WT1, podocalyxin, and P-cadherin mRNA expression were significantly higher in cells at high density (Fig. 7A). These findings suggest the factors associated with confluence other than cell cycle arrest also contribute to the expression of these four mRNAs.
Fig. 7.
Cell density and not only cellular quiescence contributes to synaptopodin, WT1, podocalyxin, and P-cadherin mRNA expression. Podocytes transformed with CDK4 were plated at high (H) or low (L) density, and growth medium supplemented with 10% FBS was replaced with medium containing 0.1% serum at 48 h. Cells were cultured for a further 96 h until analysis. mRNA for nephrin, synaptopodin, WT1, P-cadherin, podocalyxin, and MYH9 were measured by quantitative RT-PCR (A), and BrdU incorporation was quantified by enzyme-linked immunosorbent assay (B). Synaptopodin, WT1, podocalyxin, and P-cadherin mRNA expression were higher in cells at H than L. In contrast, BrdU incorporation was mostly equal between H and L. The cellular density of each (H) and (L) was 1.87 cells/cm2 (100% confluence) and 0.54 cells/cm2 (50% confluence). The data were normalized to β-actin mRNA (A). Relative expression to cells at H is shown as means ± SD of triplicate samples (A, B). **P < 0.01 and *P < 0.05.
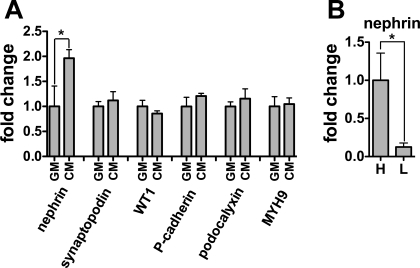
To investigate a possible role for soluble autocrine and/or paracrine factors that might accumulate in high-density cultures, we cultured CDK4-podocytes in conditioned medium (CM) or growth medium (GM) for 24 h. Only nephrin mRNA was higher in cells treated with CM compared with GM (Fig. 8A). To control for possible effects of soluble factors, we cultured cells at high and low density, replacing medium every 12 h. Nephrin mRNA was still increased in high-density cultures compared with low-density cultures, suggesting either that soluble factors accumulate rapidly or that nephrin expression is particularly dependent on cell density.
Fig. 8.
Soluble autocrine/paracrine factors as well as cell-cell contact induce nephrin mRNA expression. A: conditioned medium (CM) was collected from confluent podocytes transformed by CDK4 (CDK4 podocytes) after 24 h of incubation. CDK4-podocytes were cultured in CM or normal growth medium (GM) for 24 h, and mRNA expression of nephrin, synaptopodin, WT1, P-cadherin, podocalyxin, and MYH9 was determined. Nephrin mRNA was higher in cells cultured in CM than GM, whereas other markers were not significantly upregulated. B: cells were plated at H or L and growth medium was replaced every 12 h for 72 h. Cells at H exhibited higher expression of nephrin mRNA than L. The data were normalized to β-actin mRNA. Gene expression relative to podocytes cultured in GM (A) or at H (B) are presented as means ± SD of triplicate samples. *P < 0.05.
Taken together, these results suggest the following: 1) cell density, possibly acting via cell-cell contact, appears to contribute to the expression of five podocyte mRNAs other than MYH9, 2) growth arrest may also contribute to the particular expression pattern seen with synaptopodin and podocalyxin mRNA, and 3) soluble factors may contribute to expression of nephrin mRNA.
DISCUSSION
In the present report, we describe mouse podocyte cultures transformed by CDK4 as a nonviral immortalizing gene (22) and found the CDK4-transformed cells exhibited a distinct phenotype, compared with podocytes transformed by tsT. In addition, we found that cell density had a prominent effect on podocyte marker genes in CDK4-podocytes. Our studies are consistent with the interpretation that cell-cell contact was the dominant factor regulating the podocyte marker gene expression in CDK-podocyte cultures, with a contributing role for growth arrest (synaptopodin, podocalyxin mRNA) and possibly for soluble factors released from cells (nephrin mRNA).
As compared with primary podocytes and CDK4-podocytes, nephrin mRNA expression in tsT-podocytes was low and did not recover after growth restriction (Fig. 2A). This resembles the findings in our recent studies describing generation of mouse podocyte cell line transformed by H2Kb-tsA58 (13) and human podocyte cell lines by tsT and hTERT (24). SV40 T inhibits the function of the tumor suppressor Rb protein and consequently activates transcriptional factor E2F family, which in turn promotes G1/S transition; this function is shared with CDK4. Furthermore, SV40 T is known to bind to tumor suppressor p53 and block its ability to activate gene expression (21). In addition to a regulation of gene expression by suppressing these tumor suppressors, SV40 T interacts directly with the cellular transcriptional machinery through diverse mechanisms (7, 12, 15) and the regulation is cell-type specific (5).
