Abstract
Obesity and type 2 diabetes are associated with insulin resistance (IR), increased circulating proinflammatory cytokines, and hypertriglyceridemia, the latter being caused by overproduction of hepatic very low density lipoprotein (VLDL). One cytokine strongly linked with development of hepatic IR is interleukin-6 (IL-6). Our objective was to evaluate IL-6 effects on hepatic apolipoprotein B (apoB) and VLDL secretion and to examine possible linkages between cytokine signaling and insulin-suppressive effects on lipoprotein secretion. Of the cytokines examined, only IL-6 stimulated secretion of apoB-containing lipoproteins in a dose-dependent manner. Both B100 and B48 secretion were significantly increased in VLDL and in lipoproteins with a density >1.019 g/ml. The ability of insulin to suppress hepatic apoB secretion was maintained in hepatocytes treated with IL-6. Pulse-chase studies indicated that enhanced apoB synthesis was the primary mechanism for increased lipoprotein secretion, which corresponded with higher abundance of apoB mRNA. Because IL-6 did not alter the decay rate of apoB mRNA transcripts, results support that increased apoB mRNA levels are the result of enhanced apob gene transcription. Increased apoB-lipoprotein secretion was also detected with oncostatin M (OSM), supporting involvement of the signal-transducing protein, gp130. Increased suppressor of cytokine signaling (SOCS) 3 expression negated IL-6 and OSM effects and significantly reduced cellular apoB mRNA abundance. We conclude that IL-6 favors secretion of apoB-containing lipoproteins by increasing availability of apoB through changes in apob gene transcription. These changes may contribute to hypersecretion of VLDL associated with obesity, particularly under conditions where SOCS3 is not overexpressed to an extent capable of overcoming IL-6-stimulated apob gene transcription.
Keywords: interleukin-6, suppressor of cytokine signaling 3 and hepatocyte lipoprotein secretion, very low density lipoprotein
metabolic syndrome is a clustering of conditions that include abdominal obesity, hypertension, hypertriglyceridemia, and insulin resistance (14) and is associated with increased risk of cardiovascular disease and type 2 diabetes (2). Hypertriglyceridemia results from hepatic hypersecretion of very low density lipoprotein (VLDL) and apolipoprotein B (apoB), an integral protein component of VLDL (1). Numerous studies have linked hepatic insulin resistance with overproduction of apoB-containing lipoproteins (11), and, recently, it has been shown that hepatic insulin resistance is sufficient to produce hypersecretion of VLDL and subsequent dyslipidemia (7). Hypersecretion of VLDL and apoB by the liver can be the result of increased expression of any of the three rate-limiting components required for VLDL assembly, including triglyceride (TG), microsomal triglyceride transfer protein (MTP), and apoB (44).
Insulin regulates the production of VLDL by liver by limiting apoB availability through presecretory intracellular degradation (48) which leads to temporary TG storage and balances intestinal and hepatic lipoprotein metabolism during the transition from fasting to fed states (44, 49, 50). Insulin-suppressive effects on apoB are mediated by the insulin receptor and depend upon activation of phosphatidylinositol 3-kinase via insulin receptor substrates (34). With the development of hepatic insulin resistance, the suppressive effect of insulin on apoB secretion is lost (12, 13), and the incremental increase in available apoB may be one possible mechanism responsible for hepatic hypersecretion of apoB under these conditions. Hepatic VLDL production can be further augmented by increased fatty acid delivery to the liver released from adipose tissue lipolysis (56) along with induction and enhanced expression and activity of MTP (26).
Both obesity and metabolic syndrome are strongly associated with chronic inflammation (20, 40). As individuals become obese, there is an increase in proinflammatory cytokines secreted by adipose tissue (31). The liver also reflects inflammatory gene changes with increasing adiposity (9). The relationship of obesity and adipocyte cytokine release with hypertriglyceridemia suggests the possibility that hypersecretion of hepatic VLDL may be mechanistically linked to proinflammatory cytokine signaling. The current study evaluated the specific stimulatory effect of interleukin 6 (IL-6) on apoB-containing lipoprotein assembly and secretion by rat hepatocytes (RH). Interestingly, the stimulatory effect of IL-6 was not through derepression of insulin inhibition, but rather through IL-6-dependent increases in hepatic apoB mRNA. The induction of cellular apoB mRNA was counterbalanced by expression of suppressor of cytokine signaling 3 (SOCS3), which downregulated cellular apoB mRNA transcripts. Overall, studies expand our knowledge of the balance of regulatory pathways for modulating hepatic TG by induction of secretion of apoB-containing TG-rich lipoproteins.
METHODS
Animals and reagents.
Sprague-Dawley rats (180–250 g) were from Harlan Teklad (Indianapolis, IN). Purified bovine serum albumin (BSA) was obtained from Serologicals Proteins (Bayer, Kankakee, IL). Waymouth's MB 752/1 medium, penicillin, streptomycin, Igepal CA-630, and 5,6-dichlorobenzimidazole were obtained from Sigma Chemical (St. Louis, MO). PD-98059 was from Tocris Bioscience (Ellisville, MO). Recombinant mouse tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β1 were from EMD Chemicals (San Diego, CA). Recombinant mouse IL-6, rat IL-1β, and oncostatin M (OSM) were obtained from Invitrogen (Carlsbad, CA). Recombinant leptin was obtained from R & D Systems (Minneapolis, MN). Percoll was obtained from Pharmacia Fine Chemicals (Piscataway, NJ). Goat polyclonal (IgG) anti-rat fibrinogen was from Immunology Consultants Laboratory (Newberg, OR). Adenoviral vectors (Ad Type5 dE1/E3) containing human (h) SOCS3, and Ad-CMV-GFP were purchased from Vector Biolabs (Philadelphia, PA). 14C molecular weight marker proteins were purchased from GE Healthcare (Buckinghamshire, UK).
Cell culture.
RH were prepared by collagenase perfusion of livers derived from fed rats between 0800 and 1000 as previously described (48). Viable cells were isolated by Percoll gradient centrifugation (47). After removal of Percoll by pelleting and resuspension of cells, RH were seeded on dishes previously coated with rat tail collagen (1 × 106 cells/ml, 2 ml/60-mm dish) and incubated in an humidified atmosphere of 95% air-5% CO2 (vol/vol) at 37°C for 2–4 h. Nonadherent cells were removed by rinsing monolayers three times in 0.2% (wt/vol) BSA/HBSS, and cells were reincubated for various time periods in Waymouth's medium containing 0.2% (wt/vol) BSA and insulin (0.1 nM), hereafter referred to basal medium, and various cytokines or inhibitors. For adenoviral infection, adenovirus (Ad) stocks were first diluted to a concentration of 1 × 109 multipliticies of infection (MOI)/ml in 0.2% BSA/HBSS, and aliquots were stored frozen at −80°C. After RH were seeded, cells were infected with Ad at a concentration of 5 MOI unless indicated otherwise followed by overnight incubation. Toxicity was monitored by lactate dehydrogenase (LDH) release in medium, and, at 5 MOI, no significant LDH release was observed. ApoB secretion rates were unaffected following infection of RH with Ad-CMV-GFP control vector compared with uninfected RH.
Lipoprotein apoB assay.
Concentrations of cellular apoB from detergent-solubilized hepatocytes, medium apoB, and apoB of ultracentrifuged lipoproproteins were measured by competitive RIA employing a mouse monoclonal antibody prepared against rat apoB (45). ApoB standard curves were prepared using rat VLDL diluted in culture medium or cell lysis buffer. ApoB concentrations were normalized to cell protein per dish as assayed using either Bradford protein assay (8) or bicinchoninic acid protein assay (Pierce, Rockford, IL). For the determination of apoB in VLDL fractions, media from five 60-mm plates were pooled (10 ml); salt density was adjusted to 1.019 g/ml by addition of a solution of sodium bromide (density = 1.495 g/ml) containing 5 mM EDTA, and divided into two ultracentrifuge tubes. Ultracentrifugation was carried out at 50,000 rpm × 18 h at 14°C in a 70.1 Ti rotor (230,000 g) in a L-70 Optima Ultracentrifuge (Beckman Coulter, Fullerton, CA). The VLDL fraction was isolated by tube slicing, and infranatant proteins were resuspended. ApoB concentrations of VLDL (density <1.019 g/ml) and infranatant fractions (density >1.019 g/ml) were determined by RIA and normalized to cell protein.
Lipoprotein apolipoprotein secretion.
