Abstract
The major short-chain fatty acid (SCFA) butyrate is produced in the colonic lumen by bacterial fermentation of dietary fiber. Butyrate serves as primary fuel for the colonocytes and also ameliorates mucosal inflammation. Disturbed energy homeostasis seen in inflamed mucosa of inflammatory bowel disease patients has been attributed to impaired absorption of butyrate. Since sodium-coupled monocarboxylate transporter 1 (SMCT1, SLC5A8) has recently been shown to play a role in Na+-coupled transport of monocarboxylates, including SCFA, such as luminal butyrate, we examined the effects of proinflammatory TNF-α on SMCT1 expression and function and potential anti-inflammatory role of probiotic Lactobacillus species in counteracting the TNF-α effects. Rat intestinal epithelial cell (IEC)-6 or human intestinal Caco-2 cells were treated with TNF-α in the presence or absence of Lactobacilli culture supernatants (CS). TNF-α treatments for 24 h dose-dependently inhibited SMCT1-mediated, Na+-dependent butyrate uptake and SMCT1 mRNA expression in IEC-6 cells and SMCT1 promoter activity in Caco-2 cells. CS of L. plantarum (LP) stimulated Na+-dependent butyrate uptake (2.5-fold, P < 0.05), SMCT1 mRNA expression, and promoter activity. Furthermore, preincubating the cells with LP-CS followed by coincubation with TNF-α significantly attenuated the inhibitory effects of TNF-α on SMCT1 function, expression, and promoter activity. In vivo, oral administration of live LP enhanced SMCT1 mRNA expression in the colonic and ileal tissues of C57BL/6 mice after 24 h. Efficacy of LP or their secreted soluble factors to stimulate SMCT1 expression and function and to counteract the inhibitory effects of TNF-α on butyrate absorption could have potential therapeutic value.
Keywords: intestinal epithelial cell-6, SLC5A8, short-chain fatty acid, inflammatory bowel disease
the short-chain fatty acid (SCFA) butyrate is a major end product of colonic luminal microbial fermentation of dietary fiber. Butyrate is an important energy nutrient for intestinal epithelial cells (IECs). Beyond its role as a nutrient, butyrate is key to many aspects of health and integrity of intestinal mucosa (19). Indeed, butyrate has an important role in ameliorating mucosal inflammation (19, 37). The ability of butyrate to elicit its beneficial effects on IECs requires its absorption into these cells. Therefore, factors with negative effects on butyrate absorption by the IECs could be detrimental to intestinal health and integrity.
Two major carrier-mediated mechanisms have so far been identified for the transport of butyrate across the luminal membranes of colonocytes. Monocarboxylate transporter 1 (MCT1), a member of SLC16 gene family, mediates the H+-coupled transport of butyrate into colonocytes (18, 34). Another transporter belonging to SLC5 solute-linked carrier family has recently been characterized as a Na+-coupled transporter of butyrate and, therefore, known as sodium-coupled MCT1 (SMCT1) (16, 40). Our laboratory's previous studies demonstrated the role of MCT1 in butyrate transport (18), its membrane localization along the length of the human intestine (15), and its transcriptional or posttranslational modulation by the transcription factor upstream stimulatory factor (17), butyrate (9), PKC-ζ (35), somatostatin (36), and in response to enteropathogenic E. coli infection (7). With respect to MCT1 expression in disease, it has been shown that, during transition from normalcy to malignancy, MCT1 expression is downregulated in colonic tissues (24). Furthermore, MCT1 is also downregulated in inflamed mucosa of inflammatory bowel disease (IBD) patients, in animal models of inflammation, and in response to proinflammatory agents IFN-γ and TNF-α (42). Compared with our knowledge on regulation of MCT1 function and expression, information on regulation of SMCT1 is very limited. Recent studies have shown that SMCT1 expression is silenced in colorectal carcinoma and colon cancer cell lines (14). However, there is no information on the modulation of SMCT1 activity and expression in intestinal inflammatory disorders. Several studies have reported impaired utilization of the SCFA butyrate in inflamed mucosa of patients with IBDs (41). This impairment, caused by either inadequate production of butyrate in the lumen, or reduced intracellular availability due to impaired butyrate absorption, could limit its protective effects toward IBD (42). Therefore, agents that stimulate butyrate absorption and/or counteract the effects of proinflammatory agents may alleviate intestinal inflammation. In this regard, probiotics, live microorganisms that confer health benefits to host, have shown promising effects in ameliorating gut disorders, including IBD (21, 44). In fact, IBD has recently been an important arena of testing efficacy of probiotics (12). Since butyrate is known to ameliorate inflammation, enhancement of butyrate absorption may represent a distinct mechanism of anti-inflammatory effect of probiotics.
In this report, we have demonstrated that the culture supernatant (CS) of the probiotic Lactobacillus plantarum (LP) enhances SMCT1 expression and function and counteracts the inhibitory effects of TNF-α on SMCT1 activity.
MATERIALS AND METHODS
Materials.
Caco-2/IEC-6, and probiotic Lactobacillus species were obtained from American Type Cell Collection (ATCC), 14C-butyrate was obtained from American Radiochemicals (St. Louis, MO), and TNF-α was purchased from Sigma (St. Louis, MO).
Cell lines, cell culture, and treatments.
IEC-6 cells were grown at 37°C in an atmosphere of 5% CO2. Cells were maintained in DMEM with 4.5 g/l glucose, 50 U/ml penicillin, 5 μg/ml streptomycin, 2 μg/ml gentamicin, and 10% FBS. Caco-2 cells were grown in 20% FBS. Na+-coupled butyrate uptake studies were performed in fully differentiated IEC-6 cells grown 12-days postplating on 24-well plastic supports or 12-well transwell inserts.
Bacterial culture and preparation of conditioned CS.
