Abstract
The Crohn's disease candidate gene, protein tyrosine phosphatase nonreceptor type 2 (PTPN2), has been shown to regulate epidermal growth factor (EGF)-induced phosphatidylinositol 3-kinase (PI3K) activation in fibroblasts. In intestinal epithelial cells (IECs), EGF-induced EGF receptor (EGFR) activation and recruitment of PI3K play a key role in regulating many cellular functions including Ca2+-dependent Cl− secretion. Moreover, EGFR also serves as a conduit for signaling by other non-growth factor receptor ligands such as the proinflammatory cytokine, IFN-γ. Here we investigated a possible role for PTPN2 in the regulation of EGFR signaling and Ca2+-dependent Cl− secretion in IECs. PTPN2 knockdown enhanced EGF-induced EGFR tyrosine phosphorylation in T84 cells. In particular, PTPN2 knockdown promoted EGF-induced phosphorylation of EGFR residues Tyr-992 and Tyr-1068 and led subsequently to increased association of the catalytic PI3K subunit, p110, with EGFR and elevated phosphorylation of the downstream marker, Akt. As a functional consequence, loss of PTPN2 potentiated EGF-induced inhibition of carbachol-stimulated Ca2+-dependent Cl− secretion. In contrast, PTPN2 knockdown affected neither IFN-γ-induced EGFR transactivation nor EGF- or IFN-γ-induced phosphorylation of ERK1/2. In summary, our data establish a role for PTPN2 in the regulation of EGFR signaling in IECs in response to EGF but not IFN-γ. Knockdown of PTPN2 directs EGFR signaling toward increased PI3K activation and increased suppression of epithelial chloride secretory responses. Moreover, our findings suggest that PTPN2 dysfunction in IECs leads to altered control of intestinal epithelial functions regulated by EGFR.
Keywords: EGF receptor, phosphatidylinositol 3′-kinase, IFN-γ, carbachol
phosphorylation and dephosphorylation of specific amino acid residues, such as tyrosine residues, represents a fundamental mechanism for the activation and inactivation of intracellular signaling molecules. Dephosphorylation is carried out by a large number of different protein phosphatases. One important family of such proteins is the protein tyrosine phosphatases. Members of this family play an essential role in the regulation of critical cell signaling events, i.e., proliferation, differentiation, and cell survival (46). The gene locus encoding one member of this protein family, protein tyrosine phosphatase nonreceptor type 2 (PTPN2), has recently been associated with Crohn's disease (CD), ulcerative colitis (UC), and Type 1 diabetes (17, 51a). Nevertheless, a functional role for PTPN2, also known as T cell protein tyrosine phosphatase, in the pathophysiology of CD or UC has not been identified. Among PTPN2 substrates are the epidermal growth factor receptor (EGFR) (27, 44, 45), the insulin receptor (18), and the signal transducers and activators of transcription 1 and 3, which are important signaling mediators of IFN-γ (42, 52, 57).
EGFR, also known as ErbB1, is a member of the ErbB receptor family and plays a major role in cell growth and wound repair. EGFR can be activated directly by a member of the EGF family of ligands or indirectly by transactivation stimulated by non-EGFR ligands such as carbachol (24) or IFN-γ (11, 48), which can occur in an EGFR ligand-dependent or -independent manner (12, 16, 48). Upon ligand binding, EGFR undergoes autophosphorylation via its tyrosine kinase activity and forms catalytically active homo- or heterodimers with other ErbB members such as ErbB2 (56). The recruitment of downstream signaling pathways by EGFR depends on the mode of receptor activation (i.e., direct vs. transactivation), the type of receptor dimer formed (homodimer vs. heterodimers), and the pattern of phosphorylation of specific EGFR tyrosine residues (19, 32). Important downstream signaling pathways, originating at the EGFR, include mitogen-activated protein kinases (MAPK), such as extracellular signal-regulated kinase 1/2 (ERK1/2), and phosphatidylinositol 3′-kinase (PI3K) (5, 32). In addition to the four major autophosphorylation sites of EGFR, Tyr-1068, Tyr-1086, Tyr-1148, and Tyr-1173, the minor autophosphorylation site Tyr-992 serves as a phospholipase C-γ binding site (34, 50). Although Tyr-1068 is a binding site for the adaptor protein, Grb2, that is involved in the recruitment of MAPK pathways (4, 26), increased phosphorylation of the residues Tyr-992 and Tyr-1068 has also been associated with elevated activity of PI3K and Akt (29, 45). Moreover, our laboratory has previously demonstrated that dephosphorylation of these residues by protein tyrosine phosphatase 1B mediates differential recruitment of PI3K by EGF but not G protein-coupled receptor-induced transactivation, even though both mechanisms of EGFR activation lead to inhibition of intestinal epithelial calcium-dependent chloride secretion (24, 28, 46, 47).
