Abstract
Learning and behavioral abnormalities are among the most common clinical problems in children with the neurofibromatosis-1 (NF1) inherited cancer syndrome. Recent studies using Nf1 genetically engineered mice (GEM) have been instructive for partly elucidating the cellular and molecular defects underlying these cognitive deficits; however, no current model has shed light on the more frequently encountered attention system abnormalities seen in children with NF1. Using an Nf1 optic glioma (OPG) GEM model, we report novel defects in non-selective and selective attention without an accompanying hyperactivity phenotype. Specifically, Nf1 OPG mice exhibit reduced rearing in response to novel objects and environmental stimuli. Similar to children with NF1, the attention system dysfunction in these mice is reversed by treatment with methylphenidate (MPH), suggesting a defect in brain catecholamine homeostasis. We further demonstrate that this attention system abnormality is the consequence of reduced dopamine (DA) levels in the striatum, which is normalized following either MPH or l-dopa administration. The reduction in striatal DA levels in Nf1 OPG mice is associated with reduced striatal expression of tyrosine hydroxylase, the rate-limited enzyme in DA synthesis, without any associated dopaminergic cell loss in the substantia nigra. Moreover, we demonstrate a cell-autonomous defect in Nf1+/− dopaminergic neuron growth cone areas and neurite extension in vitro, which results in decreased dopaminergic cell projections to the striatum in Nf1 OPG mice in vivo. Collectively, these data establish abnormal DA homeostasis as the primary biochemical defect underlying the attention system dysfunction in Nf1 GEM relevant to children with NF1.
INTRODUCTION
While individuals with the inherited cancer predisposition syndrome neurofibromatosis-1 (NF1) are prone to the development of benign and malignant tumors, one of the most challenging clinical problems for school-age children with NF1 involves learning disabilities and attention system dysfunction. These specific learning tasks and attention problems limit overall school performance in standard classroom settings (1–7). The spectrum of learning deficits includes abnormalities in visual-motor integration, visual-spatial judgment and visual-perceptual skills, such that children with NF1 perform worse than control children on the Judgment of Line Orientation test, the Beery Visual-Motor Integration Test and the Recognition-Discrimination Test (8). Moreover, these children frequently perform poorly on tasks of reading, spelling and mathematics (5), and exhibit deficits in receptive and expressive language as well as verbal and visual memory (3).
In addition to problems with learning and memory, there is a higher prevalence of attention deficits in children with NF1 (1,2,9). In these studies, 63–67% of children with NF1 had sustained attentional difficulties, while 38–50% fulfilled the diagnostic criteria for attention-deficit hyperactivity disorder (ADHD) (4,10). While both attentional abnormalities and hyperactivity co-exist in children with NF1, disturbances in attention, rather than hyperactivity, predominate (11). In this regard, only 1.2% of children with NF1 exclusively have the hyperactive-impulsive ADHD subtype. In light of the prevalence of ADHD abnormalities in children with NF1, methylphenidate (MPH) treatment was evaluated in a cohort of children with NF1 (12): Significant improvements in performance on tests of attention as well as learning ability were observed in the MPH-treated group.
While MPH and other stimulant medications that influence dopamine (DA) homeostasis are commonly prescribed for children with NF1, the relationship between NF1, attention system dysfunction and DA regulation is unclear. In this report, we employ a unique Nf1 genetically engineered mouse (GEM) strain to uncover novel behavioral abnormalities in rodent attention system function, and show that the DA uptake inhibitor, MPH, and l-dopa ameliorate this attention system dysfunction in vivo. We demonstrate that Nf1 mutant mice exhibit reduced DA levels in the striatum, which reflects lower levels of the rate-limiting enzyme in the synthesis of DA [tyrosine hydroxylase (TH)], with no associated loss of dopaminergic neurons in the substantia nigra. Moreover, Nf1+/− dopaminergic neurons exhibit cell-autonomous reductions in growth cone area and neurite extension in vitro, which results in decreased dopaminergic cell projections to the striatum in Nf1 optic glioma (OPG) mice in vivo. Together, these observations establish a mechanistic connection between Nf1 gene expression, attention system function and dopaminergic pathway integrity, and support the use of DA pathway-directed stimulant medication in children with significant NF1-associated attention deficits.
RESULTS
Nf1 OPG mice exhibit abnormal locomotor activity levels and exploratory behaviors, compromised spatial learning and memory, but intact sensorimotor capabilities
Pioneering studies by Silva et al. (13) have previously revealed mild spatial learning and memory deficits in conventional heterozygous Nf1 knockout (Nf1+/−) mice compared with their wild-type littermate controls. We were specifically interested in developing an experimental platform to assess the impact of brain tumor (glioma) treatment on cognitive function in NF1, and since Nf1+/− mice do not develop gliomas, we employed a GEM strain in which Nf1+/− mice with Nf1 inactivation in GFAP+ cells (glia) develop optic glioma (OPG). Consistent with prior observations using Nf1+/− mice, Nf1 OPG mice also exhibit mild spatial learning/memory deficits in the Morris water maze, but more interestingly, have marked defects in attention system function.
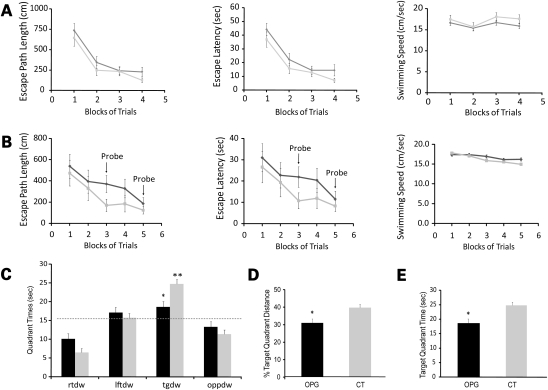
Testing in the Morris water maze involved initial cued (visible platform) training. No significant effects of genotype or genotype by blocks of trials interactions were observed. As a group, the performance of Nf1 OPG mice was not different from their control littermates with respect to path length, latency and swimming speeds (Fig. 1A), arguing against the presence of non-associative deficits (e.g. sensorimotor, visual or motivational disturbances) that could impair their swimming performance or their ability to swim to a cued location. Next, the mice underwent place condition training using a submerged, non-visible platform to evaluate spatial learning capabilities. No significant overall effects were found, although there were trends suggesting reduced performance midway through acquisition training in the Nf1 OPG mice (Fig. 1B). Results from a probe trial conducted at this time (1 h after the last place trial on day 3; Block 3), when the platform was removed from the pool, demonstrated clear differences in spatial learning and/or retention. In these experiments, Nf1 OPG mice spent significantly less time in the target quadrant that had previously contained the platform (Fig. 1C) compared with control littermate mice [F(1,33) = 11.09, P = 0.002]. Moreover, the results of one-way repeated measures ANOVA/MANOVA analyses conducted within each group [controls: F(3,48) = 40.46, P < 0.00005; Hotelling–Lawley trace F(3,14) = 51.99, P < 0.00005], followed by within-subjects contrasts of quadrant times, showed that the control mice exhibited a spatial bias for the target quadrant by spending significantly more time in that quadrant compared with the times spent in the other quadrants (P < 0.0002). In contrast, Nf1 OPG mice showed no such bias (Fig. 1C). Similarly, the percentage of the distance swum in the target quadrant (out of the total distance traveled in the entire pool during the probe trial) was significantly reduced (Fig. 1D) in Nf1 OPG mice [F(1,33) = 7.53, P = 0.01]. Nf1 OPG and control mice showed no differences with respect to the number of times they passed directly over where the platform had been located—a measure of more highly resolved retention of platform location (Fig. 1E). Although the Nf1 OPG mice exhibited deficits during probe trial 1, these differences were no longer present when probe trial 2 was conducted at the end of acquisition training (data not shown). These results demonstrate a transient mild abnormality in spatial memory and learning.
Figure 1.
Nf1 optic glioma (OPG) mice exhibit spatial learning deficits. (A) Control mice (CT; gray squares) and Nf1 OPG mice (black diamonds) show equivalent performance on cued trials for path length (course taken to platform), latency (time to reach platform) and swimming speed. (B) Although Nf1 OPG mice tended to exhibit inferior performance relative to the littermate control (CT) group during the place trials with regard to the distance traveled and time taken to navigate to the platform, these differences were not statistically significant. (C) However, when the platform was removed during probe trial 1, the Nf1 OPG mice spent significantly less time in the target quadrant (tg) compared with the littermate control (CT) group (*P = 0.002). Nf1 OPG mice showed little preference for the target quadrant (in terms of time spent in) compared with the right, left and opposite quadrants (rt, lt, opp), whereas the littermate control (CT) mice showed a significant spatial bias for the target quadrant as shown by them spending significantly more time in the target quadrant versus the time spent in the other quadrants (**P < 0.0002).The dotted line represents the time spent in each quadrant expected by chance alone. (D) In addition, the percentage of the distance swum in the target quadrant (out of the total distance traveled in the entire pool during the probe trial) was significantly lower in Nf1 OPG mice (*P = 0.01). (E) While not statistically significant, Nf1 OPG mice showed a strong trend toward fewer platform crossings compared with the littermate control (CT) group.