Podocin mRNA was absent and nephrin protein expression was low in CDK4-podocytes, which represents a limitation of this approach. Podocin mRNA was not detected even in primary cultures. Nephrin and podocin mRNA were reported to decrease after subculture in rat primary podocytes (14). In the present study, to obtain sufficient amount of cells, primary podocytes were used for qRT-PCR after three subcultures, and transformation was performed after two subcultures. These serial passages of primary podocytes could have diminished podocin mRNA expression. Primary culture methods that allow podocytes to retain the in vivo phenotype better than the current approach are needed. We note that our culture methods were similar to those used by other investigators (14).
Another limitation of our study is the use of heterogeneous cell cultures of CDK4-podocytes and tsT-podocytes. We acknowledge heterogeneity of gene expression among the cells and possible contamination of other type of cells could affect our results. Clones obtained by single cell cloning could be prepared for the future use, especially for sensitive assays like microarray, although clone-to-clone variation may be a problem. Healthy podocytes in vivo are generally held to be terminally differentiated growth-arrested cells. Proliferation of dysregulated podocytes (or perhaps podocyte progenitor cells) underlies pathogenesis of collapsing glomerulopathy (3, 26). Synaptopodin mRNA expression was suppressed in tsT-podocytes under growth-permissive conditions and CDK4-podocytes compared with primary podocytes (Fig. 2, B and D). However, synaptopodin mRNA expression was upregulated consistently in tsT-podocytes after growth restriction (Fig. 2, B and D) and after a serum starvation in CDK4-podocytes (Fig. 5, B and D), suggesting that cellular quiescence promoted expression of this gene. This result was congruent with our previous study using a mouse podocyte cell line immortalized by thermosensitive SV40 T in which synaptopodin was profoundly increased after growth restriction (13). Our findings are also supported by a report by Wu et al. (31) that G0/G1 growth arrest promoted differentiation of podocytes, as determined by high synaptopodin expression. We note that in the present work, the transgene is consistently expressed in CDK4-podocytes and cells are not quiescent unless deprived of serum, while SV40 T is inactivated in tsT-podocytes under growth-restricted conditions.
Nephrin mRNA in the CDK4-podocytes was upregulated by incubation with podocyte-conditioned medium. Soluble factors including cytokines, retinoids, and steroid hormones have been reported to upregulate nephrin gene expression (17, 28, 32). We recently identified secreted proteins using conditioned medium derived from human podocyte culture by a proteomics approach (18). Further study will be required to identify autocrin and/or paracrine activators for nephrin gene expression.
Cell-cell contact has been shown to regulate gene expression in several cell types (2, 6, 9). More recently, Hwang et al. (10) showed that microRNA expression was globally activated by cell-cell contact, using cell culture approaches very similar to those that we have taken to isolate the effects of cell-cell contact. In our study, we found that expression of nephrin, synaptopodin, WT1, P-cadherin, and podocalyxin mRNA was significantly higher in confluent CDK4-podocytes than subconfluent or less confluent cells (Fig. 4). Cell-cell contact, cellular quiescence, and soluble factors were considered as possible contributors to this high expression. Among these three factors, cellular quiescence was found to promote synaptopodin and podocalyxin expression. When an effect of cellular proliferation was eliminated, an effect of cell density was apparent for synaptopodin, WT1, podocalyxin, and P-cadherin. We also found soluble factors may contribute to nephrin mRNA expression. Under conditions in which the effect of soluble factors was minimized, an effect of cell density for nephrin mRNA expression was still observed. Thus, we believe cell-cell contact played a role in mRNA expression of synaptopodin, WT1, podocalyxin, P-cadherin, and possibly nephrin.
In conclusion, we generated CDK4-podocytes, shown that these cells have a distinct phenotype from tsT-podocytes, and demonstrated that cell-cell contact is an important regulator of gene expression. It is well-recognized that podocyte loss initiates and promotes glomerular disease progression (16, 20). While cultured podocytes lack normal cell-cell podocyte contacts, including slit diaphragms, our results are compatible with a feed-forward model in which normal cell-cell contact between podocytes initiates signals that maintain a differentiated phenotype, and conversely loss of these signals degrades podocyte phenotype.
GRANTS
This study was supported by Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health under Grant ZO1-DK-043308.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank H. Takahashi for technical assistance, Dr. J. Shay for providing the CDK4 expression construct, and Dr. S. Rane for review of the manuscript.
