Abstract
The correct organization of cells within an epithelium is essential for proper tissue and organ morphogenesis. The role of Decapentaplegic/Bone morphogenetic protein (Dpp/BMP) signaling in cellular morphogenesis during epithelial development is poorly understood. In this paper, we used the developing Drosophila pupal retina – looking specifically at the reorganization of glial-like support cells that lie between the retinal ommatidia – to better understand the role of Dpp signaling during epithelial patterning. Our results indicate that Dpp pathway activity is tightly regulated across time in the pupal retina and that epithelial cells in this tissue require Dpp signaling to achieve their correct shape and position within the ommatidial hexagon. These results point to the Dpp pathway as a third component and functional link between two adhesion systems, Hibris-Roughest and DE-cadherin. A balanced interplay between these three systems is essential for epithelial patterning during morphogenesis of the pupal retina. Importantly, we identify a similar functional connection between Dpp activity and DE-cadherin and Rho1 during cell fate determination in the wing, suggesting a broader link between Dpp function and junctional integrity during epithelial development.
Keywords: Adhesion, BMP, Dpp, Epithelia, Patterning
INTRODUCTION
Guiding cells to their correct positions within a patterned epithelium involves an intricate combination of cellular events. These events are typically coordinated by ‘organizer cells’ within the epithelium that act to set pattern across the tissue. Bone morphogenetic proteins (BMPs) are members of the Transforming growth factor-β (TGF-β) superfamily of proteins, which regulate a wide range of biological processes (Attisano and Wrana, 2002; Hogan, 1996; Ozdamar et al., 2005). Although much is known of the basic signaling pathway, the role of BMPs in cell morphogenesis remains poorly understood. In this paper we use the Drosophila pupal retina to explore how temporal and cell type-specific regulation of BMP signaling regulates the positioning of cells within developing epithelia.
TGF-β-family proteins activate signaling by binding Type I and Type II serine-threonine kinase receptors, which in turn recruit and phosphorylate receptor SMADs to regulate transcription of target genes (Shi and Massague, 2003). Dpp is the Drosophila ortholog of vertebrate BMP2/4. Its potential receptors include the Type II receptors Punt (Put) and Wishful Thinking (Wit) and the Type I receptors Thickveins (Tkv) and Saxophone (Sax), which activate the downstream target Mad (Letsou et al., 1995; Marques et al., 2002; Newfeld et al., 1997; Penton et al., 1994; Xie et al., 1994). The Dpp signaling pathway regulates multiple developmental processes including dorsoventral patterning of the embryo, gut morphogenesis, growth, patterning and differentiation of the imaginal discs, and epithelial morphogenetic processes such as dorsal closure and imaginal disc spreading (Ferguson and Anderson, 1992; Firth and Baker, 2005; Greenwood and Struhl, 1999; Neumann and Cohen, 1997; Panganiban et al., 1990; Rogulja and Irvine, 2005; Affolter et al., 1994; Martin-Blanco et al., 2000).
Recently, strong loss of Dpp signaling in the wing has been demonstrated to cause the release of cells from the epithelium and the establishment of a basal cyst (Gibson and Perrimon, 2005; Shen and Dahmann, 2005). This suggests that Dpp pathway activity is required to maintain epithelial integrity. Epithelial integrity and tissue morphogenesis are mediated through dynamic regulation of the apical junctions (Schock and Perrimon, 2002). Dpp signaling is also precisely regulated during development, and one possibility is that it regulates epithelial patterning or maturation through an association with apical junctions.
The Drosophila pupal retina has proven a useful system for studying epithelial patterning. Its precise pattern emerges through a series of morphogenetic processes that include changes in cell shape, cell position and programmed cell death (Cagan and Ready, 1989b). Formation of correct cell contacts and selective cell adhesion – collectively known as cell sorting – are also key events during patterning of the pupal retina (Bao and Cagan, 2005; Grzeschik and Knust, 2005; Hayashi and Carthew, 2004; Reiter et al., 1996). The adhesion molecule Roughest (Rst) is the ortholog of vertebrate NEPH1 (also known as KIRREL1) and a member of the immunoglobulin superfamily. Mutations in the rst gene result in impaired cell sorting and subsequent blockade in programmed cell death during pupal retinal development (Reiter et al., 1996; Wolff and Ready, 1991). Rst regulates patterning of the pupal retina through selective heterophilic adhesion with Hibris (Hbs) and formation of cell junctions (Bao and Cagan, 2005). Additionally, the adhesion molecule DE-cadherin has been proposed to regulate Rst during stages of maximal cell rearrangements in the pupal retina (Grzeschik and Knust, 2005), although the precise relationship between these two adhesion molecules remains to be elucidated.
In this paper, we demonstrate an essential role for the Dpp pathway in regulating epithelial cell shape and patterning in the pupal retina. We provide evidence that Dpp pathway activity is regulated dynamically across time and that it acts as a new component and functional link between two adhesion systems, Hbs-Rst and DE-cadherin. Our data support a novel role for temporal and cell-type specific Dpp/BMP signaling to direct shape and positioning of individual cells into an emerging epithelial pattern.
MATERIALS AND METHODS
Fly lines
All crosses and staging were conducted at 25°C unless otherwise noted. Wild-type (Canton-S), GMR-gal4, dpp-lacZP10638, tkvk16713, Rho172F and putP flies were kindly provided by the Bloomington Drosophila Stock Center, dppe90 by Kristi Wharton (Brown University, Providence, RI), UAS-tkvQ253D (constitutively active) by Michael O’Connor (University of Minnesota, Minneapolis, MN), tkv4 by Nick Baker (Albert Einstein College of Medicine, Bronx, NY), tkv8 and Mad12 by Laurel Raftery (Massachusetts General Hospital, Charlestown, MA), shgR69 by Richard Carthew (Northwestern University, Evanston, IL), UAS-CD4:GFP, hs-FLP; Gal80, FRT40 by Andreas Bergmann (UT M.D. Anderson Cancer Center, Houston, TX), scalloped-gal4 by Sarah Bray (University of Cambridge, Cambridge, UK) and rstCT by Karl Fischbach (Albert-Ludwigs-Universitaet, Freiburg, Germany). The rst3 allele was described previously (Tanenbaum et al., 2000). We constructed two independent UAS-tkv-IR (tkv-IR) lines by subcloning inverted repeats into pGEM-WIZ (Bao and Cagan, 2006): tkv-IR1, which targets part of the 5′ half; and tkv-IR2, directed towards the 3′ half of the tkv mRNA.
RNA extraction from pupal retinas and RT-PCR
Retinal-brain complexes from 10–15 pupae per genotype were dissected under RNAse-free conditions. Retinas were separated from the brains using a sterile surgical razor blade and subject to RNA extraction using TRIzol (Invitrogen). The RNA was then used to detect tkv transcript levels by RT-PCR.
Temperature-sensitive experiments and clonal analysis
To examine the role of dpp in pupal patterning, dppe90 homozygous flies were kept at 18°C (permissive temperature), pupae were selected at 42 hours [equivalent to approximately 20 hours after puparium formation (APF)] at 25°C, and then switched to 25°C (restrictive temperature) for 22 hours and dissected. As a control, dppe90 pupae were held at the permissive temperature until dissection.
Whole eye mutants for puntP were generated using the EGUF system (Stowers and Schwarz, 1999) using pupae with the following genotypes: (1) control: ey-Gal4, UAS-FLP; FRT82B, cl, GMR-hid/FRT82B lacZ; (2) experimental: ey-Gal4, UAS-FLP; FRT82B, cl, GMR-hid/FRT82B puntP.
Discrete tkv and Mad mutant clones were generated by FRT-mediated recombination (Golic and Lindquist, 1989; Xu and Rubin, 1993). Recombinant clones were induced by heat shocking larvae 72 hours after egg laying for 1 hour at 37°C. For MARCM tkv8 clones (Lee and Luo, 1999), third instar larvae were heat shocked for 30 minutes at 37°C. Clonal analysis was performed in pupae with the following genotypes: (1) tkv8 clones: hs-FLP; tkv8 FRT40/ub-nGFP FRT40; (2) tkv4 clones: hs-FLP; tkv4 FRT40/ub-nGFP FRT40; (3) Mad12 clones: hs-FLP; Mad12 FRT40/ubnGFP FRT40; (4) tkv8 single-cell clones: UAS-CD4:GFP, hs-FLP; Gal80, FRT40/tkv8 FRT40; tubulin-Gal4/+.
Immunostaining and imaging
Pupal retinas and wings were processed as described previously (Bao and Cagan, 2005; Blair and Ralston, 1997). Antibodies used were: mouse anti- Armadillo and rat anti-DE-cadherin (1:3 and 1:10, respectively, from the Developmental Studies Hybridoma Bank at the University of Iowa); mouse anti-Rst (1:50, from Karl Fischbach); rabbit anti-β-galactosidase (1:2000, Cappel); rabbit anti-luminal Tkv (1:10, from Marcos Gonzalez-Gaitán, Max Planck Institute, Dresden, Germany); rabbit anti-GFP (1:2000, from Pam Silver, Harvard Medical School, Boston, MA); mouse anti-Rho1 and mouse anti-Tubulin (E7) (1:10, from the Developmental Studies Hybridoma Bank at the University of Iowa); mouse anti-Srf (1:50, from M. Gilman, Cold Spring Harbor Laboratory, NY); and rabbit anti-p-Mad (1:5000, from Tetsuya Tabata, University of Tokyo, Tokyo, Japan). Alexa488- and Alexa568-conjugated secondary antibodies were used (1:1000, Molecular Probes).
Whole-mount in situ hybridization was carried out as previously described (Bao and Cagan, 2005). Cell surface-associated Tkv was visualized with an antibody directed against the extracellular domain of Tkv (Kruse et al., 2004); dissected tissue was incubated with the antibody at 4°C prior to fixation (Strigini and Cohen, 2000). The antibody did not work when added after fixation and permeabilization.
Images were captured with a Zeiss Axiophot microscope equipped with a Quantix CCD camera (Photometrics) and Image Pro Plus software. Images were processed with Photoshop (Adobe). Confocal xzy projections were taken on a Leica confocal microscope using the Leica confocal software. For scanning electron microscopy, flies were prepared by ethanol fixation followed by critical-point drying. Images were captured using a Hitachi S-2600H scanning electron microscope.