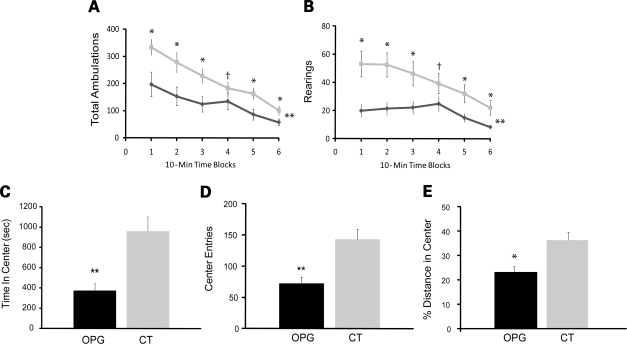
To assess general activity and attention system function, Nf1 OPG mice and littermate controls were evaluated using a 1 h locomotor activity/exploration test. Compared with littermate control (CT) mice, Nf1 OPG mice had markedly reduced general ambulatory activity and exploratory behaviors (Fig. 2A and B). Specifically, significant main effects of genotype and genotype by time interactions were found for both total ambulations [F(1,33) = 17.47, P = 0.0002; F(5,165) = 3.16, P = 0.02, respectively] and rearings [F(1,33) = 12.73, P = 0.001; F(5,165) = 4.36, P = 0.003, respectively] across the test sessions. Interestingly, differences between Nf1 OPG and control mice were maximal early during the test session (first two time blocks), suggesting that Nf1 OPG mice had muted reactions to the novelty of the test situation. In addition, Nf1 OPG mice also showed altered emotionality (Fig. 2C and D), and spent significantly less time and made fewer entries into the center of the test field [F(1,33) = 14.17, P = 0.0007; F(1,33) = 19.51, P = 0.0001, respectively]. Although these differences in center-of-the-field behaviors could reflect differences in general ambulatory activity, Nf1 OPG mice still showed reduced entries and ambulations relative to control mice [F(1,33) = 12.64, P = 0.001] when distances traveled in the center were normalized to the total distance traveled (Fig. 2E), supporting the existence of altered emotionality beyond simple differences in general activity.
Figure 2.
Nf1 OPG mice exhibit reduced ambulatory activity and exploratory behaviors in the 1h locomotor activity test and show evidence of attention deficits. (A) Total ambulations, as a measure of general activity in a novel environment, were significantly lower in Nf1 OPG mice relative to littermate control (CT) mice (P = 0.008). (B) Rearing, as a measure of exploration and non-selective attention to environmental change, was also significantly reduced in Nf1 OPG mice compared with littermate control (CT) mice (P = 0.0039). Note that differences were greatest early on in the test session, suggesting an attenuated response to novelty on the part of the Nf1 OPG mice. (C) Nf1 OPG mice also showed evidence of altered emotionality by exhibiting fewer entries into the center than littermate control (CT) mice (P = 0.01). (D) Similarly, Nf1 OPG mice spent less time exploring the center compared with littermate control (CT) mice (P = 0.0001). (E) When normalized to the total distance traveled during the test session, there is still a significant reduction in distance traveled in the center for Nf1 OPG mice (P = 0.001).
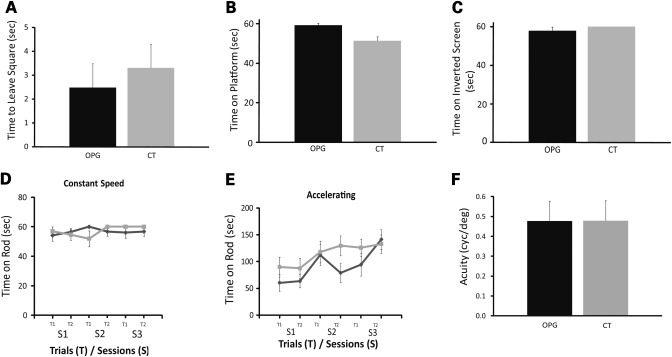
In contrast to the activity and behavioral exploration data, Nf1 OPG mice did not demonstrate impairments on any of the seven measures used within the sensorimotor battery, arguing against the existence of gross deficits in initiation of movement (Fig. 3A), balance (Fig. 3B) or strength (Fig. 3C). Moreover, no differences between Nf1 OPG mice and littermate controls were observed on stationary (data not shown), constant speed (Fig. 3D) or accelerating (Fig. 3E) rotarod testing. To exclude any potential contribution of reduced visual function to the behavioral deficits found in Nf1 OPG mice, we employed the virtual optomotry system (VOS) method to quantify visual (grating) acuity and contrast threshold under photopic conditions. No differences in visual contrast thresholds (data not shown) or visual acuity thresholds were detected between control and Nf1 OPG mice (Fig. 3F), and the values obtained were consistent with previously published values for C57BL/6 mice. Collectively, these data demonstrate that the learning/memory and attention system abnormalities observed in Nf1 OPG mice did not result from gross deficits in balance, strength, coordination, movement initiation or vision.
Figure 3.
Nf1 OPG mice have no abnormalities in sensorimotor function or visual impairment. (A) Walking initiation is unaffected in Nf1 OPG mice. (B) Nf1 OPG mice were not impaired relative to littermate control (CT) mice on the small platform (balance) test. (C) There were no differences between littermate control (CT) and Nf1 OPG mice on the inverted screen test, suggesting that grip strength was intact in Nf1 OPG mice. Nf1 OPG mice performed equivalently to littermate control (CT) mice on rotarod tests of balance and dexterity involving (D) constant and (E) accelerating speeds. (F) No significant differences between littermate control (CT) and Nf1 OPG mice were observed using VOS to measure visual acuity (P = 0.96).
Nf1 OPG mice exhibit reduced selective and non-selective attention system function
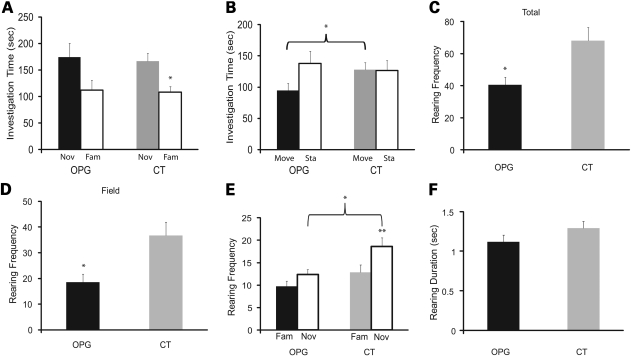
Rearing in response to environmental change is often considered an index of non-selective attention in rodents (14,15). In this model, rearing frequency is thought to reflect the level of orientation to environmental stimuli, while rearing duration is believed to represent the time spent scanning the environment and processing information. Since attention deficits are cardinal symptoms of NF1 patients and Nf1 OPG mice exhibit an abnormally low frequency of rearing in response to a novel environment, we examined rearing and other related exploratory behaviors in greater detail. Previous studies by Silva and colleagues (16) revealed selective attention deficits in Nf1+/− mice using the lateralized reaction-time task, which is a highly structured, operant-based procedure. To assess both selective and non-selective attention, we assessed spontaneously occurring behaviors in response to environmental events using a modified version of our object recognition test procedure (17). We further differentiated total rearing into rearing in the ‘field’ away from the objects and rearing during object investigation. In this paradigm, rearing during object investigation likely reflects selective attention, similar to other aspects of object investigation, while ‘field' rearing away from the objects is used as an index of non-selective attention.
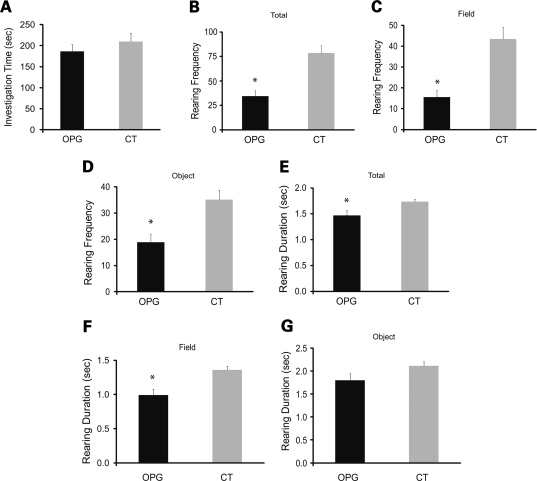
During the sample trial when the mice were presented with two identical objects (Fig. 4A), no differences were observed between groups with regard to the amount of time spent actively investigating the objects (selective attention). However, similar to the findings from the 1 h locomotor activity test, Nf1 OPG mice exhibited a lower frequency of total rearing (Fig. 4B) during the 10min session [F(1,33) = 20.76, P = 0.0001]. We next quantified rearing frequency in the ‘field' away from the objects (Fig. 4C) as well as rearing observed during object investigation (Fig. 4D). Again, Nf1 OPG mice reared less often than control littermates in the field [F(1,33) = 19.57, P = 0.0001] and during object investigation [F(1,33) = 10.72, P = 0.0025]. In addition, we analyzed the average rearing duration for each episode (Fig. 4E and F), and found that Nf1 OPG mice had lower rearing durations with regard to the total number of rearing episodes [F(1,33) = 4.88, P = 0.034] and rearings in the field [F(1,33) = 11.66, P = 0.0017]. Interestingly, however, the average rearing durations between the two groups during object investigation were not significantly different. No effects of gender were found.
