Abstract
Sodium/hydrogen exchangers (NHEs) are a family of proteins that transport sodium ions into the cells by moving protons out of the cells. They play a major role in sodium absorption, cell volume regulation, and intracellular pH regulation. Three out of nine identified NHEs (NHE2, NHE3, and NHE8) are expressed on the apical membrane of intestinal epithelial cells. Glucocorticoids have been found to regulate NHE3 function in the intestine, but it is unknown if they have a similar function on NHE8 expression. Interestingly, high glucocorticoid levels in the intestine coincide chronologically with the change from high expression of NHE8 to high expression of NHE3. Studies were performed to explore the role of glucocorticoids on NHE8 expression during intestinal maturation. Brush-border membrane vesicles were isolated from intestinal epithelia, and Western blotting was performed to determine NHE8 protein expression of suckling male rats treated with methylpredisolone. Real-time PCR was used to quantitate NHE8 mRNA expression in rats and Caco-2 cells. Human NHE8 promoter activity was characterized through transfection of Caco-2 cells. Gel mobility shift assays (GMSAs) were used to identify the promoter sequences and the transcription factors involved in glucocorticoid-mediated regulation. Our results showed that the expression of NHE8 mRNA and protein was decreased in glucocorticoid-treated rats and human intestinal epithelial cells (Caco-2). The activity of the human NHE8 gene promoter transfected in Caco-2 cells was also reduced by glucocorticoid treatment. GMSAs suggested that the reduction in promoter activity in the presence of glucocorticoids was due to enhanced transcription factor Pax5 binding on the NHE8 proximal promoter region. In conclusion, this study showed that glucocorticoids inhibit NHE8 gene expression by increasing Pax5 binding on NHE8 gene promoter, suggesting an important role for Pax5 during intestinal maturation.
Keywords: intestine, sodium/hydrogen exchanger 8, Caco-2, steroids
sodium is an electrolyte that plays a vital role in the body. Sodium absorption is mediated by several transporter families, including sodium/hydrogen exchangers (NHEs). The NHEs are plasma membrane-bound antiporters that mediate the movement of extracellular sodium into cells in exchange for intracellular H+. To date, nine mammalian NHEs have been discovered. These proteins have broad physiological functions, including intracellular pH homeostasis, cell volume regulation, acid-base regulation, and electroneutral NaCl transport. In the intestine, five NHEs (NHE1–4 and 8) are identified, and they have different membrane localization and functions (30, 32). Targeted interruption of NHE1–4 results in various phenotypes ranging from decreased growth rate to impaired gastrointestinal function (2, 3, 11, 19, 21, 24). The latest identified NHE family member, NHE8, has distinct transporter kinetics compared with other NHEs. It is also highly expressed in the intestine before adulthood and plays an important role in sodium absorption during early development (29, 30).
NHE function is closely regulated by many physiological factors, including steroids (18, 33). Glucocorticoids are a class of steroid hormones that bind to the glucocorticoid receptor to regulate a wide range of body functions. In medical practice, glucocorticoids are frequently used as a part of anti-inflammatory or immune suppression therapies. Interestingly, glucocorticoids have been shown to increase intestinal sodium absorption by stimulating NHE3 expression in rat and rabbit (8, 18, 27). Because glucocorticoid levels increase after weaning (13) and intestinal NHE8 expression is significantly decreased after weaning, we hypothesize that elevated glucocorticoid levels may mediate the reduction of NHE8 expression during intestinal maturation.
In the presented study, we explored the effect of glucocorticoids on NHE8 expression in suckling rats and the mechanism of the effect on NHE8 expression in human intestinal cells (Caco-2). Our results showed that glucocorticoids reduced intestinal NHE8 expression in suckling rats and in Caco-2 cells by decreasing NHE8 gene transcription. We also found that inhibition of NHE8 gene transcription in Caco-2 cells involved the transcription factor Pax5. So, increased Pax5 interaction on NHE8 gene promoter may be the reason for suppression of NHE8 transcription. These findings suggest that Pax5 could be an important player in glucocorticoid-mediated transcriptional regulation of the intestinal NHE expression during intestinal maturation.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (17 days old) received subcutaneous injections of methylprednisolone (40 μg/g body wt; Sigma-Aldrich, St. Louis, MO) or vehicle (PBS) one time a day for 2 days. After the last injection (18 h), rats were killed, and intestinal mucosa was harvested for mRNA purification and brush-border membrane vesicle (BBMV) isolation. All animal work was approved by the University of Arizona Institutional Animal Care and Use Committee. The experiments were repeated four times with different groups of animals (4 rats/group).
Cell culture.
Human intestinal epithelial cells (Caco-2) were purchased from the American Type Culture Collection (ATCC) and cultured according to ATCC guidelines. Rat intestinal epithelial (RIE) cells were a gift from Dr. Raymond DuBois (Vanderbilt University School of Medicine, Nashville, TN) and were cultured in DMEM containing 10% FBS and 1% ampicillin/streptomycin. Cells were cultured at 37°C in a 95% air-5% CO2 atmosphere and passaged every 48–72 h. For dexamethasone (Sigma-Aldrich) treatment experiments, cells were incubated with 100 nM dexamethasone for 18 h before they were harvested.
Protein purification and Western blot analysis.
