Abstract
BACKGROUND
The outcomes of gene therapy to correct congenital immunodeficiencies are unknown. We reviewed long-term outcomes after gene therapy in nine patients with X-linked severe combined immunodeficiency (SCID-X1), which is characterized by the absence of the cytokine receptor common γ chain.
METHODS
The nine patients, who lacked an HLA-identical donor, underwent ex vivo retrovirus-mediated transfer of γ chain to autologous CD34+ bone marrow cells between 1999 and 2002. We assessed clinical events and immune function on long-term follow-up.
RESULTS
Eight patients were alive after a median follow-up period of 9 years (range, 8 to 11). Gene therapy was initially successful at correcting immune dysfunction in eight of the nine patients. However, acute leukemia developed in four patients, and one died. Transduced T cells were detected for up to 10.7 years after gene therapy. Seven patients, including the three survivors of leukemia, had sustained immune reconstitution; three patients required immunoglobulin-replacement therapy. Sustained thymopoiesis was established by the persistent presence of naive T cells, even after chemotherapy in three patients. The T-cell–receptor repertoire was diverse in all patients. Transduced B cells were not detected. Correction of the immunodeficiency improved the patients’ health.
CONCLUSIONS
After nearly 10 years of follow-up, gene therapy was shown to have corrected the immunodeficiency associated with SCID-X1. Gene therapy may be an option for patients who do not have an HLA-identical donor for hematopoietic stem-cell transplantation and for whom the risks are deemed acceptable. This treatment is associated with a risk of acute leukemia. (Funded by INSERM and others.)
The cytokine receptor common γ chain, which is encoded by the interleukin-2 receptor subunit gamma (IL2RG) gene, is a critical functional component of the receptors for interleukin-2, interleukin-4, interleukin-7, interleukin-9, interleukin-15, and interleukin-21.1 Naturally occurring mutations in IL2RG are responsible for X-linked severe combined immunodeficiency (SCID-X1) disease. This condition is characterized by the complete lack of T cells and natural killer cells, whereas B cells are present.2,3 Hematopoietic stem-cell transplantation is a lifesaving therapy. Despite associated improvements in the survival rate, however, non–HLA-identical hematopoietic stem-cell transplantation has a number of drawbacks. For example, reconstitution of T-cell function is delayed in patients receiving non–HLA-identical hematopoietic stem-cell transplants, as compared with patients receiving HLA-identical transplants; graft-versus-host disease (GVHD) requires post-transplantation immunosuppression (a therapy that is associated with high rates of infectious disease and death); and a decrease in thymopoiesis over the long term has been reported in some cases,4–7 although this outcome is not universal.6,8 We previously described the efficacy of ex vivo γ-chain retrovirus-mediated gene transfer into autologous hematopoietic precursor cells in five patients with SCID-X1, with a follow-up period of 2.5 years.9,10 Our results were consistent with those of a similar trial performed in London.11 Both groups reported the occurrence of leukemia (in 5 of 20 treated patients in the two studies combined), which was associated with oncogene transactivation by the vector’s transcriptional control elements.12,13 Here, we describe the long-term outcomes (at 8 to 11 years) for patients treated in our center.
METHODS
Nine patients with confirmed SCID-X1 who lacked an HLA-identical donor were eligible for enrollment in a γ-chain gene-therapy trial at the Necker–Enfants Malades Hospital between March 1999 and April 2002. An additional patient (Patient 9) was treated in Australia and described in a case report14 (see the Supplementary Appendix, available with the full text of this article at NEJM.org). The patients’ genetically modified CD34+ bone marrow cells were prepared as described elsewhere.9,10
The protocol was approved by the French Drug Agency and the local investigational review board, and the study was conducted in accordance with the protocol. The patients’ parents were informed that an alternative treatment based on hematopoietic stem-cell transplantation was available and that this new, experimental gene therapy carried a potential risk of leukemia. Written informed consent was obtained from the parents. All patients were placed in sterile isolation and received nonabsorbable antibiotics and anti-infective agents, including intravenous immune globulin. In Patient 3, the T-cell compartment10 was not reconstituted because of persistent splenomegaly (see the Supplementary Appendix). Clinical and immunologic investigations were subsequently performed at regular intervals. The end date for the analysis was March 1, 2010. The criterion for discontinuing intravenous immune globulin therapy was a combination of a T-cell count above 1000 per cubic millimeter and an absence of infection. Details of the study methods are included in the Supplementary Appendix.
