Abstract
Acute lung injury (ALI) and the development of the multiple organ dysfunction syndrome (MODS) are major causes of death in trauma patients. Gut inflammation and loss of gut barrier function as a consequence of splanchnic ischemia-reperfusion (I/R) have been implicated as the initial triggering events that contribute to the development of the systemic inflammatory response, ALI, and MODS. Since hypoxia-inducible factor (HIF-1) is a key regulator of the physiological and pathophysiological response to hypoxia, we asked whether HIF-1 plays a proximal role in the induction of gut injury and subsequent lung injury. Utilizing partially HIF-1α-deficient mice in a global trauma hemorrhagic shock (T/HS) model, we found that HIF-1 activation was necessary for the development of gut injury and that the prevention of gut injury was associated with an abrogation of lung injury. Specifically, in vivo studies demonstrated that partial HIF-1α deficiency ameliorated T/HS-induced increases in intestinal permeability, bacterial translocation, and caspase-3 activation. Lastly, partial HIF-1α deficiency reduced TNF-α, IL-1β, cyclooxygenase-2, and inducible nitric oxide synthase levels in the ileal mucosa after T/HS whereas IL-1β mRNA levels were reduced in the lung after T/HS. This study indicates that prolonged intestinal HIF-1 activation is a proximal regulator of I/R-induced gut mucosal injury and gut-induced lung injury. Consequently, these results provide unique information on the initiating events in trauma-hemorrhagic shock-induced ALI and MODS as well as potential therapeutic insights.
Keywords: hemorrhagic shock, inflammation, multiple organ dysfunction syndrome, acute lung injury
in patients sustaining major trauma, the development of the systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction (MODS) is a major clinical problem resulting in 50–80% of all deaths in surgical intensive care units. Since the pathophysiology of this syndrome remains incompletely understood and therapy remains largely supportive (16), studies focusing on the basic biology of trauma-induced SIRS, organ injury/dysfunction, and MODS have been major areas of investigation. These mechanistic studies have generated several working hypotheses, one of which is the gut hypothesis of MODS. A key element in the gut hypothesis of MODS is that a splanchnic ischemia-reperfusion (I/R) insult leading to gut inflammation and loss of barrier function is the initial triggering event that turns the gut into the “motor” of MODS (19). However, the exact mechanisms by which gut I/R leads to intestinal injury and how an intestinal ischemic insult is transduced into a systemic inflammatory response remains incomplete. To date, the majority of the molecular and cellular studies investigating shock-induced gut injury and gut-induced MODS have focused primarily on the reperfusion phase of the intestinal I/R insult and the consequent production of proinflammatory mediators such as inducible nitric oxide synthase (iNOS)-derived nitric oxide (22), reactive oxygen species, cytokines (IL-6) (48), transcription factors (NF-κB, AP-1) (72), cyclooxygenase-2 (COX-2) (31), and poly(ADP-ribose) polymerase (44). Yet, because the induction of many of these factors is secondary to or accentuated by hypoxia and because ischemia precedes reperfusion, it seemed likely that the molecular response triggered by the ischemic component of the I/R insult is a critical step in initiating the sequence of events that lead to the development of gut injury and MODS.
The adaptive response to hypoxia or ischemia has been shown to be primarily regulated by hypoxia-inducible factor (HIF-1). The HIF-1 heterodimer consists of an oxygen-labile HIF-1α subunit and a constitutively expressed HIF-1β subunit that mediates a wide spectrum of physiological and cellular adaptive responses such as angiogenesis, metabolic adaption, erythropoiesis, and vascular tone (67). In the presence of oxygen, prolyl hydroxylation of the oxygen-dependent degradation domain of HIF-1α marks it for ubiquitin-proteasomal degradation (34, 36) and asparaginyl hydroxylation of the COOH-terminal transactivation domain of HIF-1α prevents interaction with the transcriptional coactivator p300 (42, 47).
In the context of ischemic injury, HIF-1 was originally shown to protect organs that are highly sensitive to energy deprivation such as the brain, heart, and kidney from ischemic damage in I/R preconditioning models (23). Thus one accepted role for HIF-1 is that it acts as an adaptive and survival factor for cells exposed to hypoxia or cells undergoing stress such as ischemic injury, especially in models of ischemic preconditioning (23, 45). However, in response to prolonged ischemic stress as well as in various nonpreconditioning models, HIF-1 may be deleterious because of its ability to augment both apoptotic (1, 26) and inflammatory processes (13, 45), especially via upregulation of iNOS. In fact, our earlier studies demonstrated that the in vivo intestinal mucosal response to gut I/R was associated with a prolonged increase in HIF-1α expression that was not rapidly lost during reperfusion when the gut becomes reoxygenated (41). These results were surprising since cellular HIF-1 levels rapidly decrease to normoxic levels within minutes of reoxygenation (32). This relatively unique prolonged HIF-1α response to I/R in the intestine appeared to be mediated by bacteria and bacterial products within the gut lumen coming into direct contact with the stressed intestinal mucosa (41). In this study, we hypothesized that prolongation of the intestinal HIF-1 response could be potentially maladaptive or injurious and contribute to loss of gut barrier function and the development of distant organ injury. Using partially HIF-1α-deficient mice, we tested the functional significance of HIF-1 in a trauma hemorrhagic shock (T/HS)-mediated gut I/R injury model.
MATERIALS AND METHODS
Mice.
The generation and genotyping of HIF-1α+/− and HIF-1α+/+ mice on a C57B6/129 genetic background was described previously (35) and provided by G. L. Semenza (John Hopkins University School of Medicine, Baltimore, MD). All animal procedures were approved by the University of Medical and Dentistry of New Jersey Animal Care and Use Committee and maintained in accordance with the recommendations of the “National Institutes of Health Guide for Care and Use of Laboratory Animals.”
T/HS model.
Male mice were anesthetized with pentobarbital (60–80 mg/kg ip) and under strict asepsis, a 2.5-cm midline laparotomy was performed. Isoflurane was given if needed to maintain surgical level of anesthesia. Blood was withdrawn from the jugular vein until a mean arterial pressure (MAP) between 35 and 40 mmHg was obtained and maintained for 60 min. After 60 min, the mice were resuscitated with their shed blood for 3 h. Sham-shock animals (T/SS) underwent cannulation of the femoral artery and jugular vein followed by a laparotomy; however, no blood was withdrawn and the MAP was kept within normal limits.
Morphological analysis of gut and lung injury.
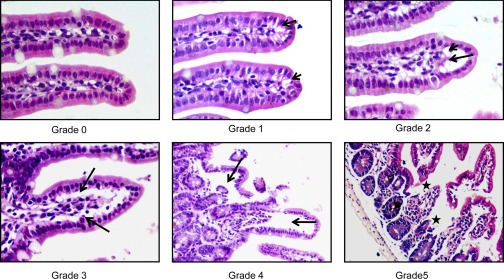
At euthanasia, terminal ileum or lung was fixed in 10% buffered formalin, processed, stained with hematoxylin and eosin, and examined by light microscopy. For each animal, the percentage of villous injury was a gross assessment of the number of damaged villus tips per 200 villus tips (∼20–25 visual fields at ×100 magnification), which ranged from blunting and vacuolation of villus tips to denuded villi and mucosal ulceration. According to the grading systems for villous injury as described by Refs. 9, 49, 74, the numerical scores were the following: 0 = normal mucosa, 1 = development of subepithelial Gruenhagen's space and vacuolization at the villus tip, 2 = extension of the subepithelial space with moderate lifting of epithelial layer from the lamina propria, 3 = massive subepithelial lifting/sloughing and increased vacuolization from the tip to midportion of villi, 4 = epithelial lifting and vacuolization from the tip to lower portion of villi, and 5 = mucosal ulceration and disintegration of the lamina propria (Fig. 1). Similarly, lung injury was determined by using a modified lung injury grading scale (10) that assessed the magnitude of interstitial edema (0–2), pulmonary edema (0–2), and alveolar integrity (0–1). In this grading system, 0 indicates no injury and the lung injury score is calculated by adding the injury grades of each component (maximal lung injury score is 5). All slides were evaluated by two examiners in a blinded fashion.
