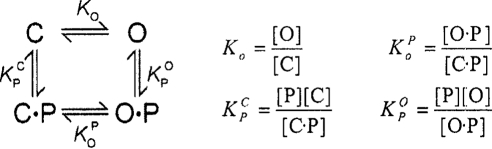Abstract
Proteases activate the epithelial sodium channel (ENaC) by cleaving the large extracellular domains of the α- and γ-subunits and releasing peptides with inhibitory properties. Furin and prostasin activate mouse ENaC by cleaving the γ-subunit at sites flanking a 43 residue inhibitory tract (γE144-K186). To determine whether there is a minimal inhibitory region within this 43 residue tract, we generated serial deletions in the inhibitory tract of the γ-subunit in channels resistant to cleavage by furin and prostasin. We found that partial or complete deletion of a short segment in the γ-subunit, R158-N171, enhanced channel activity. Synthetic peptides overlapping this segment in the γ-subunit further identified a key 11-mer tract, R158-F168 (RFLNLIPLLVF), which inhibited wild-type ENaC expressed in Xenopus laevis oocytes, and endogenous channels in mpkCCD cells and human airway epithelia. Further studies with amino acid-substituted peptides defined residues that are required for inhibition in this key 11-mer tract. The presence of the native γ inhibitory tract in ENaC weakened the intrinsic binding constant of the 11-mer peptide inhibitor, suggesting that the γ inhibitory tract and the 11-mer peptide interact at overlapping sites within the channel.
Keywords: ion channels, channel inhibitor, prostasin, amiloride
the epithelial sodium channel (ENaC) resides in the apical membrane of high-resistance epithelia in a wide array of tissues including the airway and kidney (4). In the aldosterone-sensitive distal nephron, ENaC participates in the fine control of sodium and fluid balance (16, 41). ENaC also participates in the maintenance of airway surface liquid volume, aiding mucociliary clearance of inhaled particles and pathogens (7, 30). Perturbations of ENaC activity have been described in pathophysiologic states that have an altered extracellular volume. For example, volume regulatory hormone excess (hyperaldosteronism) and ENaC mutations that impair channel endocytosis (Liddle syndrome) heighten ENaC activity in the kidney leading to volume expansion and hypertension (5, 16, 42).
ENaC is cleaved and activated by specific serine proteases including furin (a proprotein convertase that resides primarily in the trans-Golgi network), prostasin [a GPI-anchored serine protease also known as channel-activating protease 1 (CAP1)], TMPRSS4 (known as CAP2), matriptase (CAP3), trypsin, neutrophil elastase, pancreatic elastase, and plasmin (1, 6, 8, 9, 14, 20, 21, 23, 36, 43). ENaC modulation by proteases may be relevant to specific disease states including volume depletion, glomerular injury, and cystic fibrosis (26). Proteases and protease inhibitors have been shown to regulate surface liquid volume in airway epithelial cell cultures by modulating ENaC activity (8, 33, 44). Furthermore, excessive proteolytic activation of ENaC is believed to promote unregulated Na+ and water absorption in the cystic fibrosis airway and thereby compromise effective mucociliary clearance (4, 29, 30). Enhanced cleavage of ENaC subunits in the aldosterone-sensitive distal nephron occurs in animal models of sodium avid states such as hyperaldosteronism, low-salt diet, and proteinuria (19, 25, 31). Plasmin, a serine protease that can cleave and activate ENaC, has been implicated in the pathogenesis of volume expansion in nephrotic syndrome and was found in the urine of humans and animals with proteinuria, but was absent in the urine of control subjects (35, 36, 43).
Furin and prostasin activate ENaC by liberating inhibitory tracts from the extracellular regions of the α- and γ-subunits (6, 13, 23, 24). Within the α-subunit, furin cleaves ENaC twice at sites flanking a 26 residue inhibitory tract (13, 23). Within the γ-subunit, furin cleaves ENaC once following γR143 (in the mouse sequence) and a number of other serine proteases have been shown to cleave the γ-subunit at sites distal to the furin site. For example, γ-subunit processing by furin and prostasin results in the release of a 43-mer residue inhibitory tract (γE144-K186 in mouse ENaC) (6). Proteolytic processing does not affect the number of channels at the membrane. Instead, processing of ENaC increases the open probability (PO) of the channel. Fully processed channels, lacking α and γ inhibitory tracts, have an PO approaching 1.0 when expressed in Xenopus laevis oocytes, in contrast to unprocessed channels that have a PO below 0.1 (39). Channels with only the α-subunit processed have an intermediate PO of ∼0.3–0.4 (6, 28), whereas channels that have the γ inhibitory tract removed but retain the α inhibitory tract are highly active (11). Other factors such as phosphatidylinositides also have important roles in modulating ENaC PO (27, 37).
ENaC activity is greatly reduced in mutant channels that lack furin-dependent cleavage sites in the α-subunit, but can be restored to wild-type levels if the intervening 26 residue inhibitory tract (αD206-R231) is deleted (13). We identified an eight amino acid minimal inhibitory tract within this 26 residue fragment (αL211-L218) (12). Synthetic peptides that correspond to either the 26 residue α inhibitory tract (αD206-R231) or to the eight key residues within the α inhibitory tract, LPHPLQRL, reduced ENaC currents in X. laevis oocytes, mouse cortical collecting duct cells (mpkCCDs), and human airway epithelia (HAE) (12, 13). In an analogous manner, a synthetic peptide corresponding to the inhibitory tract released from the γ-subunit by furin and prostasin (γE144-K186) inhibited endogenous ENaC activity in mpkCCD cells and HAE (6).
In this report, we identified an 11-mer residue tract (γR158-F168) that retains the inhibitory properties of the region extending between the furin- and prostasin-dependent cleavage sites in the γ-subunit (γE144-K186). Further studies with synthetic peptides bearing substitutions at specific sites defined important residues required for inhibition of wild-type channels by the 11-mer peptide. Our studies suggest that the tract γR158-F168, when present, interacts with the extracellular region of the γ-subunit reducing the PO of the channel.