The influence of IL-6 on secretion of B100 and B48 as well as other apolipoproteins in various lipoprotein density fractions was determined using l-[14C]leucine steady-state labeling conditions as previously described (13). Briefly, hepatocytes were incubated with and without 0.5 nM IL-6 (5–60 mm dishes/condition) for 16 h in leucine-free, Waymouth's basal medium containing 15 μM added l-leucine and 3.3 μCi/ml l-[14C]leucine (sp. act. ∼315 mCi/mmol, CFB 67; Amersham Biosciences, Piscataway, NJ). Media from each condition (5 dishes, 10 ml) were pooled, and 2 ml of freshly prepared rat serum were added to act as carrier. The solution was then divided into duplicate ultracentrifuge tubes (6 ml each), and VLDL (density <1.019 g/ml), low density lipoprotein (LDL) (1.019 g/ml < density <1.063 g/ml), and high density lipoprotein (HDL) (1.063 g/ml < density <1.225 g/ml) fractions were isolated by flotation by sequential density ultracentrifugation at 14°C at 50,000 rpm for 18, 22, and 40 h, respectively. Density adjustments were made by the addition of a solution of NaBr (density = 1.495 g/ml) containing 5 mM l-leucine, 1% (vol/vol) aprotinin, and 5 mM EDTA. After isolation, lipoprotein fractions were dialyzed against PBS containing 5 mM EDTA using 3500 MWCO membranes (Spectra/Por; Spectrum Labs, Rancho Dominguez, CA). After dialysis, apolipoproteins were concentrated by precipitation during the process of delipidation in chloroform-methanol-diethyl ether (5:5:10, vol/vol/vol). Delipidated apolipoprotein pellets were air-dried and then dissolved in 2× Laemmli's buffer (29) containing freshly added 10–25 mM dithiothreitol. 14C-labeled apolipoproteins were separated by SDS-PAGE on 3.5% (wt/vol) to 26% (wt/vol) gradient acrylamide/Acrylaide gels cast on GelBond PAG film (FMC BioProducts, Rockland, ME). After electrophoresis, gels were briefly rinsed in distilled water, heat-fixed in a convection oven, and 14C-labeled apolipoproteins were visualized by PhosphorImager analysis. Specific apolipoproteins were identified by characteristic molecular weights through comparison with coelectrophoresed 14C molecular weight marker proteins.
RNA isolation and analysis.
Total RNA was isolated from four to five (60-mm) dishes of RH using 3 ml TRIzol reagent/dish according to the manufacturer's recommendation (Invitrogen). Purified RNA was dissolved in 200 μl of FORMAzol (Molecular Research Center, Cincinnati, OH) for long-term storage at −80°C. An aliquot of each RNA sample was removed to measure ratios of absorbance at 260 and 280 nm, which were consistently >1.9. For Northern blotting, liver RNA was separated on 0.8 or 1.2% (wt/vol) agarose gels containing formaldehyde. Ratios of 28S to 18S RNA ranged from 1.8 to 2.0 as analyzed on ethidium bromide-stained gels. Separated RNAs were transferred to Nytran Super Charge nylon membranes (Schleicher and Schuell Bioscience, Keene, NH) using a turboblotter (Schleicher and Schuell), and RNAs were immobilized by ultraviolet cross-linking. Membranes were prehybridized in ExpressHyb (BD Biosciences Clontech, Palo Alto, CA) and then hybridized with [32P]cDNA probes (35). cDNAs were labeled using Ready-To-Go DNA-labeling beads (minus dCTP) (Amersham Biosciences) and [α-32P]dCTP (3,000 Ci/mmol; PerkinElmer Life and Analytical Sciences, Boston, MA). The apoB probe used for Northern blotting was a 5′ probe kindly provided by Dr. Alana Mitchell (University of Melbourne, Australia). Probes for cyclophilin and 18S RNA were obtained from Ambion (Austin, TX). After hybridization with labeled cDNAs, membranes were washed, and binding was evaluated by PhosphorImager analysis (Molecular Dynamics, Sunnyvale, CA) using ImageQuant software. To control for gel loading, membranes were stripped by incubation in 0.5% (wt/vol) SDS for 10 min at 100°C and rehybridized with labeled cyclophilin or 18S cDNA probes as indicated. Gene expression changes were also determined by quantitative real-time PCR (Q-RT-PCR). First-strand cDNA synthesis was performed by reverse transcription using total RNA recovered following ethanol precipitation of RNA stored in Formazol. Real-time PCR (RT-PCR) was performed on the PRISM 7500 sequence detection system (Applied Biosystems, Foster City, CA). Assays employed were as follows: rat apoB (Rn01499050_g1), β-actin (Rn4352340E), and hSOCS3 (Hs00165177_m1). RT-PCR results are reported as relative quantitation where the PCR signal of the target transcript in a treatment group was normalized as a ratio to the untreated control representing fold differences in mRNA abundance.
Pulse-chase experiments.
Hepatocytes were incubated in basal medium with and without added 0.5 nM IL-6 for 12 h. Afterward, cells were rinsed three times in l-methionine-free and l-cysteine-free Waymouth's medium (depletion medium) and reincubated in depletion medium for 45 min containing appropriate additions. To each dish was then added 160 μCi EXPRE35S35S-Protein Labeling Mix (1,175 Ci/mmol, NEG-072; PerkinElmer), and incubation was continued for exactly 30 min. Label incorporation into peptides was terminated by rapid addition of an equivalent volume of cold (4°C) quench medium containing 20 mM l-methionine and 5 mM l-cysteine with rapid mixing. Quenched media were rapidly removed by aspiration, and cells were then washed three times with chase medium containing 10 mM l-methionine and 2.5 mM l-cysteine and reincubated in chase medium with and without IL-6. Cells were terminated after 25, 60, 90, and 120 min of chase by collecting medium, washing plates three times in ice-cold HBSS, and freezing the cells in liquid nitrogen. Frozen cells were stored at −80°C until further analysis. Immunoprecipitation and SDS-PAGE analysis of cellular and media 35S-labeled B100 and B48 were carried out as previously described (13) using rabbit anti-rat apoB antibody prepared in our laboratory. Labeled apoB was separated by SDS-PAGE on 4% (wt/vol) acrylamide-Acrylaide minigels cast on GelBond PAG film (FMC BioProducts). Following electrophoresis, gels were rinsed in distilled water and heat-fixed, and label incorporated into B100 and B48 were determined by PhosphorImager analysis of dried gels.
Statistics.
Values are presented as means ± SD for independent results from n liver perfusions. For experiments where multiple independent liver perfusions (n) were performed and multiple dishes (3–5) were evaluated for each test condition, results are expressed as average of averages ± SE. Statistical differences between means were evaluated by Student's t-test or ANOVA. P values <0.05 were considered significant.
RESULTS
IL-6 specifically stimulates apoB secretion.
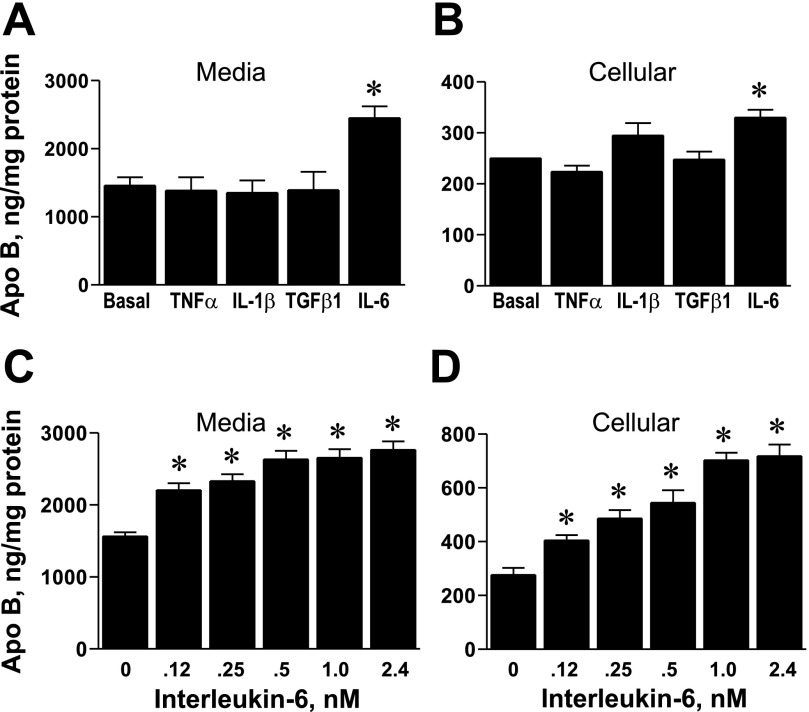
Effects of various cytokines on lipoprotein apoB secretion and cellular apoB levels were examined using primary RH (Fig. 1). Concentrations of cytokines were used at levels known to elicit appropriate downstream effects on target proteins (37, 39, 42, 52). Results indicate that, in our culture system, there is a specific stimulatory effect of IL-6 on apoB secretion (Fig. 1A) and cellular apoB (Fig. 1B) compared with other cytokines. The effect of IL-6 was dose-dependent in stimulating the accumulation of apoB-containing lipoproteins in medium (Fig. 1C) and increasing cellular apoB content (Fig. 1D).