The following Lactobacilli species, with ATCC strain numbers given in parentheses, were grown in MRS broth (Difco, Detroit, MI) for 24 h at 37°C, without shaking: L. acidophilus (4357), L. rhamnosus (53103), LP (14917), and L. casei (393). The cultures were then centrifuged at 3,000 g at 4°C for 10 min. The supernatant, filtered through a 0.22-μm filter (Millex, Millipore, Bedford, MA) to sterilize and remove all bacterial cells, was designated as CS. For treating the cell monolayers, the CS was diluted 1:50 in DMEM cell culture media.
Measurement of Na+-dependent 14C-butyrate uptake.
SMCT1 function was assessed by measuring Na+-dependent 14C-butyrate uptake, as described previously in rat thyroid follicular FRTL-5 cells (11), with minor modifications, in postconfluent IEC-6 monolayers. Briefly, cell monolayers were washed twice with the tracer-free uptake buffer containing (in mM) 10 HEPES (pH 7.4), 137 NaCl, 5.4 KCl, 2.8 CaCl2, and 1.2 MgCl2. A time course experiment was initially performed to determine the linearity of uptake by incubating the cells for 0–10 min with sodium-containing or sodium-free (137 mM choline chloride replaces NaCl) uptake buffer with 5 mM 14C-butyrate (1 mCi/ml). Uptake was terminated by washing the cells twice with 1 × PBS. Cells were solubilized with 0.5 N NaOH, and radioactivity was counted in a Tri-CARB 1600-TR liquid scientillation counter. Protein concentrations were measured by the method of Bradford (10). Results were expressed as nanomoles of butyrate per milligram protein.
Preparation of human intestinal apical and basolateral membranes.
Apical and basolateral membranes were prepared from various regions of the human intestine obtained from organ donors according to the methods previously described by us (15). The purified membrane fractions were used to measure SMCT1 protein levels by immunoblotting.
Generation of anti-SMCT1 antibodies.
Polyclonal antibodies against the COOH-terminal sequences of the human (amino acids 591–610, AFNHIELNSDQSGKSNGTRL) and rodent (amino acids 596–611, VELNFTDHSGKINGTRL) SMCT1 proteins were raised in rabbits by the Research Resource Facility of the University of Illinois.
Immunoblotting.
Proteins in the human intestinal purified membranes or lysates of mouse intestinal mucosa were separated by SDS-PAGE on a 8% gel. Proteins were transferred to a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ) and probed with the SMCT1 antibodies raised in rabbits. Immunoreactive bands were visualized using the ECL detection kit (Amersham).
RNA isolation and real-time RT-PCR.
RNA was isolated from human and rat IECs and mouse intestinal tissues using RNeasy Mini Kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. An equal amount of RNA for each sample was reverse transcribed and amplified in one-step reaction using Brilliant SYBR Green QRT-PCR master mix kit (Stratagene, La Jolla, CA) and using Mx 3000 (Stratagene). The gene-specific primers for human, mouse, and rat SMCT1 used for the RT-PCR reactions are shown in Table 1. The quantitation of the amplification was expressed as a ratio of 2ΔCt-SMCT1/2ΔCt-β-actin, where ΔCt-SMCT1 and ΔCt-β-actin represent the difference between the threshold cycle of amplification of SMCT1 and β-actin, respectively.
Table 1.
Primers used for real-time RT-PCR analysis and cloning experiments
| Gene | Accession No. | Primer Sequence |
|---|---|---|
| Primers for real-time RT-PCR | ||
| SLC5A8 (human) | NM_145913 | (F): 5′-CCCTATCAGTCCAGGGCATA-3′ |
| (R): 5′-CCCAGGGCAATAAACAGAAA-3′ | ||
| SLC5A8 (mouse) | NM_145423 | (F): 5′-TGCCATTTCCTTATGGGTAGG-3′ |
| (R): 5′-AGTGGAGTCCTTTCCGCATTA-3′ | ||
| SLC5A8 (rat) | NM_576209 | (F): 5′-GCGACTGGCAGCGG-3′ |
| (R): 5′-AGCATGCCTGCGAA-3′ | ||
| Primers for cloning promoter region of Human SLC5A8 | ||
| SLC5A8 (human) | NM_145913 | (F): 5′-ATCCTCGAGAGCCATAACTTACAGTAGCCC-3′ |
| (R): 5′-ATCCTCGAGTGAGCACTCAGGGCAGCGGGT-3′ | ||
F, forward; R, reverse.
Cloning of 5′-untranslated region of SMCT1 gene (SLC5A8) and measurement of promoter activity.
A 2,015-bp fragment of the 5′-untranslated region of slc5a8 gene and several 5′ deletion fragments were cloned into the pGL2 reporter plasmid (Promega, Madison, WI) between XhoI and HinDIII sites upstream of the luciferase reporter gene. We used Elongase Amplification System (Invitrogen) and PCR method to clone this fragment using human genomic DNA as the template. The gene-specific primer pair used to clone the 2,015-bp fragment is shown in Table 1. A touch-down long-PCR method was used with the following amplification conditions: heating at 94°C for 30 s, followed by 40 cycles, 30 s each, of varying annealing temperatures (5 × 83°C, 5 × 80°C, 5 × 75°C, 5 × 70°C, 20 × 68°C) and then elongation at 68°C for 10 min. The PCR products were purified using the gel extraction kit (Qiagen), digested with XhoI and HinDIII, and ligated to the corresponding sites in pGL2. For the present study, we used the longest fragment (2,015 bp). To determine the promoter activity of this fragment, Caco-2 cells were transfected with the promoter fragment using Lipofectamine 2000 (Invitrogen), and the transfected cells were grown on 12-well transwell inserts. Twenty four hours after transfection, cells were treated with TNF-α added in the basolateral medium, with or without probiotics CS in the apical medium, for an additional 24 h. Subsequently, promoter activity was determined by measuring luciferase activity according to the procedure described previously (9). Promoter activity was expressed as relative luciferase units that were normalized with the corresponding β-galactosidase units as internal control for transfection efficiency.
Statistical analyses.
The data presented are means ± SE of three to four independent experiments. Differences between controls vs. various treatments were analyzed using one-way ANOVA, with Dunnett's multiple-comparison tests for repeated comparisons to the control. Differences were considered significant at P < 0.05.
