Abstract
Rationale
The recently discovered PHLPP-1 (PH domain leucine-rich repeat protein phosphatase-1) selectively dephosphorylates Akt at Ser473 and terminates Akt signaling in cancer cells. The regulatory role of PHLPP-1 in the heart has not been considered.
Objective
To test the hypothesis that blockade/inhibition of PHLPP-1 could constitute a novel way to enhance Akt signals and provide cardioprotection.
Methods and Results
PHLPP-1 is expressed in neonatal rat ventricular myocytes (NRVMs) and in adult mouse ventricular myocytes (AMVMs). PHLPP-1 knockdown by small interfering RNA significantly enhances phosphorylation of Akt (p-Akt) at Ser473, but not at Thr308, in NRVMs stimulated with leukemia inhibitory factor (LIF). The increased phosphorylation is accompanied by greater Akt catalytic activity. PHLPP-1 knockdown enhances LIF-mediated cardioprotection against doxorubicin and also protects cardiomyocytes against H2O2. Direct Akt effects at mitochondria have been implicated in cardioprotection and mitochondria/cytosol fractionation revealed a significant enrichment of PHLPP-1 at mitochondria. The ability of PHLPP-1 knockdown to potentiate LIF-mediated increases in p-Akt at mitochondria and an accompanying increase in mitochondrial hexokinase-II was demonstrated. We generated PHLPP-1 knockout (KO) mice and demonstrate that AMVMs isolated from KO mice show potentiated p-Akt at Ser473 in response to agonists. When isolated perfused hearts are subjected to ischemia/reperfusion, p-Akt in whole-heart homogenates and in the mitochondrial fraction is significantly increased. Additionally in PHLPP-1 KO hearts, the increase in p-Akt elicited by ischemia/reperfusion is potentiated and, concomitantly, infarct size is significantly reduced.
Conclusions
These results implicate PHLPP-1 as an endogenous negative regulator of Akt activity and cell survival in the heart.
Keywords: Akt, PHLPP, phosphatase, heart, protection
Numerous studies have demonstrated that activation of Akt contributes to the cardioprotective effects of receptors tyrosine kinases,1,2 glycoprotein 130–linked receptors,3–5 and G protein–coupled receptors.6,7 These receptors activate phosphatidylinositol 3-kinase (PI3K) and the resultant increase in phosphatidylinositol (3,4,5) triphosphate (PIP3) levels drives Akt translocation to the plasma membrane. Akt is subsequently activated through phosphorylation at Thr308 by the upstream kinase phosphoinositide-dependent kinase 1 (PDK1) and phosphorylation at Ser473 by a mechanism that depends on both TORC2 and the intrinsic catalytic activity of Akt.8,9
The lipid phosphatase PTEN, which dephosphorylates PIP3 to PIP2, has been shown to limit Akt activation by decreasing PIP3. Deletion or mutation of PTEN is observed in many types of tumors and is accompanied by high Akt activity.10 A recent study identified a PH domain-only protein, PHLDA3, that competes with the PH domain of Akt for binding of PIP3.11 These molecules regulate the activation of Akt via various mechanisms but far less is known about mechanisms involved in terminating Akt activity by its dephosphorylation.
Protein phosphatase (PP)2A has been shown to dephosphorylate Akt at Thr308 and/or Ser473 in noncardiac cells.12,13 A pharmacological study also suggests that in retina PP2B (calcineurin) can dephosphorylate Akt at both sites.14 A more specific Akt-directed novel PP2C family member protein phosphatase, PHLPP (PH domain leucine-rich repeat protein phosphatase),15–17 has been recently identified. Two isoforms of PHLPP, PHLPP-1 and PHLPP-2, have been shown to selectively dephosphorylate the hydrophobic motif of Akt (Ser473), terminating Akt signaling.15,16 PHLPP levels are markedly reduced in several cancer cell lines, resulting in elevated Akt activation.17 Conversely heterologous expression of PHLPP in cancer cells can prevent Akt activation and promote apoptotic death.15,16
In cardiac myocytes, overexpression of PTEN has been shown to be proapoptotic, whereas genetic deletion of PTEN rescues hearts from ischemia/reperfusion (I/R) injury.18,19 These data support observations made in noncardiac cells which demonstrate that modulation of Akt activity regulates cell survival. A recent article showed that either PP2A or PP2B (calcineurin) can dephosphorylate Akt and thereby, regulate insulin signaling in cardiomyocytes.20 It has been generally believed that phosphatases such as PP2A and PP2B have poor substrate selectivity, eliciting dephosphorylation of diverse target molecules. In contrast, PHLPP has been reported to be a selective Akt phosphatase.15–17 In this study, we demonstrate a role for endogenous PHLPP-1 in regulation of cardiomyocyte Akt activity and survival in vitro and in vivo.
Methods
PHLPP-1 knockout (KO) mice were generated in the C57BL/6 strain as described previously.21 All mice used in the present study were male at 8 to 10 weeks of age. All procedures were performed in accordance with NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. To knockdown PHLPP-1, predesigned PHLPP-1 ON-TARGETplus small interfering (si)RNA for rat and control siRNA were purchased from Thermo Scientific. NRVMs were transfected with siRNA using DharmaFECT-I transfection reagent (Thermo Scientific) based on the instructions of the manufacturer, with additional details in the expanded Methods section (Online Data Supplement, available at http://circres.ahajournals.org). Results are reported as averages±SEM. Statistical significance was determined using ANOVA followed by the Tukey post hoc test. P<0.05 was considered statistically significant. For additional details regarding the methods used, see the Online Data Supplement.
