Abstract
Platelet endothelial cell adhesion molecule-1 (PECAM-1) has been implicated in endothelial cell motility during angiogenesis. Although there is evidence that SHP-2 plays a role in PECAM-1-dependent cell motility, the molecular basis of the activity of SHP-2 in this process has not been defined. To investigate the requirement of SHP-2 in PECAM-1-dependent cell motility, studies were done in which various constructs of SHP-2 were expressed in cell transfectants expressing PECAM-1. We observed that the levels of PECAM-1 tyrosine phosphorylation and SHP-2 association with PECAM-1 were significantly increased in cells expressing a phosphatase-inactive SHP-2 mutant, suggesting that the level of PECAM-1 tyrosine phosphorylation, and thus SHP-2 binding are regulated in part by bound, catalytically active SHP-2. We subsequently found that expression of PECAM-1 stimulated wound-induced migration and the formation of filopodia (a morphological feature of motile cells). These activities were associated with increased mitogen-activated protein kinase (MAPK) activation and the dephosphorylation of paxillin (an event implicated in the activation of MAPK). The phosphatase-inactive SHP-2 mutant, however, suppressed these PECAM-1-dependent phenomena, whereas the activity of PECAM-1 expressing cells was not altered by expression of wild-type SHP-2 or SHP-2 in which the scaffold/adaptor function had been disabled. Pharmacological inhibition of SHP-2 phosphatase activity also suppressed PECAM-1-dependent motility. Furthermore, PECAM-1 expression also stimulates tube formation, but none of the SHP-2 constructs affected this process. These findings therefore suggest a model for the involvement of SHP-2 in PECAM-1-dependent motility in which SHP-2, recruited by its interaction with PECAM-1, targets paxillin to ultimately activate the MAPK pathway and downstream events required for cell motility.
Keywords: mitogen-activated protein kinase, angiogenesis, leukocyte transendothelial migration
platelet endothelial cell adhesion molecule-1 (PECAM-1) is a transmembrane glycoprotein that is expressed on endothelial cells where it concentrates at intercellular junctions (25, 34). It is also expressed, albeit at lower levels, on platelets and leukocytes. In terms of its endothelial functions, PECAM-1 regulates leukocyte transendothelial migration (50), participates in the molecular sensing of fluid sheer stress (46), and confers resistance to apoptotic or endotoxic stresses (10, 31). PECAM-1 is also involved in angiogenesis (8, 9, 15, 16, 41, 52), where it has been implicated in endothelial cell motility (8, 9, 20, 28, 35). Although initially described as a mediator of cell-cell adhesion, subsequent studies established that PECAM-1 also participates in intracellular signaling (18, 23, 25). This signaling activity is mediated in part by tyrosine residues 663 and 686 of the cytoplasmic domain, each of which falls within a conserved signaling sequence known as the immunoreceptor tyrosine-based inhibitory motif (ITIM) (5, 36). As with other ITIM-containing receptors, phosphorylation of these two tyrosine residues by protein-tyrosine kinases (e.g., Src, Csk, and Fps/Fes family kinases) (7, 27, 30, 47) generates docking sites for the binding and activation of several cytosolic signaling molecules containing Src homology-2 (SH2) domains, including the tyrosine phosphatase SHP-2 (7, 24, 30, 38).
SHP-2 is a ubiquitously expressed 68-kDa nonreceptor protein tyrosine phosphatase composed of two amino-terminal SH2 domains, a catalytic phosphatase domain and COOH-terminal tail containing two tyrosines Y542 and Y580, that form docking sites for SH-2 domain-bearing proteins (11, 14, 45). SHP-2 is activated by the binding of tyrosine-phosphorylated proteins to the NH2-terminal SH2 domain causing exposure of the active catalytic phosphatase site (2). It has been suggested that the COOH-terminal SH2 domain functions to stabilize associations made by the NH2-terminal SH2 domain (1). In addition, a number of SH2 domain-containing adapter proteins associate with SHP-2 after receptor activation, including Grb-2 (growth factor receptor-bound protein 2), SHIP-1 [SH2 (Src homology 2)-containing inositol phosphatase-1], and SIT (SHP2-interacting transmembrane adaptor protein) (11, 14), which link surface receptors to intracellular signaling pathways. A large amount of genetic and biochemical data demonstrate that SHP-2 promotes mitogen-activated protein kinase (MAPK) activation in response to diverse agonists. As a result it is a positive component of many growth factors, cytokine and extracellular matrix signaling pathways, and thus plays a role in diverse cellular processes, including proliferation, survival, differentiation, and migration (11, 14). The importance of SHP-2 in vivo is evident by the fact that the loss of expression of functional SHP-2 in developing mice results in death between days 8.5–10.5 of gestation. These fetuses display multiple defects in mesodermal patterning as well as impaired hematopoietic development in embryonic stem cells (43, 44, 48). In addition, genetic mutations in PTPN11 that cause hyperactivation of SHP-2 phosphatase activity have been identified in the Noonan syndrome, a human developmental disorder (43, 48), and in various childhood leukemias (29, 44). Activating mutations in SHP-2 have also been detected in sporadic solid tumors (11).
There is evidence that the binding of SHP-2 to PECAM-1 following PECAM-1 tyrosine phosphorylation is involved in the enhancement of cell motility that results from the expression of PECAM-1 (35). The molecular basis of SHP-2's activity in this phenomenon is unknown, particularly, whether the enhanced cell migration mediated by PECAM-1 is due to the activity of SHP-2 as a phosphatase and/or an adaptor/scaffolding protein. Using cell transfectants expressing human PECAM-1, we therefore studied the effects of coexpressing various constructs of SHP-2, as well as the effects of pharmacological inhibition of SHP-2 phosphatase activity, on PECAM-1-dependent cell motility. The results of these studies suggest a model for the involvement of SHP-2 in PECAM-1-dependent endothelial cell motility in which SHP-2 binds to phosphorylated PECAM-1, becomes catalytically active, and dephosphorylates PECAM-1, leading to the release of SHP-2 from PECAM-1. The liberated, but now membrane localized, SHP-2 subsequently targets paxillin to ultimately activate the ERK/MAPK pathway and downstream events required for cell motility.
MATERIALS AND METHODS
Reagents and chemicals.
All reagents and chemicals were obtained from Sigma (St. Louis, MO) unless otherwise specified. The SHP-2 inhibitor NSC-87877 (13) was obtained from Calbiochem (San Diego, CA). Pfu Ultra High-Fidelity DNA polymerase with reaction buffer was purchased from Stratagene (La Jolla, CA). Restriction enzymes and reaction buffers were purchased from Promega (Madison, WI). Rapid DNA Ligation Kit was purchased from Roche Applied Science (Indianapolis, IN). Kits for DNA plasmid purification and agarose gel extraction were purchased from Qiagen (Valencia, CA). Sequencing and PCR primers were synthesized by Integrated DNA Technologies (Coralville, IA). Competent bacteria and dNTP mix were obtained from Invitrogen (Carlsbad, CA).
Antibodies.