In vivo visualization
In vivo imaging was performed in pupae with the following genotypes: (1) experimental: GMR-gal4/+; UAS-αCatenin-GFP/tkv8; UAS-tkv-IR1(2X)/UAS-tkv-IR1(2X) (Fig. 3; see Movie 2 in the supplementary material); (2) control: GMR-gal4/+; UAS-αCatenin-GFP/+ (see Movie 1 in the supplementary material). Larvae were allowed to pupate at 29°C (0 hours APF). Pupae were then collected and staged at 25°C until 25 hours APF. The pupal case was removed in the head area and the animal was placed with the eye region pressed against a coverslip. Temperature and humidity were controlled and images were captured every 15 minutes.
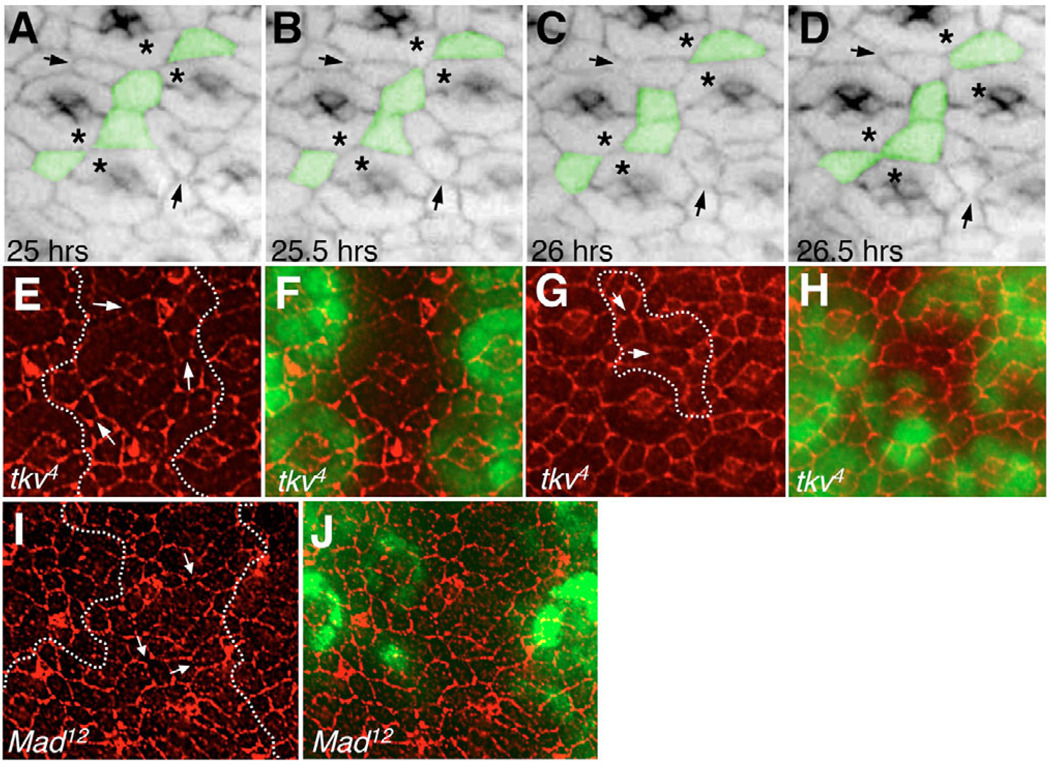
Fig. 3. Aberrant IPC morphogenesis and unstable IPC-IPC junctions in retinas with reduced Dpp signaling.
(A–D) In vivo imaging of a Drosophila retina with reduced Tkv activity [GMR-gal4/+; UAS-αCatenin-GFP/tkv8; UAS-tkv-IR1(2x)/UAS-tkv-IR1(2X)] (see also Movie 2 in the supplementary material). Hours APF are indicated. Pseudo colored in green are examples of IPCs that transiently lose their apical contact and leave primary pigment cells from adjacent ommatidia (asterisks) in direct, aberrant contact. Arrows point to examples of adherens junctions, which disappear as the IPC-IPC surface contact decreases. (E–J) Clones of tkv4 (E–H) and Mad12 (I,J) dissected at 25 hours APF and stained with anti-DE-cadherin (red; E–J). Clonal tissue is marked by the absence of GFP (green; F,H,J) or outlined by dotted lines (E,G,I). Arrows point to IPC-IPC junctions with abnormally low-to-undetectable DE-cadherin staining.
RESULTS
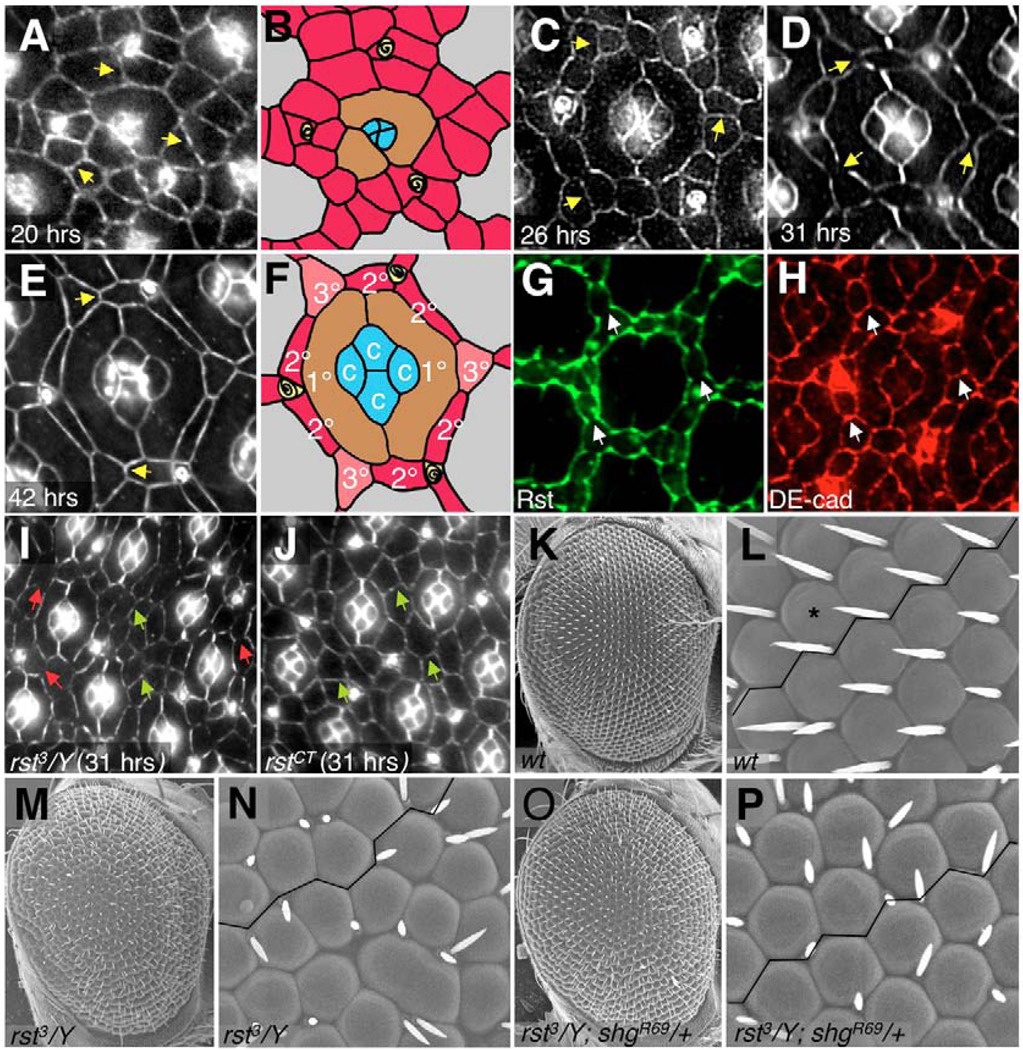
The Drosophila retina develops from a monolayer epithelium composed of approximately 750 unit eyes, or ‘ommatidia’ (Fig. 1E,F,K,L). Each ommatidium has a core composed of four cone cells (Fig. 1F), two primary pigment cells (1°; Fig. 1F) and eight underlying photoreceptors. Secondary and tertiary pigment cells (2° and 3°, respectively; Fig. 1F) interweave between the ommatidial cores to form a precise honeycomb pattern (Cagan and Ready, 1989b) with three sensory bristles at alternating vertices. We will collectively refer to mature interommatidial cells as ‘2°/3°s’. This precise interommatidial pattern emerges 18–31 hours after puparium formation (APF) (Fig. 1A,C,D). ‘Interommatidial precursor cells’ (IPCs) are precursors to the 2°/3°s (Fig. 1A,B). They undergo dynamic cell rearrangements that are necessary to direct them into a precise 2°/3° hexagonal array (Fig. 1E,F). In an early-stage pupal retina, IPCs are initially arranged in double and triple rows between ommatidia (Fig. 1A,B). These cells then rearrange into single-cell rows (Fig. 1C–E). Throughout this patterning process, excess IPCs are eliminated by programmed cell death (Rusconi et al., 2000).
Fig. 1. Morphogenesis of the Drosophila pupal retina and the role of cell adhesion.
(A–F) Time course of retinal development. Apical cell profiles were visualized with anti-Armadillo to highlight adherens junctions. Anterior is to the right; times refers to hours after puparium formation (APF). Unpatterned arrays of IPCs (A,B) sort to single file (C); IPC number continues to decrease as patterning tightens (D) until the final pattern is achieved (E,F). B and F are schematics of the central ommatidium from A and E, respectively; cone cells (c, blue shading), primary pigment cells (1°, brown), IPCs (red), 2° (red), 3° (pink) and bristles (yellow) are indicated. Arrows in A,C,D,E point to IPC:IPC adherens junctions. (G,H) 26 hours APF retina stained with anti-Rst (G) and anti-DE-cadherin (H). Arrows point to the IPC:IPC junctions, where DE-cadherin is expressed and Rst is absent. (I,J) 31 hours APF rst mutant retinas; magnification is reduced to show additional ommatidia. Green arrows point to IPC:IPC junctions that failed to clear (compare with wild type, D). This defect correlated with the failure in mutant IPCs to sort out into single-cell rows, as observed in mutants for the hypomorphic allele rst3, which is subject to position effect variegation (I). Red arrows in I point to rst3 regions where IPCs have sorted out into single layers and have also cleared out their junctions; these are likely to contain normal levels of Rst protein. (K–P) Scanning electron micrographs of adult eyes (genotypes as listed) taken at 180× (K,M,O) and 800× (L,N,P). K and L show a wild-type adult eye; a single ommatidium is indicated with an asterisk. Note the straight ommatidial rows, highlighted by a line drawn between ommatidia. The aberrant ommatidial packing observed in an rst3 eye (M,N) is rescued by removing a single functional copy of shg (O,P).