Results
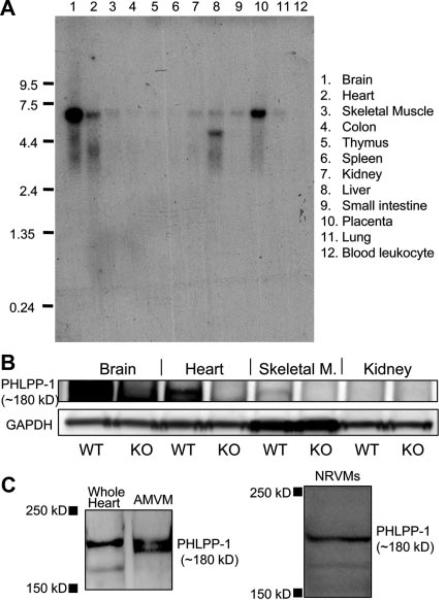
Northern blotting of various human tissues indicates that PHLPP-1 mRNA is most highly expressed in the brain, as previously reported,22 but is also present at significant levels in the heart (Figure 1A). To determine that PHLPP-1 protein is expressed in mouse, we evaluated PHLPP-1 expression by Western blotting in adult mouse tissues from wild-type (WT) and PHLPP-1 KO mice. PHLPP-1 appeared at the expected molecular weight of ≈180 kDa and was highly expressed in the brain. PHLPP-1 was also clearly detected in the heart (Figure 1B), in isolated adult mouse ventricular myocytes (AMVMs) and in neonatal rat ventricular myocytes (NRVMs) (Figure 1C). PHLPP-2 mRNA was also present in isolated AMVMs. PHLPP-2 knockdown in NRVMs did not significantly affect phosphorylation of Akt at Ser473 (Online Figure I); thus, we focused our attention on examining the regulatory role of PHLPP-1 in cardiomyocytes.
Figure 1. Expression of PHLPP-1 in heart and cardiomyocytes.
A, Northern blot analysis of PHLPP-1 expression in human tissues. B, Western blot of PHLPP-1 in WT and PHLPP-1 knockout mice tissues. C, Western blot of PHLPP-1 in adult mouse ventricle homogenate, in isolated AMVMs and in NRVMs.
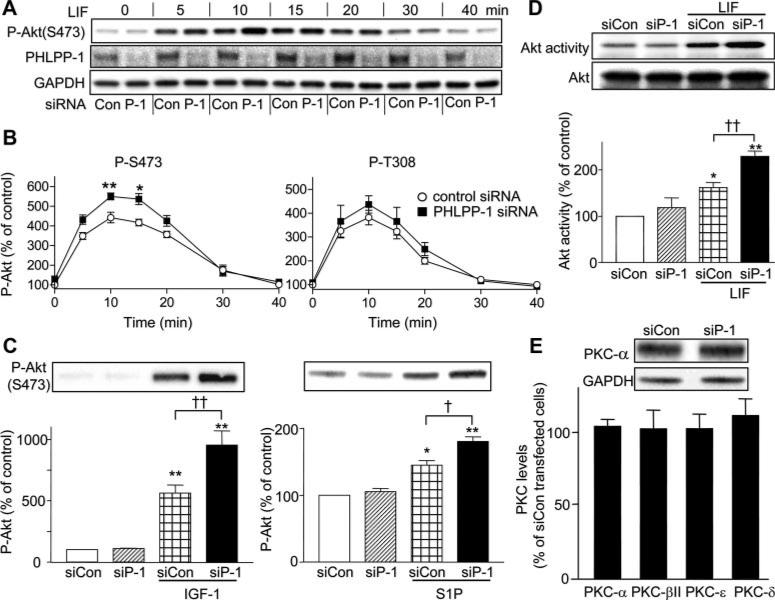
To determine whether PHLPP-1 regulates Akt phosphorylation in cardiomyocytes, PHLPP-1 expression was inhibited using siRNA. Significant knockdown of PHLPP-1 was achieved by siRNA treatment of NRVMs (Figure 2A). To evaluate the functional effect of PHLPP-1 knockdown, NRVMs were stimulated with leukemia inhibitory factor (LIF) and levels of Akt phosphorylation at both Ser473 and Thr308 were examined (Figure 2B). LIF treatment elicited a greater than 3- to 4-fold peak increase in phosphorylation of Akt at Ser473 and Thr308 at 10 minutes and this declined to basal levels by 40 minutes. The magnitude of the response monitored by Akt Ser473 phosphorylation was significantly greater in cardiomyocytes treated with PHLPP-1 siRNA. Notably, LIF-induced phosphorylation of Akt at Thr308 was not significantly changed by the knockdown of PHLPP-1. PHLPP-1 knockdown-mediated potentiation of Akt phosphorylation at Ser473 was also observed in NRVMs stimulated with insulin-like growth factor (IGF)-1 or sphingosine-1-phosphate (S1P) (Figure 2C), although T308 phosphorylation was not potentiated (data not shown). These data suggest that endogenous PHLPP-1 selectively regulates the extent of Akt phosphorylation at Ser473 in response to agonist, consistent with the initial study demonstrating that PHLPP-1 phosphatase activity is selective for Akt dephosphorylation at Ser473.15
Figure 2. PHLPP-1 knockdown potentiates agonist-induced Akt phosphorylation at Ser473 and Akt catalytic activity in NRVMs.
NRVMs transfected with control siRNA (siCon) or PHLPP-1 siRNA (siP-1) were treated with agonists, harvested, and subjected to Western blotting. A, Representative blots of LIF-induced (10 nmol/L) phosphorylated Akt (S473), PHLPP-1, and GAPDH (loading control). B, Quantitative analysis of LIF-induced phosphorylation of Akt at S473 and at T308 in control- or PHLPP-1 siRNA–treated NRVMs (n=7). C, PHLPP-1 knockdown enhances IGF-1–mediated (1 nmol/L, 5 minutes) or S1P-mediated (1 μmol/L, 5 minutes) Akt phosphorylation at Ser473. D, Akt catalytic activity was enhanced by PHLPP-1 knockdown (n=7). E, Expression of PKCs (α, βII, ε, and δ) in control and PHLPP-1 siRNA–treated NRVMs (n=5). *P<0.05, **P<0.01 vs control; †P<0.05; ††P<0.01 vs control+agonist.