RESULTS
CLINICAL OUTCOME
Nine patients with γ-chain deficiency underwent gene therapy at a median age of 7 months (range, 1 to 11) (Table 1). The patients did not undergo a conditioning regimen. They received an infusion of autologous bone marrow–derived CD34+ cells transduced with the γ chain–containing retroviral vector (median dose of CD34+ γ-chain+ cells per kilogram of body weight, 4×106; range, 1×106 to 22×106).
Table 1.
Characteristics of the Patients.*
| Patient No. | Age at Treatment | Clinical Status before Treatment | γ-Chain Expression before Treatment | Infused γ-Chain+ CD34+ Cells | Severe Adverse Events | Clinical Status after Treatment | Follow-up | Growth (SD)† | Immune Globulin Therapy |
|---|---|---|---|---|---|---|---|---|---|
| mo | no. per kg of body weight (×10−6) | yr | |||||||
| 1 | 11 | Pneumocystis jirovecii pneumonitis, protracted diarrhea, failure to thrive | Yes | 3 | ND | Well | 11.0 | +2 | No |
| 2 | 8 | P. jirovecii pneumonitis, protracted diarrhea, GVHD-like lesions, failure to thrive | No | 5 | ND | Well | 10.9 | +1 | No |
| 3 | 8 | Disseminated BCG infection, pulmonary adenovirus and RSV infection, protracted diarrhea, failure to thrive | No | 5 | ND | Well, allogeneic hematopoietic stem-cell transplantation at 7 mo | ND | NA | No |
| 4 | 1 | Well | No | 18 | T-ALL (at 30 mo) | Died (at 60 mo) | NA | ND | |
| 5 | 3 | GVHD-like lesions | No | 20 | T-ALL (at 34 mo) complete remission | Well | 10.1 | Median | No |
| 6 | 6 | Disseminated VZV infection with encephalitis, failure to thrive | No | 1 | ND | Well | 9.1 | −1 | Yes |
| 7 | 11 | Disseminated B-cell lymphoproliferative disease, failure to thrive | No | 4 | T-ALL (at 68 mo) complete remission | Well | 9.1 | Median | Yes |
| 8 | 6 | Pulmonary BCG infection | No | 22 | ND | Well | 8.5 | +1 | No |
| 10 | 9 | Pulmonary infection, protracted diarrhea, failure to thrive | No | 11 | T-ALL (at 33 mo) complete remission | Well | 8.0 | Median | Yes |
Patient 9 was treated in Australia; data on this patient were not included in the analysis.
BCG denotes bacille Calmette–Guérin, GVHD graft-versus-host disease, NA not applicable, ND not determined, RSV respiratory syncytial virus, T-ALL T-cell acute lymphoblastic leukemia, and VZV varicella–zoster virus.
Curves for weight (in kilograms) and height (in centimeters) as a function of age were based on a large population of healthy French children and were expressed as medians ±2 SD according to the Tanner growth curves.15 There were no discrepancies between weight and height growth.
All treated patients left the sterile unit between days 45 and 90, and none subsequently had any severe opportunistic infections (see the Supplementary Appendix). Varicella–zoster virus (VZV) infections that did not require hospitalization developed in all patients and were not associated with severe adverse events. Patients 1, 2, and 7 had recurrent rhinitis. Skin manifestations of chronic human papillomavirus infection did not develop in any of the patients, although two patients had transient warts. Four patients (Patients 4, 5, 7, and 10) had the same severe adverse event (i.e., the development of T-cell acute lymphoblastic leukemia); the details of this outcome have been described previously.12 One of the four patients died, and the other three recovered after chemotherapy (Table 1 in the Supplementary Appendix). Immunoglobulin-replacement therapy was reinitiated in Patient 6, because of low antibody responses combined with recurrent otitis at 48 months, and in Patients 7 and 10, because of T-cell acute lymphoblastic leukemia and chemotherapy with the occurrence of bronchitis at 69 and 34 months, respectively. All patients had normal growth with respect to weight and height (Table 1) and attended regular schools. All patients except one did not have delays in their progression through school grades.
