Abstract
The tumor suppressor gene PTEN (phosphatase and tensin homolog deleted on chromosome 10) and the androgen receptor (AR) play important roles in tumor development and progression in prostate carcinogenesis. Among many functions, PTEN negatively regulates the cytoplasmic phosphatidylinositol-3-kinase/AKT anti-apoptotic pathway; and nuclear PTEN affects the cell cycle by also negatively regulating the MAPK pathway via cyclin D. Decreased PTEN expression is correlated with prostate cancer progression. Over-expression of AR and upregulation of AR transcriptional activity are often observed in the later stages of prostate cancer. Recent studies indicate that PTEN regulates AR activity and stability. However, the mechanism of how AR regulates PTEN has never been studied. Furthermore, resveratrol, a phytoalexin enriched in red grapes, strawberries and peanuts, has been shown to inhibit AR transcriptional activity in prostate cancer cells. In this study, we use prostate cancer cell lines to test the hypothesis that resveratrol inhibits cellular proliferation in both AR-dependent and -independent mechanisms. We show that resveratrol inhibits AR transcriptional activity in both androgen-dependent and -independent prostate cancer cells. Additionally, resveratrol stimulates PTEN expression through AR inhibition. In contrast, resveratrol directly binds epidermal growth factor receptor (EGFR) rapidly inhibiting EGFR phosphorylation, resulting in decreased AKT phosphorylation, in an AR-independent manner. Thus, resveratrol may act as potential adjunctive treatment for late-stage hormone refractory prostate cancer. More importantly, for the first time, our study demonstrates the mechanism by which AR regulates PTEN expression at the transcription level, indicating the direct link between a nuclear receptor and the PI3K/AKT pathway.
INTRODUCTION
Prostate cancer is the most common cancer and the second leading cause of cancer mortality among men in the western world. In 2009, there were192 280 estimated new cases and 27 360 deaths in the USA (1). Prostate tumors are initially dependent on androgens for growth, but the majority of patients treated with anti-androgen therapy progress to androgen independence characterized by resistance to such treatment, portending a poor prognosis. The mechanism of progression to androgen independence remains unclear and, so far, there is no effective treatment for hormone-refractory prostate cancer.
The androgen receptor (AR) belongs to the nuclear receptor superfamily and can act as a transcription factor. AR is known to play important roles in reproductive system development and prostate cancer progression. In its inactive form, AR forms a complex with Hsp90/70 in the cytoplasm (2). Presence of ligand, such as dihydrotestosterone (DHT), induces AR phosphorylation and conformational change, resulting in its nuclear translocation and target-gene regulation. Over-expression of AR and upregulation of its transcriptional activity are often observed in advanced prostate cancer (3,4). Teleologically, this provides prostate cancer cells with a potential survival advantage under the low androgen levels after androgen deprivation treatment, and so, leads to progression to hormone refractory disease.
The tumor suppressor gene PTEN (phosphatase and tensin homolog deleted on chromosome 10), located on chromosome sub-band 10q23, is one of the most frequently mutated genes in a broad variety of human cancers (5). Through its phospholipid 3-phosphatase activity, PTEN negatively regulates the (PI3K)/AKT pathway, which is involved in cell proliferation, migration and apoptosis. Recently, it has been shown that PTEN can translocate into the nucleus, functioning as a protein phosphatase (6). Loss of PTEN expression is frequently found in prostate cancer cell lines and human non-cultured tumor specimens (7). As a result, AKT phosphorylation and activity are significantly increased, especially in androgen-independent prostate cancers (8). PTEN inhibits phosphorylation of AKT that, in turn, stimulates AR phosphorylation and activity (9). In addition, PTEN also directly interacts with the AR DNA-binding domain/Hinge domain and inhibits AR nuclear translocation and AR-mediated transcriptional activity (10). However, the inverse correlation of AR and PTEN expression in prostate cancers is not fully understood, which led us to hypothesize that AR must regulate PTEN to complete a feedback loop. Resveratrol (3,4′,5-trihydroxystilbene), a phytoalexin, commonly exists in a wide variety of fruits and plants, such as grapes, peanuts and raspberries (11). Among them, black-grape skin contains high concentrations of resveratrol, with the latter a major constituent of red wine. Epidemiologic studies have demonstrated the positive effect of resveratrol on lowering the risk of cardiovascular disease and certain cancers (12,13). In vitro, resveratrol has also been shown to inhibit proliferation of several types of cancer cells (14–16). There is sufficient promise that clinical trails are being carried out to determine the effectiveness of resveratrol treatment for skin and colon cancer.
We showed in our previous report that resveratrol increases PTEN expression in breast cancer cell lines and inhibits proliferation (17), together with recent observations that resveratrol inhibits AR transcriptional activity in androgen-dependent LNCaP prostate cancer cells (18). Therefore, we sought to first determine the role of resveratrol on prostate cancer cell proliferation in the context of AR-dependent and -independent pathways, and then to utilize resveratrol to interrogate the precise mechanism of AR regulation of PTEN and prostate cancer cell proliferation.
RESULTS
Resveratrol inhibits proliferation in both androgen-dependent and -independent prostate cancer cell lines
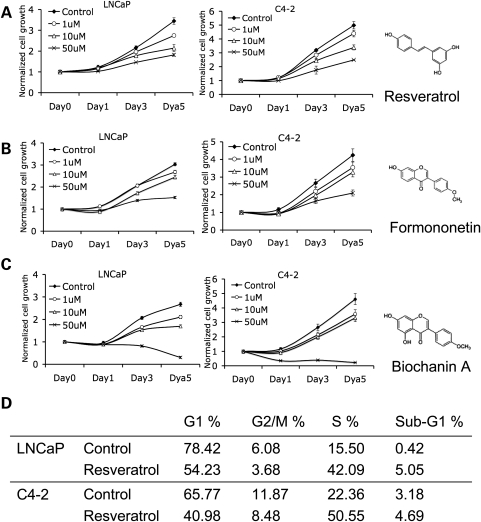
We first began by analyzing the effect of resveratrol on prostate cancer cell proliferation. We treated androgen-dependent LNCaP cells and its androgen-independent sub-clone, C4-2 cells, with three different dosages of resveratrol for 5 days, and measured growth each day. The chosen dosages, which were based on reported resveratrol concentration in red wine, have been widely used in previous studies (19,20). Growth of both androgen-dependent and -independent prostate cancer cells was inhibited by resveratrol, an effect that was obvious by Day 3 (Fig. 1A). We have selected three doses of resveratrol to include the believed physiological concentration after consumption of one to two glasses of red wine (usually accepted as 10–20 µm) and two points, one at either extreme. Accordingly, we have selected 10 µm to represent post-red wine consumption, and 1 µm for the lower end and 50 µm the upper extent. To determine whether other isoflavones affect prostate cancer cell growth, we treated LNCaP and C4-2 cells with Formononetin (Fig. 1B) or Biochanin A (Fig. 1C). Both drugs inhibited cell proliferation (Fig. 1B and C). Because there was evidence that 25 µm resveratrol induces S phase arrest in normal prostate epithelial cells (13), we treated LNCaP and C4-2 cells with this dose of resveratrol for 48 h followed by flow cytometry. This revealed S phase arrest in both androgen-dependent and -independent prostate cancer cells, while no changes in apoptosis capacity were noted (Fig. 1D). We also analyzed the cell cycle and apoptosis after 24 h of resveratrol exposure, with similar results (data not shown).