Fig. 1.
Effects of various cytokines, including interleukin (IL)-6 on apolipoprotein B (apoB) in primary rat hepatocytes (RH). A: RH were incubated overnight (16 h) in Waymouth's medium containing 2 nM tumor necrosis factor (TNF)-α (n = 3), 2 nM IL-1β (n = 6), 1 nM transforming growth factor (TGF)-β1 (n = 3), or 0.5 nM IL-6 (n = 6). Afterward, apoB accumulation in the medium was determined by RIA. Results are the average apoB concentration (ng/mg cell protein) ± SE for 3–5 replicate dishes/condition from n independent rat liver experiments. *P < 0.001 vs. basal medium. B: corresponding changes in cellular apoB levels as determined by RIA. C and D: dose-dependent effects of IL-6 on accumulation of apoB in medium and cellular apoB content, respectively. Results are the average apoB concentration of 5 plates/condition ± SD from one study. *Significant difference from basal conditions at a probability level of at least P < 0.05.
IL-6 stimulates the secretion of apoB-containing lipoproteins.
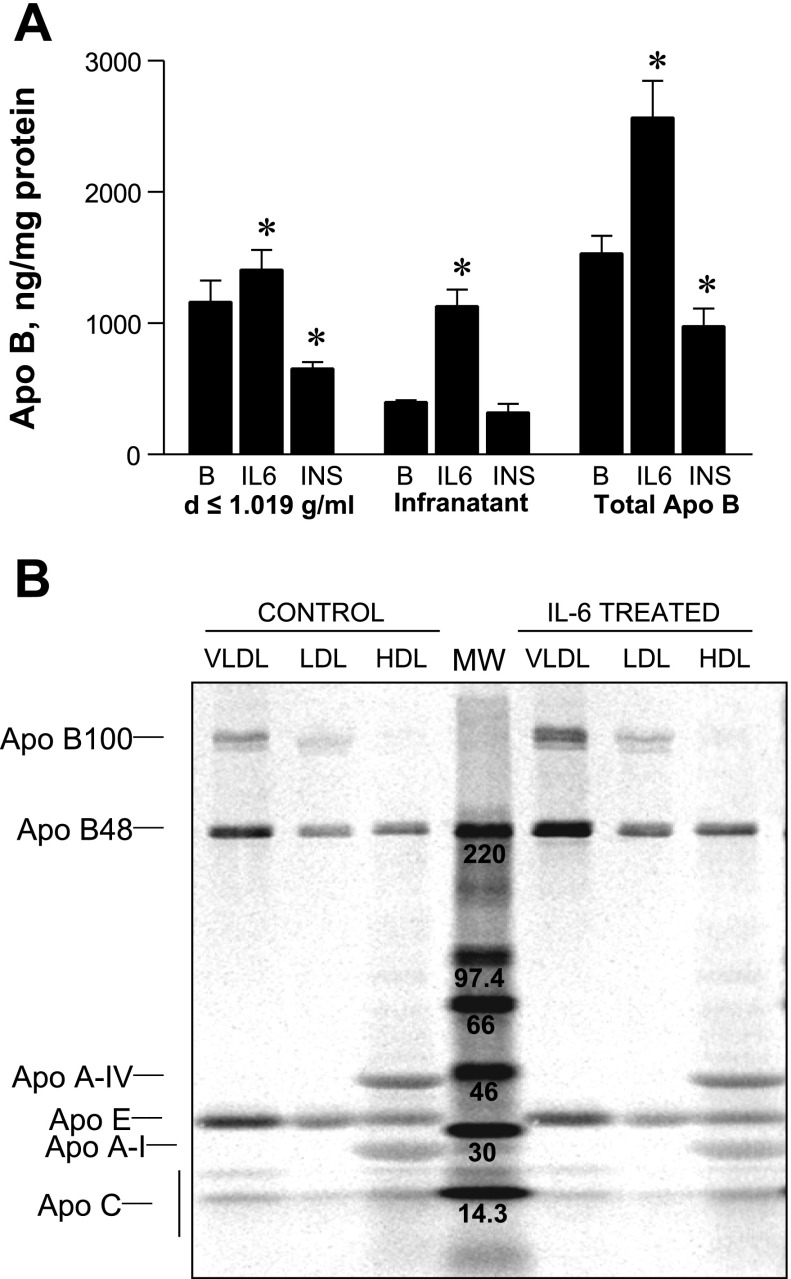
RH secrete apoB as a component of VLDL as well as denser lipoproteins. To determine which lipoproteins are most affected by IL-6, media was fractionated by ultracentrifugation into VLDL (density <1.019 g/ml) and infranatant lipoprotein fractions (density >1.019 g/ml), and apoB content of each fraction was measured by RIA (Fig. 2A). Compared with basal conditions, IL-6 significantly increased the secretion of VLDL-apoB on average by 35%. This contrasts with the known suppressive effect of insulin on VLDL-apoB, which was reduced on average by 40%. IL-6 treatment markedly increased apoB-containing lipoprotein secreted by RH with densities >1.019 g/ml by an average of 150% compared with basal conditions. RH secrete both B100- and B48-containing lipoproteins (43), and, to determine whether IL-6 treatment favored the secretion of one over the other apoB variant, RH were incubated with [14C]leucine in the presence and absence of IL-6 to label secretory proteins. Media lipoproteins were then fractionated into VLDL, LDL, and HDL by sequential density ultracentrifugation. Following delipidation, 14C-labeled apolipoproteins of each density class were separated by SDS-PAGE and identified by their known molecular weights (Fig. 2B). IL-6 stimulated the secretion of both 14C-labeled B100 and B48 in VLDL and LDL and B48 in HDL lipoproteins. Compared with apoB, secreted 14C-labeled apoA-IV, apoE, and apoA-I were not stimulated by IL-6 treatment.
Fig. 2.
Effect of IL-6 on secretion of apoB in various lipoprotein density fractions. A: effect of IL-6 (0.5 nM) or insulin (100 nM) on the secretion of very low density lipoprotein (VLDL) apoB (density <1.019 g/ml) and infranatant apoB-containing lipoproteins (density >1.019 g/ml). VLDL was isolated by ultracentrifugation of pooled media, and VLDL and infranatant fractions were assayed for apoB content by RIA. Results are averages ± SD of 4 pools derived from 5 plates each from 2 independent rat liver experiments. *Means differ from basal conditions (B) compared with IL-6 or insulin (INS) conditions at a probability level of at least P < 0.05. B: effect of IL-6 on secretory 14C-labeled apolipoproteins derived from isolated VLDL (density <1.019 g/ml), low density lipoprotein (LDL) (1.019 g/ml < density <1.063 g/ml), and high density lipoprotein (HDL) (1.063 g/ml < density <1.225 g/ml) following separation by SDS-PAGE and visualization using the phosphorimager. 14C molecular weight (MW) markers (center lane) are shown with corresponding molecular masses (kDa).
Effect of IL-6 on insulin-mediated suppression of apoB secretion.
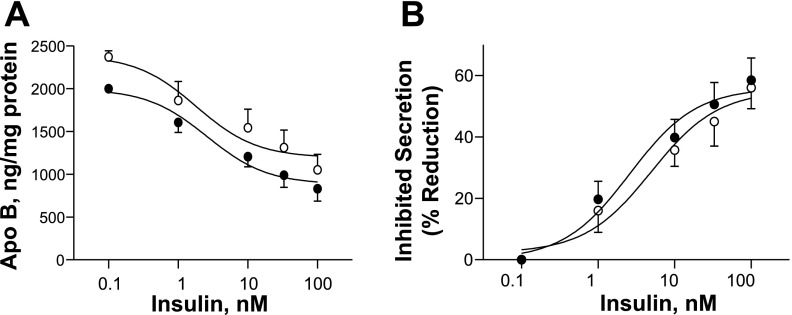
IL-6 has been shown to induce insulin resistance in HepG2 cells and primary rodent hepatocytes (39). A possible explanation for the observed increase in lipoprotein apoB secretion with IL-6 treatment is loss of insulin-mediated suppression. To address this possibility, insulin dose-response curves were prepared in RH treated with and without IL-6, and lipoprotein apoB secretion of the treatment groups were compared (Fig. 3A). Insulin reduced apoB secretion in a dose-dependent manner both in the presence and in the absence of IL-6. Insulin (100 nM) maximally suppressed apoB secretion by an average of 58.8 ± 7.2% in the absence of IL-6 and by 56 ± 6.8% in the presence of IL-6 (mean ± SE, n = 5). Dose-response curves based on percent apoB inhibited by insulin with and without IL-6 were similar with a detectable shift-to-the-right with IL-6 treatment (Fig. 3B), suggesting a small decrease in insulin sensitivity. Overall, these results support that the increase in lipoprotein apoB secretion induced by IL-6 is not due to the loss of insulin-mediated lipoprotein suppression, since insulin maintains its inhibitory effect on apoB secretion even in the presence of IL-6.
