Abstract
Background
End-stage liver disease due to hepatitis C virus (HCV) is the most common indication for liver transplantation in U.S. veterans. We investigated the influence of HCV genotypes on the incidence and timing of recurrent HCV hepatitis, survival, infectious morbidity, and response to interferon-α therapy in this unique patient population.
Methods
HCV genotype was determined by direct sequencing of the NS5 region of HCV with type-specific primers.
Results
Genotype 1a (66%, 32/47) was the predominant genotype. Type 1b was found in 25% (12/47) of patients and type 2b was found in 9% (4/47). Histopathologically recurrent HCV hepatitis developed in 53% (25/47) of the patients after transplantation. This group included 45% (14/31) of the patients with type 1a, 67% (8/12) of the patients with type 1b, and 25% (1/4) of the patients with type 2b (P>0.5). The time to recurrence and the severity of HCV recurrence as defined by aminotransferase levels or Knodell scores were not different among the three genotypes. There was a trend toward a higher incidence of major infections in patients with type 1b (75%) versus type 1a (48%) and type 2b (50%) (P=0.11). The response to interferon-α therapy did not differ significantly among the genotypes. Mortality at 5 years was 16% (5/31) in patients with genotype 1a, 42% (5/12) in patients with genotype 1b, and 50% (2/4) in patients with genotype 2b (P=0.06).
Conclusions
The incidence, time to recurrence, and response to interferon-α therapy did not differ between the various genotypes in our liver transplant recipients. However, there was a trend toward higher infectious morbidity and overall mortality in patients with genotype 1b after transplantation.
End-stage liver disease due to hepatitis C virus (HCV*) has emerged as one of the leading indications for orthotopic liver transplantation (1–4). Persistent posttransplant viremia has been documented in up to 95% of recipients, and histopathologic recurrence has been observed in 30–70% of patients with HCV within the first year after transplantation (1–4). The precise reason for the disparity between the near universal recurrence of HCV infection and clinical (histopathologic) recurrence is not known, although the intensity of posttransplant immunosuppression, donor-recipient HLA matching, level of pre- and posttransplant viremia, and viral genotype have been proposed as contributory variables (5–11).
Simmonds et al. (12) have identified 6 major HCV genotypes and 11 subtypes based upon the phylogenetic analysis of the NS5 region of the HCV genome. The prevalence of HCV genotypes shows significant geographic variation (13). In the United States and Western Europe, genotypes 1a and 1b are common, whereas subtypes 2a, 2b, and 3a occur infrequently. In Southeast Asia, genotypes 1b, 2a, and 2b are common, whereas infection with type 1a is rare.
Prognostic implications for HCV genotypes in liver transplant recipients have thus far been assessed in only two reports published from Europe (5, 7). Both studies demonstrated that genotype 1b was the most prevalent genotype, and infection with genotype 1b was associated with more aggressive posttransplant HCV recurrence. An association between genotypes and severity of posttransplant recurrence was not observed in a U.S. study published in abstract form (6). To our knowledge, no other study has assessed the association between HCV genotypes and outcome in U.S. patients undergoing liver transplantation.
HCV genotypes have been shown to be variably responsive to interferon-α therapy for HCV hepatitis. In the nontransplant setting, genotype 1b has been associated with poor response to interferon-α therapy (14–22). In contrast, patients infected with type 2 have demonstrated a better response to interferon-α. An association between response to interferon-α and HCV genotypes has never been assessed in liver transplant recipients.
A higher incidence of infections, particularly due to pathogens associated with depressed cell-mediated immunity, has been demonstrated in patients with recurrent HCV hepatitis after liver transplantation (23). However, the effect of distinct viral genotypes on the incidence and the types of infections after transplantation is not known.
End-stage liver disease due to HCV has been documented in 53% of U.S. veterans undergoing liver transplantation at the Pittsburgh Veterans Affairs Medical Center; recurrent HCV hepatitis has developed in 42% of these patients. In this study, we assessed the impact of HCV genotypes on incidence and severity of recurrent HCV hepatitis. incidence of infections, response to interferon-α, and outcome in U.S. veterans undergoing liver transplantation.