Figure 1.
Resveratrol inhibits prostate cancer cell proliferation. (A) Effect of resveratrol on LNCaP (left panel) and C4-2 (right panel) cell proliferation. Fifteen thousand cells were plated in each of 24-well plates in complete medium (FBS) and treated with DMSO (control) or different concentrations of resveratrol. Growth rates of the cells were assessed by MTT assay over a period of 5 days. Each growth-data point represents a mean value of three experiments and the error bars indicate the standard deviation, unless otherwise indicated. A Jonckheere–Terpstra trend test was performed to evaluate alternatives of ordered class differences by dose concentrations (P < 0.001). All statistical tests were two sided. Both cell lines show growth inhibition in response to increasing resveratrol doses. (B) Effect of Formononetin (P < 0.01) and (C) Biochanin A (P < 0.001) on LNCaP and C4-2 cell growth rates as assessed by the MTT assay. Molecular structures of the three phytoestrogens are shown as inserts on the right of the growth curves. (D) Flow cytometric analysis of the effects of resveratrol on proliferation and apoptosis (sub-G1). LNCaP and C4-2 cells were treated with 25 µm resveratrol for 48 h, ethanol-fixed, stained with propidium iodide and subjected to flow cytometry. The fraction of cells in the different phases of the cell cycle and those undergoing apoptosis was measured as described.
Resveratrol inhibits the AR pathway in prostate cancer cells
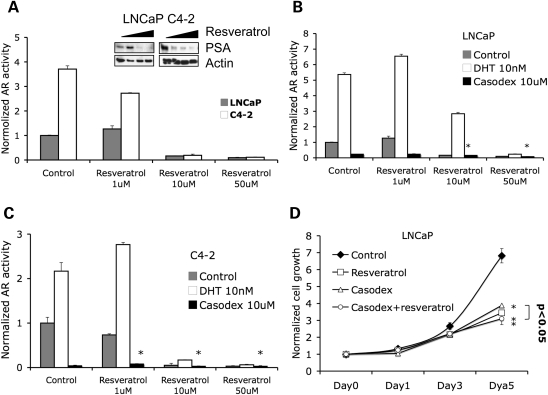
Next, we analyzed the effect of resveratrol on AR in both androgen-dependent LNCaP and androgen-independent C4-2 cells. We transfected each cell with a reporter plasmid, containing a promoter comprising six copies of androgen receptor response elements (AREs) tagged with a luciferase reporter gene. Luciferase assays showed that AR transcriptional activity was inhibited by resveratrol at both 10 and 50 µm concentrations in both LNCaP and C4-2 cells, while low concentrations of resveratrol (1 µm) slightly, but not significantly, increased AR activity in LNCaP (Fig. 2A). The expression of PSA transcript, which is regulated by AR, was inhibited by resveratrol (Supplementary Material, Fig. S1). Also, resveratrol inhibited the endogenous PSA protein expression at the two higher doses in both LNCaP and C4-2 cells (Fig. 2A, insert). DHT significantly increased AR transcriptional activity in both LNCaP and C4-2 cells, an effect that was abrogated by resveratrol (Fig. 2B and C). In contrast, an anti-AR drug bicalutamide (Casodex) inhibited AR activity, and resveratrol further enhanced this inhibitory effect (Fig. 2B and C).
Figure 2.
Resveratrol inhibits AR transcriptional activity. (A) LNCaP and C4-2 cells were co-transfected with plasmids expressing hARE-Luc and Renilla-Luc and treated with DMSO (control) and different concentrations of resveratrol for 48 h before lysis of cells for luciferase assay. Data represent mean luciferase firefly luminescence + SD (n = 3) normalized to Renilla-luminescence. Right panel shows western blots demonstrating increasing concentrations of resveratrol associated with decreasing PSA expression in LNCaP cells. Parenthetically, at the 1 µm concentration, resveratrol may have slightly increased AR transcriptional activity and PSA expression in LNCaP cells. (B) LNCaP and (C) C4-2 cells were transfected with plasmids expressing hARE-Luc and Renilla-Luc and treated as indicated. AR activity was then measured by luciferase assay (*P < 0.005). (D) LNCaP cells were treated with DMSO (control) or different concentrations of resveratrol. Growth rate of the cells was assessed by the MTT assay over a period of 5 days. Note that resveratrol and Casodex induced cell growth inhibition and the two-drug combination had the same effect as resveratrol by itself (*P < 0.01).
As both Casodex and resveratrol inhibit AR activity, we next used LNCaP cells to see their effect on cell proliferation. Although 10 µm resveratrol or 10 µm Casodex induced similar levels of AR inhibition (Fig. 2B), resveratrol had a more significant (P = 0.048) effect on cell growth (57% inhibition) compared with Casodex (46% inhibition) (Fig. 2D). We also found that the combination of resveratrol and Casodex showed the same inhibition ratio as resveratrol itself (Fig. 2D). These data imply that resveratrol may inhibit cell growth through a secondary mechanism independent of the AR pathway.
