Abstract
Background
Macroprolactin is a large, heterogeneous form of prolactin with limited bioavailability. Detection of macroprolactin by different immunoassays varies widely. The objectives of this study were to determine the immunoreactivity of macroprolactin by the Ortho Clinical Diagnostics Vitros ECi prolactin immunoassay, establish the most effective method for interpreting the prolactin concentration after PEG-precipitation, and correlate the clinical features of hyperprolactinemia with the presence of macroprolactin.
Methods
PEG-precipitation was performed on 120 hyperprolactinemic specimens. Of these, 31 specimens with a recovery <80% were fractionated by GFC. Four different approaches for identifying true hyperprolactinemia were investigated. Clinical symptoms of hyperprolactinemia were determined by chart review.
Results
Macroprolactin was detected by the Vitros ECi prolactin immunoassay. Use of a PEG-modified prolactin reference interval was effective for identifying hyperprolactinemia in the presence of macroprolactin. There was no difference in the prevalence of abnormal menses, galactorrhea, or abnormal MRI between those with and without macroprolactin (p>0.05). Accounting for macroprolactin in patients with hyperprolactinemia reduced the number of idiopathic cases.
Conclusions
The Vitros ECi prolactin immunoassay detects macroprolactin. PEG-precipitation is an acceptable surrogate to detect hyperprolactinemia in the presence of macroprolactin when using a prolactin reference interval derived from PEG-precipitated reference sera. Although testing for macroprolactin should not substitute for standard evaluation of hyperprolactinemia, identification of macroprolactin may clarify a diagnosis and direct appropriate therapy.
Key terms: prolactin, immunoassay, macroprolactin, sensitivity and specificity
Introduction
Prolactin (PRL) is a globular protein synthesized and secreted by lactotrophs in the anterior pituitary gland [1]. Three major variants of PRL can be found in the blood: monomeric PRL (monoPRL), big PRL (bigPRL), and macroprolactin (macroPRL). The monomeric form has a molecular weight of 23 kDa and accounts for most of the total PRL immunoreactivity in the serum of both normal subjects and those patients with hyperprolactinemia. BigPRL has a molecular weight of 48–56 kDa and is thought to be a covalently bound dimer of PRL, accounting for 10–15% of PRL immunoreactivity. MacroPRL has a molecular weight of 150–204 kDa and consists of an antigen-antibody complex of monoPRL and IgG [1].
While the half-life of monoPRL ranges from 26–47 minutes [1], macroPRL has substantially longer renal clearance [2] resulting in its accumulation in the serum. Owing to its large size, macroPRL is thought to be confined to the vasculature with limited bioavailability to PRL receptors. However, this remains controversial, as some studies report that patients with a high concentration of macroPRL exhibit signs or symptoms of hyperprolactinemia such as galactorrhea, menstrual irregularities, or infertility [3, 4], Other studies report that macroprolactinemia cannot be differentiated from hyperPRL based on clinical symptoms alone [5, 6]. Because several of the signs and symptoms of hyperprolactinemia are non-specific, it is possible that the occurrence of symptoms with macroprolactinemia is coincidental and that the two are not causally related [7].
From a clinical perspective, it is important to identify the presence of macroPRL as the cause of hyperprolactinemia in order to avoid inappropriate treatment with dopamine agonists or radiological investigations [8]. This is particularly important given the caveat that 5–10% of healthy individuals have a pituitary anomaly that could be interpreted as an adenoma [9]. Macroprolactinemia has been found to occur in 15–46% of hyperprolactinemic specimens [3, 10, 11] and its identification could reduce unnecessary treatment as well as the number of idiopathic cases.
The immunoreactivity of commercially available PRL immunoassays vary widely in their detection of macroPRL [5, 12, 13]. One study of nine different immunoassays demonstrated 2.3–7.8-fold differences in measured PRL concentrations in 10 sera containing predominantly macroPRL [14]. This suggests that assay variability is likely due to different combinations of capture and detection antibodies used in the various PRL immunoassays. While most of the commonly used immunoassays have been well-characterized with respect to macroPRL immunoreactivity, there is only a one report describing the Vitros ECi (Ortho Clinical Diagnostics, Raritan, NJ) PRL immunoassay [15]. In that report, a single specimen demonstrated moderate crossreactivity with macroPRL.
