Abstract
Increased intestinal bile acid absorption and expansion of the bile acid pool has been implicated in the hypercholesterolemia associated with diabetes mellitus. However, the molecular basis of the increase in bile acid absorption in diabetes mellitus is not fully understood. The ileal apical Na+-dependent bile acid transporter (ASBT) is primarily responsible for active reabsorption of the majority of bile acids. Current studies were designed to investigate the modulation of ASBT function and expression in streptozotocin (STZ)-induced diabetes mellitus in rats and to examine the effect of insulin on rat ASBT promoter by insulin. Diabetes mellitus was induced in Sprague-Dawley rats by intraperitoneal injection of low doses of STZ (20 mg/kg body wt) on five consecutive days. Human insulin (10 U/day) was given to a group of diabetic rats for 3 days before euthanasia. RNA and protein were extracted from mucosa isolated from the small intestine and ASBT expression was assessed by real-time quantitative RT-PCR and Western blotting. Our data showed that ASBT mRNA and protein expression were significantly elevated in diabetic rats. Insulin treatment of diabetic rats reversed the increase in ASBT protein expression to control levels. Consistently, ileal Na+-dependent [3H]taurocholic uptake in isolated intestinal epithelial cells was significantly increased in diabetic rats. In vitro studies utilizing intestinal epithelial Caco-2 cells demonstrated that ASBT expression and promoter activity were significantly decreased by insulin. These studies demonstrated that insulin directly influences ASBT expression and promoter activity and that ASBT function and expression are increased in rats with STZ-induced diabetes mellitus. The increase in ASBT expression may contribute to disturbances in cholesterol homeostasis associated with diabetes mellitus.
Keywords: hypercholesterolemia, insulinopenia, enterohepatic circulation
diabetes mellitus is a complex disorder resulting from insulin imbalance and characterized by dyslipidemia and hypercholesterolemia (18, 34). High levels of plasma cholesterol in diabetes mellitus represent a major risk factor for atherosclerosis and cardiovascular diseases (17). The imbalance in cholesterol homeostasis could result from either an increase in cholesterol synthesis and intake or a decrease in cholesterol removal from the body (11, 25). A major route for eliminating cholesterol from the body occurs via the conversion of the insoluble molecules of cholesterol into detergent-like bile acids that are secreted in the intestine to emulsify dietary fat (1, 31). The majority of the secreted bile acids are efficiently reabsorbed in the distal ileum and return back to the liver for resecretion in the intestine, whereas ∼5–10% of bile acids are excreted in the feces and compensated for by de novo synthesis, accounting for a major route of cholesterol removal from the body (1). The circulating bile acids play important roles as signaling molecules and are critical modulators of lipid and cholesterol metabolism (1, 26). Under pathological conditions, an increase in the amount of bile acids returning to the liver causes more inhibition of cholesterol catabolism, leading to hypercholesterolemia (1, 20). Indeed, the size of the circulating pool of bile acids has been shown to be increased in diabetic patients and in animal models of diabetes mellitus (2, 6, 7). However, the mechanism underlying this increase is not fully understood.
Intestinal absorption of bile acids has been shown to be the major determinant of the amount of bile acids circulating between the intestine and the liver (1, 24). Indeed, studies in animal models of diabetes mellitus have shown an increase in the intestinal absorption of bile acids (6). Moreover, recent clinical trials demonstrated the beneficial effects of blockers of bile acid absorption in improving the lipid and cholesterol profile in patients with diabetes mellitus (33, 34). Current therapy of inhibiting bile acid absorption is based on sequestering them by using resins such as cholestyramine and colesevelam that bind bile acids in the intestinal lumen and prevent their absorption (33, 34). However, poor patient compliance for cholestyramine and the need of high doses (several g/day) of bile acid sequestrants warrant the need for better and more efficient inhibitors of bile acid absorption (3, 22). Therefore, further investigations are needed to delineate the modulation of bile acid absorption in diabetes mellitus at the molecular level.
The first and rate-limiting step of intestinal bile acid absorption occurs via the ileal apical Na+-dependent bile acid transporter (ASBT) that is expressed on the brush-border membrane of ileal epithelial cells (1, 24). The essential role of ASBT in maintaining the recycling of bile acids is clear from studies showing the depletion of bile acid pool and the low level of plasma cholesterol in ASBT knockout mice (12). Whether ASBT expression is altered in diabetes mellitus is not known. Diabetes mellitus is characterized by hormonal dysregulation, typically insulin imbalance. Previous studies have shown that ASBT function and expression are regulated by hormones such as glucocorticoids (10, 14, 30). Whether insulin modulates ASBT expression via a mechanism that influences ASBT expression in diabetes mellitus is not known.
Therefore, we investigated the modulation of ASBT function and expression in the widely used streptozotocin (STZ)-induced model of diabetes mellitus in rats. To avoid confounding toxicity inflicted by the high dose of STZ, we used the recently described model of inducing diabetes mellitus with multiple injections of low doses of STZ (21). Our findings demonstrated an increase in ASBT function and expression in the distal ileum of diabetic rats. The increase in ASBT expression reverted back to normal by insulin treatment. Insulin decreased the promoter activity of rat ASBT in intestinal Caco-2 cells, suggesting that the lack of insulin contributes to the pathological increases in ASBT expression in diabetic rats. Our data suggest that ASBT dysregulation induced by alterations in the level of insulin may underlie the increase in bile acid pool observed in diabetes mellitus.
MATERIALS AND METHODS
Animal studies.
Sprague-Dawley rats (males, 7–8 wk of age, 200–250 g) were purchased from Charles River Laboratories (Wilmington, MA) and housed at the Veterinary Medical Unit at the Jesse Brown Veterans Affairs Medical Center (12:12-h light-dark cycle). Animals had free access to food and water and were fed regular chow diet. Diabetes mellitus was induced in the rats by low doses (20 mg·kg body wt−1·day−1) of STZ (Sigma) administered by intraperitoneal injections on five consecutive days (STZ was dissolved in citrate buffer, pH 4.5). Control animals were injected with vehicle alone. Blood samples were collected from tail snips, and the level of glucose was measured by a True-Track glucometer (Smart System). Rats were killed 2 wk after the last STZ injection, and blood and small intestinal tissues were collected. Human insulin (Humalin; Eli Lilly) was given (10 U/day) to a group of diabetic rats via subcutaneous osmotic mini pumps (Osmotic pump Model 2 microL1; Alzet) for 3 days before death. The level of insulin in the plasma was evaluated using a rat/mouse insulin ELISA kit (Millipore, Billerica, MA). Each group of experimental animals contained at least three to six rats. Immediately after euthanasia the intestine was removed and flushed with ice-cold 1× PBS, pH 7.4. The small intestine was divided into three equal parts, and the mucosa from the first proximal part (jejunum) and the distal part (ileum) was scraped, snap-frozen in liquid nitrogen, and stored at −80°C for subsequent protein and RNA extractions. Small pieces of the intestine from different regions (jejunum, ileum, and colon) were cut and were immediately snap-frozen in optimal cutting temperature embedding medium (Tissue-Tek OCT compound; Sakura) for immunofluorescence analysis and laser capture microdissection. Protocols for animal studies were approved by animal care committees at the University of Illinois at Chicago and the Jesse Brown Veterans Affairs Medical Center.