Resveratrol induces PTEN transcription by AR inhibition in prostate cancer cells
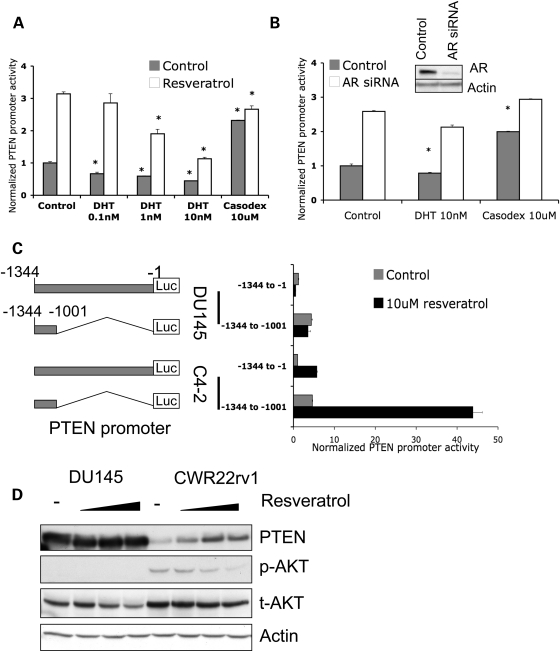
Resveratrol has recently been shown to induce PTEN protein expression in MCF-7, a breast cancer cell line (17). Clinical observations note that AR levels correlated with PTEN protein expression and that the ratio between the two proteins by immunohistochemistry is correlated with patient survival and outcome (21). At the same time, AR over-expression and hyper-activity are often observed in androgen-independent prostate cancer in response to anti-androgen therapy (3,22). Our data above demonstrate that resveratrol (often considered a phyto-estrogen), acting as a weak estrogen, inhibits AR transcriptional activity in both androgen-dependent LNCaP cells and androgen-independent C4-2 cells (Fig. 2B and C). We then set out to determine the relationship of resveratrol-regulated AR activity on PTEN transcription. Androgen-independent C4-2 cells were transfected with PTEN promoter reporter plasmid and treated with 10 µm resveratrol combined with different concentrations of DHT or with Casodex. Resveratrol significantly stimulated PTEN promoter activity 3-fold (Fig. 3A). Interestingly, we found that DHT decreased PTEN promoter activity and mitigated resveratrol-induced PTEN promoter activity in a dose-dependent manner (Fig. 3A). In contrast, Casodex alone dramatically increased PTEN promoter activity to similar levels as that of resveratrol alone or both Casodex and resveratrol (Fig. 3A). To further confirm our observations here suggesting that AR regulates the PTEN promoter, we knocked down AR by transfecting C4-2 cells with anti-AR siRNA. AR siRNA significantly decreased AR expression (Fig. 3B, insert) and increased PTEN promoter activity 2.5-fold (Fig. 3B). Furthermore, additional treatment with DHT or Casodex after AR knockdown could not further regulate PTEN promoter activity (Fig. 3B). Taken together, therefore, our data here suggest that resveratrol-induced PTEN transcription is mediated by AR inhibition, at least in prostate cancer cells.
Figure 3.
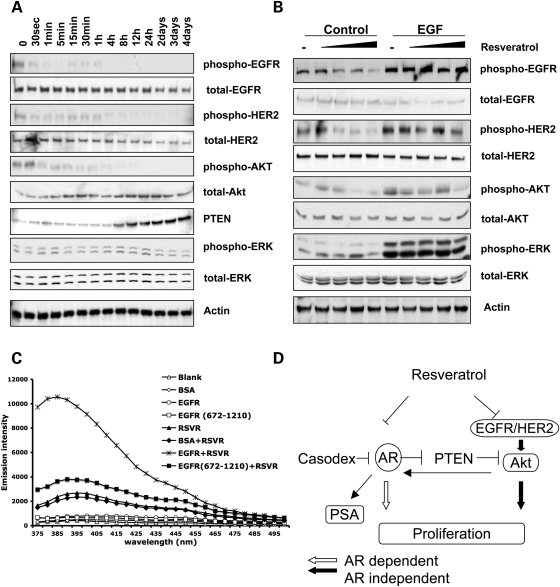
Resveratrol-induced PTEN promoter activity is mediated by AR. (A) C4-2 cells were co-transfected with plasmids expressing PTEN-Luc/Renilla-Luc and treated as indicated for 48 h. PTEN promoter activity was measured by dual luciferase assay. AR-ligand DHT inhibited PTEN promoter activity in a dose-dependent manner and resveratrol stimulated PTEN promoter activity. In contrast, AR-antagonist Casodex was shown to stimulate PTEN promoter activity, with resveratrol only adding slightly to this effect (*P < 0.005). (B) Knockdown of AR results in increased PTEN promoter activity. C4-2 cells were co-transfected with control siRNA or anti-AR siRNA and PTEN-luc/Renilla-Luc plasmids. After 48 h of treatment, PTEN promoter activity was measured by luciferase assay. The insert shows the knockdown of AR protein expression (*P < 0.005). (C) DU145 and C4-2 cells were transfected with PTEN-Luc/Renilla-Luc plasmids. Cells were treated with DMSO, or 10 µm resveratrol for 48 h. PTEN promoter activity was measured by luciferase assay. The structures of the −1134 to −1 and truncated −1134 to −1001 PTEN promoters are shown on the left panel. (D) AR-negative DU145 and AR-positive CWR22rv1 cells were treated with DMSO, 10, 20 or 50 µm resveratrol for 48 h. Western blots show PTEN, phospho-AKT, total-AKT and Actin levels. The quantification of phospho-AKT is shown in Supplementary Material, Fig. S3. Note that resveratrol did not change PTEN levels in DU145 cells.
To further confirm that resveratrol-induced PTEN promoter activity is mediated by AR, AR-positive C4-2 cells and AR-negative DU145 cells were transfected with PTEN-promoter reporter plasmids and treated with resveratrol. Reporter constructs included a full-length PTEN promoter −1344 to −1 and truncated PTEN promoter −1344 to −1001 (Fig. 3C, left panel). Both PTEN promoter constructs, the full-length as well as the −1344 to −1001 segments, could be activated by resveratrol exposure in AR-positive C4-2 cells (Fig. 3C). In contrast, these two PTEN promoter constructs did not respond to resveratrol exposure in AR-negative DU145 cells (Fig. 3C). Our data indicate that the resveratrol-associated PTEN promoter activity is AR-dependent and this regulation is mediated by the −1344 to −1001 region of the PTEN promoter. We then proceeded to compare resveratrol-regulated endogenous PTEN expression in AR-positive CWR22rv1 cells and AR-negative DU145 cells, the only two prostate cancer cell lines that express endogenous PTEN. CWR22rv1 cells treated with different concentrations of resveratrol showed increased PTEN expression and decreased AKT phosphorylation, while the PTEN levels in DU45 cell did not change after similar treatment (note DU145 cells do not express phospho-AKT) (Fig. 3D). Our observations from this series of experiments demonstrate that AR, acting as a transcriptional repressor, downregulates PTEN promoter activity in prostate cancer cells. Thus, inhibition of AR by resveratrol releases PTEN promoter activity and stimulates PTEN protein expression in the prostate cancer cells.