BBMVs were prepared from rat intestinal mucosa, as previously described (30), and 30 μg BBMV proteins were used for Western blot. Total protein was prepared from cultured Caco-2 cells with the RIPA method (8), and 40 μg total proteins were used for Western blot. Nuclear extracts were prepared from Caco-2 cells by a previously described method (31), and 15 μl nuclear protein were used for Western blot. NHE8 antibody was used as a 1:3,000 dilution in these experiments (30). A 1:5,000 dilution of the β-actin antiserum (Sigma, St. Louis, MO) was used to determine β-actin protein. A 1:2,000 dilution of the TATA binding protein (TBP) antiserum (Abcam, Cambridge, MA) was used to determine TBP. A 1:1,000 dilution of a goat polyclonal anti-Pax5 antiserum (SC-1974; Santa Cruz Biotechnology, Santa Cruz, CA) was used to detect Pax5 protein. Western detection was performed with the BM Chemiluminescence Western Blotting Kit (Roche Diagnostics, Indianapolis, IN). For NHE8 protein expression quantitation, a ratio of NHE8 protein intensity over β-actin protein intensity was used. For Pax5 protein expression quantitation, a ratio of Pax5 protein intensity over TBP protein intensity was used.
RNA purification and PCR analysis to detect NHE8 expression.
RNA was purified from rat intestinal mucosa and Caco-2 cells using Trizol (Invitrogen, Carlsbad, CA). Total RNA (500 ng) was reverse-transcribed using the qScript kit (Quanta Biosciences, Gaithersburg, MD), and 10% of the reverse transcription reaction was used for Real-Time PCR. TaqMan technology was used to determine the expression levels of NHE8 using rat and human NHE8 (hNHE8) and TBP primers from Applied Biosystems (Foster City, CA). Resulting data were analyzed using the comparative cycle threshold (Ct) method. The target gene cycle thresholds are adjusted relative to a calibrator (normalized Ct value obtained from control groups) and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin no. 2: Rev B “Relative Quantitation of Gene Expression”). TBP data were used as an endogenous reference to normalize expression levels. For detecting Pax5 mRNA expression, Pax5 specific primers were designed to cover Pax5 cDNA (NM_016734) from the 1,236- to 1,798-bp region. Forward primer (F1–1236, 5′-AGTATTCAGCCATGGCCTCG-3′) and reverse primer (R1–1798, 5′- AGCATCCAGAAGTCCAACGG-3′) were used for initial PCR with an expected PCR product of ∼563 bp. Forward primer (F2–1308, 5′-CTGACATCGGGAGCAGTGTG-3′) and reverse primer (R2–1692, 5′-TGGGCTCTCTGGCTATCTTCA-3′) were used for nest PCR with an expected PCR product of ∼385 bp. RT-PCR was performed using a previously described method (31).
Assembly of reporter gene constructs.
A series of progressively shorter hNHE8 promoter constructs were made in the pGL-3/basic luciferase reporter vector (Promega, Madison, MI) by restriction enzyme digestion or PCR as described previously (28). Briefly, the pGL3/−671 construct was made by subcloning a SacI-EcoRV fragment from pCR2.1-TOPO vector in SacI/XmaI-digested pGL3Basic vector. The pGL3b/−89 construct was made by SacI/SmaI digestion followed by blunt end reaction with Klenow treatment and subsequent ligation with T4 DNA ligase. The pGL3b/−32 construct was made by PCR. Mutations on the promoter construct were introduced by PCR with mutant primers. All promoter constructs ended at the same position at the 3′ end, and sequences were confirmed by sequencing.
Transient transfection and functional promoter analysis.
Caco-2 cells were cultured in 24-well plates. Promoter constructs containing various length of hNHE8 gene promoter region were used to identify the region that responded to glucocorticoid regulation. When cell density reached 60–70%, Caco-2 cells were transfected with the promoter constructs using Effectene (Qiagen, Valencia, CA) according to the manufacturer's instructions. Cells were harvested for promoter reporter assays 40 h after transfection. Promoter reporter assays were performed using a dual luciferase assay kit according to the manufacturer's instructions (Promega). Luciferase activities were measured with a luminometer (Femtomaster FB 12; Berthold Detection System, Pforzheim, Germany). Renilla luciferase activity driven by pRL-CMV (Promega) was used as an internal control to calculate the relative luciferase activity. To test the effect of dexamethasone on hNHE8 promoter activity, transfected cells were treated with 100 nM dexamethasone for 18 h before promoter reporter assay.
Nuclear protein isolation and gel mobility shift assay.
Nuclear extracts were prepared from Caco-2 cells treated with or without dexamethasone (100 nM, 18 h) by a previously described method (31). Synthetic double-stranded oligonucleotides were designed to span the targeted promoter region. DNA oligonucleotides were end-labeled with [32P]ATP, and 4 μg of nuclear extract were incubated with 1 ng of labeled probe in GMSA binding buffer containing 10 mM HEPES (pH 7.5), 1 mM EDTA, 50 mM NaCl, 1 mM dithiothreitol, and 50 μg/ml poly[d(I-C)]. After incubation at room temperature for 20–30 min, the mixture was electrophoresed on a 6% polyacrylamide gel in 0.25× Tris-boric acid-EDTA buffer. Gels were dried and exposed to X-ray film. For competition experiments, a 100-fold molar excess of unlabeled probe was added to the reaction mixture before the labeled probe was added. For supershift assays, the reaction mixtures were added to 4 μg of Ap2 (Abcam) or Pax5 (Santa Cruz Biotechnology) antibody.
Statistical analysis.
Student's t-test was used to compare values of the experimental data. P values <0.05 were considered significant.
RESULTS
Effect of methylprednisolone on the intestinal NHE8 protein expression in rats.