Fig. 1.
Histological criteria used to grade villous damage following trauma hemorrhagic shock (T/HS). 0 = normal mucosa, 1 = development of subepithelial Gruenhagen's space and vacuolization at the villus tip, 2 = extension of the subepithelial space with moderate lifting of epithelial layer from the lamina propria, 3 = massive subepithelial lifting/ sloughing and increased vacuolization from the tip to midportion of villi, 4 = epithelial lifting and vacuolization from the tip to lower portion of villi, and 5 = mucosal ulceration and disintegration of the lamina propria. The shorter arrows depict the presence of vacuoles, the longer arrows depict the subepithelial and epithelial lifting, and the stars depict mucosal ulceration. Magnification ×400.
In vivo intestinal permeability assay.
Under anesthetized conditions, at 30 min before euthanasia, a 5-cm segment of distal ileum was isolated, beginning at 3 cm proximal to the cecum and bilateral end of ileum, and then ligated with a 4-0 silk suture to prevent leakage of 4.4-kDa fluorescein isothiocyanate-dextran (FD-4; Sigma-Aldrich). PBS (200 μl) containing 25 mg/ml FD-4 was injected into the lumen, which was returned to the abdominal cavity and then closed. After 30 min, the mouse was euthanized and a blood sample (100 μl) was taken by cardiac puncture. The concentration of FD-4 in the plasma was measured by a fluorescence spectrophotometer.
MPO activity.
Frozen ileal mucosa and lung tissue was homogenized and processed for myeloperoxidase (MPO) activity measurement as previously described (73). The MPO activity in these supernatants was determined by measuring the H2O2-mediated oxidation of o-dianisidine hydrochloride at 460 nm. MPO activity was determined by using a standard curve from MPO derived from human leukocytes (Sigma Aldrich) and normalized to milligrams of protein determined by bicinchoninic acid protein assay (Pierce).
Bacterial translocation.
The mesenteric lymph node (MLN) complex was harvested and the level of translocating bacterial quantified as previously described (17). Briefly, using sterile technique, the MLN complex was harvested, weighed, and homogenized in 0.2 ml of sterile saline. Aliquots were plated onto both blood and MacConkey agar plates. These plates were examined at 24 h of aerobic incubation at 37°C. The colonies were counted on the plates and recorded as the number of colony forming units per gram of MLN.
Real-time PCR.
Total RNA was prepared from ileal mucosa or lung tissue by using the RNeasy kit (Qiagen) and cDNA synthesis was reverse transcribed by using the high-capacity cDNA reverse transcription kit (Applied Biosystems) according to manufacturer's protocols. cDNA was then amplified with TaqMan gene expression Master Mix and predesigned TaqMan probes for murine GLUT1, COX-2, TNF-α, IL-1β, IL-6, and IL-10 as recommended by Applied Biosystems. Within each experimental group, mRNA expression was normalized to 18S amplification (ΔCt) and then fold changes in expression relative to wild-type (WT) T/SS (ΔΔCt) was determined by use of 2−(−ΔΔCt) (54).
Western blotting.
Whole cell extracts prepared from ileal mucosal scrapings as described (41) were resolved by SDS-PAGE and detected by Western blotting. The following antibodies were used: iNOS, HIF-1α (BD Biosciences), cleaved Asp175 caspase-3, p42/p44 (Cell Signaling), and HIF-1α (Novus Biologicals). As reported by others (38), total p42/p44 was used as a our loading control since the expression of commonly used loading controls such as actin and tubulin have been shown to be altered in tissues of animals subjected to I/R injury (63) and intestinal cytoskeleton degradation has been shown to precede tight junction loss (64). Ponceau S staining of the membranes was also done to validate transfer efficiency as well as equal protein loading. The Western blots were developed with Immobilon Western chemiluminescent HRP substrate (Millipore) and analyzed by densitometry using an AlphaImager 3400 imaging system and AlphaEase FC software (Alpha Innotech).
Caspase-3 activity and caspase-3/7 activity assays.
For in vivo studies, caspase-3 activity was determined according to the instructions provided by the caspase-3/CPP32 colorimetric assay kit with DEVD-pNA substrate (Biovision). For in vitro studies, caspase-3/7 was determined according to the instructions provided by the sensoLyte homogenous Rh110 caspase-3/7 assay kit (AnaSpec).
Generation of HIF-1α stable transfectants.
Using the Nucleofector T kit (Amaxa), Caco-2 cells (ATCC) were nucleofected with p (HA)HIF-1α or pcDNA3 (empty) expression vector provided by H. F. Bunn (Harvard Medical School, Boston, MA). Pools of HIF-1α or control Caco-2 stable transfectants were selected in the presence of G418 (1 mg/ml) for 14 days. To confirm HIF-1α expression, control and HIF-1α transfectants were starved overnight in DMEM containing 0.1% FBS and exposed to normoxia or hypoxia (1% O2) for 3 h as described (41).
Statistical analysis.
Statistical significance among groups for villous injury, in vivo intestinal permeability, polymorphonuclear cell (PMN) infiltration, and MPO activity was determined by one-way ANOVA analysis followed by Tukey-Kramer or Newman-Keuls. For lung injury score and real-time PCR analysis, data were analyzed by Mann-Whitney test and bacterial translocation by χ2 analysis with Fisher exact test. P values less than 0.05 were considered statistically significant.
RESULTS
Partial HIF-1α deficiency attenuates gut and lung I/R injury.