Resveratrol inhibits EGFR family members and AKT phosphorylation in prostate cancer cells
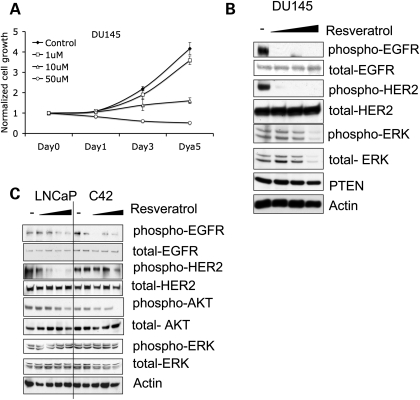
Our data above suggest that resveratrol inhibits proliferation through the AR in both androgen-dependent and -independent prostate cancer cells. In order to examine whether resveratrol has a similar effect on cell proliferation in AR-negative prostate cancer cells, we treated DU145, an AR-negative androgen-independent prostate cancer cell line, with resveratrol. After 5 days of treatment at the 10 or 50 µm concentration, cell proliferation showed significant decreases, an effect that was visible at 3 days (Fig. 4A). These observations suggest that resveratrol can induce AR-independent growth inhibition as well. The flow cytometry data also indicated that 50 µm resveratrol could induce apoptosis in DU145 (Supplementary Material, Fig. S2).
Figure 4.
Resveratrol inhibits cell proliferation in AR-negative prostate caner cells. (A) Resveratrol inhibits AR-negative DU145 cell proliferation. DU145 cells were treated with DMSO (control) or increasing concentrations of resveratrol. Growth rate of the cells was assessed by MTT assay over a period of 1–5 days. (B) DU145 cells were treated with DMSO, and 10, 20 or 50 µm resveratrol. Western blots show resveratrol decreased phosphorylation of EGFR-Tyr1068 (upper panel), HER2-Tyr1221/1222 (second panel). (C) LNCaP and C4-2 cells were treated with DMSO, and 1, 10, 20 or 50 µm resveratrol. Resveratrol decreased EGFR (Tyr1068), HER2 (Tyr1221/1222) and AKT phosphorylation. The quantifications of protein phosphorylation are shown in Supplementary Material, Fig. S4.
Upregulated AKT signaling has been correlated with both prostate cancer development and androgen-independent progression (8). Resveratrol has been shown to decrease AKT phosphorylation in LNCaP cells, but there was no evidence to support a direct effect (23). Our data above showed that resveratrol increased PTEN expression and decreased AKT phosphorylation in CWR22rv1 cells. Based on these observations and knowing that upregulation of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2)/NEU in breast cancer cells result in AKT phosphorylation and increased signaling (24,25), we sought to determine whether our AR-independent resveratrol effect in prostate cancer cells was mediated via member(s) of the EGFR family leading to downstream AKT pathway signaling. After resveratrol treatment, AR-negative DU145 cells showed significant inhibition of EGFR1 (Tyr1068) and EGFR2/HER2 (Tyr1221/1222) phosphorylation (Fig. 4B). Interestingly, we did not observe changes at other EGFR phosphorylation sites, including Tyr845, Tyr992 and Tyr1045 (data not shown). The prostate cancer cells also showed decreased extracellular signal-regulated kinase (ERK) phosphorylation likely due to decreased total ERK protein expression (Fig. 4B). We then examined resveratrol's effect on EGFR and HER2 and downstream AKT phosphorylation in AR-positive prostate cancer cells. After 48 h exposure of androgen-dependent LNCaP cells and androgen-independent C4-2 cells to resveratrol, phosphorylation of EGFR, HER2 and AKT was significantly decreased (Fig. 4C). In fact, this effect was more marked in the androgen-independent sub-clone C4-2 over the primary androgen-dependent LNCaP cells. These results suggest that resveratrol-induced downregulation of AKT phosphorylation may be independent of PTEN levels in prostate cancer cells.
Our observations above on prostate cancer cells and previous study in breast cancer cells corroborate that resveratrol treatment decreases AKT and ERK phosphorylation (17). However, the mechanism of how resveratrol regulates cell signaling is still unclear. Therefore, to determine whether resveratrol downregulates EGFR phosphorylation resulting in inhibition of its downstream pathways, C4-2 cells were treated with 10 µm resveratrol for a total 4 days (Fig. 5A). Time-course experiments revealed that phosphorylation of EGFR and HER2 was inhibited within 30 s after resveratrol treatment (Fig. 5A). Downstream AKT phosphorylation was not decreased until 1min of treatment (Fig. 5A). In addition, PTEN protein expression was significantly increased after 8 h of treatment (Fig. 5A). The AKT phosphorylation level was further decreased after PTEN upregulation. We did not observe decreased ERK phosphorylation induced by resveratrol in prostate cancer cells, a phenomenon that has been reported in breast cancer cells (26–28). The rapid effect of resveratrol suggests that resveratrol may directly inhibit EGFR and HER2 phosphorylation, resulting in the deactivation of downstream AKT pathways. To confirm resveratrol's direct effect on EGFR and HER2, we first treated C4-2 cells with 1 ng/ml of EGF combined with different concentrations of resveratrol. As we expected, EGF significantly increased EGFR and HER2 phosphorylation and its downstream AKT phosphorylation (Fig. 5B, comparing lane 6 to lane 1). In addition, EGF treatment abrogated resveratrol's inhibitory effect on the phosphorylation of those targets (Fig. 5B, comparing lanes 6–10 to lanes 1–5).
Figure 5.
Resveratrol inhibits EGFR/HER2 signaling pathway. (A) Time course of resveratrol treatment of CWR22rv1 cells. Cells were treated with 10 µm resveratrol and cell lysates were harvested at each time point indicated. EGFR, HER2 and AKT phosphorylation are significantly decreased after the treatment. Resveratrol also stimulates PTEN protein expression. (B) C4-2 cells were cultured with DMSO, and 1, 10, 20 or 50 µm resveratrol for 48 h. After 3 min of DMSO or EGF (1 ng/ml) treatment, cells were harvested and subjected to western blot. EGF abrogated resveratrol's effect on EGFR, HER2 and AKT phosphorylation. (C) Emission fluorescence of resveratrol (5 µm) in PBS in the context of water as control, 1 µm BSA, 1 µm EGFR or 1 µm EGFR (672-1210) at pH 7.0. Excitation wavelength is 334 nm and resveratrol's emission fluorescence peaked at 392 nm. (D) Schematic model depicting the mechanism by which resveratrol inhibits proliferation in both AR-dependent and -independent mechanisms. Resveratrol inhibits AR transcriptional activity and PSA expression. In contrast, it inhibits phosphorylation of EGFR and HER2, rapidly suppressing downstream AKT pathways. Resveratrol lifts AR repression of PTEN promoter resulting in PTEN transcription and a later-effect suppression of AKT signaling. The quantifications of protein phosphorylation are shown in Supplementary Material, Fig. S5.
