Abstract
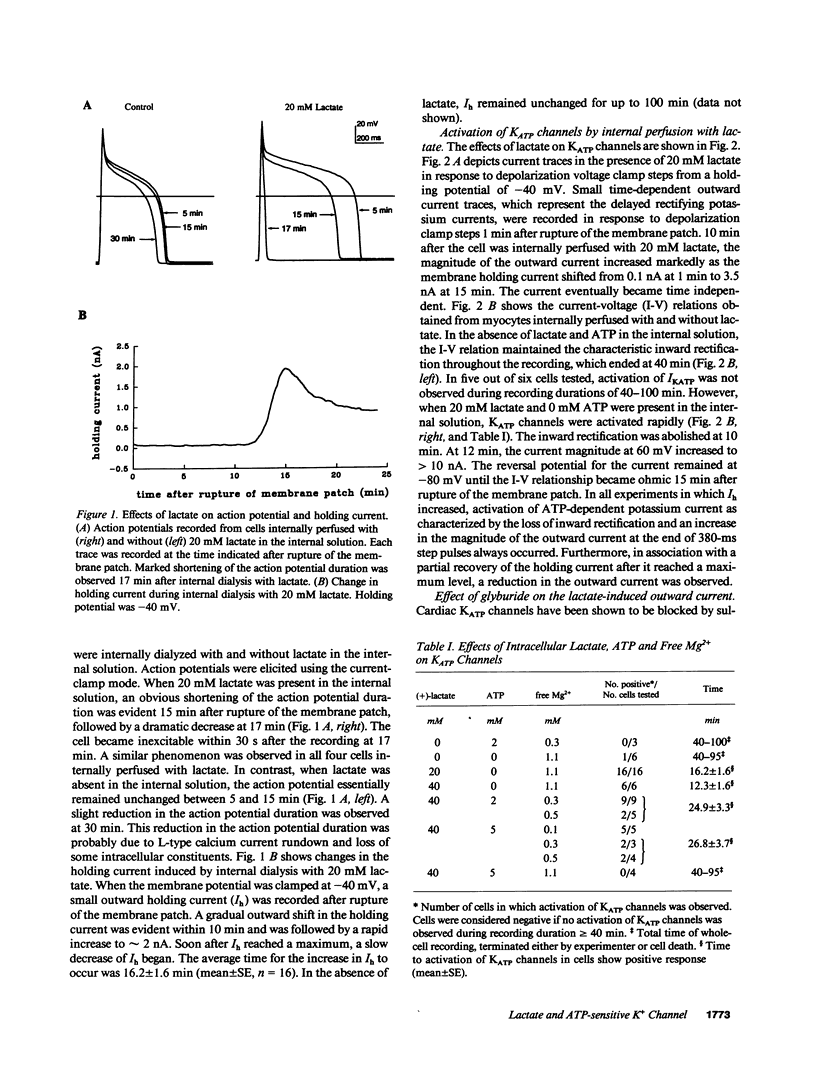
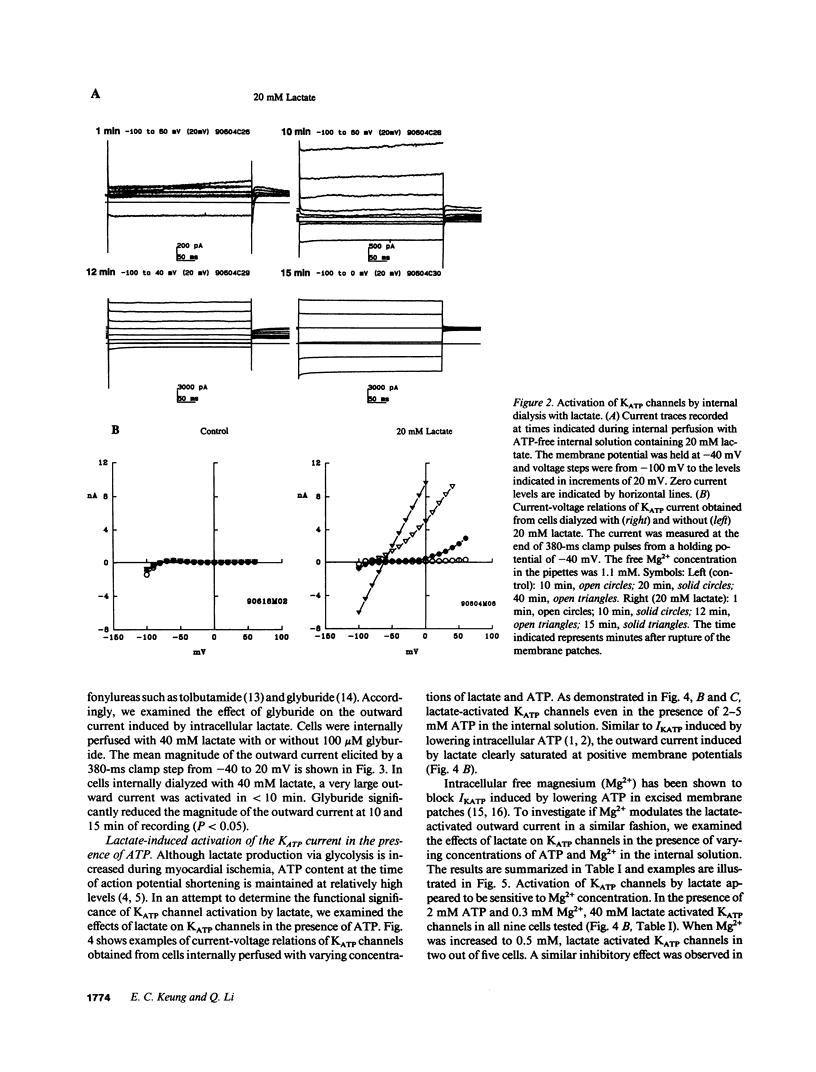
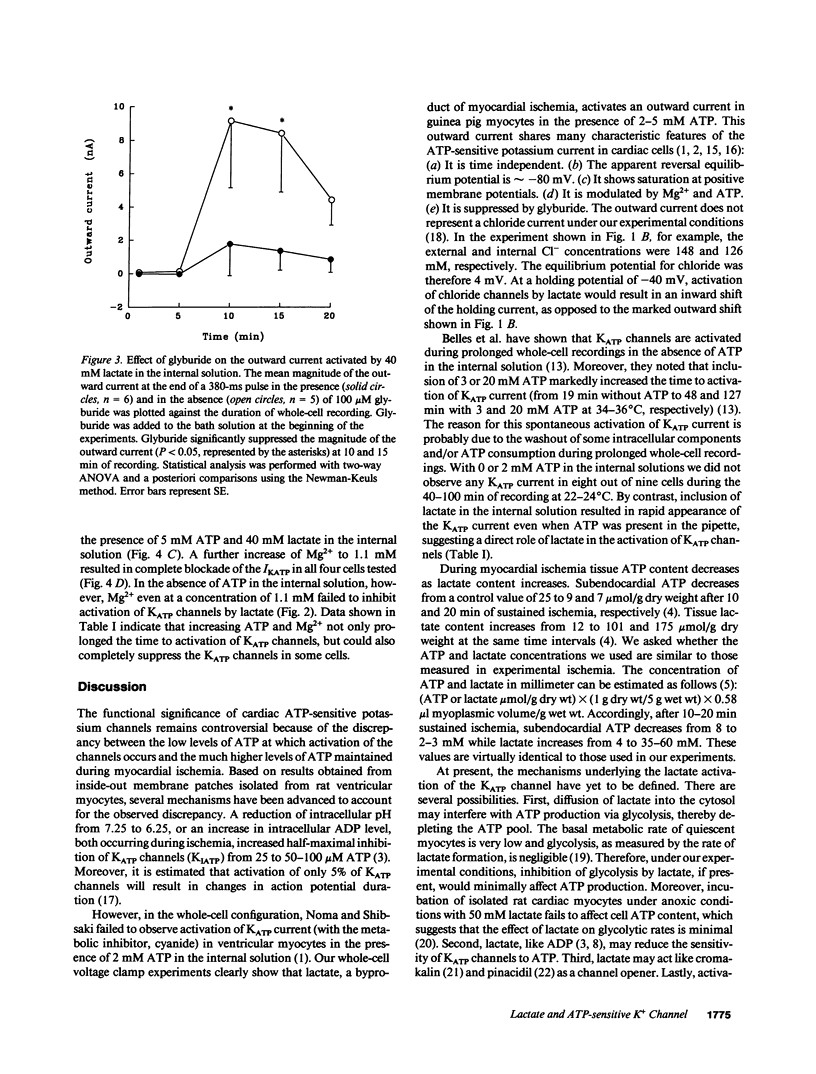
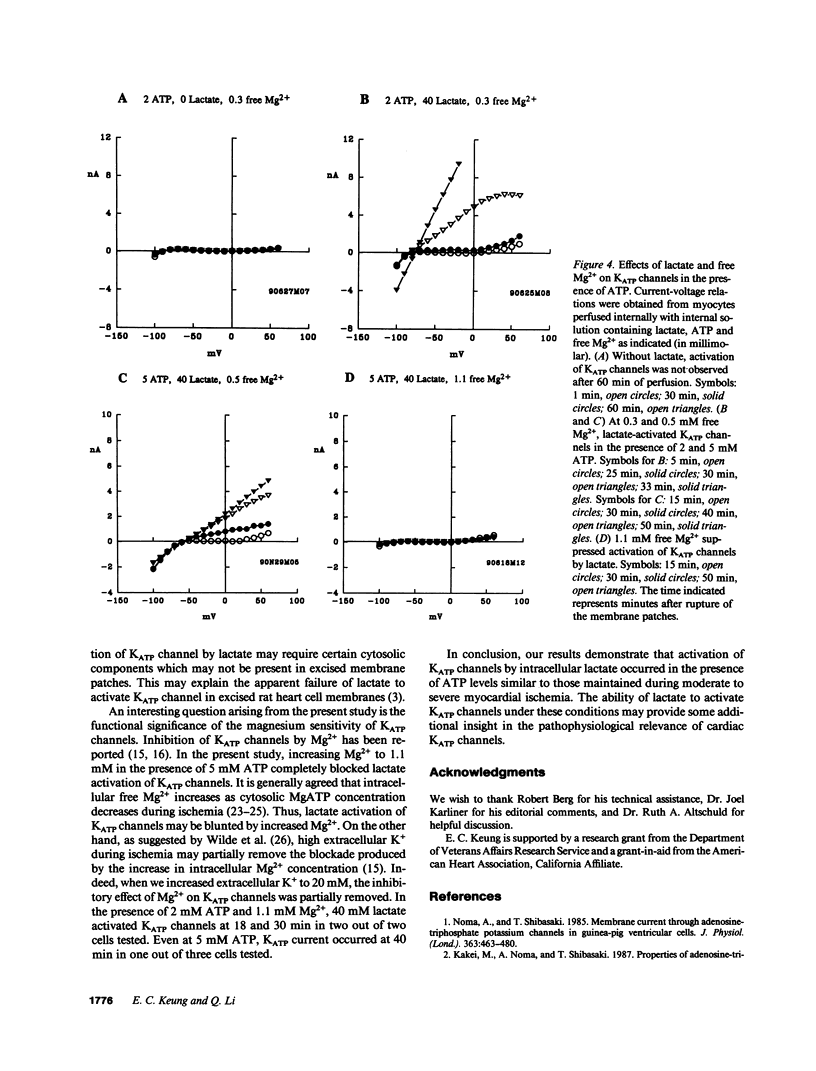
The functional significance of cardiac ATP-sensitive potassium channels remains controversial because of the discrepancy between the low levels of ATP at which activation of the channels occurs and the much higher levels of ATP maintained during myocardial ischemia. We studied the effects of (+)-lactate, which accumulates in large quantity as a result of increased glycolysis during ischemia, on ATP-sensitive potassium channels in adult guinea pig ventricular myocytes using the whole-cell patch-clamp technique. Lactate at 20-40 mM in the internal solution activated ATP-sensitive potassium channels and shortened action potential duration. Activation of the channels occurred even in the presence of 2-5 mM ATP in the internal solution and was dependent on intracellular free magnesium levels. Our results suggest that intracellular lactate may play a significant role in activating cardiac ATP-sensitive potassium channels and shortening action potential duration even at ATP levels similar to those resulting from moderate to severe myocardial ischemia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arena J. P., Kass