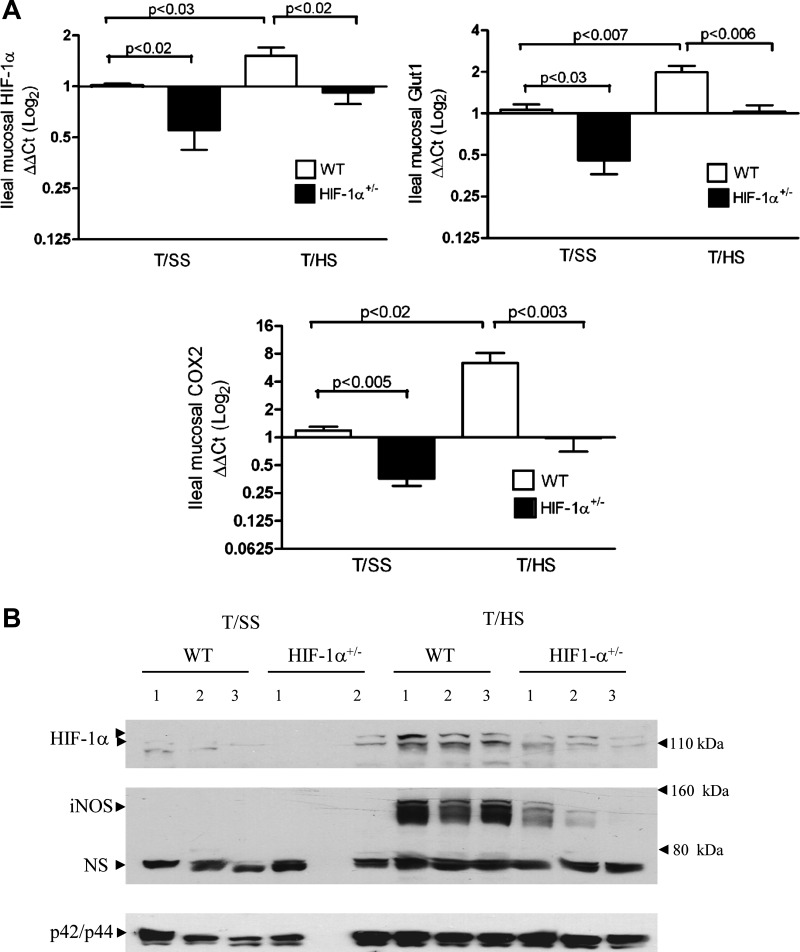
To establish the functional significance of HIF-1 in gut I/R injury, we utilized mice that were heterozygous for a knockout allele at the HIF-1α locus (HIF-1α+/−) in a combined T/HS model. The T/HS model (laparotomy plus 60 min of hemorrhagic shock at 35–40 mmHg) and 3 h of reperfusion represents a global I/R injury. The addition of a laparotomy is important because tissue injury is a component of traumatic hemorrhage and the combined insult of hemorrhage and tissue injury results in an inflammatory response that more closely mimics the clinical situation than hemorrhage alone (15). We first determined the effects of T/HS on the induction of HIF-1α mRNA and protein expression in the ileal mucosa of WT and HIF-1α+/− mice. As shown in Fig. 2A, real-time PCR analysis demonstrated a modest increase in mucosal HIF-1α expression in WT mice subjected to T/HS compared with their T/SS counterparts. In contrast, HIF-1α expression was not evident in HIF-1α+/− mice after T/HS. T/HS was also capable of inducing the expression of two HIF-1 targets, GLUT1 and COX-2, in WT but not HIF-1α+/− mice after T/HS. In agreement with earlier studies (5, 35, 52), the baseline mRNA levels of HIF-1α were significantly lower in the T/SS group of HIF-1α+/− relative to the T/SS WT group. Consistent with these findings, both HIF-1α and iNOS protein levels were increased in the ileal mucosa of WT mice after T/HS compared with T/SS-operated mice (Fig. 2B). In contrast, the HIF-1α and iNOS responses were markedly lower in the ileal mucosa of HIF-1α+/− mice after T/HS. Negligible HIF-1α and iNOS protein levels were detected in WT or HIF-1α+/− mice subjected to T/SS. We chose to characterize the iNOS response since iNOS has been identified as a HIF-1 target and is a key effector in the pathophysiology of gut barrier failure during shocked states. These findings are in agreement with earlier studies demonstrating reduced expression of endogenous HIF-1α protein and downstream targets in HIF-1α+/− cells under hypoxic conditions (5, 35, 52) and HIF-1α+/− mice manifesting impaired physiological responses to ischemia and hypoxia (5, 52, 60).
Fig. 2.
Induction of hypoxia-inducible factor (HIF)-1α- and HIF-1-dependent genes in the ileal mucosa after T/HS. Wild-type (WT) and HIF-1α+/− mice were subjected to T/HS or sham shock (T/SS) for 60 min and 3 h reperfusion. A: relative levels of HIF-1α, GLUT1, and cyclooxygenase-2 (COX-2) mRNA levels in the ileal mucosa were determined by real-time PCR using the ΔΔCt method by using a log2 scale. Mean values ± SE are shown (n = 4–15 mice/group). B: whole cell extracts (WCEs) prepared from mucosal scrapings from the distal ileum were examined for HIF-1α, inducible nitric oxide synthase (iNOS), and total MAPK expression by Western blot analysis. A nonspecific (NS) band (70 kDa) recognized by iNOS antibody also confirmed equal loading of our samples. Numbers refer to individual mice.
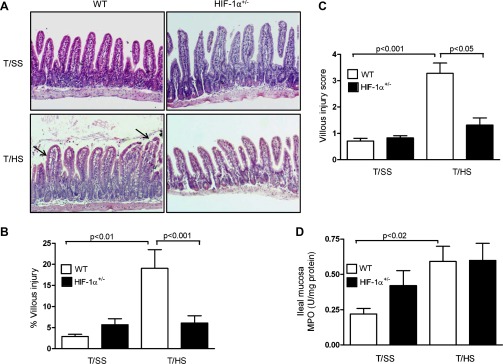
Since our findings demonstrated that T/HS induced an intestinal HIF-1α response, we assessed the effect of partial HIF-1α deficiency on T/HS-induced villous injury. Histological sections of the ileal mucosa (Fig. 3A) showed evidence of subepithelial lifting, mucosal edema, and sloughing in WT mice subjected to T/HS whereas the ileal mucosa of the T/HS-challenged HIF-1α+/− mice had minimal mucosal damage and resembled the T/SS groups, regardless of genotype. The magnitude of T/HS-induced villous injury was approximately fourfold greater in WT mice than in HIF-1α+/− mice (Fig. 3B). Although the percentage of villous injury in HIF-1α+/− mice was the same for T/SS and T/HS groups, the percentage of villous injury was twofold higher in the HIF-1α+/− mice subjected to T/SS as compared its WT counterpart. The villous injury score was also markedly higher in WT than HIF-1α+/− mice after T/HS (Fig. 3C). We also determined whether HIF-1 modulated the sequestration of PMN into the ileal mucosa after T/HS. Although MPO levels were extremely low in the ileal mucosa, T/HS elevated MPO levels in WT mice by approximately threefold compared with their T/SS counterpart (P < 0.02; Fig. 3D). However, there was no difference between MPO levels in HIF-1α+/− mice subjected to T/SS and T/HS.
Fig. 3.
Partial HIF-1α deficiency attenuates T/HS-induced gut injury. WT and HIF-1α+/− mice were subjected to T/HS or T/SS for 60 min and 3 h reperfusion. A: representative sections of hematoxylin and eosin (H&E) staining of the distal ileum (×200). Arrows depict subepithelial lifting sloughing and mucosal edema in the villi. B: percentage of villous injury. C: histological scoring of villous damage. Values in B and C are expressed as means ± SE (n = 7–11 mice/group). D: MPO activity (U/mg of protein) was measured in the ileal mucosa. Values are expressed as means ± SE (n = 5–10 mice/group).