The gold standard method for detecting macroPRL is gel filtration chromatography (GFC), a procedure that allows for quantification of all three variants of PRL [3]. Unfortunately, this method is labor-intensive and not suitable for performance in clinical laboratories. In contrast, precipitation with polyethylene glycol (PEG) is a widely used screening test for macroPRL and is easily performed in clinical laboratories. A low PRL recovery after PEG treatment indicates the presence of macroPRL. This method has been validated against GFC [16, 17] and recoveries <30–50% have been used as the thresholds for the detection of macroprolactinemia [4, 8, 18]. Unfortunately, reliance on a relative percentage makes this method subject to potential misinterpretation because low recoveries have been observed in patients with increased amounts of both monoPRL and macroPRL [3]. In consideration of this, a more rigorous definition of macroprolactinemia has been advocated requiring that concentrations of residual PRL (after removal of macroPRL) fall in the range of sera from a healthy reference population similarly treated with PEG [8]. Another suggested approach is to estimate monoPRL from the post-PEG PRL concentration from a regression equation derived from a correlation between PRL after PEG-precipitation and monoPRL determined by GFC [19]. This approach would compensate for the loss of monoPRL during PEG-precipitation and permit the use of an established reference interval PRL. There are no published studies that have compared these different methods for interpreting the residual PRL concentration after PEG-precipitation.
Materials and Methods
Study design and population
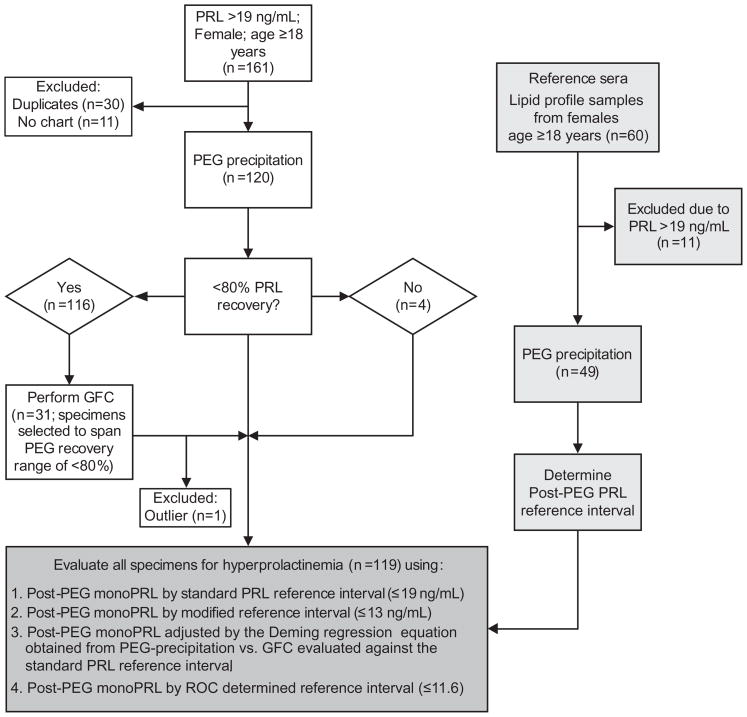
This was an observational study using serum specimens sent to the laboratory for physician-ordered PRL tests. The study population included 161 consecutive specimens obtained from females ≥18 years with hyperprolactinemia (PRL >19 ng/mL; this reference interval was validated by and used in the McLendon Clinical Laboratories at the University of North Carolina Hospitals). A total of 41 specimens were excluded; 30 were duplicates from patients included in the study and 11 were excluded due to lack of clinical notes. The remaining 120 specimens were PEG precipitated. Thirty-one of these specimens were fractionated by GFC to span a post-PEG recovery range of 10–80%. A flowchart of the study design is shown in Figure 1.
Figure 1.
Study design algorithm.
For the reference interval study, 60 serum specimens collected from females aged 18–65 years were randomly selected from specimens sent to the laboratory for lipid profile tests. There were 11 specimens excluded due to hyperprolactinemia (PRL >19 ng/mL).
Chart review was performed on all subjects in order to record clinical signs and symptoms of hyperprolactinemia. This study was approved by the Office of Human Research Ethics (OHRE) at the University of North Carolina at Chapel Hill.
Prolactin assay
All PRL tests were performed using an automated, immunometric PRL assay on an Ortho Clinical Diagnostics Vitros ECi. The assay has a total imprecision (coefficient of variation; CV) of 5.8% and 2.9% at PRL concentrations of 6.2 and 34.3 ng/mL, respectively.
PEG-precipitation
PEG-precipitation was performed by adding 150 μl of serum to an equal volume of 25% (w/v) PEG6000 (Fisher Scientific, USA) dissolved in phosphate buffered saline (PBS; 137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH of 7.4). To control for the effect of the dilution when calculating recovery, a parallel analysis was done by diluting the specimen 1:2 with PBS. After incubation for 10 min at room temperature, specimens were vortex mixed for 30 seconds and then centrifuged at 3000×g for 30 minutes. PRL recovery was calculated by dividing the post-PEG PRL result by the PRL result obtained from the 1:2 dilution with PBS. The post-PEG PRL concentration was determined by multiplying the PRL result by 2 to correct for the dilution with PEG.