EXPERIMENTAL PROCEDURES
DNA constructs, site-directed mutagenesis, and cRNAs.
We used a PCR-based approach to generate serial deletions in mouse γ-ENaC within the γE144-K186 region (2, 40). The previously described γ-ENaC construct that possesses mutations at the furin- and prostasin-dependent cleavage sites (R143A and RKRK186QQQQ) was used as a template to create these deletion mutations (6). The γ-ENaC (γR143AΔE144-K186 and γΔE144-K186) constructs and α-ENaC construct that has furin-dependent cleavage site mutations (αR205A/R231A) were previously described (6, 12). cRNAs for α-, β-, and γ-ENaC subunits (wild-type and mutant) were synthesized using T3 mMessage mMachine (Ambion, Austin,TX).
Peptides.
Synthetic peptides with acetylated NH2 termini and amidated COOH termini were produced and HPLC purified (>90% purity) by Genscript (Piscataway, NJ) or by the Genomics and Proteomics Core Laboratories of the University of Pittsburgh (Pittsburgh, PA).
Functional expression in X. laevis oocytes.
The protocol for harvesting oocytes from X. laevis was approved by the University of Pittsburgh's Institutional Animal Care and Use Committee. α-, β-, and γ-ENaC cRNAs were injected at a concentration of 2 ng·subunit−1·oocyte−1 into stage V–VI X. laevis oocytes (10). Electrophysiological measurements were performed using two-electrode voltage clamp (TEV) as previously described (10). The recording solution contained (in mM) 110 NaCl, 2 KCl, 1.54 CaCl2, 10 HEPES, pH 7.4. For Na+ self-inhibition experiments, the low-Na+ TEV solution contained (in mM) 1 NaCl, 109 N-methyl-d-glucamine, 2 KCl, 2 CaCl2, and 10 HEPES, pH 7.4. Na+ self-inhibition was measured by rapidly increasing the bath [Na+] from 1 to 110 mM (28). The amiloride-insensitive component of the whole cell current was determined by perfusion with recording solution supplemented with amiloride (10 μM). For the peptide dose-response curves, currents were measured at −60 mV. In all other experiments, currents were measured at −100 mV before and after amiloride. ENaC-mediated Na+ currents were defined as the amiloride-sensitive component of the current.
mpkCCD cell culture and short-circuit current measurements.
mpkCCD(cl4) cells were grown on permeable filter supports (0.4-μm-pore size, polycarbonate membrane, 1-cm2 surface area Snapwell filters; Corning, Corning, NY). Medium was changed every 2 days and short-circuit currents (Isc) were measured 4 to 6 days after cells were placed on filters (3). Filters containing the cell monolayers were mounted in modified Ussing chambers and continuously short-circuited using a VCC MC6 or MC8 voltage/current clamp system (Physiologic Instruments, San Diego, CA) (13). The bath solution contained (in mM) 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose, pH 7.4. A gas mixture of 95% O2-5% CO2 at 37°C was constantly infused into the bath solution. The resistance of the monolayers was measured every 60 s using a 5-mV bipolar pulse.
Primary cultures of HAE cells.
As approved by the University of Pittsburgh Institutional Review Board, HAE were obtained from excess tissue remaining from lung transplantation or organ donor lungs not suitable for transplant. Cells were isolated from second through sixth generation bronchi and grown on permeable Transwell filters (Corning) as previously described (15). HAE cultures were used for Isc experiments following 3–6 wk of culturing on an air-liquid interface.
Data and statistical analyses.
Data are expressed as means ± SE (n), where n is the number of independent experiments analyzed. Statistical comparisons between groups were performed using Kruskal-Wallis nonparametric ANOVA followed by Dunn's multiple comparisons posttest. A P < 0.05 was considered statistically significant. The IC50 was expressed as a mean with 95% confidence interval. The IC50 was estimated using normalized amiloride-sensitive currents and plotted as a function of peptide concentration fitted by the equation y = t[1+10(log X−log IC50)], where X is the concentration of peptide. The IC50 is the concentration of peptide that inhibits amiloride-sensitive currents (y) halfway between the baseline (t) and maximal response. Statistical comparisons and fitting of data points to dose-response curves were performed using GraphPad 5.0 (GraphPad Software, San Diego, CA) and Igor Pro (Wavemetrics, Portland, OR) (12).
RESULTS
Activating deletions in protease-resistant channels implicate a small inhibitory region within γE144-K186.
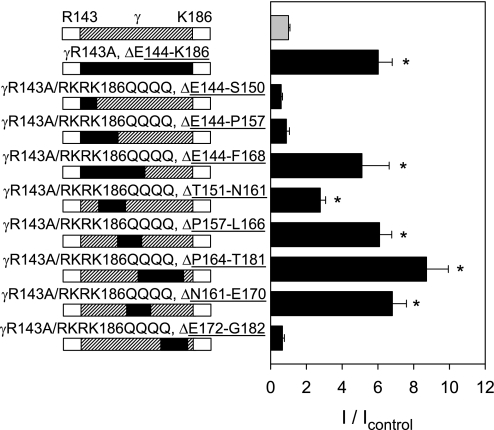
ENaC activity is modulated by proteolytic processing of its α- and γ-subunits (6, 13, 14, 23, 24). Mutations at the furin- and prostasin-dependent cleavage sites in the γ-subunit (γR143A and γRKRK186QQQQ) prevented the activation of the channel that results from cleavage of the γ-subunit (6). However, deletion of the 43-mer residue intervening inhibitory tract between the furin- and prostasin-dependent cleavage sites resulted in channel activation (6, 11). We hypothesized that the 43-mer residue tract interacts with the extracellular region of the γ-subunit, affecting the gating of the channel. To identify the key functional residues within the γ 43-mer inhibitory tract necessary for channel inhibition, we generated serial deletions within this tract (γE144-K186) in channels bearing mutations at the furin- and prostasin-dependent cleavage sites. As these γ constructs had furin- and prostasin-dependent cleavage sites mutated, only deletion of key residues required for inhibition, and not cleavage, should activate the channel (6, 23). ENaCs lacking furin-dependent cleavage sites in the α- and γ-subunits have very low activity when expressed in X. laevis oocytes and deletion of the γ inhibitory tract in these mutant channels induces a large increase in ENaC activity (11). To increase the sensitivity of the assay, mutant γ-subunits were coexpressed with α-subunits that had both furin-dependent cleavage sites mutated (αR205A/R231A), and wild-type β-subunits.
