Abstract
Objective
Individuals with chronic fatigue syndrome (CFS) experience many pain symptoms. The present study examined whether pain and fatigue ratings and pain threshold and tolerance levels for cold pain differed between twins with CFS and their cotwins without CFS.
Design
Cotwin control design to assess cold pain sensitivity, pain, and fatigue in monozygotic twins discordant for CFS.
Patients and Setting
Fifteen twin pairs discordant for CFS recruited from the volunteer Chronic Fatigue Twin Registry at the University of Washington.
Results
Although cold pain threshold and tolerance levels were slightly lower in twins with CFS than their cotwins without CFS, these differences failed to reach statistical significance. Subjective ratings of pain and fatigue at multiple time points during the experimental protocol among twins with CFS were significantly higher than ratings of pain (p = 0.003) and fatigue (p < 0.001) by their cotwins without CFS.
Conclusions
These results, while preliminary, highlight the perceptual and cognitive components to the pain experience in CFS. Future studies should focus on examining the heritability of pain sensitivity and the underlying mechanisms involved in the perception of pain sensitivity in CFS.
Pain complaints are prominent and defining features of chronic fatigue syndrome (CFS). According to the Centers for Disease Control and Prevention definition (1), CFS cannot be diagnosed without at least one pain complaint. CFS also often coexists with fibromyalgia (2), a syndrome of diffuse musculoskeletal pain (3). Both CFS and fibromyalgia may be central nervous system hypersensitivity disorders, characterized by enhanced interoception (4, 5). Interoception is the perception of internal sensory phenomena, especially visceral sensations (6). While the biological underpinnings of interoception are being explored, intriguing neuroimaging studies suggest that the anterior insula may function as the interoceptive cortex and modulate this phenomenon (6).
Although the considerable overlap between CFS and fibromyalgia suggests the biopsychosocial processes underlying these conditions are interrelated (7, 8), few studies have examined the association between pain and fatigue in controlled experimental settings. In one study, cold pain threshold and tolerance levels were similar among CFS patients, non-patient healthy controls, and depressed patients (9). Likewise, in another study, chronically fatigued combat veterans and non-fatigued veterans had comparable responses to a cold pressor test (10). These studies suggest that CFS may not be associated with heightened pain reactivity. However, studies consistent with the enhanced interoception hypothesis have shown that persons with CFS may not accurately interpret body sensations, including pain, and are more aware of their internal physiological state (11, 12).
Our research group has used a co-twin control methodology to study a cohort of monozygotic (MZ) twins discordant for CFS for nearly a decade. This study design, which is a matched-pair comparison that adjusts for genetic and environmental factors not typically considered in typical case-control studies (13), offers a powerful alternative to using healthy or depressed control subjects. It is particularly valuable in studies of pain as the experience of pain and pain sensitivity may be partially genetically controlled (14–16). In this paper, we report data that examined the cold pain threshold and tolerance and subjective ratings of pain and fatigue in MZ twins discordant for CFS. We addressed 2 questions: 1) Do measures of pain threshold and tolerance differ between twins with CFS and their cotwins without CFS? and 2) Do subjective ratings of pain and fatigue during the experimental pain protocol differ between the CFS and their cotwins without CFS? We hypothesized that twin pairs would show similar cold pain threshold and tolerance levels during the cold pressor test, but that cold pressor pain and fatigue ratings would be higher in CFS twins than their cotwins without CFS.
METHODS
Participants
Twins were recruited from the volunteer Chronic Fatigue Twin Registry at the University of Washington (17). From the Registry, 22 pairs of MZ CFS-discordant twins were recruited and completed an intensive 7-day evaluation in Seattle, in which an expert physician confirmed their CFS. These twins participated in protocols to assess the association of CFS with physical, psychological, and subjective illness features (18–26). After the completion of the initial intensive evaluation, the twins were contacted to inquire about their willingness to participate in the second phase and willingness to restrict medications, caffeine, alcohol and travel to Seattle with their cotwin. Fifteen twin pairs agreed to participate in the second intensive study. Medication use was tapered and discontinued by 2 weeks prior to travel to Seattle except for those deemed by primary care physicians inappropriate to discontinue (e.g., thyroid hormones). Twins then returned to Seattle a second time, 3–5 years after their initial evaluation, for another intensive battery of tests to further examine the genetic and environmental factors involved in the development of CFS. Throughout the study, all investigators and technicians were blind to the health status of the twins. Written informed consent was obtained from all twins in accordance with regulations of the University of Washington Human Subjects Office.