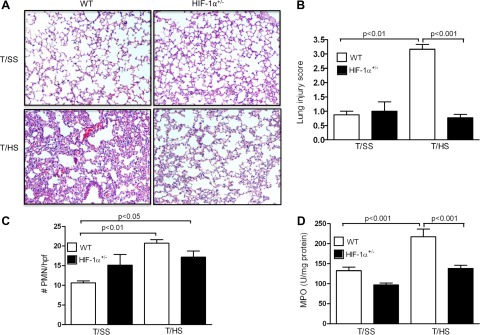
On the basis of earlier studies demonstrating that T/HS-induced gut injury leads to the development of a systemic inflammatory response and lung injury, we asked whether HIF-1 played a maladaptive role in T/HS-induced lung injury. As a consequence of T/HS, loss of alveolar integrity, increased inflammation, and red blood cell congestion are evident in the lungs of WT mice but not of HIF-1α+/− mice (Fig. 4A). No evidence of lung injury is seen in WT or HIF-1α+/− mice subjected to T/SS. The lung injury score (Fig. 4B), the number of infiltrating PMN per high-power field (Fig. 4C), and MPO levels (Fig. 4D) were markedly elevated in WT mice subjected to T/HS compared with T/SS. In contrast, there was no significant difference in lung injury and PMN influx in the lungs of HIF-1α+/− mice subjected to T/HS or T/SS. Thus partial HIF-1α deficiency significantly attenuated T/HS-induced gut and lung injury, thereby supporting the concept that elevation of HIF-1α after gut I/R is deleterious and contributes to both local gut and distant lung organ damage.
Fig. 4.
Partial HIF-1α deficiency attenuates T/HS-induced lung injury. WT and HIF-1α+/− mice were subjected to T/HS or T/SS for 60 min and 3 h reperfusion. A: representative section of H&E staining of the lung. B: lung injury score. C: number of infiltrating polymorphonuclear cells (PMNs) per high-power field (hpf). D: MPO activity (U/mg of protein) was measured in the lung. Values in B–D are expressed as means ± SE (n = 6–13 mice/group).
Persistent HIF-1 activation is linked to loss of gut barrier function.
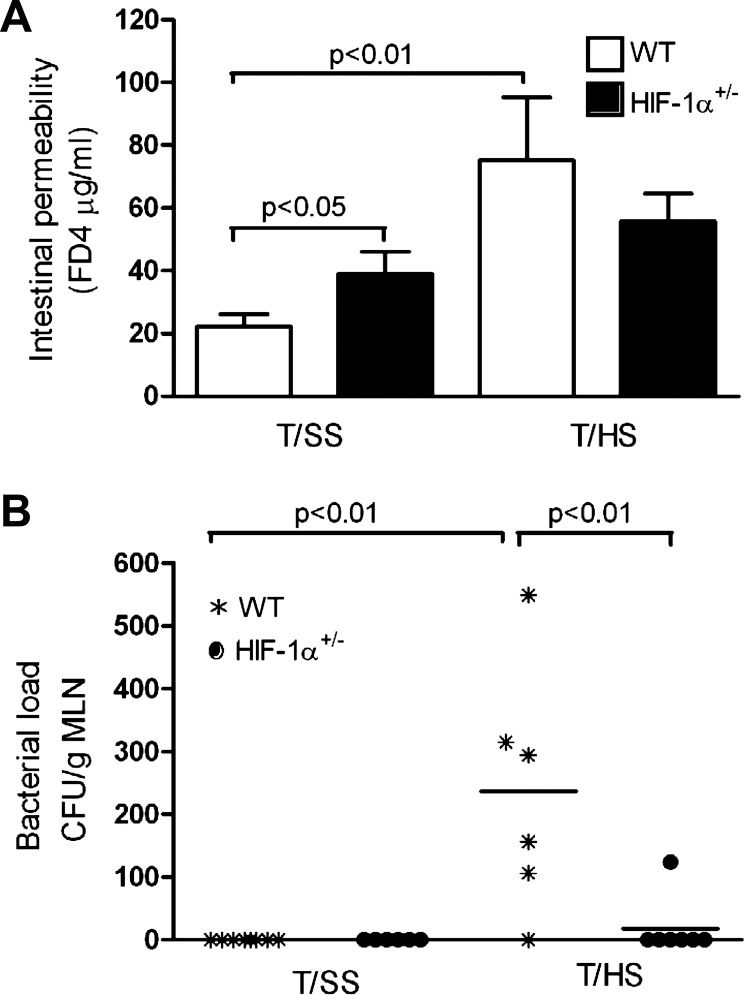
Since functional studies of intestinal barrier function have demonstrated that intestinal permeability increases after gut I/R (57, 70), we tested the hypothesis that HIF-1α activation contributes to T/HS-induced increased intestinal permeability. With use of an in vivo FD-4 intestinal permeability assay, T/HS increased intestinal permeability by 3.8-fold in WT mice compared with their T/SS counterparts (Fig. 5A). However, T/HS was only capable of increasing intestinal permeability by 1.8-fold in HIF-1α+/− mice vs. their T/SS counterparts. The baseline level of intestinal permeability for the T/SS HIF-1α+/− group was approximately twofold higher than the WT T/SS group (P < 0.05).
Fig. 5.
HIF-1 directly impairs gut barrier function. A: in vivo intestinal permeability was assessed in WT and HIF-1α+/− mice subjected to T/SS or T/HS for 60 min and 3 h reperfusion by quantifying 4.4-kDa fluorescein isothiocyanate-dextran (FD-4) concentration (μg/ml) in the serum. Values are expressed as means ± SE (n = 5–8 mice/group). B: bacterial translocation to the mesenteric lymph nodes (MLN) was measured in WT and HIF-1α+/− mice subjected to T/SS or T/HS. Number of colony forming units of bacteria per gram MLN (CFU/g) and values are expressed as means ± SD (n = 6–8 mice/group).
Consistent with these results, bacterial translocation to the mesenteric lymph node was markedly increased in WT mice subjected to T/HS relative to their T/SS group (Fig. 5B). In contrast, no translocation was evident in HIF-1α+/− mice after T/HS and as expected no translocation was found in either WT or HIF-1α+/− mice after T/SS. Taken together, our findings suggest that partial HIF-1α deficiency ameliorated intestinal barrier dysfunction during T/HS, thereby supporting the concept that HIF-1 mediates gut injury.
HIF-1 promotes apoptosis during gut I/R injury.