Channels composed of αR205A/R231A, wild-type β and wild-type γ retained their α and γ inhibitory tracts and have low activity (Fig. 1, top bar, n = 55). αR205A/R231Aβγ (control) had similar levels of functional expression compared with channels containing protease resistant α- and γ-subunits, αR205A/R231AβγR143A/RKRK186QQQQ (7 ± 2 and 4 ± 2% of wild-type αβγ current, respectively, Student's t-test, P = 0.14, αR205A/R231Aβγ vs. αR205A/R231AβγR143A/RKRK186QQQQ). Channels that retained their α inhibitory tract but had the γ -subunit inhibitory tract deleted as well as a mutated γ-subunit furin site (αR205A/R231AβγR143A/ΔE144-K186) had significantly higher activity than controls (αR205A/R231Aβγ; P < 0.001, n = 61; Fig. 1). In the setting of protease-resistant α- and γ-subunits, channels with γ-subunit deletions ΔE144-S150, ΔE144-P157, and ΔE172-G182 did not exhibit enhanced ENaC activity, suggesting that the tracts E144-P157 and E172-G182 were not required for inhibition (Fig. 1). In contrast, channels with the γ-subunit deletions ΔE144-F168, ΔT151-N161, ΔP157-L166, ΔP164-T181, and ΔN161-E170 showed significantly enhanced ENaC activity, compared with αR205A/R231Aβγ (*P < 0.001; see Fig. 1; Kruskal-Wallis nonparametric ANOVA followed by Dunn's multiple comparisons posttest, n = 10–61 oocytes). Our data suggest that partial or complete deletion of the intervening tract γR158-N171 is sufficient to activate the channel (Fig. 1). For instance, deletions of γT151-N161 and γP164-T181 do not overlap, but both resulted in enhanced activity.
Fig. 1.
Activating deletions in protease-resistant channels implicate a short inhibitory tract within γE144-K186. Two-electrode voltage clamp was performed in Xenopus laevis oocytes expressing ENaCs 36–48 h postinjection. Wild-type or mutant γ-subunits were coinjected with αR205A/R231A and wild-type β-subunits. Data are expressed as the ratio of the amiloride-sensitive currents of ENaCs containing mutant γ-subunits relative to controls (αR205A/R231Aβγ; I/Icontrol). Amiloride-sensitive currents in oocytes expressing αR205A/R231Aβγ and αR205A/R231AβγR143AΔ144–186 were −500 ± 55 nA (n = 55) and −3,270 ± 500 nA (n = 61), respectively. Statistically significant differences in the relative activity compared with controls are indicated as *P < 0.001 (Kruskal-Wallis nonparametric ANOVA followed by Dunn's multiple comparisons posttest). Experiments were performed with 10–61 oocytes per experimental group. Black bars within the illustrations on the left indicate residues that were deleted within the tract γR144-K186. Hatched bars indicate residues that were retained within the tract γR144-K186. Activating deletions involved all or part of the tract γR158-N171.
11-mer peptide corresponding to the tract γR158-F168 inhibits endogenous channels in mpkCCD and primary HAE cells.
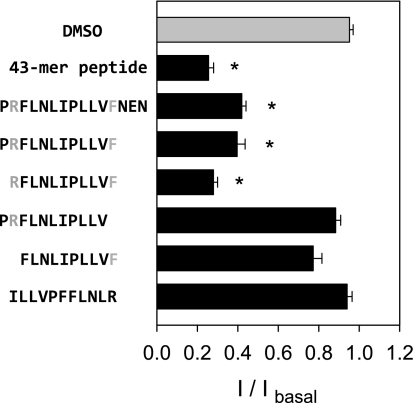
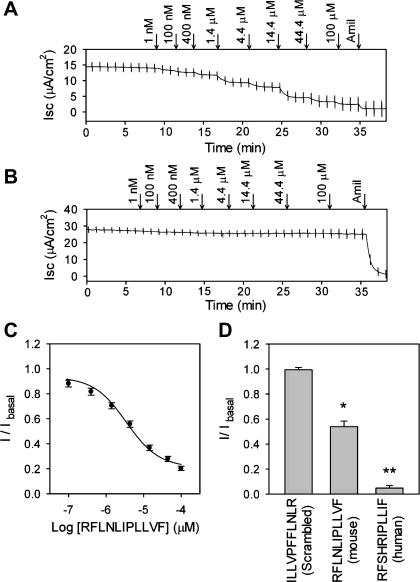
To define the minimal tract in the γ-subunit required for ENaC inhibition, we examined the effect of a series of synthetic peptides corresponding to all or part of the region γR158-N171 on endogenous ENaCs in mpkCCD monolayers (Fig. 2). Isc was determined before and following the addition of the peptides at a final concentration of 44 μM. DMSO (vehicle alone) served as a negative control in these experiments. The 43-mer peptide corresponding to the entire γ inhibitory tract, released by furin and prostasin cleavage, inhibited endogenous channels in mpkCCD cells (6). We found that a short peptide corresponding to γP157-N171 (PRFLNLIPLLVFNEN), encompassing the smaller region identified in the deletion experiments above, also significantly inhibited endogenous channels in mpkCCD cells (P < 0.001, n = 26; Fig. 2). The shortest peptide that inhibited endogenous channels was RFLNLIPLLVF corresponding to γR158-F168. Peptides lacking the proximal R158 or distal F168, such as FLNLIPLLVF and PRFLNLIPLLV, lacked inhibitory properties. The 11-mer RFLNLIPLLVF inhibited amiloride-sensitive currents in mpkCCD cells with an IC50 of 3.5 μM (95% CI 2.7–4.5 μM, n = 22; Fig. 3C). This value is similar to the previously described IC50 of the 43-mer peptide measured in mpkCCD cells (6). A peptide containing a scrambled version of this sequence (ILLVPFFLNLR) had no inhibitory effect (Figs. 2 and 3B). Representative tracings of the Isc of an mpkCCD monolayer at increasing concentrations of RFLNLIPLLVF or scrambled peptide are shown in Fig. 3, A and B. We also tested the peptide RFSHRIPLLIF, corresponding to the 11-mer human ortholog, on cultured HAE cells (Table 1). At a single dose of 100 μM, this peptide reduced amiloride-sensitive currents in HAE cells by 95.2 ± 2% (Fig. 3D). By comparison, the mouse 11-mer peptide, RFLNLIPLLVF, inhibited Isc in HAE cells by 46.0 ± 4.3%, suggesting a degree of species specificity. A scrambled version of the mouse 11-mer peptide had no effect in HAE monolayers.