R. S. Enhancement of potassium-sensitive current in heart cells by pinacidil. Evidence for modulation of the ATP-sensitive potassium channel. Circ Res. 1989 Aug;65(2):436–445. doi: 10.1161/01.res.65.2.436. [DOI] [PubMed] [Google Scholar]
- Belles B., Hescheler J., Trube G. Changes of membrane currents in cardiac cells induced by long whole-cell recordings and tolbutamide. Pflugers Arch. 1987 Aug;409(6):582–588. doi: 10.1007/BF00584657. [DOI] [PubMed] [Google Scholar]
- Borchgrevink P. C., Bergan A. S., Bakøy O. E., Jynge P. Magnesium and reperfusion of ischemic rat heart as assessed by 31P-NMR. Am J Physiol. 1989 Jan;256(1 Pt 2):H195–H204. doi: 10.1152/ajpheart.1989.256.1.H195. [DOI] [PubMed] [Google Scholar]
- Elliott A. C., Smith G. L., Allen D. G. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ Res. 1989 Mar;64(3):583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- Escande D. The pharmacology of ATP-sensitive K+ channels in the heart. Pflugers Arch. 1989;414 (Suppl 1):S93–S98. doi: 10.1007/BF00582255. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Findlay I. Effects of ADP upon the ATP-sensitive K+ channel in rat ventricular myocytes. J Membr Biol. 1988;101(1):83–92. doi: 10.1007/BF01872823. [DOI] [PubMed] [Google Scholar]