Zygosity
Monozygosity was initially determined using previously validated self-report methods (27, 28), then confirmed with analysis of restriction fragment length polymorphisms. DNA samples were extracted and digested with the restriction endonuclease Hae III. The restriction fragments were separated by molecular size in agarose gel, Southern blotted onto nylon membrane, and hybridized with a variable number of tandem repeat probes. Following six probes, the probability of monozygosity can be ascertained with 99.9% certainty (29).
Cold Pressor Test
The cold pressor test is a widely used measure of acute stress with well-established methodology (30–32). The cold-pressor apparatus is a container divided into 2 compartments by a wire screen. Ice cubes are placed in the smaller part and water in the larger part. The water temperature is maintained at 1–2°C via a submerged pump that circulates the water around the ice. Twins were instructed to place their non-dominant hand and forearm in a cradle in the water compartment and to inform the research assistant when the cold sensation had become pain (threshold) and when the pain was no longer tolerable (tolerance). Twins were asked to withdraw their arm at tolerance or 5 minutes, whichever comes first. Time in seconds was used as a measure of threshold and tolerance.
Ratings of Pain and Fatigue
Immediately before beginning the cold pressor test (“pre-test”), participants rated their pain and fatigue on a 100 mm visual analog scale (VAS), anchored at 0 (no pain/fatigue) and 100 (worst pain/fatigue ever). Pain and fatigue were rated again at the threshold and tolerance points, and again 15 minutes after reaching tolerance (“post-test”). In addition, at the beginning of the day before starting any tests, each twin rated his or her fatigue and pain levels on similar 100 mm VAS. These daily ratings were used as adjustment variables in statistical modeling.
Statistical analyses
We calculated descriptive means and standard deviations for all measures. In order to examine the difference in threshold and tolerance, we used paired t-tests to compare mean seconds to threshold and mean seconds to tolerance in the twin pairs. We used mixed effects linear regression models to compare means for the twins adjusting for initial fatigue and pain levels from the beginning of the day before beginning any tests. These models included a random effect for twin pair and a fixed effect for CFS. To evaluate the ratings of pain and fatigue, we examined the main effect of CFS in mixed effects linear regression models for the four longitudinal pain and fatigue ratings taken immediately before, during (threshold and tolerance points), and 15 minutes after the cold pressor test. These models included a random effect for twin pair and assumed unstructured correlation between the four outcome measures. We adjusted for the initial fatigue and pain ratings taken early in the day, and for time point (pre-test, threshold, tolerance, post-test) as a categorical covariate. We considered an alpha error rate of 0.05 as the threshold for statistical significance. Analyses were conducted with the SAS version 9.0 statistical software package (33).
RESULTS
Demographic and Descriptive Characteristics
On average, twins were 45 years old at the time of testing and had 14 years of education. All twins were White and 93% were female. The fatigued twins reported an average fatigue duration of 9.5 years.
Cold Pain Threshold and Tolerance
Table 1 presents the samples’ pain and fatigue characteristics. Paired t-tests failed to show statistically significant differences between twins with and without CFS in mean seconds to threshold (p = 0.55) or to tolerance (p = 0.13). These results persisted after adjusting for initial daily fatigue and pain ratings in the mixed effects linear regression model.
Table 1.
Pain and fatigue characteristics of monozygotic twins discordant for chronic fatigue syndrome.