RESULTS
Expression of SMCT1 in the human and mouse intestine.
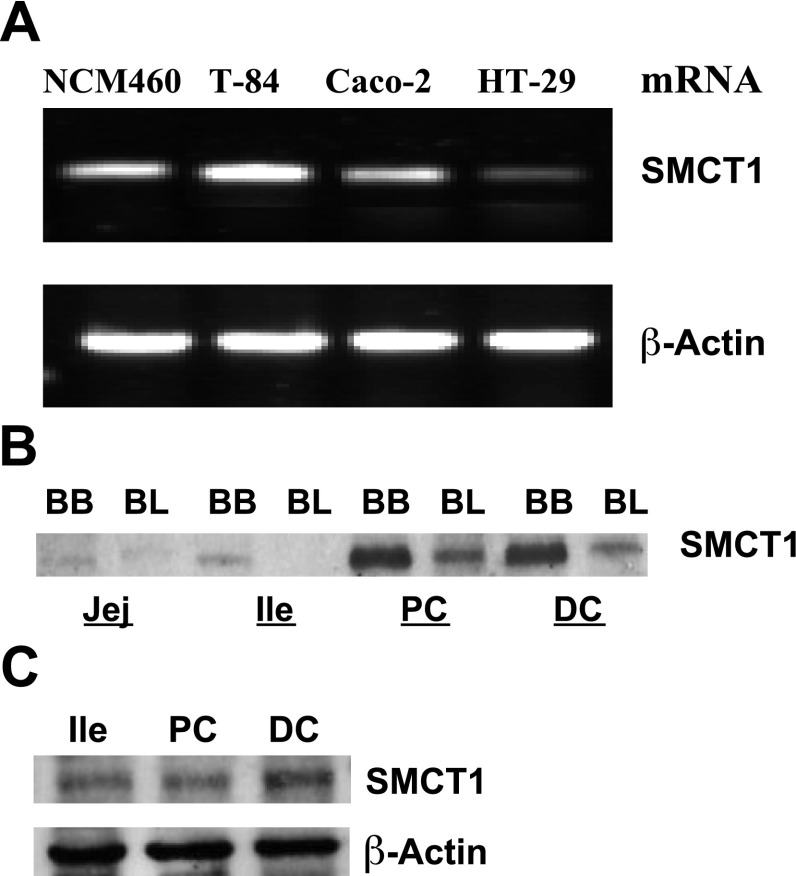
SMCT1 (SLC5A8) has recently been implicated in Na+-coupled transport of butyrate across the luminal membrane of the intestinal epithelium. Therefore, we examined the expression of SMCT1 mRNA in various IEC lines using real-time PCR and SMCT1 protein along the lengths of the human and mouse intestines by immunoblotting. Results in Fig. 1A show that NCM460 and T-84 cells express higher levels of SMCT1 mRNA compared with Caco-2, whereas HT-29 cells showed minimal level of SMCT1 mRNA. In both human (Fig. 1B) and mouse (Fig. 1C), SMCT1 protein levels were highest in distal colon, followed by proximal colon and ileum. Furthermore, SMCT1 was predominantly localized to the apical membrane in the human ileum and colon (Fig. 1B).
Fig. 1.
Expression of sodium-coupled monocarboxylate transporter 1 (SMCT1) in human and mouse intestine. A: SMCT1 mRNA levels in human intestinal epithelial cells (IECs). SMCT1 protein levels in brush border (BB) and basolateral (BL) membranes in human jejunum (Jej), ileum (Ile), proximal colon (PC), and distal colon (DC; B) and in mouse Ile, PC, and DC (C) are shown. Immunoblots for protein are representatives of three experiments.
Measurement of Na+-coupled butyrate uptake (SMCT1 function) in IEC-6 cells.
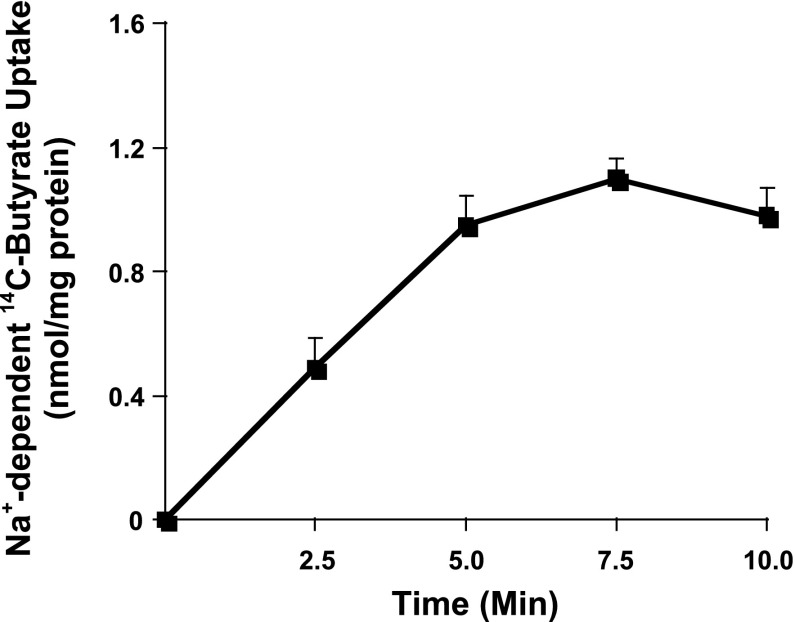
Since previous studies reported silencing of SMCT1 in colon cancer cell lines (14), we used IEC-6, a nontransformed rat intestinal cell line, as a cell culture model to standardize the method to measure SMCT1-mediated, Na+-coupled butyrate uptake and its modulation in response to TNF-α and/or probiotics treatments. Several other investigators have also utilized IEC-6 cells for intestinal transport studies (2, 31). We used 12th day postplating cell monolayers grown on transwell inserts to measure uptake of 14C-butyrate in the presence or absence of sodium in the incubation buffer, for different time periods. Linearity of Na+-coupled uptake of 14C-butyrate was observed for up to 5 min (Fig. 2), and, therefore, for subsequent studies, 5-min time point was used. These studies showed that IEC-6 cells express a functional SMCT1 and, therefore, could be utilized as a model to study regulation of SMCT1.