At least two adhesion systems are involved in directing IPC patterning: Hbs-Rst and DE-cadherin. Reducing the activity of Rst or Hbs led to a failure of IPC cell movement (Bao and Cagan, 2005; Reiter et al., 1996). Rst is found primarily at the junction between IPCs and 1°s and is excluded from IPC:IPC junctions (Reiter et al., 1996; Bao and Cagan, 2005) (Fig. 1G,H). Rst regulates patterning of the pupal retina through selective heterophilic adhesion with Hbs and formation of DE-cadherin-rich adherens junctions (Bao and Cagan, 2005).
The relationship between these adhesion systems is complex. Experiments that altered the activity or expression of DE-cadherin suggested that DE-cadherin is required to drive Rst to the adherens junctions (Grzeschik and Knust, 2005). Conversely, overexpression of Rst led to an increase in DE-cadherin (Bao and Cagan, 2005). Additionally, we observed that mutations in rst disrupted the dynamic localization of IPC:IPC adherens junctions (Fig. 1I,J). Normally, the adherens junctions between IPCs are strongly reduced by 31 hours APF (Bao and Cagan, 2005; Grzeschik and Knust, 2005) (Fig. 1, compare D with A,C,E). Retinas from rst mutants failed to clear these junctions (Fig. 1I,J). Consistent with the previous result, taking away one functional copy of shotgun (shg), the gene encoding DE-cadherin, significantly suppressed the rough eye phenotype of rst mutants (Fig. 1K–P). Together, these data emphasize the complexity of the relationship and epistatic order between Hbs-Rst and components of the adherens junctions. To better understand this relationship, we examined other potential regulators of IPC patterning.
Dpp is required for patterning the pupal retina
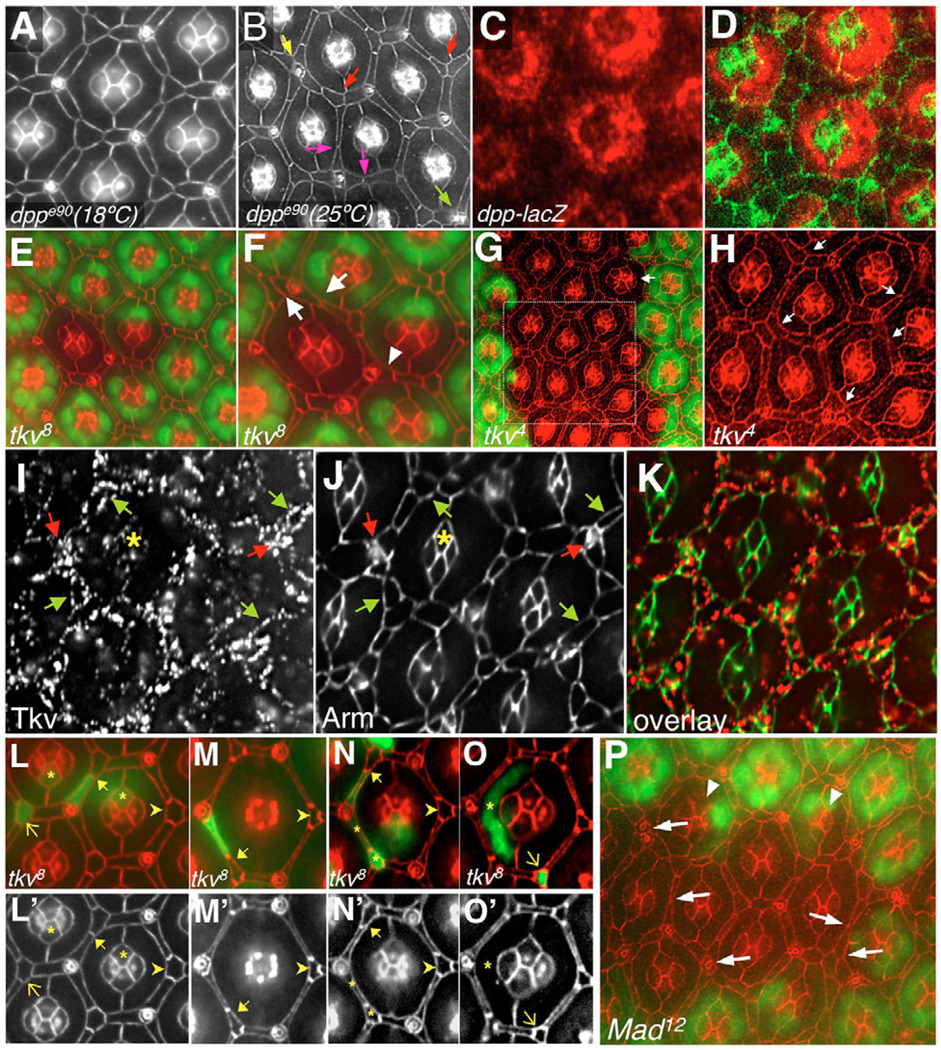
To explore the role of Dpp specifically in the pupa, we utilized the temperature-sensitive allele dppe90 (Fig. 2A,B). Genotypically dppe90 animals were kept either at 18°C (Fig. 2A) or switched to the non-permissive temperature at 20 hours APF just prior to the stage of cell rearrangements in the pupal retina (Fig. 2B). Downregulation of dpp resulted in an abnormal hexagonal pattern due to disruption in the shape and patterning of 2°/3°s as assessed with the junctional marker Armadillo (Fig. 2B). The main 2°/3° defects we observed included: (1) abnormal IPC:IPC contacts; (2) a failure of 3°s to establish a correct position within the vertex of the hexagon; (3) 2°/3°s that were abnormally arranged around sensory bristles; and (4) misplaced bristle organs. As previously reported (Wharton et al., 1996), there was some variability in the penetrance and expressivity of the phenotype (for quantification of defects, see Table S1 in the supplementary material).
Fig. 2. Dpp signaling regulates IPC patterning in the Drosophila pupal retina.
All retinas are 42 hours APF and apical membrane profiles are highlighted with anti-Armadillo, except where noted. (A,B) Retinas from animals carrying the temperature-sensitive allele dppe90 raised at the permissive (A) or non-permissive (B) temperature. (B) Mutant retinas show: abnormal IPC:IPC contacts (pink arrows); 3°s, which failed to establish a correct position as a vertex of the hexagon (red arrows); 2°/3°s abnormally arranged around sensory bristles (yellow arrow); and aberrant bristle-bristle contacts (green arrow). (C,D) dpp was expressed in primary pigment cells at 26 hours APF (red). (E,F) tkv8 clone marked by the absence of nuclear GFP (green). (F) Magnification of the clonal tissue in E. Arrows in F point to typical 2°/3°s patterning defects; arrowhead points to ectopic 2°/3°. (G,H) tkv4 clone (cells marked as in E,F). H is a magnified view of the boxed region in G. Arrows in H indicate examples of typical 2°/3° defects. Arrow in G indicates a rare cone cell defect (five versus four). (I–K) Tkv localization at 26 hours APF. (I) Tkv protein was found primarily at the surface of IPCs (green arrows), sensory bristles (red arrows) and at lower levels in cone cells (asterisk). (L–O′) tkv8 single-cell clones were marked by the presence of GFP (green). Full arrows point to cases where tkv mutant 2°s failed to fully expand into their proper niche, as evidenced by their shortened apical profile, while wild-type neighboring 3°s elongated to compensate. Arrowheads point to examples of the apical profile characteristic of wild-type 3°s. Asterisks in N and N′ indicate how neighboring mutant cells typically show normal apical profiles. Thin arrows and asterisks in L,L′,O,O′ point to 3°s, cone cells and primary pigment cells, whose shape was not affected by the absence of Tkv activity. (P) A Mad12 clone marked by the absence of nuclear GFP (green). The arrows point to a subset of the 2°/3°s patterning defects and aberrant bristle-bristle contacts within the clone. Arrowheads indicate rare, abnormal cone cell clusters.
Our previous cell ablation experiments indicated that primary pigment cells act locally to direct patterning of neighboring IPCs (Miller and Cagan, 1998). A dpp-lacZ reporter line indicated that Dpp was expressed exclusively in all primary pigment cells at the time of active IPC morphogenesis (Fig. 2C,D). Together, these results suggest a model in which Dpp is provided by primaries to regulate patterning of neighboring IPCs.
Type I and Type II receptors are required for patterning the pupal retina
We next utilized the hs-FLP/FRT system (Golic and Lindquist, 1989; Xu and Rubin, 1993) to generate clonal patches bearing reduced activity of the Type I receptor Tkv, or the Type II receptor Punt, in the pupal retina. Clones of the null allele tkv8 (Nellen et al., 1994) (Fig. 2E,F) or of the hypomorphic allele tkv4 (Penton et al., 1994) (Fig. 2G,H), led to defects in the shape and patterning of 2°/3°s similar to those observed when Dpp activity was reduced (Fig. 2B). Additionally, we observed cases of ectopic 2°/3°s. Whole eye clones of genotypically puntP tissue showed patterning defects that were similar to, but milder than, those seen in dpp and tkv mutants, and discrete puntP clones showed infrequent defects (data not shown). Weaker phenotypes of punt versus tkv clones have previously been documented in the wing disc (Burke and Basler, 1996), perhaps reflecting the hypomorphic nature of available punt alleles. Clones of null alleles of wit or sax, which encode alternative Type II and Type I receptors, respectively, gave no mutant phenotype (data not shown).
In situ hybridization experiments and a tkv-lacZ reporter indicated that tkv and punt transcripts were expressed in IPCs and cone cells during stages of IPC patterning (data not shown). Antibody staining (Kruse et al., 2004) detected surface-exposed Tkv in puncta along the surface of IPCs, cone cells and sensory bristles (Fig. 2I–K). Therefore, Dpp and its receptors Tkv and Punt are mainly expressed in complementary cell types, supporting a model in which Dpp from primary pigment cells binds to Tkv and Punt in the IPCs to regulate their shape and positioning.
Tkv regulates cell shape autonomously in the pupal retina
To more closely explore Dpp pathway activity, we generated single-cell clones of tkv8 using the MARCM system (Lee and Luo, 1999). Each cell within the pupal retina has a stereotyped apical profile (Fig. 1E,F), and deviations are readily observed. The apical profiles of isolated, genotypically tkv8 cells failed to stretch out and fill their proper niche within the hexagon (65%, n=30; Fig. 2L–N). Instead, their shortened profile was typically compensated for by a wild-type neighbor, which expanded to fill the unoccupied space (Fig. 2L–N). Interestingly, when two tkv8 cells were juxtaposed they typically exhibited normal apical profiles (88%, n=40; Fig. 2N), indicating that relative levels of Dpp signaling between neighboring cells determine cell shape.