| CFS Twins1 | Non-CFS Twins | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Pain threshold, seconds | 8.1 (6.6) | 9.6 (6.4) |
| Pain tolerance, seconds | 23.1 (6.5) | 31.7 (17.5) |
| Cold pressor fatigue rating2 | ||
| Pre-test | 52.3 (19.5) | 16.8 (8.3) |
| Threshold | 57.7 (22.2) | 14.8 (8.3) |
| Tolerance | 68.2 (25.2) | 18.3 (10.8) |
| Post-test | 59.4 (23.1) | 11.5 (8.5) |
| Cold pressor pain rating2 | ||
| Pre-test | 39.3 (24.8) | 6.1 (5.1) |
| Threshold | 63.1 (20.4) | 34.0 (16.4) |
| Tolerance | 83.1 (17.3) | 65.1 (16.8) |
| Post-test | 48.1 (25.9) | 13.3 (22.3) |
| Daily fatigue rating2 | 46.2 (30.3) | 13.3 (9.5) |
| Daily pain rating2 | 43.7 (32.5) | 11.1 (17.2) |
CFS = chronic fatigue syndrome;
Visual analog rating scale of 0–100 mm, with 0 = no pain/fatigue and 100 = worst pain/fatigue ever.
Ratings of Pain and Fatigue
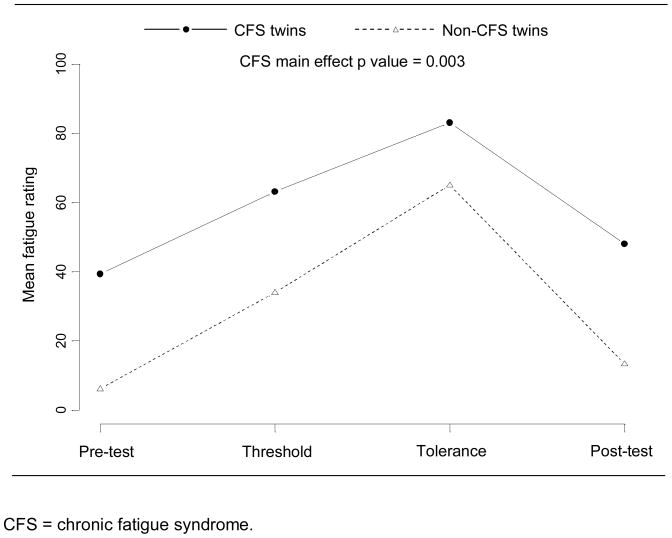
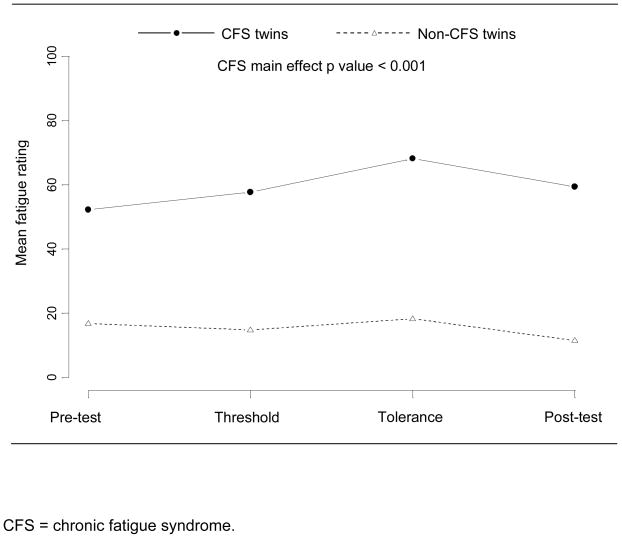
The linear regression models showed a strong association between CFS status and VAS pain (p = 0.003) and fatigue (p < 0.001) ratings before, during, and after the cold stressor test. This association persisted for both pain and fatigue ratings after adjusting for time point and initial daily fatigue and pain levels. Figures 1 and 2 present the unadjusted mean values of pain and fatigue over the four time points for the twins with and without CFS. The VAS pain ratings went up during the cold stressor test and then dropped at the 15 minute post-test time point for both groups, but the twins with CFS consistently rated their pain higher than the twins without CFS at all time points. VAS fatigue ratings did not change substantially for either group over the course of the cold pressor test nor at the 15 minute post-test time point, but again twins with CFS consistently reported higher fatigue levels than their cotwins without CFS.
Figure 1.
Mean visual analog scale pain ratings for monozygotic twins discordant for chronic fatigue syndrome, before, during, and after the cold pressor test.
Figure 2.
Mean visual analog scale fatigue ratings for monozygotic twins discordant for chronic fatigue syndrome, before, during, and after the cold pressor test.