Fig. 2.
Measurement of SMCT1 function in IEC-6 cells. Na+-dependent 14C-butyrate uptake was measured in postconfluent IEC-6 monolayers as a function of time. Values represent difference between 14C-butyrate uptake in presence and absence of sodium in the uptake buffer. Values are means ± SE; n = 4.
TNF-α inhibits SMCT1 function and expression in IEC-6 cells.
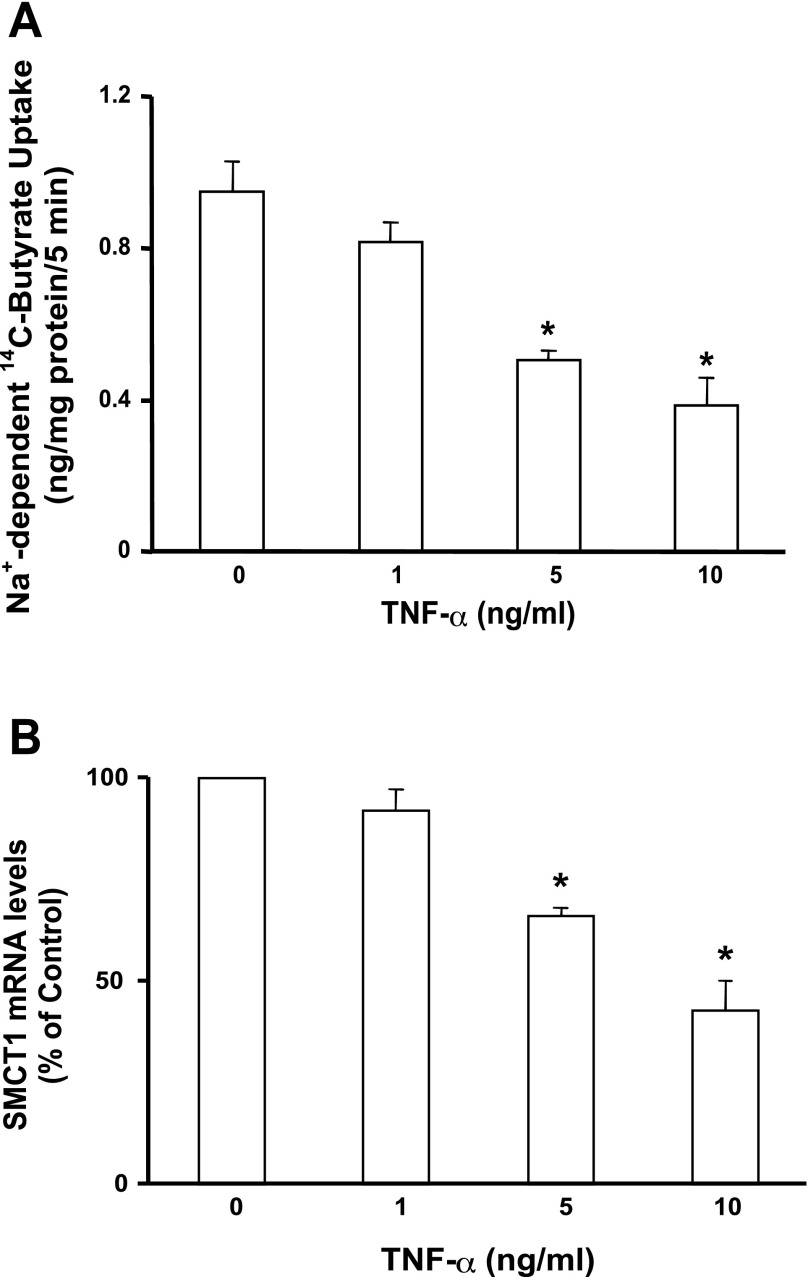
Previous studies have shown that reduced expression of MCT1 mRNA and protein in HT-29 cells in response to TNF-α treatments correlated with a decrease in MCT1 function (42). In parallel studies, we measured Na+-coupled butyrate uptake and SMCT1 mRNA levels in IEC-6 cells in response to TNF-α treatments. Earlier studies have shown TNF-α effects on IEC-6 cells, indicating expression of TNF-α receptors in this cell line (4, 23). Our results showed that TNF-α inhibited Na+-coupled butyrate uptake in a dose-dependent manner (Fig. 3A), which was correlated with a dose-dependent inhibition of SMCT1 mRNA expression (Fig. 3B).
Fig. 3.
TNF-α inhibits SMCT1 function and expression in IEC-6 cells. The cells grown on transwell inserts were treated basolaterally with indicated concentrations of TNF-α for 24 h. A: Na+-dependent 14C-butyrate uptake was measured in control or TNF-α-treated cells. B: total RNA was isolated from control or TNF-α-treated cells, and SMCT1 mRNA was measured by real-time quantitative PCR. Values are means ± SE; n = 3. *Different from control, P < 0.001.
Long-term treatment with LP-CS stimulates Na+-coupled butyrate uptake in IEC-6 cells.