Gel filtration chromatography
GFC fractionation was performed with PBS buffer at room temperature using a Superdex™ 75 16/60 120 mL chromatography column (GE Healthcare, USA) with a perfusion pump and an automatic fraction collector. Prior to analysis, the column was calibrated using Dextran Blue 2000 to determine the dead volume and the expected elution fraction for macroPRL. Following the application of 500 μL of serum, fractions of 0.3 mL were collected and analyzed for PRL. The relative amount of monoPRL, bigPRL, and macroPRL in each specimen was determined by calculating the percent area under curve (AUC) using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). The reproducibility of PRL fractionation by GFC was determined by analyzing two serum specimens in duplicate over three days; the interassay CV for the AUC was 5.9%.
Performance comparison of various methods for the detection of monomeric hyperprolactinemia
To determine the most effective method for identifying true hyperprolactinemia following PEG-precipitation, the performance characteristics of four different approaches were evaluated: 1) the standard PRL reference interval (≤19 ng/mL); 2) a post-PEG modified reference interval (≤13 ng/mL; see Results); 3) estimated monoPRL concentration derived from a Deming regression (obtained from a comparison between PEG-precipitation and GFC) and then evaluated against the standard PRL reference interval (≤19 ng/mL); and 4) a post-PEG modified reference interval determined by receiver operator characteristic (ROC) curve analysis (≤11.6 ng/mL; see Results). The diagnostic sensitivity and specificity for identifying hyperprolactinemia using each of these four approaches was determined using GFC as the gold standard to determine hyperprolactinemia. Hyperprolactinemia was defined as the combined concentrations of monoPRL and bigPRL >19 ng/mL which were determined by multiplying the AUCs of each by the neat PRL concentration.
Chart review
Chart review was performed for all patients to record clinical signs and symptoms associated with hyperprolactinemia, including galactorrhea, irregular menses, and infertility. In addition, patient charts were reviewed for evidence of pregnancy, pituitary adenoma by imaging studies, or drug-induced hyperprolactinemia. In cases where there was no attributable cause of hyperprolactinemia, patients were defined as having idiopathic hyperprolactinemia. Testing for macroPRL had not been done for clinical purposes on any of the patients included in the study.
Statistics
Data analysis was performed using GraphPad Prism software. The agreement between monoPRL following PEG-precipitation and GFC fractionation was assessed using Deming linear regression and Spearman correlation. One sample was excluded from the analysis as an outlier due to a >40% difference between GFC and PEG-precipitation. The optimal percent recovery cutoff for the detection of macroprolactinemia by PEG-precipitation was determined by ROC curve analysis using GFC as the gold standard where macroprolactinemia was defined as ≥50% macroPRL; this cutoff has previously been used to define macroprolactinemia [4]. ROC curve analysis was also used to determine the optimal post-PEG PRL concentration cutoff to identify true hyperprolactinemia. For this analysis, the GFC classification of specimens were used as the gold standard where true hyperprolactinemia was defined as GFC PRL >19 ng/mL. The post-PEG PRL reference interval was determined by using the entire range of PRL results obtained from the reference sera following precipitation with PEG. Continuous data were compared between groups using ANOVA and discrete data were compared using Fisher’s exact probability test.
Results
PRL recovery for the 120 specimens with hyperprolactinemia assayed after PEG-precipitation ranged from 10–110% (supplemental Figure S1). Using the most commonly published cutoff of <40% PRL recovery as the definition of macroPRL [10, 18], there was a 17% (20/120) prevalence of macroprolactinemia in the study population. The average recovery was 63% among samples without significant levels of macroPRL (post-PEG recovery ≥40%).
As evident by GFC fractionation, all PRL variants (macroPRL, bigPRL, and monoPRL) were immunoreactive in the Vitros ECi PRL assay (supplemental Figure S2). Based on the peak separation, the GFC analysis demonstrated the ability to differentiate between various PRL variants.
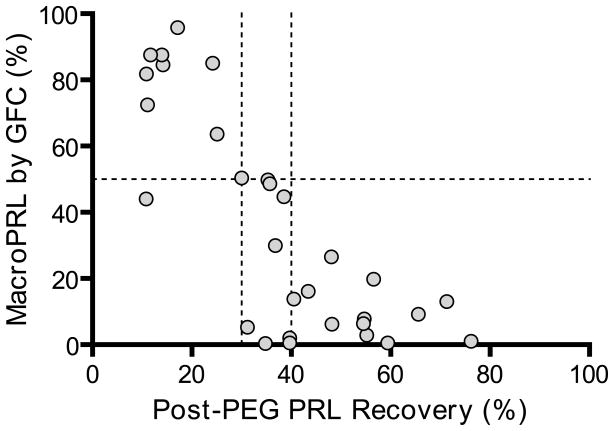
A PRL recovery cutoff of 40% following PEG precipitation detected 100% of specimens with macroPRL (defined as ≥50% macroPRL as determined by GFC) but was only 57% specific. Decreasing the post-PEG PRL recovery cutoff to 30% maintained 100% detection but increased the specificity to 95% (Figure 2).
Figure 2.