Protein extraction and Western blotting.
Frozen intestinal mucosa was cut, weighed (5–30 mg), and immediately transferred to ice-cold lysis buffer. The tissues were then homogenized by douncing in the lysis buffer containing 10 mM Tris-HEPES, pH 7.4, 150 mM NaCl, 1% Triton X-100, and protease cocktail inhibitors (Roche). The homogenates were then centrifuged at 3,000 rpm to pellet the nuclei and other debris. The clear supernatant was removed, and the concentration of protein was assessed by the method of Bradford (4). Equal amounts of protein (80 μg) were solubilized in Laemmli sample buffer (2% SDS, 10% glycerol, 100 mM dithiothreitol, 60 mM Tris, pH 6.8, 0.01% bromphenol blue), separated on 10% Tris-glycine SDS polyacrylamide gel, and electrotransferred to nitrocellulose membranes. Western blotting was performed by washing the nitrocellulose membranes three times and then blocking overnight in PBS containing 5% nonfat dry milk. The blots were then incubated for 3 h at room temperature or left overnight at 4°C with anti-rat ASBT antibodies (Santa Cruz) diluted (1:500) in the blocking solution. Blots were washed with PBS containing 0.1% Tween 20 and then incubated with the same buffer containing donkey anti-goat antibodies (Santa Cruz). The bands were visualized by an enhanced chemiluminescence kit according to the manufacturer's instructions (Amersham, Arlington Heights, IL).
Immunofluorescence analysis.
Snap-frozen tissues were sectioned (5 μm) using cryostat, mounted on the slides, and preserved at −80°C until further use. For immunostaining, the sections were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature followed by blocking in PBS containing 5% donkey serum and 0.3% Triton X-100 for 45 min at room temperature. Sections were then incubated with goat, anti-rat ASBT antibodies (dilution 1:100) and rabbit, anti-villin (1:100) for 2 h in the blocking buffer. After PBS washes, the sections were incubated with the secondary antibodies, Alexa Fluor 488-conjugated donkey anti-goat IgG (green) and Alexa Fluor 568-conjugated goat anti-rabbit IgG (red) for 60 min, and then washed and mounted with slow-fade DAPI (blue, nuclei) by using cover slips. Microscopy was performed with a ×63 oil immersion objective of a Zeiss immunofluorescence microscope (Observer Z1) equipped with deconvolution software (AxioVision).
Laser capture microdissection.
Frozen intestinal tissue sections (9 μm) were mounted on special stainless steel slide carrier Pet Foil (W. Nuhsbaum, McHenry, IL) suitable for laser capture microdissection. The tissues were stained with hematoxylin and eosin, and the cells were visualized with a Leica laser capture microscopy system. Epithelial cell size was estimated, and equal numbers of epithelial cells (∼4,000 cells) were cut from ileal tissues obtained from control and diabetic rats. Total RNA was then extracted, and the relative level of ASBT mRNA expression was evaluated by real-time RT-PCR as outlined below.
RNA extraction and quantitative real-time RT-PCR analysis.
Total RNA was extracted from tissues and cells using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. Total RNA was treated with DNase I to avoid genomic DNA contamination. Equal amounts of RNA from both treated and control samples were reverse transcribed and amplified in a one-step reaction using a Brilliant SYBR Green QRT-PCR Master Mix Kit (Stratagene). Primers used for the current studies are mentioned in Table 1.
Table 1.
Primers used for real-time PCR
| Primer | |
|---|---|
| Rat ASBT | Forward: 5′-GGTTGCGCTTGTTATTCCTGT-3′ |
| Reverse: 5′-GGTTCAATGATCCAGGCACTT-3′ | |
| Rat Ost α | Forward: 5′-CCCTCATACTTACCAGGAAGAAGCTAC-3′ |
| Reverse: 5′-CCATCAGGAATGAGAAACAGGC-3′ | |
| Rat Ost β | Forward: 5′-TATTCCATCCTGGTTCTGGCAGT-3′ |
| Reverse: 5′-CGTTGTCTTGTGGCTGCTTCTT-3′ | |
| Rat 18S | Forward: 5′-CCCAGTAAGTGCGGGTCATAA-3′ |
| Reverse: 5′-GATCCGAGGGCCTCACTAAAC-3′ | |
| Human ASBT | Forward: 5′-GCCCCAAAAAGCAAAGATCA-3′ |
| Reverse: 5′-GCTATGAGCACAATGAGGATGG-3′ | |
| Human actin | Forward: 5′-CATGTTTGAGACCTTCAACAC-3′ |
| Reverse: 5′-CCAGGAAGGAAGGCTGGAA-3′ |
ASBT, apical Na+-dependent bile acid transporter; Ost, organic solute transporter.
Because the amplification efficiencies were approximately equal, the quantitation was expressed as a ratio of 2ΔCt(target) gene/2ΔCt(internal control), where ΔCt represents the difference between the threshold cycle of amplification of RNA from different experimental groups for target gene and internal control gene (18S or actin).
Isolation of intestinal epithelial cells and bile acid uptake.
Epithelial cells were isolated from the distal ileum as previously described (29). Briefly, intestinal fragment was washed with ice-cold PBS, opened, and sliced into small pieces (∼1 cm). The intestinal pieces were then washed with Hanks' solution supplemented with 2% BSA and 2% glucose. The intestinal pieces were then incubated for 15 min at 37°C with shaking in Hanks' solution containing 0.5 mM dithiothreitol and 1.5 mM EDTA to isolate the epithelial cells. The cells were collected with low-speed centrifugation at 500 g for 5 min, resuspended in the uptake buffer, and immediately used for assessment of bile acid uptake. Cell viability was determined by trypan blue exclusion. A fraction of the same amount of cells was used for protein extraction and Western blotting analysis to determine the amount of villin (the epithelial cell marker) per milligram protein of isolated cells. Equal amounts of solution containing isolated intestinal epithelial cells were then used for Na+-dependent [3H]taurocholic acid (TC) transport. Cells were incubated at 37°C with buffer containing (in mM) 110 NaCl (with Na+) or choline chloride (without Na+), 4 KCl, 1 MgSO4, 1 CaCl2, 50 mannitol, and 10 HEPES (pH 7.4) as well as 10 μM of [3H]TC (Perkin Elmer, Boston, MA) for the designated period of time. The transport process was terminated by quick centrifugation followed by two washes with ice-cold PBS. Cells were then solubilized with 0.5 N NaOH for at least 4 h. The protein concentration was measured by the method of Bradford (4), and the radioactivity was counted by a Packard liquid scintillation analyzer Tri-CARB 1600-TR (Packard Instrument, Downers Grove, IL). Na+-dependent TC uptake was expressed as picomoles per milligram protein, and the uptake values were normalized to the number of viable cells and the density of villin obtained by Western blotting.