Fig. 3.
Effect of IL-6 on insulin-dependent suppression of apoB secretion. RH were incubated overnight (16 h) in medium with IL-6 (0.5 nM, ○) and without IL-6 (●) with various concentrations of insulin (0.1, 1, 10, 33, and 100 nM). Afterward, apoB accumulation in the medium was measured by RIA. A: average medium apoB (ng/mg cell protein) ± SE from 5 independent rat liver experiments (3–5 plates/condition) is plotted against insulin dose. B: average apoB secretion inhibited by insulin (%reduction) is plotted against insulin dose in the presence (○) and in the absence (●) of IL-6 in studies carried out as described in A.
IL-6 increases B100 and B48 synthesis.
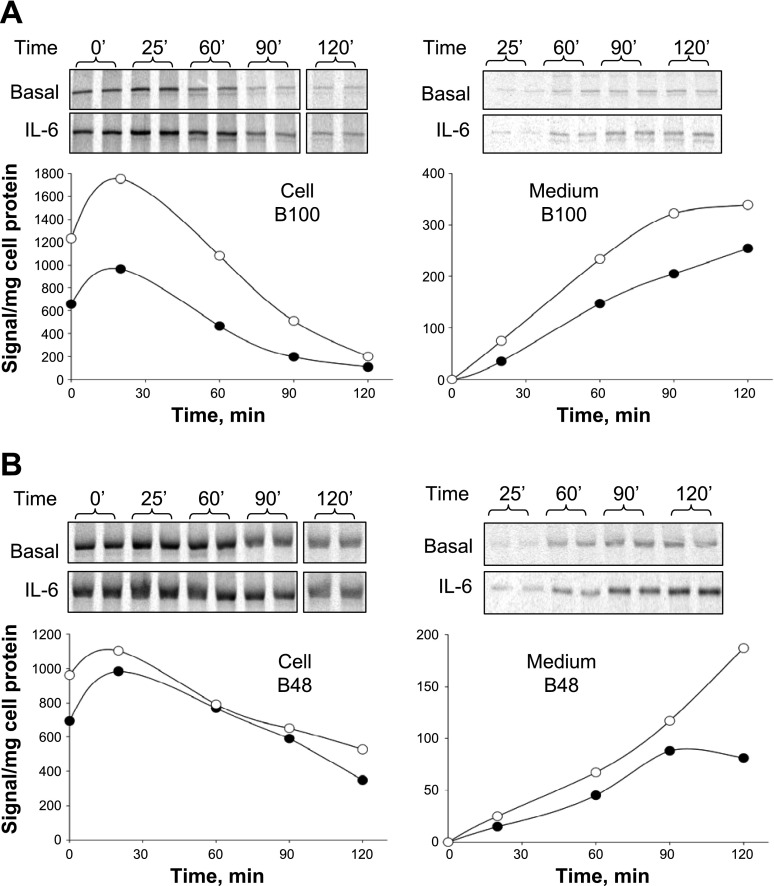
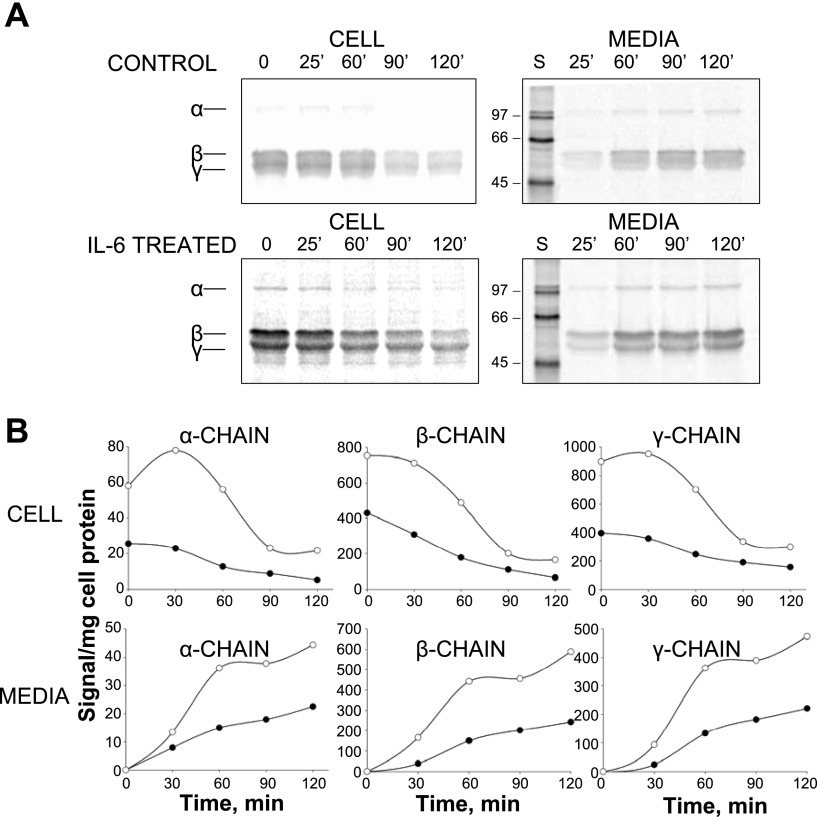
In hepatocytes, apoB available for VLDL assembly is mainly regulated by posttranslational apoB degradation (19). To determine whether IL-6 treatment diminished apoB degradation as the mechanism responsible for the observed increase in lipoprotein apoB secretion, pulse-chase studies were performed (Fig. 4, A and B). The most significant effect of IL-6 was on the amount of label incorporated during the pulse period (synthesis) seen as 35S-labeled apoB at the initiation of the chase period (time 0) and after 25 min of chase. Cellular B100 synthesis was increased 125% by IL-6 (Fig. 4A), whereas B48 synthesis was increased by 38% (Fig. 4B). Newly synthesized B100 recovered after 120 min of chase was similar in control and IL-6-treated RH, averaging 36.6 and 31.1% of that synthesized during the pulse, respectively. Somewhat more B48 was recovered in IL-6-treated RH than in control, averaging 61.6 vs. 43.0%. We also examined the effect of IL-6 on the synthesis and secretion of fibrinogen, a known IL-6 gene target (Fig. 5, A and B). RH treated with IL-6 demonstrated increased levels of fibrinogen subunits synthesized and secreted in the medium compared with untreated RH, consistent with known effects of IL-6 in the acute phase response (APR).
Fig. 4.
Effect of IL-6 on cellular transit and secretion of pulse-labeled apoB. RH were incubated in basal medium or in medium containing 0.5 nM IL-6 for 12 h before performing pulse-chase experiments. RH were labeled with 35S label for 30 min followed by incubation in chase medium for 0, 25, 60, 90, and 120 min. Cellular and medium 35S-labeled apoB at each time point were isolated by immunoprecipitation, and 35S-labeled B100 and B48 were separated by SDS-PAGE and quantified by phosphorimager analysis. After normalization for cell protein, cellular (left) and medium (right) 35S-labeled B100 (A) and B48 (B) were plotted against the time of chase. Results are a representative study performed in 2 independent rat liver experiments showing essentially identical results.
Fig. 5.
Effect of IL-6 on cellular transit and secretion of pulse-labeled fibrinogen subunits. RH were incubated in basal medium or in medium containing 0.5 nM IL-6 for 12 h before performing pulse-chase analysis as described in Fig. 4. A: cellular (left) and secreted (right) fibrinogen subunits were isolated by immunoprecipitation; subunits were separated by SDS-PAGE and quantified by phosphorimager analysis. B: after normalization to cell protein, cell and medium 35S-fibrinogen subunits were plotted against time of chase. Results are from a control study.
IL-6 treatment increases cellular apoB mRNA levels.