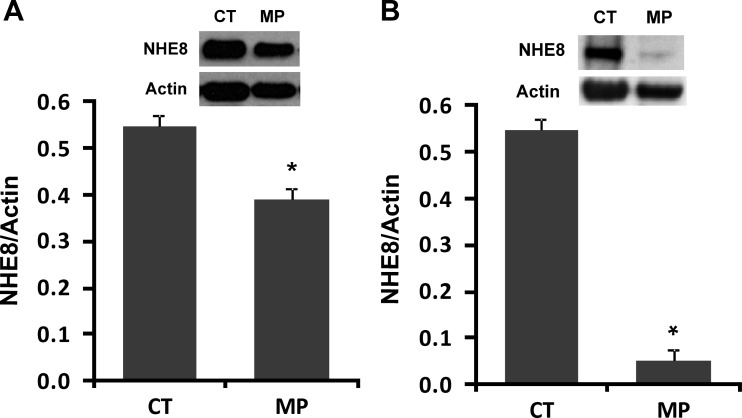
Male rats (17 days old) were given methylprednisolone subcutaneously (40 μg/kg body wt, one time a day for 2 days). After the last methylprednisolone administration (18 h), rats were killed, and BBMV was isolated from intestinal mucosa. BBMV protein was then used for Western blot to determine NHE8 protein abundance. NHE8 immunoreactive protein abundance was calculated by dividing the optical densities of the NHE8 band by that of the β-actin band. In the jejunum, methylprednisolone administration inhibited NHE8 immunoreactive protein abundance by ∼30% (0.55 ± 0.02 in control rats; 0.39 ± 0.02 in methylprednisolone rats, P ≤ 0.01, n = 4) (Fig. 1A). In the ileum, methylprednisolone significantly reduced NHE8 protein expression by ∼90% (0.55 ± 0.02 in control rats; 0.05 ± 0.03 in methylprednisolone rats, P ≤ 0.01, n = 4) (Fig. 1B).
Fig. 1.
Effect of methylprednisolone on the intestinal NHE8 protein expression in rats. Brush-border membrane vesicles (BBMVs) were isolated from the intestinal mucosa of control or methylprednisolone-treated rats. BBMV protein (30 μg) was loaded on SDS-PAGE and immunoblotted. Rat NHE8 antibody and β-actin antibody were used to detect NHE8 and β-actin, respectively. The expression of NHE8 protein was calculated by dividing the optical density of the NHE8 band over that of the β-actin band. Bars show the NHE8 protein expression indicated as means ± SE in the sum of 4 independent experiments. *P ≤ 0.01 for control (CT) groups vs. methylprednisolone (MP) groups. Inset: the corresponding Western blot image. A: effect of methylprednisolone on NHE8 protein expression in rat jejunum. B: effect of methylprednisolone on NHE8 protein expression in rat ileum.
Effect of methylprednisolone on the intestinal NHE8 mRNA expression in rats.
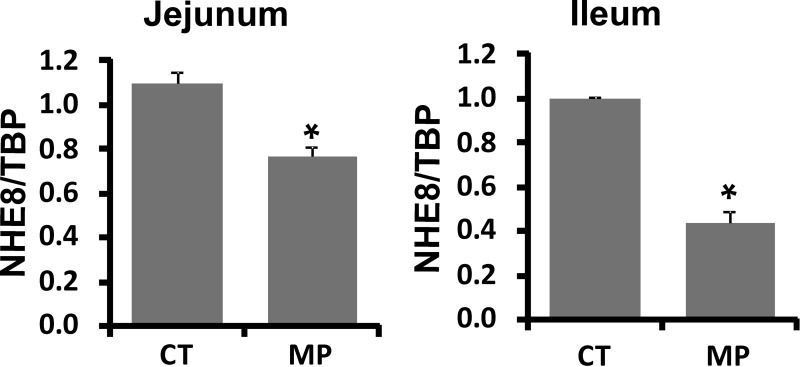
Because NHE8 protein abundance was reduced in the rat intestine, we tested NHE8 mRNA in the intestine. Mucosa from the jejunum and ileum were collected from rats 18 h after the last methylprednisolone administration. RNA was purified and used for Real-Time PCR to determine the abundance of NHE8 mRNA. Similar to protein expression changes, NHE8 mRNA expression in the jejunum decreased by ∼28% from 1.10 ± 0.02 in control rats to 0.79 ± 0.03 in methylprednisolone-treated rats (P ≤ 0.001, n = 4) (Fig. 2A). In the ileum, NHE8 mRNA expression decreased by ∼45% in methylprednisolone-treated rats (P ≤ 0.001, n = 4) (Fig. 2B).
Fig. 2.
Effect of methylprednisolone on the intestinal NHE8 mRNA expression in rats. RNA was isolated from the intestinal mucosa of control (CT) or methylprednisolone-treated (MP) rats and used for Real-Time PCR. NHE8 mRNA and TATA binding protein (TBP) mRNA were amplified with rat-specific NHE8 and TBP primers. The changes in NHE8 gene expression were analyzed by the comparative cycle threshold (Ct) method. Data are means ± SE from a total 18 rats (9 for the methylprednisolone group, 9 for the control group). *P ≤ 0.01 for the control group vs. the methylprednisolone group. Left: effect of methylprednisolone on NHE8 mRNA expression in rat jejunum. Right: effect of methylprednisolone on NHE8 mRNA expression in rat ileum.
Effect of dexamethasone on NHE8 expression in Caco-2 cells.