VECTOR COPY NUMBER IN T CELLS VERSUS B CELLS
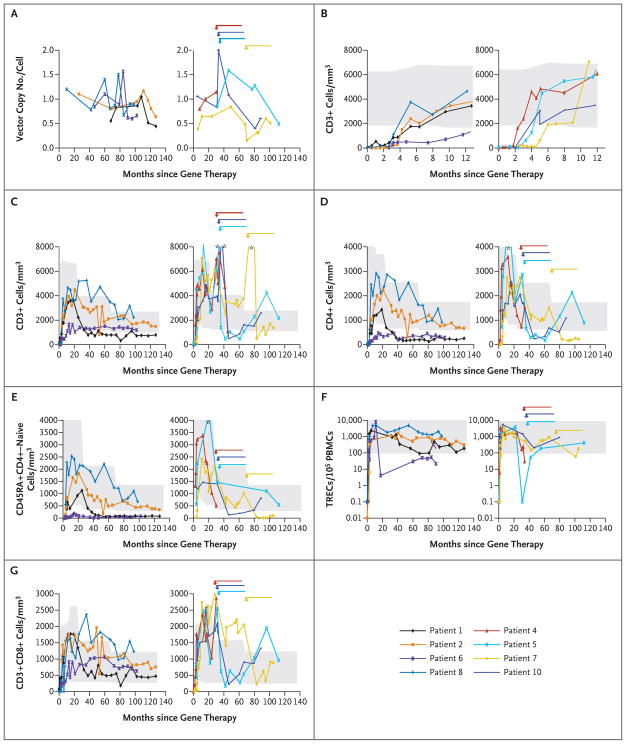
Longitudinal quantitative determination of the vector copy number revealed that in all patients, samples of T cells that were not blastic included approximately 0.5 to 1.5 copies per cell, with a seemingly stable pattern over time (Fig. 1A). In contrast, the proportion of transduced B cells decreased from approximately 1.0% during the first 2 years to less than 0.1% 6 to 10 years after therapy10 (Table 2 in the Supplementary Appendix).
Figure 1. Vector Copy Numbers and T-Cell Subgroups after Gene Therapy.
Patients were categorized into two groups: those in whom severe adverse events did not develop (Patients 1, 2, 6, and 8; left side of each panel) and those in whom severe adverse events did develop (Patients 4, 5, 7, and 10; right side of each panel). Panel A shows the vector copy number in peripheral-blood mononuclear cells over time. In Patient 10, blast cells contained two copies, as previously reported.12 Panel B shows short-term and Panel C shows long-term T-cell reconstitution as evidenced by absolute CD3+ lymphocyte counts in whole blood, measured with the use of flow cytometry. Panel D shows the change over time in absolute numbers of CD4+ T cells, and Panel E shows the change over time in absolute numbers of CD45RA+CD4+–naive T lymphocytes in whole blood, as measured with the use of flow cytometry. Panel F shows the change over time in the number of T-cell–receptor excision circles (TRECs) in peripheral-blood mononuclear cells (PBMCs). Panel G shows the change over time in the absolute numbers of CD3+CD8+ T cells in whole blood, as measured with the use of flow cytometry. In Panels B, C, D, E, F, and G, the shaded areas indicate reference values for age-matched controls. In Panels A, C, D, E, F, and G, the arrows indicate the occurrence of leukemia and the duration of chemotherapy.
T-CELL RECONSTITUTION
T-cell counts reached normal values for age between 2 and 5 months after therapy. There was a trend (P = 0.11) toward higher T-cell counts at 1 year after therapy in the patients in whom leukemia developed (Fig. 1B). Flow-cytometric analysis of the patients’ CD3+ population revealed γ-chain expression similar to that of control T cells9,10,12 (data not shown). Between 7.3 and 10.7 years after treatment, T-cell counts were within the normal range for age in six patients and were slightly below the normal range in one patient (Fig. 1C and Table 2). The T-cell pool returned to normal values in the three survivors of leukemia (Patients 5, 7, and 10) 66 months, 37 months, and 14 months after completion of chemotherapy, respectively (Fig. 1C and Table 2).