DISCUSSION
We examined cold pain sensitivity in a sample of MZ twins discordant for CFS and failed to detect differences on cold pressor pain threshold and tolerance levels between twins with and without CFS. This finding suggests that there are no alterations in cold pain sensitivity in those with CFS. Prior research has produced similar findings for cold sensitivity (5, 6). Additionally, we found that even after controlling for premorbid (beginning of day) pain and fatigue ratings, CFS twins reported significantly greater pain and fatigue in response to the aversive sensory stimulation of the cold pressor test than their cotwins without CFS. These elevated ratings, despite lack of differences in pain threshold and tolerance levels, could be in part attributed to cognitive or perceptual differences between twins with and without CFS. Persons with CFS may experience a heightened perception of pain and fatigue perhaps due to the severe underlying fatigue. For example, individuals with CFS have been shown to experience somatic perceptual distortions, including misinterpretations of the nature and gravity of body sensations such as pain (34), with resultant physical and psychosocial dysfunction (35). Alternatively, failure to discover differences between twins with and without CFS may be attributable to inadequacies of the cold pressor paradigm. More advanced techniques for exploring abnormalities in sensory systems, such as quantitative sensory testing (QST; 36), have been used in a number of pain conditions (37, 38). With regard to future research, QST may have the potential for more effectively characterizing differences in somatosensory profiles between persons with versus without CFS than the cold pressor task.
These findings also are consistent with the theory of enhanced interoception in CFS. A central nervous system hypersensitivity disorder would be consistent with subjective pain sensitivity, sensitivity to exercise, and other neuropsychological disturbances that are often reported by patients with CFS (7). In this regard, the primary interoceptive representation in the anterior insula cortex engenders distinct bodily sensations including pain, temperature, itch, sensual touch, muscular and visceral sensations, and vasomotor activity (39). Neuroimaging studies relevant to interoception and the feeling of self are rapidly accumulating and underscore the biological underpinnings of this phenomenon. Functional neuroimaging during performance of sensory tasks may have the potential for further illuminating the neurophysiological processes underlying CFS. Recent functional magnetic resonance imaging (fMRI) studies have suggested that persons with CFS evidence abnormal neuroimaging during motor (40), auditory (41), and visual (42) tasks. A potentially fruitful area of future research is the use of fMRI in relation to standardized laboratory pain tasks.
While this study failed to find differences between twins in terms of the cold pressor measures of threshold and tolerance, both twins with and without CFS showed lower cold pressor tolerance than those reported previously for other populations (32–35). In the present sample, cold pressor tolerance was 23.1 seconds among twins with CFS, and 31.7 seconds for twins without CFS. This contrasts with cold pressor tolerance values ranging from 54.6–132 seconds reported for community samples (43, 44), or 90.9–99.7 seconds among individuals with chronic pain (45, 46). Minute differences in cold pressor methodology could account for the differences in cold pressor tolerance levels found in this study in comparison with previous findings (47). Alternately, the lowered cold pain tolerance in these cotwins could be explained by the influence of heritable factors on this index of pain sensitivity (14–16).
Studies of illness discordant twins who were raised together control for genetic and familial effects (13). Thus, twin studies are particularly useful in identifying the behavioral, clinical, or laboratory factors most strongly associated with CFS. Additionally, studies of MZ and DZ twin pairs are useful in estimating the unique genetic and environmental contribution to an illness. Recent twin studies have shown that chronically fatiguing illnesses are, in part, under genetic control (48–50). Additionally, several genes have been associated with pain sensitivity (14, 16), and twin studies have addressed the heritability of painful conditions. For example, a large population-based study of twins found an estimated genetic effect of 44% for neck pain, which gradually decreased with age (51). Alternately, the only twin study of pain sensitivity in which measurement of pressure pain threshold was obtained from adult twin pairs, concluded that shared familial environment was a significant determinant of pain sensitivity (52). Findings from this study, however, should be interpreted cautiously since twin pairs underwent the experimental protocol together, increasing the possibility of inflated pain threshold correlations among twins. Nonetheless, taken together, these findings underscore the importance of genetic and familial factors in understanding the experience of pain in those with CFS.