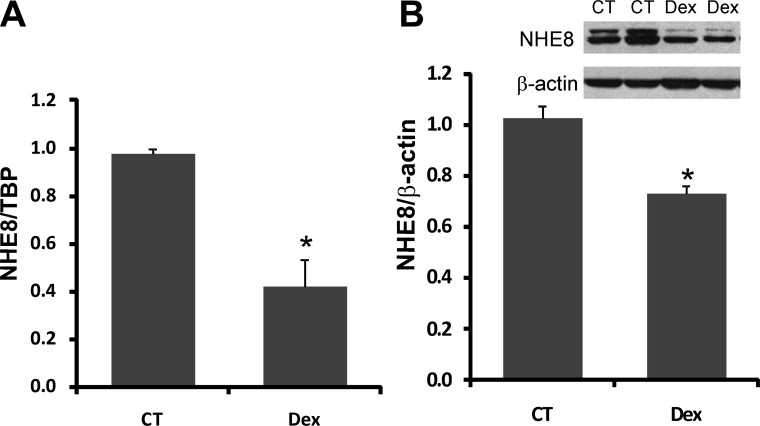
The expression of NHE8 mRNA in Caco-2 cells exposed to standard or dexamethasone-containing medium was assessed by Real-Time PCR. The expression of NHE8 protein in Caco-2 cells was determined by Western blotting. Dexamethasone treatment (100 nM, 18 h) reduced NHE8 gene expression by 60% in Caco-2 cells compared with untreated cells (n = 4, P < 0.01) (Fig. 3A). On the protein level, dexamethasone treatment also reduced NHE8 protein expression by ∼27% in Caco-2 cells (1.0 ± 0.05 in control cells; 0.73 ± 0.03 in dexamethasone cells; n = 3, P < 0.05) (Fig. 3B). The reduction in NHE8 expression levels by this synthetic glucocorticoid are in agreement with the observation in methylprednisolone-injected rats.
Fig. 3.
Effect of dexamethasone (Dex) on the endogenous NHE8 expression in human intestinal epithelial cells (Caco-2). Caco-2 cells were cultured in standard medium or dexamethasone-containing medium (100 ng/ml) for 18 h before harvest. RNA was isolated from these cells and used for RT-PCR. Real-Time PCR was performed with human NHE8 or TBP primers in separate reactions. Total protein was prepared from cells and used for Western blot. Results are means ± SE from 3–5 separate experiments. *P < 0.01 for control vs. dexamethasone treatment. A: effect of dexamethasone on NHE8 mRNA expression in Caco-2 cells. B: effect of dexamethasone on NHE8 protein expression in Caco-2 cells.
Effect of dexamethasone on hNHE8 gene promoter activity.
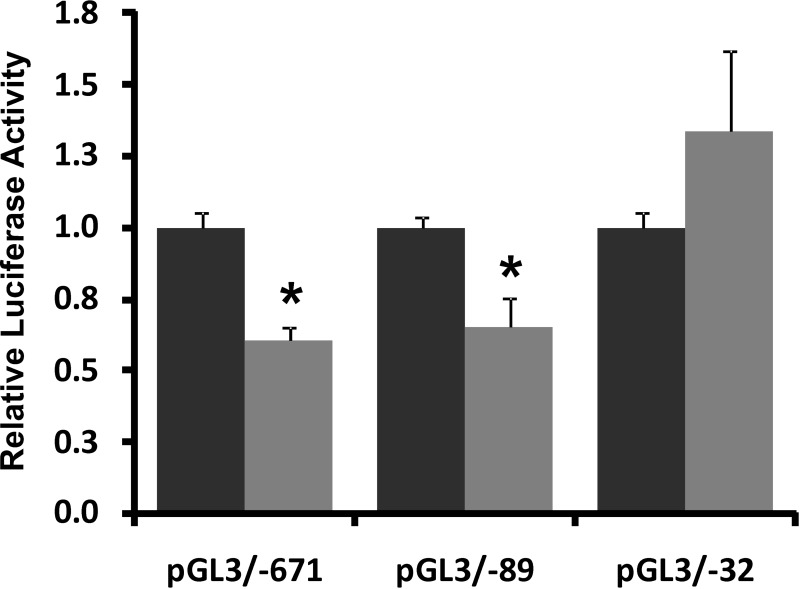
To explore if dexamethasone-mediated NHE8 expression downregulation is due to reduced gene transcription, Caco-2 cells were transfected with hNHE8 gene promoter constructs followed by 18 h of treatment with 100 nM dexamethasone before analyzing promoter activity. As shown in Fig. 4, NHE8 promoter activity was significantly reduced in dexamethasone-treated Caco-2 cells (n = 8; P < 0.01). Approximately 40% reduction of the promoter activity was seen in hNHE8 gene promoter constructs pGL3B/−671 and pGL3B/−89, but not in pGL3B/−32.
Fig. 4.
Effect of dexamethasone on human NHE8 gene promoter activity. Cells were cotransfected with pRL-CMV and pGL3 basic (pGL3b) or human NHE8 promoter constructs (pGL3b/−671, pGL3b/−89, or pGL3b/−32). Dexamethasone was applied 18 h before measuring promoter activities. Promoter activity is shown as a relative activity, a ratio of firefly luciferase activity driven by NHE8 promoter over Renilla luciferase activity driven by CMV promoter. The degree of inhibition is shown as the ratio of luciferase activity in dexamethasone-treated cells (shaded bars) over luciferase activity in vehicle-treated cells (solid bars). Results are means ± SE from 6 separate experiments. *P < 0.01 for control vs. dexamethasone treatment.
Identification of trans-factor and cis-element involved in dexamethasone response of the hNHE8 promoter.