Fig. 2.
11-mer peptide RFLNLIPLLVF inhibits ENaC. Synthetic peptides were generated based on the tract γR158-N171 identified by the activating deletions in protease-resistant channels. Synthetic peptides were applied apically at 44 μM to mpkCCD cells grown on permeable supports and mounted in an Ussing chamber. The relative inhibition of the ENaC-mediated Isc (I/Ibasal) was estimated as the ratio of the amiloride-sensitive component of Isc following application of peptide, relative to the basal amiloride-sensitive Isc. The amiloride-sensitive Isc after application of the 43-mer peptide was 6.7 ± 0.6 μA/cm2, n = 24 (I/Ibasal was 0.26 ± 0.03), after RFLNLIPLLVF was 5.8 ± 0.7 μA/cm2, n = 22 (I/Ibasal was 0.28 ± 0.02), after scrambled peptide (ILLVPFFLNLR) was 24.5 ± 2.2 μA/cm2, n = 6 (I/Ibasal was 0.94 ± 0.02), and after an equivalent volume of DMSO (vehicle alone) was 27.1 ± 2.0 μA/cm2, n = 25 (I/Ibasal was 0.95 ± 0.02). Statistically significant differences in the response to the inhibitory peptides compared with control (DMSO) are indicated as *P < 0.001(Kruskal-Wallis nonparametric ANOVA followed by Dunn's multiple comparisons posttest). Experiments were performed with 6–26 filters per experimental group. Light gray letters mark the beginning and end of the 11-mer RFLNLIPLLVF.
Fig. 3.
Inhibition of endogenously expressed ENaCs in mpkCCD and human airway epithelia (HAE) cells by the 11-mer peptide RFLNLIPLLVF. Representative recordings of the effect of the 11-mer (A) and scrambled peptide (B) on Isc measured in mpkCCD monolayers. Arrows indicate the addition of peptide (in μM) or amiloride (Amil) at 10 μM. C: inhibition of ENaC in mpkCCD cells by the 11-mer peptide RFLNLIPLLVF. A dose-response curve for the peptide RFLNLIPLLVF was generated in mpkCCD cells. I/Ibasal represents the ratio of the amiloride-sensitive component of the Isc following apical application of peptide, relative to the basal amiloride-sensitive current (I/Ibasal). ENaC-mediated Isc was inhibited with an IC50 of 3.5 μM, 95% confidence interval (CI) 2.7–4.5 μM (n = 22). D: human ortholog of γR158-F168 inhibits endogenous ENaC in HAE cells. Inhibition was estimated as the ratio of the amiloride-sensitive Isc following apical application of peptide (100 μM) relative to the basal amiloride-sensitive Isc (I/Ibasal). Human and mouse peptides significantly inhibited amiloride-sensitive Isc compared with scrambled peptide (*P < 0.01 and **P < 0.001; Kruskal-Wallis nonparametric ANOVA followed by Dunn's multiple comparisons posttest). Experiments were performed with 3–11 filters per experimental group.
Table 1.
Alignment of orthologous 11-mer peptide sequences
| Species | 11-Mer Sequence |
|---|---|
| Mus musculus | (158) RFLNLIPLLVF (168) |
| Rattus norvegicus | (153) RFFKLIPLLVF (163) |
| Homo sapiens | (153) RFSHRIPLLIF (163) |
| Equus caballus | (155) KFLNLVPLLAF (165) |
| Canis lupus | (155) KFLRLVPLMVF (165) |
| Monodelphis domestica | (155) KFINSLPLVVF (165) |
| Oryctolagus cuniculus | (155) KFLNLVPLLIF (165) |
| Bos taurus | (155) KFLNLAPLMAF (165) |
| Xenopus laevis | (158) IFLKQIPLFRL (168) |
The Xenopus laevis sequence is listed for comparison.
Substitutions of residues within the 11-mer peptide reveal structural requirements for inhibition.