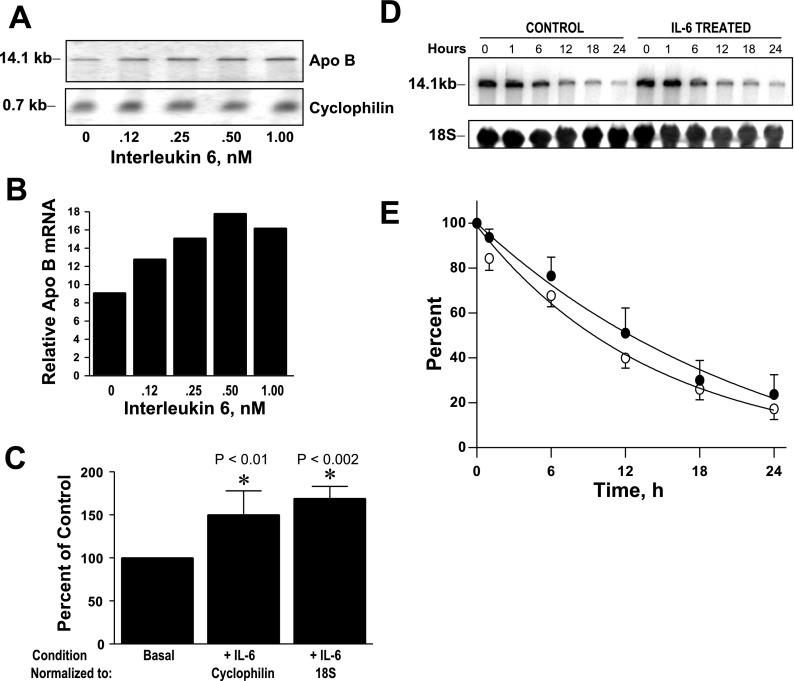
To address whether the mechanism responsible for the observed increase in apoB synthesis with IL-6 in pulse-chase studies was related to changes in apoB mRNA abundance, we measured apoB mRNA levels by Northern blotting (Fig. 6). RH were incubated for 16 h with increasing concentrations of IL-6 (0.12–1 nM); total RNA was isolated, and Northern blotting was performed (Fig. 6, A and B). Cellular apoB mRNA levels showed a dose-dependent increase with increasing IL-6 concentration. With 0.5 nM IL-6, the average increase in abundance was 50 ± 27.9 and 69 ± 14% (mean ± SD) over the no IL-6 condition using cyclophilin (n = 6) and 18S (n = 4), respectively, to normalize blots (Fig. 6C). To determine whether the increase in apoB mRNA abundance was due to stabilization of mRNA transcripts, we also determined cellular apoB mRNA half-lives following IL-6 treatment (Fig. 6, D and E). The cellular half-life of apoB mRNA was relatively long, averaging 10 h in RH treated with IL-6 compared with 16 h under basal conditions (Fig. 6E), the latter being consistent with previous reports obtained in HepG2 cells (36). These results support the conclusion that the increase in apoB synthesis, cellular apoB, and lipoprotein apoB secretion with IL-6 treatment is due to IL-6-dependent increases in apob gene transcription rather than stabilization of apoB mRNA transcripts.
Fig. 6.
Effects of IL-6 on apoB mRNA and apoB mRNA decay by Northern blotting. A: RH were incubated with increasing concentrations of IL-6 (0–1 nM) for 16 h, and RNA was isolated and analyzed by Northern blotting. B: apoB mRNA abundance relative to cyclophilin mRNA was quantified by phosphoimager analysis. This is a representative experiment that was repeated with essentially identical results. C: average percentage change ± SD in apoB mRNA abundance with IL-6 treatment normalized to cyclophilin in 6 independent rat liver experiments and normalized to 18S rRNA in 4 independent rat liver experiments. *The mean percent of IL-6 mRNA relative to normalization control is significantly greater than control after normalization. D: to assess cellular stability of apoB mRNA, RH were incubated with and without IL-6 (0.5 nM) followed by addition of 5,6-dichlorobenzimidazole (DRB) to arrest mRNA synthesis. Cellular RNA (5 plates/time point) was isolated before addition of DRB (0 h) and various times thereafter (1, 6, 12, 18, and 24 h). Cellular apoB mRNA levels were determined by Northern blotting and normalized using 18S rRNA hybridization signal in 3 independent rat liver experiments. E: average percent of cellular apoB mRNA remaining ± SD (n = 3) at each time point with IL-6 treatment (○) and without IL-6 treatment (●) was plotted against time after addition of DRB.
MEK inhibition does not prevent IL-6-mediated changes in apoB mRNA.
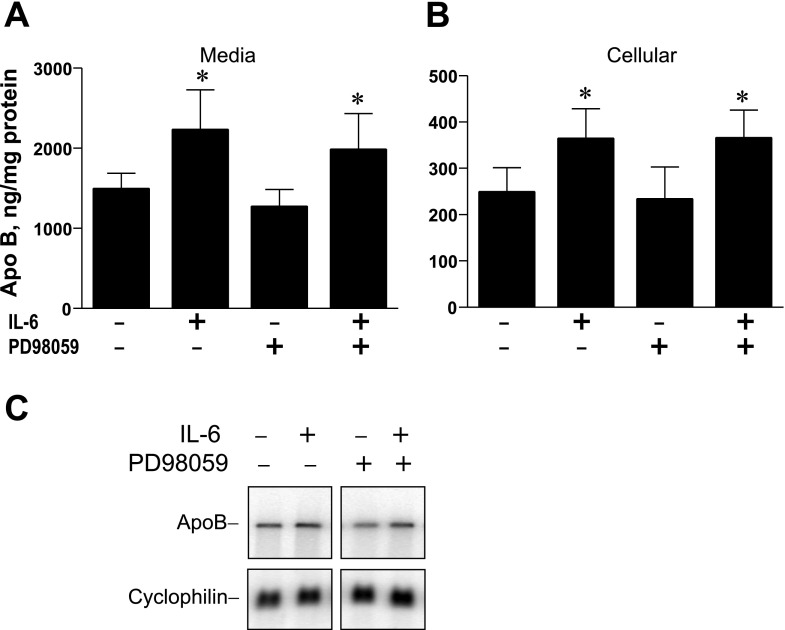
IL-6 signaling has been shown to activate the mitogen-activated protein kinase (MAPK) pathway in addition to the JAK/STAT pathway (21). To determine the role of MAPK activation, RH were preincubated for 2 h with the MEK inhibitor PD-98059 followed by addition of IL-6, and media apoB secretion and cellular apoB levels were assayed (Fig. 7). In two independent studies, IL-6 increased apoB secretion by an average of 56% in RH pretreated with PD-98059 and by 49% in RH treated with vehicle alone (Fig. 7A). Cellular apoB levels were increased by 46% with IL-6 incubations and were also increased in RH pretreated with PD-98059, with increases averaging 58% (Fig. 7B). Northern blotting analysis indicated that increased levels of apoB mRNA stimulated by IL-6 were maintained in RH pretreated with the MEK inhibitor followed by IL-6 (Fig. 7C). These results suggest that the effect of IL-6 on induction of apoB mRNA does not appear to involve activation of the MEK-MAPK pathway.
Fig. 7.
Effect of preincubation of RH with the MEK inhibitor PD-98059 on apoB protein expression and mRNA levels. RH were preincubated with 100 μM PD-98059 or vehicle (DMSO) and then IL-6 was added (0.5 nM) to half of the dishes, and incubations were continued for 16 h. Afterward, apoB contents of media (A) and in cells (B) were determined by RIA and normalized to plate protein. Results are averages ± SD from 8 individual plates of hepatocytes from 2 independent rat liver experiments. *The average secreted or cellular apoB in incubations with IL-6 are significantly greater than that in the absence of IL-6. Increases are not affected by preincubation with PD-98059. C: cellular RNA was isolated from RH treated as described above and subjected to Northern blotting to determine levels of apoB mRNA with cyclophilin mRNA for normalization.
Leptin induces similar increases in apoB secretion.