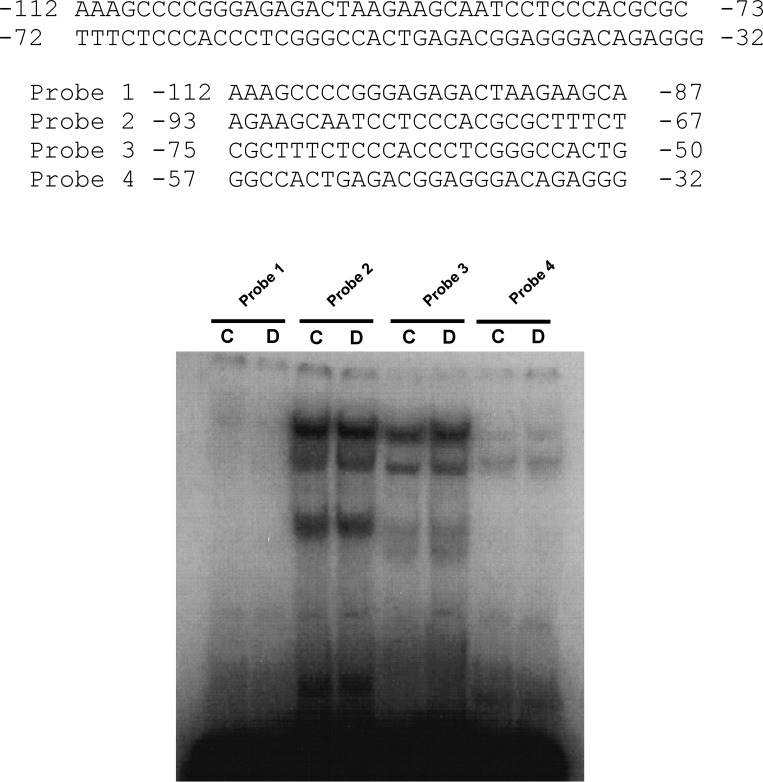
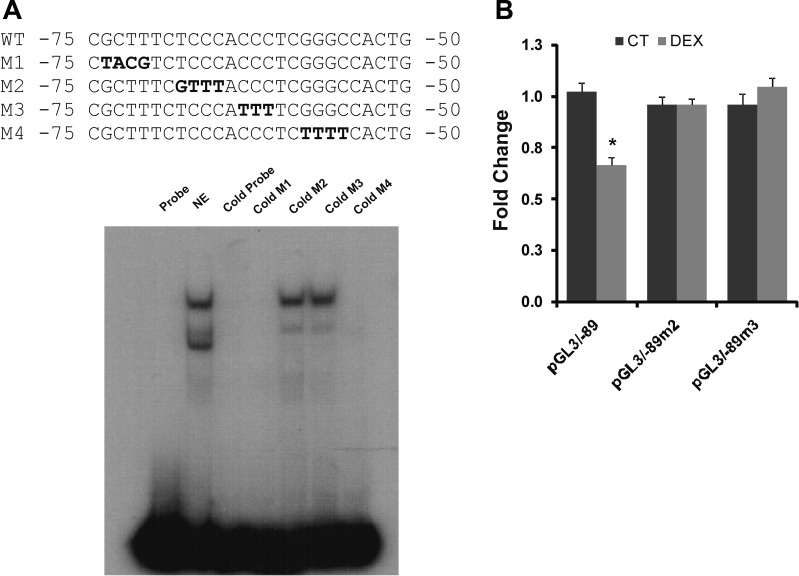
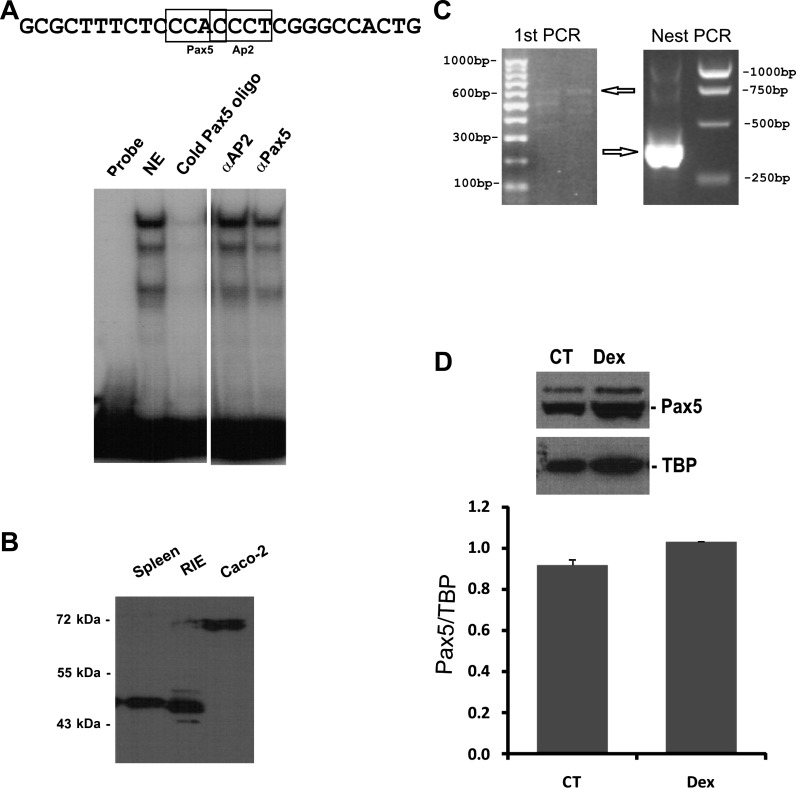
Gel mobility shift assay (GMSA) was used to study the DNA-protein interaction involved in the dexamethasone response. Because the glucocorticoid-responsive region is located between −89 to −32 bp of the hNHE8 promoter, the DNA sequences and the corresponding DNA-binding protein responsive to dexamethasone should also be within this part of the promoter region. We first identified the precise DNA sequences of the human intestinal NHE8 gene promoter by performing GMSAs using DNA oligos (oligo 1, 2, 3, and 4) designed between the −112/−32 bp region. As shown in Fig. 5, strong DNA-protein interactions were detected with labeled oligo 2 and oligo 3 probes. An increased DNA-protein interaction was only observed in the presence of oligo 3 probes, but not in the presence of oligo 2 probes. At the oligo 3 region, mutant oligos M1 and M4 could compete the protein binding on labeled oligo 3 probe but not mutant oligos M2 and M3 (Fig. 6A). Transfection with the mutant promoter constructs (pGL3b/−89M2 and pGL3b/−89M3) showed that the response of hNHE8 gene promoter to dexamethasone was abolished compared with wild-type promoter construct pGL3b/−89 (Fig. 6B). GMSAs indicated that cold Pax5 oligos competed the binding of nuclear protein to the labeled oligo 3 probes. Supershift indicated that Pax5 antibody partially blocked the formation of DNA-protein complex, whereas Ap2 antibody did not affect the DNA-protein interaction (Fig. 7A). Western blotting further confirmed the presence of Pax5 protein in Caco-2 cells and in RIE cells (Fig. 7B). RT-PCR also detected the expression of Pax5 mRNA in Caco-2 cells (Fig. 7C). Furthermore, dexamethasone treatment did not significantly alter Pax5 protein distribution in the nuclei in Caco-2 cells (Fig. 7D).