Transient transfection and luciferase assay.
For in vitro studies, human intestinal Caco-2 cells were obtained from American Type Culture Collection and were cultured in minimum essential medium (Eagle) adjusted to contain 1.5 g/l sodium bicarbonate, 0.1 mM nonessential amino acids, and 1.0 mM sodium pyruvate and supplemented with FBS (20%). Caco-2 cells were transiently cotransfected with the 3-kb rat ASBT promoter construct (9), pCMVβ vector expressing β-galactosidase, and insulin receptor expression vector (generous gift from Dr. Michael Quon from the National Institutes of Health, National Center for Complimentary and Alternative Medicine) by electroporation using Amaxa technology (Amaxa). Briefly, ∼2 × 106 cells were harvested and then were electroporated in 100 μl of solution T (supplied by Amaxa) along with 10 μg of DNA and then transferred to full media and plated on Transwell inserts (12-well plate). Cells were then incubated (4 h after transfection) with insulin (Humalin) for 16–20 h. Cells were then washed with PBS and lysed in 1× of the reporter lysis buffer from Promega. The activities of both Firefly Luciferase and β-galactosidase were measured by luminometer using kits from Promega and Clontech, respectively, according to the manufacturer's instructions. The promoter activity was expressed as a ratio of luciferase to β-galactosidase activity in each sample.
Statistical analysis.
Results are expressed as means ± SE. Student's t-test was used in statistical analysis. P ≤ 0.05 was considered statistically significant.
RESULTS
Induction of diabetes mellitus by multiple low doses of STZ.
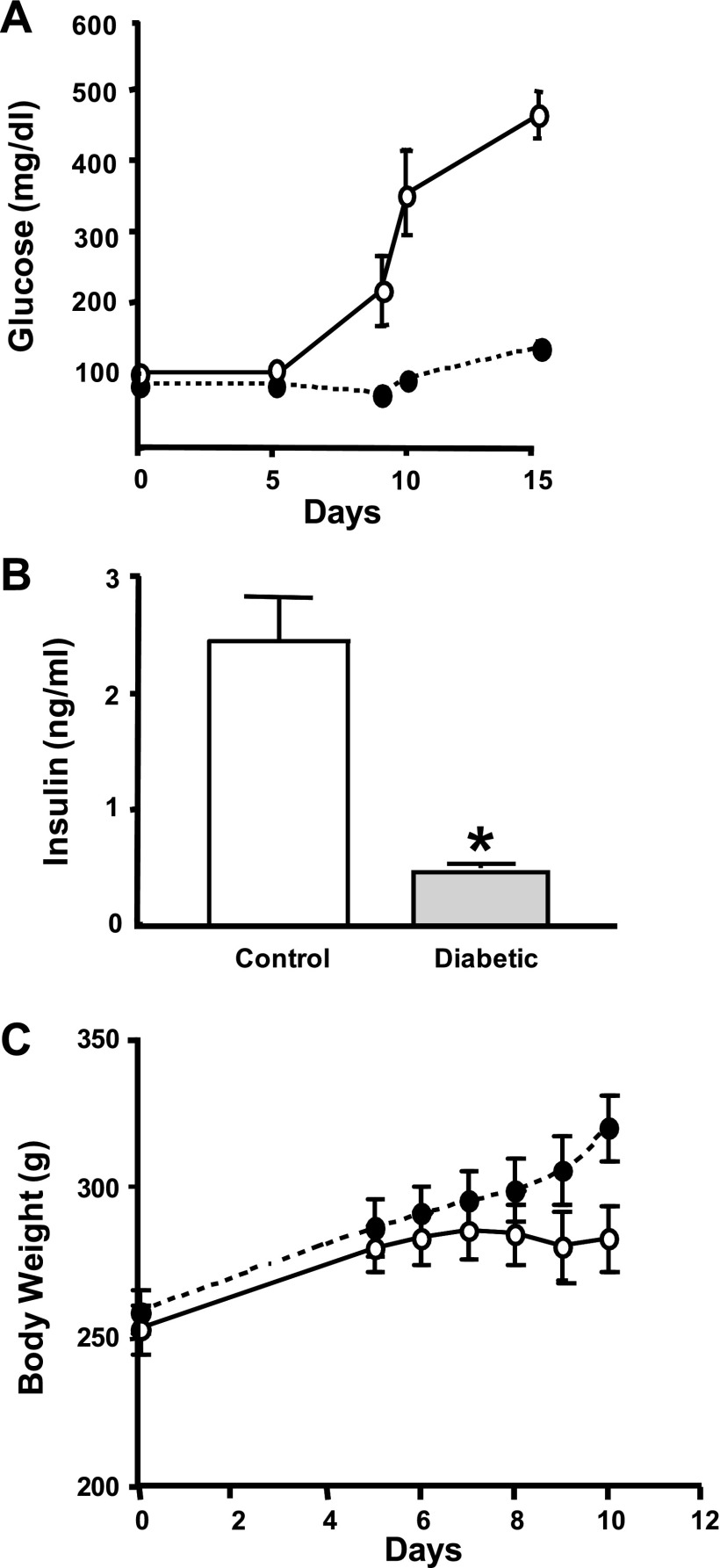
Insulin-dependent diabetes mellitus in rats is commonly induced by a single high dose (60–80 mg/kg body wt) of intraperitoneal injection of STZ. Recent studies, however, indicated that high toxicity and severe weight loss caused by the high dose of STZ might confound the results obtained from this model (21). An alternative approach that uses multiple injections of low doses of STZ (20 mg/kg body wt) has been shown to be equally effective and less toxic (21). To investigate alterations in ASBT expression in diabetes mellitus, we used the rat model with multiple low doses of STZ. Because the results of STZ injections could differ between different strains of rats, we first examined whether low-dose injections of STZ (20 mg/kg body wt) over five consecutive days are sufficient to produce diabetes mellitus with minimal toxic effects in Sprague-Dawley rats obtained from Charles River Laboratories International. Rats (7–8 wk old) were injected (ip) with 20 mg/kg body wt every day for five consecutive days. As shown in Fig. 1A, the multiple low doses of STZ induced hyperglycemia similar to that previously reported in the model with a high dose of STZ. Also, the level of insulin after 14 days of the last injection was significantly decreased, further confirming insulinopenia in these rats (Fig. 1B). However, the diabetic rats maintained weight throughout the course of the experiments, as shown in Fig. 1C, indicating that multiple low doses of STZ are less toxic compared with previously reported weight loss caused by the high-dose model (21). To further characterize the model of diabetes mellitus induced by multiple low doses of STZ, we measured the levels of plasma cholesterol in control and experimental rats. Our data showed that cholesterol levels were significantly increased in diabetic rats compared with control rats and that the high levels of cholesterol reverted back to normal with insulin treatment [67.38 ± 2.3 mg/dl in control, 102.1 ± 12.9 mg/dl in diabetic rats (P < 0.05), and 72.3 ± 1.7 mg/dl in diabetic rats with insulin treatment (P < 0.05)]. These data are consistent with previous studies (13) showing an increase in plasma levels of cholesterol in rats with diabetes mellitus induced with a single high dose of STZ.