The effect of phosphorylation of the Ser473 regulatory sites on Akt function is controversial. Accordingly, we assessed the effect of PHLPP-1 knockdown on Akt catalytic activity in our system using an in vitro kinase assay with a glycogen synthase kinase (GSK)3-α/β peptide as substrate. As shown in Figure 2D, LIF increased Akt activity to a significantly greater extent in PHLPP-1 siRNA–treated cells compared to control cells. Treatment with PHLPP-1 siRNA did not affect expression of total Akt or of gp130, the receptor for LIF (data not shown). Thus, the relative increase in LIF stimulated Akt activity in PHLPP-1 knockdown cells compared to control cells appears to result from the increase in Ser473 phosphorylation rather than upregulation of total Akt or the receptor. It has been demonstrated that PHLPP-1 deletion increased levels of conventional and novel protein kinase (PK)Cs (α, βII, and ε) in non cardiac cells.23 However, there were no significant changes in PKCα, -βII, -δ, and -ε in NRVMs transfected with PHLPP-1 siRNA (Figure 2E).
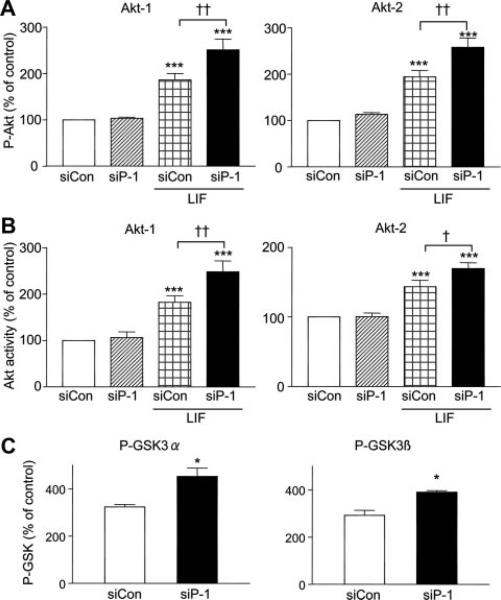
Previous work from the Newton laboratory demonstrated that in cancer cell lines PHLPP-1 has selectivity for Akt-2 versus Akt-1.16 To determine whether PHLPP-1 differentially dephosphorylates Akt-1 and Akt-2 in cardiomyocytes, we immunoprecipitated either the Akt-1 or Akt-2 isoform from cells stimulated with LIF before Western blotting with P-Ser473 antibody (Figure 3A). Unexpectedly, PHLPP-1 knockdown induced comparable enhancement in Ser473 phosphorylation of Akt-1 and Akt-2, indicating that in cardiomyocytes PHLPP-1 dephosphorylates both Akt isoforms. The kinase activity assay likewise demonstrated that LIF-induced increases in both Akt-1 and Akt-2 catalytic activities are enhanced by PHLPP-1 knockdown (Figure 3B). It has also been reported that Akt-1 and Akt-2 have different substrate specificity, with GSK-3α preferentially phosphorylated by Akt-2 in noncardiac cells.16 We observed, however, that LIF-induced phosphorylation of GSK-3α and -3β were both significantly enhanced by PHLPP-1 knockdown (Figure 3C). These data indicate that PHLPP-1 can affect activation of and substrate phosphorylation by both of the major cardiac Akt isoforms.
Figure 3. PHLPP-1 knockdown equally potentiates Akt-1 and Akt-2 signals.
Akt-1 or Akt-2 were immunoprecipitated from NRVMs transfected with control siRNA (siCon) or PHLPP-1 siRNA (siP-1) and stimulated by 10 nmol/L LIF for 10 minutes. A, Potentiation of phosphorylation of Akt-1 and Akt-2 by PHLPP-1 knockdown (n=8). B, Potentiation of Akt-1 and Akt-2 kinase catalytic activities by PHLPP-1 knockdown (n=5). C, LIF-induced phosphorylation of GSK-3α and GSK-3β were potentiated in PHLPP-1 siRNA–treated cardiomyocytes (n=6 to 7). *P<0.05, ***P<0.001 vs control; †P<0.05, ††P<0.01 vs control+LIF.
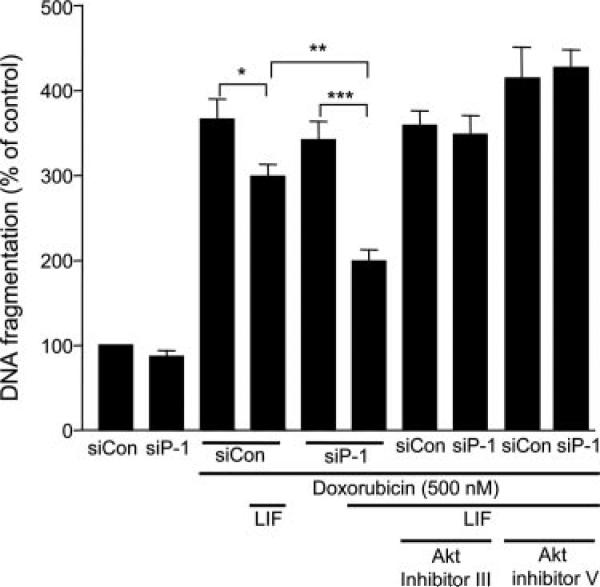
To determine whether the increased Akt activity provided by PHLPP-1 knockdown translates into enhanced protection of cardiomyocytes, cells were transfected with control or PHLPP-1 siRNA and treated with doxorubicin, a chemotherapeutic agent known to exert significant cardiotoxic effects. Several published reports have demonstrated that doxorubicin-induced cell death is prevented by agonists that stimulate Akt, including LIF4,24; thus, we determined whether PHLPP-1 knockdown enhances LIF-mediated protection against doxorubicin. A robust apoptotic response was induced by 18-hour treatment with 500 nmol/L doxorubicin, and LIF treatment was protective (Figure 4). The LIF-mediated protection was enhanced significantly in cardiomyocytes in which PHLPP-1 was knocked down. In the presence of Akt inhibition, the protective effects of LIF treatment and PHLPP-1 knockdown were reversed, indicating that the observed effects of LIF and PHLPP-1 siRNA treatment are mediated through changes in Akt activity. These data provide evidence that the enhanced Akt phosphorylation and activity achieved by PHLPP-1 knockdown is functionally important in increasing the protective effects of an Akt activating ligand.
Figure 4. Enhancement of LIF-induced cardiomyocyte protection by PHLPP-1 knockdown.