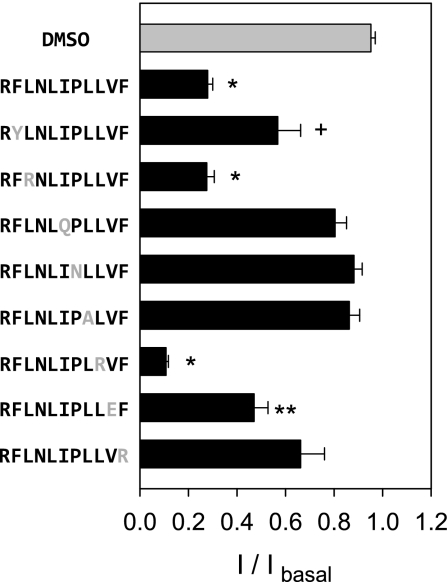
Table 1 shows an alignment of 11-mer sequences of γ-subunits from various species. The mouse and human sequences have notable differences at positions 3–5 (LNL vs. SHR). A peptide corresponding to the human tract sequence, RFSHRIPLLIF, inhibited endogenous mouse ENaCs in mpkCCD cells with an IC50 of 6.7 μM (95% CI 4.9–9.2 μM), suggesting that residues LNL at positions 3–5 are not required to inhibit the mouse channel (Table 2). The orthologous 11-mer peptide of X. laevis, IFLKQIPLFRL, showed reduced apparent affinity for endogenous channels in mpkCCD cells with an IC50 of 17.4 μM (95% CI 11.2–27.1 μM), compared with the mouse 11-mer peptide (Table 2). To further examine structural requirements for channel inhibition, we generated peptides with substitutions at specific positions within the 11-mer peptide. We investigated the effect of the peptides (44 μM) on the amiloride-sensitive component of the Isc in mpkCCD cells (Fig. 4). The 11-mer (RFLNLIPLLVF) reduced currents by 72 ± 2% at 44 μM. Peptides with substitutions of L by R at positions 3 or 9 retained inhibitory properties. In contrast, peptides with substitutions at positions 6, 7, 8, or 11 did not inhibit ENaC currents in mpkCCD monolayers and were not statistically different than DMSO (vehicle alone). The substitutions at positions 2 and 10 reduced the efficacy of the 11-mer peptide, compared with RFLNLIPLLVF (Student's t-test, P < 0.001). Taken together, our data suggest that the key residues for inhibition are RFXXXIPLXVF, where X is a site where an amino acid substitution did not significantly reduce the efficacy of the 11-mer peptide to inhibit ENaC. A review of mammalian orthologs listed in Table 1 suggests that positions 6 and 10 may also allow for other nonpolar amino acid substitutions, while the first position may require a basic residue.
Table 2.
Summary of peptide IC50s
| Cell System | ENaC | 11-Mer Peptide (Species) | IC50, μM (CI) |
|---|---|---|---|
| mpkCCD | Endogenous channels | RFLNLIPLLVF (mouse) | 3.5 (2.7–4.5) (n = 22) |
| mpkCCD | Endogenous channels | RFSHRIPLLIF (human) | 6.7 (4.9–9.2) (n = 6) |
| mpkCCD | Endogenous channels | IFLKQIPLFRL (X. laevis) | 17.4 (11.2–27.1) (n = 7) |
| X. laevis oocytes | αβγ | RFLNLIPLLVF (mouse) | 2.7 (1.3–3.0) (n = 10) |
| X. laevis oocytes | αβγR143A | RFLNLIPLLVF (mouse) | 2.3 (0.9–2.9) (n = 10) |
| X. laevis oocytes | αβγΔE144-K186 | RFLNLIPLLVF (mouse) | 3.5 (3.1–3.9) (n = 10) |
| X. laevis oocytes | αβγR143AΔE144-K186 | RFLNLIPLLVF (mouse) | 3.2 (1.8–3.1) (n = 10) |
| X. laevis oocytes | αR205A/R231AβγΔE144-K186 | RFLNLIPLLVF (mouse) | 0.56 (0.43–0.60) (n = 10) |
Fig. 4.
Structure-activity analyses of the inhibitory peptide RFLNLIPLLVF. Synthetic peptides with single amino acid substitutions (in light gray letters) were applied apically at a concentration of 44 μM to mpkCCD cells grown on permeable supports and mounted in Ussing chambers. The magnitude of inhibition was estimated as the ratio of the amiloride-sensitive Isc following application of peptide relative to the basal amiloride-sensitive Isc (I/Ibasal). An equivalent volume of DMSO (vehicle alone) had no effect on amiloride-sensitive currents (27.1 ± 2.0 μA/cm2, n = 25) compared with amiloride-sensitive currents remaining after RFLNLIPLLVF (5.8 ± 0.7 μA/cm2, n = 22). Responses were compared with the DMSO control using Kruskal-Wallis nonparametric ANOVA followed by Dunn's multiple comparisons posttest (*P < 0.001, **P < 0.01, +P < 0.05). Experiments were performed with 6–25 filters per experimental group.
Effects of proteolytic processing on 11-mer peptide efficacy.
When expressed in X. laevis oocytes, ENaC γ-subunits are primarily cleaved at the furin-dependent cleavage site (γR143), resulting in retention of the γ inhibitory tract (23). Wild-type channels exhibited an PO of ∼0.3–0.4 (6, 28). Deletion of the γ 43-mer inhibitory tract (γΔE144-K186) resulted in channels with an PO approaching 1, representing fully processed channels (6, 11). We examined the effects of the 11-mer peptide (RFLNLIPLLVF) on ENaC expressed in oocytes, where furin cleavage of the γ-subunit was blocked (γR143A mutant). In addition, the γ 43-mer inhibitory tract was either retained or deleted (γ vs. γΔE144-K186). In the presence or absence of the γ inhibitory tract, peptide inhibition was unaffected by cleavage at γR143, with the four possible combinations of conditions having IC50 values of 2.3–3.5 μM (Table 2).