Fig. 5.
Narrowing down the DNA region involved in dexamethasone regulation. Nuclear proteins were isolated from dexamethasone-treated (D) and nontreated (C) Caco-2 cells. Probes 1, 2, 3, and 4 for gel mobility shift assays (GMSAs) were made with [32P]ATP end-labeled DNA oligos 1, 2, 3, and 4, respectively. The oligos span the proximal promoter region of the human NHE8 gene. Results shown are representative of 3 separate experiments.
Fig. 6.
Identification of the DNA sequences on the proximal promoter region of the human NHE8 gene involved in the dexamethasone regulation. A: identification of the DNA sequences involved in dexamethasone regulation. DNA oligo 3 was end-labeled with [32P]ATP and used as a probe in GMSAs. Nuclear protein was isolated from Caco-2 cells, and GMSAs were performed as described in materials and methods. Mutant oligos (oligos M1, M2, M3, and M4) were used to identify the precise DNA sequences of the DNA-protein interaction. Bold letters indicate the mutation site at the promoter region. GMSA image indicates that cold probe and mutant probes (M1, M4) compete with the protein binding on the labeled oligo 3 probe, but mutant oligos (M2 and M3) could not compete with the protein binding on the labeled oligo 3 probe. B: functional characterization of DNA-protein interacting sequences at NHE8 promoter region. Wild-type (WT) promoter construct (pGL3b/−89) or mutant promoter constructs (pGL3b/−89M2 and pGL3b/−89M3) were cotransfected with pRL-CMV in Caco-2 cells. Promoter reporter assay was performed 18 h after dexamethasone treatment. Promoter activity was calculated as relative activity of firefly luciferase activity driven by NHE8 promoter to Renilla luciferase activity driven by CMV promoter. Fold changes were used to compare the promoter activity in the dexamethasone group with that of the control group. Results are means ± SE from 4 separate experiments. *P ≤ 0.05 for control treatment (CT) vs. dexamethasone treatment.
Fig. 7.
Identification of Pax5 interaction with NHE8 promoter in Caco-2 cells. A: GMSAs identification of the interacting protein at the NHE8 promoter region. Oligo 3 was end labeled with [32P]ATP and used as a probe in GMSAs. Nuclear proteins were isolated from Caco-2 cells. Ap2 and Pax5 antibodies (4 μg/binding) were used for supershift experiments. GMSA indicated that Pax5 oligo competed with nuclear protein binding on the human NHE8 promoter and that Pax5 antibody (αPax5) partially blocked the interaction between Pax5 protein and the NHE8 promoter. In the same experiment, Ap2 antibody (αAp2) failed to compete with the DNA-protein interaction. B: Western blot detection of Pax5 expression in intestinal epithelial cells. Nuclear protein was extracted from Caco-2 cells and rat intestinal epithelial (RIE) cells, and 15 μg protein were used for Western blotting. Pax5 antibody (1:1,000 dilution) was used to detect Pax5 protein expression. Mouse spleen lysate was used as the positive control for Pax5 detection. C: PCR detection of Pax5 mRNA expression in Caco-2 cells. Total RNA was isolated from Caco-2 cells. RT-PCR was performed using human Pax5-specific primers. Primers F1–1236 and R1–1798 were used for the initial PCR. Primers F2–1308 and R2–1692 were used for nest PCR. Arrows indicate Pax5 PCR products. D: dexamethasone's effect on nuclear Pax5 abundance in Caco-2 cells. Nuclear protein was extracted from Caco-2 cells, and 15 μl protein extracts were used for Western blotting. Pax5 antibody and TBP antibody were used to detect Pax5 and TBP, respectively. The expression of Pax5 protein was calculated by the optical density of Pax5 band over that of TBP band. Bars show the Pax5 protein abundance in nuclear protein extracts. CT, nuclear protein from control cells; Dex, nuclear protein from dexamethasone-treated cells.
DISCUSSION
The intestinal epithelium plays an important role in barrier function and transport of water, nutrients, and electrolytes. Apically expressed NHEs (NHE2, NHE3, and NHE8) are major players for electroneutral sodium absorption, and they are expressed throughout the life of the organism. Among the three, NHE8 is the predominant isoform when the organism is young (7, 8, 30).
Glucocorticoids are involved in many physiological processes, such as organ maturation, immunological modulation, salt absorption, tissue repair, and cancers. They are also produced in many tissue, including the intestine. Excess or lack of glucocorticoids causes major health issues. In the intestine, glucocorticoids are known to accelerate epithelial maturation and to enhance sodium absorption (9, 10, 15, 20, 26). Although glucocorticoids have been shown to stimulate intestinal NHE3 activity (6, 16, 27), it was unclear whether glucocorticoids affect intestinal NHE8 expression. To determine the role of glucocorticoids on NHE8 expression, we used young rats as an in vivo model to characterize NHE8 expression in the intestine and Caco-2 cells as an in vitro model to study the mechanism of glucocorticoid regulation on NHE8 gene expression. Although previous studies showed that methylprednisolone stimulates NHE3 activity by enhancing NHE3 gene expression in the small intestine of the rats and rabbits (18, 27), we showed that methylprednisolone significantly reduced the expression of NHE8 in the small intestine of the young rats. At the BBMV protein level, methylprednisolone inhibited ∼30% and ∼90% of NHE8 protein distribution in the jejunum and the ileum, respectively. At the mRNA level, methylprednisolone inhibited ∼28% and ∼45% of NHE8 RNA expression in the jejunum and the ileum, respectively. These observations suggest that the effect of methylprednisolone on NHE8 expression may involve different mechanisms in the proximal and the distal small intestine. Methylprednisolone most likely inhibits jejunal NHE8 expression at the gene transcription level. On the other hand, methylprednisolone may regulate ileal NHE8 expression at both the gene transcription and the protein translation levels.