Table 2.
Immunologic Characteristics at Last Follow-up.*
| Variable | Patient 1 | Patient 2 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 10 | Reference Range |
|---|---|---|---|---|---|---|---|---|
| Follow-up (yr) | 10.6 | 10.7 | 9.4 | 8.5 | 8.8 | 8.2 | 7.3 | |
| Cell count (per mm3) | ||||||||
| CD3+ | 800 | 1494 | 2175 | 1248 | 1368 | 2250 | 2573 | 1140–2812 |
| CD4+ | 250 | 670 | 900 | 320 | 230 | 1107 | 1085 | 589–1589 |
| CD8+ | 470 | 756 | 950 | 704 | 874 | 1225 | 1333 | 342–1295 |
| CD31+CD45RA+CD4+ | 48 | 213 | 333 | 38 | 66 | 500 | 705 | >60 |
| TRECs (per105 PBMCs) | 187 | 502 | 423 | 22 | 174 | 1272 | 860 | >100 |
| T-cell receptors (%) | ||||||||
| α/β | 91 | 95 | 86 | 81 | 68 | 98 | 94 | 90–95 |
| γ/δ | 8 | 5 | 14 | 18 | 32 | 2 | 6 | 5–1 |
| Natural killer T cells (per 106 CD3+ cells) | ND | 174 | ND | 412 | 673 | 3241 | ND | 150–4000 |
| CD4+, CD25+ high, Foxp3+ (%) | ND | 3.2 | ND | 3.1 | ND | 3 | 8.3 | 3–10 |
| Anti-CD3 antibody–induced proliferation (cpm×10−3) | 27 | 28 | 28 | 36 | 39 | 62 | 52 | >25 |
| Age† | 1 | 1 | ND | 2 | 1 | 2 | 1 | |
| Natural killer cells (per mm3) | 1 | 18 | 3 | 8 | 9 | 7 | 31 | 57–814 |
| CD19+ (per mm3) | 200 | 288 | 300 | 336 | 513 | 225 | 496 | 114–851 |
| CD27+CD19+ (%) | 2 | 30 | 3 | 9 | ND | 12 | 8 | 10–25 |
Patient 9 was treated in Australia; data on this patient were not included in the analysis. Data on CD3+, CD4+, CD8+, CD31+CD45RA+CD4+ cells, natural killer T cells, CD19+ cells, and CD27+CD19+ cells were from the last follow-up assessment in Patients 2, 7, and 8.
ND denotes not done, PBMC peripheral-blood mononuclear cell, and TREC T-cell–receptor excision circle.
Results were positive for any one of the following tested stimuli: tetanus toxoid, varicella–zoster virus, Candida albicans, and purified protein derivative.
T-CELL CHARACTERISTICS
Long-Term Thymic Output
In all but one of the treated patients, analysis of the CD4+ T-cell subset showed that counts increased to the normal value for age during the first 2 years after therapy. In Patient 6, who received the lowest dose of transduced progenitor cells, CD4+ counts were below the normal range (Fig. 1D). Over the long term (i.e., 7.3 to 10.7 years after treatment), CD4+ T-cell counts were within the normal range in four patients and slightly below the normal range in three patients. In Patient 1, CD4+ T-cell counts also decreased below the normal range 2 years after therapy but then stabilized. Up to 7.3 to 10.7 years after therapy, naive CD45RA+CD4+ T-cell counts were within the normal range in four patients and were low but detectable in three others (Patients 1, 6, and 7) (Fig. 1E). Naive T cells were also measured by quantifying T-cell–receptor excision circles (TRECs). As shown in Figure 1F, levels of TRECs remained within the normal range except in Patient 6. These data correlated well with the CD31+CD45RA+CD4+ naive T-cell counts (Table 2). Naive T cells were newly detected after chemotherapy in the three survivors of leukemia, indicating a persistent potential for T-cell lymphopoiesis (Fig. 1E and 1F and Table 2).