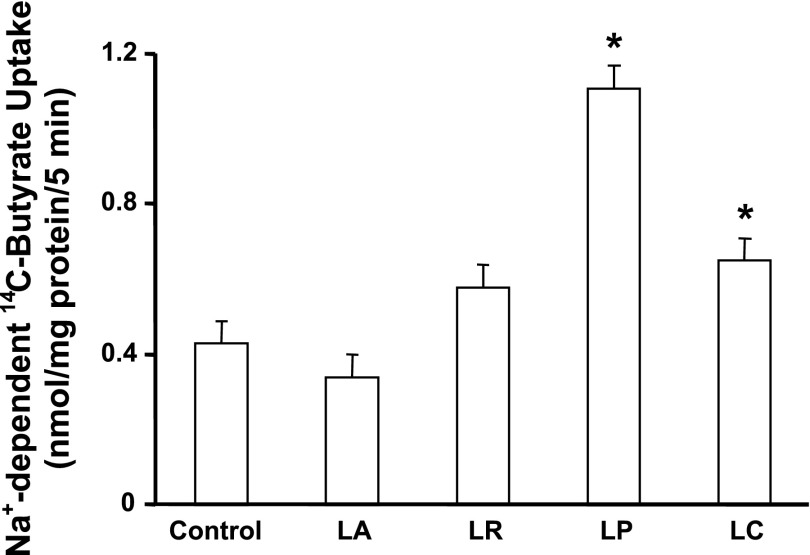
The CS of four Lactobacillus species described in materials and methods were tested for their effects on Na+-coupled butyrate uptake in IEC-6 cells. The CS prepared as described in materials and methods and diluted 1:50 in DMEM were used to treat the cells for 3 and 24 h before butyrate uptake was measured. As shown in Fig. 4, the CS of LP significantly enhanced (∼2.5-fold) Na+-coupled butyrate uptake after 24 h (but not at 3 h;f not shown). The CS of L. casei also showed a marginal, but significant stimulatory effect, whereas the other two species were ineffective.
Fig. 4.
Long-term treatment with Lactobacillus culture supernatant (CS) stimulates SMCT1 function. Postconfluent IEC-6 cells were treated with CS (diluted 1:50 in DMEM) of L. acidophilus (LA), L. rhamnosus (LR), L. plantarum (LP), and L. casei (LC) for 24 h, and Na+-dependent 14C-butyrate uptake was measured. Values are means ± SE; n = 3. *Different from control, P < 0.001.
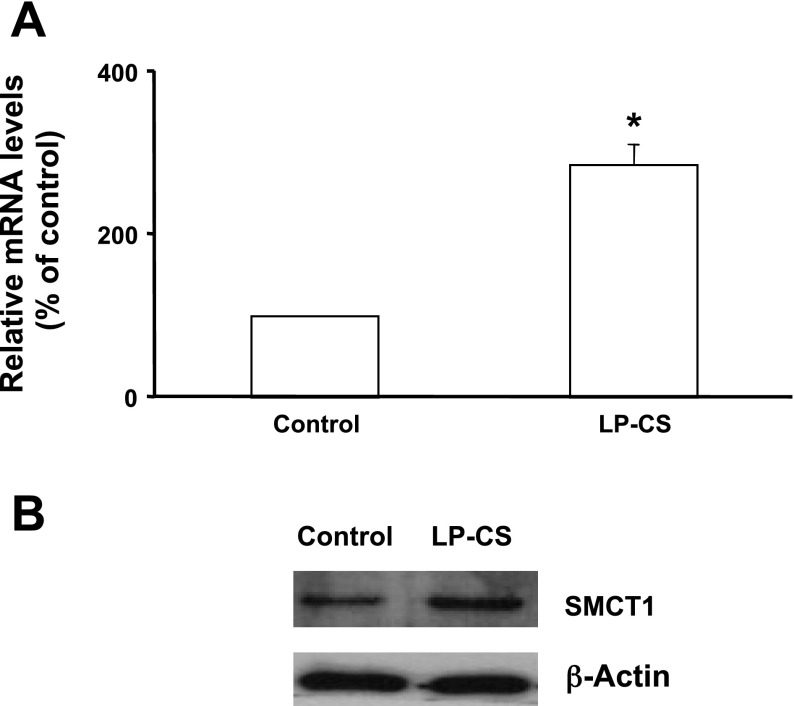
CS of LP increases SMCT1 mRNA and protein levels in IEC-6 cells.
We also measured SMCT1 mRNA levels in response to long-term treatment (24 h) of IEC-6 cells with LP-CS. LP-CS significantly increased SMCT1 mRNA (Fig. 5A) and protein levels (Fig. 5B).
Fig. 5.
LP-CS enhances SMCT1 mRNA and protein expression in IEC-6 cells. Cells treated with LP-CS for 24 h were used for RNA isolation and lysate preparation. SMCT1 mRNA (A) and protein levels (B) in control and LP-CS-treated cells are shown. Values are means ± SE; n = 3. *Different from control, P < 0.001.
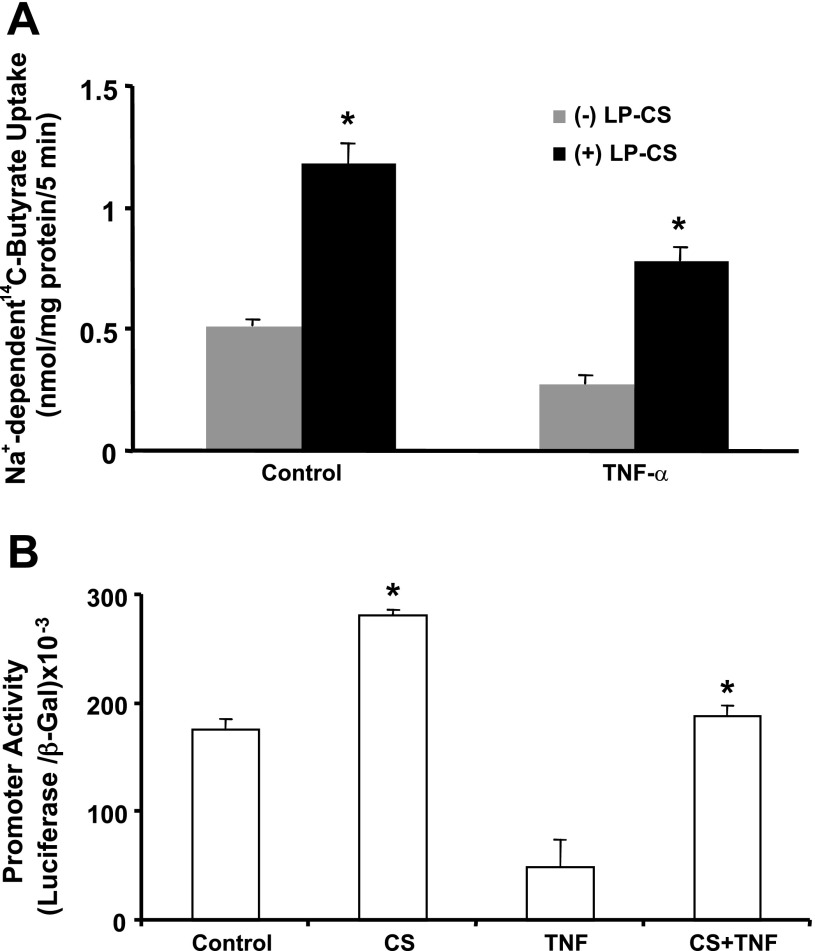
CS of LP blocks TNF-α-induced inhibition of Na+-coupled butyrate uptake in IEC-6 cells.