METHODS
Study population
Between October 1989 and October 1995, 140 orthotopic liver transplantations were performed in 130 consecutive U.S. veterans under primary tacrolimus (Prograf, Fujisawa USA, Inc., Deerfield, IL)-based immunosuppression. Fifty-three percent (68/130) of the patients underwent transplantation for HCV. All of these patients were male with a mean age of 46 years (range, 31–63 years). A history of ethanol use was present in 43% (29/68) of the patients with HCV. The diagnosis of HCV infection was established by the presence of anti-HCV antibody (EIA-I was used before March 1992 and EIA-II thereafter) and confirmed by a RIBA-II assay. Beginning in April 1990, a preoperative serum sample was collected and frozen at −80°C in all patients. Recurrent hepatitis was diagnosed histopathologically using previously reported criteria and required the presence of portal lymphoid follicles, mononuclear lobular infiltrate, and hepatocyte necrosis with degeneration and ballooning in the absence of cytomegalovirus (CMV), herpes simplex virus, hepatitis B virus, and rejection (8, 24). Patients were followed until death or for a median period of 38 months (range, 6–74 months).
Of 68 liver transplant recipients with HCV, a preoperative serum sample was unavailable for genotyping in 10 patients and HCV was not typeable in 11. Therefore, genotype analysis was available in 47 patients, who comprised the study population. All donors except one were seronegative for HCV. Data on demographics, service in Southeast Asia, timing of posttransplant HCV recurrence, severity of HCV recurrence (Knodell scores), incidence of major bacterial infections, fungal infections, CMV infection and CMV disease, receipt of interferon-α, duration of interferon-α therapy, mortality, and cause of death were assessed in all patients.
Immunosuppression
All patients received tacrolimus and low-dose prednisone as immunosuppressive agents. One gram of methylprednisolone was given immediately after revascularization of the graft. Twenty milligrams of methylprednisolone were given intravenously daily until the oral route was established, at which time 20 mg of prednisone were administered daily. During the subsequent months, prednisone was slowly tapered. Rejection episodes were treated with 1 g of methylprednisolone bolus with or without steroid cycles (methylprednisolone given intravenously in four divided doses daily, with tapering of the dose from 200 mg to 20 mg a day over 6 days). OKT3 was used for steroid-resistant rejection.
Infectious morbidity
Infections were defined as reported previously (25). Infections were characterized as either major or minor. Major infections included bacteremia, intra-abdominal abscess, peritonitis, wound infection, pneumonia, Clostridium difficile colitis, cholangitis. invasive fungal infection, and symptomatic CMV infection (CMV disease). Minor infections included mucocutaneous herpes simplex or zoster infections, cystitis, and asymptomatic CMV shedding.
Interferon-α therapy
Response to interferon-α was assessed using criteria that have been previously used in liver transplant recipients. Complete response was defined as normalization of both aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Patients with any abnormality of either AST, ALT, or both were considered nonresponders. Quantitative HCV RNA levels were not available for all patients during the interferon-α treatment interval. Early responders were defined as patients demonstrating complete response by 6 months of therapy. Late responders were those who did not demonstrate a response at 6 months but responded with normalization of AST and ALT after 6 months of therapy. Nonresponders were patients who did not respond at either 6 months or at latest follow-up.
HCV genotyping assay
HCV was extracted from sera by the modified guanidinium thiocyanate-phenol/chloroform technique using a commercially available kit (RNAzol B. Biotecx Laboratories. Houston, TX). The HCV RNA was then reverse-transcribed into cDNA and amplified by polymerase chain reaction as described previously; appropriate measures were taken to prevent polymerase chain reaction product contamination (26). Oligonucleotides specific for the NS5 region of the HCV genome were used as primers: 5′ GGCGGAAT-TCCTGGTCATAGCCTCCGTGAA 3′ and 5′ TGGGGATCCGTAT-GATACCCGCTGTTTGA 3′. Polymerase chain reaction-positive samples were sequenced directly by the Sanger dideoxynucleotide termination method and a modified T7 DNA polymerase (Sequenase version 2.0 kit; United States Biochemical Corp., Cleveland, OH) using internal primers published by Simmonds et al. (27). For some reactions, the external primers were used for sequencing. Altogether, a 22-basepair fragment of the NS5 region was read and used for comparison, as described by Simmonds et al. (27). Of 47 samples, 22 were genotyped using the type-specific primers for the C region (28).