In all patients, CD8+ T-cell counts were within the normal range up to 7.3 to 10.7 years after therapy (Fig. 1G). A balanced distribution between T-cell receptor αβ and T-cell receptor γδ was observed throughout the follow-up period (Table 2). Furthermore, natural killer T-cell counts in Patients 2, 6, 7, and 8 were within the normal range for age (Table 2). The other patients were not tested for this T-cell population. Moreover, 7 to 10 years after gene therapy, the proportion of CD4+, CD25+ high, forkhead box P3 (Foxp3+) T cells (a measure of the regulatory T-cell subgroup), assayed in Patients 2, 6, 8, and 10, was within the normal range (Table 2).
Repertoire Diversity of T-Cell–Receptors
Complementary determining region 3 (CDR3) length expression profiles (i.e., coding segments) for the 22 tested T-cell–receptor Vβ families showed a gaussian distribution in all patients (Fig. 1 in the Supplementary Appendix). Repertoire diversity returned in the survivors of leukemia, attesting to the persistence of transduced progenitor cells. The CDR3 length distribution for all 33 Vα families also showed a normal gaussian distribution in evaluated patients (Fig. 2 in the Supplementary Appendix). Finally, the Vγ and Vδ distribution in γδ T-cell populations strictly mirrored control values, with the predominance of Vγ9 Vδ2 cells (Fig. 3 in the Supplementary Appendix).
Long-Term Immune Function
Lymphocyte proliferation in response to phyto-hemagglutinin and anti-CD3 monoclonal antibodies was restored to normal levels in most patients 3 to 6 months after treatment and remained present throughout the follow-up period. Patient 6 had a mild decrease in T-cell proliferative capacity (Table 2, and Fig. 4A in the Supplementary Appendix). Patient 7 had discontinued chemotherapy 14 months before his cells were evaluated, making assessment of his immune function more difficult. Similarly, T-cell proliferation in response to at least one foreign antigen (tetanus toxoid, Candida albicans, VZV, or purified protein derivative) was positive in all tested patients (Patients 1, 2, 6, 7, 8, and 10) (Table 2).
LONGITUDINAL EVOLUTION OF THE NATURAL KILLER–CELL SUBGROUP
Normal levels of natural killer cells (CD56+CD16+ CD3− cells, which were found to express γ chain) were observed in the peripheral blood after gene therapy in patients who were 1 to 2 months of age.9 The counts remained stable for the first 18 months but decreased markedly thereafter (Table 2, and Fig. 4B in the Supplementary Appendix). Counts of natural killer cells increased to levels that were two to five times as high as baseline levels (to 150 to 250 cells per cubic millimeter) in Patients 4, 5, and 7 a few months before clonal T-cell proliferation, suggesting an expansion stimulated by the abnormal clone (Fig. 4B in the Supplementary Appendix).
B-CELL ANTIBODY RESPONSES
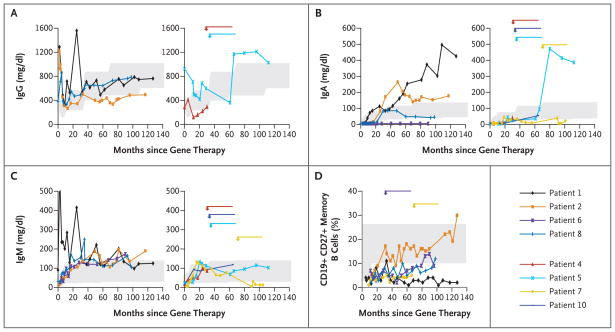
After gene therapy, B-cell counts, which were typically elevated at presentation in the infants with SCID-X1, decreased to normal values (Table 2). Although virtually no transduced B cells were detected in the blood of these patients 6 to 10 years after therapy, only three patients (Patients 6, 7, and 10) required immunoglobulin-replacement therapy to prevent bacterial infections. As shown in Figure 2, IgG serum levels were close to normal values in four patients, IgA levels were high or normal in five patients and low in two patients, and IgM levels were normal in seven of eight patients. Antibodies against polioviruses and tetanus and diphtheria toxoids were first detected at protective values 3 months after the third immunization in all patients except Patient 5. However, normal titers were not consistently detected in any of the tested patients at later time points, despite repeated immunization (Table 2 in the Supplementary Appendix); this was also the case for anti-VZV antibodies, which were not detected in five patients 3 to 6 years after varicella infection. A specific antibody response to polysaccharide antigens was detected after immunization against Streptococcus pneumoniae, with an increase in the titer in most patients that was more than four times as high as the titer before immunization; this elevated level persisted in three patients (Table 2 in the Supplementary Appendix). Isohemagglutinins also persisted in three of four patients tested (Table 2 in the Supplementary Appendix). A CD19+CD27+ memory B-cell subpopulation was present but constituted a small proportion of B cells (<10%) in all but one patient (Fig. 2D) 7.3 to 10.7 years after therapy. The γ chain–dependent response to interleukin-21 was lacking in B cells from six tested patients as indicated by the lack of IgA production after stimulation by the CD40 ligand and interleukin-21 (Table 2 in the Supplementary Appendix). Furthermore, switched B cells (μ–δ–CD27+ B cells) were detected, albeit in low proportions, in Patients 2 and 5 (5% and 3%, respectively, vs. >10% in controls) but could not be detected in Patient 1.