We next examined whether the CS of LP can counteract the inhibitory effects of TNF-α on SMCT1 function. Postconfluent IEC-6 monolayers were pretreated with LP-CS from the apical surface for 8 h, which was continued for another 16 h, with and without TNF-α (10 ng/ml) added basolaterally. Na+-dependent 14C-butyrate uptake was then measured. As before, TNF-α treatment significantly inhibited and LP-CS stimulated Na+-dependent 14C-butyrate uptake. Further, LP-CS also completely blocked the TNF-α-induced inhibition of butyrate uptake, which was even higher than the control value (Fig. 6A).
Fig. 6.
LP-CS stimulates SMCT1 promoter activity and attenuates TNF-α inhibition of SMCT1 function and promoter activity. A: postconfluent IEC-6 monolayers were pretreated with LP-CS from the apical surface for 8 h, which was continued for another 16 h, with and without TNF-α (10 ng/ml) added basolaterally. Na+-dependent 14C-butyrate uptake was then measured. B: Caco-2 cells were transfected with the SMCT1 promoter construct in pGL2. Twenty-four hours after transfection, cells were pretreated with LP-CS from the apical surface for 8 h, which was continued for another 16 h, with and without TNF-α (10 ng/ml) added basolaterally. Promoter activity was measured as luciferase units and normalized with the corresponding β-galactosidase units for transfection efficiency. Values are means ± SE; n = 4. *Different from control, P < 0.001.
CS of LP stimulates SMCT1 promoter activity and attenuates TNF-α inhibition of promoter activity in Caco-2 cells.
Since long-term treatment with LP-CS enhanced both SMCT1 function and expression, we next examined the effects of LP-CS on SMCT1 promoter activity. We have cloned a 2,015-bp fragment from the 5′-untranslated region of the human SMCT1 gene, which showed high promoter activity when transfected into human intestinal Caco-2 cells. We did not use IEC-6 cells for promoter studies, as the transfection efficiency of this promoter fragment was very low in this cell line. In Caco-2 cells, treatment with CS of LP (24 h) significantly enhanced promoter activity (Fig. 6B). These results suggest involvement of transcriptional mechanisms in probiotic-mediated stimulation of SMCT1. Furthermore, TNF-α (10 ng/ml, 24 h) inhibited promoter activity (∼65%), and this effect of TNF-α was blocked in cells pre- and cotreated with CS of LP (Fig. 6B).
Oral administration of live LP enhances intestinal SMCT1 expression.
Live LP or vehicle was gavaged to C57BL/6 mice. Twenty-four hours after gavage, mice were killed, intestine was removed, and mucosa scraped from ileal and colonic regions were utilized for RNA isolation. As shown in Fig. 7, both ileal and colonic SMCT1 mRNA levels were significantly increased in response to LP administration. Thus the efficacy of LP to enhance SMCT1 expression has also been observed in vivo.
Fig. 7.
Oral administration of live LP increases SMCT1 mRNA and protein in mouse ileum and colon. C57BL/6 mice were gavaged with live LP in sterile PBS containing 1 × 10−9 colony-forming units/ml of the bacteria. After 24 h, mice were killed, intestine removed, and mucosa was scraped from ileum and colon for RNA isolation and preparation of cell lysate. SMCT1 mRNA was measured by real-time PCR using gene specific primers (A) and SMCT1 protein levels by immunoblotting (B).
DISCUSSION
It is well established that butyrate, a product of colonic luminal fermentation, serves as the primary energy source for the colonic mucosa and also ameliorates mucosal inflammation and diarrhea associated with IBDs (5, 19, 20). The importance of butyrate is further evident from disturbed energy homeostasis of chronically inflamed mucosa in IBD, attributed to its impaired absorption (41). Butyrate anions are suggested to be primarily absorbed into the colonocytes by two distinct SCFA transporters: the H+-coupled MCT1 (18, 34) and the Na+-coupled SMCT1 (16, 40). Our laboratory and others have extensively characterized the MCT1-mediated H+-coupled butyrate transport and also the regulation of MCT1 in health and disease (1, 7, 9, 17, 35, 36). Recent studies have shown that mucosal deficiency of butyrate during intestinal inflammation is secondary to downregulation of MCT1 expression and function (40). However, studies pertaining to the functional role of SMCT1 in the normal intestine and regulation of its function and expression during inflammation are limited. The focus of this study was to examine the expression of SMCT1 in various regions of human and mouse intestine and to characterize its SCFA transport function in a model intestinal cell line. For this purpose, we have chosen rat intestinal IEC-6, a nontransformed cell line, because SMCT1 expression has been reported to be silenced in colon cancer cell lines (14). We have demonstrated that IEC-6 cells express a functional SMCT1, mediating butyrate uptake in a Na+-dependent manner. Therefore, this cell line may be utilized to study regulation of SMCT1 expression and function. We also examined whether the inhibition of butyrate uptake by the proinflammatory agent TNF-α, as reported in earlier studies (42), involves dysregulation of SMCT1 expression and function. We demonstrate that the proinflammatory cytokine TNF-α, produced abundantly during intestinal inflammation, downregulates SMCT1 expression in rat IEC-6 cells, which is also functionally reflected by a decrease in Na+-dependent butyrate uptake. TNF-α also inhibits SMCT1 promoter activity, suggesting that TNF-α downregulates SMCT1 expression via a transcriptional mechanism. Our results, therefore, support the previous studies suggesting that butyrate deficiency in inflamed tissues in IBD could result from impaired absorption of butyrate.