Scatterplot of PRL recovery after PEG-precipitation vs. macroPRL by GFC. The dotted lines represent 30% and 40% recovery cutoffs (x-axis) and 50% macroPRL (y-axis).
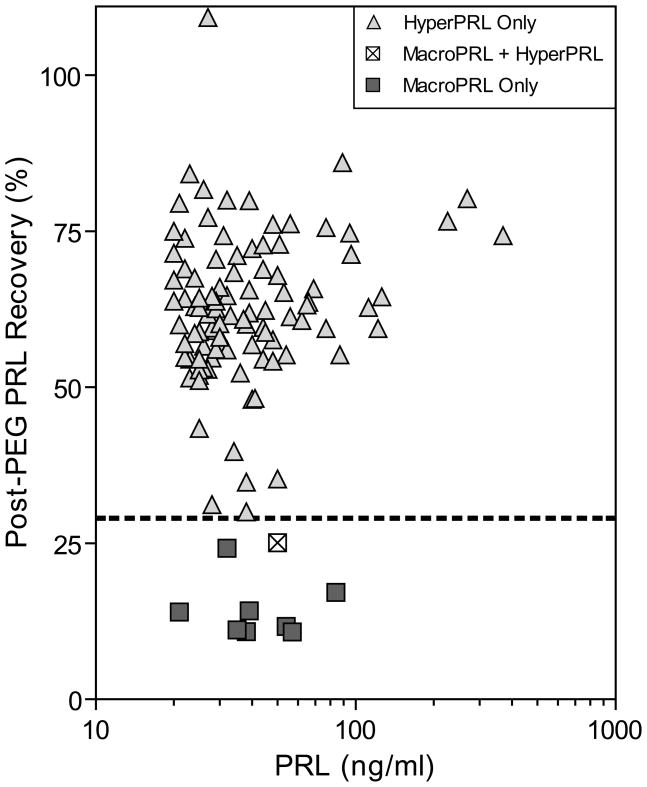
Spearman correlation was used to assess the relationship between the total PRL concentration and the PRL recovery following PEG-precipitation (Figure 3). The two were not correlated (r=0.07, p=0.22) and macroPRL was not present in any specimens with a PRL concentration >85 ng/mL.
Figure 3.
Scatterplot of PRL concentration vs. PRL recovery after PEG-precipitation. HyperPRL only was defined as a post-PEG recovery >30% and post-PEG PRL concentration >13 ng/mL. MacroPRL and HyperPRL was defined as post-PEG PRL recovery ≤30% at a post-PEG PRL concentration >13 ng/mL. MacroPRL only was defined as a post-PEG recovery ≤30% and post-PEG PRL ≤13 ng/mL. The dotted line indicates the 30% PRL recovery cutoff used to detect macroPRL.
To define a post-PEG PRL reference interval, PRL was measured in 60 specimens from apparently healthy females aged 18–65 years. Eleven specimens were excluded that had a PRL concentration >19 ng/mL. The post-PEG PRL concentration range in the remaining 49 specimens was 3.8–13.0 ng/mL and the mean recovery was 70.6 ± 14.6% (SD).
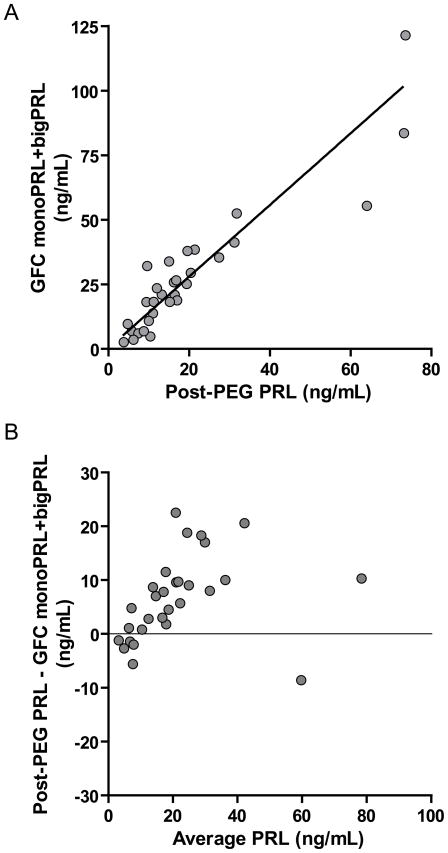
Deming regression analysis between PRL following PEG-precipitation and monoPRL + bigPRL determined by GFC showed good correlation (r=0.90) although results determined by GFC were higher than by PEG precipitation (slope 1.4; y-intercept 0.2) (Figure 4A). This is consistent with the loss of some monoPRL by PEG precipitation. A Bland-Altman difference plot demonstrated a positive bias for post-PEG PRL that was proportional to the PRL concentration (Figure 4B).
Figure 4.