Furthermore, we used a fluorescence assay to analyze the physical interaction between resveratrol and EGFR. As reported previously, resveratrol, containing a stilbene structure, has an emission fluorescence peak at 392 nm and the excitation wavelength of 344 nm (29,30). After incubation with 1 µm EGFR, resveratrol's fluorescence intensity was significantly increased, while the same concentration of BSA did not affect its fluorescence emission (Fig. 5C). This result suggests that EGFR directly binds multiple resveratrol molecules, resulting in the enhanced fluorescence intensity. Interestingly, truncated EGFR (672-1210), without the extracellular ligand-binding domain and transmembrane domain, did not interfere with resveratrol's fluorescence emission. As a strong hydrophilic compound, resveratrol is unlikely to interact with the hydrophobic transmembrane domain of EGFR, indicating that the ligand-binding domain of EGFR is necessary for resveratrol binding (Fig. 5C). Overall, our observations indicate that resveratrol may directly inhibit EGFR signaling, which, in turn, downregulates the AKT pathway.
DISCUSSION
For many decades, anecdotal observations and clinical epidemiologic studies confirm that red wine has beneficial effects in lowering the risk of cardiovascular diseases and some cancers (31–33). Recent studies revealed that resveratrol, an important component of red wine, also has the ability to inhibit growth and induce apoptosis in various kinds of tumor cells, such as prostate cancer, breast cancer and melanoma (15,34,35). Resveratrol commonly exists in many types of food, such as peanuts, cranberries and grapes, making it a good candidate as a natural chemopreventive or therapeutic adjunct against cancer (11). Several potential targets of resveratrol have been reported, including NF-kappa B, AP-1, p53, Bcl-2 and Bax (15,16,35,36). However, the mechanism by which resveratrol inhibits cancer cell growth is still not fully understood. For certain cancers, epidemiologic studies have not been conclusive whether red wine is beneficial or detrimental (37–39). If the opposing studies are well controlled, then one may surmise from these observations that the local milieu, whether hormonal or otherwise, might be important.
In this study, we used prostate cancer cells to demonstrate that resveratrol inhibits proliferation via both AR-dependent and -independent mechanisms (Fig. 5D). Our data showed that resveratrol not only inhibits AR activity but also inhibits EGFR and HER2 phosphorylation with consequent decreased AKT phosphorylation in both androgen-dependent and -independent prostate cancer cells. Notably, we have shown, for the first time, that resveratrol stimulates PTEN promoter activity via AR, resulting in upregulation of both PTEN transcript and protein expression.
One of the biggest challenges in the treatment of advanced prostate cancer is lack of effective therapy for the hormone refractory tumor, which is resistant to androgen deprivation therapy. Previous reports showed that androgen-independent prostate cancers have a hyper-activated PI3K/AKT pathway resulting in increased proliferation (40,41). As a result, these carcinoma cells attain a growth advantage after hormone deprivation with cellular proliferation becoming independent of androgen levels. We show here that resveratrol inhibits proliferation in both AR-positive and -negative prostate cancer cells through not only AR-dependent but also, and importantly, AR-independent pathways, suggesting that resveratrol may be a plausible candidate for adjunctive therapy in hormone-independent advanced prostate cancers.
Recent studies found that resveratrol inhibits AKT and MAPK cell signaling pathways (23,27). However, the mechanism of how resveratrol regulates those pathways remains unclear. A previous study showed that resveratrol is able to reduce HCMV-induced EGFR auto-phosphorylation in human embryonic lung fibroblasts (42). Here, we found that resveratrol inhibits EGFR and HER2 phosphorylation, resulting in decreased AKT phosphorylation and this effect is independent of AR. Interestingly, we noticed that high dose of resveratrol inhibited ERK phosphorylation, most likely due to the decreased total protein level in DU145 cells but not other cell lines we tested. Further study is needed to investigate the mechanism. EGFR family members play critical roles in cancer development and progression. After binding with EGF, EGFR undergoes dimerization, auto-phosphorylation and activation in response to ligand binding, resulting in activation of its downstream cell signaling pathways, such as those of AKT and MAPK. Phosphorylation of EGFR provides binding sites for Grb2 and Gab1 (43,44). Grb2 then induces downstream MAPK/ERK cascades, while Gab1 induces p85 phosphorylation, resulting in the activation of AKT (45,46). HER2/NEU activation leads to activation of PI3K/AKT pathway, which has been shown to phosphorylate AR at Ser213 and Ser791 (9). Meanwhile, HER2/NEU could also regulate AR through activating MAPK pathway (25). Notably, we have shown, for the first time, that resveratrol binds directly to multiple docking sites of the extracellular portion of EGFR, thus explaining the rapid dephosphorylation of EGFR on Tyr-1068, with consequent downregulation of AKT signaling. We also found that phosphorylation of HER2 (Tyr1221/1222) and HER3 (Tyr1289) (data not shown) was decreased by resveratrol. However, we did not observe any change in ERK phosphorylation, which has been reported in at least one other study (27). In contrast, resveratrol stimulates PTEN protein expression 8–12 h after exposure, suggesting that consequent decrease in AKT phosphorylation (1min after treatment) is a result of signaling mainly from resveratrol's effect on dowregulating EGFR. Taken together, these observations in toto suggest a bimodal temporal AKT response to resveratrol exposure, an AR-independent rapid response via direct binding to and downregulation of EGFR, and a later response via AR-mediated upregulation of PTEN. We also recognize the challenges for using resveratrol in vivo in direct patient treatment because of its low bioavailability due to rapid metabolism and elimination. After oral intake of 25 mg of trans-resveratrol, the absorption was at least 70%, with peak plasma levels of resveratrol and metabolites of 2 µm and a plasma half-life of 9 h (47). However, only trace amounts of resveratrol (<5 ng/ml) could be detected in plasma, while its glucuronic acid sulfate conjugates were recovered in urine (47). Therefore, a further study to identify stable forms of resveratrol or its analogy is critical for practical clinic use.