The following antibodies targeting human proteins were employed: 4G6, a mouse anti-PECAM-1 antibody (a generous gift from Dr. Steven Albelda, University of Pennsylvania, Philadelphia, PA); 1.3, a mouse anti-PECAM-1 antibody (a generous gift from Dr. Peter Newman, Blood Center of Southeastern Wisconsin, Milwaukee, WI); a rabbit anti-SHP-2 antibody and a mouse anti-phospho-ERK antibody from Cell Signaling Technology (Danvers, MA); a rabbit anti-phosphotyrosine antibody and a mouse anti-paxillin antibody from BD Biosciences, Transduction Laboratories (Lexington, KY); a rabbit anti-ERK antibody from Promega; a rabbit anti-phospho-(Tyr-31)-paxillin antibody from Santa Cruz Biotechnology (Santa Cruz, CA); a mouse anti-GAPDH antibody from Chemicon-Millipore (Temecula, CA); and a mouse anti-FLAG antibody from Sigma.
Generation of SHP-2 constructs.
The following constructs of SHP-2 with a FLAG-tag were generated: wild-type SHP-2 (WT-SHP-2); phosphatase inactive SHP-2 (cysteine 459 mutated to serine; CS-SHP-2), and SHP-2 in which the scaffold/adaptor function has been disabled (tyrosines 542 and 580 mutated to phenylalanines; 2YF-SHP-2). Briefly, a wild-type SHP-2 construct (Addgene plasmid 8381: pCMV-WT-SHP-2, MA) was subcloned into pcDNA3.1(+) vector with HindIII and EcoRV to obtain pcDNA-WT-SHP-2 without an hemogglutin antibody (HA) tag. A PCR product of 3xFLAG fragment with restriction digestion sites of NheI and KpnI was generated from pBICEP-CMV-2 (Sigma). 3xFLAG was subcloned into pcDNA-WT-SHP-2 to generate pcDNA-3xFLAG-WT-SHP-2 (WT-SHP-2). The resulting construct was then subjected to site-directed mutagenesis using the GeneTailor Site-Directed Mutagenesis System (Invitrogen) according to the manufacturer's instructions. Cys459 of SHP-2 was mutated to Ser459 by PCR using the forward primer 5′-CAGGGCCGGTCGTGGTGCACAGCAGTGCTGGAATTGGC-3′ with the reverse primer 5′-GTGCACCACGACCGGCCCTGCATCCATGATGC-3′ to generate pcDNA-3xFLAG-CS-SHP-2 (CS-SHP-2). Point mutations converting Tyr542 and Tyr580 to Phe542 and Phe580 were generated by PCR using the following primer sets: for Y542 to F542, the forward primer 5′-GCAAGAGGAAAGGGCACGAATTTACAAATATTAAGTAT-3′ with the reverse primer 5′TTCGTGCCCTTTCCTCTTGCTTTTCTGCTCTT-3′; for Y580 to F580, the forward primer 5′-GAGAAGACAGTGCTAGAGTCTTTGAAAACGTGGGCCTG-3′ with the reverse primer 5′-GACTCTAGCACTGTCTTCTCTCATTTCTGCAC-3′ to generate pcDNA-3xFLAG-2YF-SHP-2 (2YF-SHP-2). These recombinant wild-type and mutant SHP-2 constructs were then subcloned into pIRESpuro3 expression vector (Clontech Laboratories, Mountain View, CA). The sequences of all constructs were verified by automated DNA sequencing.
Cell culture and transfection.
The human mesothelioma cell line REN (40) was cultured in RPMI media supplemented with 10% FBS, penicillin-streptomycin, and 2 mM l-glutamine. REN cell transfectants stably expressing human PECAM-1 (REN-HP) were cultured in the same media with G418 (0.5 mg/ml; GIBCO-BRL, Grand Island, NY) (8, 35). REN or REN-HP cells were transfected with SHP-2 constructs described above using Lipofectamine 2000 (Invitrogen). Stable transfectants were obtained by selection with Puromycin (1 mg/ml) for REN cells and with Puromycin (1 mg/ml) plus G418 (0.5 mg/ml) for REN-HP cells, respectively. Positive clones expressing transfected SHP-2 constructs were confirmed by Western blot analysis.
Intracellular SHP-2 phosphatase activity.
The intracellular SHP-2 phosphatase activity of lysates from stably transfected cell lines was determined using DuoSet IC (IntraCellular) Assay Development Systems (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions. Briefly, cell lysates were aliquoted, frozen in liquid nitrogen, and stored at −80°C. Protein concentration was determined using the BCA Protein Assay kit (Thermo Fisher Scientific, Rockford, IL). Fifty microliters of each standard in triplicate were used to construct a standard curve, and the phosphate released over 30 min by 5 μg total protein for each sample was assayed by the manufacturer's protocol. Absorbances were measured on 620 nm with the Beckman Coulter DTX880 Multimode Detector (Beckman Coulter, Fullerton, CA), and the phosphatase activity was calculated with the accompanying software.
In vitro cell proliferation.
REN cells and REN cell transfectants were cultured for 24–48 h in 96-well plates (4,000 cells/well), and the number of viable cells were determined using the Promega Cell Titer96 Aqueous Non-Radioactive Cell Proliferation Assay (Madison WI). This is a colorimetric assay with the absorbances measured at 490 nm.
In vitro wound-induced migration assay.
The wounding of confluent cell monolayers was modified from previously published procedures (8, 35). Twenty thousand REN cells and REN cell transfectants were added to 24-well tissue culture plates and allowed to grow to confluence. Linear defects were then made in the monolayer. The wounded culture was washed with PBS and then incubated for 24 h in complete media. With the use of computer-assisted image analysis and the MetaMorph software (Molecular Devices, Sunnyvale, CA), images were obtained immediately after wounding and then 24 h later, and the distance migrated by cells at the wound edge was determined. For each cell type three to five wounds were analyzed.
In vitro tube formation assay.
In vitro tube formation was studied using previously described procedures (35). REN cells and REN cell transfectants were plated on ECMatrix gel (a Matrigel equivalent) obtained from Chemicon. Fifty microliters of the solution were added to each well of a 96-well plate and allowed to form a gel at 37°C for 1 h. Twenty thousand cells in 200 μl of complete media were subsequently added to each well and incubated for 8 h at 37°C in 5% CO2. The wells were washed and the gel and its cells fixed with 3% paraformaldehyde. Total tube length per well was determined by computer-assisted image analysis using the MetaMorph software package.
Immunoprecipitation and immunoblotting.
Cultures of confluent cells were serum starved overnight and then incubated for 2 h in medium with or without 0.5 mM sodium orthovanadate at 37°C before scratch wounds were made in the monolayer. For our biochemical analyses, multiple parallel wounds, 1 mm in width were made in the confluent monolayer of cells by drawing a specially fabricated squeegee, with comb-like teeth, across the culture surface of a T-25 flask. We have visually confirmed that with this procedure all of the remaining, attached cells are adjacent to a cell-free zone into which they able are to migrate. One hour after wounding, the cells were washed with ice-cold PBS containing 0.5 mM sodium orthovanadate and and then lysed in TNC (0.01 M Tris-acetate, pH 8.0, 0.5% Nonidet-40, 0.5 mM Ca2+) with 1 mM sodium orthovanadate, 2 mM PMSF, and protease inhibitor cocktail (Sigma) for 20 min on ice. Extracts were clarified for 10 min at 4°C, and the supermatant protein concentrations were determined by the BCA assay kit (Pierce). Equal amounts (100 μg) of protein were incubated with 2 mg of 4G6 anti-PECAM-1 or anti-paxillin antibody with rocking movement overnight at 4°C. Protein A Sepharose beads (GE Healthcore Bio-Sciences, Piscataway, NJ) were added and incubated for an additional 2 h. After binding and collection were completed, the beads were washed three times with DOC wash (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 5% deoxycholate, and 0.1% SDS), dissolved in reducing loading buffer to release the immunoprecipitant. Proteins from immunoprecipitation or total cell lysates were resolved by SDS-PAGE (Novex; Invitrogen), followed by transfer onto nitrocellulose membranes using the iBlot Dry Blotting System (Invitrogen), which employs semidry electrotransfer. Membranes were washed in 1× TTBS for 2–3 min, blocked with 2% BSA, and incubated with the primary antibody in 2% BSA for 1 h at room temperature or overnight at 4°C. Unbound antibodies were washed off with TTBS before membranes were incubated with horseradish peroxidase-labeled species-specific secondary antibodies for 1 h at room temperature. After the membranes were again washed with PBS, bound antibody signals were detected by ECL substrate and documented on X-ray film. The chemiluminescent signals were quantified by densitometry (ImageQuant; Amersham, Piscataway, NJ).