Strikingly, the effects of losing tkv activity was specific to 2°s. No cell profile defects were observed when tkv activity was reduced in cone cells, primaries or 3°s (Fig. 2L,O). These data suggest that the defects in 3° positioning frequently observed in larger dpp and tkv clones is an indirect consequence of the patterning defects in the neighboring 2°s, and that Dpp activity is required autonomously in 2°s to direct proper overall hexagonal patterning.
IPC patterning defects are due to a failure in proper cell movement and morphogenesis
To better assess the role of Dpp signaling we used in vivo imaging analysis (Monserrate and Baker Brachmann, 2007; Vidal et al., 2006) to observe morphogenesis progression between 25 and 30 hours APF (Fig. 3A–D; see Movies 1, 2 in the supplementary material). To facilitate these studies, we generated two transgenic lines that reduced tkv activity through RNA interference: tkv-IR1 targets within the 5′ region and tkv-IR2 within the 3′ region of the tkv mRNA. The phenotypes of the two lines were identical, except that the phenotypes observed in flies with tkv-IR1 were consistently stronger than in those with tkv-IR2 (data not shown). The following observations further validated the specificity of our tkv-IR constructs: (1) expressing either tkv-IR line with the wing pouch driver scalloped-gal4 (sd>tkv-IR) or the eye driver (GMR>tkv-IR) phenocopied dpp and tkv loss-of-function phenotypes (Terracol and Lengyel, 1994) (Fig. 2); (2) removing a functional genomic copy of tkv significantly enhanced the sd>tkv-IR2 phenotype; and (3) wing imaginal discs from sd>tkv-IR larvae showed significant downregulation of the levels of the phosphorylated form of Mad (p-Mad) in the wing pouch region (data not shown).
To visualize tkv development in living tissues, multiple copies of the transgene were targeted specifically to the eye (GMR>tkv-IR). The most common and striking phenotype observed within developing GMR>tkv-IR eyes was a failure to maintain stable IPC:IPC contacts (see Movie 2 in the supplementary material). Neighbors established contact but then broke apart leading to direct contact between primary pigment cells from adjacent ommatidia (Fig. 3A–D; see Movie 2 in the supplementary material) in a manner that was not seen in control retinas (see Movie 1 in the supplementary material). This failure to maintain contact was briefly preceded by dissolution of the visible IPC:IPC adherens junction (Fig. 3A–D; see Movie 2 in the supplementary material). Further, these abnormal IPC:IPC interactions were accompanied by aberrant changes in cell shape that included abnormal expansions and/or reductions of their apical profiles. IPC:IPC contacts were often later reformed, reducing the severity of the final phenotype (Fig. 3D). These aberrant phenotypes repeated themselves across the retina over the time of visualization (see Movie 2 in the supplementary material). They were consistent with the abnormal IPC:IPC contacts observed in dpp, tkv and Mad mutants (Fig. 2), and in 25 hours APF tkv and Mad mutant clones (Fig. 3E–J), which frequently exhibited premature clearing of the IPC-IPC DE-cadherin junctions suggestive of junction dissolution. RT-PCR results indicated that shg expression levels were not detectably altered (data not shown). Together, these results indicate that Dpp signaling is required to maintain normal IPC:IPC contacts, junction stability and cell shape during morphogenesis of the pupal retina.
Dpp signaling activity is tightly regulated in IPCs
Activation of Tkv leads to phosphorylation of the conserved transcription factor Mad, promoting multimerization and transcriptional activity (Newfeld et al., 1997; Sekelsky et al., 1995). Clones of the null allele Mad12 led to 2°/3° patterning defects that mimicked those observed in dpp and tkv mutants (Fig. 2P). These results indicate that Dpp-dependent IPC patterning in the pupal retina requires classical pathway signaling that includes Mad activity.
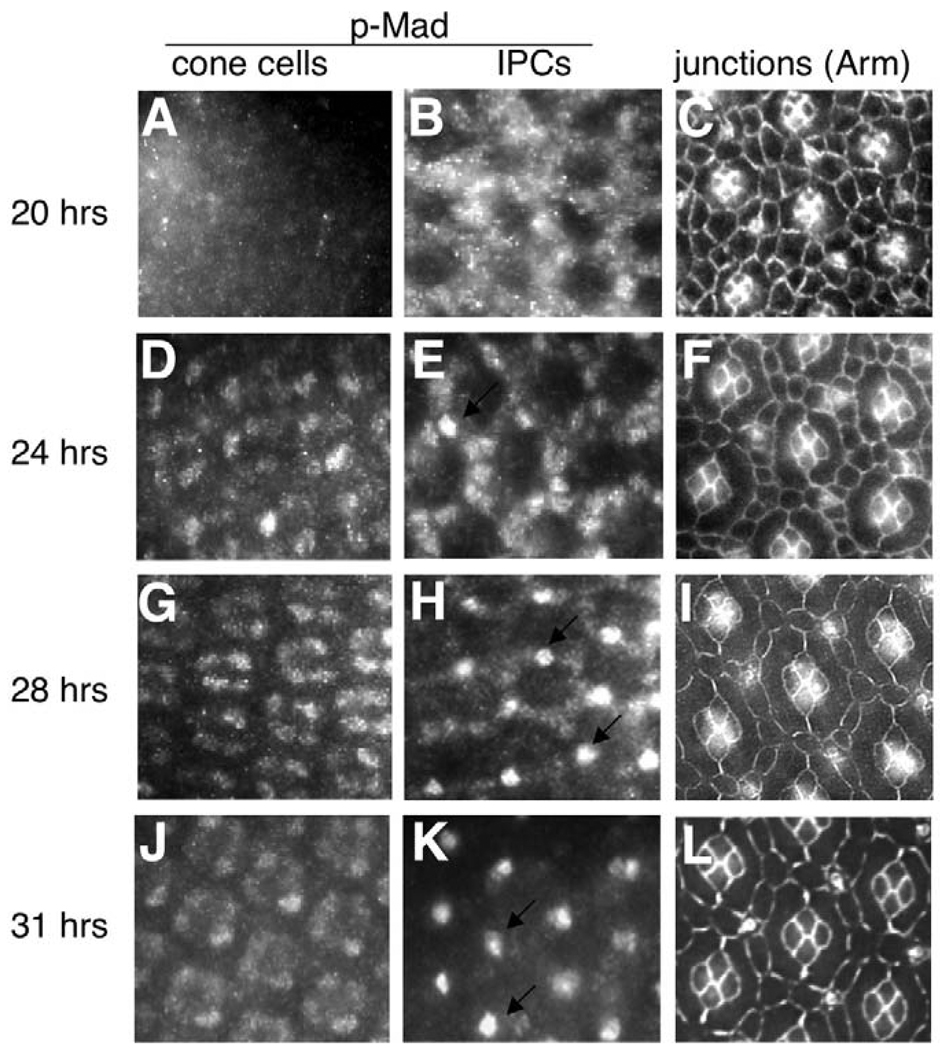
Nuclear levels of p-Mad serve as a readout of Dpp signal transduction activity (Tanimoto et al., 2000). Our data suggest that, in the context of the pupal retina, primary pigment cells might act as a source of Dpp that is then provided to surrounding cells to influence their patterning. To further test this hypothesis, we utilized an antibody specific for p-Mad (see Fig. S1 in the supplementary material) to identify the cells that exhibit active Dpp signaling. Fig. 4 presents a time course of Dpp pathway activity in the pupal retina. Consistent with our ligand/receptor expression pattern and phenotypic results, p-Mad was detected in cone cells, IPCs and sensory bristles but not in primary pigment cells. IPCs contained high levels of p-Mad during the period of maximal IPC patterning (20–26 hours APF; Fig. 4B,E; data not shown). Subsequently, IPC levels decreased at 28 hours APF and were undetectable by 31 hours APF (Fig. 4H,K). Mad activity in cone cells and bristles was evident after 20 hours APF and remained unchanged through all stages examined (Fig. 4A,D,G,J); we did not observe consistent defects when dpp activity was reduced in these cells and the functional relevance of Mad activation in either cell type is unclear.
Fig. 4. Dynamic Dpp signaling activity in the Drosophila pupal retina.
(A–L) Dpp pathway activity is visualized in pupal retinas at different developmental stages using anti-p-Mad antibody. (A,D,G,J) p-Mad staining at the level of the cone cell nuclei; (B,E,H,K) p-Mad staining at the level of the IPC nuclei. (C,F,I,L) The maturing IPC pattern from age-matched retinas as development proceeded; cell membranes were stained with anti-Armadillo antibody. Time refers to hours APF. Arrows in E,H,K point to nuclear p-Mad in the sensory bristles.
Consistent with our phenotypic analysis, therefore, IPCs exhibited a dynamic pattern of Dpp activity that was highest at the time of active cell rearrangement and was then rapidly downregulated.
Dpp signaling works in opposition to Rst during IPC patterning
No significant genetic modifier interactions were observed (data not shown) between components of the Dpp pathway and Notchfa-g, EgfrEl or winglesscx4 which were previously implicated in IPC patterning (Cagan and Ready, 1989a; Cordero et al., 2004; Freeman, 1996; Miller and Cagan, 1998).
The results from our phenotypic analysis and in situ visualization indicated that mutations in the Dpp pathway affected cell shape, cell movements and cell-cell contacts, making Rst an attractive candidate to mediate Dpp function during IPC patterning. Consistent with this view, removing one genomic copy of tkv strongly suppressed the rough eye phenotype of rst3 mutants; removing one copy of Mad also produced a milder but significant suppression (Fig. 5). Independent tkv and Mad alleles gave similar results (data not shown). These results suggest that Rst and Dpp are functionally linked and that they act in opposition during patterning of the pupal retina.