The present study had several limitations. First, we examined a small sample of 15 twin pairs, raising questions about the generalizability of our findings, and the adequacy of statistical power to detect differences between twins with and without CFS. The sample size was determined by logistical constraints, and could not be based on a priori power calculations. Thus, our findings are preliminary and should be replicated. Second, we did not include a healthy control group in order to directly compare findings from CFS-discordant twins with those of community samples. Finally, we relied on a single sensory stressor within one domain, which may have not been adequate in identifying the range of perceptual or cognitive alterations associated with CFS. Prior researchers have found that persons with CFS may be distinguished by unusually blunt patterns of reactivity to mental, but not sensory, stressors (10).
In summary, we found that despite similarities in threshold and tolerance levels, our twins with CFS experienced greater subjective pain and fatigue in response to the cold pressor test. CFS may be associated with a heightened perception of pain and fatigue in reaction to stressful or painful situations. Our findings also highlight the possible role of genetic and familial factors in the association between CFS and pain sensitivity. Future studies should focus on examining the heritability of pain sensitivity and the underlying mechanisms involved in the perception of pain sensitivity in this population.
Acknowledgments
This work was supported by grant U19AI-038429 from the National Institutes of Health (Dr. Buchwald, PI).
References
- 1.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–59. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald D, Garrity D. Comparison of patients with chronic fatigue syndrome, fibromyalgia, and multiple chemical sensitivities. Arch Intern Med. 1994;154:2049–53. [PubMed] [Google Scholar]
- 3.Wolfe F, Smythe H, Yunus M, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 4.Cameron OG, Minoshima S. Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosom Med. 2002;64:851–861. doi: 10.1097/01.psy.0000038939.33335.32. [DOI] [PubMed] [Google Scholar]
- 5.White PD. What causes chronic fatigue syndrome? BMJ. 2004;329:928–929. doi: 10.1136/bmj.329.7472.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig AD. Human feelings: why are some more aware than others? Trends in Cognitive Sciences. 2004;8:239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? Lancet. 1999;354:936–39. doi: 10.1016/S0140-6736(98)08320-2. [DOI] [PubMed] [Google Scholar]
- 8.Hudson JI, Pope HG., Jr Affective spectrum disorder: does antidepressant response identify a family of disorders with a common pathophysiology? Am J Psychiatry. 1990;147:552–64. doi: 10.1176/ajp.147.5.552. [DOI] [PubMed] [Google Scholar]
- 9.Schmaling KB, Hamilos DL, DiClementi JD, Jones JF. Pain perception in chronic fatigue syndrome. J Chronic Fatigue Syndr. 1998;4:13–22. [Google Scholar]
- 10.Peckerman A, LaManca JJ, Smith SL, et al. Cardiovascular stress responses and their relation to symptoms in Gulf War veterans with fatiguing illness. Psychosom Med. 2000;62:509–16. doi: 10.1097/00006842-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry. 2003;160:221–36. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 12.Moss-Morris R, Chalder T. Illness perceptions and levels of disability in patients with chronic fatigue syndrome and rheumatoid arthritis. J Psychosom Res. 2003;55:305–308. doi: 10.1016/s0022-3999(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 13.Hubrec Z, Robinette CD. The study of human twins in medical research. N Engl J Med. 1984;310:435–41. doi: 10.1056/NEJM198402163100706. [DOI] [PubMed] [Google Scholar]