Figure 2. Serum Immunoglobulin Levels and Memory B-Cell Counts after Gene Therapy.
The changes over time in levels of serum IgG (Panel A), IgA (Panel B), and IgM (Panel C) in Patients 1, 2, 6, and 8 (left side of each panel) and Patients 4, 5, 7, and 10 (right side of each panel) are shown. Data on IgG levels in Patients 6, 7, and 10 (who received immunoglobulin-replacement therapy) are not shown. Panel D shows the change over time in the proportion of CD19+CD27+ memory B cells as measured with the use of flow cytometry in Patients 1, 2, 6, 7, and 8. In all panels, arrows indicate the occurrence of leukemia and the duration of chemotherapy. The shaded areas indicate reference values for age-matched controls.
INTEGRATION SITES IN NAIVE AND MEMORY T CELLS
We compared the diversity and abundance of integration sites in naive (CD45RA) and memory (CD45RO) CD4+ T cells obtained from Patient 2 at 102 months and from Patient 8 at 73 months. Pyrosequencing was used to determine 875 unique integration sites in Patient 2 and 2087 integration sites in Patient 8. There were no major differences between naive cells and memory cells in terms of overall diversity or distribution of integration sites (Fig. 5 in the Supplementary Appendix). The percentages of unique integration sites common to both naive and memory cells in Patients 2 and 8 were 21% and 13%, respectively. In Patient 2, an integration site within the CCND2 locus was abundant in naive cells (18%) but less so in memory cells (3%).
DISCUSSION
We describe the long-term results of retrovirus-mediated gene therapy in nine patients with SCID-X1, with a median follow-up period of 9 years (range, 8 to 11). Common γ-chain gene transfer into autologous CD34+ cells led to sustained reconstitution of the T-cell pool and protection from infections, but at the risk of the development of T-cell acute lymphoblastic leukemia. All children except one, including the three survivors of T-cell acute leukemia, could live normally in a nonprotected environment and cope with microorganisms (e.g., VZV) without harmful consequences while growing normally. We documented the sustained presence of T cells (including the various effector and regulatory subgroups) with near-normal functional characteristics. Furthermore, the chemotherapy-induced immunodeficiency in the three survivors of leukemia resolved spontaneously without additional therapeutic intervention. These data provide strong evidence of the persistence of T-lymphocyte progenitors for at least 3 to 6 years after therapy. We noted some individual variability in T-cell reconstitution, which appeared to correlate strongly with the number of transduced cells injected and the diversity of integration sites within the T-cell pool.16 These data were consistent with a minimum required threshold for the number of injected, transduced cells of 1×106 to 3×106 CD34+ cells per kilogram.