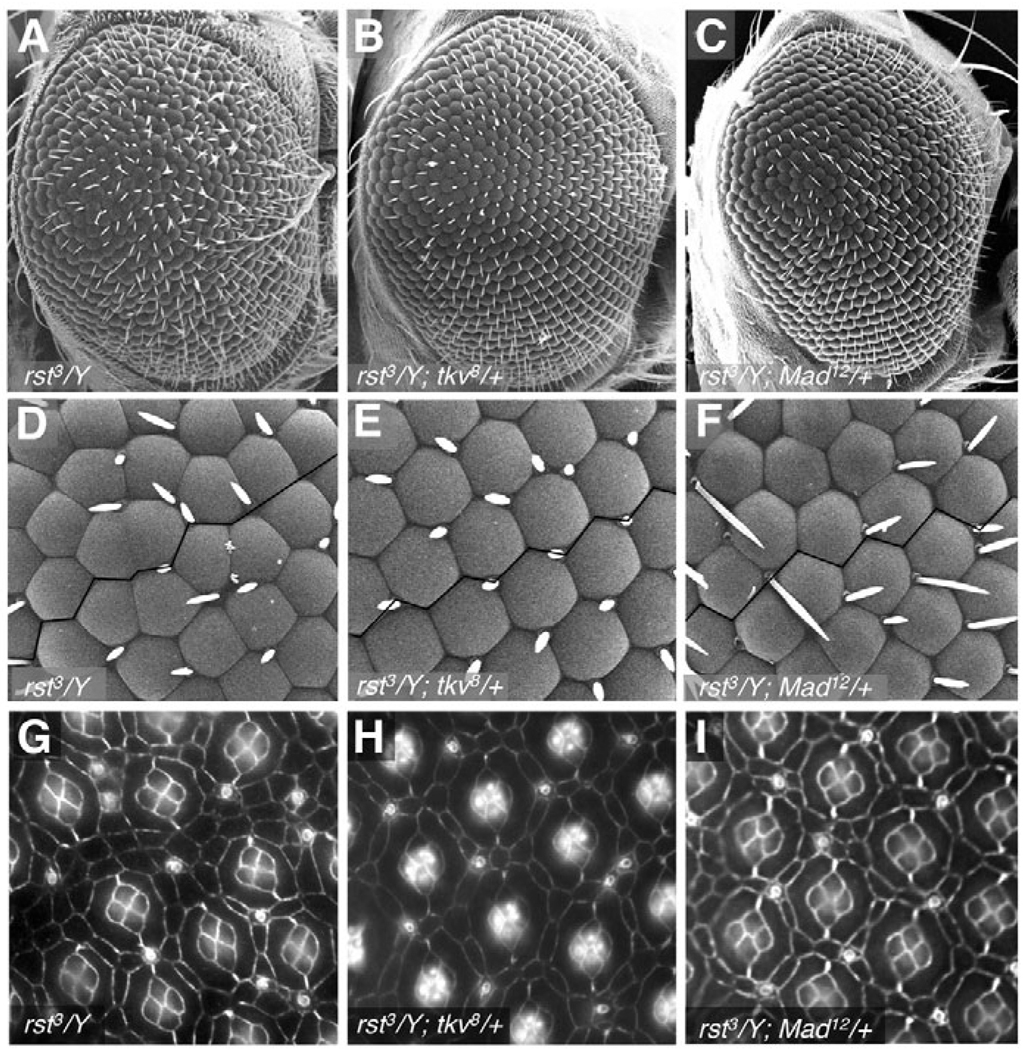
Fig. 5. Dpp signaling and Rst work in opposition in the Drosophila pupal retina.
Genetic interactions between dpp pathway components and rst. (A–F) Scanning electron micrographs of adult eyes taken at (A–C) 180× and (D–F) 800×. (G–I) 42 hours APF retinas stained with anti-Armadillo antibody. Removing a single functional genomic copy of tkv (B,E,H) or Mad (C,F,I) suppressed the rough eye phenotype of rst3 mutants (A,D,G).
Next, we assessed the epistatic relationship between Rst and Dpp signaling. We found no changes in Rst protein levels or localization when Dpp pathway activity was reduced (see Fig. S2A,B in the supplementary material). By contrast, we observed a striking failure of genotypically rst3 and rstCT pupal eyes to properly downregulate Dpp pathway activity: the normal reduction in p-Mad within IPCs at 28 hours APF did not occur (n=7 retinas for each genotype; Fig. 6, compare E,H with B). We could not unambiguously compare early-stage p-Mad in IPCs owing to its normally high levels. Levels of p-Mad in cone cells and bristle organs were not affected, providing a further, internal control. Together, these data indicate that Rst acts as a negative regulator of Dpp signaling activity as IPC patterning in the pupal retina progresses. In this scheme, loss of Rst activity leads to heightened Dpp pathway activity that in turn leads to defects in IPC patterning. Further supporting this model, expression of activated Tkv (GMR>tkvQ253D) in the developing eye led to a robust and fairly specific phenotype in the IPCs that at least partially phenocopied the defects observed in rst mutant retinas (Fig. 6J–L).
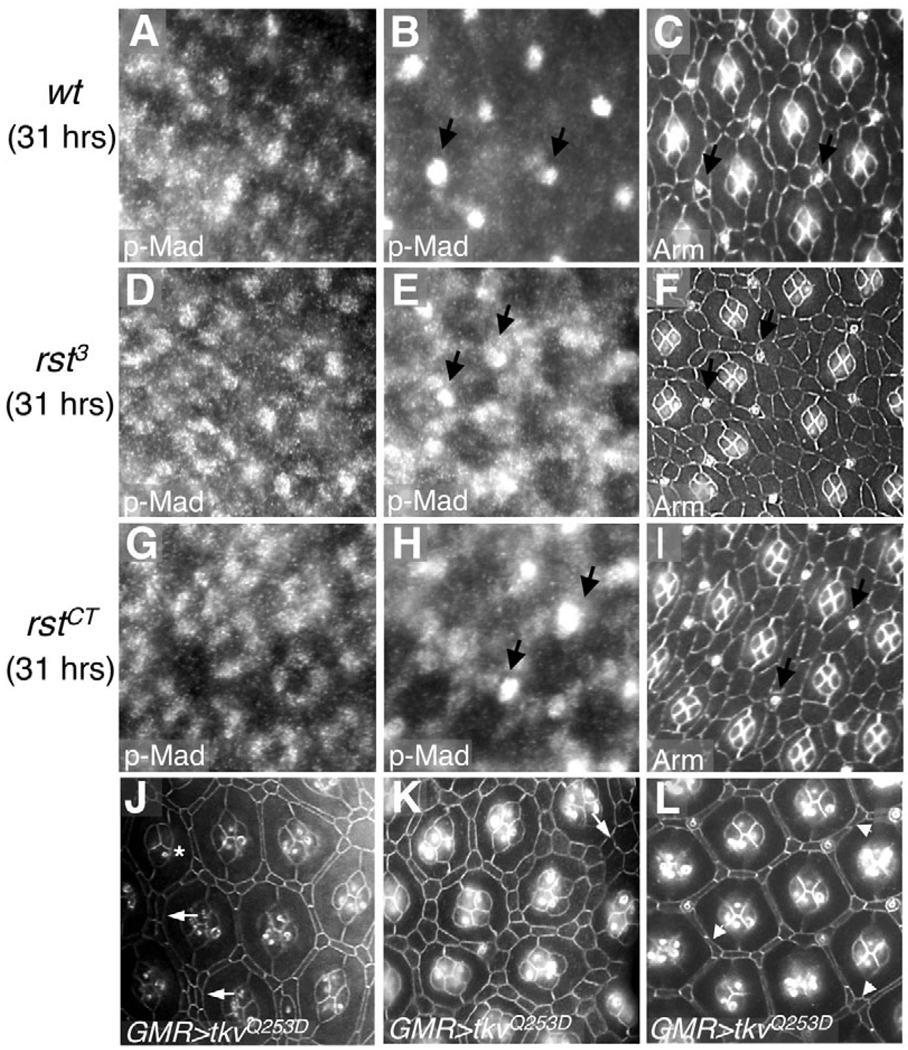
Fig. 6. Rst function is required to achieve proper Dpp signaling activity in the Drosophila pupal retina.
(A,B,D,E,G,H) p-Mad staining in 31 hours APF retinas from wild-type (A,B) and rst mutant animals (D,E,G,H). (C,F,I) Anti-Armadillo staining of the retinas to visualize the cells. Abnormally high levels of p-Mad were observed in genotypically rst IPCs (E,H; compare with B). Arrows point to sensory bristles. (J–L) 42 hours APF retinas overexpressing activated Tkv (GMR>tkvQ253D). The predominant phenotype observed (J,K, arrows point to examples of multi-layered IPCs), except for the most-anterior part of the retina which showed only minor IPC patterning defects (L, arrowheads).
We next sought to determine at what level Rst acts to downregulate Dpp signaling. Pathway activation has been shown to directly correlate with the levels of surface-associated Tkv (Jekely and Rorth, 2003). Indeed, we observed that Tkv surface presence correlated with p-Mad levels: the levels of IPC surface-associated Tkv were highest during the period of maximal IPC patterning and decreased subsequently (Fig. 7A–F). In rst mutant retinas, surface Tkv protein failed to decrease as patterning progressed (Fig. 7G–L). Mutant retinas for the null allele rstCT showed uniform IPC patterning defects accompanied by general upregulation of cell surface Tkv (Fig. 7G–I). Mutants for the hypomorphic allele rst3, which is subject to position effect variegation (PEV) (Tanenbaum et al., 2000), showed highest levels of cell surface Tkv in the areas with strongest IPC patterning defects (Fig. 7J–L). Co-immunoprecipitation experiments from tissue failed to detect physical interaction between Rst and Tkv (not shown), suggesting that the regulation of Tkv protein by Rst might require additional intermediaries. Furthermore, we saw no change in the levels of tkv transcript in control versus rst mutant retinas (see Fig. S3 in the supplementary material). Together, these data suggest that Rst opposes Dpp signaling by regulating the levels of cell surface-associated Tkv protein.