- 14.Mogil JS, Miermeister F, Seifer F, et al. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci. 2005;102:12938–43. doi: 10.1073/pnas.0503264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogil JS, Sternberg WF, Marek P, Sadowski B, Belknap JK, Liebeskind JC. The genetics of pain and pain inhibition. Proc Natl Acad Sci. 1996;93:3048–55. doi: 10.1073/pnas.93.7.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fillingim RB, Kaplan L, Staud R, et al. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6:159–67. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Buchwald D, Herrell R, Ashton S, Belcourt M, Schmaling K, Goldberg J. The Chronic Fatigue Twin Registry: method of construction, composition, and zygosity assignment. Twin Res. 1999;2:203–11. doi: 10.1375/136905299320565870. [DOI] [PubMed] [Google Scholar]
- 18.Watson NF, Jacobsen C, Goldberg J, Kapur V, Buchwald D. Subjective and objective sleepiness in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2004;27:973–77. doi: 10.1093/sleep/27.5.973. [DOI] [PubMed] [Google Scholar]
- 19.Mahurin RK, Claypoole KH, Goldberg JH, Arguelles LM, Ashton S, Buchwald D. Cognitive processing in monozygotic twins discordant for chronic fatigue syndrome. Neuropsychology. 2004;18:232–39. doi: 10.1037/0894-4105.18.2.232. [DOI] [PubMed] [Google Scholar]
- 20.Ball N, Buchwald DS, Schmidt D, Goldberg J, Ashton S, Armitage R. Monozygotic twins discordant for chronic fatigue syndrome: Objective measures of sleep. J Psychosom Res. 2004;56:207–12. doi: 10.1016/S0022-3999(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 21.Watson NF, Kapur V, Arguelles LM, et al. Comparison of subjective and objective measures of insomnia in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2003;26:324–28. doi: 10.1093/sleep/26.3.324. [DOI] [PubMed] [Google Scholar]
- 22.Koelle DM, Barcy S, Huang ML, et al. Markers of viral infection in monozygotic twins discordant for chronic fatigue syndrome. Clin Infect Dis. 2002;35:518–25. doi: 10.1086/341774. [DOI] [PubMed] [Google Scholar]
- 23.Sabath DE, Barcy S, Koelle DM, Zeh J, Ashton S, Buchwald D. Cellular immunity in monozygotic twins discordant for chronic fatigue syndrome. J Infect Dis. 2002;185:828–32. doi: 10.1086/339194. [DOI] [PubMed] [Google Scholar]
- 24.Lewis DH, Mayberg HS, Fischer ME, et al. Monozygotic twins discordant for chronic fatigue syndrome: regional cerebral blood flow SPECT. Radiology. 2001;219:766–73. doi: 10.1148/radiology.219.3.r01jn18766. [DOI] [PubMed] [Google Scholar]
- 25.Claypoole K, Mahurin R, Fischer ME, et al. Cognitive compromise following exercise in monozygotic twins discordant for chronic fatigue syndrome: fact or artifact? Appl Neuropsychology. 2001;8:31–40. doi: 10.1207/S15324826AN0801_5. [DOI] [PubMed] [Google Scholar]
- 26.Poole J, Herrell R, Ashton S, Goldberg J, Buchwald D. Results of isoproterenol tilt table testing in monozygotic twins discordant for chronic fatigue syndrome. Arch Intern Med. 2000;160:3461–8. doi: 10.1001/archinte.160.22.3461. [DOI] [PubMed] [Google Scholar]
- 27.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam era twin registry: an approach using questionnaires. Clin Genet. 1989;35:423–32. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 28.Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genetics Medicine. 1979;28:225–36. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- 29.Keith L, Machin G. Zygosity testing: current status and evolving issues. J Reprod Med. 1997;42:699–707. [PubMed] [Google Scholar]
- 30.Hines EA, Brown JB. The cold pressor test for measuring the reactibility of the blood pressure: Data concerning 571 normal and hypertensive subjects. The Am Heart J. 1936;11:1–9. [Google Scholar]
- 31.Patil PG, Apfelbaum JL, Zacny JP. Effects of a cold-water stressor on psychomotor and cognitive functioning in humans. Physiol Behav. 1995;58:1281–86. doi: 10.1016/0031-9384(95)02071-3. [DOI] [PubMed] [Google Scholar]
- 32.Turk DC, Meichenbaum D, Genest M. Pain and behavioral medicine: A cognitive-behavioral perspective. NY: Guilford; 1983. [Google Scholar]
- 33.SAS Institute Inc. SAS version 9.0 statistical software package. SAS Institute Inc; Cary, NC, USA: [Google Scholar]