The proteolytic processing of the γ-subunit could have an effect on the measured 11-mer peptide efficacy by several mechanisms. First, excising the innate inhibitory tract may enhance accessibility to the peptide-binding site. Second, changing the open state-closed state equilibrium of the channel may affect the efficacy of the peptide. Determining the effect of release (or deletion) of the innate γ inhibitory tract on 11-mer peptide efficacy is complicated by the effect that tract release has on channel PO. As the 11-mer peptide is an allosteric inhibitor that drives the channel to the closed state, increasing the channel PO through γ inhibitory tract release will weaken measured 11-mer peptide affinity independent of its “true” binding affinity. In a pathway-independent equilibrium scheme
O and C are the open and closed states, respectively, P is the peptide, and O·P and C·P are peptide-bound states to the open (O·P) and closed (C·P) channel. The apparent peptide affinity K′ is given by
| (1) |
Eq. 1 simplifies to eq. 2 assuming that [C·P] is much greater than [O·P] at equilibrium
| (2) |
If the open state is highly favored, in the absence of peptide and giving a large Ko value, the value of K′ is also large despite the value of the intrinsic binding constant KPC. Since PO = [O]/([O] + [C]), Ko can be expressed as PO/(1 − PO). Thus, small changes in PO near PO values of 1 will have dramatic effects on measured K′. Conversely, changes in PO below values of ∼0.8 will have more modest effects.
To determine the PO effect on measured 11-mer peptide affinity, we compared 11-mer inhibition in two groups of channels lacking the γ inhibitory tract that have different PO (αβγΔE144-K186 vs. αR205A/R231AβγΔE144-K186). We measured both Na+ self-inhibition and 11-mer inhibition in these channels (Fig. 5, A and B) and estimated the intrinsic binding constant KPC based on these data. Measurements of Na+ self-inhibition can be used to approximate PO (28). Na+ self-inhibition represents a reduction in channel PO in response to external Na+. In general, this response is observed when channels are initially in a low-[Na+] bath, where we showed that they have a high PO (39). When the bath [Na+] is acutely raised, measured current immediately increases commensurate with the increased driving force for Na+ entry reaching a peak current value (Ipeak), which subsequently declines as the channel arrives at a new equilibrium at a lower PO [current steady-state (Iss)] due to Na+ self-inhibition. The ratio of (Ipeak − Iss)/Ipeak represents the magnitude of the Na+ self-inhibition response.
Fig. 5.
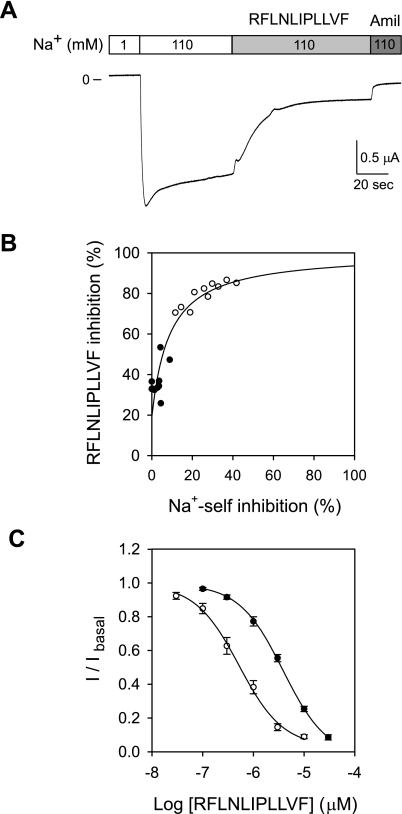
Effects of cleavage, open probability, and presence of the inhibitory tract on 11-mer peptide affinity. A: representative tracing showing the Na+ self-inhibition and peptide response in an oocyte expressing αR205A/R231AβγΔE144-K186. Oocytes were perfused with two-electrode voltage clamp (TEV) solution containing 1 mM Na+ and then rapidly switched to a solution containing 110 mM Na+ to determine Na+ self-inhibition. The oocytes were subsequently perfused with TEV solution containing 110 mM Na+ and 2 μM peptide, followed by TEV solution containing 110 mM Na+ and 10 μM amiloride. B: analysis of the relationship between Na+ self-inhibition and peptide inhibition of mutant ENaC channels. Circles represent individual X. laevis oocytes expressing αβγΔE144-K186 (●) and αR205A/R231AβγΔE144-K186 (○). Inhibition by the peptide RFLNLIPLLVF (2 μM) was determined as the percent reduction in amiloride-sensitive whole cell Na+ current at the plateau. Na+ self-inhibition was estimated as previously described (28). A relationship exists between Na+ self-inhibition (ratio of Ipeak − Iss/Ipeak), a surrogate for PO, and the magnitude of peptide inhibition. Data were fitted with eq. 3. C: dose-response curves showing peptide block of αβγΔE144-K186 channels (●, IC50 = 3.5 μM, 95% CI 3.1–3.9 μM, n = 10) and of αR205A/R231AβγΔE144-K186 channels (○, IC50 = 0.56 μM, 95% CI 0.43–0.60 μM, n = 10). The relative inhibition was estimated as the ratio of the amiloride-sensitive component of the whole cell Na+ current following application of peptide relative to the basal amiloride-sensitive current (I/Ibasal).
Na+ self-inhibition was measured by rapidly increasing the bath [Na+] from 1 to 110 mM, while 11-mer inhibition was measured at a single dose of 2 μM after whole cell currents had stabilized in the 110 mM Na+ bath (Fig. 5A). Oocytes expressing αβγΔE144-K186 had minimal Na+ self-inhibition (6 ± 2%, n = 10), reflecting the expected high PO for these channels, and 38 ± 2% 11-mer inhibition of whole cell Na+ currents by 2 μM peptide. We also performed these experiments with channels that lacked the γ inhibitory tract but retain the α inhibitory tract (αR205A/R231AβγΔE144-K186), under the premise that this α-subunit mutation would reduce PO without altering the 11-mer putative binding site in the γ-subunit. We found that these channels had increased Na+ self-inhibition (25 ± 4%, n = 10), consistent with a decrease in PO, and increased 11-mer inhibition (84 ± 2%), consistent with the relationship described by eq. 2. We then estimated PO based on the Na+ self-inhibition response, using the previously described linear relationship between PO and Na+ self-inhibition (28). We fit these data to eq. 3 derived from the equilibrium scheme:
| (3) |
where IP and Imax are the observed peptide inhibition and maximal predicted peptide inhibition, respectively, and calculated the intrinsic binding constant (KPC) of the 11-mer peptide for channels lacking the γ inhibitory tract at 0.17 ± 0.04 μM. This relationship between Na+ self-inhibition and 11-mer inhibition in channels lacking the γ inhibitory tract (αβγΔE144-K186 vs. αR205A/R231AβγΔE144-K186) is illustrated in Fig. 5B.