Figure 4.
Nf1 OPG mice have reduced rearing frequency and duration during the sample object trial in the object recognition test. (A) Nf1 OPG (n = 20) and littermate control (CT) (n = 17) mice did not differ in the times devoted to investigating the two identical sample objects. However, the total rearing frequency (B) and rearing frequency in the field away from the objects (C) were both significantly reduced in Nf1 OPG mice (P = 0.0001) during the sample objects trial. (D) Rearing frequency during investigation of the sample objects was also significantly reduced in Nf1 OPG mice (P = 0.0025). The average rearing duration per episode was also significantly lower in Nf1 OPG mice in terms of (E) total rearings and (F) rearings in the field (P = 0.034 and 0.0017, respectively). (G) In contrast, average rearing duration was not different between groups during sample object investigation.
During the test trials involving the presentation of familiar and novel objects in the test field (Fig. 5A), a significant effect of object type (familiar versus novel) was observed [F(1,33) = 7.84, P = 0.009]. Whereas control mice spent significantly more time investigating the novel object compared with the familiar one [F(1,16) = 15.01, P = 0.0013], Nf1 OPG mice exhibited no significant differences in object investigation times (Fig. 5A). OPG mice exhibited reduced investigation of moved objects compared to CT littermates (Fig. 5B). Similarly, Nf1 OPG mice had significantly lower frequencies of rearing compared with control mice in terms of total rearing (Fig. 5C) [F(1,33) = 8.52, P = 0.006)] and rearing in the field away from the objects (Fig. 5D) [F(1,33) = 9.75, P = 0.004)]. Nf1 OPG mice also exhibited reduced rearing frequency [F(1,33) = 7.62, P = 0.009] compared with controls (Fig. 5E). Also, within-groups analyses showed that both the Nf1 OPG and control mice exhibited more rearing when investigating the novel object compared with the familiar object, [F(1,19) = 6.61, P = 0.019 and F(1,16) = 12.89, P = 0.002, respectively]. The Nf1 OPG mice also exhibited a trend towards reduced rearing duration while investigating the novel object (Fig. 5F; P = 0.051). In addition, a planned comparison showed that Nf1 OPG mice spent less time investigating a moved object compared with littermate control mice (Fig. 5B), and spent a smaller percentage of total investigation time exploring a moved object (data not shown). Together, these data demonstrate defects in both non-selective (Figs 2B, 4B, C, E and F, 5C and D) and selective (Figs 4D and 5A, B and E) attention in Nf1 OPG mice.
Figure 5.
Nf1 OPG mice exhibit object recognition retention impairments and attention abnormalities. (A) Littermate control (CT) mice spent significantly more time investigating the novel object versus the familiar object during the test trial (P = 0.0013), while the object investigation times (novel versus familiar) were not different for the Nf1 OPG mice. (B) Nf1 OPG mice spent significantly less time investigating the moved object compared with the littermate control (CT) group during the second test trial (P = 0.044). Nf1 OPG mice showed significantly reduced rearing frequency of (C) total rearings and (D) rearings in the field (P = 0.006 and 0.004, respectively) during the novel versus familiar test trial. (E) Nf1 OPG mice reared significantly less often than the littermate control (CT) group during investigation of the novel object (P = 0.009), but the groups did not differ in terms of rearing frequency shown during investigation of the familiar object. Both the littermate control (CT) and Nf1 OPG mice reared significantly more often when investigating the novel object compared with the familiar one (P = 0.002 and 0.019, respectively). (F) Nf1 OPG mice showed a non-significant trend toward decreased average rearing duration compared with the littermate control (CT) group during the novel versus familiar test trial.
MPH and l-dopa significantly increase exploratory behavior in Nf1 OPG mice
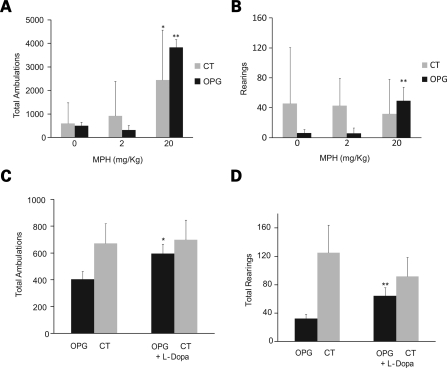
Given the depressed exploratory and attentional behaviors in the Nf1 OPG mice, and the obvious relevance to the attention system abnormalities common in children with NF1, we next sought to determine whether treatments used for attention deficit disorder in the pediatric population would reverse these behavioral deficits in Nf1 OPG mice. We initially investigated the effect of MPH on the exploratory and attentional behaviors of Nf1 OPG mice, and found that 20 mg/kg MPH increased both total ambulations (Fig. 6A) and rearings (Fig. 6B) (P = 0.02, P = 0.05; n = 5). Statistically significant changes in total ambulations, but not rearings, were observed in control littermate mice following MPH administration (Fig. 6A and B).
Figure 6.
Treatment with methylphenidate (MPH) or l-dopa improves exploratory and attention behaviors in Nf1 OPG mice. (A) Total ambulations of Nf1 OPG and littermate control (CT) mice increased after a single IP injection of MPH (20 mg/kg) (P = 0.02). (B) Rearings in only Nf1 OPG mice increased after MPH administration (P = 0.05). (C) While not as strong an inducer of activity as MPH, l-dopa (50 mg/kg) also increased total ambulations (P = 0.04). (D) Rearings were increased in Nf1 OPG mice following l-dopa injection (P = 0.02).
Since MPH is known to affect both the serotonergic and dopaminergic pathways, we next treated mice with l-dopa to target the dopaminergic pathway alone. This choice was based on previous rodent behavioral studies suggesting that serotonin may be more important for controlling general activity levels, whereas DA is related to attention system function (18–20). Following l-dopa treatment, Nf1 OPG mice exhibited increased exploratory (Fig. 6C) and attention-related (rearing) behaviors (Fig. 6D) (P = 0.04, n = 13; P = 0.02, n = 15), whereas there were no statistically significant changes in control littermates after l-dopa administration (Fig. 6C and D). These findings demonstrate that the abnormal exploratory behaviors observed in Nf1 OPG mice primarily reflect defects in DA pathway function.
Nf1 OPG mice have reduced DA levels in the striatum
To provide direct evidence for a DA defect in Nf1 OPG mice, we measured DA levels as well as its primary breakdown products (Dopac and HVA) by reverse-phase high-performance liquid chromatography (HPLC) in the corpus striatum, the major site of DA release. Consistent with the MPH and l-dopa studies, Nf1 OPG mice had lower DA and DA breakdown products than their control littermates (P = 0.01) (Table 1). Moreover, following MPH and l-dopa treatment in vivo, Nf1 OPG mice had near normalization of their DA, Dopac and HVA levels, whereas control littermates showed no change (Table 1). In contrast, neither Nf1+/− mice nor mice lacking Nf1 expression in GFAP+ cells (Nf1GFAPCKO mice) had reduced DA or DA breakdown products by HPLC compared with littermate controls (data not shown).
Table 1.