- 34.Kim H, Neubert J, Rowan J, Brahim J, Iadarola M, Dionne R. Comparison of experimental and acute clinical pain responses in humans as pain phenotypes. J Pain. 2004;5:377–84. doi: 10.1016/j.jpain.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Moss-Morris R, Petrie KJ. Cognitive distortions of somatic experiences: revision and validation of a measure. J Psychosom Res. 1997;43:293–306. doi: 10.1016/s0022-3999(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 36.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain. doi: 10.1016/j.pain.2006.01.041. In press. [DOI] [PubMed] [Google Scholar]
- 37.Lang PM, Schober GM, Rolke R, Wagner S, Hilge R, Offenbacher M, Treede RD, Hoffmann U, Irnich D. Sensory neuropathy and signs of central sensitization in patients with peripheral arterial disease. Pain. doi: 10.1016/j.pain.2006.04.011. in press. [DOI] [PubMed] [Google Scholar]
- 38.Boivie J. Central pain and the role of quantitative sensory testing (QST) in research and diagnosis. Eur J Pain. 2003;7(4):339–43. doi: 10.1016/S1090-3801(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 39.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 40.de Lange FP, Kalkman JS, Bleijenberg G, Hagoort P, van der Werf SP, van der Meer JW, Toni I. Neural correlates of the chronic fatigue syndrome--an fMRI study. Brain. 2004;27:1948–57. doi: 10.1093/brain/awh225. [DOI] [PubMed] [Google Scholar]
- 41.Lange G, Steffener J, Cook DB, Bly BM, Christodoulou C, Liu WC, Deluca J, Natelson BH. Objective evidence of cognitive complaints in Chronic Fatigue Syndrome: a BOLD fMRI study of verbal working memory. Neuroimage. 2005;26:513–24. doi: 10.1016/j.neuroimage.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka M, Sadato N, Okada T, Mizuno K, Sasabe T, Tanabe HC, Saito DN, Onoe H, Kuratsune H, Watanabe Y. Reduced responsiveness is an essential feature of chronic fatigue syndrome: a fMRI study. BMC Neurol. 2006;6:1471–1486. doi: 10.1186/1471-2377-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heijmans MJ. Coping and adaptive outcomes in chronic fatigue syndrome: importance of illness cognitions. J Psychosom Res. 1998:39–51. doi: 10.1016/s0022-3999(97)00265-1. [DOI] [PubMed] [Google Scholar]
- 44.Lowery D, Fillingim RB, Wright RA. Sex differences and incentive effects on perceptual and cardiovascular responses to cold pressor pain. Psychosom Med. 2003;65:284–91. doi: 10.1097/01.psy.0000033127.11561.78. [DOI] [PubMed] [Google Scholar]
- 45.Greenberg J, Burns JW. Pain anxiety among chronic pain patients: Specific phobia or manifestation of anxiety sensitivity? Behav Res Ther. 2003;41:223–40. doi: 10.1016/s0005-7967(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 46.Johnson MH, Petrie SM. The effects of distraction on exercise and cold pressor tolerance for chronic low back pain sufferers. Pain. 1997;69:43–48. doi: 10.1016/s0304-3959(96)03272-1. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell LA, MacDonald RA, Brodie EE. Temperature and the cold pressor test. J Pain. 2004;5:233–38. doi: 10.1016/j.jpain.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Buchwald D, Herrell R, Ashton S, Belcourt M, Schmaling K, Sullivan P, Neale M, Goldberg J. A twin study of chronic fatigue. Psychosom Med. 2001;63:936–943. doi: 10.1097/00006842-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Hickie IB, Bansa AS, Kirk KM, Lloyd AR, Martin NG. A twin study of the etiology of prolonged fatigue and immune activation. Twin Res. 2001;4:94–102. doi: 10.1375/1369052012209. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan PF, Evengard B, Jacks A, Pedersen NL. Twin analyses of chronic fatigue in a Swedish national sample. Psychol Med. 2005;35:1327–1336. doi: 10.1017/S0033291705005222. [DOI] [PubMed] [Google Scholar]
- 51.Fejer R, Hartvigsen J, Kyvik KO. Heritability of neck pain: a population-based study of 33,794 Danish twins. Rheumatol. doi: 10.1093/rheumatology/kei224. in press. [DOI] [PubMed] [Google Scholar]
- 52.MacGregor AJ, Griffiths GO, Baker J, Spector TD. Determinants of pressure pain threshold in adult twins: evidence that shared environmental influences predominate. Pain. 1997;73:253–57. doi: 10.1016/S0304-3959(97)00101-2. [DOI] [PubMed] [Google Scholar]