There were no trends toward a decline in T-cell counts or loss of repertoire diversity over the follow-up period in most patients (see the Supplementary Appendix). T-cell reconstitution was similar to that seen in patients who have undergone hematopoietic stem-cell transplantation, in terms of phenotypic and functional characteristics. Six of seven patients with SCID-X1 had normal TREC levels 7.3 to 10.7 years after therapy; these values were similar to those observed 10 years after hematopoietic stem-cell transplantation in 9 of 11 surviving patients with SCID-X1.6
In contrast to the findings in the first few years after treatment,9,10 transduced B lymphocytes and myeloid cells were no longer detected 6 to 10 years after gene therapy, indicating that no transduced functional progenitors or stem cells persisted in the patients’ bone marrow. Competition of a few transduced progenitors with untransduced stem cells could also be involved in B-cell development, since the γ-chain–negative B-cell lineage can be normally generated. However, the persistence of TREC+ naive T cells and their reappearance in patients who had undergone chemotherapy 3 to 6 years after gene therapy strongly suggest that T-cell progenitors persist and ensure sustained thymopoiesis. We speculate that these cells either commit to a T-cell lineage or maintain multipotency.17 Gene therapy for adenosine deaminase (ADA) deficiency has also been shown to restore T-cell counts and functions, with full restoration reported in five patients and partial restoration in another five patients.18 T-cell reconstitution in the latter patients was somewhat slower than that observed in our patients with SCID-X1. Furthermore, long-term T-cell counts appear to be somewhat lower in ADA-deficient patients. These data indicate that the quality of immune reconstitution depends on the genetic disease and the resulting physiological environment. We analyzed integration sites in sorted naive and memory T cells in two patients to assess and compare the potential effect of provirus integration on the capacity of these populations to proliferate or differentiate. Our data did not indicate deviation over time in clonal diversity or abundance. Integration patterns were similar in the two populations; this finding suggested an absence of selection bias and the maintenance of diversity in memory T cells. Overall, these results suggest that genome modification of T-cell precursors by means of retroviral insertion did not significantly skew T-cell differentiation, and they seem to argue against the hypothesis that vector insertion close to certain sets of genes favors the selection of clones that are more fit.16,19
All patients with SCID-X1 had a long-term (albeit incomplete) deficiency of natural killer cells. This finding mimics the long-term outcome of allogeneic hematopoietic stem-cell transplantation in patients with SCID-X1 in the absence of myeloablation4,5,7 and emphasizes that the conditions of this intervention do not favor the development and survival of natural killer cells as effectively as they do the T-cell lineage. Despite the lack of sustained transduced B-cell counts, four patients did not require immunoglobulin-replacement therapy. This outcome strongly suggests that in vivo B-cell immunity was preserved to some extent, as shown by the sustained presence of all serum immunoglobulin isotypes, detectable antibody responses to polysaccharide antigens (in some patients), and the presence of memory B cells with somatic mutations in their immunoglobulin-variable-region genes. These data may reflect the persistence of residual, transduced B cells that could not be detected in the blood, the ability of γ-chain–negative B cells to be activated by T-cell signals and cytokines (with the important exception of interleukin-21), or both.20–22
Overall, the present study confirms the expectation that ex vivo gene therapy for SCID-X1 can result in long-term correction of the SCID phenotype. However, the risk of the development of a malignant condition related to treatment, which involved the use of a retroviral vector containing a competent enhancer of long terminal repeats, cannot be ignored. Alternatives to gene therapy include transplantation from unrelated donors (who may not be identified for a considerable amount of time), from cord blood, or from haploidentical parents. With all three alternatives, GVHD and related infections remain a major cause of death. For instance, in our experience, after our gene-therapy protocol was closed in 2002, a total of 3 of 10 consecutive patients with γ-chain deficiency died from infections 2 to 4 months after undergoing haploidentical hematopoietic stem-cell transplantation; this survival rate is consistent with the 72% survival rate recently reported from the European database for SCID-X1.23 Our study showed substantial improvement of immune function in seven of nine patients treated with gene therapy; however, a potentially fatal treatment-related complication developed in four of the nine patients (44%), and one patient (11% of the total) died. Thus, gene therapy may be another option for patients with SCID-X1 who lack an HLA-identical donor. Our results set the stage for trials with safer vectors in the treatment of SCID-X1 and other severe forms of inherited diseases of the hematopoietic system.