Statistical analysis
Demographics and genotyping were entered into a database (PROPHET Statistics, BBN Systems and Technologies, Cambridge, MA). Patients were compared as follows: continuous variables (episodes of infection, rejection, etc.) were compared using the t test or, when a normal distribution could not be assumed, the Mann-Whitney test. Categorical data (presence or absence of CMV, residence in Southeast Asia, etc.) were compared using the chi-square or Fisher’s exact test. A three-way comparison of age was done using the analysis of variance test. A Kaplan-Meier probability curve was constructed using the date of HCV recurrence as the end point. The Mantel-Cox test was used to compare probability curves.
RESULTS
Of 47 liver transplant recipients with HCV, 66% (31/47) had genotype 1a, 25% (12/47) had genotype 1b, and 9% (4/47) had genotype 2b (Table 1). The median age of the patients with genotype 1a or 1b was 45 years and that of patients with type 2b was 55 years; patients with genotype 2b were significantly older than other patients (P=0.01) (Table 1). Forty-five percent (14/31) of the patients with genotype 1a, 58% (7/12)of patients with type 1b, and 25% (1/4) with type 2b (P>0.5) had served and resided in Southeast Asia during the Vietnam War.
Table 1.
Demographic and clinical variable in patients with the three HCV genotypes
| Variable | HCV |
p | ||
|---|---|---|---|---|
| Genotype 1a (n=31) | Genotype 1b (n=12) | Genotype 2b (n=4) | ||
| Prevalence in patients with HCV, % (n) | 66% (3/147) | 25% (12/47) | 9% (4/47) | |
| Age, median (range) | 45 (36–63) | 45 (31–54) | 55 (47–64) | 0.01a |
| Residence in Southeast Asia | 55% (14/31) | 58% (7/12) | 25% (1/4) | NSb |
| Recurrent HCV hepatitis post-Tx | 52% (16/31) | 67% (8/12) | 25% (1/4) | NS |
| Rejectionc episodes before recurrenced (mean) | 0.54 | 1.1 | NS | |
| Incidence of rejectionc | 31% | 57% | NS | |
| Percent of patients receiving boluses/mean no. of steroid boluses | 54%/1 | 57%/0.71 | NS | |
| Percent of patients receiving steroid recycles/mean no. of steroid recycles | 23%/0.46 | 14%/0.14 | NS | |
| Mortality | 45% (14/31) | 58% (7/12) | 25% (1/4) | NA |
Patients with genotype 2b were significantly older than other patients.
NS, not significant, P>0.05.
Rejection episodes refer to biopsy-proven rejection.
Mean values for rejection and immunosuppression are not given for genotype 2b because only one patient in this group developed recurrence.
Histopathologically recurrent HCV hepatitis after transplantation developed in 53% (25/47) of the patients. The recurrence rate was 52% (16/31) in patients with genotype 1a, 67% (8/12) in patients with type 1b, and 25% (1/4) in patients with type 2b (P>0.5). Figure 1 depicts the Kaplan-Meier probability of recurrent HCV hepatitis in the three genotypes. The median time to recurrence after transplantation was 301 days (range, 87–994 days) for type 1a, 198 days (range, 72–714 days) for type 1b, and 111 days for type 2b (Table 2); the time to recurrence did not differ significantly among the HCV genotypes (P=0.5). Severity of HCV recurrence, as assessed by aminotransferase levels and Knodell scores at the onset of recurrent HCV hepatitis, was not different for the three genotypes (Table 2). Incidence of rejection and intensity of immunosuppression, as assessed by the number of corticosteroid boluses and recycles received, before recurrent HCV hepatitis did not differ between patients with genotype 1a or 1b who developed recurrence. None of the study patients received OKT3 (Table 1).
Figure 1.