Assessing the morphology of plated cells.
REN cells or REN cell transfectants, resuspended in complete M199 medium, were lightly seeded into uncoated two-well Lab-Tek Chamber slides (Nunc, Thermo Fischer Scientific, Rochester, NY) followed by culturing for 2–3 days. Digital images were then captured. With the use of computer-assisted image analysis, the points on either side of the base of a filopodium, at which the stalk of the filopodium angles with the cell surface, were located and a line was drawn between these two points. A second line was then drawn from the center of the first line through the center of filopodium to its tip, the length of which was taken as the length of the filopodium.
Statistical analyses.
Differences among groups were analyzed using one-way ANOVA. Results are presented as means ± SE. When statistically significant differences were found (P < 0.05), individual comparisons were made using the Bonferroni-Dunn test.
RESULTS
Expression of SHP-2 constructs in REN cell transfectants.
The expression of PECAM-1 in the REN cell mesothelioma line (40) has provided a useful system for modeling the function of endothelial PECAM-1 (8, 19, 26, 33, 35, 42, 49). Admittedly, there are potential limitations to use of REN cells as a model for endothelial cells, given the tumor cell origin of these cells. REN cells, however, do have a number of features that make them an attractive system for modeling the endothelium. First, they form cobblestone cell monolayers reminiscent of endothelial cells. Second, REN cells do not express PECAM-1 yet have several relevant endothelial surface molecules (e.g., αvβ3, ICAM-1, VCAM-1, and VEGFR-1). Third, in both endothelial cells and REN cell transfectants expressing human PECAM-1 (REN-HP): 1) PECAM-1 concentrates at cell-cell junctions (35); 2) tube-like structures form on Matrigel (8, 35); 3) H2O2 activates a calcium-permeant, nonselective cation current (26); and 4) wound-induced cell migration is associated with PECAM-1 tyrosine phosphorylation and SHP-2 association (35). Finally, REN-HP cells and human endothelial cells behave similarly with respect to the internalization and intracellular trafficking of surface-bound anti-PECAM-1 antibodies (19, 33, 49).
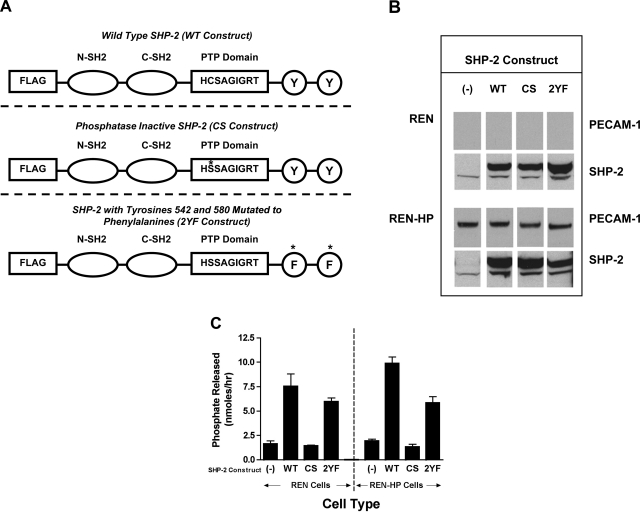
To define the requirements of SHP-2 in PECAM-1-dependent cell motility, the following constructs of SHP-2 with a FLAG-tag were expressed in REN or REN-HP cells: wild-type SHP-2 (WT-SHP-2); phosphatase inactive SHP-2 (cysteine 459 mutated to serine; CS-SHP-2); and SHP-2 in which the scaffold/adaptor function has been disabled (tyrosines 542 and 580 mutated to phenylalanines; 2YF-SHP-2) (Fig. 1A). These constructs were successfully expressed at comparable levels in REN and REN-HP cells (Fig. 1B). As expected, the SHP-2 phosphatase activity was increased in the cells expressing WT-SHP-2 or 2YF-SHP-2 but not CS-SHP-2 (Fig. 1C). Furthermore, the expression levels of these constructs were such that they would exceed and displace the endogenous SHP-2. In the cells transfected with the SHP-2 constructs, we observed that the amount of endogenous SHP-2 was somewhat increased over that found in cells without these constructs. This suggests that the processes that degrade or turnover SHP-2 in the cell have a defined capacity and that the SHP-2 mutants, because of their abundance, out compete the endogenous SHP-2 for these processes.
Fig. 1.
Expression of Src homology-1 phosphatase (SHP-2) constructs in REN cells and REN cells expressing human platelet endothelial cell adhesion molecule-1 (PECAM-1) (REN-HP). A: three constructs that were expressed in REN and REN-HP cells: wild-type SHP-2 (WT), phosphatase-inactive SHP-2 (CS), and SHP-2 with tyrosines 542 and 580 mutated to phenylalanines (2YF). B: cell lysates from REN and REN-HP cells expressing the various SHP-2 constructs, demonstrating both the presence of the endogenous SHP-2 (lower molecular weight band) and the transduced SHP-2 in the transfectants expressing a SHP-2 construct. (The data are from the same experiment and the same gel for each antibody and are representative of 3 experiments). C: intracellular SHP-2 phosphatase activities of each cell line as determined by the amount of phosphate released by 5 μg of cell lysate (see materials and methods) (n = 2). (The phosphatase data are representative of 2 experiments).
Increased PECAM-1 tyrosine phosphorylation and SHP-2 association in PECAM-1 transfectants expressing phosphatase-inactive SHP-2.