A) Deming regression analysis demonstrating the relationship between “monoPRL + bigPRL by GFC” and “post-PEG PRL” (y=1.4x +0.20; Spearman r= 0.90; n=30). B) Bland-Altman plot of GFC and post-PEG PRL results.
ROC curve analysis was used to determine the optimal post-PEG PRL concentration for the assessment of hyperprolactinemia after PEG-precipitation (supplemental Figure S3). Specimens were classified as either normoprolactinemic (monoPRL ≤19 ng/mL) or hyperprolactinemic (monoPRL >19 ng/mL) by GFC. The optimal post-PEG PRL cutoff for identifying hyperprolactinemia was ≤11.6 ng/mL. This cutoff produced a sensitivity of 94% (95% CI 71–100%) and a specificity of 84% (95% CI 55–98%). The area under the ROC curve was 0.93.
Clinically, the presence of macroPRL is less important than the presence of true hyperprolactinemia. Therefore, we compared the four different approaches described above to evaluate residual PRL concentrations following PEG-precipitation (Table 1). Each of these methods was compared against the GFC gold standard where true hyperprolactinemia was defined as a monoPRL + bigPRL concentration >19 ng/mL. Of the four approaches, the standard PRL reference interval of ≤19 ng/mL (Vitros ECi PRL immunoassay) had the lowest sensitivity (59%) for identifying true hyperprolactinemia but was 100% specific. By comparison, the post-PEG modified PRL reference interval (≤13 ng/mL) and the regression estimated monoPRL had higher sensitivities (88 and 82%, respectively) but at a lower specificity (85%). The reference interval determined by ROC analysis (≤11.6 ng/mL) was the most efficient producing a sensitivity of 94% and a specificity of 85%.
Table 1.
Performance characteristics of various approaches for the evaluation of hyperprolactinemia following PEG-precipitation.
| Method | Sensitivity (%) | 95% CI (%) | Specificity (%) | 95% CI (%) | Efficiency * (%) |
|---|---|---|---|---|---|
| Standard reference interval (≤19 ng/mL) | 59 | 33–81 | 100 | 72–100 | 77 |
| Post-PEG modified reference range (≤13 ng/mL) | 88 | 62–98 | 85 | 54–97 | 87 |
| GFC regression estimate with use of the standard reference interval (≤19 ng/mL) | 82 | 56–95 | 85 | 54–97 | 83 |
| Post-PEG cutoff determined by ROC analysis (≤11.6 ng/mL) | 94 | 71–99 | 85 | 55–98 | 90 |
Percent correctly classified ([true positives + true negatives])/total # of patients)
From a practical standpoint, the post-PEG modified PRL reference interval (≤13 ng/mL) is simplest and the most cost effective of the aforementioned four approaches. Accordingly, the clinical data were interpreted using this reference interval to stratify patients by PRL status (Table 2). Patients were stratified into one of three groups as follows: 1) all individuals included in the study; 2) patients with true hyperprolactinemia (defined as PRL recovery >30% and having a post-PEG PRL concentration >13 ng/mL); and 3) patients with only macroPRL (defined as PRL recovery of ≤30% and having a post-PEG PRL concentration ≤13 ng/mL). There were no significant differences in the number of patients with abnormal menses, galactorrhea, infertility, or abnormal MRI findings between any of the three groups (p>0.05). Similarly, the number of patients treated with dopamine agonists and those who showed a response to treatment were not significantly different between groups. A diagnosis of idiopathic hyperprolactinemia was more frequent in patients with only macroPRL (i.e. hyperprolactinemia was not attributable to drug therapy, prolactinomas, pregnancy, or lactation). None of these patients were treated with dopamine agonists.
Table 2.
Clinical characteristics of patients with hyperprolactinemia, and macroprolactinemia.
| All patients1 | True HyperPRL2 | Only macroPRL3 | P-value | |
|---|---|---|---|---|
| 119 | 98 | 8 | ||
| Mean PRL, ng/mL (SD) | 45 (46) | 48 (49) | 45 (20) | 0.86 |
| Mean patient age, years (SD) | 36 (13) | 37 (13) | 37 (12) | 1.00 |
| Abnormal menses, % (# tested) | 64 (65/101) | 65 (55/84) | 83 (5/6) | 0.66 |
| Galactorrhea, % (# tested) | 49 (50/102) | 49 (42/85) | 57 (4/7) | 1.00 |
| Infertility, % (# tested) | 33 (39/119) | 31 (30/98) | 13 (1/8) | 0.43 |
| Abnormal MRI % (# tested) | 65 (49/75) | 69 (44/64) | 60 (3/5) | 0.65 |
| Treated with dopamine agonists, % (# tested) | 17 (19/111) | 17 (16/93) | 43 (3/7) | 0.13 |
| Decrease PRL and symptom response on treatment, %(# tested) | 88 (49/56) | 88 (43/49) | None Treated4 | 1.00 |
| Idiopathic hyperprolactinemia, % (# tested) | 39 (47/119) | 34 (33/98) | 50 (4/8) | 0.44 |
One patient had both macroPRL and hyperPRL.