HPLC quantitation of DA and DA breakdown products in Nf1 mutant mice
| DA | SD | Dopac | SD | HVA | SD | N | |
|---|---|---|---|---|---|---|---|
| CT | 24.3 | 9.0 | 1.7 | 0.31 | 1.9 | 0.39 | 7 |
| OPG | 14.5 | 4.5 | 1.1 | 0.22 | 1.2 | 0.23 | 6 |
| CT + MPH | 20.8 | 4.3 | 1.7 | 0.53 | 2.0 | 0.50 | 8 |
| CT + l-dopa | 19.0 | 5.7 | 1.8 | 0.45 | 1.6 | 0.35 | 9 |
| OPG + MPH | 21.6 | 5.7 | 1.5 | 0.43 | 2.0 | 0.48 | 6 |
| OPG + l-dopa | 19.6 | 11.3 | 2.6 | 1.47 | 2.0 | 0.85 | 6 |
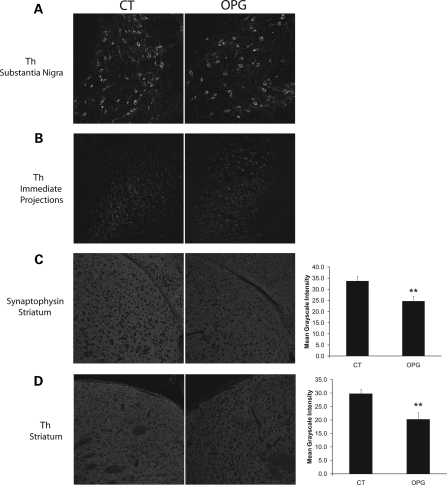
To determine whether the reduced DA in the striatum of Nf1 OPG mice reflected increased neuronal cell loss, we quantitated the number of TH-immunoreactive cells (DA neurons) and total cells (TO-PRO3) in the substantia nigra. No differences in the number of TH-positive cells (Fig. 7A) or total cells (data not shown) were observed in Nf1 OPG relative to control littermate mice. Since dopaminergic neuronal loss did not account for the lower DA levels in Nf1 OPG mice, we next measured the expression of TH, the rate-limiting step in DA synthesis, in the striatum of Nf1 OPG and control littermate mice. We found that Th mRNA expression was reduced by 40% in Nf1 OPG mice as assessed by real-time quantitative PCR observed (P = 0.02; n = 7) (Fig. 7B). Collectively, these results elucidate the mechanism underlying the reduced exploratory behavior in Nf1 OPG mice, and suggest that reduced DA synthesis by neurofibromin is a major etiologic factor.
Figure 7.
Nf1 OPG mice have normal numbers of dopaminergic neurons, but express reduced TH mRNA levels in the striatum. (A) TH-positive neuronal cell counts in the substantia nigra were similar in Nf1 OPG mice and littermate control (CT) mice (n = 6). (B) qPCR analysis of Th mRNA levels in the corpus striatum showed a significant decrease in Nf1 OPG mice compared with littermate controls (P = 0.02).
The reduced DA levels in Nf1 OPG mice reflect impaired Nf1+/− dopaminergic neuron striatal innervation
The observed reductions in Th mRNA expression and DA levels in Nf1 OPG mice could also reflect abnormalities in dopaminergic neuronal circuit integrity (21,22). Since reduced DA levels were observed in Nf1 OPG mice, but not in mice heterozygous for an inactivating mutation in the Nf1 gene (Nf1+/− mice), we employed Rosa reporter mice to determine whether Cre-mediated inactivation of neurofibromin occurred in Nf1 OPG mice. For these experiments, the GFAP-Cre strain used to generate the Nf1 OPG mice was crossed with Rosa-GREEN reporter mice (23). In GFAP-Cre/Rosa-GREEN double-transgenic mice, green fluorescent protein (GFP) expression should only be detected in cells arising from progenitors in which Cre recombinase was expressed. However, no TH-immunoreactive cells were GFP-positive by double-labeling immunofluorescence (Supplementary Material, Fig. S1), demonstrating that dopaminergic neurons in Nf1 OPG mice were heterozygous for an inactivating mutation in the Nf1 gene, and were not deficient for neurofibromin expression.
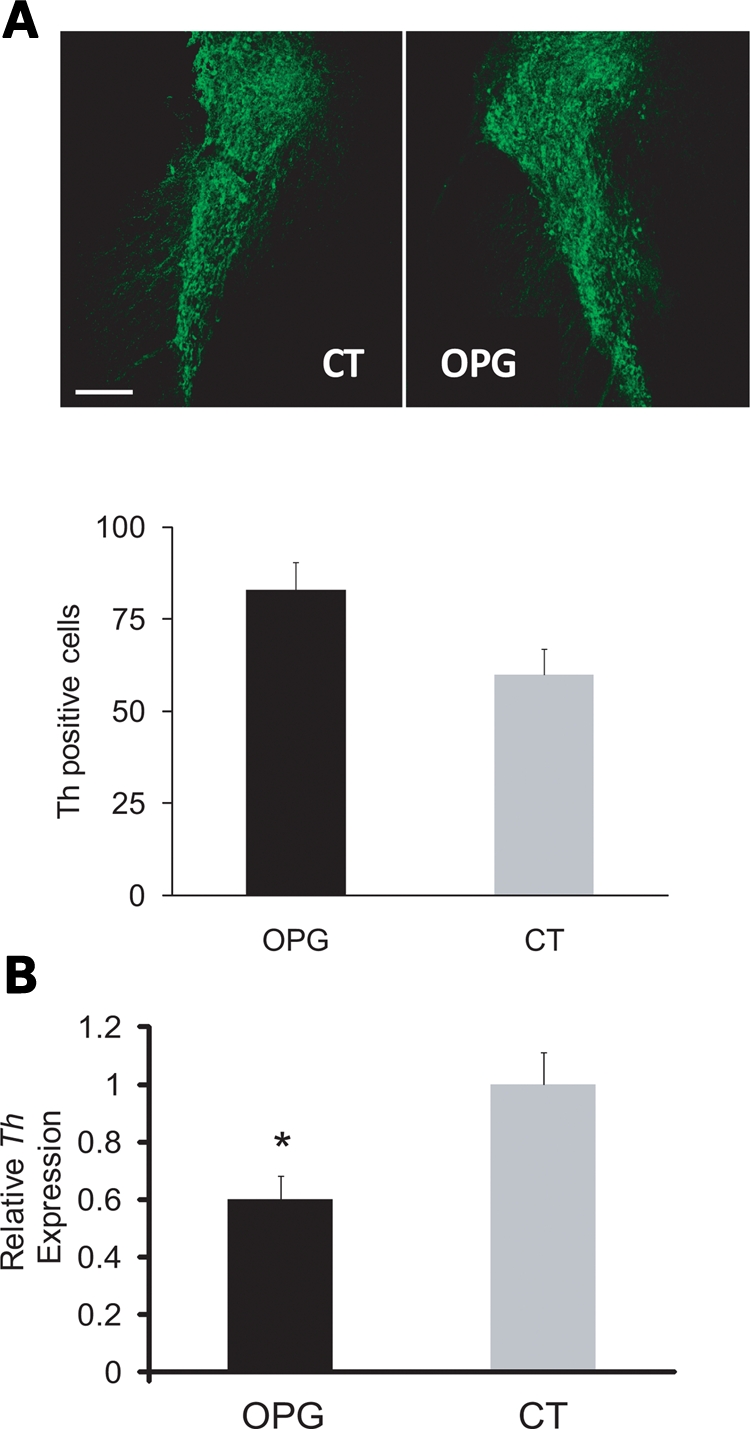
Since the dopaminergic neurons in Nf1 OPG mice are Nf1+/− cells, we next sought to determine whether a cell-autonomous defect in neuronal integrity existed. Similar to our previous observations using Nf1+/− retinal ganglion and hippocampal neurons (24), TH-immunoreactive Nf1+/− neurons from the ventral midbrain had shorter neurite lengths (∼50% decrease, P = 0.0001; Fig. 8A) and smaller growth cone areas (∼50% decrease, P = 0.001; Fig. 8B) compared with their wild-type counterparts. These results support the existence of a cell-autonomous neurite projection defect in Nf1+/− dopaminergic neurons.
Figure 8.