Fig. 1.
Multiple low doses of streptozotocin (STZ) are not associated with weight loss. Sprague-Dawley rats obtained from Charles River were given 5 ip injections (○) with multiple low doses (20 mg·kg body wt−1·day−1). Control rats (●) were injected with vehicle alone (citrate buffer, pH 4.5). Glucose was measured at the indicated day (A), and insulin level was assessed after the animals were euthanized (B). Body weight was measured at the indicated day (C), demonstrating that the diabetic rats did not lose weight throughout the experiment. Values are expressed as means ± SE from 4 animals. *P ≤ 0.05 compared with control.
Expression of intestinal bile acid transporter is increased in diabetic rats.
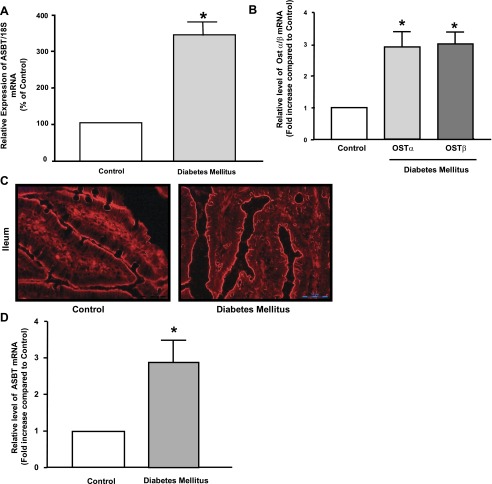
Previous studies have shown an increase in the bile acid pool in diabetic patients and rats with experimentally induced diabetes mellitus (2, 6, 7). Because intestinal bile acid absorption is one of the major processes that determine the amount of circulating bile acids (1), we investigated the expression of intestinal bile acid transporters. Total RNA was extracted from intestinal mucosal scrapings from diabetic and control rats, and the level of ileal ASBT expression was assessed by real-time QRT-PCR. As shown in Fig. 2A, the relative level of ASBT mRNA was significantly increased in diabetic rats compared with control. Furthermore, the expression of bile acid organic solute transporters (Ost) α and β, responsible for the transport of bile acids across the basolateral membrane of intestinal epithelial cells, was also significantly increased in diabetes mellitus (Fig. 2B). It has been previously reported that hyperplasia and hypertrophy might contribute to the increase in the absorptive processes observed in rats with diabetes mellitus induced by a high dose of STZ (15). In the current model of multiple low doses of STZ-induced diabetes mellitus, the intestinal epithelia appear to be hyperplastic with no sign of hypertrophy compared with control rats (Fig. 2C). We used the laser capture microdissection technique to further determine that hyperplasia is not contributing to the observed increase in ASBT mRNA expression in diabetic rats. Tissues harvested from the distal ileum of diabetic and control rats were embedded in optimal cutting temperature media, and frozen tissues were sectioned (∼9 μm). Hematoxylin- and eosin-stained tissues where then visualized under the microscope, and an equal number of epithelial cells were cut by the laser from tissues of diabetic and control rats. Cells were then collected, and total RNA was extracted. As depicted in Fig. 2D, ASBT mRNA expression normalized to the same number of epithelial cells was significantly increased in ileum of diabetic rats compared with control. Collectively, these data indicate that the expression of ileal ASBT and other intestinal bile acid transporters is significantly elevated in rats with STZ-induced diabetes mellitus.
Fig. 2.
Apical Na+-dependent bile acid transporter (ASBT) expression is increased in diabetes mellitus. Distal ileum was removed from control and diabetic animals, and mucosa was scraped as mentioned in materials and methods. Total RNA was extracted, and the relative expression of ASBT mRNA (A) and organic solute transporter (Ost) α and β mRNA (B) was assessed by real-time RT-PCR using SYBR green. The level of mRNA expression for each target gene was normalized to the expression of 18S mRNA in the same sample. In C, immunofluorescence staining for actin (red) in optimal cutting temperature (OCT)-embedded sections of rat ileum from control and diabetic rats showing long villi but no apparent change in the size of epithelial cells. In D, ileal segments were embedded in OCT media, sectioned to 9 μm thickness. The tissues were then mounted on special slides suitable for laser capture microdissection as mentioned in materials and methods. Ileal sections were stained with hematoxylin-eosin (H&E), and epithelial cells were visualized under the microscope. Equal number of cells was cut by laser from tissues from control and diabetic rats. Cells were collected, and total RNA was extracted. The relative expression of ASBT mRNA normalized to 18S mRNA was evaluated by real-time RT-PCR. Values represent means ± SE from at least 3–5 animals. *P ≤ 0.05 compared with control.
Insulin treatment decreases the elevated expression of ASBT in diabetic rats.
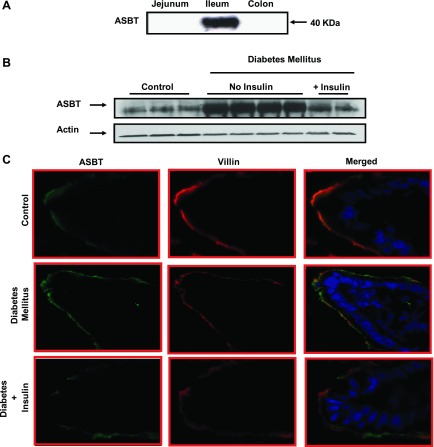
To further examine the role of insulin deficiency in the observed increase in ASBT expression, we treated a group of diabetic rats with human insulin supplied continuously from a subcutaneous osmotic pump. Replacement of insulin for 3 days from the osmotic pump at the rate of 10 U/day was sufficient to decrease the high levels of glucose to almost normal levels. We next investigated the effect of insulin treatment on ASBT protein expression in rats with STZ-induced diabetes mellitus. Total protein lysates were prepared from mucosal scrapings obtained from rat distal ileum and investigated for ASBT protein expression by Western blotting. As shown in Fig. 3A, anti-ASBT antibodies detected the expected size band in distal ileum but not in jejunum or colon, indicating their specificity. Similar to the increase in ASBT mRNA, Fig. 3B shows a significant increase in ASBT protein expression in the distal ileum of diabetic compared with control rats. Furthermore, ASBT protein expression in diabetic rats was reverted back to control levels after 3 days of insulin treatment. In addition, immunofluorescence analysis demonstrated an increase in ASBT staining (green) in epithelial cells of the distal ileum from diabetic rats compared with ASBT staining in tissues obtained from control and diabetic rats with insulin treatment (Fig. 3C). These findings suggest that insulin may directly modulate ASBT expression in intestinal epithelial cells.