The intestinal epithelium is responsible for the uptake of nutrients and the absorption and secretion of electrolytes and fluids. The driving force for the intestinal secretion of ions and water is the secretion of chloride. Dysregulation of chloride secretion can result in the hypersecretion of chloride and excessive loss of salt and water into the lumen, as occurs in secretory diarrhea (35), or insufficient chloride secretion as occurs in cystic fibrosis (7) and chronic inflammatory states such as inflammatory bowel disease (IBD) (33, 50). Therefore, precise regulation of the intestinal epithelial chloride secretion is crucial.
Because PTPN2 is involved in regulating both EGFR and IFN-γ signaling in nonepithelial systems, this led us to the hypothesis that PTPN2 might be involved in modifying the ability of these pathways to regulate intestinal epithelial chloride secretion. Therefore, the aim of this study was to determine whether PTPN2 regulates EGFR-mediated signaling and, subsequently, epithelial chloride secretion in intestinal epithelial cells. We found that PTPN2 promotes EGF, but not IFN-γ-induced EGFR activation, and potentiates EGF-induced PI3K activity and inhibition of intestinal epithelial chloride secretion.
EXPERIMENTAL PROCEDURES
Materials.
Human IFN-γ (Roche, Mannheim, Germany), recombinant human EGF (Genzyme, Cambridge, MA), AG1478 (Sigma, St. Louis, MO), LY294002 (Sigma), carbachol (Sigma), mouse anti-PTPN2 antibody that detects both the 45-kDa and the 48-kDa splice forms of PTPN2 (45) (Calbiochem, San Diego, CA), phosphorylation site-specific rabbit anti-EGFR phosphotyrosine-antibodies (Biosource, Camarillo, CA), mouse anti-phosphotyrosine PY20 antibody (BD Biosciences, Santa Cruz, CA), rabbit anti-Akt antibody and mouse anti-EGFR-neutralizing antibody (clone LA1) (Upstate, Lake Placid, NY), rabbit anti-lamin A/C antibody, rabbit anti-PI3K p110 antibody and rabbit anti-ERK1/2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and rabbit anti-EGFR antibody, rabbit anti-phospho-ERK1/2 antibody and rabbit anti-phospho-Akt (Ser473) antibody (Cell Signaling Technologies, Danvers, MA) were obtained from the sources noted. All other reagents were of analytical grade and acquired commercially.
Cell culture.
Human colonic T84 epithelial cells were cultured in a humidified atmosphere with 5% CO2 as described previously (48) in Dulbecco's modified Eagle's/F-12 medium (Mediatech, Herndon, VA) supplemented with 5% newborn calf serum. Cells were separated by trypsinization and 1 × 106 cells were seeded onto 12 mm Millicell-HA semipermeable filter supports (Millipore, Bedford, MA). T84 cells used for the experiments were between passages 22 and 35. When seeded on filters, T84 cells develop monolayers with the polarized phenotype of native colonic crypt epithelial cells (25). According to the localization of their receptors, IFN-γ (1,000 U/ml), EGF (100 ng/ml), and carbachol (100 μM) were added basolaterally. EGFR inhibitor AG1478 (10 μM) and PI3K inhibitor LY294002 (20 μM) were added bilaterally.
Preparation of cell lysates.
After stimulation, T84 cells were washed three times with ice-cold Ringer solution (140 mM Na+, 5.2 mM K+, 1.2 mM Ca2+, 0.8 mM Mg2+, 120 mM Cl−, 25 mM HCO3−, 2.4 mM H2PO4−, 0.4 mM HPO42−, 10 mM glucose) and lysed in ice-cold lysis buffer (1% Triton X-100, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml antipain, 100 μg/ml phenylmethylsulfonylfluoride, 1 mM sodium vanadate, 1 mM sodium fluoride, and 1 mM EDTA in PBS) for 45 min. T84 cells were scraped from the filters, transferred to a microcentrifuge tube, and centrifuged for 10 min at 13,000 g. Cell lysate supernatants were assayed for protein content via a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) and adjusted to ensure that each sample contained an equal amount of protein. An aliquot of each lysate was mixed with an equal volume of 2× gel loading buffer (50 mM Tris, pH 6.8, 2% SDS, 200 mM dithiothreitol, 40% glycerol, 0.2% bromophenol blue) and boiled for 4 min.
Immunoprecipitation.