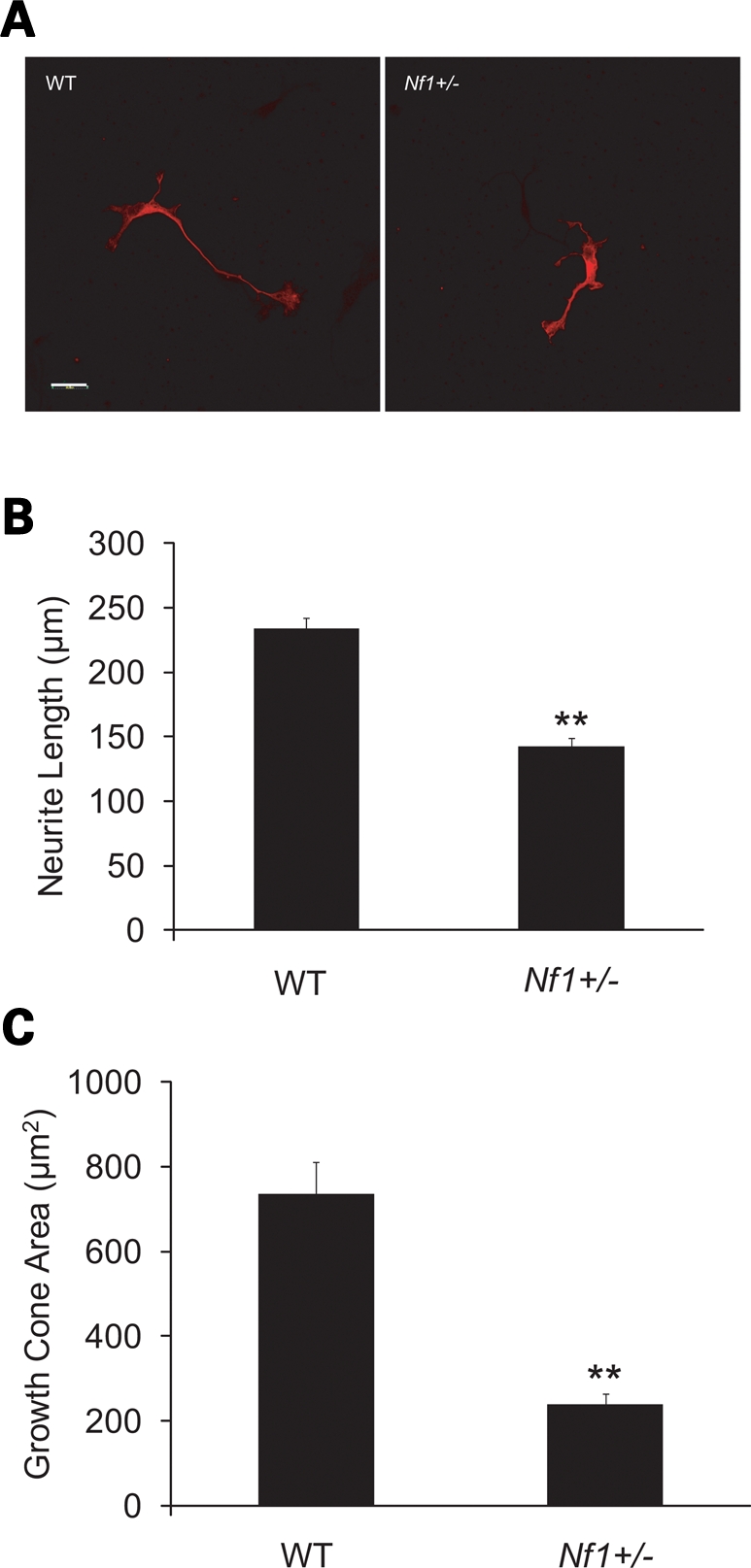
Nf1+/− dopaminergic neurons have reduced neurite lengths and smaller growth cone areas. (A) TH-immunoreactive neurons from the ventral midbrain of E13 wild-type (WT) and Nf1+/− mice. (B) Nf1+/− TH-immunoreactive neurons have shorter neurite lengths compared with WT controls (P = 0.001; n = 30). (C) Nf1+/− TH-immunoreactive neurons have smaller growth cone areas relative to WT controls (P = 0.001; n = 30). Scale bars = 50 µm.
To determine whether the reduction in Nf1+/− dopaminergic neurite extension was also observed in Nf1 OPG mice in vivo, we examined dopaminergic and total neuron densities using TH and synaptophysin immunostaining, respectively. Since dopaminergic neurons project from the substantia nigra and synapse on neurons in the striatum, we quantified the immunostaining intensity in both the substantia nigra (cell bodies) and the striatum (neurite projections). There was no decrease in the intensity of TH labeling in the cell bodies of dopaminergic neurons in the substantia nigra (Fig. 9A) or their immediate projections (Fig. 9B) in Nf1 OPG mice relative to littermate controls. In contrast, we found reduced synaptophysin (9.5% decrease; P = 0.0001, n = 8; Fig. 9C) and TH (9% decrease; P = 0.0001, n = 8; Fig. 9D) staining in the striatum of Nf1 OPG mice compared with littermate controls. Collectively, these results support a model in which a cell-autonomous defect in Nf1+/− dopaminergic neuronal projections results in reduced DA levels in the brains of mice with Nf1 loss in glial cells.
Figure 9.
Nf1 OPG mice have reduced dopaminergic projections in the striatum. (A) No change in the numbers of TH-immunoreactive neurons was observed in the substantia nigra of Nf1 OPG mice relative to littermate controls. (B) No differences in TH staining intensity were observed in the immediate neuronal projections from the substantia nigra in Nf1 OPG mice relative to littermate controls. (C) Synaptophysin immunolabeling of neurons in the striatum demonstrated a 9.5% reduction in mean intensity in Nf1 OPG mice relative to littermate controls (P = 0.0001; n = 8). (D) TH immunolabeling of neurons in the striatum demonstrated a 9% reduction in mean intensity in Nf1 OPG mice relative to littermate controls (P = 0.0001; n = 8). Scale bars = 100 µm.
DISCUSSION
While NF1 is primarily considered a tumor predisposition syndrome, one of the most devastating complications of this disorder is poor scholastic performance. This is partly the result of specific learning disabilities, including problems with spatial memory and learning, verbal memory, motor speed and spatial relations. However, one of the most frequently overlooked co-morbid abnormalities involves attention system dysfunction, present in 50–80% of children with NF1 (7,25). Children with NF1 exhibit problems with sustained attention, switching attention, selective attention and divided attention (11). In addition, some of these children will also manifest a hyperactivity phenotype, prompting treatment with stimulants such as MPH. The profound problems with attention experienced by these children interfere with their cognitive performance, and may underlie the dramatic discrepancies in verbal and performance IQ (1,2,5,11).
In this report, we uncover new attention system deficits in Nf1 OPG mice and define an underlying neurochemical defect that may contribute to these and other cognitive disturbances. We showed that Nf1 OPG mice retain the mild spatial learning/memory impairments in the water maze first described in Nf1+/− mice without brain tumors (13). Similar to Nf1+/− F1 mice generated from 129sv ×C57BL/6 crosses, Nf1 OPG mice exhibit compromised spatial memory. However, it is worth noting that the probe trial performance deficits observed in Nf1 OPG mice were transient in nature, such that Nf1 OPG mice performed as well as control mice on the second probe trial at the end of acquisition training. These results indicate that the Nf1 GEM model spatial learning/memory deficits do not reflect an inability to learn, but rather that these mice require additional acquisition training to perform as well as their control littermates. This reduced learning/memory performance is similar to that observed in children with NF1, where learning certain problems can be overcome with additional training (11).
In addition, we identified novel attention dysfunction in Nf1 OPG mice without an associated hyperactivity phenotype. Previous studies revealed selective attention deficits in Nf1+/− mice, as evidenced by impaired performance on the lateralized reaction-time task, a highly structured test utilizing operant behavior techniques (16). Our studies confirm and extend these observations through the analysis of spontaneously occurring behaviors in response to novel environmental events. Using a battery of behavioral tests, we identified reduced rearing frequency during the 1 h locomotor activity test in Nf1 OPG mice compared with control littermates. This reduced rearing was particularly striking during the early stages of the test session, indicative of an attenuated response to novelty. Consistent with this interpretation, additional testing revealed that control mice exhibited increased exploration of the novel object compared with the familiar one, whereas Nf1 OPG mice did not. Furthermore, the frequency of rearing during investigation of the novel object was significantly lower in Nf1 OPG mice compared with controls. However, Nf1 OPG mice also demonstrated deficits in non-selective attention, including reduced exploratory behaviors, rearing frequency and rearing duration in the field. Together, these findings support the existence of deficits in both selective and non-selective attention function.
Since the Nf1 GEM strain used in the present work develops a low-grade glial cell neoplasm affecting the prechiasmatic optic nerve and chiasm (26), which results in abnormal visual-evoked responses on electrophysiologic testing and low levels of retinal ganglion cell apoptosis (26,27), we formally evaluated performance on behaviorally based tests of visual function. In these studies, Nf1 OPG mice were indistinguishable from control mice on visual optomotry system tests of visual (grating) acuity and contrast thresholds under photopic conditions. The results of the VOS testing are consistent with those from the cued condition in the Morris water maze, and collectively indicate that the observed performance deficits were unlikely to reflect poor vision. Similarly, swimming and rearing can be affected by compromised sensorimotor performance, prompting us to conduct rotarod testing to evaluate subtle coordination and balance dysfunction in Nf1 OPG mice. The results of the rotarod testing were in complete agreement with data from the sensorimotor battery, arguing against the presence of motor, coordination or balance abnormalities in Nf1 OPG mice.