Fig. 3.
ASBT protein expression is upregulated in diabetic rats. Total protein extracts were prepared from mucosal scrapings from rat colon and small intestine. Equal amounts of protein were separated on 10% SDS-PAGE and electrotransferred to nitrocellulose blots. Blots were then probed with anti-rat ASBT antibodies, and bands corresponding to ASBT fusion proteins were visualized as described in materials and methods. Blot in A shows that ASBT expression is detected in rat ileum but not jejunum or colon. In B, a representative blot demonstrating an increase in ASBT protein expression in diabetic rat ileum compared with control is shown. Insulin treatment to diabetic rats reversed the increase in ASBT protein expression to the level of control rats. In C, immunofluorescence staining for ASBT (green) and villin (red) with blue-stained nuclei in OCT-embedded sections of rat ileum from control, diabetic, and insulin-treated diabetic rats is shown. Data are representative of 3 different experiments.
ASBT function is increased in isolated ileal enterocytes.
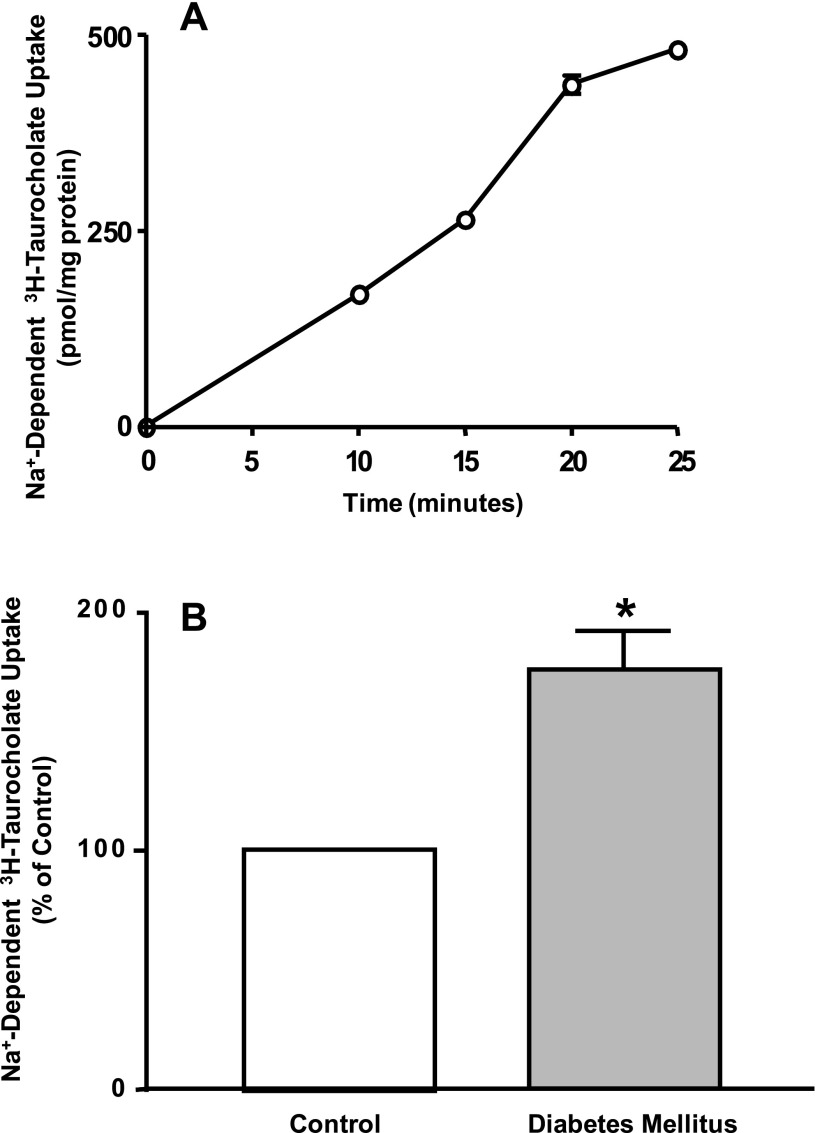
To examine changes in ASBT function in diabetic rats, we measured the Na+-dependent transport of [3H]TC in isolated epithelial cells. Intestinal epithelial cells were isolated from the distal ileum as previously described (29). Cells in suspension were then incubated with the uptake buffer containing 10 μM [3H]TC in the presence and the absence of Na+. Cell viability was determined by trypan blue exclusion. The amount of villin (as a marker for epithelial cells) in each sample of isolated epithelial cells was estimated by Western blotting to ensure equal loading of epithelial cells in each uptake sample per milligram of protein. As shown in Fig. 4A, the Na+-dependent uptake in isolated enterocytes was linear as a function of time. Figure 4B shows Na+-dependent uptake was significantly increased in cells isolated from diabetic rats compared with control animals. Taken together, these data suggest that the insulin deficiency resulted in an increase in ASBT function and expression.
Fig. 4.
ASBT function is elevated in rats with diabetes mellitus. Intestinal epithelial cells were isolated from ileal segments, washed, and immediately resuspended in uptake buffer containing 10 μM of [3H]taurocholic acid (TC) in the presence or absence of Na+. Uptake was terminated at the indicated time point by two washes in ice-cold PBS buffer and was expressed as pmol/mg protein. Na+-dependent [3H]TC uptake was linear up to 20 min of incubation with uptake buffer (A). Na+-dependent [3H]TC uptake was evaluated in isolated epithelial cells from control and diabetic rats (B). Results are presented as means ± SE of uptake values obtained from 3 different animals. Where not shown, error bars are smaller than symbol. *P ≤ 0.05 compared with control.
Insulin decreases rat ASBT promoter activity in intestinal epithelial cells.