NRVMs were cultured for 18 hours in the presence or absence of 500 nmol/L doxorubicin±LIF (10 nmol/L). Akt inhibitor III (SH-6) (2 μmol/L) or Akt inhibitor V (triciribine) (1 μmol/L) was added to some samples before LIF treatment. DNA fragmentation was determined by ELISA-based assay (sandwich enzyme-immunoassay using mouse monoclonal antibodies directed against DNA and histones) (n=6 to 8). *P<0.05, **P<0.01, ***P<0.001.
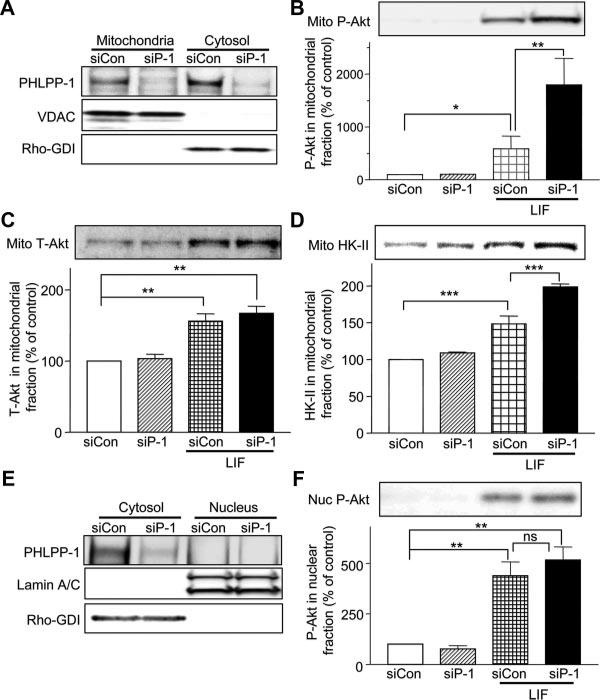
We recently reported that activated Akt translocates to mitochondria where it protects mitochondria against loss of function and attenuates cardiomyocyte cell death.5 We hypothesized that PHLPP-1 might also localize at mitochondria where it could locally regulate Akt activity and mitochondrial integrity. To explore this possibility, mitochondrial and cytosolic fractions were prepared as described in our previous work5 (Figure 5A). A significant amount of PHLPP-1 protein was found in the mitochondrial fraction. Treatment with PHLPP-1 siRNA was used to confirm the identity of the immunoreactive protein and also demonstrated that the protein detected in both fractions could be significantly downregulated.
Figure 5. Mitochondrial distribution of PHLPP-1 and regulation of Akt at mitochondria.
A, NRVMs transfected with control siRNA (siCon) or PHLPP-1 siRNA (siP-1) were fractionated into mitochondrial and cytosolic fractions; equal portion of the total from each fractions were subjected to Western blotting with PHLPP-1. Voltage-dependent anion channel (VDAC) and Rho-GDI were used as mitochondrial and cytosolic markers, respectively. B, LIF-induced increase in phosphorylated Akt (S473) in the mitochondrial fraction is potentiated by PHLPP-1 knockdown (n=5). C, LIF induces total Akt (T-Akt) increases in mitochondrial fraction (n=5). D, LIF-induced increase in mitochondrial HK-II is further enhanced by PHLPP-1 knockdown (n=4). E, Cytosol/nuclear fractionation in NRVMs. PHLPP-1 was not detectable in the nuclear fraction. Lamin A/C and Rho-GDI were used as nuclear and cytosolic markers, respectively. F, LIF-induced phosphorylated Akt (S473) increase in the nuclear fractions is not significantly enhanced by PHLPP-1 knockdown (n=6). *P<0.05, **P<0.01, ***P<0.001.
To determine whether mitochondrial Akt activation is enhanced by PHLPP-1 knockdown, cardiomyocytes were treated with LIF, fractionated and Ser473 phosphorylated Akt levels examined. LIF increased phosphorylation of Ser473 in mitochondria of control cardiomyocytes and this response was ≈3-fold greater in PHLPP-1 siRNA–treated cells (Figure 5B). Increase in total Akt in mitochondrial fraction induced by LIF was not enhanced by PHLPP-1 knockdown (Figure 5C). Our previous study demonstrated that Akt increased the phosphorylation and association of hexokinase (HK)-II with mitochondria.5 Here, we demonstrate that PHLPP-1 knockdown also enhances the LIF-induced increase in HK-II association with mitochondria (Figure 5D). IGF-1–mediated increases in phosphorylated Akt and HK-II in the mitochondrial fraction were also significantly potentiated by PHLPP-1 knockdown (Online Figure II). These data support the notion that mitochondrial Akt activity is regulated by endogenous PHLPP-1. It has been demonstrated that nuclear Akt activity is increased in response to agonist stimulation.25 We determined whether nuclear Akt activity is controlled by PHLPP-1 by nuclear/cytosolic fractionation. PHLPP-1 was not detectable in the nuclear fraction (Figure 5E) and increase in phosphorylated Akt in the nuclear fraction induced by LIF was not potentiated by PHLPP-1 knockdown (Figure 5F).
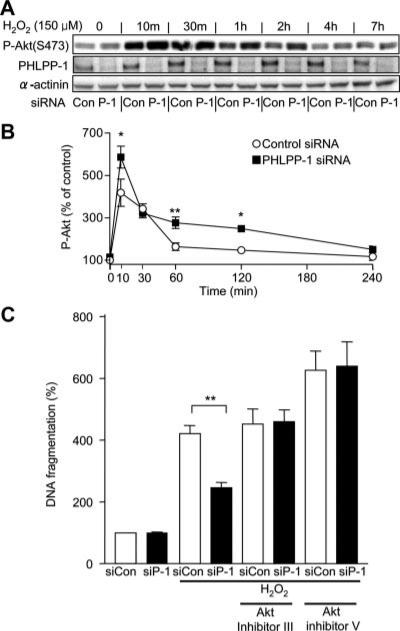
Oxidative stress and I/R activate Akt and H2O2 treatment mimics this response in cardiomyocytes. Activation of Akt by H2O2 in cardiomyocytes is increased by PHLPP-1 knockdown, as demonstrated by enhanced and prolonged Akt Ser473 phosphorylation (Figure 6A). PHLPP-1 expression was not affected by H2O2 (Online Figure III). Phosphorylation of Akt at Thr308 was increased by H2O2, which was unaffected by PHLPP-1 knockdown (data not shown). H2O2-induced apoptosis was assessed after 20 hours of H2O2 treatment by measuring DNA fragmentation and found to be significantly attenuated by PHLPP-1 knockdown (Figure 6C). Addition of Akt inhibitors fully prevented the protective effect of PHLPP-1 knockdown, demonstrating that Akt activation is responsible for the effect of PHLPP-1 knockdown. H2O2 induced activation of caspase-9 and caspase-3 was also significantly attenuated by PHLPP-1 knockdown (Online Figure IV).