Having established a robust attention deficit though multiple behavioral tests in Nf1 OPG mice, we next sought to determine whether abnormalities in DA system function were directly responsible for these deficits. Since children with NF1 and attention system dysfunction are commonly treated with MPH, we initially demonstrated that the abnormal rearing behaviors in Nf1 OPG mice could be ameliorated with a single injection of MPH. Although MPH increases ambulations in control mice, it does not increase rearing behavior. However, in Nf1 OPG mice, both total ambulations (exploration) and rearings were significantly increased following MPH administration. Since MPH increases catecholamine levels of DA and norepinephrine through blockade of their reuptake transporters (28) as well as potentially affecting the serotonin pathway (28,29), we demonstrated that administration of l-dopa rescues the exploration and rearing behavior deficits in Nf1 OPG mice similar to MPH, arguing that serotonin is unlikely to be responsible for this attention phenotype. This was confirmed by direct HPLC measurements of DA in the striatum of Nf1 OPG mice, where 40% reductions in DA and DA breakdown product levels were found. Consistent with the reversal of the behavioral deficits in Nf1 OPG mice following MPH and l-dopa treatment, these treatments also restored DA levels in the striata of Nf1 OPG mice.
Several possibilities could explain the reduced DA levels in Nf1 OPG mice. First, DA degradation might be increased. However, the unchanged ratios of DA to its breakdown products in Nf1 OPG compared with control mice do not support a defect in DA degradation. Second, reduced DA levels could reflect loss of dopaminergic neurons. We quantified the number of dopaminergic neurons in the substantia nigra, which supplies most of the DA projections for the striatum, and found no reduction in TH-immunoreactive or total cell numbers in Nf1 OPG mice relative to control mice. Third, it is possible that reduced DA levels result from decreased DA synthesis. We therefore measured the expression of TH, the rate-limiting enzyme for DA synthesis, and found attenuated mRNA expression in Nf1 OPG mice, suggesting that an abnormality in DA production might underlie these attention system abnormalities (30,31).
The reduction in Th mRNA expression could alternatively reflect defects in the integrity of dopaminergic neuronal connections. Previous studies from our laboratory have revealed a cell-autonomous reduction in neurite lengths and growth cone areas in Nf1+/− retinal ganglion and hippocampal neurons (24). Consistent with the observed reductions in striatal DA levels, we found reduced neurite lengths and growth cone areas in Nf1+/− dopaminergic neurons in vitro and reduced dopaminergic synaptic density in Nf1 OPG mice in vivo. Collectively, these findings support a model in which dopaminergic neurons with reduced neurofibromin expression exhibit impairments in neurite function, which likely culminate in decreased dopaminergic synapses in the striatum. These defects in DA homeostasis could result from abnormalities in the function of existing neurons or the birth of new neurons from neuroglial progenitors. In this regard, neurons differentiating from Nf1+/− or Nf1−/− neural stem cells also exhibit reduced neurite lengths (32). Future studies will be required to explore the impact of Nf1 gene dosage on neurogenesis relevant to learning and behavior in adult mice (33–35).
Finally, while we observed a cell-autonomous defect in neuronal function in Nf1+/− neurons, Nf1+/− mice exhibit no reduction in DA levels. Similarly, mice with GFAP-Cre-mediated loss of neurofibromin expression in astrocytes (Nf1GFAPCKO mice) also have normal DA levels. The fact that only Nf1 OPG mice (Nf1+/− mice with Nf1 loss in GFAP+ cells) have reduced DA levels suggests that reduced striatal DA homeostasis requires cooperation between Nf1+/− neurons and Nf1−/− glial cells. This obligate interaction between Nf1+/− neurons and Nf1−/− astrocytes is also observed in the optic pathway, where Nf1+/− neurons exhibit increased cell death only when coupled with Nf1 loss in astrocytes or in response to injury in vivo (24).
In summary, we have established a new GEM model of NF1-associated attention deficits and have identified the neurochemical defect underlying this abnormality. The discovery of reduced dopaminergic pathway integrity and resulting decreased DA levels provides a mechanistic explanation for the observed attention system abnormalities in these mice, and supports the clinical utility of MPH and related drugs for the treatment of attention system dysfunction in children with NF1.
MATERIALS AND METHODS
Mice
Nf1+/−GFAPCKO, Nf1+/−, GFAP-Cre and Nf1GFAPCKO mice were generated as described previously (36–38) and maintained on an inbred C57BL/6 background. Littermate controls were used for all experiments. Rosa-GREEN mice were obtained from the Jackson Laboratories (23). Nf1flox/flox mice are identical to wild-type mice. Mice had ad libitum access to food and water. All experiments were performed under active Animal Studies Committee protocols at the Washington University School of Medicine.
Behavioral experiments
Mice were evaluated behaviorally on the following tests described below in greater detail: (i) 1 h locomotor activity and exploration, (ii) a battery of sensorimotor measures (walking initiation, ledge, inclined and inverted screens and pole), (iii) the Morris water navigation task to assess spatial learning and memory, (iv) the object recognition test plus contemporaneous analysis of rearing behavior to study selective and non-selective components of attention and their relation to memory retention, (v) the rotarod procedure to evaluate complex motor coordination and balance and (vi) the VOS to quantify visuospatial thresholds.
One-hour locomotor activity/exploratory behavior
Locomotor and exploratory activity was evaluated over a 1 h period by placing individual mice into transparent (47.6 × 25.4 × 20.6 cm high) polystyrene enclosures as described previously (39). Each enclosure was surrounded by a frame containing a 4 × 8 matrix of photocell pairs, the output of which was fed to an online computer (Hamilton-Kinder, LLC, Poway, CA, USA). The system software (Hamilton-Kinder, LLC) was used to define a 33 × 11 cm central zone and a peripheral or surrounding zone that was 5.5 cm wide, with the sides of the cage being the outermost boundary. Another frame that contained eight pairs of photocells, and which was raised ∼7 cm above the floor of the enclosure, was used to quantify vertical rearing activity. Variables that were analyzed included the total number of ambulations (whole body movements) and vertical rearings, as well as the distance traveled in the center and peripheral zone, and the time spent in and entries made into the center.
Sensorimotor measures
A battery of sensorimotor tests was administered to assess balance, strength, coordination and initiation of movement using previously published methods (39). The battery included ledge and platform tests that involved determining how long a mouse could remain on an elevated, narrow (0.75 cm wide) plexiglas ledge or on an elevated small circular wooden platform (1.0 cm thick, 3.0 cm diameter), respectively. A walking initiation test was also performed by determining the time it took a mouse to move out of a small square (21 × 21 cm) outlined on a black tabletop, while general coordination and strength were studied through the use of the pole and inclined and inverted screen tests. The pole test involved placing a mouse ‘head up’ on top of a finely textured rod (diameter 8 mm; height 55 cm) and determining the time it took the mouse to turn and climb down the pole. In 60° and 90° inclined screen tests, mice were placed in the middle of elevated wire mesh grids at two different inclinations (60° or 90°) with their heads oriented down, and the time for the mouse to turn and climb to the top of the screen was determined. In the inverted screen test, a mouse was placed on the 60° inclined screen, which was then inverted, and the time that the mouse remained upside down on the screen before falling was determined. Two trials were administered for each test in the battery, and means were computed for each mouse.
Spatial learning and memory
Spatial learning and memory were evaluated using the Morris water navigation test, which involved using methods similar to those described previously (39). The procedure included cued trials (visible platform, varied location, four trials/day, 2 days) to determine whether non-associative factors were likely to affect acquisition performance, followed by place trials (submerged platform, fixed location, four trials/day, 5 days), with probe trials being conducted 1 h after the last place trial on day 3 and day 5. Escape path length (distance traveled to the platform) and latency (time to reach the platform) for cued and place trials were recorded by a computerized tracking system and swimming speeds were also calculated. Time spent in the target quadrant where the platform had been located, the percent of distance traveled in the target quadrant out of the total distance traveled during the trial, spatial bias for the target quadrant (target quadrant time versus times in each of the other quadrants) and number of crossing made over the platform location served as dependent variables for assessing probe trial performance.
Object recognition
Object recognition was evaluated using a variant of our previously published procedure (17) which presently also included contemporaneous analysis of rearing behavior. Mice were habituated to the test arena (8.5 × 17 × 8 in.) for 20 min per day, for 2 days before testing. Each mouse received a sample trial and two test trials. During the sample trial, mice were placed in a familiar arena with two copies of the same object and allowed to explore for 10 min, and then returned to their home cage. Following a 50 min delay, the mice were placed back in the arena, where a novel object was presented along with the copy of the familiar object (position counterbalanced) that had been explored during the sample trial, and exploration was allowed for 10 min. Fifty minutes later, the familiar object was moved to an opposite corner of the arena and exploration was allowed for 10 min. Objects and test arena were cleaned with 70% ethanol after each trial. Sessions were video recorded for later analysis. The amount of time each animal spent actively investigating the objects was manually scored using Stopwatch and software (CBN, Emory University, Atlanta, GA, USA). Rearing behavior was also scored for each animal in relation to the objects and in the field away from the objects and during object investigation. The amount of time the mouse spent investigating the novel object, compared with the familiar object, was analyzed as a measure of recognition memory as described previously (17,40).