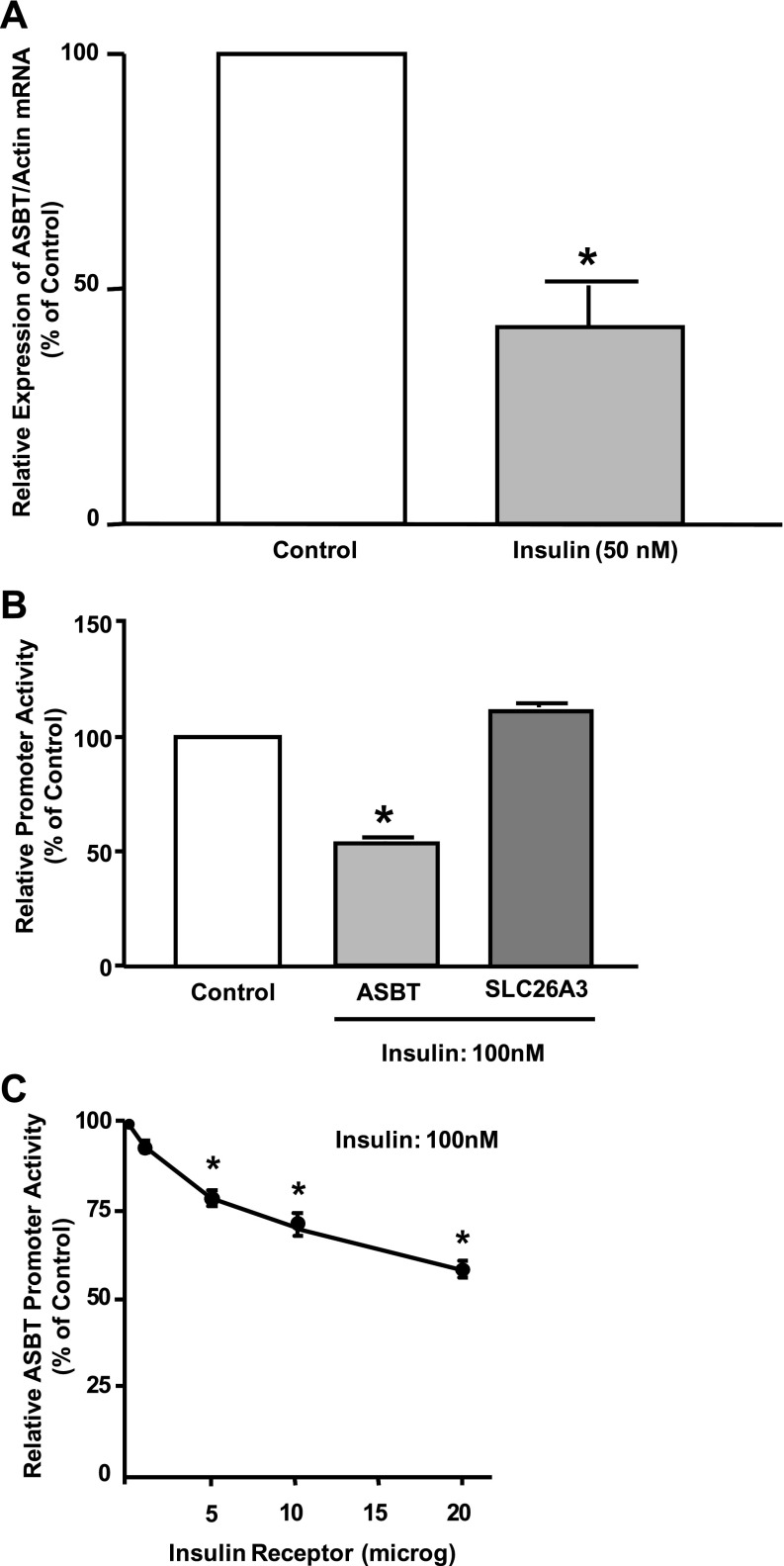
Because insulin treatment to diabetic animals reduced the elevated level of ASBT protein, we hypothesized that insulin directly modulates ASBT expression in intestinal epithelial cells. Therefore, the modulation of ASBT mRNA expression by insulin was investigated using Caco-2 cells. Cells were plated on Transwell inserts and exposed to insulin from the basolateral side. As shown in Fig. 5A, ASBT mRNA expression was significantly decreased by incubation with 50 nM insulin. We next investigated the effects of insulin on the activity of a previously characterized 3 kb of rat ASBT promoter (9). Caco-2 cells were transiently cotransfected with rat ASBT promoter by electroporation using Amaxa technology along with mammalian expression vector for human insulin receptor. As shown in Fig. 5B, incubation with 100 nM insulin for 24 h significantly decreased rat ASBT promoter activity in Caco-2 cells by ∼45–50%. Insulin effects on rat ASBT promoter activity were specific, since activation of insulin receptor in Caco-2 cells did not alter the promoter activity of the SLC26A3 anion transporter (Fig. 5B). Also, the observed decrease was dependent on the amount of the expressed insulin receptor, as shown in Fig. 5C. These data clearly indicate that insulin directly downregulates ASBT expression and promoter activity in intestinal epithelial cells.
Fig. 5.
Rat ASBT promoter activity is decreased by insulin. A: intestinal Caco-2 cells were plated on Transwell inserts and were exposed to 50 nM insulin for 24 h from the basolateral compartment. Total RNA was extracted, and ASBT mRNA expression was assessed by real-time RT-PCR normalized to actin mRNA level (internal control). Control cells were treated with vehicle only. B: Caco-2 cells were transiently cotransfected by electroporation (Amexa) with rat ASBT or the promoter or human SLC26A3 transporter along with expression vector of human insulin receptor and CMV β for β-galactosidase to normalize for the transfection efficiency. Cells were plated on Transwell, treated with 100 nM insulin for 24 h, and harvested for firefly luciferase and β-galactosidase assays to assess the promoter activity. Control cells are cells treated with vehicle only. C: Caco-2 cells were transfected with rat ASBT promoter along with different amounts of the expression vector of human insulin receptor. Cells were plated on Transwell, treated with 100 nM insulin from 24 h, and harvested for firefly luciferase and β-galactosidase assays. Control cells are transfected with rat ASBT promoter and β-galactosidase vectors only without insulin receptor expression vector. Results are presented as % of control and represent means ± SE for 6–9 determinations performed on 3 separate occasions. *P ≤ 0.05 compared with control.
DISCUSSION
Diabetes mellitus is associated with a high risk of developing cholesterol-related disorders such as atherosclerosis (17, 18, 34). A major factor causing hypercholesterolemia has been suggested to be an increase in the circulating pool of bile acids reported in diabetic patients and animal models of diabetes mellitus (2, 6, 7). However, the mechanism(s) involved in the expansion of the bile acid pool in diabetes mellitus is not fully defined. The current studies present novel data demonstrating an increase in the function and expression of ileal bile acid transporter ASBT in rats with STZ-induced diabetes mellitus. The increase in ASBT expression was reversed by insulin treatment of diabetic rats. Furthermore, we demonstrated that insulin directly modulated rat ASBT promoter activity in intestinal epithelial cells. Our data suggest that insulin deficiency is involved in the observed upregulation of ASBT, which may underlie the associated enlargement in the circulating pool of bile acids in STZ-induced diabetes mellitus.
STZ has been widely used to induce diabetes mellitus in rodents. STZ is an antibiotic that destroys β-cells in the pancreas, causing insulinopenia, hyperglycemia, and a disease process similar to type 1 diabetes mellitus (23, 37). Conventionally, diabetes mellitus in rats is induced by a single intraperitoneal injection of a high dose of STZ (60–80 mg/kg body wt) (21, 37). A high dose of STZ, however, has been shown to cause rapid and severe weight loss with other toxic effects influencing gastric motility and the function of gastrointestinal neuroendocrine cells (5, 21). Therefore, observations pertaining to the gastrointestinal tract obtained from the high-dose model of STZ-induced diabetes mellitus should be interpreted with caution. On the contrary, recent studies using multiple low doses of STZ have shown less toxicity and similar efficiency of induction of diabetes mellitus compared with the single high dose (21). Our current data confirmed these observations in Sprague-Dawley rats (obtained from Charles River), demonstrating efficient induction of insulinopenia and hyperglycemia by five consecutive injections with a low dose of STZ (20 mg·kg body wt−1·day−1). Noticeably, diabetic rats did not lose weight as opposed to the drastic loss of weight associated with the high dose of STZ. Upon insulin treatment from subcutaneous osmotic pumps, glucose levels were restored to normal (data not shown), indicating the efficiency of insulin treatment and suitability of the model to investigate the role of insulin in the modulation of intestinal bile acid absorption associated with insulin deficiency.