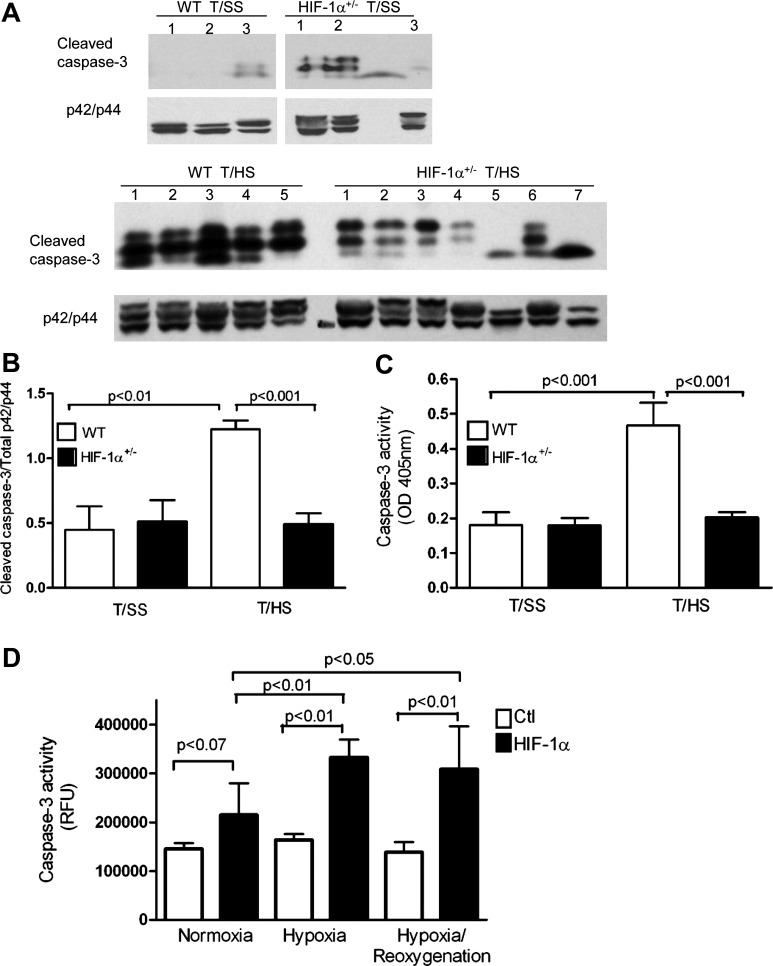
We next studied caspase-3 activation since it has been implicated in gut injury models of T/HS (39, 40), hypoxia (20), and ischemia (7, 25) and the caspase-3 promoter contains a functional HIF-1 response element (65). A significant decrease in the cleavage of caspase-3 (Fig. 6, A and B) and caspase-3/7 activity (Fig. 6C) was found in the ileal mucosa of HIF-1α+/− mice subjected to T/HS compared with their WT counterparts. Similarly, a progressive increase in caspase-3 activity was found in our HIF-1α Caco-2 transfectants exposed to normoxia, hypoxia, or hypoxia/reoxygenation compared with control Caco-2 transfectants (Fig. 6D). These findings suggest that HIF-1 also manifests a proapoptotic role during T/HS-induced gut injury.
Fig. 6.
HIF-1 promotes apoptosis during gut ischemia-reperfusion (I/R) injury. A and B: WCEs were prepared from ileal mucosa of WT and HIF-1α+/− mice subjected to T/HS or T/SS for 60 min and 3 h reperfusion. A: cleaved caspase-3 and total p42/p44 expression was determined by Western blotting. B: densitometry was performed to quantify cleaved caspase-3 and total p42/p44 expression. Data are represented as means ± SE (4–6 mice/group). C: caspase-3 activity is expressed as absorbance at 405 nm per 50 μg of protein. Values are expressed as means ± SE (n = 4–5/group). D: caspase-3/7 activity assay using WCEs derived from HIF-1α and control (Ctl) Caco-2 transfectants exposed to normoxia (N), hypoxia (H) for 3 h, or H followed by reoxygenation for 3 h (H/R). RFU, relative fluorescence units. Mean values ± SE are shown (n = 3–4/condition).
HIF-1 differentially regulates proinflammatory cytokines in the ileal mucosa and lung after T/HS.
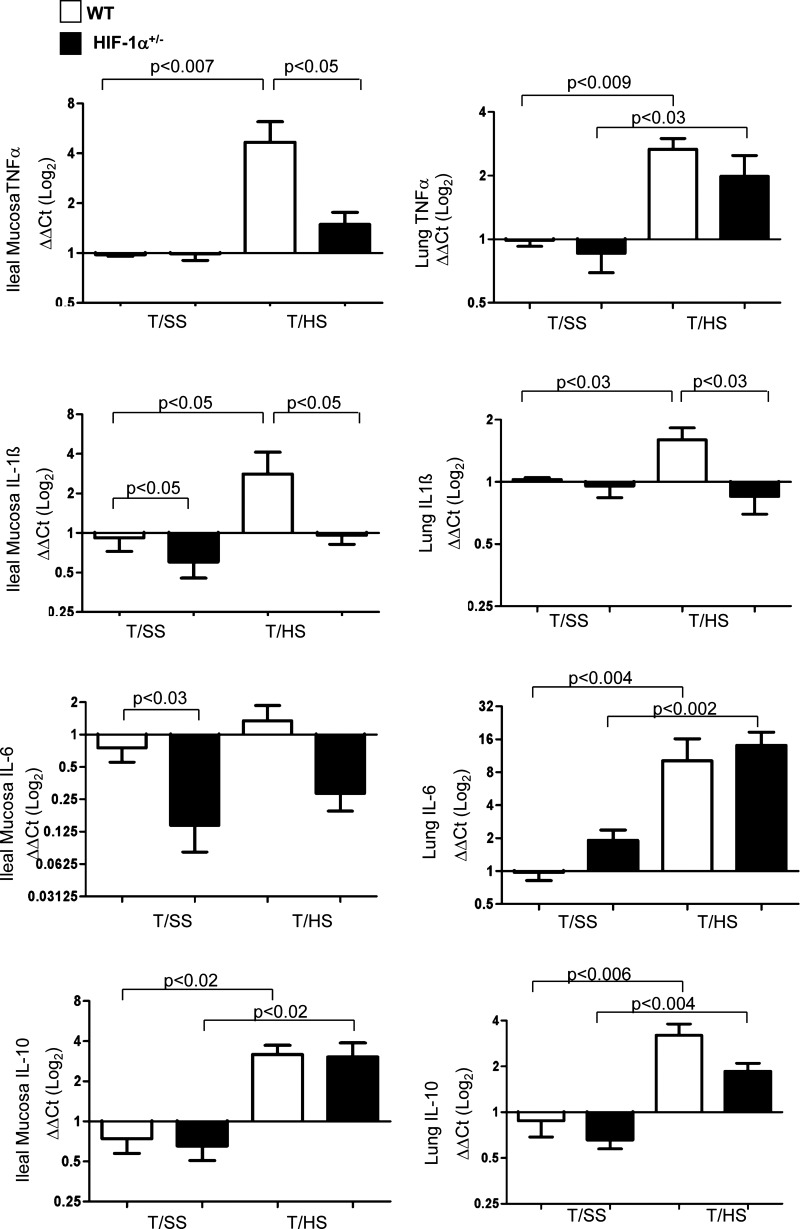
In T/HS models, loss of gut barrier function has been associated with a gut inflammatory response (18, 21). The translocation of bacteria or their products have also been shown to trigger or sustain gut inflammation (21, 62), and our recent studies have demonstrated that bacteria and LPS upregulated HIF-1 expression in intestinal epithelial cells under normoxic conditions (41). Given that HIF-1 is capable of modulating the innate immune and inflammatory responses in hematopoietic and nonhematopoietic cells, we determined whether HIF-1 activation modulated the intestinal and lung inflammatory response after T/HS. As shown in Fig. 7, T/HS increased TNF-α and IL-1β mRNA levels in the ileal mucosa of WT mice compared with their T/SS group (P < 0.007 and P < 0.05, respectively). In contrast, partial HIF-1α deficiency attenuated the induction of mucosal TNF-α and IL-1β gene expression after T/HS compared with WT mice after T/HS (P < 0.05). Ileal IL-6 gene expression was not modulated after T/HS in either genotype, but its baseline level of expression as well as IL-1β were significantly lower in HIF-1α+/− mice than in WT mice (P < 0.05). We then asked whether HIF-1 activation affected the pulmonary inflammatory response. Lung TNF-α, IL-1β, and IL-6 levels were markedly higher in WT mice after T/HS. However, in contrast to the intestinal inflammatory response, only the increase in T/HS-induced lung IL-1β expression was prevented in HIF-1α+/− mice (P < 0.03). IL-10 levels were also measured to determine whether T/HS induced higher levels of IL-10 in HIF-1α+/− mice compared with WT mice since IL-10 was described as an anti-inflammatory cytokine (46, 68). IL-10 levels were equally elevated in both the ileum and lungs of WT and HIF-1α+/− mice subjected to T/HS compared with their T/SS counterparts. Thus the attenuation of gut and subsequent lung injury by partial HIF-1α deficiency was not due to an increased IL-10 response. Taken together, our results suggest that HIF-1 differentially regulates intestinal and lung-derived inflammation after T/HS.