Post-PEG recovery >30% and hyperprolactinemic by modified reference range (post-PEG PRL >13 ng/mL).
Post-PEG recovery ≤30% and normoprolactinemic by modified reference range (post-PEG PRL ≤13 ng/mL).
No patients with macroPRL were on dopamine agonists at the time of sample collection.
Discussion
The data in this study indicate that macroPRL is readily detected by the Vitros ECi PRL immunoassay. Based on proficiency testing survey results from the College of American Pathologists there are at least 100 laboratories using the Vitros ECi PRL assay [20]. The ECi platform has previously been implicated to cross-react with macroPRL in a brief report describing measurement of PRL in a single specimen with macroPRL [15]. Compared with other PRL tests, the Vitros ECi PRL immunoassay recognized macroPRL with an affinity close to the mean of all methods tested [15]. The present study provides definitive evidence that the Vitros ECi PRL immunoassay can detect macroPRL.
The regression analysis demonstrates that PEG-precipitation correlates well with GFC to and can be used to identify patients with macroprolactinemia. From a practical perspective, PEG-precipitation is preferable to GFC as it much less labor intensive and a more cost effective method for the detection of macroPRL. For example, a minimum of 20 PRL tests were needed to accurately establish the presence of macroPRL using GFC while PEG-precipitation required only two PRL tests. Other reports have also advocated the use of PEG-precipitation as a surrogate method for macroPRL detection [16, 18, 21]. While a variety of different recovery cutoffs have been used to classify patients as having macroprolactinemia, we used ROC analysis to establish a cutoff and found that a recovery ≤30% after PEG-precipitation was 100% sensitive at a specificity of 95% when using the Vitros ECi PRL immunoassay. Because of the variability between immunoassays, it appears that laboratories using different PRL methods need to establish their own percent recovery cutoff for macroPRL in PEG precipitated samples. The data in this study supports using ROC analysis to determine the optimal cutoff.
Although macroPRL is a potential cause of unnecessary treatment for hyperprolactinemia, we considered whether it could co-exist in patients with clinically important, true hyperprolactinemia. Consistent with this idea, we explored the use of PEG-precipitation as a means to identify patients with true hyperprolactinemia in the presence of macroPRL. Four different approaches were used to classify patients with true hyperprolactinemia as follows: 1) the standard interval (PRL ≤19 ng/mL); 2) a post-PEG modified interval (≤13 ng/mL); 3) Deming regression estimation of monoPRL compared against the standard PRL reference interval; and 4) a ROC analysis determined cutoff (≤11.6 ng/mL). Each of these approaches was compared to the GFC gold standard classification of true hyperprolactinemia (monoPRL + bigPRL >19 ng/mL). The cutoff determined by ROC curve analysis proved to be the most sensitive and efficient means to identify patients as having true hyperprolactinemia (sensitivity 94%, efficiency 90%). Despite its excellent performance, the ROC method has not been previously applied as a method for classification of patients with hyperprolactinemia in the presence of macroprolactin. However, this approach required the analysis of numerous specimens by both GFC and PEG-precipitation which makes it a non-viable option for most clinical laboratories. The post-PEG modified PRL reference interval had the next best performance with slightly lower sensitivity (88%). Given that the performances of the different classification methods were not statistically different, we propose that a post-PEG modified reference range would be the best means to accurately identify patients with true hyperprolactinemia. This supports the conclusions of a recent study advocating the use of a post-PEG modified interval [22].
Despite the extensive literature on the detection of macroPRL, the clinical significance of this PRL variant remains unclear. Several papers have proposed routine laboratory screening for macroPRL to eliminate unnecessary investigation and/or treatment of patients with apparent hyperprolactinemia [8, 21], while other groups recommend further clinical evaluation prior or in addition to assessment for macroPRL [4, 5, 11, 23, 24]. We found that the majority of hyperprolactinemic specimens were collected from patients with known causes of hyperprolactinemia. At the time of collection during the study, we found that none of the patients with macroprolactinemia were being treated with dopamine agonists. This is in spite the fact that none of these patients had previously been screened for macroPRL or identified as macroprolactinemic.
Based on the relationship between the concentration of PRL and percent PRL recovery after PEG-precipitation (Figure 3), no specimens with a PRL concentration >85 ng/mL met the definition of macroprolactinemia. This suggests that when the PRL concentration is >85 ng/mL clinicians should not be concerned with macroPRL, but focus on routine evaluation of hyperprolactinemia. Accordingly, testing for macroPRL would only be useful in patients with a moderately elevated PRL concentration (19–85 ng/mL) in whom no other cause of hyperprolactinemia could be identified. The guidelines of the Pituitary Society for the diagnosis and management of prolactinomas, state that high concentrations of PRL (>150 ng/mL) are usually consistent with an underlying adenoma and it would be reasonable to consider the presence of macroPRL in patients with atypical symptoms with PRL values between 25–150 ng/mL [23]. The findings in this study support that these guidelines are are applicable to users of the Vitros ECi PRL immunoassay.