We estimated apparent 11-mer peptide affinity by measuring the dose response in these mutant channels (Fig. 5C). The measured IC50 values of the 11-mer for αβγΔE144-K186 and αR205A/R231AβγΔE144-K186 were 3.5 μM (95% CI 3.1–3.9 μM) and 0.56 μM (95% CI 0.43–0.60 μM), respectively. Based on these values and the KPC value for channels lacking the γ inhibitory tract determined above, we estimated the PO values of αβγΔE144-K186 and αR205A/R231AβγΔE144-K186 as 0.95 and 0.70, respectively. By comparison, wild-type ENaC expressed in oocytes had an 11-mer IC50 value of 2.7 μM (95% CI 1.3–3.0 μM). Since wild-type ENaC has a PO of ∼0.3–0.4, its KPC value is estimated at ∼1.8 μM, which is 10-fold weaker than for channels lacking the γ inhibitory tract (0.17 ± 0.04 μM). As the presence of the γ inhibitory tract weakens the KPC, these data suggest that the γ inhibitory tract and the 11-mer occupy overlapping sites on ENaC.
DISCUSSION
We identified an essential 11-mer residue tract (γR158-F168) within the γ-subunit of ENaC that encompasses the key functional residues of the γ inhibitory tract (γE144-K186) (6). Whole cell Na+ currents were enhanced in channels bearing partial or complete deletion of the 11-mer residue tract (γR158-F168), compared with controls. The synthetic peptide, RFLNLIPLLVF, corresponding to this tract inhibited ENaC expressed endogenously in mpkCCD cells and heterologously in X. laevis oocytes (IC50 = 3.5 and 2.7 μM, respectively). The inhibitory efficacy of the 11-mer peptide is similar to the previously described γ-subunit derived 43-mer peptide (corresponding to γE144-K186) (6). The orthologous human 11-mer, RFSHRIPLLIF, inhibited endogenous channels in HAE cells as well.
The region between the furin- and prostasin-dependent cleavage sites is poorly conserved in mammalian γ-subunits. Within the 11-mer tract, all mammalian sequences have an initial basic residue, F at positions 2 and 11, and PL at positions 7–8. There is otherwise species variability within the 11 amino acid sequence, particularly at positions 3–5. Our data suggest that the residues at positions 3–5 may not have an important role regarding channel inhibition, as both human and mouse 11-mer peptides, which diverge at positions 3–5 (SHR for human and LNL for mouse), had similar IC50 values when applied to mpkCCD cells. Eleven residues is the minimal length required for inhibition as determined by sequence-activity analyses. Deletion of R in position 1 or F in position 11 resulted in loss of inhibition. Comparison of mammalian sequences revealed that the R in position 1 could be replaced with another basic residue, K. Replacement of F at position 11 with a charged residue disrupted peptide function. The IPL tract at positions 6–8 was essential for inhibition. As this P is highly conserved, we speculate that this residue introduces a functionally important bend in the peptide. There appears to be only limited sequence similarities between this 11-mer inhibitory peptide derived from the γ-subunit and the previously defined α-subunit derived, eight residue inhibitory tract (αL211-L218), LPHPLQRL (12), suggesting distinct peptide-specific binding sites within the channel. Both peptides have P residues that are required for their inhibitory properties (12).
Although the X. laevis ortholog of the 11-mer peptide (IFLKQIPLFRL) inhibited ENaC with reasonable efficacy, it shares only partial sequence similarity with the mouse 11-mer peptide. Residues at position 2, 7, and 8 that are conserved within mammals are also present in the X. laevis ortholog (see Table 1). At position 11, F is substituted by L in X. laevis. Interestingly, we found that replacement of L by F at position 1 in the 8-mer α inhibitory peptide did not alter its efficacy (12). The sites within the channel that bind the 11-mer γ peptide are unknown. However, if the 11-mer peptide binds to a hydrophobic pocket, replacement of F by L may only have a modest effect on the efficacy of inhibition as both amino acids are relatively hydrophobic.
The presence or absence of the inhibitory tract in the γ-subunit did not significantly alter the measured IC50 for the 11-mer peptide (Table 2). However, channels that lacked the γ inhibitory tract have a high PO that will reduce the 11-mer apparent affinity. Taking into account the effect of the channel PO on the efficacy of peptide inhibition, our results suggest the presence of the γ inhibitory tract weakened the intrinsic peptide affinity by ∼10-fold. This observation is consistent with the notion that the 43-mer γ inhibitory tract and the 11-mer peptide have overlapping binding sites. However, we cannot rule out the possibility of noncompetitive binding, as the concentration of the γ inhibitory tract within the γ-subunit cannot be experimentally altered.
Differences in the response to the external application of proteases have been observed in isolated collecting ducts when compared with cultured kidney epithelial cell lines. For example, trypsin did not affect amiloride-sensitive currents in A6, M1, or mpkCCD cell lines, suggesting that ENaC subunits are fully processed under the conditions that these cultured cell lines were maintained (34, 45). In contrast, trypsin increased ENaC activity and Na+ absorption in isolated CCDs (18, 32, 34), suggesting that a population of channels with subunits that are unprocessed or partially processed by proteases is expressed at the apical membrane in vivo. While γ-subunits that migrate at a molecular mass consistent with proteolytic processing have been described in rodent kidney (18, 31), evidence of multiple cleavage events leading to inhibitory tract release is lacking.