- Geisbuhler T. P., Rovetto M. J. Lactate does not enhance anoxia/reoxygenation damage in adult rat cardiac myocytes. J Mol Cell Cardiol. 1990 Nov;22(11):1325–1335. doi: 10.1016/0022-2828(90)90068-d. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Clark C. D., Hume J. R. Chloride current in mammalian cardiac myocytes. Novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol. 1990 Jun;95(6):1077–1102. doi: 10.1085/jgp.95.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A., Moss R. L. Metabolic cost of the stimulated beating of isolated adult rat heart cells in suspension. Circ Res. 1983 Mar;52(3):342–351. doi: 10.1161/01.res.52.3.342. [DOI] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung E. C. Calcium current is increased in isolated adult myocytes from hypertrophied rat myocardium. Circ Res. 1989 Apr;64(4):753–763. doi: 10.1161/01.res.64.4.753. [DOI] [PubMed] [Google Scholar]
- Keung E. C., Karliner J. S. Complex regulation of calcium current in cardiac cells. Dependence on a pertussis toxin-sensitive substrate, adenosine triphosphate, and an alpha 1-adrenoceptor. J Clin Invest. 1990 Mar;85(3):950–954. doi: 10.1172/JCI114524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkels J. H., van Echteld C. J., Ruigrok T. J. Intracellular magnesium during myocardial ischemia and reperfusion: possible consequences for postischemic recovery. J Mol Cell Cardiol. 1989 Nov;21(11):1209–1218. doi: 10.1016/0022-2828(89)90697-4. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Steenbergen C., Levy L. A., Raju B., London R. E. Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem. 1989 Apr 5;264(10):5622–5627. [PubMed] [Google Scholar]
- Murry C. E., Richard V. J., Reimer K. A., Jennings R. B. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990 Apr;66(4):913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Scott A. L., Zingaro G. J., Siegl P. K. BRL 34915 (cromakalim) activates ATP-sensitive K+ current in cardiac muscle. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8360–8364. doi: 10.1073/pnas.85.21.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. N., Lamp S. T. Cardiac ATP-sensitive K+ channels. Evidence for preferential regulation by glycolysis. J Gen Physiol. 1989 Nov;94(5):911–935. doi: 10.1085/jgp.94.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A. A., Escande D., Schumacher C. A., Thuringer D., Mestre M., Fiolet J. W., Janse M. J. Potassium accumulation in the globally ischemic mammalian heart. A role for the ATP-sensitive potassium channel. Circ Res. 1990 Oct;67(4):835–843. doi: 10.1161/01.res.67.4.835. [DOI] [PubMed] [Google Scholar]