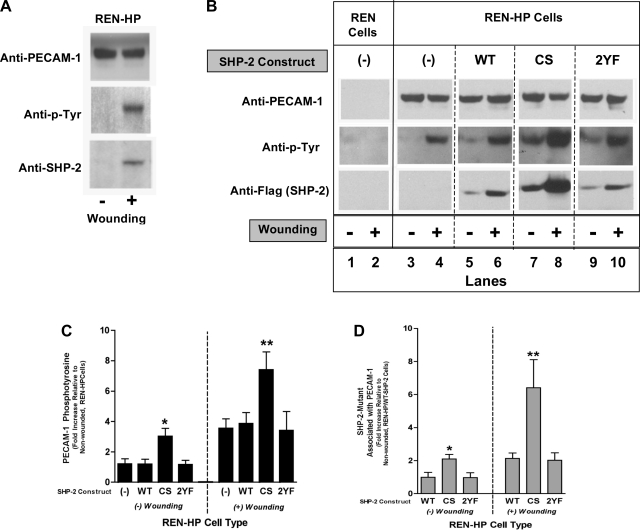
The wounding of confluent monolayers of PECAM-1-expressing cells such as HUVEC or REN-HP cells induces the tyrosine phosphorylation of PECAM-1 (on Y663 and Y686) and the subsequent binding of SHP-2 to PECAM-1 (35). PECAM-1 tyrosine phosphorylation was therefore investigated in REN-HP transfectants expressing the SHP-2 constructs (Fig. 2). As noted previously (35), the wounding of REN-HP cells induced PECAM-1 tyrosine phosphorylation and recruitment of the endogenous SHP-2 (Fig. 2A). The wounding of REN-HP cells expressing WT-SHP-2 (Fig. 2B, lanes 5 and 6) or 2YF-SHP-2 (Fig. 2B, lanes 9 and 10) induced PECAM-1 tyrosine phosphorylation that was comparable to control REN-HP transfectants (Fig. 2B, lanes 3 and 4) and accompanied by increased SHP-2 binding to PECAM-1. The behavior of the REN-HP cells expressing CS-SHP-2 (Fig. 2A, lanes 7 and 8), however, differed significantly from the other SHP-2 transfectants in two important respects. First, quiescent (unwounded) REN-HP/CS-SHP-2 cells demonstrated PECAM-1 tyrosine phosphorylation and SHP-2 association that exceeded the levels of resting WT-SHP-2 and 2YF-SHP-2 transfectants (Fig. 2B, lanes 7 compared with lanes 5 and 9; and Fig. 2, C and D). Second, while wounding did in fact stimulate PECAM-1 tyrosine phosphorylation and SHP-2 binding (Fig. 2B, lanes 7 and 8), the levels of tyrosine phosphorylation and SHP-2 binding associated with wounded REN-HP/CS-SHP-2 cells were two to three times that of the other REN-HP transfectants (Fig. 2A, lane 8 compared with lanes 6, and 10; and Fig. 2, C and D). These data suggest that the level of PECAM-1 tyrosine phosphorylation, and thus SHP-2 binding, are regulated in part by bound, phosphatase-active SHP-2. This is consistent with a model in which SHP-2 binds phosphorylated PECAM-1, becomes catalytically active, and dephosphorylates PECAM-1 leading to the release of SHP-2.
Fig. 2.
PECAM-1-SHP-2 association in REN-HP transfectants. Cell lysates from nonwounded or wounded monolayers of REN cells, REN-HP cells, or REN-HP cells expressing WT-SHP-2 (WT), CS-SHP-2 (CS), or 2YF-SHP-2 (2YF) were immunoprecipitated with anti-PECAM-1 and then blotted with anti-PECAM-1, anti-phospho-tyrosine (anti-p-Tyr), anti-SHP-2, or anti-FLAG (to detect SHP-2 constructs). In the REN-HP cells wounding induced PECAM-1 tyrosine phosphorylation that was associated with increased SHP-2/PECAM-1 association (A). The same phenomenon was observed for REN-HP cells expressing the various SHP-2 mutants (B, lanes 6, 8, 10). However, the amounts of SHP-2 associated with PECAM-1, both at rest and after wounding, were higher in the cells expressing the CS-SHP-2 construct (lanes 7 and 8) compared with the other cell lines. (The data are from the same experiment and the same gel for each antibody. They are representative of 5–6 experiments). Densitometry confirmed that PECAM-1 tyrosine phosphorylation (C) (expressed as fold increase compared with nonwounded REN-HP cells) and PECAM-1-SHP-2 association (D) (expressed as fold increase compared with nonwounded REN-HP/WT-SHP2 cells) were increased by coexpression of CS-SHP-2. Data were initially normalized to total PECAM-1 and are presented as means ± SE (n = 5–6; *P < 0.05, compared with nonwounded REN-HP expressing WT-SHP-2 cells; **P < 0.05 compared with wounded REN-HP expressing WT-SHP-2 cells).
Decreased cell migration, but preserved proliferation, in PECAM-1 transfectants expressing phosphatase-inactive SHP-2.
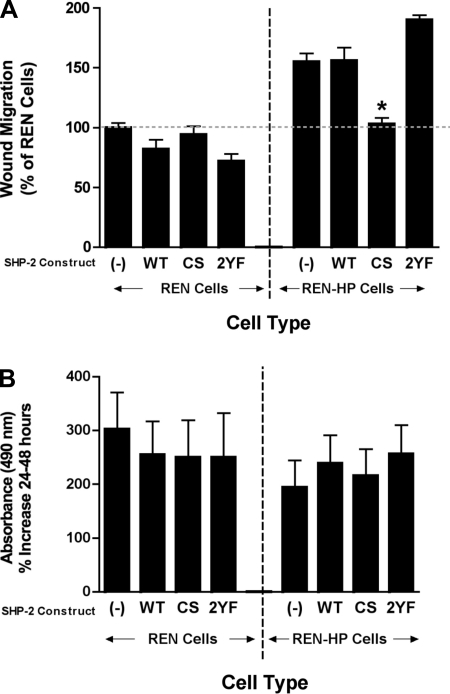
The expression of PECAM-1 in REN cells stimulates an increase in wound-induced cell migration, and SHP-2 has been implicated in this process (35). Wound-induced migration was therefore studied in REN and REN-HP cells expressing the SHP-2 constructs (Fig. 3A). The expression of PECAM-1 in REN cells induced a 50% increase in cell motility, which was not augmented further by the presence of WT-SHP-2. This suggests that the endogenous levels of SHP-2 are fully sufficient to mediate this phenomenon. Significantly, the enhanced motility observed in REN-HP cells was completely lost if CS-SHP-2 was simultaneously expressed, whereas the augmented migration was preserved in the 2YF-SHP-2 transfectants. The expression of WT-SHP-2, CS-SHP-2, or 2YF-SHP-2 did not significantly alter the motility of REN cells lacking PECAM-1 (Fig. 3A). These motility data are not related to changes in cell proliferation as the expression of PECAM-1 in REN cells does not significantly alter cell proliferation. Furthermore, the presence of WT-SHP-2, CS-SHP-2 or 2YF-SHP-2 did not inhibit the proliferation of REN or REN-HP cells (Fig. 3B). Together, these data suggest that PECAM-1-dependent cell migration is mediated by catalytically active SHP-2.
Fig. 3.
Effects of the expression of SHP-2 constructs on the migration and proliferation of REN and REN-HP cells. Wound-induced migration (A) and cell proliferation (B) were studied in REN and REN-HP cells expressing WT, CS, or 2YF constructs. The expression of CS-SHP-2 abrogated the stimulation of cell motility mediated by the expression of PECAM-1, whereas cell proliferation was not affected by the presence of any of the constructs. Data are presented as means ± SE (n = 3–4, for the proliferation studies; n = 6 for the migration studies; *P < 0.0001). The data presented are representative of least 3 experiments.
Decreased filopodia formation in PECAM-1 transfectants expressing phosphatase-inactive SHP-2.