REFERENCES
- 1.Abe Y, Sakairi T, Kajiyama H, Shrivastav S, Beeson C, Kopp J. Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol 299: C464–C476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akao M, Ueshima S, Okada K, Fukao H, Seki T, Ariga T, Matsuo O. Cellular density regulation of plasminogen gene expression in mouse hepatocytes. Life Sci 72: 1695–1704, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Barisoni L, Schnaper HW, Kopp JB. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2: 529–542, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Blain SW. Switching cyclin D-Cdk4 kinase activity on and off. Cell Cycle 7: 892–898, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Cantalupo PG, Saenz-Robles MT, Rathi AV, Beerman RW, Patterson WH, Whitehead RH, Pipas JM. Cell-type specific regulation of gene expression by simian virus 40 T antigens. Virology 386: 183–191, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YC, Zheng W, Jefcoate CR. Disruption of cell-cell contact maximally but transiently activates AhR-mediated transcription in 10T1/2 fibroblasts. Toxicol Appl Pharmacol 199: 220–238, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Damania B, Alwine JC. TAF-like function of SV40 large T antigen. Genes Dev 10: 1369–1381, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Douillard-Guilloux G, Mouly V, Caillaud C, Richard E. Immortalization of murine muscle cells from lysosomal alpha-glucosidase deficient mice: a new tool to study pathophysiology and assess therapeutic strategies for Pompe disease. Biochem Biophys Res Commun 388: 333–338, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Hershman KM, Levitan ES. Cell-cell contact between adult rat cardiac myocytes regulates Kv1.5 and Kv42 K+ channel mRNA expression. Am J Physiol Cell Physiol 275: C1473–C1480, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Hwang HW, Wentzel EA, Mendell JT. Cell-cell contact globally activates microRNA biogenesis. Proc Natl Acad Sci USA 106: 7016–7021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jat PS, Sharp PA. Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol Cell Biol 9: 1672–1681, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston SD, Yu XM, Mertz JE. The major transcriptional transactivation domain of simian virus 40 large T antigen associates nonconcurrently with multiple components of the transcriptional preinitiation complex. J Virol 70: 1191–1202, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kajiyama H, Titus S, Austin CP, Chiotos K, Matsumoto T, Sakairi T, Kopp JB. Tetracycline-inducible gene expression in conditionally immortalized mouse podocytes. Am J Nephrol 29: 153–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsuya K, Yaoita E, Yoshida Y, Yamamoto Y, Yamamoto T. An improved method for primary culture of rat podocytes. Kidney Int 69: 2101–2106, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Larminie CG, Sutcliffe JE, Tosh K, Winter AG, Felton-Edkins ZA, White RJ. Activation of RNA polymerase III transcription in cells transformed by simian virus 40. Mol Cell Biol 19: 4927–4934, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD. Podocytopenia and disease severity in IgA nephropathy. Kidney Int 61: 1475–1485, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol 172: 299–308, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto T, Hess S, Kajiyama H, Sakairi T, Saleem M, Mathieson P, Nojima Y, Kopp J. Proteomic analysis identifies insulin-like growth factor binding protein-related protein 1 as a podocyte product. Am J Physiol Renal Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pipas JM, Levine AJ. Role of T antigen interactions with p53 in tumorigenesis. Semin Cancer Biol 11: 23–30, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, Milchgrub S, Smith AL, Souza RF, Gilbey L, Zhang X, Gandia K, Vaughan MB, Wright WE, Gazdar AF, Shay JW, Minna JD. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res 64: 9027–9034, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Roig AI, Eskiocak U, Hight SK, Kim SB, Delgado O, Souza RF, Spechler SJ, Wright WE, Shay JW. Immortalized epithelial cells derived from human colon biopsies express stem cell markers and differentiate in vitro. Gastroenterology 138: 1012–1021, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Sakairi T, Abe Y, Kajiyama H, Bartlett LD, Howard LV, Jat PS, Kopp JB. Conditionally immortalized human podocyte cell lines established from urine. Am J Physiol Renal Physiol 298: F557–F567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Srivastava T, Garola RE, Singh HK. Cell cycle regulatory proteins in the podocyte in collapsing glomerulopathy in children. Kidney Int 70: 529–535, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Stamps AC, Davies SC, Burman J, O'Hare MJ. Analysis of proviral integration in human mammary epithelial cell lines immortalized by retroviral infection with a temperature-sensitive SV40 T-antigen construct. Int J Cancer 57: 865–874, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Takano Y, Yamauchi K, Hayakawa K, Hiramatsu N, Kasai A, Okamura M, Yokouchi M, Shitamura A, Yao J, Kitamura M. Transcriptional suppression of nephrin in podocytes by macrophages: roles of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett 581: 421–426, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaughan MB, Ramirez RD, Andrews CM, Wright WE, Shay JW. H-ras expression in immortalized keratinocytes produces an invasive epithelium in cultured skin equivalents. PLoS One 4: e7908, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu DT, Bitzer M, Ju W, Mundel P, Bottinger EP. TGF-beta concentration specifies differential signaling profiles of growth arrest/differentiation and apoptosis in podocytes. J Am Soc Nephrol 16: 3211–3221, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Yamauchi K, Takano Y, Kasai A, Hayakawa K, Hiramatsu N, Enomoto N, Yao J, Kitamura M. Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int 70: 892–900, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.