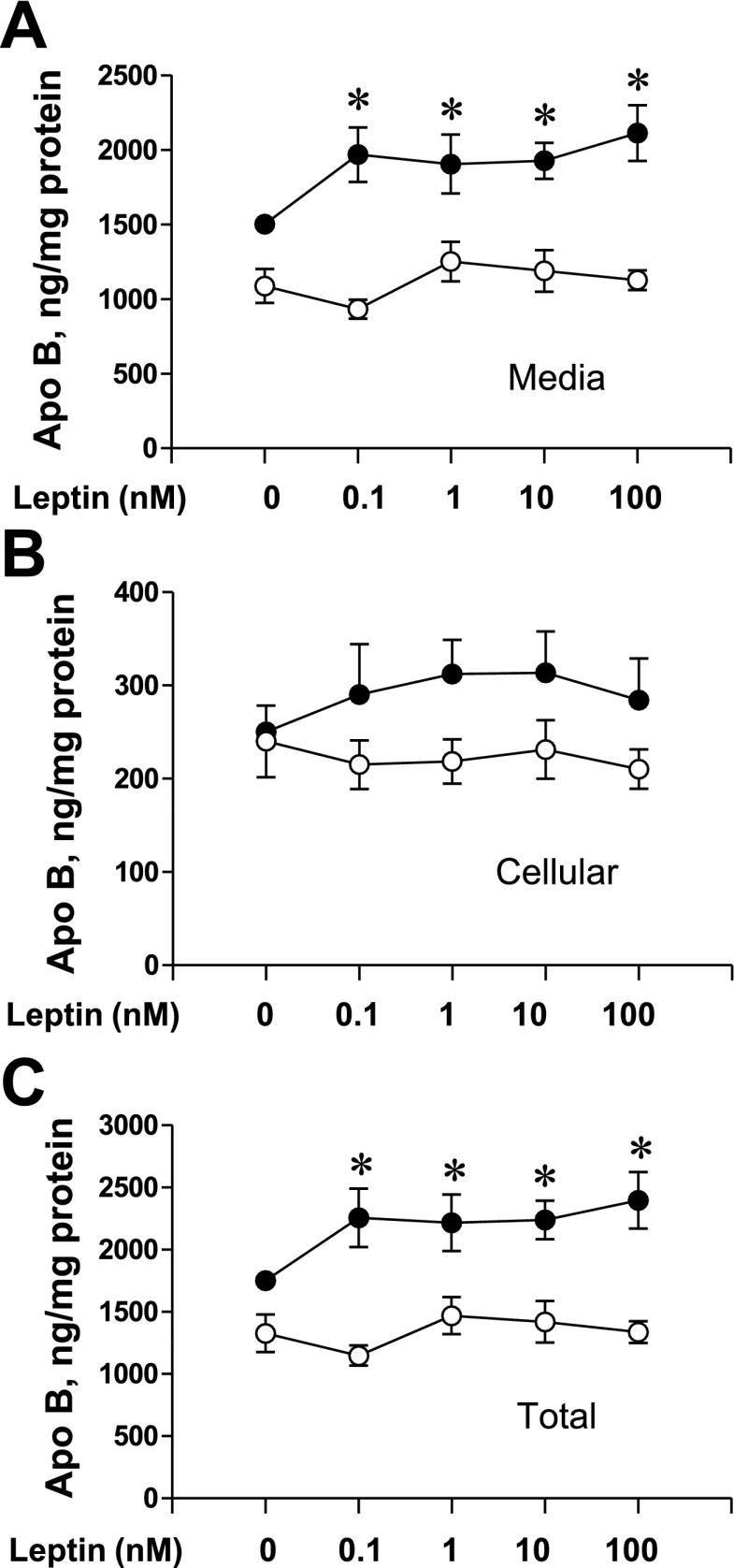
Leptin, a member of the type 1 cytokine family, is capable of signaling through IL-6-type cytokine receptors (6). To examine the potential for leptin action on apoB, we examined the effect of increasing concentrations of leptin on apoB secretion, cellular apoB, and total apoB (cellular + medium) (Fig. 8). Both secreted apoB (Fig. 8A) and total apoB (Fig. 8C) were increased with leptin incubations while the cellular apoB content trended upward. As observed with IL-6, insulin-suppressive effects were observed at all concentrations of leptin. These results support that leptin has similar effects to IL-6 on apoB without changing the ability of insulin to suppress apoB secretion.
Fig. 8.
Effect of leptin on media, cellular, and total apoB under basal and insulin conditions. RH were incubated overnight (16 h) in medium under basal conditions (0.1 nM insulin, ●) or with 100 nM insulin added (○) with increasing concentration of recombinant leptin. After incubation, apoB accumulation in the medium and cellular apoB were measured by RIA and normalized to plate protein. A: average medium apoB (ng/mg cell protein) ± SE from 4 independent rat liver experiments (3–5 plates/condition) is plotted against leptin dose. B: average cellular apoB (ng/mg cell protein) is plotted against leptin dose. C: total apoB (cell + media) is plotted against leptin dose. *Means differ from the 0 leptin condition at a probability level of at least P < 0.05.
SOCS3 balances IL-6 induction of cellular apoB mRNA abundance.
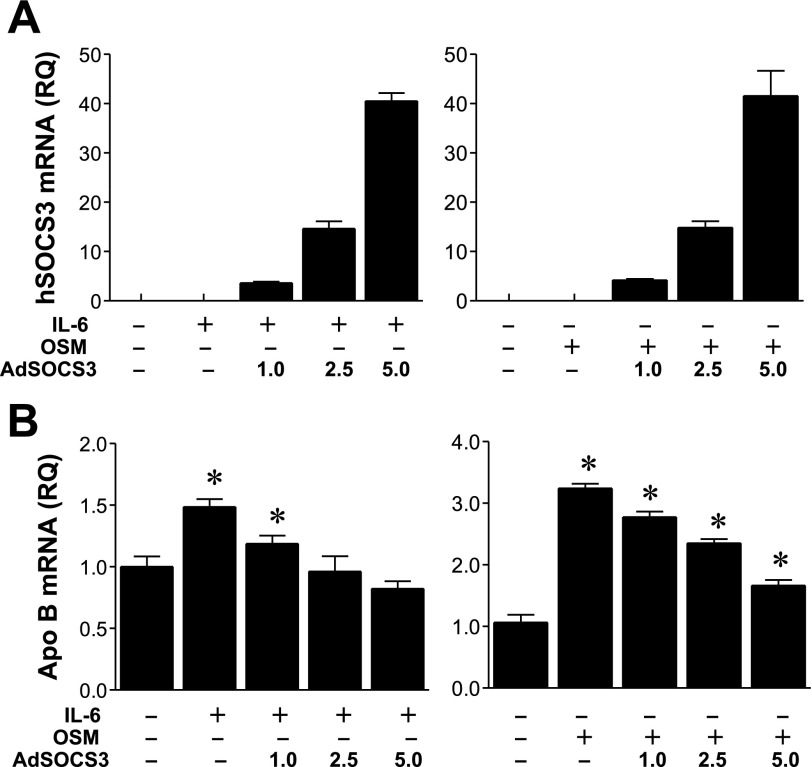
A known negative regulator of IL-6-type cytokines, including leptin, is SOCS3 (24), and we therefore examined whether SOCS3 expression was able to modulate apoB mRNA levels induced by IL-6 (Fig. 9, A and B). We treated RH with increasing MOI of Ad-CMV-hSOC3 (1, 2.5, and 5 MOI) in the presence of IL-6. RH were also incubated with OSM, a structurally and functionally related cytokine to IL-6 that utilizes the same signal-transducing molecule, gp130 (21) (Fig. 9, right). Compared with control incubations, both IL-6 and OSM significantly increased cellular apoB mRNA abundance as determined by Q-RT-PCR, with average increases of 48 and 218%, respectively, over control levels (Fig. 7B). Increasing expression of hSOCS3 demonstrated a progressive reversal of IL-6 and OSM induction of apoB mRNA levels, supporting the conclusion that increases in apoB mRNA levels stimulated by IL-6-type cytokines are negatively regulated by SOCS3 expression.
Fig. 9.
Effect of various levels of ectopic human (h) suppressor of cytokine signaling (SOCS) 3 expression on IL-6 and oncostatin M-induced increases in apoB mRNA abundance. A: expression level of hSOCS3 mRNA in RH following adenoviral infection at 1, 2.5, and 5.0 multiplicities of infection (MOI) for 16 h as assessed by quantitative real-time PCR (Q-RT-PCR). Results are the average of triplicate PCR reactions ± SD. B: apoB mRNA levels were determined by Q-RT-PCR of RNA isolated from RH treated with IL-6 (0.5 nM) or oncostatin M (0.5 nM) at various levels of expression of hSOCS3. Ad, adenovirus; RQ, relative quantitation. Results are averages of 3 separate PCR reactions ± SD. *The mean apoB mRNA level is significantly different from the control (no IL-6, no OSM, no SOCS3).
DISCUSSION
Obesity is associated with chronic subacute inflammation that may be promoted by the release of specific cytokines by an enlarging adipose tissue mass (23). Adipose tissue cytokines, through paracrine mechanisms, impact hepatic metabolism and have been implicated in the development of hepatic insulin resistance and steatosis (40). Hypertriglyceridemia is commonly associated with obesity and is caused primarily by hypersecretion of VLDL by the liver (11). Whether specific cytokines are able to play a direct role in stimulating hypersecretion of VLDL and whether increased lipoprotein secretion relates to reversal of insulin-mediated suppression of VLDL are issues addressed by the current study. TNF-α, IL-1β, and TGF-β1 were shown to have minimal effects in 16-h incubations on secretion of apoB-containing lipoproteins by RH and cellular apoB levels. IL-6, in contrast, significantly stimulated both cellular apoB content and the secretion of apoB-containing lipoproteins in a dose-dependent manner, suggesting direct effects of IL-6 on lipoprotein production. Insulin suppression of apoB-containing lipoprotein secretion was maintained in the presence of IL-6, suggesting that the observed increase in lipoprotein secretion was not due to derepression of insulin action on apoB. Pulse-chase studies indicated that IL-6 significantly stimulated B100 and B48 synthesis as well as the synthesis of fibrinogen, a known IL-6 target protein. The enhancement in apoB synthesis with IL-6 correlated with a significant increase in cellular apoB mRNA transcripts that was not due to mRNA stabilization, thereby supporting a transcriptional mechanism. Our results, however, cannot exclude the possibility that the inhibition of transcription necessary to perform mRNA decay studies blocked the synthesis of a modulator of apoB mRNA stabilization. Expression of SOCS3, which mediates a negative feedback loop on cytokine signaling, mitigated the stimulatory effect of IL-6 and OSM, an IL-6-like cytokine, on apoB mRNA levels. Together, these data support that IL-6 is a direct positive regulator of apob gene expression that leads to increased apoB mRNA abundance and stimulated synthesis and secretion of apoB-containing lipoproteins by the liver.