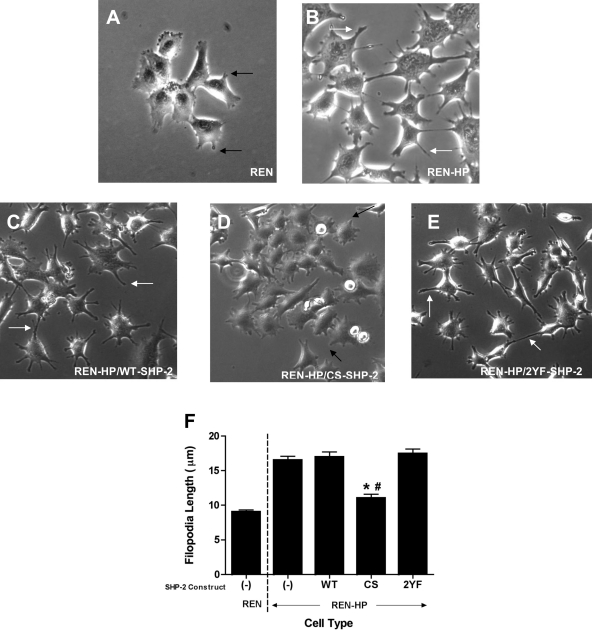
Filopodia are a feature of actively motile cells, mediating several functions required for cell migration (21, 32). We have previously demonstrated that PECAM-1 increases the rate and/or efficiency of filopodia formation (9). Therefore, this activity may be one of the mechanisms by which PECAM-1 promotes cell motility. Our finding that the expression of CS-SHP-2 abrogated the enhanced motility observed after transfection with PECAM-1 led us to also explore the effects of our SHP-2 constructs upon filopodia formation by REN-HP cells (Fig. 4). REN cell transfectants expressing PECAM-1 are characterized by numerous, long filopodia (Fig. 4B). Although REN-HP transfectants expressing WT-SHP-2 or 2YF-SHP-2 were morphologically similar to control REN-HP cells (Fig. 4, C and E), transfectants expressing CS-SHP-2 had filopodia morphology that was reminiscent of the nontransfected REN cells (Fig. 4, A and D). The mean filopodia length was determined for the various cell lines and was found for the REN-HP cells to be twice that of the nontransfected REN cells (Fig. 4F). Although the mean filopodia lengths for the REN-HP/WT-SHP-2 and REN-HP/2YF-SHP-2 cells were comparable to the REN-HP cells, the mean filopodia length for the REN-HP/CS-SHP-2 transfectants was not significantly different from that of the nontransfected REN cells. Filopodia formation in REN cells was not affected by the expression of the SHP-2 constructs (data not shown). These findings parallel what we observed for cell migration (Fig. 3) and implicate PECAM-1/SHP-2 interactions in the formation of filopodia during PECAM-1-dependent cell motility.
Fig. 4.
Effects of the expression of SHP-2 constructs on the formation of filopodia in REN and REN-HP cells. Shown are REN cells (A), REN-HP cells expressing no SHP-2 constructs (B), and REN-HP cells expressing WT-SHP-2 (C), CS-SHP-2 (D), or 2YF-SHP-2 (E) constructs. Filopodia were longer in the REN-HP cells (B, white arrows) compared with those of the REN cells (A, black arrows). The morphology of the filopodia on the REN-HP cells expressing WT-SHP-2 (C) and 2YF-SHP-2 (E) were similar to the REN-HP cells (B), whereas the REN-HP/CS-SHP-2 cells (D, black arrows) had a morphology that was reminiscent of the nontransfected REN cells (A). The mean filopodia length was determined for each cell line (F). For the REN-HP/CS-SHP-2 cells, the mean filopodia length was significantly less than that of the REN-HP, REN-HP/WT-SHP-2, and REN-HP/2YF-SHP-2 cells and not significantly different from the nontransfected REN cells. Data are presented as means ± SE (n = 100; *P < 0.0001, compared with REN-HP cells; #P > 0.05, compared with REN cells). The data presented are representative of 3 experiments.
Pharmacological inhibition of SHP-2 phosphatase activity inhibits PECAM-1-dependent motility and filopodia formation.
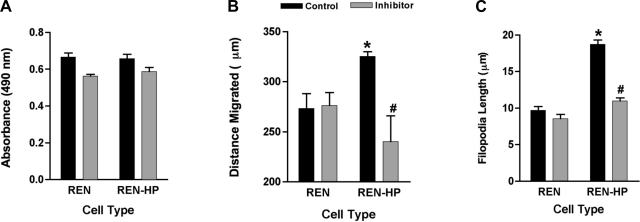
The data presented above, based on a molecular approach, provided evidence that PECAM-1-dependent cell motility and filopodia formation are mediated through the phosphatase activity of SHP-2. To confirm these findings with an alternative strategy, we also studied the effects of NSC-87877, a potent and specific inhibitor SHP-2 phosphatase activity, on the behavior of REN and REN-HP cells (13). NSC-87877 (20 μM) induced a very modest and comparable (∼10%) inhibition of proliferation in both cell lines (Fig. 5A). In contrast, the enhanced cell migration and filopodia formation induced by the presence of PECAM-1 in REN-HP cells were completely abrogated by NSC-87877, without any effects on the motility and filopodia of the control REN cells (Fig. 5, B and C). A similar pattern of effects was seen with HUVEC treated with this inhibitor (data not shown). These data thus provide pharmacological confirmation of the importance of the SHP-2 phosphatase activity in PECAM-1-dependent cell motility.
Fig. 5.
Effects of NCS-87877 on the proliferation, cell migration, and filopodia formation of REN and REN-HP cells. Proliferation (A) (n = 4), wound-induced migration (B) (n = 3), and filopodia formation (C) (n = 100) were assessed in REN and REN-HP cells cultured in media without or with NSC-87877 (20 μM). Although the inhibitor induced a very modest decrease in proliferation for both cell types (A), it specifically suppressed the enhancements in wound migration and filopodia length that were stimulated by the expression of PECAM-1 in REN-HP cells. Data are presented as means ± SE. When compared with control REN cells, *P < 0.01, for the proliferation and migration studies and <0.0001 for the filopodia experiments; #P > 0.05, compared with inhibitor-treated REN cells.
Expression of PECAM-1 increases ERK activation, an effect inhibited by expression of phosphatase-inactive SHP-2.
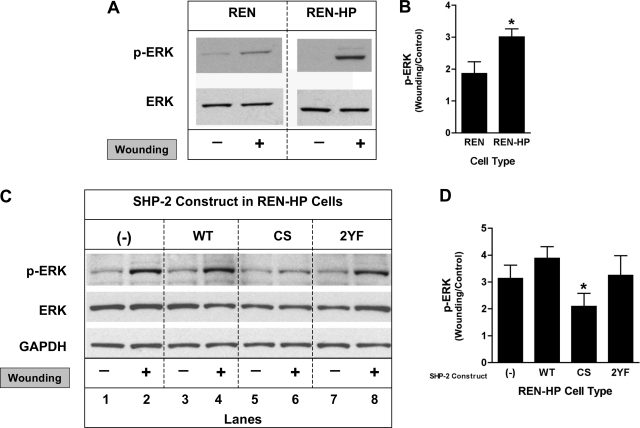
MAPK has been shown to play a role in cell motility and in the formation of filopodia (6, 14, 22, 37, 51). The activation of extracellular signal-regulated kinase (ERK) was therefore determined in REN and REN-HP cells (Fig. 6). We found that ERK activation following wounding of confluent cell monolayers was increased in the REN-HP cells compared with the control REN cells (Fig. 6, A and B). The increase in ERK activation resulting from the presence of PECAM-1 in REN-HP cells was not enhanced further by the expression of WT-SHP-2 but was abrogated by the expression of CS-SHP-2 (Fig. 6, B and C). PECAM-1-dependent ERK activation was not altered by the presence of 2YF-SHP-2. ERK phosphorylation in REN cells during wound-induced migration was not significantly altered by the expression of these SHP-2 constructs (data not shown). This suggests that catalytically active SHP-2 in the context of PECAM-1-dependent cell motility mediates an activation of the ERK/MAPK pathway.