In conclusion, we identified an 11-mer residue stretch within the γ inhibitory tract that is essential for its inhibitory function. Aerosolized amiloride analogs offer an approach to inhibit ENaC in the airways of individuals with disorders such as cystic fibrosis (22, 38, 46), where aberrant proteolytic activation of ENaC has been implicated in the pathogenesis of mucociliary dysfunction (7, 8, 29, 30, 33). External peptides may provide an alternative therapeutic approach to inhibit ENaC.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK-065161, P30-DK-079307, F32-DK-080574, K01-DK-078734, and K08-HL-087932. M. D. Carattino was supported by a Carl W. Gottschalk award from the American Society of Nephrology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Dr. A. Vandewalle provided the mpkCCD cells.
REFERENCES
- 1.Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of gamma ENaC mediates elastase activation of Na+ transport. J Gen Physiol 130: 611–629, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Alternate protocol: introduction of a point mutation by sequential PCR steps. In: Current Protocols in Molecular Biology. New York: John Wiley and Sons, 1995, p. 8.5.8. [Google Scholar]
- 3.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Botero-Velez M, Curtis JJ, Warnock DG. Brief report: Liddle's syndrome revisited–a disorder of sodium reabsorption in the distal tubule. N Engl J Med 330: 178–181, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Caci E, Melani R, Pedemonte N, Yueksekdag G, Ravazzolo R, Rosenecker J, Galietta LJ, Zegarra-Moran O. Epithelial sodium channel inhibition in primary human bronchial epithelia by transfected siRNA. Am J Respir Cell Mol Biol 40: 211–216, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol 288: L813–L819, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol 286: C190–C194, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Carattino MD, Hill WG, Kleyman TR. Arachidonic acid regulates surface expression of epithelial sodium channels. J Biol Chem 278: 36202–36213, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem 283: 25290–25295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carattino MD, Passero CJ, Steren CA, Maarouf AB, Pilewski JM, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the α-subunit of the epithelial sodium channel. Am J Physiol Renal Physiol 294: F47–F52, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem 281: 18901–18907, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol 111: 127–138, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devor DC, Pilewski JM. UTP inhibits Na+ absorption in wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol 276: C827–C837, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Eaton DC, Malik B, Saxena NC, Al-Khalili OK, Yue G. Mechanisms of aldosterone's action on epithelial Na+ transport. J Membr Biol 184: 313–319, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci USA 93: 15370–15375, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frindt G, Ergonul Z, Palmer LG. Surface expression of epithelial Na channel protein in rat kidney. J Gen Physiol 131: 617–627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frindt G, Palmer LG. Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium. Am J Physiol Renal Physiol 297: F1249–F1255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Caballero A, Dang Y, He H, Stutts MJ. ENaC proteolytic regulation by channel-activating protease 2. J Gen Physiol 132: 521–535, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC. A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem 282: 58–64, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Hirsh AJ. Altering airway surface liquid volume: inhalation therapy with amiloride and hyperosmotic agents. Adv Drug Deliv Rev 54: 1445–1462, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem 278: 37073–37082, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kastner C, Pohl M, Sendeski M, Stange G, Wagner CA, Jensen B, Patzak A, Bachmann S, Theilig F. Effects of receptor-mediated endocytosis and tubular protein composition on volume retention in experimental glomerulonephritis. Am J Physiol Renal Physiol 296: F902–F911, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J Biol Chem 277: 7641–7644, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Maarouf AB, Sheng N, Chen J, Winarski KL, Okumura S, Carattino MD, Boyd CR, Kleyman TR, Sheng S. Novel determinants of epithelial sodium channel gating within extracellular thumb domains. J Biol Chem 284: 7756–7765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Mall MA. Role of the amiloride-sensitive epithelial Na+ channel in the pathogenesis and as a therapeutic target for cystic fibrosis lung disease. Exp Physiol 94: 171–174, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol 291: F663–F669, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem 281: 27942–27949, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Nesterov V, Dahlmann A, Bertog M, Korbmacher C. Trypsin can activate the epithelial sodium channel (ENaC) in microdissected mouse distal nephron. Am J Physiol Renal Physiol 295: F1052–F1062, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Passero CJ, Hughey RP, Kleyman TR. New role for plasmin in sodium homeostasis. Curr Opin Nephrol Hypertens 19: 13–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem 283: 36586–36591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pochynyuk O, Bugaj V, Stockand JD. Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr Opin Nephrol Hypertens 17: 533–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roomans GM. Pharmacological treatment of the ion transport defect in cystic fibrosis. Expert Opin Investig Drugs 10: 1–19, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol 290: F1488–F1496, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sheng S, Li J, McNulty KA, Avery D, Kleyman TR. Characterization of the selectivity filter of the epithelial sodium channel. J Biol Chem 275: 8572–8581, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Staub O, Verrey F. Impact of Nedd4 proteins and serum and glucocorticoid-induced kinases on epithelial Na+ transport in the distal nephron. J Am Soc Nephrol 16: 3167–3174, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, Jespersen B, Jensen BL, Korbmacher C, Skott O. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol 20: 299–310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol 127: 591–604, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607–610, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Zhou Z, Treis D, Schubert SC, Harm M, Schatterny J, Hirtz S, Duerr J, Boucher RC, Mall MA. Preventive but not late amiloride therapy reduces morbidity and mortality of lung disease in betaENaC-overexpressing mice. Am J Respir Crit Care Med 178: 1245–1256, 2008 [DOI] [PubMed] [Google Scholar]