Figure 6. PHLPP-1 knockdown potentiates H2O2-induced Akt activation and attenuates H2O2-induced apoptosis.
NRVMs were treated with 150 μmol/L H2O2. A, Potentiation of phosphorylation of Akt (Ser473). B, Quantitative analysis of H2O2-induced phosphorylation of Akt at S473. *P<0.05, **P<0.01 vs control. C, Cardiomyocytes were cultured for 20 hours in the presence or absence of 150 μmol/L H2O2±LIF. Akt inhibitor III (SH-6) (2 μmol/L) or Akt inhibitor V (triciribine) (1 μmol/L) were added before LIF treatment. DNA fragmentation was determined by an ELISA-based assay (n=4 to 9). **P<0.01.
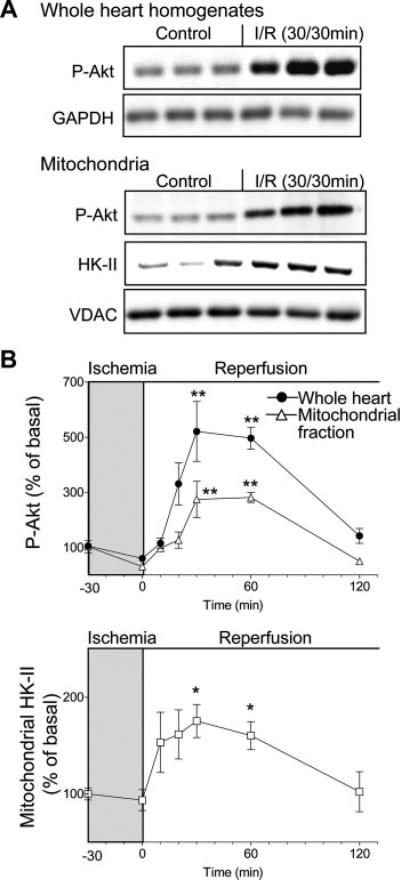
To extend our findings on the role of PHLPP-1 in response to oxidative stress, we examined the participation of PHLPP-1 in the isolated perfused heart subject to I/R. Robust time-dependent increases in phosphorylation of Akt at Ser473 were evident in Langendorff-perfused mouse hearts subject to ex vivo I/R (Figure 7). The peak increase was seen at 30 minutes of reperfusion and phosphorylation declined to basal levels by 120 minutes. Phosphorylation of Akt at Thr308 and GSK3 was also increased by ex vivo I/R in mouse hearts, suggesting increases in Akt kinase activity (data not shown). Phosphorylation of Akt was also observed in the mitochondrial fraction and showed kinetics similar to that observed in whole-heart homogenates. Mitochondrial association of HK-II, used as a readout for Akt activation at mitochondria, was also increased by I/R and over a similar time course (Figure 7B). Thus, data obtained from adult mouse hearts confirm those from NRVMs indicating that Akt is activated in response to oxidative stress, distributes to mitochondria, and increases mitochondrial HK-II association.
Figure 7. Ischemia/reperfusion in isolated perfused mouse hearts elicits Akt activation.
Hearts were perfused in the Langendorff mode and subsequently subjected to no-flow (global) ischemia followed by reperfusion. Whole-heart homogenate and mitochondrial fraction were prepared for Western blotting. A, Representative blots of phosphorylated Akt (Ser473) or HK-II in whole-heart homogenates and mitochondrial fraction after 30 minutes of reperfusion following 30 minutes ischemia. B, Quantitative analysis of time course of increase in phosphorylation of Akt at Ser473 and HK-II induced by I/R (n=3 to 5). *P<0.05, **P<0.01 vs basal.
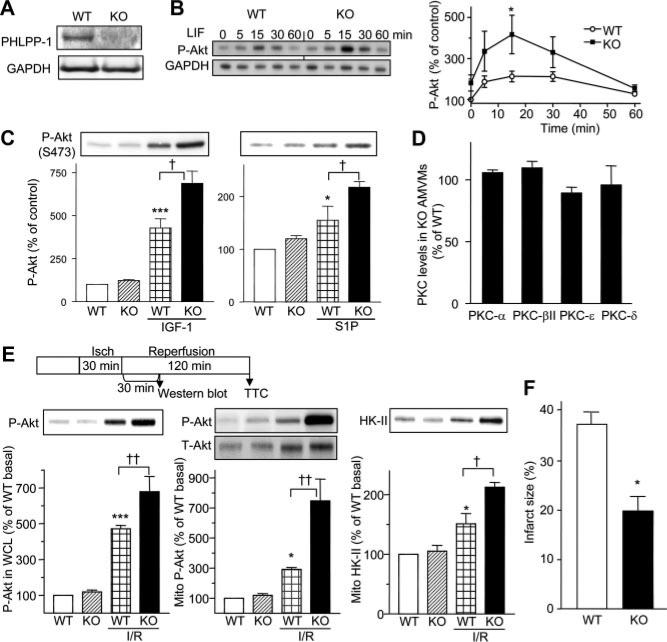
PHLPP-1 knockout mice were recently generated in our laboratories.21 These mice lack PHLPP-1 expression in the heart (Figures 1B and 8A) but have no overt basal cardiac phenotype. AMVMs isolated from WT and PHLPP-1 KO mice were stimulated with LIF for various times to assess phosphorylation of Akt. There was a marked increase in LIF-induced Akt phosphorylation at Ser473 in AMVMs isolated from PHLPP-1 KO mice (Figure 8B), whereas increase in phosphorylation of Akt at Thr308 was not changed (data not shown). PHLPP-1 deletion also enhanced phosphorylated Akt at S473 induced by IGF-1 or S1P (Figure 8C), without affecting phosphorylation of Akt at T308. As observed in NRVMs transfected with PHLPP-1 siRNA, levels of PKCs were not changed in AMVMs isolated from KO and WT, in the presence or absence of LIF (Figure 8D; also Online Figure V).