Fig. 6.
Effects of the expression of SHP-2 constructs on wound-induced ERK activation in REN and REN-HP cells. Cell lysates from nonwounded or wounded monolayers of REN or REN-HP cells were immunoblotted with anti-phopho-ERK and anti-ERK antibodies. A: expression of PECAM-1 in REN cells resulted in increased phospho-ERK levels after wounding compared with nontransfected REN cells. B: densitometry confirmed that ERK activation following wounding of REN cells (expressed as the ratio of wounding/control) was increased by the expression of PECAM-1. Data are presented as means ± SE (n = 4; *P < 0.05, compared with REN cells). C: expression of the CS-SHP-2 (CS) construct in REN-HP cells inhibited ERK activation (lanes 5 and 6 compared with lanes 1 and 2) following wounding, while the presence of WT-SHP-2 (WT) or 2 YF-SHP-2 (2YF) did not suppress PECAM-1-dependent, wound-induced, ERK activation (lanes 3 and 4, and lanes 7 and 8, compared with lanes 1 and 2). D: densitometry confirmed that the ERK activation mediated by PECAM-1 (expressed as the ratio of wounding/control) was suppressed by coexpression of CS-SHP-2. Data are presented as means ± SE (n = 3; *P < 0.05, compared with REN-HP/WT cells). The densitometry were all initially normalized to total ERK expression. (The data presented in A and C are from the same experiment and the same gel for each antibody. They are representative of 3–4 experiments).
Expression of PECAM-1 suppresses paxillin tyrosine phosphorylation, an effect reversed by expression of phosphatase-inactive SHP-2.
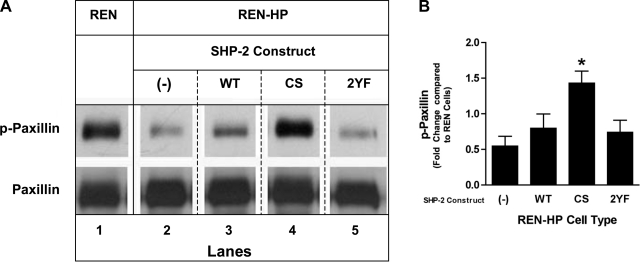
One model for the activation of ERK involves the tyrosine dephosphorylation of paxillin and the subsequent release of Src kinase from paxillin-dependent inhibition (14, 37). We therefore assessed the effects on paxillin tyrosine phosphorylation of expressing the SHP-2 constructs in REN-HP cells (Fig. 7). As has been previously reported (35), we found that the overall level of paxillin tyrosine phosphorylation following wounding was significantly reduced in REN-HP cells compared with control REN cells (Fig. 7A, lane 2 compared with lane 1). In REN-HP cells that expressed WT-SHP-2 or 2YF-SHP-2, the levels of paxillin tyrosine phosphorylation were similarly reduced (Fig. 7A, lanes 3 and 5 compared with lane 2). In contrast, when CS-SHP-2 was expressed in REN-HP cells, paxillin tyrosine phosphorylation was preserved and even enhanced compared with that of nontransfected REN cells (Fig. 7A, lane 4 compared with lane 1 and Fig. 7B). Phosphopaxillin levels in REN cells during wound-induced migration were not increased by the presence of the CS mutant (data not shown). These data provide evidence of a mechanistic role for SHP-2 in the stimulation of PECAM-1-dependent cell motility through the dephosphorylation of paxillin and the subsequent activation of the ERK/MAPK pathway.
Fig. 7.
Effects of the expression of SHP-2 constructs on wound-induced paxillin phosphorylation in REN and REN-HP cells. A: cell lysates from wounded monolayers of REN cells, REN-HP cells, and REN-HP cells expressing WT, CS, or 2YF constructs were immunoprecipitated with anti-paxillin antibody and then immunoblotted with anti-phospho-paxillin and anti-paxillin antibodies. In the REN-HP cells, wounding induced paxillin dephosphorylation (lane 2 compared with lane 1) that was reversed by expression of CS-SHP-2 (lane 4 compared with lanes 1 and 2). The presence of WT-SHP-2 or 2YF-SHP-2 did not reverse PECAM-1-dependent, wound-induced, paxillin dephosphorylation (lanes 3 and 5, and 1 and 2). (The data are from the same experiment and the same gel for each antibody. They are representative of 5 experiments). B: densitometry confirmed that the dephosphorylation of paxillin mediated by PECAM-1 (expressed as fold change compared with REN cells) was reversed by coexpression of CS-SHP-2. Data are presented as means ± SE (n = 5; *P < 0.05, compared with REN-HP cells). Paxillin levels in the nonwounded cells were comparable and were not altered by the expression of any of the SHP-2 constructs (data not shown).
PECAM-1-dependent tube formation is not inhibited by the expression of phosphatase-inactive SHP-2.
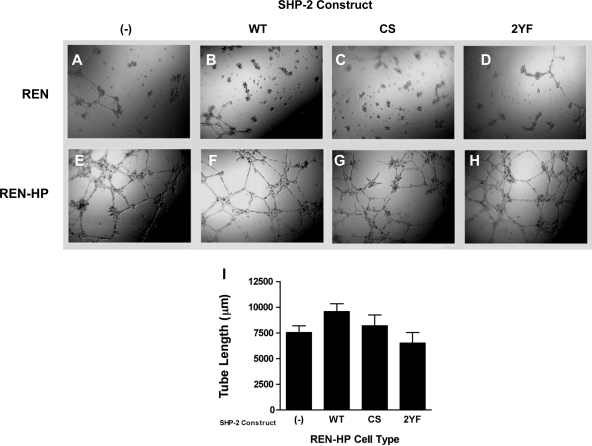
A number of studies have demonstrated that the presence of PECAM-1 promotes the in vitro formation of cord-like/tubular networks by endothelial cells or cellular transfectants expressing PECAM-1 (8, 35, 52). We therefore studied the ability of PECAM-1 transfectants expressing our SHP-2 mutants to form tubular structures on Matrigel (Fig. 8). We found that cord/tube formation was similar in control REN-HP transfectants and transfectants expressing the SHP-2 constructs (Fig. 8, E–I). In particular, although the CS-SHP-2 mutant inhibited PECAM-1-dependent motility (Fig. 3A), it did not suppress PECAM-1-dependent cord/tube formation (Fig. 8, G and I). This suggests that the stimulation of cord-like/tubular networks mediated by the expression of PECAM-1 is not due merely to an enhancement in cell motility and may be less dependent on PECAM-1/SHP-2 interactions.
Fig. 8.
Cord/tube formation on Matrigel by REN cells and REN-HP cells expressing SHP-2 constructs. Shown are REN cells (A–D) and REN-HP cells (E–H), expressing no SHP-2 constructs (A and E), WT-SHP-2 (B and F), CS-SHP-2 (C and G), or 2YF-SHP-2 (D and H) plated on Matrigel. REN cells did not form cord/tube-like networks on Matrigel, whereas all of the REN-HP cell transfectants did form cord/tube-like networks at comparable levels. Cord/tube formation was quantitated using image analysis (I). There were no significant differences between the various cell lines (n = 6). The data presented are representative of 3 experiments.