Intestinal bile acid absorption is a major determinant of the enterohepatic circulation of bile acids (1, 24). The increase in bile acid absorption causes an enlargement in the circulating pool, whereas a reduction in the intestinal absorption leads to depletion of the bile acid pool (1, 20). Ileal ASBT mediates the first and the rate-limiting step of intestinal bile acid absorption (1, 24). Bile acids are then transported by the intracellular ileal bile acid-binding protein and exit the cell across the basolateral membrane via the action of a heterodimer of Ost α and β (1, 24). Studies in knockout mice provided compelling evidence demonstrating that ASBT represents the essential intestinal component of the enterohepatic circulation of bile acids (12). Furthermore, the fact that plasma cholesterol is reduced in ASBT knockout mice strongly indicates the role of ASBT in cholesterol homeostasis (12). Our findings showed an increase in ASBT and Ost α and β mRNA expression in rats with STZ-induced diabetes mellitus. It could be argued that the observed increase in intestinal bile acid transporters is a result of the associated hypertrophy and hyperplasia in the intestinal epithelia (15). While histological examination of the intestinal epithelia in our model revealed an increase in the length of the villi, there were no apparent changes in the size of epithelial cells excluding hypertrophy. Furthermore, the amount of DNA extracted per milligram protein harvested from the distal ileum of diabetic and control rats showed no significant changes (data not shown), ruling out a possible epithelial hypertrophy. Additionally, data obtained by laser capture microdissection from an equal number of ileal cells collected from diabetic and control rats show a significant increase in ASBT mRNA expression at the cellular level and therefore minimize a role of epithelial hyperplasia or hypertrophy in the observed changes.
Our data also show that ASBT protein expression was significantly increased in diabetes mellitus as judged by the following two complementary approaches: Western blotting and immunofluorescence. The specificity of rat ASBT antibodies (obtained from Santa Cruz) is confirmed by the fact that the expected size band is detected in ileum but not in jejunum and colon similar to ASBT pattern of expression. ASBT staining was predominantly present on the apical membranes of epithelial cells as indicated by the colocalization with villin (the marker for the apical membrane of epithelial cells). It should be noted that the level of villin expression observed by immunostaining was not altered in diabetic rats compared with normal rats, indicting that the observed changes are specific for ASBT. Along with the increase in ASBT mRNA and protein expression, our findings showed that ASBT activity was significantly increased in diabetes mellitus. ASBT function in the current studies was examined as Na+-dependent [3H]TC uptake in isolated intestinal epithelial cells. The increase in ASBT activity in isolated epithelial cells further rules out the involvement of hypertrophy or hyperplasia in the observed increase in ASBT function and expression in STZ-induced diabetes mellitus.
Insulin deficiency is the main characteristic of STZ-induced diabetes mellitus in rats (21). Interestingly, the increase in ASBT protein expression demonstrated both by Western blotting and immunofluoresce was decreased back to the normal level by insulin treatment. This observation suggested the direct involvement of insulin in the regulation of ASBT expression. In this regard, the role of insulin in the regulation of a number of intestinal epithelial processes is well established. For example, insulin has been shown to stimulate the function of oligopeptide transporter, Pept-1 (36), to decrease the transepithelial resistance (28), and to abrogate calcium-dependent chloride secretion in T84 intestinal epithelial cells (8). Additionally, previous studies have shown that insulin acutely decreased glucose flux across rat jejunum and ileum (27). Indeed, our data showed that insulin directly downregulated ASBT expression in an in vitro model using intestinal Caco-2 cells. Furthermore, our data demonstrate that insulin has a negative effect on rat ASBT promoter activity, indicating alterations at the level of gene transcription. In this regard, insulin is known to stimulate several downstream effector molecules in various cells types. For example, PKCζ isoform activation was shown to mediate the effects of insulin on the translocation of GLUT4 to the plasma membrane of rat adipocytes (35). Interestingly, the role of PKCζ in the transcriptional and posttranscriptional regulation of ASBT has been recently shown (16, 32). Therefore, future studies will focus on determining the role of PKCζ and other signaling molecules in the regulation of ASBT expression and promoter activity by insulin. Also, preliminary sequence analysis identified a potential binding site ∼1,577 bp upstream of the transcription initiation site for the CCAAT/enhancer binding protein that has been previously shown to mediate the effects of insulin on gene transcription (19). Future studies should focus on determining the role of this transcription factor in mediating the regulation of ASBT expression and promoter activity by insulin.
In summary, the current studies demonstrated that ASBT function and expression are increased in a STZ-induced rat model of diabetes mellitus. The increase in ASBT expression may contribute to the enlargement in the circulating pool of bile acids and high plasma level of cholesterol occurring in diabetes mellitus. Interestingly, recent clinical trials have shown the beneficial effects of bile acid sequestrants in improving lipid profile and hyperglycemia in patients with diabetes mellitus. The use of bile acid sequestrants to block bile acid absorption, however, is problematic because of the unpleasant nature of cholestyramine and the need of high dose (in grams) for effective treatment. The data described in the current studies provide a unique experimental tool to further investigate the impact of ASBT inhibition on the associated hypercholesterolemia.
GRANTS
These studies were supported by grants from the Department of Veteran Affairs (W. A. Alrefai and R. D. Kineman), National Institute of Diabetes and Digestive and Kidney Diseases Grants F32 DK-79542 (F. Annaba), DK-54165 (B. L. Shneider), DK-74459 (R. K. Gill), and DK-71596 (W. A. Alrefai), and the Crohn's and Colitis Foundation of America CCFA Ref. no. 1942 (S. Saksena).