Each sample was incubated with the immunoprecipitating antibody overnight at 4°C followed by incubation with protein A agarose beads for 1 h at 4°C. The beads were centrifuged for 3 min at 13,000 g at 4°C, washed three times with ice-cold Ringer, resuspended in 2× gel loading buffer, and boiled for 4 min.
Western blotting.
Proteins were separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Millipore). Membranes were blocked with 1% blocking solution and an appropriate concentration of primary antibody was added in 1% blocking buffer over night. Membranes were washed with Tris-buffered saline containing 1% Tween 20 (1% TBST) for 1 h, horseradish peroxidase-labeled secondary anti-mouse or anti-rabbit IgG antibody (BD Biosciences, Santa Cruz, CA) in 1% blocking solution (1:2,500) was added for 30 min and membranes were washed for 1 h with 1% TBST. Finally, immunoreactive proteins were detected using an enhanced chemiluminescence detection kit (GE Healthcare, Little Chalfont, UK). Densitometric analysis of Western blots was performed by NIH Image software.
Electrophysiological studies.
T84 cell monolayers were mounted in Ussing chambers with a window area of 0.6 cm2 and bathed in oxygenated (95% O2-5% CO2) Ringer solution at 37°C. By using short-circuit current (Isc) the monolayers were continuously voltage-clamped to zero potential difference. A specific finding for T84 cells is that changes in Isc (ΔIsc) under these conditions are exclusively due to changes in electrogenic chloride secretion (13).
Transepithelial electrical resistance (TER) across T84 monolayers was assessed by voltohmmeter (WPI, Sarasota, FL) and companion electrodes (Millipore). Measurements were calculated in ohms times centimeters squared.
siRNA transfection.
2 × 106 T84 cells were seeded 3 days before transfection and grown to 50–70% confluency in T75 flasks. Three different annealed Silencer predesigned siRNA oligonucleotides targeting PTPN2 were obtained from Applied Biosystems (Foster City, CA). For transfection reactions, a total amount of 100 pmol of the three gene-specific small interfering RNA (siRNA) oligonucleotides was transfected into T84 cells by using the Amaxa nucleofector system (Amaxa, Gaithersburg, MD) according to manufacturer's instructions. After transfection, T84 cells were cultured on filter membranes for 48 h before further treatment for Western blotting experiments and for 96 h for electrophysiological studies. A nonspecific control siRNA SMARTpool (100 pmol, Upstate Biotechnology/Dharmacon, Chicago, IL) was used as a negative control.
Statistical analysis.
Data are presented as means ± SE for a series of n experiments. Data are expressed as a percentage of the respective control. Statistical analysis was performed by ANOVA followed by Student-Newman-Keuls post hoc test. P values < 0.05 were considered significant.
RESULTS
Knockdown of PTPN2 enhances EGF-induced EGFR tyrosine phosphorylation.
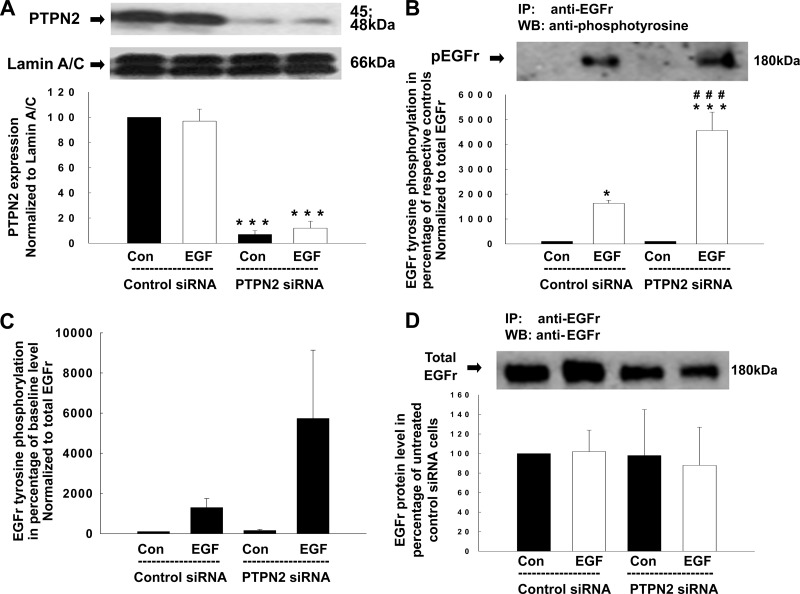
EGFR regulates a variety of intracellular signaling pathways. We have previously validated that treatment with EGF at a concentration of 100 ng/ml for 5 min is optimal to cause EGFR tyrosine phosphorylation in T84 cells (24, 27, 29, 45, 47). Using human fibroblasts, it has been elucidated that PTPN2 dephosphorylates, and thereby inactivates, EGFR following EGF treatment (45). Therefore, our first goal was to investigate whether PTPN2 also regulates EGF-induced EGFR tyrosine phosphorylation in human T84 intestinal epithelial cells. To address this issue, we performed PTPN2 knockdown studies and analyzed EGFR tyrosine phosphorylation in EGF-treated T84 cells by Western blotting. T84 cells were transfected with either nonspecific control siRNA or specific siRNA targeting PTPN2 and subsequently stimulated with EGF (100 ng/ml) for 5 min. As shown in Fig. 1A, PTPN2-specific siRNA caused a dramatic reduction of PTPN2 protein expression. Densitometric analysis revealed a maximal decrease in PTPN2 protein of 93 ± 3% (Fig. 1A). Equivalent