DISCUSSION
The studies in this paper were done with the overall goal of furthering our understanding of the role of PECAM-1 as a mediator of endothelial cell motility during angiogenesis. The mechanisms by which PECAM-1 enhances cell migration still remain to be determined, although previous studies have indirectly implicated a role for SHP-2 in the ability of PECAM to stimulate endothelial cell motility (35). In these prior investigations, the ability of PECAM-1 to promote cell migration was eliminated by mutations of Y663 and Y686 in the cytoplasmic domain that led to the loss of the molecule's ability to bind to SH2 domain containing proteins such as SHP-2 (35). In this report we specifically demonstrate that overexpression of catalytically inactive SHP-2, but not wild-type SHP-2 or SHP-2 in which the adaptor/scaffold functions have been disabled, eliminates the ability of PECAM-1 to enhance cell migration (Fig. 3). Given that the various SHP-2 constructs were expressed at comparable levels, the effects of the catalytically inactive SHP-2 are unlikely to be related to “flooding” the cells with excess SHP-2. In addition, the enhanced motility mediated by the presence of PECAM-1 was completely abrogated by a specific pharmacological inhibitor of SHP-2 phosphatase activity (Fig. 5). Consequently, our studies now provide direct evidence for the involvement of SHP-2 in PECAM-1-dependent motility. These data also point to the importance of the phosphatase activity of SHP-2, rather than its adaptor/scaffold functions, in this process.
Although there is still more to be learned, our data offer some clues regarding the activity of SHP-2 as a mediator of PECAM-1-dependent motility. First, in REN-HP cells expressing the phosphatase-inactive SHP-2 mutant, both at rest or following wounding, we found that the levels of PECAM-1 tyrosine phosphorylation and SHP-2 association were significantly increased when compared with control REN-HP cells. In contrast, significant changes were not induced by the expression of the other constructs (Fig. 2). These data are consistent with a dynamic interaction between SHP-2 and PECAM-1 in which the level of PECAM-1 tyrosine phosphorylation, and thus SHP-2 binding, are regulated in part by bound, catalytically active SHP-2.
Second, we found that the stimulation of migration and filopodia formation induced by the expression of PECAM-1 was associated with increased ERK activation that was suppressed by coexpression of catalytically inactive SHP-2 (Fig. 6). There are a number of downstream targets of activated ERK (e.g., MLCK, FAK, and calpain) whose phosphorylation promotes processes required for efficient cell locomotion (12, 14, 22). For certain stimuli, SHP-2 may mediate the activation of ERK (14, 37). Our finding that PECAM-1-dependent cell migration was associated with increased ERK activation, which was inhibited by phosphatase-inactive SHP-2 (Fig. 5), is therefore consistent with previous reports. Furthermore, these data suggest that PECAM-1 in the context of endothelial cell motility, through SHP-2, may play a role in the upstream regulation of ERK.
Finally, our data suggest a mechanism for this PECAM-1-dependent ERK activation. During wound-induced migration we observed that PECAM-1 mediated a significant dephosphorylation of paxillin that was reversed by coexpression of phosphatase-inactive SHP-2 (Fig. 7). This is significant because there is evidence that Csk, by binding to phosphorylated paxillin, mediates inhibitory signals that repress ERK activation (14, 37). In initial studies, we have observed that the amount of Csk associated with paxillin during wound-induced migration was always significantly greater in the cells expressing the CS-SHP-2 mutant compared with the other transfectants (data not shown). Consequently, in the setting of PECAM-1-stimulated cell migration, dephosphorylation of paxillin by SHP-2, which was recruited and potentially activated by PECAM-1, may serve to trigger an ERK/MAPK-dependent signaling cascade by relieving Csk-mediated inhibition.
Our finding that expression of the catalytically inactive SHP-2 construct in REN-HP suppresses PECAM-1-dependent cell motility without inhibiting the capacity of these cells to form cord-like/tubular networks (Fig. 8) suggests that the ability of PECAM-1 to promote in vitro cord/tube formation is independent of its ability to stimulate cell migration. Additionally, in previous studies we have shown that loss of the ability of PECAM-1 to bind to SH2 domain containing proteins inhibits PECAM-1-dependent in vitro tube formation, implicating molecules such as SHP-2 in this process (15). Our data, however, suggest this inhibition is not due to the loss of a PECAM-1/SHP-2 interaction, pointing to the possibility that other PECAM-1-binding, SH2 domain containing proteins, may substitute for or be more important than SHP-2 in mediating the ability of PECAM-1 to stimulate to the formation of tubular/cord-like networks. We would stress that our data neither preclude some other molecular interaction with PECAM-1 that might contribute to junctional stabilization, nor do they exclude the involvement of PECAM-1 in the maintenance of junctional stability during quiescence.
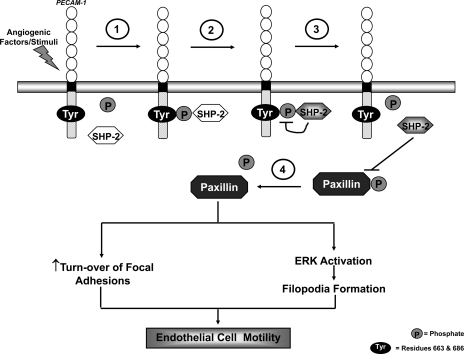
Although a number of elements remain to be confirmed, we propose a model (Fig. 9) for the involvement of PECAM-1 in endothelial motility during angiogenesis in which angiogenic factors and/or stimuli trigger the tyrosine phosphorylation of PECAM-1 and the subsequent binding of SHP-2 to PECAM-1. This PECAM-1/SHP-2 interaction accomplishes two things: the recruitment of SHP-2 to the membrane and the activation of its phosphatase activity, possibly by conformational changes stimulated by PECAM-1-binding. With its phosphatase activity now “turned on,” SHP-2 then dephosphoylates the PECAM-1 molecule to which it is bound, resulting in the release of SHP-2. The liberated SHP-2, now localized at the membrane, promotes the turnover of focal adhesions by dephosphorylating constituent proteins such as paxillin (35). The membrane-localized SHP-2 also mediates dephosphoylation events that activate the ERK pathway, a consequence of which is the enhanced formation of filopodia. The net effects of these events are increased cell motility and a role for PECAM-1 in facilitating these processes through the recruitment and activation of SHP-2.
Fig. 9.
A proposed model for the involvement of PECAM-1 in endothelial cell motility during angiogenesis. Angiogenic factors and/or conditions stimulate PECAM-1 tyrosine phosphorylation and the binding of SHP-2 to PECAM-1 (step 1). This association of SHP-2 with PECAM-1 results in activation of its phosphatase activity (step 2). The activated SHP-2 dephosphorylates the PECAM-1 molecule to which it is bound, leading to its release from PECAM-1 (step 3). The liberated SHP-2 subsequently targets paxillin, dephosphorylating it (Step 4), to trigger events such as the turnover of focal adhesions and ERK-mediated activation of filopodia formation, which ultimately promote endothelial cell motility. (Tyr = residues 663 and 686; P = phosphate).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
This work was supported by grants from the Department of Defense (PR043482) and the National Heart, Lung, and Blood Institute (HL-079090).