Figure 8. PHLPP-1 knockout mouse hearts show increased Akt and decreased infarct following I/R.
A, Western blot of PHLPP-1 in WT and PHLPP-1 KO mouse hearts. B, LIF-induced Akt phosphorylation at Ser473 is potentiated in AMVMs isolated from KO (n=16 to 17). C, IGF-1–mediated (1 nmol/L, 5 minutes) or S1P–mediated (1 μmol/L, 5 minutes) Akt phosphorylation at Ser473 were potentiated in AMVMs isolated from PHLPP-1 KO (n=8 to 9). D, Levels of PKCs (α, βII, δ, and ε) were not changed in AMVMs isolated from PHLPP-1 KO (n=6). E, Ex vivo I/R in PHLPP-1 KO mouse hearts. Scheme shows I/R durations and harvest timing for biochemical assays and infarct size measurement. Left and Middle, Phosphorylation of Akt at Ser473 induced by I/R is potentiated. Right, Mitochondrial association of HK-II is increased by I/R and this is potentiated in PHLPP-1 KO mouse hearts. *P<0.05, ***P<0.001 vs WT control; †P<0.05, ††P<0.01 vs WT I/R (n=6 to 9). F, Infarct size measured by TTC staining is significantly smaller in KO mice after 30 minutes ischemia/120 minutes reperfusion. *P<0.05 vs WT (n=7).
We then examined the effect of PHLPP-1 deletion on Akt phosphorylation in response to ex vivo I/R (Figure 8E). Akt phosphorylation was increased in response to I/R (30 minutes/30 minutes) and there was a significant enhancement of Akt Ser473 phosphorylation in the whole-heart homogenates from PHLPP-1 KO (Figure 8E, left). To determine whether phosphorylated Akt and HK-II at mitochondria are enhanced by PHLPP-1 deletion, mitochondria were isolated and subjected to Western blotting. Increases in phosphorylated Akt at Ser473 and HK-II in the mitochondrial fraction induced by I/R were markedly enhanced in PHLPP-1 KO (Figure 8E, middle and right). Mitochondrial total Akt was also increased in response to I/R but this was not increased by PHLPP-1 deletion. These results suggest that Akt activity is regulated by PHLPP-1 at mitochondria. To determine the functional importance of PHLPP-1 deletion, infarct size after 120 minutes reperfusion was measured by TTC staining. Remarkably, infarct size was smaller (by 45%), evidence that potentiation of Akt activation by PHLPP-1 deletion protects the heart against I/R injury (Figure 8F).
Discussion
Akt is an established survival signal in the heart. One approach to manipulating this pathway would be to increase Akt activation, but an equally feasible and potentially more selective approach would be to slow its inactivation. Mechanisms regulating the termination of Akt signals have, until recently, been poorly documented. PHLPP-1 was recently discovered to be an Akt phosphatase that selectively dephosphorylates Ser473 on Akt and can regulate tumor cell survival.15–17 The functional significance of PHLPP-1 expression in regulating physiological and pathophysiologic responses of other cell and tissue types has not, however, been examined. We demonstrate here that PHLPP-1 is expressed in cardiomyocytes, that it negatively regulates Akt activity through dephosphorylation of Akt at Ser473 and that it has functional effects on cardiomyocyte survival in vitro and in the ex vivo heart. Interestingly, our results suggest that PHLPP-1 distributes not only to cytosol but also to mitochondria where the extent of Akt activation can be locally regulated.
Analyzing constructs of Akt with Ala at one or the other of the phosphorylation positions (T308A or S473A), Alessi et al reported that mutation of either Ser473 or Thr308 reduced the rate of Akt activity by 85% and 95%, respectively, compared to WT Akt phosphorylated at both sites.26 Consistent with this, Akt selectively dephosphorylated at Ser473 by PHLPP has markedly reduced activity in in vitro assays using GSK3 as substrate.15 Thus, the intrinsic catalytic activity of Akt is reduced >80% in the absence of phosphorylation of Ser473. The data presented here confirm that increases in p-Akt at Ser473 are induced by PHLPP-1 knockdown or genetic deletion, in the absence of concomitant increases in p-Akt at Thr308, and that this results in significant increases in Akt catalytic activity, supporting the significance of phosphorylation of Ser473 in regulating in vivo Akt kinase activity.
PHLPP-1 knockdown/knockout potentiates Akt phosphorylation at S473 induced by agonists (LIF, IGF-1 and S1P), suggesting the general importance of PHLPP-1 in regulation of Akt activity in cardiomyocytes. Knockdown of PHLPP-1 in NRVMs increases the LIF-induced Ser473 phosphorylation and activity of Akt-1 to the same extent as it affects Akt-2. PHLPP-1 knockdown also potentiates LIF-induced phosphorylation of both Akt-1 and Akt-2 substrates (GSK3-α and -β) in cardiomyocytes. In previous work, PHLPP-1 showed specificity for Akt-2.20 This may reflect differences in the properties of terminally differentiated cardiomyocytes versus tumor cells. It might also be related to our observations that PHLPP-2, although present in cardiomyocytes, is unable to regulate LIF-induced phosphorylation of Akt at Ser473, as indicated by the results of PHLPP-2 knockdown. With regard to the functions of Akt-1 and Akt-2, a protective role of Akt-1 has been well established in the heart,1,2 but genetic deletion of Akt-2 likewise revealed an involvement in protection against myocardial infarction.27 Thus, although the enhanced protective effects of PHLPP-1 knockdown observed in this study likely result from increased activity of Akt-1, it could reflect increases in activity of both Akt isoforms. It has been reported in colon cancer and normal breast epithelial cell lines that PHLPP-1 dephosphorylates PKCs, such as PKCα, -βII, -δ, and -ε, promoting degradation of the enzymes23 and this could contribute to the protection because protective role of PKC, especially PKCε, has been demonstrated.28 However, we did not observe changes in the levels of PKCs (α, βII, δ, and ε) in NRVMs transfected with PHLPP-1 siRNA or in AMVMs from PHLPP-1 KO, suggesting that potentiation of Akt signaling plays an important role in protective effects of PHLPP-1 knockdown/deletion.