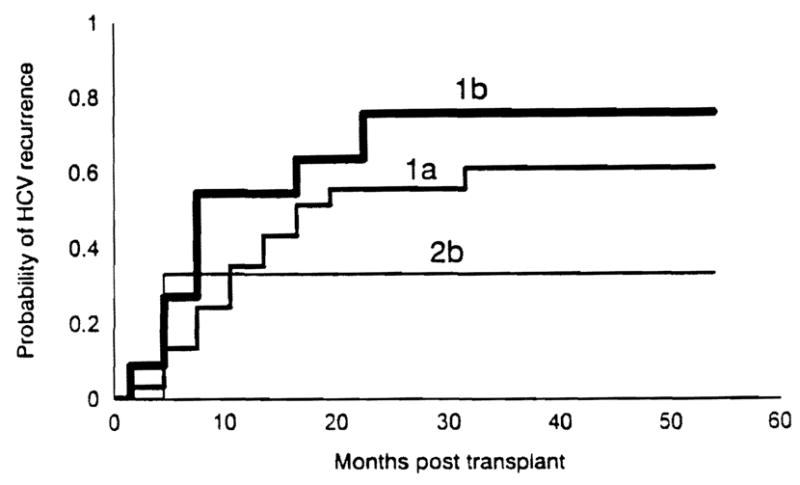
This Kaplan-Meier curve depicting the probability of recurrent HCY hepatitis in patients with three Hey genotypes; the rate of recurrence did not differ significantly among the three genotypes.
Table 2.
Incidence, timing, and severity of HCV recurrence in patients with three HCV genotypesa
| Variable | Genotype 1a | Genotype 1b | Genotype 2b |
|---|---|---|---|
| Rate of recurrence | 52% (16/31) | 67% (8/12) | 25% (1/4) |
| Time to recurrence, median (range) in days | 301 (87–994) | 198 (72–174) | 111 |
| Mean AST level (IU/L) | 116 | 88 | 150 |
| Mean ALT level (IU/L) | 124 | 87 | 224 |
| Mean Knodell score | 5.0 | 5.8 | 2 |
None of the variables differed significantly among the three genotypes.
Response to interferon-α
The response to interferon-α was assessable in 18/25 patients with HCV recurrence who received >6 months of interferon-α treatment (4 patients received <1 month of -αinterferon before they died, 2 patients did not receive interferon-α because of contraindications, and 1 patient started interferon-α therapy only 1 month ago). At 6 months, 25% (3/12) of patients with genotype 1a and 17% (1/6) of patients with type 1b were biochemical responders (P>0.2). Six of the nine nonresponders with type 1a and four of six nonresponders with type 1b continued interferon-α beyond 6 months (median, 14 months: range, 8–48 months). At current follow-up, 48% (5/12) of the patients with type 1a and 50% (3/6) of the patients with type 1b were responders.
Infections
Major infections after transplantation were observed in 48% of the patients with genotype 1a. 75% of the patients with type 1b (P=0.11), and 50% of those with type 2b. The number of infections per patient, the rate of major bacterial infections, major fungal infections, CMV infections, and CMV disease did not differ significantly among the three genotypes. Likewise, when infectious complications were analyzed only in patients with recurrent HCV hepatitis (i.e., patients with genotype 1a versus those with genotype 1b), the incidences of infections (50% vs. 75%, respectively), major bacterial infections (38% vs. 62%), major fungal infections (19% vs. 0%), CMV infection (50% vs. 62%), and CMV disease (31% vs. 25%) were not significantly different (P>0.20 for all infections) (Table 3).
TABLE 3.
Major infections in patients with the three HCV genotypes
| Genotype 1a | Genotype 1b | Genotype 2b | |
|---|---|---|---|
| Infections in the study population (n=47) | |||
| No. of patients | 31 | 12 | 4 |
| Any major infectiona | 48% | 75% | 50% |
| Mean no. of infections | 1.0 | 1.67 | 1.0 |
| Major bacterial infections | 39% | 58% | 50% |
| Major fungal infections | 13% | 8% | 0 |
| CMV infection | 35% | 50% | 50% |
| CMV disease | 16% | 25% | 25% |
| Infections in patients with recurrent HCV hepatitisb (n=25) | |||
| No. of patients | 16 | 8 | 1 |
| Any major infection | 50% | 75% | |
| Major bacterial infection | 38% | 62% | |
| Major fungal infection | 19% | 0 | |
| CMV infection | 50% | 62% | |
| CMV disease | 31% | 25% | |
The incidence of infections in the study population and in patients with recurrent HCV hepatitis only did not differ significantly among the genotypes; however, HCV patients with genotype 1b had a trend toward a higher incidence of major infection as compared with patients with genotype 1a (P=0.11).
Data presented for genotypes 1a and 1b because only one patient with genotype 2b had HCV recurrence.