Supplementary Material
Acknowledgments
Supported by grants from INSERM, Association Française contre les Myopathies (AT0203), the European Commission (QLK3-CT-1999-00859), the Concerted Safety and Efficiency Evaluation of Retroviral Transgenesis in Gene Therapy of Inherited Diseases (005242), Gene Therapy of Hematopoietic Stem Cells for Inherited Diseases (QLK3-CT-2001-00427), Agence Nationale de la Recherche (05-MRAR-004), Programme Hospitalier de Recherche Clinique of the Health Ministry (PHRC AOM 08064-P071204), Assistance Publique–Hôpitaux de Paris, Fondation de l’Avenir, Institut Pasteur and College de France, and the National Institutes of Health (AI52845 and AI082020, to Dr. Bushman). Dr. Hauer was supported by a scholarship from the Akademie der Naturforscher Leopoldina (BMBF-LPD 9901/8-149).
We thank the families of the patients for their continuous support of the study, the medical and nursing staff of Unité d’Immunologie et d’Hématologie Pédiatriques, Hôpital des Enfants Malades for patient care, and M. Forveille for technical help.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Sugamura K, Asao H, Kondo M, et al. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 2.Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–8. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 3.Noguchi M, Yi H, Rosenblatt HM, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–57. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 4.Buckley RH, Schiff SE, Schiff RI, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–16. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 5.Antoine C, Müller S, Cant A, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361:553–60. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 6.Cavazzana-Calvo M, Carlier F, Le Deist F, et al. Long-term T-cell reconstitution after hematopoietic stem-cell transplantation in primary T-cell-immunodeficient patients is associated with myeloid chimerism and possibly the primary disease phenotype. Blood. 2007;109:4575–81. doi: 10.1182/blood-2006-07-029090. [DOI] [PubMed] [Google Scholar]
- 7.Neven B, Leroy S, Decaluwe H, et al. Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood. 2009;113:4114–24. doi: 10.1182/blood-2008-09-177923. [DOI] [PubMed] [Google Scholar]
- 8.Sarzotti-Kelsoe M, Win CM, Parrott RE, et al. Thymic output, T-cell diversity, and T-cell function in long-term human SCID chimeras. Blood. 2009;114:1445–53. doi: 10.1182/blood-2009-01-199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–72. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 10.Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–93. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 11.Gaspar HB, Parsley KL, Howe S, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–7. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 12.Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–42. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe SJ, Mansour MR, Schwarzwaelder K, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–50. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginn SL, Curtin JA, Kramer B, et al. Treatment of an infant with X-linked severe combined immunodeficiency (SCID-X1) by gene therapy in Australia. Med J Aust. 2005;182:458–63. doi: 10.5694/j.1326-5377.2005.tb06785.x. [DOI] [PubMed] [Google Scholar]
- 15.Tanner JM. Normal growth and techniques of growth assessment. Clin Endocrinol Metab. 1986;3:411–51. doi: 10.1016/s0300-595x(86)80005-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang GP, Berry CC, Malani N, et al. Dynamics of gene-modified progenitor cells analyzed by tracking retroviral integration sites in a human SCID-X1 gene therapy trial. Blood. 2010 March 12; doi: 10.1182/blood-2009-12-257352. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weerkamp F, Baert MR, Brugman MH, et al. Human thymus contains multi-potent progenitors with T/B lymphoid, myeloid, and erythroid lineage potential. Blood. 2006;107:3131–7. doi: 10.1182/blood-2005-08-3412. [DOI] [PubMed] [Google Scholar]
- 18.Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–58. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 19.Baum C. Insertional mutagenesis in gene therapy and stem cell biology. Curr Opin Hematol. 2007;14:337–42. doi: 10.1097/MOH.0b013e3281900f01. [DOI] [PubMed] [Google Scholar]
- 20.Brière F, Servet-Delprat C, Bridon JM, Saint-Remy JM, Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med. 1994;179:757–62. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Punnonen J, Aversa G, Cocks BG, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avery DT, Deenick EK, Ma CS, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–71. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gennery AR, Slatter MA, Grandin L, et al. Transplantation of haematopoietic stem cells and long term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. doi: 10.1016/j.jaci.2010.06.015. (in press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.