levels of the loading control, lamin A/C, confirmed that the transfection had no nonspecific effects on cellular protein expression (Fig. 1A). To study the effect of PTPN2 knockdown on EGF-induced EGFR phosphorylation, whole cell lysates were immunoprecipitated with an anti-EGFR antibody and immunoblotted for phosphotyrosine. Densitometric analysis of Western blots revealed that EGF treatment of control siRNA transfected T84 cells increased EGFR tyrosine phosphorylation significantly, and this effect was further enhanced in PTPN2-deficient cells (P < 0.001; Fig. 1B). In Fig. 1B, we assessed the level of EGF-induced EGFR phosphorylation in relation to the respective untreated control cells. Therefore, the extent of EGFr phosphorylation in untreated cells transfected with control siRNA or PTPN2 siRNA, respectively, was regarded as 100% and the extent of EGFR phosphorylation in EGF-treated cells transfected with control siRNA or PTPN2 siRNA respectively, was calculated as a percentage of each of the respective controls. We then performed a secondary analysis to demonstrate the magnitude of EGFR induction from baseline. As the baseline level, we used the extent of EGFR phosphorylation in untreated cells that were transfected with control siRNA constructs. All of the other EGFR phosphorylation values were then calculated in relation to the extent of EGFR phosphorylation in untreated cells that had been transfected with control siRNA. We found that EGFR phosphorylation in untreated PTPN2-deficient cells differed only to a very limited extent from the level of EGFR phosphorylation in untreated PTPN2-competent cells, especially when this difference was compared with the massive increase in EGFR phosphorylation in response to EGF (Fig. 1C). In contrast and as shown by densitometric analysis, PTPN2 knockdown did not significantly affect the expression of EGFR protein (Fig. 1D). EGF treatment had no effect on PTPN2, lamin A/C, or EGFR protein levels (Fig. 1, A and D). These data demonstrate that PTPN2 likely dephosphorylates the EGF-activated EGFR in T84 intestinal epithelial cells.
Fig. 1.
Protein tyrosine phosphatase nonreceptor type 2 (PTPN2)-specific small interfering RNA (siRNA) decreases PTPN2 protein expression and promotes epidermal growth factor (EGF)-induced EGF receptor (EGFr) tyrosine phosphorylation. T84 cells were transfected with either nonspecific siRNA or PTPN2-specific siRNA and subsequently treated with EGF (100 ng/ml) for 5 min. A: representative Western blots and their densitometric analysis below show protein levels of PTPN2 and the nuclear envelope protein lamin A/C, used as a loading control (Con), in whole cell lysates (n = 3). B–D: whole cell lysates were immunoprecipitated with an anti-EGFr antibody, blotted for phosphotyrosine, stripped, and reblotted for EGFr. B: representative Western blot shows tyrosine-phosphorylated EGFr. The histogram below demonstrates densitometric analysis of 3 similar experiments. Data are presented as percentage of the respective controls (EGF-treated control siRNA cells as percentage of untreated control siRNA cells and EGF-treated PTPN2 siRNA cells as a percentage of untreated PTPN2 siRNA cells). C: we performed a secondary densitometric analysis to assess the effect of PTPN2 siRNA on EGFr phosphorylation. This analysis demonstrates the magnitude of EGFr phosphotyrosine induction above that present in untreated cells that were transfected with control siRNA constructs, described in the y-axis legend as “baseline.” All of the other EGFr phosphorylation values in C were then calculated in relation to the extent of EGFr phosphorylation in these cells. D: representative Western blot and the respective densitometric analysis shows EGFr protein level (n = 3). IP, immunoprecipitation; WB, Western blotting. Data are presented as a percentage of the respective controls. Significant difference vs. the respective control: *P < 0.05, ***P < 0.001. ###P < 0.001 vs. EGF-treated T84 cells transfected with control siRNA.