There is increasing evidence that normal gut microflora is altered in IBD and other forms of intestinal inflammation, with a reduction in the population of beneficial bacteria (38). Probiotics are a heterogeneous group of nonpathogenic microorganisms with diverse beneficial effects on host health. Studies of probiotics functionality in recent years have provided strong evidence that these beneficial bacteria counteract gastrointestinal inflammation by exerting several positive effects on epithelial cell functions. For example, they are known to improve barrier function, modulate inflammatory cytokine production, inhibit pathogen adherence, and modulate mucosal immune functions (3, 6, 25–27, 33, 44). Various earlier studies reported the efficacy of Lactobacillus species to counteract intestinal inflammatory responses (21, 44). Studies from our laboratory have suggested that probiotics could exert ameliorating effects on inflammation-associated diarrheal disorders by virtue of their ability to modulate electrolyte absorption. For example, we have shown that L. acidophilus stimulated Cl−/HCO3−(OH−) exchange activity and enhanced the expression of downregulated in adenoma (SLC26A3), the apical membrane Cl−/HCO3− (OH−) exchanger in in vitro and in vivo models (8, 30). In the present report, we have shown that the bacteria-free CS of the probiotic LP stimulates Na+-dependent butyrate uptake, enhances SMCT1 expression and promoter activity, and counteracts the inhibitory effects of TNF-α on SMCT1 expression and function. It is noteworthy that L. acidophilus, which stimulated Cl−/HCO3− (OH−) exchange activity, had no effects on the expression and function of SMCT1, suggesting species-specific effects of Lactobacillus in modulating expression and activity of different intestinal transporters. It is also important to note that the CS of LP was effective in blocking the inhibitory effects of TNF-α on SMCT1 activity and expression, and live bacteria were not necessary. These results, therefore, provide evidence for the role of probiotic-secreted soluble factors in mediating the beneficial effects of probiotics. Although some protective effects of probiotics require direct interactions of epithelial cells with live bacteria, various recent reports have shown that even the cellular components of these agents, or their secreted soluble effector molecules, may just be as effective and considerably safer for the host (8, 22, 28, 29, 39). The CS of L. plantarum, a common Lactobacillus species in the gut of healthy human, has recently been shown to exert anti-inflammatory effects in colonic epithelial cells by inhibiting multiple NF-κB pathways (28). In this regard, the SCFA butyrate is also known to ameliorate intestinal inflammation by virtue of its ability to regulate NF-κB pathway. Therefore, the effects of L. plantarum-secreted soluble factor(s) in stimulating intestinal butyrate absorption via modulation of SMCT1 expression and function under normal and inflammatory conditions could represent a distinct mechanism of the anti-inflammatory properties of this probiotic bacterium. It will be important in our future studies to characterize the soluble factor(s) in Lactobacillus CS that mediate their positive effects on intestinal absorptive processes. With regard to the mechanisms of counteracting the TNF-α effects, it is unlikely that the soluble factors in the LP-CS prevented the cytokine from binding to their receptors. This is because the supernatant was added apically, whereas TNF-α was added to the basolateral medium, as the receptors for TNF-α are known to be localized to the basolateral membranes of polarized IECs (32, 43). Therefore, the activity of the LP-CS to counteract the TNF-α effects on SMCT1 expression and function appears to involve mechanisms independent of TNF-α binding to its receptors.
SMCT1 (SLC5A8) was originally described as a candidate tumor suppressor gene (13). In view of the expression and apical membrane localization of the protein in colonic epithelial cells, SMCT1 has recently been suggested to play an important role in the transport of luminal butyrate (16). However, no studies to date have characterized SMCT1 function showing Na+-dependent butyrate transport in the native human intestine, or human intestinal cell lines (41). Although we found rat intestinal IEC-6 cells to express a functional SMCT1, our studies utilizing Caco-2 cells, or apical membrane vesicles prepared from human colonic mucosa of organ donors, did not show Na+-dependent uptake of 14C-butyrate. It is possible that this transporter is not active under basal conditions, but may be a regulatable SCFA-absorbing protein in response to either luminal or hormonal effector molecules. Therefore, the future establishment of the precise functional roles of SMCT1 in the human intestine will be important to further understand its roles in the normal physiology of the human intestine.
Overall, our results demonstrate expression of a functional SMCT1 protein in the apical membrane of rat intestinal IEC-6 cells, which appeared to be downregulated in inflammation. The soluble factor(s) present in the CS of LP specifically enhanced SMCT1 levels with a concomitant stimulation of its transport function to mediate Na+-dependent absorption of luminal butyrate. Furthermore, the soluble factors also attenuated the proinflammatory cytokine-induced dysregulation of SMCT1 expression and activity. Our data demonstrating stimulatory effects of probiotic Lactobacillus species on butyrate absorption support the proabsorptive role of probiotics. Furthermore, our data also extend the spectrum of beneficial effects of probiotics or probiotic-derived molecules on intestinal epithelial function and potential ameliorating effects in inflammatory disorders.