Cytotoxic interventions such as doxorubicin have been shown to be counteracted by agonists such as LIF or IGF-1 which activate Akt.4,24,29 We demonstrate here that PHLPP-1 knockdown potentiates the cytoprotective effects of these ligands against doxorubicin toxicity through its effects on Akt. Oxidative stress, as induced by H2O2, is accompanied by activation of Akt.30,31 Akt has been considered as a reperfusion injury salvage kinases (RISK), counteracting cell damage through a compensatory protective pathway.32 This protective signaling response is also shown here to be potentiated by PHLPP-1 knockdown leading to enhanced cardiomyocyte survival. A more physiological form of oxidative stress is induced by exposing perfused hearts to global I/R. Akt has been shown to be activated in the adult heart by reperfusion following no flow ischemia33 and we demonstrate here that there is enhanced Akt activation by I/R when PHLPP-1 is genetically deleted. These findings suggest a physiological role for this phosphatase, whereas the concomitantly diminished infarct size demonstrates its functional importance. Thus, our results not only confirm that Akt is activated in response to oxidative stress30,31 and ex vivo I/R,33 but indicate that PHLPP-1 plays a role in this pathway and that the magnitude of this self-protecting signal can be enhanced by PHLPP-1 downregulation. These data suggest that PHLPP-1 inhibitors could have therapeutic potential for protecting cardiomyocytes against I/R injury.
The mitochondrial death pathway plays a crucial role in heart diseases induced by I/R and doxorubicin toxicity.34,35 There is growing evidence that mitochondrial integrity can be regulated by reversible phosphorylation controlled by resident mitochondrial kinases/phosphatases or by kinases that translocate to mitochondria.36,37 We and others have shown that Akt activated at the plasma membrane redistributes to several cellular compartments including mitochondria and nucleus.5,25,38,39 More specifically, our previous work demonstrated that increases in mitochondrial Akt are responsible for preservation of mitochondrial integrity following stress induced by elevated Ca2+ and ROS.5 This is attributable, at least in part, to increases in mitochondrial association of HK-II, a putative component of the permeability transition pore. We determined that HK-II has an Akt phosphorylation consensus sequence and is directly phosphorylated by Akt, providing a mechanism for increased association of HK-II with mitochondria.5 In the present study, we demonstrate for the first time that ex vivo I/R increases the accumulation of phosphorylated Akt at mitochondria. As evidence that the mitochondrial Akt is active, we find that the increase in phosphorylated Akt is accompanied by increased mitochondrial HK-II association. Our subcellular fractionation studies also reveal that PHLPP-1 can localize at mitochondria and that the accumulation of phosphorylated Akt at mitochondria is significantly increased by knockdown/knockout of PHLPP-1. Indeed, potentiation of Akt Ser473 phosphorylation by PHLPP-1 knockdown or deletion is considerably more prominent in the mitochondrial fraction than in whole cell/heart lysate. The observation that increase in mitochondrial HK-II following Akt activation is significantly enhanced in PHLPP-1 knockdown/knockout further suggest that PHLPP-1 locally regulates Akt activity and thereby mitochondrial integrity. These results provide a previously undescribed mechanism by which mitochondrial function and integrity can be regulated through a dynamic balance between kinases and phosphatases.
Little is known about the mechanisms that regulate PHLPP, but changes in its expression level rather than its activation state may be most important in controlling Akt activity. Interestingly, we observed that 48 hours of LIF treatment upregulated both PHLPP-1 and PHLPP-2 expression in NRVMs (Online Figure VI). These increases were inhibited by treatment with an Akt inhibitor, implicating an Akt mediated feedback mechanism in regulation of PHLPP-1 and PHLPP-2 expression. Indeed, such a feedback mechanism was recently reported by Gao and coworkers, who demonstrated that that Akt activation prevents PHLPP-1 degradation by inhibiting its ubiquitination.17 Consistent with this, we also observed increased PHLPP-1 and PHLPP-2 expression in hearts from IGF-1 transgenic mice, which have elevated Akt activity (Online Figure VI). Further studies will be needed to determine whether these increases in PHLPP protein are attributable to transcriptional upregulation or to protein stabilization and whether they affect Akt activation and cardioprotection.
In conclusion, we demonstrate for the first time that PHLPP-1 is expressed in the heart and provide data indicating that it dephosphorylates Ser473 of Akt-1 and Akt-2, decreasing Akt kinase activity and facilitating cardiomyocyte death. Inhibition of Akt activity by PHLPP-1 can be observed at the level of mitochondria, contributing to control of mitochondrial integrity. Taken together, these data suggest that inhibition of PHLPP-1 would be beneficial in limiting ischemic heart diseases. Although therapeutic strategies leading to sustained Akt activation may have cardioprotective effects, they may also increase in cancer risk. In contrast, controlling Akt activity through pharmacological inhibition of PHLPP-1 for a relatively short time period after cardiac events should have beneficial effects by preventing the onset of cardiomyocyte loss and subsequent pathophysiological remodeling.
Supplementary Material
Acknowledgments
We thank Angela Yuen and Melissa Ridaout for technical assistance.
Sources of Funding
This work was supported by NIH grants HL085577 (to J.H.B.), GM067946 (to A.C.N.), and HL097037 (to S.M.).
Non-standard Abbreviations and Acronyms
- AMVM
adult mouse ventricular myocyte
- GSK3
glycogen synthase kinase-3
- HK-II
hexokinase II
- IGF-1
insulin-like growth factor 1
- I/R
ischemia/reperfusion
- KO
knockout
- LIF
leukemia inhibitory factor
- NRVM
neonatal rat ventricular myocyte
- PHLPP-1
PH domain leucine-rich repeat protein phosphatase-1
- PIP3
phosphatidylinositol (3,4,5) triphosphate
- PKC
protein kinase C
- PP2A
protein phosphatase 2A
- S1P
sphingosine-1-phosphate
- siRNA
small interfering RNA
- WT
wild type
Footnotes
Disclosures
None.