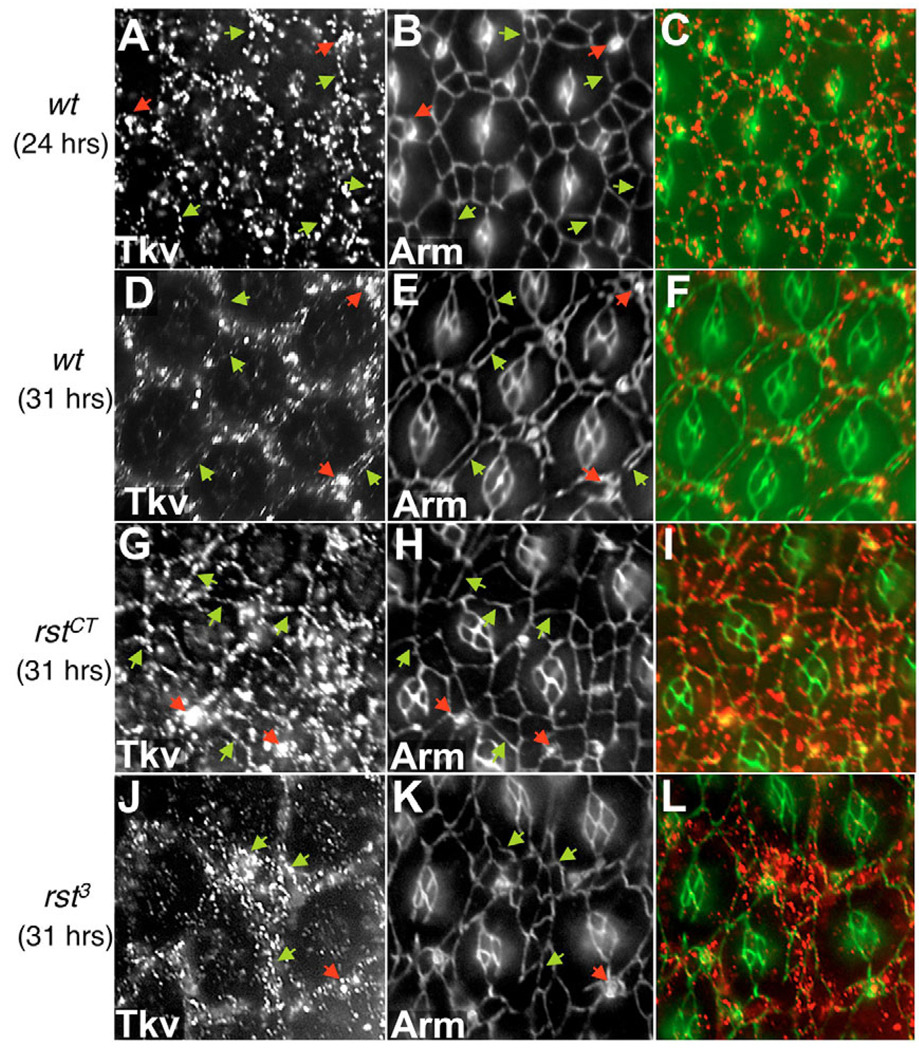
Fig. 7. Rst function is required to regulate cell surface levels of Tkv in the Drosophila pupal retina.
(A,D,G,J) Levels of cell-surface Tkv in pupal retinas. (B,E,H,K) Matched anti-Armadillo staining to visualize cells. (C,F,I,L) Overlay. Genotype and developmental stages (hours APF) are indicated at the left; wt, wild type. Green arrows indicate cell membranes where Tkv is localized. Red arrows indicate sensory bristles, which accumulated high levels of Tkv. Mutants in rst failed to downregulate surface Tkv protein in IPCs (G,J; compare with D).
Dpp signaling works in conjunction with DE-cadherin and Rho1 during IPC patterning
DE-cadherin, along with α-catenin and Armadillo (also known as β-catenin), constitute the core components of the adherens junctions and can help mediate cell-cell adhesion and cell rearrangement (Peifer and Wieschaus, 1990; Tepass et al., 1996; Uemura et al., 1996). Notably, we identified defects in adherens junction coherence and function when Dpp pathway activity was reduced (Fig. 3; see Movie 2 in the supplementary material). To further explore the relationship between Dpp and DE-cadherin, we tested null alleles of components of the Dpp pathway in trans to a null allele of shotgun (shgR69). Control retinas heterozygous for tkv8, Mad12 or shgR69 were essentially wild type except for infrequent 2°/3° defects (Fig. 8C). The trans heterozygous combinations tkv8/shgR69 and Mad12/shgR69 led to a significant increase in the percentage of 2°/3° defects (Fig. 8A–C). This genetic enhancement is consistent with our observation that DE-cadherin and Rst act in opposition (Fig. 1I–P) and, together with the junction phenotype observed in tkv and Mad mutant retinas (Fig. 3; see Movie 2 in the supplementary material), further supports a model in which Dpp signaling regulates IPC patterning at least in part by regulating DE-cadherin-mediated cell adhesion in the retina.
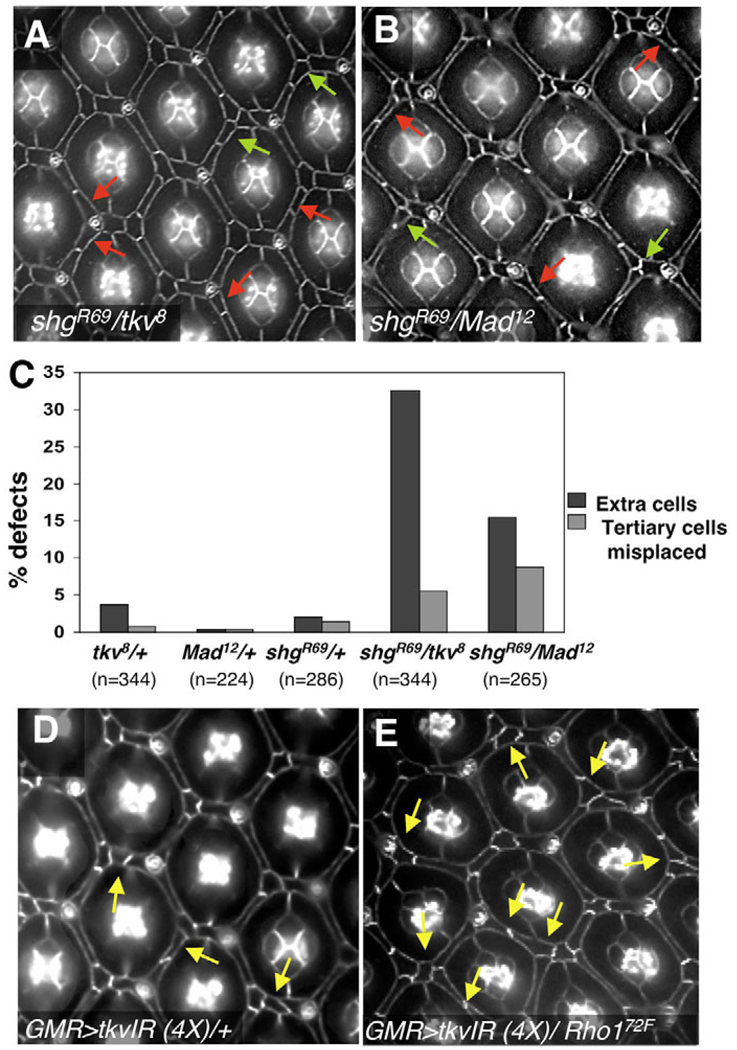
Fig. 8. Dpp signaling works together with DE-caderin and Rho1 to pattern the Drosophila pupal retina.
(A,B) Removing one genomic copy of shg and either tkv (A) or Mad (B) resulted in a significant increase in the incidence of ectopic 2°/3°s (red arrows) and misplaced 3°s (green arrows). Most misplaced 3°s were accompanied by an extra cell; conversely, we observed many cases of extra cells without a misplaced tertiary (red arrow). (C) Quantification of 2°/3°s defects. The data are expressed as the percentage of ommatidia with defects out of the total number of ommatidia counted. n=number of ommatidia counted from at least four different animals for each genotype. (D,E) Removing one genomic copy of Rho1 in retinas expressing four copies of tkv-IR enhanced the frequency and severity of IPC patterning defects (arrows, compare D with E). The full genotypes were: (D) GMR-gal4/+; UAS-tkv-IR2(2X)/+; UAS-tkv-IR1(2X)/+; (E) GMR-gal4/+; UAS-tkv-IR2(2X)/Rho172F; UAS-tkv-IR1(2X)/+.
Members of the Rho family of small GTPases – Rac, Rho and Cdc42 – have been linked to regulation of the actin cytoskeleton in diverse organisms (Van Aelst and D’Souza-Schorey, 1997). Rho1 interacts with DE-cadherin-associated proteins and regulates cadherin-based cell junctions (Magie et al., 2002). Using a lower copy number of tkv-IR (GMR>tkv-IR) to direct a mild IPC patterning phenotype (Fig. 8D), we found that removing one copy of Rho1 (Rho172F) led to significant enhancement of IPC patterning defects (Fig. 8E; 60% of at least 15 retinas scored). This functional relationship is not due to regulation of Rho1 expression by Tkv activity, as Rho1 protein or transcript levels were not altered in a tkv mutant background (see Fig. S2C,D in the supplementary material; data not shown). Conversely, loss of Rho1 (or DE-cadherin) activity did not alter p-Mad levels (data not shown). This effect was specific for Rho1 because removing one copy of Cdc42 or a Rac1, Rac2, Mtl triple heterozygote did not detectably modify the tkv-IR phenotype (not shown). Together, these results indicate that Dpp signaling cooperates with DE-cadherin and Rho1 to regulate dynamic IPC morphogenesis, movement and cell-cell contacts during morphogenesis of the pupal retina.
Dpp signaling works in conjunction with DE-cadherin and Rho1 during wing patterning
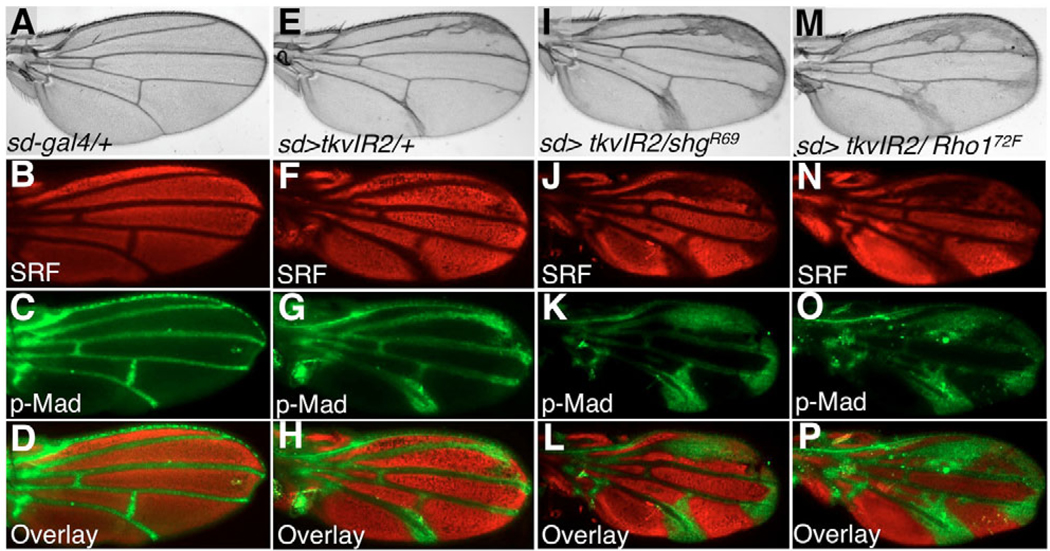
Dpp signaling is a well-known regulator of the cell fate decisions involved in the formation of wing veins (O’Connor et al., 2006). Driving tkv-IR using the wing pouch driver scalloped-gal4 (sd>tkv-IR) yielded a classical tkv loss-of-function wing vein phenotype (Terracol and Lengyel, 1994) that included thickening of wing veins L2 and L5 and of the intersection between L5 and the posterior cross vein (Fig. 9E–H; data not shown). Removing one genomic copy of either shg (Fig. 9I–L) or Rho1 (Fig. 9M–P) significantly enhanced these wing vein phenotypes to levels comparable to those observed when removing one genomic copy of tkv (data not shown). These phenotypes were a result of defects in cell fate determination as assessed with molecular markers specific for vein and intervein cells (Fig. 9). As in the eye, removing one copy of Cdc42 or a Rac1, Rac2, Mtl triple mutant did not detectably modify the tkv-IR phenotype in the wing (not shown). These data suggest that the functional connection between Dpp signaling and DE-cadherin and Rho1 is not exclusive to the eye, but is also relevant to the role of this pathway during cell fate decisions in the wing.