EGFR phosphotyrosine residues Tyr-992 and Tyr-1068 are targets of PTPN2.
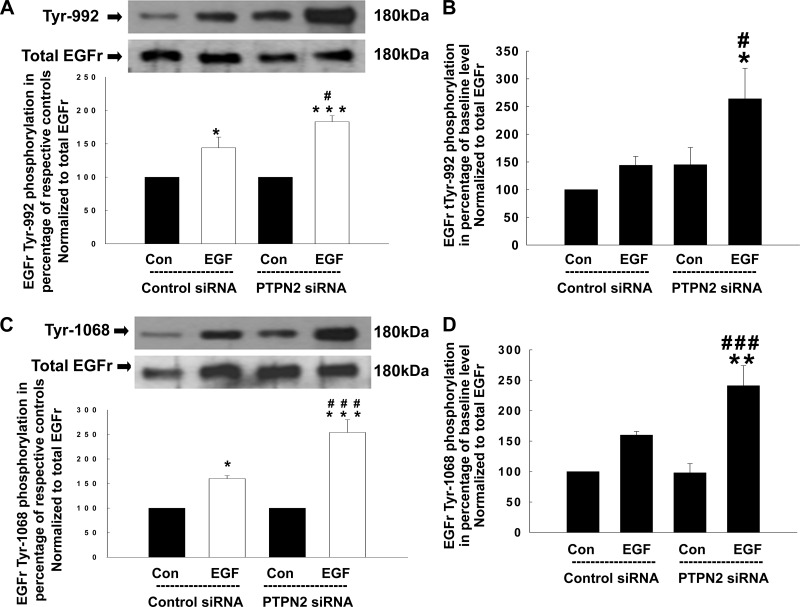
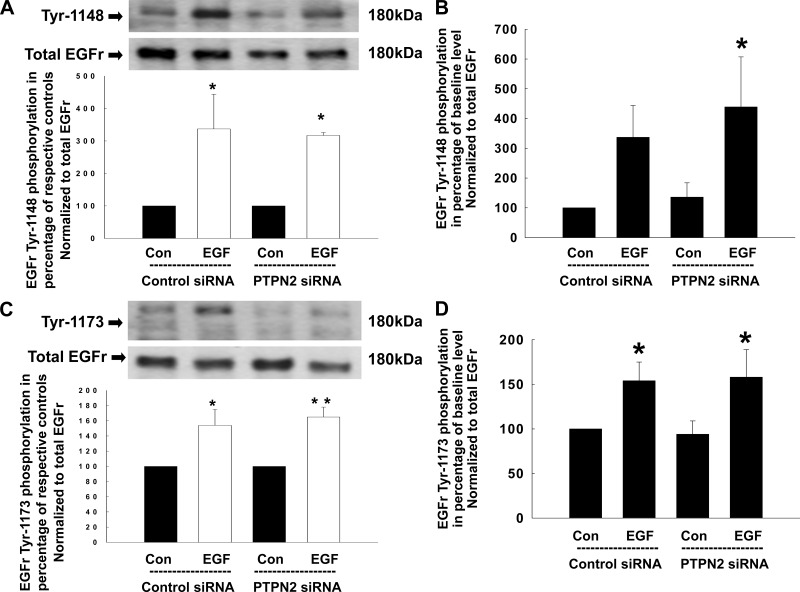
Having shown that PTPN2 knockdown promotes EGF-induced EGFR tyrosine phosphorylation, we next investigated which specific EGFR tyrosine residues are targeted by the phosphatase. T84 cells were transfected either with control siRNA or PTPN2 siRNA, treated with EGF (100 ng/ml) for 5 min and analyzed for EGFR phosphorylation patterns using phosphotyrosine-specific antibodies by Western blotting. As shown in Fig. 2A, EGF treatment alone significantly increased phosphorylation of Tyr-992, and this effect was further enhanced in PTPN2-deficient cells (144 ± 16 vs. 183 ± 9%; P < 0.05). Interestingly, loss of PTPN2 led to an increase in baseline EGFR Tyr-992 phosphorylation to a similar extent as EGF treatment of PTPN2-competent cells. However, this effect was statistically not significant (Fig. 2B). Although phosphorylation of Tyr-1068 was also induced in response to EGF and further enhanced by PTPN2 knockdown (160 ± 6 vs. 254 ± 26%; P < 0.001; Fig. 2C), baseline Tyr-1068 phosphorylation levels were not altered in PTPN2-deficient cells (Fig. 2D). In contrast, PTPN2 knockdown did not significantly affect baseline phosphorylation or EGF-induced phosphorylation of Tyr-1148 (Fig. 3, A and B) or Tyr-1173 (Fig. 3, C and D) in T84 cells as assessed by densitometric analysis of Western blots. Of interest, the phosphorylated form of Tyr-1173 seemed to appear as a double band. However, the lower band was detected at a molecular weight of ∼130 kDa (EGFR: 180 kDa) and is thus unlikely to represent an EGFR doublet, although we cannot exclude that it may represent a truncated form of the EGFR. These data demonstrate that PTPN2 activity selectively modulates the pattern of EGFR tyrosine phosphorylation and may, subsequently, play a role in the regulation of downstream signaling originating at the EGFR.