Mortality
Overall mortality was 16% (5/31) in patients with genotype 1a, 42% (5/12) in patients with genotype 1b, and 50% (2/4) in patients with type 2b (P=NS). Death was attributable to recurrent HCV hepatitis in 20% (1/5) of the patients with genotype 1a, 40% (2/5) of the patients with genotype 1b, and 0% (0/2) of those with type 2b. When compared with non-HCV patients, actuarial survival for patients with and without HCV recurrence was not significantly different at 12, 24, 36. and 48 months after transplantation. Survival at 5 years was lower but not significantly different for patients with HCV recurrence (72%) when compared with those without recurrence (77%) and non-HCV patients (84%) (P=NS).
DISCUSSION
HCV genotype has been shown to be an important predictor of the severity of liver disease independent of the level of HCV viremia. These data suggest that HCV genotypes may differ intrinsically with respect to virulence. In the nontransplant setting, it has been observed that genotype 1b is associated with greater severity of liver disease as well as non-responsiveness to interferon-α therapy (14–22). In French liver transplant recipients, genotype 1b was the predominant genotype, occurring in 68% of the patients. Acute and chronic recurrent HCV hepatitis was observed in 77% and 59% of the patients, respectively, after 3 years, as compared with only 40% and 22% in patients with other genotypes (5). Genotype 1b was also the most prevalent genotype in liver transplant recipients in the United Kingdom and was associated with more severe allograft injury because of HCV recurrence as compared with the other genotypes (7). In contrast, Zhou et al. (6) did not observe a significant difference in the incidence or severity of posttransplant recurrent hepatitis according to genotype; the predominant genotype in this U.S. study was also type 1b.
We studied a unique group of patients (U.S. veterans) with a high incidence of end-stage liver disease due to HCV hepatitis. Since nearly half (23/47) of our study patients with HCV had served in Southeast Asia, we hypothesized that we would observe a high prevalence of genotypes known to be endemic in Southeast Asia and Japan (types 1b, 2a, and 2b). Surprisingly. 66% of our patients were infected with type 1a, a genotype not endemic in Southeast Asia. Twenty-five percent of our patients had type 1b and only 9% had type 2b. This suggests that our patients likely either had HCV infection before their service or acquired it in the U.S. afterward. In contrast to the findings of the European studies, histopathologic recurrence was not higher in our patients with type 1b. Likewise, the time to recurrence or severity of recurrence as assessed by aminotransferase levels and Knodell scores was not significantly greater in patients with genotype 1b.
The intensity of immunosuppression has been shown to correlate with recurrent HCV hepatitis after liver transplantation (8, 11). As the HCV genome undergoes mutation during HCV infection, it is conceivable that the different genotypes interact differently with the host immune response. Differences in rate or severity of HCV recurrence among the three genotypes in our study could not be explained by the intensity of immunosuppression, since the number of rejection episodes and the cumulative immunosuppression did not differ significantly among genotypes. To our knowledge, no previous study has assessed immunosuppression as a contributory variable to differences in severity of recurrence among various genotypes.
Patients with recurrent HCV hepatitis have been shown to be susceptible to late-occurring infections after transplantation. Indeed, a recent study in the nontransplant setting showed that T cell-derived cytokines were significantly elevated in patients with HCV as compared with normal control subjects (29). Whether cytokine levels and the infectious morbidity varies among genotypes, however, is not known. We observed a trend toward a higher incidence of major infections in patients with genotype 1b (75%) as compared with genotype 1a (48%), although statistical significance was not attained (P=0.11). Patients with genotype 1b or 2b had a trend toward higher mortality (42% and 50%, respectively) as compared with patients with genotype 1a (16%, P=0.11). Notably, fungal infections and bacteremias and not overt graft failure due to recurrent HCV was the predominant cause of death in these patients.
A number of studies have shown that genotype 1b is notably refractory to interferon-α therapy in nontransplant patients (14–16). Other virologic parameters, such as viral RNA levels and anti-core IgM HCV antibodies titers, also have been shown to be important predictors of response to interferon-α (17–22). Quantitative HCV RNA levels were not available for all of our study patients; however, this would likely not have altered our conclusions, since HCV genotype has been shown to be a predictor of response to interferon-α independent of HCV RNA levels. Although our numbers were small, the response rate to interferon-α did not differ significantly among the genotypes; at 6 months of therapy, 25% of the patients with genotype 1a and 17% of patients with type 1b were responders. With long-term maintenance interferon-α therapy, 48% of the patients with genotype 1a and 50% of the patients with genotype 1b had completely normalized their aminotransferase levels. A Japanese study in nontransplant HCV patients has also shown that prolonged (up to 52 weeks) interferon-α therapy appears to suppress relapse after cessation of therapy (19). We have previously reported that long-term therapy with interferon-α for posttransplant recurrent HCV is well tolerated and did not appear to precipitate rejection (30).