Fig. 9. Dpp signaling works together with DE-Caderin and Rho1 during Drosophila wing patterning.
(A,E,I,M) Adult wings. (B–D,F–H,J–L,N–P) 24–36 hours APF pupal wings were dissected and stained with anti-Srf (red; B,F,J,N) and anti-p-Mad (green; C,G,K,O) antibodies to label intervein and vein cells, respectively; (D,H,L,P) overlay. Removing one genomic copy of shg (I–L) or Rho1 (M–P) dramatically enhanced the cell fate defects of sd>tkv-IR wings (E–H). The full genotypes were: (A–D) sd-gal4/+; (E–H) sd-gal4, UAS-tkvIR2/+; +/+; (I–L) sd-gal4, UAS-tkvIR2/+; shgR69/+; (M–P) sd-gal4, UAS-tkvIR2/+; Rho172F/+.
DISCUSSION
Dpp signaling is a novel, essential regulator of IPC patterning during morphogenesis of the pupal retina
Loss of Dpp pathway activity results in a loss of epithelial integrity (Gibson and Perrimon, 2005; Shen and Dahmann, 2005), but the function of Dpp signaling during maturation of developing epithelia is not fully understood. Here, we show that reducing the activity of components of the Dpp pathway leads to abnormal IPC shape and organization within the ommatidial hexagonal pattern (Fig. 2). This activity is linked to fine regulation of apical junction components and is required to maintain stable cell-cell contacts during cell movements within the epithelium. The expression of Dpp in primary pigment cells (Fig. 2C,D) and the segregation of its receptors to the neighboring IPCs suggest a model in which Dpp acts in the primaries to organize local IPCs through the dynamic control of apical junctions. This view is supported by the dynamic changes in p-Mad activity in the neighboring IPCs, which is highest during the stage (20–26 hours APF) when IPCs rearrangements are maximal (Fig. 4).
The role of Dpp in cellular morphogenesis during epithelial development is poorly understood. Therefore, we took advantage of the unique stereotyped pattern of the pupal retina to study cell behavior as morphogenesis progresses, focusing on events at the single-cell level. In situ visualization experiments suggest that IPCs with reduced Tkv activity are incapable of maintaining their cell-cell contacts and are subject to aberrant changes in their cell shape (Fig. 3; see Movie 2 in the supplementary material). Further emphasizing the link with cellular adhesion, this function of Dpp signaling involves DE-cadherin and Rho1 (Fig. 8), which are essential regulators of cell adhesion and cell shape (Magie et al., 2002).
IPCs require a balance between Rst and Dpp signaling
We provide several lines of evidence indicating that Rst is a negative regulator of Dpp signaling (Figs 5–7). Previous work has demonstrated that Rst directs IPC movements through selective cell adhesion: IPCs seek to maximize their Rst-mediated contacts with primaries while decreasing contacts with their neighbors (Bao and Cagan, 2005) (Fig. 1A–F). Additionally, reducing Rst activity leads to a failure of initial cell movement (D.E.L., S. Bao and R.C., unpublished). Consistent with these results, Rst activity opposes DE-cadherin-mediated cell adhesion (Fig. 1I–P). One model to account for these observations is that cells require a balance between cell movement provided by Hbs-Rst and the stability of cell-cell contacts provided by Dpp signaling. Our live visualization supports the view that reducing Dpp activity leaves cells with an imbalance, as IPCs move toward their proper positions but fail to stabilize cell-cell contacts or lock stably into their final positions (Fig. 3; see Movie 2 in the supplementary material). Furthermore, downregulation of Dpp signaling leads to unstable DE-cadherin IPC-IPC junctions (Fig. 3). Conversely, loss of rst results in loss of cell movements, which can be compensated by either reducing cell adhesion (Fig. 1M–P) or Dpp signaling activity (Fig. 5), again supporting the importance of maintaining a balance between the Hbs-Rst and the Dpp–DE-cadherin systems. Perhaps Dpp (and, by extension, BMP) activity is utilized in the adult for similar functions – for example, as a ‘proof-reading’ mechanism to remove aberrant cells from an epithelium.
Is Dpp signaling a general regulator of cell adhesion and cell shape?
Our results in the wing raise the interesting possibility that regulation of DE-cadherin and Rho1-dependent cell shape and cell adhesion might be a characteristic of Dpp pathway activity common to other biological systems. Similar to the pupal retina, epithelial cells in the wing disc with reduced Dpp signaling displayed abnormal morphologies and were unable to maintain their positions. In the case of the wing, these defects were manifested as viable cysts of mutant cells that were basally excluded from the epithelium (Gibson and Perrimon, 2005; Shen and Dahmann, 2005). The mechanisms involved in such cell behaviors remain unknown. Our results suggest that the role of Dpp signaling during wing patterning also involves DE-cadherin and Rho1 (Fig. 9). Our experiments do not distinguish whether the defects in wing cell fates are a direct or a secondary effect of altered cell adhesion, although altering DE-cadherin activity by itself was not sufficient to cause such defects (data not shown). Cell adhesion and cell fate have been related previously: for example, Rho-dependent cell shape changes can influence fate decisions in stem cells (McBeath et al., 2004). Despite the commonalities observed, tissue-specific factors are likely to regulate Dpp-dependent epithelial patterning: for example, Rst does not appear to have a role in wing development, and we did not observe changes in retinal Tubulin distribution reported for the wing (Gibson and Perrimon, 2005) (see Fig. S2E–H in the supplementary material).
Dpp is the closest ortholog of vertebrate BMP2/4, and it appears to be active during cellular morphogenesis in a number of contexts including the developing vertebrate eye (Belecky-Adams et al., 2002; Furuta and Hogan, 1998). Interestingly, and similar to our observations for IPCs (Fig. 4), fiber cells in the developing vertebrate lens show high levels of p-SMAD activity during the period of cell elongation. Loss of the Type I receptor ALK3 (also known as BMPR1A) or expression of the inhibitor noggin led to abnormal morphogenesis of these fiber cells including mispositioning and failure to elongate (Beebe et al., 2004); requirements for E-cadherin (also known as cadherin 1) and RHOA function have not been explored.
Finally, Rst does regulate developmental processes other than IPC patterning. For example, Rst is expressed in retinal axons and is required for correct targeting of those axons into the larval brain lobes (Schneider et al., 1995). Interestingly, Dpp signaling also has a role in this process (Yoshida et al., 2005). We have observed genetic interactions between rst3 and members of the Dpp pathway in the arrangement of these descending axons (L. Wickline and R.C., unpublished), raising the intriguing possibility that the two systems act together in axon targeting as well.
Summary and future directions
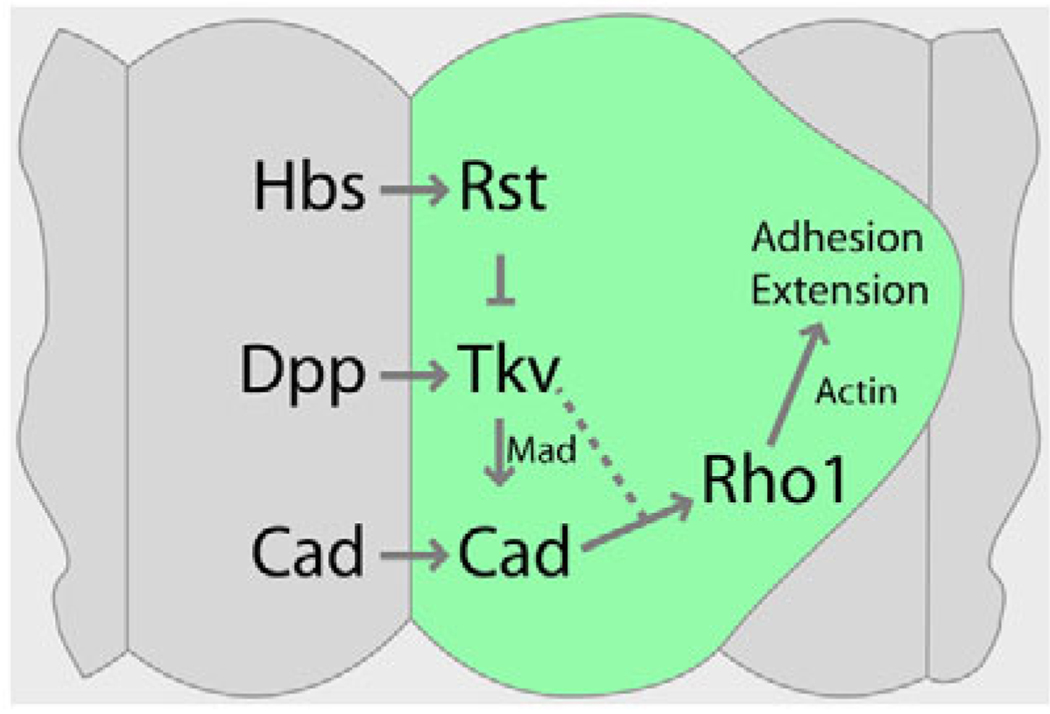
Our results provide evidence to support a model in which the Dpp pathway acts as an intermediary between the Rst and DE-cadherin adhesion systems. A balanced interplay between these three systems is essential to regulate epithelial cell movements, cell shape and cell-cell contacts during morphogenesis of the pupal retina (Fig. 10).