GRANTS
These studies were supported by the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK 54016 (P. K. Dudeja), DK 81858 (P. K. Dudeja), and the Program Project Grant DK 067887 (P. K. Dudeja, K. Ramaswamy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Alrefai WA, Tyagi S, Gill R, Saksena S, Hadjiagapiou C, Mansour F, Ramaswamy K, Dudeja PK. Regulation of butyrate uptake in Caco-2 cells by phorbol 12-myristate 13-acetate. Am J Physiol Gastrointest Liver Physiol 286: G197–G203, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Balamurugan K, Said HM. Role of reduced folate carrier in intestinal folate uptake. Am J Physiol Cell Physiol 291: C189–C193, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Barrett KE. Building better bugs to deliver biologics in intestinal inflammation. Gut 59: 427–428, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Bhattacharya S, Ray RM, Johnson LR. Prevention of TNF-alpha-induced apoptosis in polyamine-depleted IEC-6 cells is mediated through the activation of ERK1/2. Am J Physiol Gastrointest Liver Physiol 286: G479–G490, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol 72: 297–313, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Boirivant M, Strober W. The mechanism of action of probiotics. Curr Opin Gastroenterol 23: 679–692, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G, Dudeja PK. Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol 290: G30–G35, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: Involvement of NF-kappaB pathway. J Cell Biochem 103: 1452–1463, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 11. Cui D, Morris ME. The drug of abuse gamma-hydroxybutyrate is a substrate for sodium-coupled monocarboxylate transporter (SMCT) 1 (SLC5A8): characterization of SMCT-mediated uptake and inhibition. Drug Metab Dispos 37: 1404–1410, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dotan I, Rachmilewitz D. Probiotics in inflammatory bowel disease: possible mechanisms of action. Curr Opin Gastroenterol 21: 426–430, 2005. [PubMed] [Google Scholar]
- 13. Ganapathy V, Gopal E, Miyauchi S, Prasad PD. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem Soc Trans 33: 237–240, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, Prasad PD. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J 10: 193–199, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol 289: C846–C852, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci 78: 2419–2425, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Hadjiagapiou C, Borthakur A, Dahdal RY, Gill RK, Malakooti J, Ramaswamy K, Dudeja PK. Role of USF1 and USF2 as potential repressor proteins for human intestinal monocarboxylate transporter 1 promoter. Am J Physiol Gastrointest Liver Physiol 288: G1118–G1126, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279: G775–G780, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27: 104–119, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Hijova E, Chmelarova A. Short chain fatty acids and colonic health. Bratisl Lek Listy 108: 354–358, 2007. [PubMed] [Google Scholar]
- 21. Isaacs K, Herfarth H. Role of probiotic therapy in IBD. Inflamm Bowel Dis 14: 1597–1605, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Kataria J, Li N, Wynn JL, Neu J. Probiotic microbes: do they need to be alive to be beneficial? Nutr Rev 67: 546–550, 2009. [DOI] [PubMed] [Google Scholar]
- 23. Kolinska J, Lisa V, Clark JA, Kozakova H, Zakostelecka M, Khailova L, Sinkora M, Kitanovicova A, Dvorak B. Constitutive expression of IL-18 and IL-18R in differentiated IEC-6 cells: effect of TNF-alpha and IFN-gamma treatment. J Interferon Cytokine Res 28: 287–296, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Lambert DW, Wood IS, Ellis A, Shirazi-Beechey SP. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br J Cancer 86: 1262–1269, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15: 300–310, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Oelschlaeger TA. Mechanisms of probiotic actions: a review. Int J Med Microbiol 300: 57–62, 2010. [DOI] [PubMed] [Google Scholar]
- 27. Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 298: G807–G819, 2010. [DOI] [PubMed] [Google Scholar]
- 28. Petrof EO, Claud EC, Sun J, Abramova T, Guo Y, Waypa TS, He SM, Nakagawa Y, Chang EB. Bacteria-free solution derived from Lactobacillus plantarum inhibits multiple NF-kappaB pathways and inhibits proteasome function. Inflamm Bowel Dis 15: 1537–1547, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 127: 1474–1487, 2004. [DOI] [PubMed] [Google Scholar]
- 30. Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of slc26a3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rajgopal A, Sierra EE, Zhao R, Goldman ID. Expression of the reduced folate carrier SLC19A1 in IEC-6 cells results in two distinct transport activities. Am J Physiol Cell Physiol 281: C1579–C1586, 2001. [DOI] [PubMed] [Google Scholar]
- 32. Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology 130: 731–746, 2006. [DOI] [PubMed] [Google Scholar]
- 33. Resta-Lenert SC, Barrett KE. Modulation of intestinal barrier properties by probiotics: role in reversing colitis. Ann N Y Acad Sci 1165: 175–182, 2009. [DOI] [PubMed] [Google Scholar]
- 34. Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter in pig and human colon: its potential to transport L-lactate as well as butyrate. J Physiol 513: 719–732, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saksena S, Dwivedi A, Gill RK, Singla A, Alrefai WA, Malakooti J, Ramaswamy K, Dudeja PK. PKC-dependent stimulation of the human MCT1 promoter involves transcription factor AP2. Am J Physiol Gastrointest Liver Physiol 296: G275–G283, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saksena S, Theegala S, Bansal N, Gill RK, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms underlying modulation of monocarboxylate transporter 1 (MCT1) by somatostatin in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 297: G878–G885, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Segain JP, Raingeard de la Bletiere D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottiere HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut 47: 397–403, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut 53: 1–4, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 290: C1018–C1030, 2006. [DOI] [PubMed] [Google Scholar]
- 40. Thangaraju M, Cresci G, Itagaki S, Mellinger J, Browning DD, Berger FG, Prasad PD, Ganapathy V. Sodium-coupled transport of the short chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J Gastrointest Surg 12: 1773–1781, 2008. [DOI] [PubMed] [Google Scholar]
- 41. Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: A transport deficiency. Inflamm Bowel Dis 16: 684–695, 2010. [DOI] [PubMed] [Google Scholar]
- 42. Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, Mosnier JF, Galmiche JP, Shirazi-Beechey S, Segain JP. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology 133: 1916–1927, 2007. [DOI] [PubMed] [Google Scholar]
- 43. Treede I, Braun A, Jeliaskova P, Giese T, Fullekrug J, Griffiths G, Stremmel W, Ehehalt R. TNF-alpha-induced up-regulation of pro-inflammatory cytokines is reduced by phosphatidylcholine in intestinal epithelial cells. BMC Gastroenterol 9: 53, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vanderpool C, Yan F, Polk DB. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis 14: 1585–1596, 2008. [DOI] [PubMed] [Google Scholar]