Fig. 2.
Loss of PTPN2 enhances phosphorylation of the EGFr tyrosine residue Tyr-992 and Tyr-1068 in response to EGF. Either control siRNA- or PTPN2 siRNA-transfected T84 cells were treated with EGF (100 ng/ml) for 5 min. Analyses were performed using whole cell lysates. A: representative Western blots show phosphorylation of the EGFr residue Tyr-992 and expression of total EGFr. Below is the densitometric analysis of 3 similar experiments. Data are presented as percentage of the respective controls. B: secondary densitometric analysis demonstrates the magnitude of Tyr-992 induction from baseline level (analysis was performed as described in Fig. 1C) in percentage of untreated cells transfected with control siRNA. C: phosphorylation of the EGFr residue Tyr-1068 in response to EGF and expression of total EGFr is demonstrated by representative Western blots. The densitometric analysis of 5 similar experiments is shown in the histogram below. Data are presented as percentage of the respective controls. D: secondary densitometric analysis demonstrates the magnitude of Tyr-1068 induction from baseline level (analysis was performed as described in Fig. 1C) in percentage of untreated cells transfected with control siRNA. Significant difference vs. the respective control: *P < 0.05, **P < 0.01, ***P < 0.001. #P < 0.05, ###P < 0.001 vs. EGF treatment of T84 cells transfected with control siRNA.
Fig. 3.
Phosphorylation of the EGFr tyrosine residue Tyr-1148 and Tyr-1173 in response to EGF is not affected by PTPN2 knockdown. Either control siRNA- or PTPN2 siRNA-transfected T84 cells were treated with EGF (100 ng/ml) for 5 min. Analyses were performed using whole cell lysates. A: representative Western blots show phosphorylation of the EGFr residue Tyr-1148 and expression of total EGFr. Below is the densitometric analysis of 3 similar experiments. Data are presented as percentage of the respective controls. B: secondary densitometric analysis demonstrates the magnitude of Tyr-1148 induction from baseline level (analysis was performed as described in Fig. 1C) in percentage of untreated cells transfected with control siRNA. C: phosphorylation of the EGFr residue Tyr-1173 in response to EGF and expression of total EGFr is demonstrated by representative Western blots. The densitometric analysis of 4 similar experiments is shown by the histogram below. Data are presented as percentage of the respective controls. D: secondary densitometric analysis demonstrates the magnitude of Tyr-1173 induction from baseline level (analysis was performed as described in Fig. 1C) in percentage of untreated cells transfected with control siRNA. Significant difference vs. the respective control: *P < 0.05, **P < 0.01.
PTPN2 regulates EGF-induced PI3K, but not ERK1/2 activation.
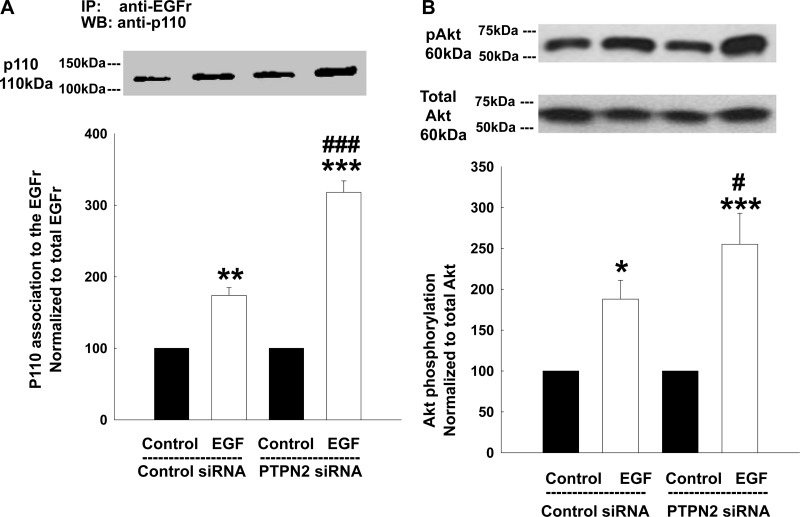