Rotarod
Motor coordination and balance were further studied using our previously published rotarod procedures (41), which included three conditions: a stationary rod; a rod that rotated at a constant speed (5 rpm for 60 s maximum); and a rod that rotated at an accelerating speed (5–20 rpm over 1–180 s maximum). The protocol consisted of three training sessions, with each session including one stationary rod trial, two constant speed rotarod trials and two accelerating rotarod trials. Sessions were separated by 4 days to minimize motor learning, and time spent on the rod was used as the dependent variable.
Virtual optomotry system
Visual acuity and contrast thresholds were assessed using the VOS as described previously (42). The apparatus consists of a virtual cylinder with a vertical sine-wave grating, which was projected in three-dimensional coordinate space on computer monitors arranged in a quadrangle (square) around a testing arena containing a central platform. A camera (FireWire iSight; Apple Computer Corp., Mountain View, CA, USA) was positioned directly above the platform to observe the behavioral responses of a mouse. Visual stimuli (light and dark bars) were projected on the walls of the cylinder to give the appearance of rotating in a clockwise or counterclockwise direction around the mouse. This perceived rotation induced optokinetic head/body tracking movements in the mice. Using psychophysical methods (staircase or methods of limits), thresholds for visuospatial acuity were generated by increasing the frequency of the sine-wave grating until individual bars were no longer perceived, thus eliminating the optokinetic tracking response. An observer, who was ‘blinded' to the genotype of the mouse and the direction of grating rotation, judged the presence or absence (and direction) of the optokinetic response by watching the mouse on a computer screen. Similar methods were used to derive contrast thresholds by varying the illumination levels of the bars. The speed of rotation, geometry of the cylinder and the spatial frequency and contrast of the grating stimuli were controlled by the system software, which also enabled live video feedback of the testing arena. Contrast thresholds were measured at a frequency of 0.128 cycles/degree and speed of 5.4°/s, and all testing was done under photopic conditions (1.8 log cd/m2).
Drug treatments
At 3 months of age, when nearly 100% of the mice develop OPG, OPG and littermate control mice received single intraperitoneal injections of l-dopa (50 mg/kg; Sigma, St Louis, MO, USA) dissolved in 2.5 mg/ml solution of ascorbic acid in PBS (43) 3 h prior to euthanasia by decapitation. Brains were then removed for analysis. Alternatively, mice received intraperitoneal injections of MPH (20 mg/kg; Sigma) in saline. Saline (0.9% NaCl) was given as an injection control. For the behavior tests, mice were injected with l-dopa and tested 3 h after injection or injected with MPH and tested immediately after injection.
DA measurements
The right corpora striata specimens were immediately homogenized in lysis buffer (0.1 N perchloric acid, 0.4 mm Na2S2O5) for HPLC analysis of DA levels. DA levels were determined 3 h after l-dopa or saline treatment and 15 min after MPH treatment. Samples were diluted 1:20 in HPLC buffer (0.1 M NaH2PO4, 0.25 g/l heptanesulfonic acid, 0.08 g/l EDTA, 6% methanol, pH 2.5), separated on a catecholamine ESA (Chelmsford, MA, USA) HR-80 column, using the same HPLC buffer for the mobile phase, and analyzed by electrochemical detection (ESA). Samples were analyzed in triplicate.
TH-positive cell quantitation
Fifty micron frozen sections were serially cut, beginning at the level of the substantia nigra and continuing throughout the length of the brainstem. Each section was rinsed three times for 5 min in PBS, permeabilized with 0.3% Triton X-100 in PBS for 30 min at room temperature and incubated in 10% serum blocking solution for 1 h at room temperature prior to incubation of TH primary antibody (1:1000 dilution; Abcam, MA, USA) for 1 h at room temperature. After each section was rinsed three times for 5 min, Alexa Fluor 488 (1:200 dilution; Invitrogen, Eugene, OR, USA) secondary antibody was applied for 1 h at room temperature. One micromolar TO-PRO-3 (Sigma) was added for 15 min at room temperature and the sections were coverslipped for analysis. TH-immunoreactive cells were quantified by counting all the cell bodies in the substantia nigra from at least three serial sections collected from each mouse brain at 20× objective magnification.
Real-time quantitative reverse transcription-PCR
Total RNA was extracted from the hippocampus, freshly isolated from E15 mice using Trizol reagent, as recommended by the manufacturer (Invitrogen). cDNA was synthesized after NanodropTM analysis from total RNA samples using the Omniscript kit (Qiagen). Real-time PCR was performed using SYBR Green detection (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. Target amplification was performed in 96-well plates in a real-time sequence detection system instrument (CFX98 Bio-RAD, CA, USA). The primer set used for TH was: (S) 5′-AAC CCA TTG GAG GCT GTG GTA T-3′ and (AS) 5′-ACT TTC AAA GCC CGA GAC AGT GAG-3′. Bio-RAD system software was used to convert the fluorescent data into cycle threshold measurements. The ΔΔCT method was used to calculate changes in fold expression relative to Nf1+/+ hippocampus samples using β-actin as an internal control.
Cell culture
Dopaminergic neuronal cell cultures were prepared as described previously (44) using Hibernate-E for dissection media. The ventral midbrain was dissociated in HBSS containing 1% papain (Worthington Biochemicals, Lakewood, NJ, USA) and 5 U/ml DNase (Gibco), transferred to a solution containing 1% ovomucoid (Worthington Biochemicals) and plated in DMEM + 10% fetal calf serum for 4 h before the media were switched to neural basal medium with B27 and 2 mm l-glutamine for an additional 3 days.
Structural analysis of growth cone areas and neurite lengths
Growth cone areas were measured using Image-J, starting at the neck and tracing around the growth cone (24). The mean, standard error and P-value were calculated from at least 30 randomly selected growth cones of TH-immunopositive neurons per genotype. Neurite lengths were measured using Image-J, and only TH-immunopositive neurons with intact growth cones were included (24). Neurites were traced starting at the outer edge of the cell body, so that the cell body size would not skew the measurements. As before, at least 30 randomly selected neurites per genotype were used to calculate the mean, standard error and P-value. The investigators were always blinded to the genotype of the neurons.
Immunofluorescence labeling and quantification
Thirty micron sections were prepared as described previously (26). Images were acquired using an Olympus confocal or an inverted microscope equipped with a Cooke Sensicam at the same level of section for each animal. Immunostaining with TH (Abcam; 1:1000 dilution) or synaptophysin (Millipore; 1:100 dilution) was performed using established protocols in our laboratory. Images acquired using phase confocal microscopy were analyzed for staining intensity using Image-J, where eight random fields within an image were quantitated to obtain a mean intensity.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported in part by grants from the National Cancer Institute (U01-CA141549-01 to D.H.G.) and Department of Defense (D.H.G. and D.F.W.), and by an NIH Neuroscience Blueprint Interdisciplinary Center Core Grant, P30 NS057105 (D.F.W.).