REFERENCES
- 1.Araki T, Nawa H, Neel BG. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J Biol Chem 278: 41677–41684, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Barford D, Neel BG. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure 6:249–254, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84: 869–901, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bentires-Alj M, Paez JG, David FS, Keilhack H, Halmos B, Naoki K, Maris JM, Richardson A, Bardelli A, Sugarbaker DJ, Richards WG, Du J, Girard L, Minna JD, Loh ML, Fisher DE, Velculescu VE, Vogelstein B, Meyerson M, Sellers WR, Neel BG. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res 64:8816–8820, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Billadeau DD, Leibson PJ. ITAMs versus ITIMs: striking a balance during cell regulation. J Clin Invest 109: 161–168, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmbhatt AA, Klemke RL. ERK and RhoA differentially regulate pseudopodia growth and retraction during chemotaxis. J Biol Chem 278: 13016–13025, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Cao MY, Huber M, Beauchemin N, Famiglietti J, Albelda SM, Veillette A. Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J Biol Chem 273: 15765–15772, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Cao G, O'Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A, DeLisser HM. The involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol Cell Physiol 282: C1181–C1190, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Cao G, Fehrenbach M, Williams J, Finklestein J, Zhu JX, DeLisser HM. Angiogenesis in PECAM-1-null mice. Am J Pathol 175: 903–915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol 166: 185–196, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan G, Kalaitzidis D, Neel BG. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev 27: 179–192, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature 410: 37–40, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Sung S, Yip LR, Lawrence HR, Ren Y, Guida WC, Sebti SN, Lawrence NJ, Wu J. Discovery of a novel Shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol 70: 562–570, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Dance M, Montagner A, Salles JP, Yart A, Raynal P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK½) pathway. Cell Signal 20: 453–459, 2008 [DOI] [PubMed] [Google Scholar]
- 15.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol 151: 671–677, 1997 [PMC free article] [PubMed] [Google Scholar]
- 16.Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. Dev Biol 315: 72–88, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol 162: 3022–3030, 1999 [PubMed] [Google Scholar]
- 18.Fujiwara K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J Intern Med 259: 373–380, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Garnacho C, Albelda SM, Muzykantov VR, Muro S. Differential intra-endothelial delivery of polymer nanocarriers targeted to distinct PECAM-1 epitopes. J Control Release 130: 226–33, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gratzinger D, Canosa S, Engelhardt B, Madri JA. Platelet endothelial cell adhesion molecule-1 modulates endothelial cell motility through the small G-protein Rho. FASEB J 17: 1458–1469, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE 400: re5, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Huang C, Jacobson Schaller, MD K. MAP kinases and cell migration. J Cell Sci 117: 4619–4628 [DOI] [PubMed] [Google Scholar]
- 23.Ilan N, Madri JA. PECAM1: old friend, new partners. Curr Opin Cell Biol 15: 515–524, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J Biol Chem 272: 6986–6993, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Jackson DE. The unfolding tale of PECAM-1. FEBS Lett 540: 7–14, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Ji G, O'Brien CD, Feldman M, Manevich Y, Lim P, Sun J, Albelda SM, Kotlikoff MI. PECAM-1 (CD31) regulates a hydrogen peroxide-activated nonselective cation channel in endothelial cells. J Cell Biol 157: 173–84, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kogata N, Masuda M, Kamioka Y, Yamagishi A, Endo A, Okada M, Mochizuki N. Identification of Fer tyrosine kinase localized on microtubules as a platelet endothelial cell adhesion molecule-1 phosphorylating kinase in vascular endothelial cells. Mol Biol Cell 14: 3553–3564, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo S, Scheef EA, Sheibani N, Sorenson CM. PECAM-1 isoform-specific regulation of kidney endothelial cell migration and capillary morphogenesis. Am J Physiol Cell Physiol 292: C2070–C2083, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Loh ML, Vattikuti S, Schubbert S, Reynolds MG, Carlson E, Lieuw KH, Cheng JW, Lee CM, Stokoe D, Bonifas JM, Curtiss NP, Gotlib J, Meshinchi S, Le Beau MM, Emanuel PD, Shannon KM. Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood 103: 2325–2331, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Lu TT, Barreuther M, Davis S, Madri JA. Platelet endothelial cell adhesion molecule-1 is phosphorylatable by c-Src, binds Src-Src homology 2 domain, and exhibits immunoreceptor tyrosine-based activation motif-like properties. J Biol Chem 272: 14442–14446, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol 288: H159–H164, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 9: 446–454, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci USA 96:2379–2384, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman PJ. The biology of PECAM-1. J Clin Invest 99: 3–8, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Brien C, Cao G, Makringiannakis A, DeLisser HM. The Role of immunoreceptor tyrosine-based inhibitory motifs of platelet endothelial cell adhesion molecule (PECAM-1) in PECAM-1 dependent cell migration. Am J Physiol Cell Physiol 287: C1103–C1113, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science 290: 84–89, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Ren Y, Meng S, Mei L, Zhao ZJ, Jove R, Wu J. Roles of Gab1 and SHP2 in paxillin tyrosine dephosphorylation and Src activation in response to epidermal growth factor. J Biol Chem 279: 8497–8505, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Sagawa K, Kimura T, Swieter M, Siraganian RP. The protein-tyrosine phosphatase SHP-2 associates with tyrosine-phosphylated adhesion molecule PECAM-1 (CD31). J Biol Chem 272: 31086–31091, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J 16: 2352–2364, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smythe WR, Hwang HC, Amin KM, Eck SL, Davidson BL, Wilson JM, Kaiser LR, Albelda SM. Use of recombinant adenovirus to transfer the herpes simplex virus thymidine kinase (HSVtk) gene to thoracic neoplasm: an effective in vitro drug sensitization system. Cancer Res 54: 2055–2059, 1994 [PubMed] [Google Scholar]
- 41.Solowiej A, Biswas P, Graesser D, Madri JA. Lack of platelet endothelial cell adhesion molecule-1 attenuates foreign body inflammation because of decreased angiogenesis. Am J Pathol 162: 953–962, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun J, Paddock C, Shubert J, Zhang HB, Amin K, Newman PJ, Albelda SM. Contributions of the extracellular and cytoplasmic domains of platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell-cell localization. J Cell Sci 113: 1459–1469, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, van der Burgt I, Crosby AH, Ion A, Jeffery S, Kalidas K, Patton MA, Kucherlapati RS, Gelb BD. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet 29: 465–468, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Tartaglia M, Niemeyer CM, Fragale A, Song X, Buechner J, Jung A, Hählen K, Hasle H, Licht JD, Gelb BD. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet 34: 148–150, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol 7: 833–846, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser HM, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Udell CM, Samayawardhena LA, Kawakami Y, Kawakami T, Craig AW. Fer and Fps/Fes participate in a Lyn-dependent pathway from FcepsilonRI to platelet-endothelial cell adhesion molecule 1 to limit mast cell activation. J Biol Chem 281: 20949–20957, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Yu WM, Zhang W, McCrae KR, Neel BG, Qu CK. Noonan syndrome/leukemia-associated gain-of-function mutations in SHP-2 phosphatase (PTPN11) enhance cell migration and angiogenesis. J Biol Chem 284: 913–20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiewrodt R, Thomas AP, Cipelletti L, Christofidou-Solomidou M, Weitz DA, Feinstein SI, Schaffer D, Albelda SM, Koval M, Muzykantov VR. Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood 99: 912–922, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol 27: 2514–2523, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Yang W, Klaman LD, Chen B, Araki T, Harada H, Thomas SM, George EL, Neel BG. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev Cell 10: 317–327, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Zhou Z, Christofidou-Solomidou M, Garlanda C, DeLisser HM. Antibody against Murine PECAM-1 Inhibits Tumor Angiogenesis in Mice. Angiogenesis 3: 181–188, 1999 [DOI] [PubMed] [Google Scholar]