Fig. 7.
HIF-1 differentially regulates proinflammatory cytokines in the ileal mucosa and lung after T/HS. RNA was prepared from ileal mucosa and lung of WT and HIF-1α+/− mice subjected to T/HS or T/SS for 60 min and 3 h reperfusion. Relative mRNA expression for TNF-α, IL-1β, IL-6, and IL-10 was quantified by real-time PCR using the ΔΔCt method using a log2 scale. Mean values ± SE are shown (n = 4–7 mice/group).
DISCUSSION
The major observation of the present work is that prolonged HIF-1 activation significantly contributes to splanchnic ischemia-reperfusion-induced loss of gut barrier function, bacterial translocation, apoptosis, and gut-derived inflammatory response, subsequently resulting in villous injury. A second major observation is that resistance to gut injury was associated with resistance to lung inflammation and injury in the HIF-1α+/− mice. These observations expand our earlier studies demonstrating that gut ischemia in vivo induces a prolonged intestinal mucosal HIF-1 response that does not disappear upon reoxygenation of the intestine and that this persistence of the HIF-1 response appears to be mediated, at least in part, by intestinal bacteria and/or bacterial products, such as LPS (41). Thus the present study provides evidence that a prolonged intestinal mucosal HIF-1 response, which persists after gut reperfusion, plays a role in the pathogenesis of gut-mediated I/R injury. These results are of potential clinical importance because gut injury and loss of normal intestinal barrier function have been associated with the development of SIRS, acute respiratory distress syndrome (ARDS), and MODS as well as worse clinical outcomes in severely injured as well as intensive care unit patient populations (18). The reason why gut injury and loss of barrier function appear to be clinically important in these patient populations is related to its increased susceptibility to injury during stress states (11, 18) and the fact that the gut and its contents are a major source of factors that contribute to the development of a systemic inflammatory state and distant organ including acute lung injury (19).
Although cytokines (58), bacteria (41, 51), and LPS (41, 59) as well as hypoxia have been shown to activate HIF-1 in enterocytes, our knowledge of HIF-1 activation and its functional sequelae in gut injury models is limited and to some extent controversial. For example, in contrast to our acute gut I/R results, HIF-1α was found to be protective in more chronic gut injury models utilizing colon-specific HIF-1α-deficient mice. These models included 2,4,6-trinitrobenzene sulfonic acid -induced colitis (12, 37, 56), chronic hypoxia (24), and Yersinia enterocolitica orogastric infection (28). In the context of gut I/R injury, one study found that mice administered the hydroxylase inhibitor, dimethyloxalglycine (DMOG) was protective in a localized gut I/R injury model (superior mesenteric artery occlusion for 15 min and 3 h reperfusion), and HIF-1 mediated its protective effects via CD73 (27). One possible explanation for this discrepancy may be due to differences in our models of gut I/R injury and a second explanation is that the pretreatment of mice with DMOG may have preconditioned their intestine to subsequent gut I/R injury. However, consistent with our studies, HIF-1α was found to be detrimental in necrotizing enterocolitis (2) and in dextran sulfate sodium-induced colitis in von Hippel-Lindau-deficient mice characterized for their constitutive HIF-1 expression (61). Thus the role of HIF-1, as protective or deleterious, may depend on whether the insult is acute or chronic, the segments of the intestine injured, as well as on whether the loss of HIF-1α is partial or total. For example, the chronic studies, where HIF-1 was protective, utilized conditional intestinal and colonic epithelial HIF-1α mice (28, 37, 56), whereas our studies were performed in mice with a partial HIF-1α deficiency that involves the entire gut. These differences may be important, since the segment of the gut that is susceptible to acute gut I/R-mediated injury is the distal small intestine (33, 69). This increased susceptibility of the ileum to I/R-mediated injury is consistent with the observation that the levels of proinflammatory genes, such as iNOS, and apoptotic cell death were markedly increased after hemorrhagic shock in the ileum compared with duodenum, jejunum, and colon (33). Thus acute and chronic gut injury models differ not only in their mechanism of injury, but also in the segments of the guts that are at highest risk of injury. Additionally, the use of partially HIF-1α+/−-deficient mice has the advantage that only a partial HIF-1α deficiency exists whereas complete deficiency of HIF-1α in a specific cell type, as with the conditional deletion of HIF-1α in intestinal and colonic cells, may result in a phenotype that is not as clinically relevant, since such a state is unlikely to exist in any human disease state. Thus although HIF-1 appears to be involved in the regulation of intestinal homeostasis, it appears to have dichotomous roles in gut inflammatory diseases in that it can be injurious or protective depending on the exact physiological conditions studied as well as the nature and duration of the insult.
This dichotomous effect of HIF-1 activation has also been observed in studies focusing on the heart and brain as well as the gut. For example, HIF-1 has been implicated as a cardioprotective factor in ischemic preconditioning models, since cardioprotection by intermittent ischemia was impaired in partially deficient HIF-1α mice (6). However, while HIF-1 is cardioprotective in preconditioning models, where HIF-1 is only transiently elevated, chronic activation of HIF-1 signaling was found to be deleterious in ischemic hearts (43). Similarly, HIF-1α was found to mediate ischemic tolerance in the neonatal rat brain (3) as well as in transient (55) and permanent (4) focal cerebral ischemia models. However, in contrast to its protective effects in models of preconditioning or tolerance, ablation of HIF-1 in the brain was associated with neuroprotection to an acute ischemic insult (30) and loss of HIF-1α in astrocytes markedly protected neurons from hypoxia-induced neuronal death (8, 66). Thus HIF-1α activation has been shown to be either beneficial or deleterious in several organ systems depending on the physiological context of the model.
There is evidence that the relationship between HIF-1 activation and inflammation appears to be bidirectional since numerous studies have shown that HIF-1 is a central regulator of inflammation and innate immunity (50), whereas at the same time proinflammatory mediators such as TNF-α, IL-1β, nitric oxide, and LPS have been shown to induce HIF-1α expression in cells, including enterocytes, even under normoxic conditions (29, 41, 58, 59). In this context, our findings suggest that the diminished intestinal TNF-α, IL-1β, and iNOS responses as well as the reduced pulmonary IL-1β response in partially deficient HIF-1α mice after T/HS may be linked to the attenuation of villous and lung injury. Support for this concept stems from earlier studies linking gut-derived factors such as IL-1β, TNF-α, and iNOS as well as enteric bacteria to intestinal barrier dysfunction and the development of both sepsis- and T/HS-mediated distant organ injury (14, 70). Additionally, HIF-1 activation has been implicated in the proinflammatory response of myeloid cells during sepsis and deletion of HIF-1α in myeloid cells afforded protection against LPS-induced mortality (53). Although ileal IL-6 levels were not significantly elevated in WT mice subjected to T/HS as reported previously (71), the baseline levels of intestinal IL-6 level and IL-1β were significantly lower in HIF-1α+/− mice than WT mice. Whether partially HIF-1α deficient mice have a less primed or less active immune status remains to be determined since we only profiled a limited number of proinflammatory cytokines. Although our findings suggest that HIF-1 activation exacerbates the T/HS-induced gut-and lung-derived inflammatory response and HIF-1 differentially regulates cytokine expression in the ileal mucosa and lung, a more extensive analysis of the HIF-1-driven intestinal and lung inflammatory response is needed to resolve this issue.