REFERENCES
- 1. Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological omplications. Pharm Res 24: 1803–1823, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Bennion LJ, Grundy SM. Effects of diabetes mellitus on cholesterol metabolism in man. N Engl J Med 296: 1365–1371, 1977. [DOI] [PubMed] [Google Scholar]
- 3. Bhat BG, Rapp SR, Beaudry JA, Napawan N, Butteiger DN, Hall KA, Null CL, Luo Y, Keller BT. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE-/- mice by SC-435. J Lipid Res 44: 1614–1621, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 5. Brenna O, Qvigstad G, Brenna E, Waldum HL. Cytotoxicity of streptozotocin on neuroendocrine cells of the pancreas and the gut. Dig Dis Sci 48: 906–910, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Caspary WF. Effect of insulin and experimental diabetes mellitus on the digestive-absorptive function of the small intestine. Digestion 9: 248–263, 1973. [DOI] [PubMed] [Google Scholar]
- 7. Caspary WF. Increase of active transport of conjugated bile salts in streptozotocin-diabetic rat small intestine. Gut 14: 949–955, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang N, Uribe JM, Keely SJ, Calandrella S, Barrett KE. Insulin and IGF-I inhibit calcium-dependent chloride secretion by T84 human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 281: G129–G137, 2001. [DOI] [PubMed] [Google Scholar]
- 9. Chen F, Ma L, Al-Ansari N, Shneider B. The role of AP-1 in the transcriptional regulation of the rat apical sodium-dependent bile acid transporter. J Biol Chem 276: 38703–38714, 2001. [DOI] [PubMed] [Google Scholar]
- 10. Chen F, Ma L, Sartor RB, Li F, Xiong H, Sun AQ, Shneider B. Inflammatory-mediated repression of the rat ileal sodium-dependent bile acid transporter by c-fos nuclear translocation. Gastroenterology 123: 2005–2016, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Cohen DE. Balancing cholesterol synthesis and absorption in the gastrointestinal tract. J Clin Lipidol 2: S1–S3, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem 278: 33920–33927, 2003. [DOI] [PubMed] [Google Scholar]
- 13. El-Batran SA, Abdel-Salam OM, Nofal SM, Baiuomy AR. Effect of rosiglitazone and nateglinide on serum glucose and lipid profile alone or in combination with the biguanide metformin in diabetic rats. Pharmacol Res 53: 69–74, 2006. [DOI] [PubMed] [Google Scholar]
- 14. Eloranta JJ, Jung D, Kullak-Ublick GA. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol Endocrinol 20: 65–79, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Fedorak RN, Chang EB, Madara JL, Field M. Intestinal adaptation to diabetes. Altered Na-dependent nutrient absorption in streptozocin-treated chronically diabetic rats. J Clin Invest 79: 1571–1578, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frankenberg T, Miloh T, Chen FY, Ananthanarayanan M, Sun AQ, Balasubramaniyan N, Arias I, Setchell KD, Suchy FJ, Shneider BL. The membrane protein ATPase class I type 8B member 1 signals through protein kinase C zeta to activate the farnesoid X receptor. Hepatology 48: 1896–1905, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garber AJ. Attenuating CV risk factors in patients with diabetes: clinical evidence to clinical practice. Diabetes Obes Metab 4, Suppl 1: S5–S12, 2002. [DOI] [PubMed] [Google Scholar]
- 18. Geethan PK, Prince PS. Antihyperlipidemic effect of d-pinitol on streptozotocin-induced diabetic Wistar rats. J Biochem Mol Toxicol 22: 220–224, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Ghosh AK, Lacson R, Liu P, Cichy SB, Danilkovich A, Guo S, Unterman TG. A nucleoprotein complex containing CCAAT/enhancer-binding protein beta interacts with an insulin response sequence in the insulin-like growth factor-binding protein-1 gene and contributes to insulin-regulated gene expression. J Biol Chem 276: 8507–8515, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Hofmann AF. Bile acids: the good, the bad, and the ugly. News Physiol Sci 14: 24–29, 1999. [DOI] [PubMed] [Google Scholar]
- 21. Kim E, Sohn S, Lee M, Jung J, Kineman RD, Park S. Differential responses of the growth hormone axis in two rat models of streptozotocin-induced insulinopenic diabetes. J Endocrinol 188: 263–270, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Kitayama K, Nakai D, Kono K, van der Hoop AG, Kurata H, deWit EC, Cohen LH, Inaba T, Kohama T. Novel non-systemic inhibitor of ileal apical Na+-dependent bile acid transporter reduces serum cholesterol levels in hamsters and monkeys. Eur J Pharmacol 539: 89–98, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Konrad RJ, Mikolaenko I, Tolar JF, Liu K, Kudlow JE. The potential mechanism of the diabetogenic action of streptozotocin: inhibition of pancreatic beta-cell O-GlcNAc-selective N-acetyl-beta-d-glucosaminidase. Biochem J 356: 31–41, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 126: 322–342, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Lammert F, Wang DQ. New insights into the genetic regulation of intestinal cholesterol absorption. Gastroenterology 129: 718–734, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89: 147–191, 2009. [DOI] [PubMed] [Google Scholar]
- 27. Madsen KL, Ariano D, Fedorak RN. Insulin downregulates diabetic-enhanced intestinal glucose transport rapidly in ileum and slowly in jejunum. Can J Physiol Pharmacol 74: 1294–1301, 1996. [DOI] [PubMed] [Google Scholar]
- 28. McRoberts JA, Riley NE. Role of insulin and insulin-like growth factor receptors in regulation of T84 cell monolayer permeability. Am J Physiol Gastrointest Liver Physiol 267: G883–G891, 1994. [DOI] [PubMed] [Google Scholar]
- 29. Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 282: 19493–19501, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Neimark E, Chen F, Li X, Magid MS, Alasio TM, Frankenberg T, Sinha J, Dawson PA, Shneider BL. c-Fos is a critical mediator of inflammatory-mediated repression of the apical sodium-dependent bile acid transporter. Gastroenterology 131: 554–567, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72: 137–174, 2003. [DOI] [PubMed] [Google Scholar]
- 32. Sarwar Z, Annaba F, Dwivedi A, Saksena S, Gill RK, Alrefai WA. Modulation of ileal apical Na+-dependent bile acid transporter ASBT by protein kinase C. Am J Physiol Gastrointest Liver Physiol 297: G532–G538, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sonnett TE, Levien TL, Neumiller JJ, Gates BJ, Setter SM. Colesevelam hydrochloride for the treatment of type 2 diabetes mellitus. Clin Ther 31: 245–259, 2009. [DOI] [PubMed] [Google Scholar]
- 34. Staels B, Kuipers F. Bile acid sequestrants and the treatment of type 2 diabetes mellitus. Drugs 67: 1383–1392, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Standaert ML, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, Sajan MP, Cenni V, Sirri A, Moscat J, Toker A, Farese RV. Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J Biol Chem 274: 25308–25316, 1999. [DOI] [PubMed] [Google Scholar]
- 36. Thamotharan M, Bawani SZ, Zhou X, Adibi SA. Hormonal regulation of oligopeptide transporter pept-1 in a human intestinal cell line. Am J Physiol Cell Physiol 276: C821–C826, 1999. [DOI] [PubMed] [Google Scholar]
- 37. van Waarde WM, Verkade HJ, Wolters H, Havinga R, Baller J, Bloks V, Muller M, Sauer PJ, Kuipers F. Differential effects of streptozotocin-induced diabetes on expression of hepatic ABC-transporters in rats. Gastroenterology 122: 1842–1852, 2002. [DOI] [PubMed] [Google Scholar]