In summary, in the U.S. veterans we studied undergoing liver transplantation for end-stage liver disease due to HCV hepatitis, genotype 1a was the predominant genotype. Although there was a trend toward greater infectious morbidity and higher overall mortality in HCV patients infected with genotype 1b, the rate of recurrent HCV hepatitis, timing to posttransplant recurrence, severity of recurrence, and response to interferon-α therapy did not differ among genotypes. However, we caution that because of our small sample size, our findings should be validated in other larger studies. Nevertheless, our preliminary observations suggest that HCV genotype may not be a significant factor influencing the posttransplant course of U.S. patients with HCV hepatitis.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CMV
cytomegalovirus
- HCV
hepatitis C virus
References
- 1.Wright TL, Donegan E, Hsu HH, et al. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology. 1992;103:317. doi: 10.1016/0016-5085(92)91129-r. [DOI] [PubMed] [Google Scholar]
- 2.Muller H, Otto G, Gosser T, Arnold J, Pfaff E, Teilman L. Recurrence of hepatitis C viral infection in liver transplant recipients. Transplantation. 1992;54:743. [PubMed] [Google Scholar]
- 3.Konig V, Bauditz J, Lobeck H, Lusebrin R, Neuhaus P. Hepatitis C virus reinfection in allografts after orthotopic liver transplantation. Hepatology. 1992;16:1137. doi: 10.1002/hep.1840160506. [DOI] [PubMed] [Google Scholar]
- 4.Ascher NL, Lake JR, Emond J, Roberts J. Liver transplantation for hepatitis C virus-related cirrhosis. Hepatology. 1994;20:245. doi: 10.1016/0270-9139(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 5.Feray C, Gigou M, Samuel D, et al. Influence of the genotypes of hepatitis C virus on the severity of recurrent disease after liver transplantation. Gastroenterology. 1995;108:1089. doi: 10.1016/0016-5085(95)90207-4. [DOI] [PubMed] [Google Scholar]
- 6.Zhou S, Kim M, Ferrell L, Wright TL. HCV genotyping of liver transplant (OLT) recipients: relation to viremia and histology [Abstract] Hepatology. 1996;20(13):815. [Google Scholar]
- 7.Gane EJ, Portman BC, Naumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Gayowski T, Wagener MM, Mdimbe OK, Nedjar S, Yu VL. Recurrent hepatitis C virus hepatitis in liver recipients: association with increased immunosuppression post-transplantation. Surgery. 1996;119(4):452. doi: 10.1016/s0039-6060(96)80147-4. [DOI] [PubMed] [Google Scholar]
- 9.Rosen HR, Martin P, Shackelton CR, Farmer DA, Holt C, Busuttil RW. OKT3 use associated with diminished graft and patient survival in patients transplanted for chronic hepatitis [Abstract] Hepatology. 1996;22 (suppl):132A. [Google Scholar]
- 10.Gane EJ, Naumov M, Quian R, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996;110:166. doi: 10.1053/gast.1996.v110.pm8536853. [DOI] [PubMed] [Google Scholar]
- 11.Shreiner CP, Schwartz ME, Mor E, et al. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology. 1995;21:30. [PubMed] [Google Scholar]
- 12.Simmonds P, Alberti A, Alter HJ, et al. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology. 1994;19:13. [PubMed] [Google Scholar]
- 13.Dusheiko G, Schmilovitz-Weiss H, Alberti A, et al. Hepatitis C virus genotypes: an investigation of type-specific differences in geographic origin and disease. Hepatology. 1994;1994:13. [PubMed] [Google Scholar]
- 14.Finkelstein S, Sayegh R, Uchman S, Christensen S, Swalsky P. HCV undergoes extensive mutational change in N55 region in association with relapse/breakthrough following alpha-interferon therapy. Hepatology. 1992;16:132. [Google Scholar]