Both AR and PI3K/PTEN/AKT pathways play important roles regulating prostate cancer proliferation and androgen independence development. Our previous publication has reviewed the complex crosstalk between these two pathways (48). First, AKT directly phosphorylates AR, increasing its transcriptional activity and stability (9). Also, AKT indirectly regulates AR transcription through its downstream GSK (49). So far, however, little was known how nuclear steroid receptor pathways regulate the PI3K/AKT pathway in prostate cancer development or progression. Our data here demonstrated that AR represses PTEN through the −1344 to −1001 promoter region, indicating a direct link between a nuclear receptor and PI3K/AKT pathway. Our data suggest a mechanism that the loss of PTEN expression in hormone-refractory prostate cancer may be a direct result of AR overexpression and hyper-activity.
In conclusion, we showed that resveratrol inhibits both AR-dependent and -independent proliferation in prostate cancer cells. Importantly, resveratrol is able to induce PTEN expression via AR inhibition. Furthermore, we showed that, independent of AR, resveratrol directly inhibits EGFR and HER2 phosphorylation, with rapid consequent inhibition of downstream AKT signaling (Fig. 5D). In contrast, resveratrol inhibits AR-mediated repression of the PTEN promoter with a later suppressive of AKT (Fig. 5D). Therefore, resveratrol's ability of targeting multiple pathways provides us with a strategic foundation on which to frame the future management of prostate cancers, especially those that are androgen independent and AR negative.
MATERIALS AND METHODS
Cell culture and pharmacological treatments
LNCaP, CWR22rv1 and DU-145 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). AR-positive C4-2 cells (UroCor, Oklahoma City, OK, USA), an androgen-independent sub-line developed from LNCaP xenografts in castrated nude mice (originating from Dr Warren Heston). All cells were cultured in ‘complete-medium’: RPMI 1640 medium with 10% fetal bovine serum. DU-145 cells were developed from metastatic prostate cancer tissue and lack AR expression. Bicalutamide (Casodex) (Astra Zeneca, UK) and 4,5α-DHT (Sigma-Aldrich, St Louis, MO, USA) were applied per routine of the Silverman and Heston labs. Formononetin and Biochanin A are from Sigma-Aldrich.
RNA inhibition
Cells were plated in 60 mm dishes and transfected with 50 pmol siRNA. General control was a pool of 4-scrambled non-specific siRNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Anti-Androgen receptor siRNA (sc-29204, Santa Cruz Biotechnology) contains a pool of four target-specific siRNAs with the following sequences: (A) 5′-CAGUCCCACUUGUGUCAAA-3′, (B) 5′-CCUGAUCUGUGGAGAUGAA-3′, (C) 5′-GUCGUCUUCGGAAAUGUUA-3′, (D) 5′-GACAGUGUCACACAUUGAA-3′.
MTT assay
Cell growth rates were estimated by the MTT assay. Cells were plated in 24-well plates and treated as indicated. Following treatment, each well was incubated with 25 ml of 5 mg/ml 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyl-tetrazolium bromide (MTT) for 1 h in a CO2 incubator at 37°C. The medium was aspirated and 0.5 ml of DMSO was added per well. Proliferation rates were estimated by the colorimetric assay of formazan intensity in a plate reader at 560 nm.
Western blotting
Western blotting was performed as described elsewhere (8). Mouse monoclonal anti-AR and anti-beta-Actin were from Santa Cruz Biotechnology. Rabbit polyclonal anti-phospho-EGFR (Tyr1068), anti-total EGFR, anti-phospho-HER2 (Tyr1221/1222), anti-total HER2, anti-phospho-HER3 (Tyr1289), anti-phospho AKT (Ser473), anti-total AKT, anti-phospho-ERK (Thr202/Tyr204), anti-total ERK, anti-Androgen Receptor and anti-Hsp90 antibodies were from Cell Signaling Technology (Beverly, MA, USA). Mouse monoclonal anti-PSA antibody was from Neomarkers, Lab Vision Corporation (Fremont, CA, USA).
Promoter activity assay
AR transcriptional activity was measured by Cignal report assay kit (SA Bioscience, Frederick, MD, USA) as per the manufacturer's instructions. We generated PTEN promoters with variable lengths tagged with the firefly luciferase gene as described in our previous study (50). Reporter gene activity was determined by dual luciferase assay. A total of 500 000 cells were plated in 60 mm dishes, co-transfected with 5 µg of promoter-firefly-luc and 0.1 µg of Renilla-Luc using Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) according to the manufacturer's recommendations. Cells grown in complete medium were treated as required for an additional 48 h. After 48 h, cell lysates were prepared for luciferase analysis using a luciferase enzyme assay system (Promega Corp., Madison, WI, USA). Each transfection experiment was performed in triplicate on at least three separate occasions. Promoter activities were normalized to Renilla luciferase. Results represent an average of independent experiments with data presented as relative luciferase activity against the means of untreated controls.
Quantitative reverse transcription-polymerase chain reaction
Quantitative reverse transcription-polymerase chain reaction (qRT–PCR) to quantitative PSA mRNA expression was measured using SYBR Green exactly as per the manufacturer's specifications (Applied Biosystem, Foster City, CA, USA). Expression of GAPDH was used as the internal control. The primer sequences are as follows: forward: 5′-GGCAGCATTGAACCAGAGGAG-3′; reverse: 5′-GCATGAACTTGGTCACCTTCTG-3′.
Fluorescence spectroscopy assay
A Synergy-4 Hybrid Multi-Mode spectrometer (Biotek, Winooski, VT, USA) with Tungsten Halogen high-energy DPR Xenon flash and deep blocking bandpass filters was used to perform all fluorescence measurements. The excitation wavelength was set at 344 nm and the emission was measured from 375 to 500 nm. The excitation and emission spectra of resveratrol, BSA, EGFR and EGFR (672-1210) were recorded at 23°C in PBS (pH 7.0). BSA is from Thermo Fisher Scientific (Pittsburg, PA, USA). EGFR is from Sigma-Aldrich. EGFR (His672–Ala1210) is from Cell Signaling Technology.
SUPPLEMENTARY MATERIAL
FUNDING
Funding to pay the Open Access Charge was provided by the Genomic Medicine Institute.