Since SIRS, ARDS, and MODS remain common causes of morbidity and mortality, understanding the mechanisms by which shock and gut ischemic states, such as trauma, lead to gut injury and gut-induced acute lung injury are critical to advancing the care of these patients. In this context, our study showing for the first time that prolonged HIF-1 activation is the proximal regulator of T/HS-induced gut mucosal injury and inflammation provides novel insights into the biology of this clinically important syndrome as well as potential therapeutic insights.
GRANTS
This research was supported by National Institutes of Health grants 1P50GM069790 (to R. Feinman and E. A. Deitch), T32-GM069330 (to F. J. Caputo and D. Doucet) and R01-HL55338 (to G. L. Semenza).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author (s).
REFERENCES
- 1. Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell 28: 941–953, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Baregamian N, Rychahou PG, Hawkins HK, Evers BM, Chung DH. Phosphatidylinositol 3-kinase pathway regulates hypoxia-inducible factor-1 to protect from intestinal injury during necrotizing enterocolitis. Surgery 142: 295–302, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol 48: 285–296, 2000. [PubMed] [Google Scholar]
- 4. Bernaudin M, Nedelec A, Divoux D, MacKenzie E, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab 22: 393–403, 2002. [DOI] [PubMed] [Google Scholar]
- 5. Bosch-Marce M, Okuyama H, Wesley J, Sarkar K, Kimura H, Liu Y, Zhang H, Strazza M, Rey S, Savino L, Zhou Y, McDonald K, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, LaVallee T, Semenza G. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res 101: 1310–1318, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1α. Cardiovasc Res 77: 463–470, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Chaitanya GV, Babu PP. Activation of calpain, cathepsin-B and caspase-3 during transient focal cerebral ischemia in rat model. Neurochem Res 33: 2178–2186, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Chen C, Hu Q, Yan J, Yang X, Shi X, Lei J, Chen L, Huang H, Han J, Zhang JH, Zhou C. Early inhibition of HIF-1α with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobiol Dis 33: 509–517, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Chiu C, McArdle A, Brown R, Scott H, Gurd F. Intestinal mucosal lesions in low-flow states. Arch Surg 101: 478–483, 1970. [DOI] [PubMed] [Google Scholar]
- 10. Claridge JA, Enelow RI, Young JS. Hemorrhage and resuscitation induce delayed inflammation and pulmonary dysfunction in mice. J Surg Res 92: 206–213, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock 28: 384–393, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134: 156–165, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Dehne N, Brüne B. HIF-1 in the inflammatory microenvironment. Exp Cell Res 315: 1791–1797, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Deitch E, Xu DZ, Franko L, Ayala A, Chaudry I. Evidence favoring the role of the gut as a cytokine generating organ in rats subjected to hemorrhagic shock. Shock 1: 141–146, 1994. [DOI] [PubMed] [Google Scholar]
- 15. Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock 9: 1–11, 1998. [DOI] [PubMed] [Google Scholar]
- 16. Deitch EA. Multiple organ failure: pathophysiology and potential future therapy. Ann Surg 216: 117–134, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deitch EA, Berg R, RS Endotoxin promotes the translocation of bacteria from the gut. Arch Surg 122: 185–190, 1987. [DOI] [PubMed] [Google Scholar]
- 18. Deitch EA, Sambol JT. The gut origin hypothesis of MODS. In: Sepsis and Multiple Organ Dysfunction: A Multidisciplinary Approach, edited by Deitch EA, Vincent J-L, Windsor A. Philadelphia, PA: Saunders, 2002, p. 105–116. [Google Scholar]
- 19. Deitch EA, Xu D, Lu Q. Gut lymph hypothesis of early shock and trauma-induced multiple organ dysfunction: a new look at gut origin of sepsis. J Org Dys 2: 70–70, 2006. [Google Scholar]
- 20. Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, Patel PK, Jacobs ER, Medhora M. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol 294: H724–H735, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin 21: 177–196, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Forsythe RM, Xu DZ, QL, Deitch EA. LPS-induced enterocyte-derived nitric oxide induces intestinal monolayer permeability in an autocrine fashion. Shock 17: 180–184, 2002. [DOI] [PubMed] [Google Scholar]
- 23. Fraisl P, Aragones J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov 8: 139–152, 2009. [DOI] [PubMed] [Google Scholar]
- 24. Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med 193: 1027–1034, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giakoustidis AE, Giakoustidis DE, Koliakou K, Kaldrymidou E, Iliadis S, Antoniadis N, Kontos N, Papanikolaou V, Papageorgiou G, Atmatzidis K, Takoudis D. Inhibition of intestinal ischemia/reperfusion induced apoptosis and necrosis via down-regulation of the NF-kB, c-Jun and caspase-3 expression by epigallocatechin-3-gallate administration. Free Radic Res 42: 180–188, 2008. [DOI] [PubMed] [Google Scholar]
- 26. Greijer A, van der Wall E. The role of hypoxia inducible factor (HIF-1) in hypoxia induced apoptosis. J Clin Pathol 57: 1009–1014, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hart ML, Henn M, Kohler D, Kloor D, Mittelbronn M, Gorzolla IC, Stahl GL, Eltzschig HK. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J 22: 2784–2797, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, Yazdi AS, Matteoli G, Bohn E, Autenrieth IB, Karhausen J, Neumann D, Colgan SP, Kempf VAJ. Hypoxia-independent activation of HIF-1 by Enterobacteriaceae and their siderophores. Gastroenterology 134: 756–767, 2008. [DOI] [PubMed] [Google Scholar]
- 29. Hellwig-Burgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood 94: 1561–1567, 1999. [PubMed] [Google Scholar]
- 30. Helton R, Cui J, Scheel JR, Ellison JA, Ames C, Gibson C, Blouw B, Ouyang L, Dragatsis I, Zeitlin S, Johnson RS, Lipton SA, Barlow C. Brain-specific knock-out of hypoxia-inducible factor-1α reduces rather than increases hypoxic-ischemic damage. J Neurosci 25: 4099–4107, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hierholzer C, Harbrecht BG, Billiar TR, Tweardy DJ. Hypoxia-inducible factor-1 activation and cyclo-oxygenase-2 induction are early reperfusion-independent inflammatory events. Arch Orthop Trauma Surg 121: 219–222, 2001. [DOI] [PubMed] [Google Scholar]
- 32. Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 95: 7987–7992, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inoue K, Takahashi T, Uehara K, Shimuzu H, Ido K, Morimatsu H, Omori E, Katayama H, Akagi R, KM. Protective role of heme oxygenase 1 in the intestinal tissue injury in hemorrhagic shock in rats. Shock 29: 252–261, 2008. [DOI] [PubMed] [Google Scholar]
- 34. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIF-1α targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001. [DOI] [PubMed] [Google Scholar]