In this study, neither the prolactin concentration nor the clinical features of patients with macroprolactinemia were statistically different from those with true hyperprolactinemia. These data are consistent with other reports where the incidence of oligomenorrhea, amenorrhea, and galactorrhea were similar between patients with macroprolactinemia and those with true hyperprolactinemia [12, 13]. However, one group has demonstrated that there are significant differences among the same clinical features between these patient groups (hyperprolactinemia vs. macroprolactinemia) [8]. These discrepancies may reflect use of different immunoassays, populations, or definitions of macroPRL. We found that a PRL recovery ≤30% following PEG-precipitation provided the most sensitive and specific definition of macroprolactinemia.
Identification of isolated macroprolactinemia is important because it reduces the incidence of idiopathic hyperprolactinemia. In cases where a potential cause of hyperprolactinemia was not identified (i.e. not attributable to drugs, adenomas, pregnancy, or lactation), measurement of macroPRL explained the etiology in an additional 16% of patients. In this observational study, some patients did not undergo full clinical evaluation for their hyperprolactinemia and the number of unexplained cases may be overrepresented. However, such an overrepresentation would actually increase the importance of detecting macroprolactinemia, as the proportion of “idiopathic” cases explained by macroPRL would increase as other causes are found on evaluation. Based on the data presented here, distinguishing macroprolactinemia from hyperprolactinemia can clarify the diagnosis and direct appropriate therapy. The presence of combined hyperprolactinemia and macroprolactinemia, though uncommon, demonstrates the importance of a normalized reference interval for directing further evaluation and treatment of patients. Patients with elevated monoPRL should undergo routine evaluation of hyperprolactinemia regardless of the presence of macroPRL
In summary, this report provides conclusive evidence that the Vitros ECi PRL immunoassay detects macroPRL. The data show that specimens containing macroPRL can be most accurately determined using a post-PEG recovery cutoff of ≤30%, and that use of an assay-specific modified reference interval can effectively identify specimens with true hyperprolactinemia. Collectively, the findings in this study support limited testing for macroPRL as part of routine evaluation of hyperprolactinemia. Screening for macroPRL would be most beneficial in samples with moderately elevated PRL from patients without identifiable causes for hyperprolactinemia.
Supplementary Material
Acknowledgments
We would like to thank Dr. Virginia Kraus for use of gel filtration equipment and Dr. Mark Brecher for laboratory research space.
Abbreviations
- macroPRL
macroprolactin
- bigPRL
big prolactin
- PRL
prolactin
- monoPRL
monomeric prolactin
- hyperPRL
hyperprolactinemia
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Melmed S, Kleinberg D, Larsen PR, Kronenberg HM, Melmed S, Polonsky KS. Williams Textbook of Endocrinology. Philadelphia: Saunders; 2003. Anterior pituitary; pp. 177–279. [Google Scholar]
- 2.Hattori N, Inagaki C. Anti-prolactin (PRL) autoantibodies cause asymptomatic hyperprolactinemia: bioassay and clearance studies of PRL-immunoglobulin G complex. The Journal of clinical endocrinology and metabolism. 1997;82:3107–3110. doi: 10.1210/jcem.82.9.4250. [DOI] [PubMed] [Google Scholar]
- 3.Olukoga AO, Kane JW. Macroprolactinaemia: validation and application of the polyethylene glycol precipitation test and clinical characterization of the condition. Clinical endocrinology. 1999;51:119–126. doi: 10.1046/j.1365-2265.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 4.Vallette-Kasic S, Morange-Ramos I, Selim A, et al. Macroprolactinemia revisited: a study on 106 patients. The Journal of clinical endocrinology and metabolism. 2002;87:581–588. doi: 10.1210/jcem.87.2.8272. [DOI] [PubMed] [Google Scholar]
- 5.Alfonso A, Rieniets KI, Vigersky RA. Incidence and clinical significance of elevated macroprolactin levels in patients with hyperprolactinemia. Endocrine practice. 2006;12:275–280. doi: 10.4158/EP.12.3.275. [DOI] [PubMed] [Google Scholar]
- 6.Gibney J, Smith TP, McKenna TJ. The impact on clinical practice of routine screening for macroprolactin. The Journal of clinical endocrinology and metabolism. 2005;90:3927–3932. doi: 10.1210/jc.2004-2234. [DOI] [PubMed] [Google Scholar]