ACKNOWLEDGEMENTS
C.E. is the Sondra J. and Stephen R. Hardis Chair of Cancer Genomic Medicine at the Cleveland Clinic, was a Doris Duke Distinguished Clinical Scientist and is an American Cancer Society Clinical Research Professor, generously funded, in part, by the F.M. Kirby Foundation.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M.J. Cancer statistics, 2009. CA. Cancer. J. Clin. 2009;59:225–249. doi: 10.3322/caac.20006. doi:10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Solit D.B., Scher H.I., Rosen N. Hsp90 as a therapeutic target in prostate cancer. Semin. Oncol. 2003;30:709–716. doi: 10.1016/s0093-7754(03)00346-4. doi:10.1016/S0093-7754(03)00346-4. [DOI] [PubMed] [Google Scholar]
- 3.Visakorpi T., Hyytinen E., Koivisto P., Tanner M., Keinanen R., Palmberg C., Palotie A., Tammela T., Isola J., Kallioniemi O.P. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. doi:10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 4.Gregory C.W., Hamil K.G., Kim D., Hall S.H., Pretlow T.G., Mohler J.L., French F.S. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998;58:5718–5724. [PubMed] [Google Scholar]
- 5.Eng C. PTEN: one gene, many syndromes. Hum. Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. doi:10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- 6.Ginn-Pease M.E., Eng C. Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0-G1 in MCF-7 cells. Cancer Res. 2003;63:282–286. [PubMed] [Google Scholar]
- 7.Bedolla R., Prihoda T.J., Kreisberg J.I., Malik S.N., Krishnegowda N.K., Troyer D.A., Ghosh P.M. Determining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activation. Clin. Cancer Res. 2007;13:3860–3867. doi: 10.1158/1078-0432.CCR-07-0091. doi:10.1158/1078-0432.CCR-07-0091. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh P.M., Malik S.N., Bedolla R.G., Wang Y., Mikhailova M., Prihoda T.J., Troyer D.A., Kreisberg J.I. Signal transduction pathways in androgen-dependent and -independent prostate cancer cell proliferation. Endocr. Relat. Cancer. 2005;12:119–134. doi: 10.1677/erc.1.00835. doi:10.1677/erc.1.00835. [DOI] [PubMed] [Google Scholar]
- 9.Wen Y., Hu M.C., Makino K., Spohn B., Bartholomeusz G., Yan D.H., Hung M.C. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 10.Lin H.K., Hu Y.C., Lee D.K., Chang C. Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells. Mol. Endocrinol. 2004;18:2409–2423. doi: 10.1210/me.2004-0117. doi:10.1210/me.2004-0117. [DOI] [PubMed] [Google Scholar]
- 11.Shakibaei M., Harikumar K.B., Aggarwal B.B. Resveratrol addiction: to die or not to die. Mol. Nutr. Food Res. 2009;53:115–128. doi: 10.1002/mnfr.200800148. doi:10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- 12.Penumathsa S.V., Thirunavukkarasu M., Koneru S., Juhasz B., Zhan L., Pant R., Menon V.P., Otani H., Maulik N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J. Mol. Cell Cardiol. 2007;42:508–516. doi: 10.1016/j.yjmcc.2006.10.018. doi:10.1016/j.yjmcc.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson T.S., Hartle D.K., Hursting S.D., Nunez N.P., Wang T.T., Young H.A., Arany P., Green J.E. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res. 2007;67:8396–8405. doi: 10.1158/0008-5472.CAN-06-4069. doi:10.1158/0008-5472.CAN-06-4069. [DOI] [PubMed] [Google Scholar]
- 14.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. doi:10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 15.Delmas D., Lancon A., Colin D., Jannin B., Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr. Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. doi:10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y.A., Rhee S.H., Park K.Y., Choi Y.H. Antiproliferative effect of resveratrol in human prostate carcinoma cells. J. Med. Food. 2003;6:273–280. doi: 10.1089/109662003772519813. doi:10.1089/109662003772519813. [DOI] [PubMed] [Google Scholar]
- 17.Waite K.A., Sinden M.R., Eng C. Phytoestrogen exposure elevates PTEN levels. Hum. Mol. Genet. 2005;14:1457–1463. doi: 10.1093/hmg/ddi155. doi:10.1093/hmg/ddi155. [DOI] [PubMed] [Google Scholar]
- 18.Harada N., Murata Y., Yamaji R., Miura T., Inui H., Nakano Y. Resveratrol down-regulates the androgen receptor at the post-translational level in prostate cancer cells. J. Nutr. Sci. Vitaminol. (Tokyo) 2007;53:556–560. doi: 10.3177/jnsv.53.556. doi:10.3177/jnsv.53.556. [DOI] [PubMed] [Google Scholar]
- 19.Mark L., Nikfardjam M.S., Avar P., Ohmacht R. A validated HPLC method for the quantitative analysis of trans-resveratrol and trans-piceid in Hungarian wines. J. Chromatogr. Sci. 2005;43:445–449. doi: 10.1093/chromsci/43.9.445. [DOI] [PubMed] [Google Scholar]
- 20.Shi W.F., Leong M., Cho E., Farrell J., Chen H.C., Tian J., Zhang D. Repressive effects of resveratrol on androgen receptor transcriptional activity. PLoS ONE. 2009;4:e7398. doi: 10.1371/journal.pone.0007398. doi:10.1371/journal.pone.0007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Sheikh S.S., Romanska H.M., Abel P., Domin J., Lalaniel N. Predictive value of PTEN and AR coexpression of sustained responsiveness to hormonal therapy in prostate cancer—a pilot study. Neoplasia. 2008;10:949–953. doi: 10.1593/neo.08582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koivisto P., Kononen J., Palmberg C., Tammela T., Hyytinen E., Isola J., Trapman J., Cleutjens K., Noordzij A., Visakorpi T., et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 23.Aziz M.H., Nihal M., Fu V.X., Jarrard D.F., Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. doi:10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh P.M., Malik S., Bedolla R., Kreisberg J.I. Akt in prostate cancer: possible role in androgen-independence. Curr. Drug Metab. 2003;4:487–496. doi: 10.2174/1389200033489226. doi:10.2174/1389200033489226. [DOI] [PubMed] [Google Scholar]
- 25.Yeh S., Kang H.Y., Miyamoto H., Nishimura K., Chang H.C., Ting H.J., Rahman M., Lin H.K., Fujimoto N., Hu Y.C., et al. Differential induction of androgen receptor transactivation by different androgen receptor coactivators in human prostate cancer DU145 cells. Endocrine. 1999;11:195–202. doi: 10.1385/endo:11:2:195. doi:10.1385/ENDO:11:2:195. [DOI] [PubMed] [Google Scholar]