- 15.Takada M, Takase S, Enomoto M, Takada A, Dak T. Clinical backgrounds of the patients having different types of hepatitis C virus gnomes. J Hepatol. 1992;14:35. doi: 10.1016/0168-8278(92)90128-c. [DOI] [PubMed] [Google Scholar]
- 16.Nousbaum JB, Pol S, Nalpas B, et al. Hepatitis C virus type 1b (II) infection in France and Italy. Ann Intern Med. 1994;44:410. doi: 10.7326/0003-4819-122-3-199502010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Orito E, Mizorami M, Nakone T, et al. Serum hepatitis C virus RNA level as a predictor of subsequent response to interferon-alpha therapy in Japanese patients with chronic hepatitis C. J Med Virol. 1994;44:410. doi: 10.1002/jmv.1890440418. [DOI] [PubMed] [Google Scholar]
- 18.Pozzato G, Moretti M, Croce LS, et al. Interferon therapy in chronic hepatitis C virus: evidence of different outcome with respect to different viral strains. J Med Virol. 1995;4S:291. doi: 10.1002/jmv.1890450416. [DOI] [PubMed] [Google Scholar]
- 19.Kashahara A, Hayashi M, Hiramatsu M, et al. Ability of prolonged interferon treatment to suppress relapse after cessation of therapy in patients with chronic hepatitis C: a multicenter randomized controlled trial. Hepatology. 1995;21:291. [PubMed] [Google Scholar]
- 20.Martinot-Peignoux M, Marcellin P, Pouteau M, et al. Pre-treatment HCU RNA levels and HCV genotype are the main and independent prognostic factors of sustained response to alpha interferon therapy in chronic hepatitis C. Hepatology. 1995;22:1050. [PubMed] [Google Scholar]
- 21.Matsumoto A, Tanaka F, Suzuki T, Ogata H, Kiyosawa K. Viral and host factors that contribute to efficacy of interferon-alpha 2a therapy in patients with chronic hepatitis C. Dig Dis Sci. 1994;39:1273. doi: 10.1007/BF02093793. [DOI] [PubMed] [Google Scholar]
- 22.Mita E, Hayashi M, Hagiwara H, et al. Predicting interferon therapy efficacy from hepatitis C virus genotype and RMA titre. Dig Dis Sci. 1996;39:977. doi: 10.1007/BF02087547. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Gayowski T, Wagener MM, Marino IR. Increased infections in liver transplant recipients with recurrent hepatitis C virus hepatitis. Transplantation. 1996;61:402. doi: 10.1097/00007890-199602150-00014. [DOI] [PubMed] [Google Scholar]
- 24.Read AE, Donegan E, Lake JR, et al. Hepatitis C in patients undergoing liver transplantation. Ann Intern Med. 1991;114:282. doi: 10.7326/0003-4819-114-4-282. [DOI] [PubMed] [Google Scholar]
- 25.Singh N, Gayowski T, Wagener M, Yu VL. Infectious complications in liver recipients on tacrolimus: prospective analysis of 88 consecutive liver transplants. Transplantation. 1994;58:774. [PubMed] [Google Scholar]
- 26.Laskus T, Persing DH, Nowicki MJ, Mosely JW, Rakela J. Nucleotide sequence analysis of the precore region in patients with fulminant hepatitis B in the United States. Gastroenterology. 1993;105:1173. doi: 10.1016/0016-5085(93)90964-e. [DOI] [PubMed] [Google Scholar]
- 27.Simmonds P, Holmes EC, Cha T, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the MS5–5 region. J Gen Virol. 1993;74:2391. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto H, Sugiyama Y, Okada S, et al. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992;73:673. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- 29.Cacciarelli TV, Martinez OM, Gish TLG, Villaneuva JC, Krams SM. Immunoregulatory cytokines in chronic hepatitis C virus infection: pre- and posttreatment with interferon alpha. Hepatology. 1996;24(1):6. doi: 10.1002/hep.510240102. [DOI] [PubMed] [Google Scholar]
- 30.Singh N, Gayowski T, Wagener MM, Wannstedt C, Marino IR. Interferon-alpha therapy for hepatitis C virus recurrence after liver transplantation: long-term response with maintenance therapy. Clin Transplant. 1996;10:348. [PubMed] [Google Scholar]