References
- 1.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 3.Craig R, Wagner M, McCardle T, Craig AG, Glembotski CC. The cytoprotective effects of the glycoprotein 130 receptor-coupled cytokine, cardiotrophin-1, require activation of NF-kappa B. J Biol Chem. 2001;276:37621–37629. doi: 10.1074/jbc.M103276200. [DOI] [PubMed] [Google Scholar]
- 4.Negoro S, Oh H, Tone E, Kunisada K, Fujio Y, Walsh K, Kishimoto T, Yamauchi-Takihara K. Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/caspase-3 interaction. Circulation. 2001;103:555–561. doi: 10.1161/01.cir.103.4.555. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol. 2008;103:203–215. doi: 10.1007/s00395-007-0687-7. [DOI] [PubMed] [Google Scholar]
- 7.Means CK, Miyamoto S, Chun J, Brown JH. S1P1 receptor localization confers selectivity for Gi-mediated cAMP and contractile responses. J Biol Chem. 2008;283:11954–11963. doi: 10.1074/jbc.M707422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 9.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 11.Kawase T, Ohki R, Shibata T, Tsutsumi S, Kamimura N, Inazawa J, Ohta T, Ichikawa H, Aburatani H, Tashiro F, Taya Y. PH domain-only protein PHLDA3 is a p53-regulated repressor of Akt. Cell. 2009;136:535–550. doi: 10.1016/j.cell.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Padmanabhan S, Mukhopadhyay A, Narasimhan SD, Tesz G, Czech MP, Tissenbaum HA. A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell. 2009;136:939–951. doi: 10.1016/j.cell.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55α regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 14.Park CH, Kim YS, Kim YH, Choi MY, Yoo JM, Kang SS, Choi WS, Cho GJ. Calcineurin mediates AKT dephosphorylation in the ischemic rat retina. Brain Res. 2008;1234:148–157. doi: 10.1016/j.brainres.2008.07.082. [DOI] [PubMed] [Google Scholar]
- 15.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene. 2009;28:994–1004. doi: 10.1038/onc.2008.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartzbauer G, Robbins J. The tumor suppressor gene PTEN can regulate cardiac hypertrophy and survival. J Biol Chem. 2001;276:35786–35793. doi: 10.1074/jbc.M102479200. [DOI] [PubMed] [Google Scholar]
- 19.Ruan H, Li J, Ren S, Gao J, Li G, Kim R, Wu H, Wang Y. Inducible and cardiac specific PTEN inactivation protects ischemia/reperfusion injury. J Mol Cell Cardiol. 2009;46:193–200. doi: 10.1016/j.yjmcc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masubuchi S, Gao T, O'Neill A, Eckel-Mahan K, Newton AC, Sassone-Corsi P. Protein phosphatase PHLPP1 controls the light-induced resetting of the circadian clock. Proc Natl Acad Sci U S A. 2010;107:1642–1647. doi: 10.1073/pnas.0910292107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu K, Okada M, Takano A, Nagai K. SCOP, a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett. 1999;458:363–369. doi: 10.1016/s0014-5793(99)01190-4. [DOI] [PubMed] [Google Scholar]
- 23.Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of protein kinase C. J Biol Chem. 2008;283:6300–6311. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Ma W, Markovich R, Chen JW, Wang PH. Regulation of cardiomyocyte apoptotic signaling by insulin-like growth factor I. Circ Res. 1998;83:516–522. doi: 10.1161/01.res.83.5.516. [DOI] [PubMed] [Google Scholar]
- 25.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res. 2001;88:1020–1027. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 26.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 27.DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inagaki K, Begley R, Ikeno F, Mochly-Rosen D. Cardioprotection by ε-protein kinase C activation from ischemia: continuous delivery and antiarrhythmic effect of an ε-protein kinase C-activating peptide. Circulation. 2005;111:44–50. doi: 10.1161/01.CIR.0000151614.22282.F1. [DOI] [PubMed] [Google Scholar]
- 29.Taniyama Y, Walsh K. Elevated myocardial Akt signaling ameliorates doxorubicin-induced congestive heart failure and promotes heart growth. J Mol Cell Cardiol. 2002;34:1241–1247. doi: 10.1006/jmcc.2002.2068. [DOI] [PubMed] [Google Scholar]
- 30.Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H2O2 regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol. 2003;35:615–621. doi: 10.1016/s0022-2828(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 31.Pham FH, Sugden PH, Clerk A. Regulation of protein kinase B and 4E-BP1 by oxidative stress in cardiac myocytes. Circ Res. 2000;86:1252–1258. doi: 10.1161/01.res.86.12.1252. [DOI] [PubMed] [Google Scholar]
- 32.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita K, Kajstura J, Discher DJ, Wasserlauf BJ, Bishopric NH, Anversa P, Webster KA. Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circ Res. 2001;88:609–614. doi: 10.1161/01.res.88.6.609. [DOI] [PubMed] [Google Scholar]
- 34.Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol. 2007;23:15–25. doi: 10.1007/s10565-006-0140-y. [DOI] [PubMed] [Google Scholar]
- 35.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai HC, Liu TJ, Ting CT, Sharma PM, Wang PH. Insulin-like growth factor-1 prevents loss of electrochemical gradient in cardiac muscle mitochondria via activation of PI 3 kinase/Akt pathway. Mol Cell Endocrinol. 2003;205:99–106. doi: 10.1016/s0303-7207(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 37.Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, Koehler C, Chen JN, Wang Y. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21:784–796. doi: 10.1101/gad.1499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunkel MT, Ni Q, Tsien RY, Zhang J, Newton AC. Spatio-temporal dynamics of protein kinase B/Akt signaling revealed by a genetically encoded fluorescent reporter. J Biol Chem. 2005;280:5581–5587. doi: 10.1074/jbc.M411534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.