ACKNOWLEDGEMENTS
We appreciate the excellent technical assistance of Steve Harmon in the O'Malley laboratory for HPLC analyses.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Coude F.X., Mignot C., Lyonnet S., Munnich A. Academic impairment is the most frequent complication of neurofibromatosis type-1 (NF1) in children. Behav. Genet. 2006;36:660–664. doi: 10.1007/s10519-005-9040-9. [DOI] [PubMed] [Google Scholar]
- 2.Descheemaeker M.J., Ghesquiere P., Symons H., Fryns J.P., Legius E. Behavioural, academic and neuropsychological profile of normally gifted Neurofibromatosis type 1 children. J. Intellect. Disabil. Res. 2005;49:33–46. doi: 10.1111/j.1365-2788.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 3.Dilts C.V., Carey J.C., Kircher J.C., Hoffman R.O., Creel D., Ward K., Clark E., Leonard C.O. Children and adolescents with neurofibromatosis 1: a behavioral phenotype. J. Dev. Behav. Pediatr. 1996;17:229–239. [PubMed] [Google Scholar]
- 4.Hyman S.L., Shores A., North K.N. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- 5.North K.N., Riccardi V., Samango-Sprouse C., Ferner R., Moore B., Legius E., Ratner N., Denckla M.B. Cognitive function and academic performance in neurofibromatosis 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121–1127. doi: 10.1212/wnl.48.4.1121. [DOI] [PubMed] [Google Scholar]
- 6.Reimers T.S., Ehrenfels S., Mortensen E.L., Schmiegelow M., Sonderkaer S., Carstensen H., Schmiegelow K., Muller J. Cognitive deficits in long-term survivors of childhood brain tumors: identification of predictive factors. Med. Pediatr. Oncol. 2003;40:26–34. doi: 10.1002/mpo.10211. [DOI] [PubMed] [Google Scholar]
- 7.Rosser T.L., Packer R.J. Neurocognitive dysfunction in children with neurofibromatosis type 1. Curr. Neurol. Neurosci. Rep. 2003;3:129–136. doi: 10.1007/s11910-003-0064-3. [DOI] [PubMed] [Google Scholar]
- 8.Schrimsher G.W., Billingsley R.L., Slopis J.M., Moore B.D., 3rd Visual-spatial performance deficits in children with neurofibromatosis type-1. Am. J. Med. Genet. A., 2003;120A:326–330. doi: 10.1002/ajmg.a.20048. [DOI] [PubMed] [Google Scholar]
- 9.Barton B., North K. Social skills of children with neurofibromatosis type 1. Dev. Med. Child. Neurol. 2004;46:553–563. doi: 10.1017/s0012162204000921. [DOI] [PubMed] [Google Scholar]
- 10.Maddrey A.M., Bergeron J.A., Lombardo E.R., McDonald N.K., Mulne A.F., Barenberg P.D., Bowers D.C. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J. Neurooncol. 2005;72:245–253. doi: 10.1007/s11060-004-3009-z. [DOI] [PubMed] [Google Scholar]
- 11.Hyman S.L., Arthur Shores E., North K.N. Learning disabilities in children with neurofibromatosis type 1: subtypes, cognitive profile, and attention-deficit-hyperactivity disorder. Dev. Med. Child. Neurol. 2006;48:973–977. doi: 10.1017/S0012162206002131. [DOI] [PubMed] [Google Scholar]
- 12.Mautner V.F., Kluwe L., Thakker S.D., Leark R.A. Treatment of ADHD in neurofibromatosis type 1. Dev. Med. Child. Neurol. 2002;44:164–170. doi: 10.1017/s0012162201001876. [DOI] [PubMed] [Google Scholar]
- 13.Silva A.J., Frankland P.W., Marowitz Z., Friedman E., Laszlo G.S., Cioffi D., Jacks T., Bourtchuladze R. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat. Genet. 1997;15:281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- 14.Aspide R., Fresiello A., de Filippis G., Gironi Carnevale U.A., Sadile A.G. Non-selective attention in a rat model of hyperactivity and attention deficit: subchronic methylphenydate and nitric oxide synthesis inhibitor treatment. Neurosci. Biobehav. Rev. 2000;24:59–71. doi: 10.1016/s0149-7634(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 15.Vallone D., Pignatelli M., Grammatikopoulos G., Ruocco L., Bozzi Y., Westphal H., Borrelli E., Sadile A.G. Activity, non-selective attention and emotionality in dopamine D2/D3 receptor knock-out mice. Behav. Brain Res. 2002;130:141–148. doi: 10.1016/s0166-4328(01)00428-4. [DOI] [PubMed] [Google Scholar]
- 16.Li W., Cui Y., Kushner S.A., Brown R.A., Jentsch J.D., Frankland P.W., Cannon T.D., Silva A.J. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr. Biol. 2005;15:1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Yuede C.M., Zimmerman S.D., Dong H., Kling M.J., Bero A.W., Holtzman D.M., Timson B.F., Csernansky J.G. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiol. Dis. 2009;35:426–432. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borycz J., Zapata A., Quiroz C., Volkow N.D., Ferre S. 5-HT 1B receptor-mediated serotoninergic modulation of methylphenidate-induced locomotor activation in rats. Neuropsychopharmacology. 2008;33:619–626. doi: 10.1038/sj.npp.1301445. [DOI] [PubMed] [Google Scholar]
- 19.Jaber M., Bloch B., Caron M.G., Giros B. Behavioral, cellular and molecular consequences of the dopamine transporter gene inactivation. C R. Seances Soc. Biol. Fil. 1998;192:1127–1137. [PubMed] [Google Scholar]
- 20.Oades R.D. Dopamine–serotonin interactions in attention-deficit hyperactivity disorder (ADHD) Prog. Brain. Res. 2008;172:543–565. doi: 10.1016/S0079-6123(08)00926-6. [DOI] [PubMed] [Google Scholar]
- 21.Georgievska B., Kirik D., Bjorklund A. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp. Neurol. 2002;177:461–474. doi: 10.1006/exnr.2002.8006. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson D., Ramirez A., Long J., Barrezueta N., Hajos-Korcsok E., Matherne C., Gallagher D., Ryan A., Ochoa R., Menniti F., et al. Quantification of MPTP-induced dopaminergic neurodegeneration in the mouse substantia nigra by laser capture microdissection. J. Neurosci. Methods. 2007;159:291–299. doi: 10.1016/j.jneumeth.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown J.A., Gianino S.M., Gutmann D.H. Defective cyclic AMP generation underlies the sensitivity of central nervous system neurons to neurofibromatosis-1 heterozygosity. J. Neurosci. 2010;30:5579–5589. doi: 10.1523/JNEUROSCI.3994-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eldridge R., Denckla M.B., Bien E., Myers S., Kaiser-Kupfer M.I., Pikus A., Schlesinger S.L., Parry D.M., Dambrosia J.M., Zasloff M.A., et al. Neurofibromatosis type 1 (Recklinghausen's disease). Neurologic and cognitive assessment with sibling controls. Am. J. Dis. Child. 1989;143:833–837. [PubMed] [Google Scholar]
- 26.Banerjee D., Hegedus B., Gutmann D.H., Garbow J.R. Detection and measurement of neurofibromatosis-1 mouse optic glioma in vivo. Neuroimage. 2007;35:1434–1437. doi: 10.1016/j.neuroimage.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegedus B., Banerjee D., Yeh T.H., Rothermich S., Perry A., Rubin J.B., Garbow J.R., Gutmann D.H. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68:1520–1528. doi: 10.1158/0008-5472.CAN-07-5916. [DOI] [PubMed] [Google Scholar]
- 28.Markowitz J.S., DeVane C.L., Pestreich L.K., Patrick K.S., Muniz R. A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: an exploratory study. J. Child. Adolesc. Psychopharmacol. 2006;16:687–698. doi: 10.1089/cap.2006.16.687. [DOI] [PubMed] [Google Scholar]
- 29.Wilens T.E. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 2008;28:46–53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- 30.Glavan G., Zivin M. Differential expression of striatal synaptotagmin mRNA isoforms in hemiparkinsonian rats. Neuroscience. 2005;135:545–554. doi: 10.1016/j.neuroscience.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 31.Wong D.L., Tank A.W. Stress-induced catecholaminergic function: transcriptional and post-transcriptional control. Stress. 2007;10:121–130. doi: 10.1080/10253890701393529. [DOI] [PubMed] [Google Scholar]
- 32.Hegedus B., Dasgupta B., Shin J.E., Emnett R.J., Hart-Mahon E.K., Elghazi L., Bernal-Mizrachi E., Gutmann D.H. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Garthe A., Behr J., Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagace D.C., Donovan M.H., DeCarolis N.A., Farnbauch L.A., Malhotra S., Berton O., Nestler E.J., Krishnan V., Eisch A.J. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc. Natl Acad. Sci. USA. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao M., Momma S., Delfani K., Carlen M., Cassidy R.M., Johansson C.B., Brismar H., Shupliakov O., Frisen J., Janson A.M. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc. Natl Acad. Sci. USA. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bajenaru M.L., Hernandez M.R., Perry A., Zhu Y., Parada L.F., Garbow J.R., Gutmann D.H. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. [PubMed] [Google Scholar]
- 37.Bajenaru M.L., Zhu Y., Hedrick N.M., Donahoe J., Parada L.F., Gutmann D.H. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol. Cell. Biol. 2002;22:5100–5113. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brannan C.I., Perkins A.S., Vogel K.S., Ratner N., Nordlund M.L., Reid S.W., Buchberg A.M., Jenkins N.A., Parada L.F., Copeland N.G. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8:1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- 39.Wozniak D.F., Hartman R.E., Boyle M.P., Vogt S.K., Brooks A.R., Tenkova T., Young C., Olney J.W., Muglia L.J. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol. Dis. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Dere E., Huston J.P., De Souza Silva M.A. Episodic-like memory in mice: simultaneous assessment of object, place and temporal order memory. Brain Res. Protoc. 2005;16:10–19. doi: 10.1016/j.brainresprot.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Grady R.M., Wozniak D.F., Ohlemiller K.K., Sanes J.R. Cerebellar synaptic defects and abnormal motor behavior in mice lacking alpha- and beta-dystrobrevin. J. Neurosci. 2006;26:2841–2851. doi: 10.1523/JNEUROSCI.4823-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prusky G.T., Alam N.M., Beekman S., Douglas R.M. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest. Ophthalmol. Vis. Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Q.Y., Palmiter R.D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 44.Wang P., Niu L., Guo X.D., Gao L., Li W.X., Jia D., Wang X.L., Ma L.T., Gao G.D. Gypenosides protects dopaminergic neurons in primary culture against MPP(+)-induced oxidative injury. Brain. Res. Bull. 2010 doi: 10.1016/j.brainresbull.2010.06.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]