apob gene transcription has long been thought to be constitutive, since liver apoB mRNA levels in animals in vivo tend to be relatively stable under a wide variety of situations. This has led to the conclusion that most regulation of lipoprotein-apoB assembly and secretion by the liver is attributable to posttranscriptional mechanisms. Controversy related to relative apoB mRNA changes has been furthered by difficulties in quantitation of apoB mRNA in livers with steatosis and lack of an agreed-to standard for normalization of relative expression levels. The constitutive nature of apob gene expression has been challenged by a number of recent reports demonstrating transcriptional regulation of apob in rat liver in vivo by dietary manipulation (46), by diurnal cycle (32), and in hepatocyte growth factor transgenic mice (28). In vitro studies provide additional evidence that steady-state apoB mRNA levels are regulated under a number of pathophysiological conditions, including in response to endotoxin (3) and in HepG2 cells following IL-1β (55) and TGF-β stimulation (42). Transcription factors important in apob gene transcription include C/EBP, hepatocyte nuclear factor (HNF)-3, HNF-4, signaling mother against decapentaplegic peptides (42), and other nuclear transcription factors (57). Recent studies support the importance of forkhead transcription factors, including HNF3β (54) and forkhead box 01 (FoxO1) (26), in transcriptional regulation of apob; both HNF3β and FoxO1 are positive regulators that can be negatively regulated by insulin by activated AKT through nuclear exclusion. The transcription factors responsible for the induction of the apob gene by IL-6 are currently being investigated.
Inflammation results in the APR leading to major changes in the concentrations of many plasma proteins mediated primarily at the transcriptional level (10). The IL-6 family of cytokines is considered the major physiological inducer of the APR and related gene expression changes that can involve a number of transcription factors, including C/EBPβ and C/EBPδ, STAT proteins, NF-κB, HNF1α, signal protein 1, and activator protein-1. Inflammation results in profound changes in lipid and lipoprotein metabolism characterized by significant hypertriglyceridemia due to increased hepatic VLDL secretion and defective catabolism that can be reproduced in vivo with administration of IL-6 to rodents (27). C/EBPβ, a transcription factor with a binding motif originally designated as the IL-6 response element, HNF1α, and forkhead transcription factor-binding motifs are present in the 5′ proximal rat apob promoter with the potential to be involved in IL-6 induction of apob gene transcription (44). Results of the current study support that IL-6 specifically increases liver apoB mRNA abundance consistent with previous studies in HepG2 cells (55), in RH (4, 33), and in RH following endotoxin exposure (33). Current results provide additional evidence that the effect of IL-6 is transcriptionally mediated, since apoB mRNA stabilization with IL-6 treatment could not be demonstrated. Results also suggest that IL-6 released from expanding adipose tissue due to obesity has the potential to stimulate apob gene transcription in a fashion similar to IL-6 released by Kupffer cells in response to endotoxin (4). In the context of obesity and subacute inflammation, IL-6 signaling would support the development of hypersecretion of apoB-containing lipoproteins and subsequent hypertriglyceridemia.
Previous reports on the effects of cytokines on the secretion of apoB-containing lipoproteins are variable and depend upon cell type, duration of culture, culture media conditions, and species (4, 5, 37, 42, 55). We did not observe an increase in total apoB secreted in 16-h incubations with TNF-α, IL-1β, or TGF-β1. Previous reports in rodent hepatocytes indicate that both TNF-α and IL-1β stimulate the secretion of VLDL apoB by transiently increasing apoB mRNA expression (4, 5). However, in 16-h cultures, similar to the time interval employed in the current study, the enhanced secretion of VLDL apoB by TNF-α returned to control levels. No direct effect of TNF-α or IL-1β was observed in VLDL “lipid” output in the absence of extracellular fatty acids nor was there an effect on cellular MTP mRNA levels (5). In contrast, TNF-α treatment of liver and hamster hepatocytes stimulated VLDL B100 production without changes in apoB mRNA but with increases in MTP mRNA and protein (37). One major difference between the current study and those mentioned above is that we measured total apoB rather than only VLDL apoB, and it is well known that rodent hepatocytes are capable of secreting apoB in denser lipoprotein fractions, particularly B48 in HDL density. Other reasons for disparities include differences in purity of hepatocyte cultures and differences in hormone content of the culture medium (insulin, dexamethasone) as well as time course differences. Contradictory results are reported in HepG2 cells where TNF-α, IL-6, and IL-1β reduce B100 secretion with (17) and without (55) changes in apoB mRNA levels. TGF-β1 can increase apoB mRNA levels in CaCo-2 cells while decreasing mRNA levels in HepG2 cells (42). An additional issue not addressed in earlier studies is the potential for the induction of SOCS proteins by cytokine treatments. SOCS3, in particular, negatively regulates multiple cytokine-signaling pathways, including TNF-α, IL-1β, IL-6, and leptin (15, 16, 24). Because SOCS3 expression suppresses induction of apoB mRNA levels mediated by IL-6 and OSM, it is possible that variable SOCS3 expression may have contributed to reported discrepancies regarding the effects of cytokines on apoB secretion and on apoB mRNA levels.
Recent studies indicate that leptin may play a role in regulating proinflammatory cytokine production, including TNF-α and IL-6 (30). The effect of leptin action in the liver is controversial, since leptin's primary action is believed to be at the level of the brain via the long form of the receptor. However, both the long form of the leptin receptor and the short form are present in RH (38), suggesting the possibility of direct leptin signaling in hepatocytes. Recent in vivo studies indicate that leptin augments the acute suppressive effects of insulin on hepatic VLDL B100 and B48 secretion (25). We therefore tested whether leptin directly affects apoB metabolism in RH. Leptin functioned similarly to IL-6 in stimulating an increase in secretory apoB, and insulin retained its inhibitory effect on apoB secretion in the presence of leptin. These results are similar to results of in vivo studies which demonstrated that insulin-suppressive effects are retained during leptin treatment.
Conflicting results exist as to whether IL-6 is a beneficial or harmful cytokine. To the extent that IL-6 treatment does not result in sustained SOCS3 expression, our results suggest that IL-6, through a transcriptional mechanism, has the potential to enhance VLDL TG secretion by increasing the availability of apoB and thereby reducing hepatic lipid content by increased TG export. Consistent with this hypothesis are findings that IL-6 treatment resolves hepatic steatosis and normalizes serum transaminase levels in fatty liver transplants in rats and following ischemia-reperfusion injury (22, 51). The resolution of fatty livers was associated with increased export of TG and cholesterol and a small increase in mitochondrial β-oxidation. Interestingly, hepatic SOCS3 expression is high in db/db and ob/ob mice and is associated with hepatic steatosis and metabolic syndrome (53). Following treatment with SOCS3 antisense, hepatic steatosis resolves, suggesting the reduction of hepatic TG observed might also involve enhanced VLDL export. Long-term increases in hepatic VLDL secretion mediated by IL-6 may also be considered harmful, since increased VLDL production is a proximal cause of hypertriglyceridemia and is associated with enhanced atherogenic LDL formation in humans.
The role of IL-6 in enhancing apoB-lipoprotein secretion expands our understanding of the necessary and complex role of lipoproteins in balancing hepatic lipid metabolism. Shorter-term modulation of VLDL allows for the balance of TG accumulation with other hepatic pathways for TG metabolism. These include oxidation and peroxidation, which can have long-term detrimental effects and can result in development of steatohepatitis. The role of IL-6 in favoring TG export may be protective in the short term, but in the long term needs to be balanced considering the potential consequences and the complexity of these relationships with other pathways, particularly SOCS3 pathways. The novel finding in the current study is that the mechanism involved with IL-6 is a transcriptional effect on the apob gene whose gene product is a carrier for TG. A full range of regulation of apoB is now being established for hepatic apoB metabolism, which includes various forms of degradation (18), translational regulation (41), and now further evidence for transcriptional regulation (46). Considerable future research will be necessary to understand the balance of apoB metabolic regulation required for overall homeostasis as a basis for interventions specifically focused on hepatic steatosis while minimizing cardiovascular risk complications.