Having demonstrated that PTPN2 likely dephosphorylates EGFR phospho-residues Tyr-992 and Tyr-1068, we next set out to study the functional consequences of this finding. The selective activation and recruitment of pathways downstream of EGFR depends on the tyrosine phosphorylation pattern of the receptor (32). Two important signaling pathways operating downstream of the EGFR are the PI3K/Akt and the MAPK pathways (5, 32). Since both Tyr-992 and Tyr-1068 of EGFR have been associated with increased PI3K and Akt activity (29, 45), we first investigated a role for PTPN2 in regulating the activation of PI3K and its well-described downstream target, Akt (14, 17), in response to EGF. PI3K consists of a regulatory p85- and a catalytic p110-subunit. To become active, the p85-subunit must bind to specific phosphotyrosine sites of a receptor tyrosine kinase, such as EGFR. This binding allows the recruitment and activation of the p110-subunit (12, 22, 53, 54). Therefore, activation of PI3K in response to EGF can be assessed as the amount of the p110-subunit in immunoprecipitates of tyrosine-phosphorylated proteins. In this case, we examined levels of EGF-induced p110 association with EGFR by stripping the same membranes that we had previously used for EGFR analysis (cf. Fig. 1, B and D), and reprobed them for p110. As expected, EGF treatment (100 ng/ml, 5 min) increased p110 association with the EGFR in control siRNA-transfected cells (Fig. 4A). This effect was significantly enhanced in PTPN2-deficient cells (P < 0.001; Fig. 4A). Correspondingly, phosphorylation of Akt, as measured in whole cell lysates, was also elevated in EGF-treated control cells and even further promoted in PTPN2 siRNA-transfected cells (P < 0.05; Fig. 4B). These findings indicate that PTPN2, likely through effects on EGFR residues Tyr-992 and Tyr-1068, regulates EGF-induced activation of PI3K.
Fig. 4.
PTPN2-deficient T84 cells show enhanced PI3K activity and phosphorylation of Akt in response to EGF. T84 cells transfected with either control siRNA or PTPN2 siRNA were treated with EGF (100 ng/ml) for 5 min. A: whole cell lysates were immunoprecipitated with an anti-EGFr antibody. Membranes as of (Fig. 1, B and D) were stripped and reprobed for p110. Representative Western blot and densitometric analysis show association of the p110 subunit of PI3K to the EGFr in response to EGF (n = 3). B: representative Western blots show phosphorylation and expression of the PI3K downstream marker, Akt, in response to EGF in whole cell lysates. The densitometric analysis of 7 similar experiments is shown by the histogram below. Data are presented as percentage of control. Significant difference vs. the respective control: *P < 0.05, **P < 0.01, ***P < 0.001. #P < 0.05, ###P < 0.001 vs. EGF treatment of T84 cells transfected with control siRNA.
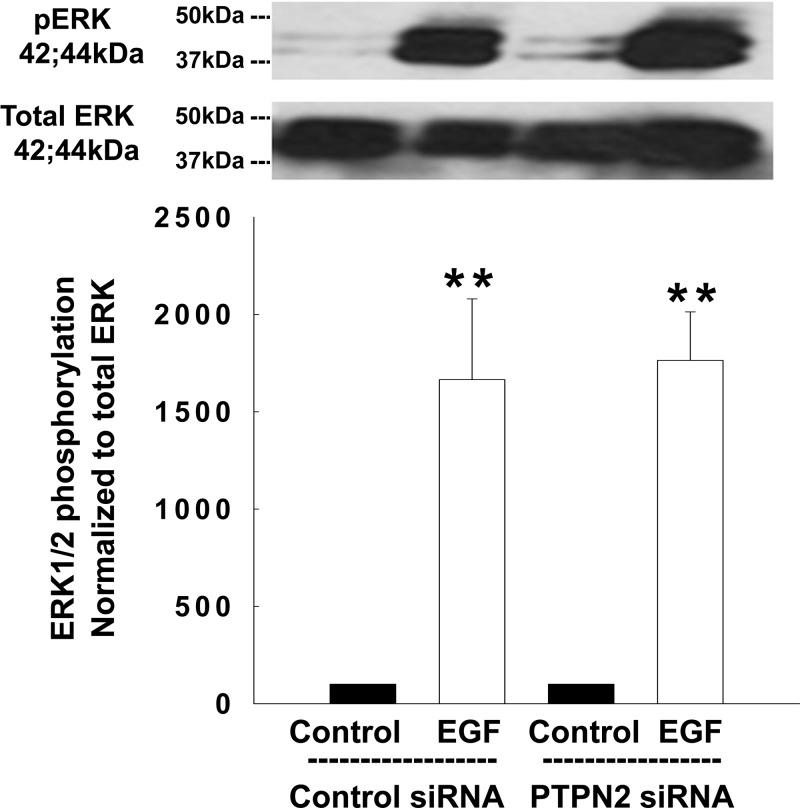
EGF activation of the ERK-isoforms (ERK1/2) of the MAPK family is well described (38). To test whether PTPN2 might also be involved in the regulation of EGF-induced activation of MAPK signaling, we examined whether EGF-induced phosphorylation of ERK1/2 was altered in PTPN2 siRNA-transfected T84 cells. Although ERK1/2 phosphorylation was strongly induced in EGF-treated control cells this effect was not altered by PTPN2 knockdown (Fig. 5). These data indicate that PTPN2 is involved in regulating the link of EGFR to the PI3K/Akt pathway, but not ERK, in response to the EGF.