- 7.Smith TP, Kavanagh L, Healy ML, McKenna TJ. Technology insight: measuring prolactin in clinical samples. Nature clinical practice. 2007;3:279–289. doi: 10.1038/ncpendmet0447. [DOI] [PubMed] [Google Scholar]
- 8.Suliman AM, Smith TP, Gibney J, McKenna TJ. Frequent misdiagnosis and mismanagement of hyperprolactinemic patients before the introduction of macroprolactin screening: application of a new strict laboratory definition of macroprolactinemia. Clinical chemistry. 2003;49:1504–1509. doi: 10.1373/49.9.1504. [DOI] [PubMed] [Google Scholar]
- 9.Aron DC, Howlett TA. Pituitary incidentalomas. Endocrinology and metabolism clinics of North America. 2000;29:205–221. doi: 10.1016/s0889-8529(05)70124-9. [DOI] [PubMed] [Google Scholar]
- 10.Fahie-Wilson MN, Soule SG. Macroprolactinaemia: contribution to hyperprolactinaemia in a district general hospital and evaluation of a screening test based on precipitation with polyethylene glycol. Annals of clinical biochemistry. 1997;34:252–258. doi: 10.1177/000456329703400305. [DOI] [PubMed] [Google Scholar]
- 11.Hauache OM, Rocha AJ, Maia AC, Maciel RM, Vieira JG. Screening for macroprolactinaemia and pituitary imaging studies. Clinical endocrinology. 2002;57:327–331. doi: 10.1046/j.1365-2265.2002.01586.x. [DOI] [PubMed] [Google Scholar]
- 12.Ellis MJ, Reed MR, Livesey JH. Cross-reactivities of macroprolactin and big-prolactin in three commercial immunoassays for prolactin: a chromatographic analysis. Clinical biochemistry. 2007;40:1285–1290. doi: 10.1016/j.clinbiochem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Fahie-Wilson M, Bieglmayer C, Kratzsch J, et al. Roche Elecsys Prolactin II assay: reactivity with macroprolactin compared with eight commercial assays for prolactin and determination of monomeric prolactin by precipitation with polyethylene glycol. Clinical laboratory. 2007;53:485–492. [PubMed] [Google Scholar]
- 14.Smith TP, Suliman AM, Fahie-Wilson MN, McKenna TJ. Gross variability in the detection of prolactin in sera containing big big prolactin (macroprolactin) by commercial immunoassays. The Journal of clinical endocrinology and metabolism. 2002;87:5410–5415. doi: 10.1210/jc.2001-011943. [DOI] [PubMed] [Google Scholar]
- 15.Fahie-Wilson MN. Detection of macroprolactin causing hyperprolactinemia in commercial assays for prolactin. Clinical chemistry. 2000;46:2022–2023. [PubMed] [Google Scholar]
- 16.Fahie-Wilson MN. Polyethylene glycol precipitation as a screening method for macroprolactinemia. Clinical chemistry. 1999;45:436–437. [PubMed] [Google Scholar]
- 17.Vieira JG, Tachibana TT, Obara LH, Maciel RM. Extensive experience and validation of polyethylene glycol precipitation as a screening method for macroprolactinemia. Clinical chemistry. 1998;44:1758–1759. [PubMed] [Google Scholar]
- 18.Leslie H, Courtney CH, Bell PM, et al. Laboratory and clinical experience in 55 patients with macroprolactinemia identified by a simple polyethylene glycol precipitation method. The Journal of clinical endocrinology and metabolism. 2001;86:2743–2746. doi: 10.1210/jcem.86.6.7521. [DOI] [PubMed] [Google Scholar]
- 19.Fahie-Wilson MN, John R, Ellis AR. Macroprolactin; high molecular mass forms of circulating prolactin. Annals of clinical biochemistry. 2005;42:175–192. doi: 10.1258/0004563053857969. [DOI] [PubMed] [Google Scholar]
- 20.Pathologists CoA, Participant Summary Survey Y-B Ligand (Special). 2008.
- 21.Kavanagh L, McKenna TJ, Fahie-Wilson MN, Gibney J, Smith TP. Specificity and clinical utility of methods for the detection of macroprolactin. Clinical chemistry. 2006;52:1366–1372. doi: 10.1373/clinchem.2005.065854. [DOI] [PubMed] [Google Scholar]
- 22.Beltran L, Fahie-Wilson MN, McKenna TJ, Kavanagh L, Smith TP. Serum total prolactin and monomeric prolactin reference intervals determined by precipitation with polyethylene glycol: evaluation and validation on common immunoassay platforms. Clinical chemistry. 2008;54:1673–1681. doi: 10.1373/clinchem.2008.105312. [DOI] [PubMed] [Google Scholar]
- 23.Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clinical endocrinology. 2006;65:265–273. doi: 10.1111/j.1365-2265.2006.02562.x. [DOI] [PubMed] [Google Scholar]
- 24.Donadio F, Barbieri A, Angioni R, et al. Patients with macroprolactinaemia: clinical and radiological features. European journal of clinical investigation. 2007;37:552–557. doi: 10.1111/j.1365-2362.2007.01823.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.