Fig. 10. Dpp signaling regulates IPC morphogenesis in the Drosophila pupal retina.
Dpp signaling is required to achieve correct cell-cell contacts, cell positions and cell shape during IPC patterning. This function requires the activity of DE-cadherin (Cad) and Rho1 and is opposed by Rst. This suggests that Dpp signaling acts as an intermediary or ‘third force’ between the Rst and DE-cadherin adhesion systems, providing a point of fine and dynamic regulation of the adherens junctions during epithelial maturation. A balanced interplay between these three systems is essential to regulate IPC patterning during morphogenesis of the pupal retina. The placement of Rho1 after DE-cadherin is speculative. See text for details.
Several questions emerge from our study. For example, our data suggest that Rst acts on Dpp signaling by regulating surface-associated Tkv. Immunoprecipitation experiments failed to identify a physical interaction between Rst and Tkv (not shown), suggesting intermediate steps remain to be identified. Also, the transcription factor Mad is required to regulate IPC patterning (Fig. 2P; Fig. 4; Fig. 5; Fig. 8B), but the transcriptional targets that link Dpp signaling to DE-cadherin and Rho1 are unknown. A better understanding of the links between these three pathways should help shed light on the mechanisms that regulate the fine cellular events required during patterning of developing epithelia.
Supplementary Material
Acknowledgments
We are grateful to our colleagues, the Developmental Studies Hybridoma Bank and the Bloomington Stock Center for kindly providing reagents. We thank Midori Seppa for producing wit−/− clones, Craig Micchelli for teaching us pupal wing dissections, and Marcos Vidal, Craig Micchelli and Midori Seppa for critical reading of the manuscript and helpful suggestions during the course of our work. We apologize to our colleagues whose work was not cited owing to space restrictions. This work was supported by the Cancer Biology Pathway (J.B.C.) and NIH grant 2R01-EY11495.
Footnotes
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/134/10/1861/DC1
References
- Affolter M, Nellen D, Nussbaumer U, Basler K. Multiple requirements for the receptor serine/threonine kinase thick veins reveal novel functions of TGF beta homologs during Drosophila embryogenesis. Development. 1994;120:3105–3117. doi: 10.1242/dev.120.11.3105. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- Bao S, Cagan R. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev. Cell. 2005;8:925–935. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bao S, Cagan R. Fast cloning inverted repeats for RNA interference. RNA. 2006;12:2020–2024. doi: 10.1261/rna.258406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D, Garcia C, Wang X, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. Contributions by members of the TGFbeta superfamily to lens development. Int. J. Dev. Biol. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Blair SS, Ralston A. Smoothened-mediated Hedgehog signalling is required for the maintenance of the anterior-posterior lineage restriction in the developing wing of Drosophila. Development. 1997;124:4053–4063. doi: 10.1242/dev.124.20.4053. [DOI] [PubMed] [Google Scholar]
- Burke R, Basler K. Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development. 1996;122:2261–2269. doi: 10.1242/dev.122.7.2261. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989a;3:1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev. Biol. 1989b;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Cordero J, Jassim O, Bao S, Cagan R. A role for wingless in an early pupal cell death event that contributes to patterning the Drosophila eye. Mech. Dev. 2004;121:1523–1530. doi: 10.1016/j.mod.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992;71:451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- Firth LC, Baker NE. Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Dev. Cell. 2005;8:541–551. doi: 10.1016/j.devcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MC, Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–1789. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- Golic KG, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Greenwood S, Struhl G. Progression of the morphogenetic furrow in the Drosophila eye: the roles of Hedgehog, Decapentaplegic and the Raf pathway. Development. 1999;126:5795–5808. doi: 10.1242/dev.126.24.5795. [DOI] [PubMed] [Google Scholar]
- Grzeschik NA, Knust E. IrreC/rst-mediated cell sorting during Drosophila pupal eye development depends on proper localisation of DE-cadherin. Development. 2005;132:2035–2045. doi: 10.1242/dev.01800. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bmps: multifunctional regulators of mammalian embryonic development. Harvey Lect. 1996;92:83–98. [PubMed] [Google Scholar]
- Jekely G, Rorth P. Hrs mediates downregulation of multiple signalling receptors in Drosophila. EMBO Rep. 2003;4:1163–1168. doi: 10.1038/sj.embor.7400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse K, Pantazis P, Bollenbach T, Julicher F, Gonzalez-Gaitan M. Dpp gradient formation by dynamin-dependent endocytosis: receptor trafficking and the diffusion model. Development. 2004;131:4843–4856. doi: 10.1242/dev.01335. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Letsou A, Arora K, Wrana JL, Simin K, Twombly V, Jamal J, Staehling-Hampton K, Hoffmann FM, Gelbart WM, Massague J, et al. Drosophila Dpp signaling is mediated by the punt gene product: a dual ligand-binding type II receptor of the TGF beta receptor family. Cell. 1995;80:899–908. doi: 10.1016/0092-8674(95)90293-7. [DOI] [PubMed] [Google Scholar]
- Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129:3771–3782. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- Marques G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O’Connor MB. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Pastor-Pareja JC, Garcia-Bellido A. JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila. Proc. Natl. Acad. Sci. USA. 2000;97:7888–7893. doi: 10.1073/pnas.97.14.7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Miller DT, Cagan RL. Local induction of patterning and programmed cell death in the developing Drosophila retina. Development. 1998;125:2327–2335. doi: 10.1242/dev.125.12.2327. [DOI] [PubMed] [Google Scholar]
- Monserrate JP, Baker Brachmann C. Identification of the death zone: a spatially restricted region for programmed cell death that sculpts the fly eye. Cell Death Differ. 2007;14:209–217. doi: 10.1038/sj.cdd.4401947. [DOI] [PubMed] [Google Scholar]
- Nellen D, Affolter M, Basler K. Receptor serine/threonine kinases implicated in the control of Drosophila body pattern by decapentaplegic. Cell. 1994;78:225–237. doi: 10.1016/0092-8674(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Neumann C, Cohen S. Morphogens and pattern formation. BioEssays. 1997;19:721–729. doi: 10.1002/bies.950190813. [DOI] [PubMed] [Google Scholar]
- Newfeld SJ, Mehra A, Singer MA, Wrana JL, Attisano L, Gelbart WM. Mothers against dpp participates in a DDP/TGF-beta responsive serine-threonine kinase signal transduction cascade. Development. 1997;124:3167–3176. doi: 10.1242/dev.124.16.3167. [DOI] [PubMed] [Google Scholar]
- O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- Panganiban GE, Reuter R, Scott MP, Hoffmann FM. A Drosophila growth factor homolog, decapentaplegic, regulates homeotic gene expression within and across germ layers during midgut morphogenesis. Development. 1990;110:1041–1050. doi: 10.1242/dev.110.4.1041. [DOI] [PubMed] [Google Scholar]
- Peifer M, Wieschaus E. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell. 1990;63:1167–1176. doi: 10.1016/0092-8674(90)90413-9. [DOI] [PubMed] [Google Scholar]
- Penton A, Chen Y, Staehling-Hampton K, Wrana JL, Attisano L, Szidonya J, Cassill JA, Massague J, Hoffmann FM. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell. 1994;78:239–250. doi: 10.1016/0092-8674(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Reiter C, Schimansky T, Nie Z, Fischbach KF. Reorganization of membrane contacts prior to apoptosis in the Drosophila retina: the role of the IrreC-rst protein. Development. 1996;122:1931–1940. doi: 10.1242/dev.122.6.1931. [DOI] [PubMed] [Google Scholar]
- Rogulja D, Irvine KD. Regulation of cell proliferation by a morphogen gradient. Cell. 2005;123:449–461. doi: 10.1016/j.cell.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Rusconi JC, Hays R, Cagan RL. Programmed cell death and patterning in Drosophila. Cell Death Differ. 2000;7:1063–1070. doi: 10.1038/sj.cdd.4400767. [DOI] [PubMed] [Google Scholar]
- Schneider T, Reiter C, Eule E, Bader B, Lichte B, Nie Z, Schimansky T, Ramos RG, Fischbach KF. Restricted expression of the irreC-rst protein is required for normal axonal projections of columnar visual neurons. Neuron. 1995;15:259–271. doi: 10.1016/0896-6273(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Schock F, Perrimon N. Molecular mechanisms of epithelial morphogenesis. Annu. Rev. Cell Dev. Biol. 2002;18:463–493. doi: 10.1146/annurev.cellbio.18.022602.131838. [DOI] [PubMed] [Google Scholar]
- Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM. Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics. 1995;139:1347–1358. doi: 10.1093/genetics/139.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–1790. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr. Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Tanenbaum SB, Gorski SM, Rusconi JC, Cagan RL. A screen for dominant modifiers of the irreC-rst cell death phenotype in the developing Drosophila retina. Genetics. 2000;156:205–217. doi: 10.1093/genetics/156.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto H, Itoh S, ten Dijke P, Tabata T. Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol. Cell. 2000;5:59–71. doi: 10.1016/s1097-2765(00)80403-7. [DOI] [PubMed] [Google Scholar]
- Tepass U, Gruszynski-DeFeo E, Haag TA, Omatyar L, Torok T, Hartenstein V. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 1996;10:672–685. doi: 10.1101/gad.10.6.672. [DOI] [PubMed] [Google Scholar]
- Terracol R, Lengyel JA. The thick veins gene of Drosophila is required for dorsoventral polarity of the embryo. Genetics. 1994;138:165–178. doi: 10.1093/genetics/138.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Oda H, Kraut R, Hayashi S, Kotaoka Y, Takeichi M. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 1996;10:659–671. doi: 10.1101/gad.10.6.659. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Vidal M, Larson DE, Cagan RL. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev. Cell. 2006;10:33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Wharton K, Ray RP, Findley SD, Duncan HE, Gelbart WM. Molecular lesions associated with alleles of decapentaplegic identify residues necessary for TGF-beta/BMP cell signaling in Drosophila melanogaster. Genetics. 1996;142:493–505. doi: 10.1093/genetics/142.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- Xie T, Finelli AL, Padgett RW. The Drosophila saxophone gene: a serine-threonine kinase receptor of the TGF-beta superfamily. Science. 1994;263:1756–1759. doi: 10.1126/science.8134837. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Soustelle L, Giangrande A, Umetsu D, Murakami S, Yasugi T, Awasaki T, Ito K, Sato M, Tabata T. DPP signaling controls development of the lamina glia required for retinal axon targeting in the visual system of Drosophila. Development. 2005;132:4587–4598. doi: 10.1242/dev.02040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.