- 35. Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12: 149–162, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jaakkola P, Mole D, Tian Y, Wilson M, Gielbert J, Gaskell S, Kriegsheim A, Hebestreit H, Mukherji M, Schofield C, Maxwell P, Pugh C, Ratcliffe P. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001. [DOI] [PubMed] [Google Scholar]
- 37. Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114: 1098–1106, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kawagoe T, Sato S, Jung A, Yamamoto M, Matsui K, Kato H, Uematsu S, Takeuchi O, Akira S. Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor-mediated immune responses but not in TCR signaling. J Exp Med 204: 1013–1024, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kiang JG, Bowman PD, Lu X, Li Y, Wu BW, Loh HH, Tsen KT, Tsokos GC. Geldanamycin inhibits hemorrhage-induced increases in caspase-3 activity: role of inducible nitric oxide synthase. J Appl Physiol 103: 1045–1055, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Kiang JG, Peckham RM, Duke LE, Shimizu T, Chaudry IH, Tsokos GC. Androstenediol inhibits the trauma-hemorrhage-induced increase in caspase-3 by downregulating the inducible nitric oxide synthase pathway. J Appl Physiol 102: 933–941, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Koury J, Deitch EA, Homma H, Abungu B, Gangurde P, Condon MR, Lu Q, Xu DZ, RF. Persistent HIF-1α activation in gut ischemia/reperfusion injury: potential role of bacteria and lipopolysaccharide. Shock 22: 270–277, 2004. [DOI] [PubMed] [Google Scholar]
- 42. Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 16: 1466–1471, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lei L, Mason S, Liu D, Huang Y, Marks C, Hickey R, Jovin IS, Pypaert M, Johnson RS, Giordano FJ. Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol Cell Biol 28: 3790–3803, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liaudet L, Szabo A, Soriano F, Zingarelli B, Szabo C, Salzman A. Poly(ADP-ribose) synthetase mediates intestinal mucosal barrier dysfunction after mesenteric ischemia. Shock 14: 142–143, 2000. [DOI] [PubMed] [Google Scholar]
- 45. Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ 15: 686–690, 2008. [DOI] [PubMed] [Google Scholar]
- 46. Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis 5: 262–270, 1999. [DOI] [PubMed] [Google Scholar]
- 47. Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 15: 2675–2686, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Michalsky MP, Deitch EA, Ding J, Lu Q, Huang Q. Interleukin-6 and tumor necrosis factor production in an enterocyte cell model (Caco-2) during exposure to Escherichia coli. Shock 7: 139–146, 1997. [DOI] [PubMed] [Google Scholar]
- 49. Murao Y, Loomis W, Wolf P, Hoyt DB, Junger WG. Effect of dose of hypertonic saline on its potential to prevent lung tissue damage in a mouse model of hemorrhagic shock. Shock 20: 29–34, 2003. [DOI] [PubMed] [Google Scholar]
- 50. Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 9: 609–617, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patel NJ, Zaborina O, Wu L, Wang Y, Wolfgeher DJ, Valuckaite V, Ciancio MJ, Kohler JE, Shevchenko O, Colgan SP, Chang EB, Turner JR, Alverdy JC. Recognition of intestinal epithelial HIF-1α activation by Pseudomonas aeruginosa. Am J Physiol Gastrointest Liver Physiol 292: G134–G142, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: essential role of hypoxia inducible factor-1α in development of lipopolysaccharide-induced sepsis. J Immunol 178: 7516–7519, 2007. [DOI] [PubMed] [Google Scholar]
- 54. Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke 34: 1981–1986, 2003. [DOI] [PubMed] [Google Scholar]
- 56. Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor (HIF) prolyl hydroxylase inhibition. Gastroenterology 134: 144–155, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rupani B, Caputo F, Watkins A, Vega D, Magnotti L, Lu Q, Xu D, Deitch E. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery 141: 481–489, 2007. [DOI] [PubMed] [Google Scholar]
- 58. Scharte M, Han X, Bertges DJ, Fink MP, Delude RL. Cytokines induce HIF-1 DNA binding and the expression of HIF-1-dependent genes in cultured rat enterocytes. Am J Physiol Gastrointest Liver Physiol 284: G373–G384, 2003. [DOI] [PubMed] [Google Scholar]
- 59. Scharte M, Han X, Uchiyama T, Tawadrous Z, Delude RL, Fink MP. LPS Increases hepatic HIF-1α protein and expression of the HIF-1-dependent gene aldolase A in rats. J Surg Res 135: 262–267, 2006. [DOI] [PubMed] [Google Scholar]
- 60. Semenza GL. Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol 91: 803–806, 2006. [DOI] [PubMed] [Google Scholar]
- 61. Shah YM, Ito S, Morimura K, Chen C, Yim SH, Haase VH, Gonzalez FJ. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology 134: 2036–2048, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 20: 495–549, 2002. [DOI] [PubMed] [Google Scholar]
- 63. Sutton TA, Wilkinson J, Mang HE, Knipe NL, Plotkin Z, Hosein M, Zak K, Wittenborn J, Dagher PC. p53 regulates renal expression of HIF-1α and pVHL under physiological conditions and after ischemia-reperfusion injury. Am J Physiol Renal Physiol 295: F1666–F1677, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thuijls G, de Haan J, Derikx J, Daissormont I, Hadfoune M, Heineman E, Buurman W. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock 31: 164–169, 2009. [DOI] [PubMed] [Google Scholar]
- 65. Van Hoecke M, Pringent-Tessier A, Garnier P, Bertrand N, Filomenko R, Bettaieb A, Marie C, Beley A. Evidence of HIF-1 functional binding activity to caspase-3 promoter after photothrombotic cerebral ischemia. Mol Cell Neurosci 34: 40–47, 2007. [DOI] [PubMed] [Google Scholar]
- 66. Vangeison G, Carr D, Federoff HJ, Rempe DA. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1α in neurons and astrocytes. J Neurosci 28: 1988–1993, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem 268: 21513–21518, 1993. [PubMed] [Google Scholar]
- 68. Wang Q, Fang CH, Hasselgren PO. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. Am J Physiol Regul Integr Comp Physiol 281: R1013–R1023, 2001. [DOI] [PubMed] [Google Scholar]
- 69. Xu DZ, Lu Q, Guillory D, Cruz N, Berg R, Deitch EA. Mechanisms of endotoxin-induced intestinal injury in a hyperdynamic model of sepsis. J Trauma 34: 676–683, 1993. [DOI] [PubMed] [Google Scholar]
- 70. Yang R, Gallo DJ, Baust JJ, Watkins SK, Delude RL, Fink MP. Effect of hemorrhagic shock on gut barrier function and expression of stress-related genes in normal and gnotobiotic mice. Am J Physiol Regul Integr Comp Physiol 283: R1263–R1274, 2002. [DOI] [PubMed] [Google Scholar]
- 71. Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol 285: G621–G629, 2003. [DOI] [PubMed] [Google Scholar]
- 72. Yeh KY, Yeh M, Glass J, Granger DN. Rapid activation of NF-kappaB and AP-1 and target gene expression in postischemic rat intestine. Gastroenterology 118: 525–534, 2000. [DOI] [PubMed] [Google Scholar]
- 73. Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of the PI-3K/Akt pathway. J Leukoc Biol 82: 774–780, 2007. [DOI] [PubMed] [Google Scholar]
- 74. Zhang HY, Radulescu A, Besner GE. Heparin-binding epidermal growth factor-like growth factor is essential for preservation of gut barrier function after hemorrhagic shock and resuscitation in mice. Surgery 146: 334–339, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]