We also showed that dexamethasone treatment reduced endogenous NHE8 gene expression in Caco-2 cells to a level comparable to the reduction in in vivo studies. To further understand the mechanism of this inhibition, we conducted promoter reporter assay in Caco-2 cells transfected with hNHE8 gene promoter constructs. When transfected cells were treated with dexamethasone, NHE8 promoter activity was reduced by ∼40%. NHE8 mRNA abundance levels were also reduced by a similar amount in dexamethasone-treated Caco-2 cells. To identify the promoter region responsive to glucocorticoids, two shortened promoter constructs were transfected in Caco-2 cells. Dexamethasone treatment significantly reduced promoter activity in pGL3b/−89 but not in pGL3b/−32 promoter constructs in Caco-2 cells, suggesting the glucocorticoid-responsive region is located between hNHE8 gene promoter −89 to −32 bp.
To locate the DNA region dexamethasone uses to downregulate NHE8 gene expression, we isolated nuclear protein from Caco-2 cells and performed GMSAs with DNA oligos spanning the proximal promoter region −112/−32 bp of the human intestinal NHE8 gene. We found that two DNA-protein interaction complexes were formed on the hNHE8 proximal promoter region. However, only one DNA-protein interaction complex, the one at oligo 3 (−75/−50 bp), increased after treatment with dexamethasone. Mutations in this region not only abolished the DNA-protein interaction but also abolished the inhibition of hNHE8 promoter activity by dexamethasone. A search for transcription factors that could interact with 5′-CCACCCT-3′ using MatInspector (http://www.genomatix.de/products/MatInspector/) found that this sequence contains binding motifs for Ap2 and Pax5. We verified MatInspector results by GMSA. Ap2 antibody could not compete with or supershift the DNA-protein interaction at oligo 3. Meanwhile, cold Pax5 oligos completely blocked the interaction of nuclear protein binding on hNHE8 promoter, suggesting that there is DNA-protein interaction between Pax5 oligos and nuclear extracts from Caco-2 cells. GMSA with an anti-Pax5 antibody also partially blocked the DNA-protein interaction on the hNHE8 promoter. These results supported the involvement of Pax5. The mutations to the dexamethasone-sensitive region mentioned before were within the Pax5-binding site.
Pax5 is a member of the paired box (PAX) family of transcriptional factors. Nine members in the Pax family have been identified in mammals (5). These Pax proteins share a common structure that contains a DNA-binding domain and the paired domain. Pax proteins are important regulators in early development. Alterations in Pax expression are linked with neoplastic transformation. PAX5 gene encodes a peptide of 391 amino acids, and it is located on the chromosome 9q13 (23, 25). Pax5 has been shown to play important roles in the early stages of B-cell differentiation and central nervous system and testis development (1, 12, 22). Deregulation of Pax5 transcription is linked with the pathogenesis of lymphomas (4, 14). Recently, Pax5 was identified to regulate c-Met transcription in small-cell lung cancer (17). However, these publications have not mentioned whether Pax5 is expressed in the intestine and what roles Pax5 could play if it were expressed in the intestine. In our studies, we successfully detected Pax5 protein expression in Caco-2 cells and RIE cells. Therefore, Pax5 protein is indeed expressed in the intestinal epithelial cells, and it facilitates glucocorticoid-mediated regulation on NHE8 expression during intestinal maturation. Interestingly, the Pax5 protein detected by Western blotting in the human intestinal epithelial cells has a molecular weight of ∼72 kDa, which is larger than the Pax5 protein detected in RIE cells and in mouse spleen cells (∼46 kDa). This discrepancy might be due to different posttranslational modifications between humans and rodents.
In summary, we have shown that the intestinal NHE8 expression is reduced in rats treated with methylprednisolone. We also showed that dexamethasone inhibited endogenous NHE8 expression in Caco-2 cells to a similar degree. Furthermore, we identified that Pax5 is a key transcriptional factor involved in glucocorticoid-mediated NHE8 gene expression downregulation in Caco-2 cells. These studies, for the first time, directly demonstrated a role of Pax5 in the intestine.