GRANTS
This work was supported by a Research Award from the American Diabetes Association (J. D. Sparks) and by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK-078131 (J. D. Sparks).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 28: 1225–1236, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Alberti KG, Zimmet P, Shaw J, Group IDFETFC. The metabolic syndrome–a new worldwide definition. Lancet 366: 1059–1062, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Aspichueta P, Perez S, Ochoa B, Fresnedo O. Endotoxin promotes preferential periportal upregulation of VLDL secretion in the rat liver. J Lipid Res 46: 1017–1026, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Bartolome N, Arteta B, Martinez MJ, Chico Y, Ochoa B. Kupffer cell products and interleukin 1beta directly promote VLDL secretion and apoB mRNA up-regulation in rodent hepatocytes. Innate Immun 14: 255–266, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Bartolome N, Rodriguez L, Martinez MJ, Ochoa B, Chico Y. Upregulation of apolipoprotein B secretion, but not lipid, by tumor necrosis factor-alpha in rat hepatocyte cultures in the absence of extracellular fatty acids. Ann NY Acad Sci 1096: 55–69, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA 93: 8374–8378, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 7: 125–134, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 9. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Trullenque R, Fabra R, Heinrich PC. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett 242: 237–239, 1989. [DOI] [PubMed] [Google Scholar]
- 11. Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am 35: 491–510, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Chirieac DV, Collins HL, Cianci J, Sparks JD, Sparks CE. Altered triglyceride-rich lipoprotein production in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab 287: E42–E49, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Chirieac DV, Davidson NO, Sparks CE, Sparks JD. PI3-kinase activity modulates apoB available for hepatic VLDL production in apobec-1−/− mice. Am J Physiol Gastrointest Liver Physiol 291: G382–G388, 2006. [DOI] [PubMed] [Google Scholar]
- 14. Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev 29: 777–822, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emanuelli B, Glondu M, Filloux C, Peraldi P, Van Obberghen E. The potential role of SOCS-3 in the interleukin-1beta-induced desensitization of insulin signaling in pancreatic beta-cells. Diabetes 53, Suppl 3: S97–S103, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem 276: 47944–47949, 2001. [DOI] [PubMed] [Google Scholar]
- 17. Ettinger WH, Varma VK, Sorci-Thomas M, Parks JS, Sigmon RC, Smith TK, Verdery RB. Cytokines decrease apolipoprotein accumulation in medium from Hep G2 cells. Arterioscler Thromb 14: 8–13, 1994. [DOI] [PubMed] [Google Scholar]
- 18. Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J Biol Chem 276: 27855–27863, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J Lipid Res Suppl 50: S162–S166, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 83: 461S–465S, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374: 1–20, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology 40: 933–941, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab 17: 365–371, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Huang W, Metlakunta A, Dedousis N, Ortmeyer HK, Stefanovic-Racic M, O'Doherty RM. Leptin augments the acute suppressive effects of insulin on hepatic very low-density lipoprotein production in rats. Endocrinology 150: 2169–2174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest 118: 2347–2364, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 45: 1169–1196, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Kosone T, Takagi H, Horiguchi N, Ariyama Y, Otsuka T, Sohara N, Kakizaki S, Sato K, Mori M. HGF ameliorates a high-fat diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol 293: G204–G210, 2007. [DOI] [PubMed] [Google Scholar]
- 29. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 30. Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J 12: 57–65, 1998. [PubMed] [Google Scholar]
- 31. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan X, Hussain MM. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J Biol Chem 282: 24707–24719, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Perez S, Aspichueta P, Ochoa B, Chico Y. The 2-series prostaglandins suppress VLDL secretion in an inflammatory condition-dependent manner in primary rat hepatocytes. Biochim Biophys Acta 1761: 160–171, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Phung TL, Roncone A, Jensen KL, Sparks CE, Sparks JD. Phosphoinositide 3-kinase activity is necessary for insulin-dependent inhibition of apolipoprotein B secretion by rat hepatocytes and localizes to the endoplasmic reticulum. J Biol Chem 272: 30693–30702, 1997. [DOI] [PubMed] [Google Scholar]
- 35. Phung TL, Sowden MP, Sparks JD, Sparks CE, Smith HC. Regulation of hepatic apolipoprotein B RNA editing in the genetically obese Zucker rat. Metabolism 45: 1056–1058, 1996. [DOI] [PubMed] [Google Scholar]
- 36. Pullinger CR, North JD, Teng BB, Rifici VA, Ronhild de Brito AE, Scott J. The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res 30: 1065–1077, 1989. [PubMed] [Google Scholar]
- 37. Qin B, Anderson RA, Adeli K. Tumor necrosis factor-alpha directly stimulates the overproduction of hepatic apolipoprotein B100-containing VLDL via impairment of hepatic insulin signaling. Am J Physiol Gastrointest Liver Physiol 294: G1120–G1129, 2008. [DOI] [PubMed] [Google Scholar]
- 38. Raman P, Donkin SS, Spurlock ME. Regulation of hepatic glucose metabolism by leptin in pig and rat primary hepatocyte cultures. Am J Physiol Regul Integr Comp Physiol 286: R206–R216, 2004. [DOI] [PubMed] [Google Scholar]
- 39. Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 51: 3391–3399, 2002. [DOI] [PubMed] [Google Scholar]
- 40. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sidiropoulos KG, Meshkani R, Avramoglu-Kohen R, Adeli K. Insulin inhibition of apolipoprotein B mRNA translation is mediated via the PI-3 kinase/mTOR signaling cascade but does not involve internal ribosomal entry site (IRES) initiation. Arch Biochem Biophys 465: 380–388, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Singh K, Batuman OA, Akman HO, Kedees MH, Vakil V, Hussain MM. Differential, tissue-specific, transcriptional regulation of apolipoprotein B secretion by transforming growth factor beta. J Biol Chem 277: 39515–39524, 2002. [DOI] [PubMed] [Google Scholar]
- 43. Sparks CE, Hnatiuk O, Marsh JB. Hepatic and intestinal contribution of two forms of apolipoprotein B to plasma lipoprotein fractions in the rat. Can J Biochem 59: 693–699, 1981. [DOI] [PubMed] [Google Scholar]
- 44. Sparks JD. FoxO1 and hepatic lipid metabolism. Curr Opin Lipidol 20: 217–226, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sparks JD, Bolognino M, Trax PA, Sparks CE. The production and utility of monoclonal antibodies to rat apolipoprotein B lipoproteins. Atherosclerosis 61: 205–211, 1986. [DOI] [PubMed] [Google Scholar]
- 46. Sparks JD, Collins HL, Chirieac DV, Cianci J, Jokinen J, Sowden MP, Galloway CA, Sparks CE. Hepatic very-low-density lipoprotein and apolipoprotein B production are increased following in vivo induction of betaine-homocysteine S-methyltransferase. Biochem J 395: 363–371, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sparks JD, Corsetti JP, Sparks CE. Liver regrowth and apolipoprotein B secretion by rat hepatocytes following partial hepatectomy. Metabolism 43: 681–690, 1994. [DOI] [PubMed] [Google Scholar]
- 48. Sparks JD, Sparks CE. Insulin modulation of hepatic synthesis and secretion of apolipoprotein B by rat hepatocytes. J Biol Chem 265: 8854–8862, 1990. [PubMed] [Google Scholar]
- 49. Sparks JD, Sparks CE. Insulin regulation of triacylglycerol-rich lipoprotein synthesis and secretion. Biochim Biophys Acta 1215: 9–32, 1994. [DOI] [PubMed] [Google Scholar]
- 50. Sparks JD, Sparks CE. Overindulgence and metabolic syndrome: is FoxO1 a missing link? J Clin Invest 118: 2012–2015, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan HN, Jaruga B, Batkai S, Hoshino S, Tian Z, Kunos G, Diehl AM, Gao B. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology 125: 202–215, 2003. [DOI] [PubMed] [Google Scholar]
- 52. Thissen JP, Verniers J. Inhibition by interleukin-1 beta and tumor necrosis factor-alpha of the insulin-like growth factor I messenger ribonucleic acid response to growth hormone in rat hepatocyte primary culture. Endocrinology 138: 1078–1084, 1997. [DOI] [PubMed] [Google Scholar]
- 53. Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci USA 101: 10422–10427, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wolfrum C, Stoffel M. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab 3: 99–110, 2006. [DOI] [PubMed] [Google Scholar]
- 55. Yokoyama K, Ishibashi T, Yi-qiang L, Nagayoshi A, Teramoto T, Maruyama Y. Interleukin-1beta and interleukin-6 increase levels of apolipoprotein B mRNA and decrease accumulation of its protein in culture medium of HepG2 cells. J Lipid Res 39: 103–113, 1998. [PubMed] [Google Scholar]
- 56. Yu YH, Ginsberg HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res 96: 1042–1052, 2005. [DOI] [PubMed] [Google Scholar]
- 57. Zannis VI, Kan HY, Kritis A, Zanni E, Kardassis D. Transcriptional regulation of the human apolipoprotein genes. Front Biosci 6: D456–D504, 2001. [DOI] [PubMed] [Google Scholar]