- 26.Filomeni G., Graziani I., Rotilio G., Ciriolo M.R. trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr. 2007;2:295–305. doi: 10.1007/s12263-007-0059-9. doi:10.1007/s12263-007-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen T.H., Mustafa F.B., Pervaiz S., Ng F.S., Lim L.H. ERK1/2 activation is required for resveratrol-induced apoptosis in MDA-MB-231 cells. Int. J. Oncol. 2008;33:81–92. [PubMed] [Google Scholar]
- 28.Stewart J.R., O'Brian C.A. Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest. New Drugs. 2004;22:107–117. doi: 10.1023/B:DRUG.0000011787.75522.ec. doi:10.1023/B:DRUG.0000011787.75522.ec. [DOI] [PubMed] [Google Scholar]
- 29.Galeano Diaz T., Duran Meras I., Airado Rodriguez D. Determination of resveratrol in wine by photochemically induced second-derivative fluorescence coupled with liquid–liquid extraction. Anal. Bioanal. Chem. 2007;387:1999–2007. doi: 10.1007/s00216-006-1007-z. doi:10.1007/s00216-006-1007-z. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Nicolas J.M., Garcia-Carmona F. Aggregation state and pKa values of (E)-resveratrol as determined by fluorescence spectroscopy and UV-visible absorption. J. Agric. Food Chem. 2008;56:7600–7605. doi: 10.1021/jf800843e. doi:10.1021/jf800843e. [DOI] [PubMed] [Google Scholar]
- 31.Das S., Santani D.D., Dhalla N.S. Experimental evidence for the cardioprotective effects of red wine. Exp. Clin. Cardiol. 2007;12:5–10. [PMC free article] [PubMed] [Google Scholar]
- 32.Ray P.S., Maulik G., Cordis G.A., Bertelli A.A., Bertelli A., Das D.K. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic. Biol. Med. 1999;27:160–169. doi: 10.1016/s0891-5849(99)00063-5. doi:10.1016/S0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 33.Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W., Fong H.H., Farnsworth N.R., Kinghorn A.D., Mehta R.G., et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. doi:10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 34.Lin H.Y., Shih A., Davis F.B., Tang H.Y., Martino L.J., Bennett J.A., Davis P.J. Resveratrol induced serine phosphorylation of p53 causes apoptosis in a mutant p53 prostate cancer cell line. J. Urol. 2002;168:748–755. doi:10.1016/S0022-5347(05)64739-8. [PubMed] [Google Scholar]
- 35.Manna S.K., Mukhopadhyay A., Aggarwal B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 36.She Q.B., Bode A.M., Ma W.Y., Chen N.Y., Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604–1610. [PubMed] [Google Scholar]
- 37.Chao C., Haque R., Van Den Eeden S.K., Caan B.J., Poon K.Y., Quinn V.P. Red wine consumption and risk of prostate cancer: the California men's health study. Int. J. Cancer. 2010;126:171–179. doi: 10.1002/ijc.24637. doi:10.1002/ijc.24637. [DOI] [PubMed] [Google Scholar]
- 38.Kaur G., Roberti M., Raul F., Pendurthi U.R. Suppression of human monocyte tissue factor induction by red wine phenolics and synthetic derivatives of resveratrol. Thromb. Res. 2007;119:247–256. doi: 10.1016/j.thromres.2006.01.020. doi:10.1016/j.thromres.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu B.L., Zhang X., Zhang W., Zhen H.N. New enlightenment of French Paradox: resveratrol's potential for cancer chemoprevention and anti-cancer therapy. Cancer Biol. Ther. 2007;6:1833–1836. doi: 10.4161/cbt.6.12.5161. [DOI] [PubMed] [Google Scholar]
- 40.Mikhailova M., Wang Y., Bedolla R., Lu X.H., Kreisberg J.I., Ghosh P.M. AKT regulates androgen receptor-dependent growth and PSA expression in prostate cancer. Adv. Exp. Med. Biol. 2008;617:397–405. doi: 10.1007/978-0-387-69080-3_38. doi:10.1007/978-0-387-69080-3_38. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Mikhailova M., Bose S., Pan C.X., deVere White R.W., Ghosh P.M. Regulation of androgen receptor transcriptional activity by rapamycin in prostate cancer cell proliferation and survival. Oncogene. 2008;27:7106–7117. doi: 10.1038/onc.2008.318. doi:10.1038/onc.2008.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evers D.L., Wang X., Huong S.M., Huang D.Y., Huang E.S. 3,4′,5-Trihydroxy-trans-stilbene (resveratrol) inhibits human cytomegalovirus replication and virus-induced cellular signaling. Antiviral Res. 2004;63:85–95. doi: 10.1016/j.antiviral.2004.03.002. doi:10.1016/j.antiviral.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Chook Y.M., Gish G.D., Kay C.M., Pai E.F., Pawson T. The Grb2-mSos1 complex binds phosphopeptides with higher affinity than Grb2. J. Biol. Chem. 1996;271:30472–30478. doi: 10.1074/jbc.271.48.30472. [DOI] [PubMed] [Google Scholar]
- 44.Laffargue M., Raynal P., Yart A., Peres C., Wetzker R., Roche S., Payrastre B., Chap H. An epidermal growth factor receptor/Gab1 signaling pathway is required for activation of phosphoinositide 3-kinase by lysophosphatidic acid. J. Biol. Chem. 1999;274:32835–32841. doi: 10.1074/jbc.274.46.32835. doi:10.1074/jbc.274.46.32835. [DOI] [PubMed] [Google Scholar]
- 45.Mattoon D.R., Lamothe B., Lax I., Schlessinger J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol. 2004;2:24. doi: 10.1186/1741-7007-2-24. doi:10.1186/1741-7007-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito T., Okada S., Ohshima K., Yamada E., Sato M., Uehara Y., Shimizu H., Pessin J.E., Mori M. Differential activation of epidermal growth factor (EGF) receptor downstream signaling pathways by betacellulin and EGF. Endocrinology. 2004;145:4232–4243. doi: 10.1210/en.2004-0401. doi:10.1210/en.2004-0401. [DOI] [PubMed] [Google Scholar]
- 47.Walle T., Hsieh F., DeLegge M.H., Oatis J.E., Jr, Walle U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. doi:10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Kreisberg J.I., Ghosh P.M. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr. Cancer Drug Targets. 2007;7:591–604. doi: 10.2174/156800907781662248. doi:10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 49.Li Y., Wang Z., Kong D., Murthy S., Dou Q.P., Sheng S., Reddy G.P., Sarkar F.H. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J. Biol. Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. doi:10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 50.Teresi R.E., Planchon S.M., Waite K.A., Eng C. Regulation of the PTEN promoter by statins and SREBP. Hum. Mol. Genet. 2008;17:919–928. doi: 10.1093/hmg/ddm364. doi:10.1093/hmg/ddm364. [DOI] [PubMed] [Google Scholar]