GRANTS
This investigation was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-073638.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Adams B, Dorfler P, Aguzzi A, Kozmik Z, Urbanek P, Maurer-Fogy I, Busslinger M. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev 6: 1589–1607, 1992. [DOI] [PubMed] [Google Scholar]
- 2. Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol 276: C788–C795, 1999. [DOI] [PubMed] [Google Scholar]
- 3. Boivin GP, Schultheis PJ, Shull GE, Stemmermann GN. Variant form of diffuse corporal gastritis in NHE2 knockout mice. Comp Med 50: 511–515, 2000. [PubMed] [Google Scholar]
- 4. Busslinger M, Klix N, Pfeffer P, Graninger PG, Kozmik Z. Deregulation of PAX-5 by translocation of the Emu enhancer of the IgH locus adjacent to two alternative PAX-5 promoters in a diffuse large-cell lymphoma. Proc Natl Acad Sci USA 93: 6129–6134, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chi N, Epstein JA. Getting your Pax straight: Pax proteins in development and disease. Trends Genet 18: 41–47, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Cho JH, Musch MW, DePaoli AM, Bookstein CM, Xie Y, Burant CF, Rao MC, Chang EB. Glucocorticoids regulate Na+/H+ exchange expression and activity in region- and tissue-specific manner. Am J Physiol Cell Physiol 267: C796–C803, 1994. [DOI] [PubMed] [Google Scholar]
- 7. Collins JF, Kiela PR, Xu H, Zeng J, Ghishan FK. Increased NHE2 expression in rat intestinal epithelium during ontogeny is transcriptionally mediated. Am J Physiol Cell Physiol 275: C1143–C1150, 1998. [DOI] [PubMed] [Google Scholar]
- 8. Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937–C1946, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Eloranta JJ, Jung D, Kullak-Ublick GA. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol Endocrinol Baltimore 20: 65–79, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Gartner H, Graul MC, Oesterreicher TJ, Finegold MJ, Henning SJ. Development of the fetal intestine in mice lacking the glucocorticoid receptor (GR). J Cell Physiol 194: 80–87, 2003. [DOI] [PubMed] [Google Scholar]
- 11. Gawenis LR, Greeb JM, Prasad V, Grisham C, Sanford LP, Doetschman T, Andringa A, Miller ML, Shull GE. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem 280: 12781–12789, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Hagman J, Wheat W, Fitzsimmons D, Hodsdon W, Negri J, Dizon F. Pax-5/BSAP: regulator of specific gene expression and differentiation in B lymphocytes. Curr Topics Microbiol Immunol 245: 169–194, 2000. [DOI] [PubMed] [Google Scholar]
- 13. Henning SJ. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol Endocrinol Metab Gastrointest Liver Physiol 235: E451–E456, 1978. [DOI] [PubMed] [Google Scholar]
- 14. Iida S, Rao PH, Nallasivam P, Hibshoosh H, Butler M, Louie DC, Dyomin V, Ohno H, Chaganti RS, Dalla-Favera R. The t(9;14)(p13;q32) chromosomal translocation associated with lymphoplasmacytoid lymphoma involves the PAX-5 gene. Blood 88: 4110–4117, 1996. [PubMed] [Google Scholar]
- 15. Jung D, Fantin AC, Scheurer U, Fried M, Kullak-Ublick GA. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut 53: 78–84, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kandasamy RA, Orlowski J. Genomic organization and glucocorticoid transcriptional activation of the rat Na+/H+ exchanger Nhe3 gene. J Biol Chem 271: 10551–10559, 1996. [DOI] [PubMed] [Google Scholar]
- 17. Kanteti R, Nallasura V, Loganathan S, Tretiakova M, Kroll T, Krishnaswamy S, Faoro L, Cagle P, Husain AN, Vokes EE, Lang D, Salgia R. PAX5 is expressed in small-cell lung cancer and positively regulates c-Met transcription. Lab Invest 89: 301–314, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kiela PR, Guner YS, Xu H, Collins JF, Ghishan FK. Age- and tissue-specific induction of NHE3 by glucocorticoids in the rat small intestine. Am J Physiol Cell Physiol 278: C629–C637, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Ledoussal C, Woo AL, Miller ML, Shull GE. Loss of the NHE2 Na+/H+ exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol 281: G1385–G1396, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Mazancova K, Kucka M, Miksik I, Pacha J. Glucocorticoid metabolism and Na+ transport in chicken intestine. J Exp Zool 303: 113–122, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Moeser AJ, Nighot PK, Ryan KA, Simpson JE, Clarke LL, Blikslager AT. Mice lacking the Na+/H+ exchanger 2 have impaired recovery of intestinal barrier function. Am J Physiol Gastrointest Liver Physiol 295: G791–G797, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 401: 556–562, 1999. [DOI] [PubMed] [Google Scholar]
- 23. Pilz AJ, Povey S, Gruss P, Abbott CM. Mapping of the human homologs of the murine paired-box-containing genes. Mamm Genome 4: 78–82, 1993. [DOI] [PubMed] [Google Scholar]
- 24. Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. [DOI] [PubMed] [Google Scholar]
- 25. Stapleton P, Weith A, Urbanek P, Kozmik Z, Busslinger M. Chromosomal localization of seven PAX genes and cloning of a novel family member, PAX-9. Nat Genet 3: 292–298, 1993. [DOI] [PubMed] [Google Scholar]
- 26. Sundaram U, Wisel S, Coon S. Neutral Na-amino acid cotransport is differentially regulated by glucocorticoids in the normal and chronically inflamed rabbit small intestine. Am J Physiol Gastrointest Liver Physiol 292: G467–G474, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Wormmeester L, Sanchez de Medina F, Kokke F, Tse CM, Khurana S, Bowser J, Cohen ME, Donowitz M. Quantitative contribution of NHE2 and NHE3 to rabbit ileal brush-border Na+/H+ exchange. Am J Physiol Cell Physiol 274: C1261–C1272, 1998. [DOI] [PubMed] [Google Scholar]
- 28. Xu H, Chen H, Dong J, Li J, Chen R, Uno JK, Ghishan FK. Tumor necrosis factor-α downregulates intestinal NHE8 expression by reducing basal promoter activity. Am J Physiol Cell Physiol 296: C489–C497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109–116, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Xu H, Inouye M, Hines ER, Collins JF, Ghishan FK. Transcriptional regulation of the human NaPi-IIb cotransporter by EGF in Caco-2 cells involves c-myb. Am J Physiol Cell Physiol 284: C1262–C1271, 2003. [DOI] [PubMed] [Google Scholar]
- 32. Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005. [DOI] [PubMed] [Google Scholar]
- 33. Zallocchi M, Igarreta P, Calvo JC, Reboucas NA, Damasco MC. The mechanisms of brush border Na+/H+ exchanger activation by corticosteroids. Med Sci Monit 9: BR85–BR90, 2003. [PubMed] [Google Scholar]