Fig. 5.
PTPN2 knockdown does not affect EGF-induced phosphorylation of ERK1/2. T84 cells transfected with either control siRNA or PTPN2 siRNA were treated with EGF (100 ng/ml) for 5 min. Analyses were performed using whole cell lysates. Representative Western blots show phosphorylation and expression of ERK1/2. The densitometric analysis of 3 similar experiments is shown by the histogram below. Data are shown as a percentage of control. Significant difference vs. the respective control: **P < 0.01.
Loss of PTPN2 enhances EGF inhibition of Ca2+-dependent chloride secretion.
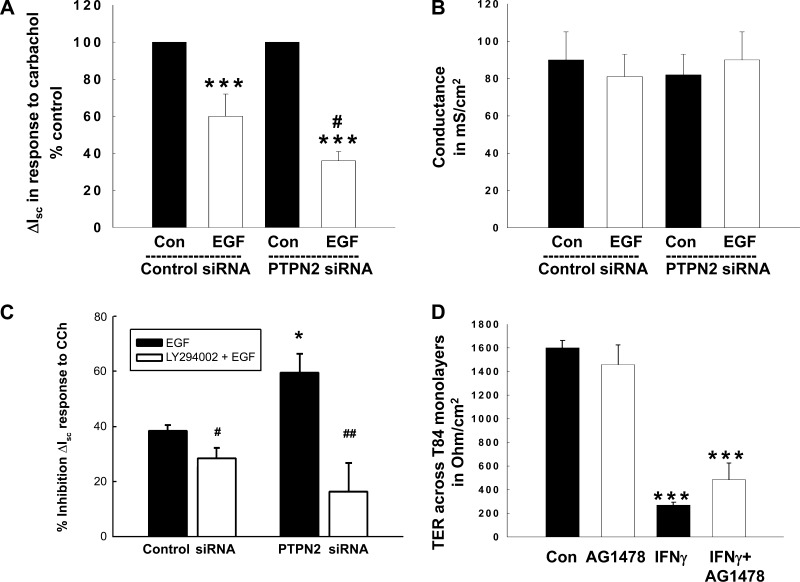
Having shown that EGF-induced activation of PI3K is enhanced in cells that lack PTPN2, we next tried to identify a functional consequence for this observation. Our laboratory has previously demonstrated that PI3K plays an essential role in mediating EGF-induced inhibition of Ca2+-dependent Cl− secretion in intestinal epithelial cells (47, 48). Therefore, we speculated that an increase in PI3K activity in PTPN2-deficient cells should promote the inhibitory effect of EGF on Cl− secretion. We performed Ussing chamber studies using control siRNA and PTPN2 siRNA-transfected T84 cells. PTPN2-specific siRNA reduced the PTPN2 protein levels under the cell growth conditions used for Ussing chamber studies clearly. After pretreatment with EGF (100 ng/ml) for 20 min, we assessed the chloride secretory response to carbachol (100 μM, basolaterally) (24, 48). As expected, EGF significantly inhibited carbachol (CCh)-stimulated chloride secretion across T84 monolayers transfected with nonspecific control siRNA (2.6 ± 0.6 vs. 4.9 ± 1.1 μA/cm2; Fig. 6A). Corresponding to the elevated PI3K activity in EGF-treated PTPN2-deficient cells (cf. Fig. 4A), the inhibitory effect of EGF on Ca2+-dependent Cl− secretion was further enhanced in PTPN2-deficient cells (2.0 ± 0.5 vs. 6.2 ± 1.6 μA/cm2; Fig. 6A). The admittedly rather small Isc responses could be due to the fact that cells were grown for only 4 days after transfection to maintain a PTPN2-deficient phenotype. Therefore, the T84 cells were likely not able to develop as tight a monolayer as “conventionally” grown T84 cells that are usually seeded for 10–14 days before conducting electrophysiological experiments. This was confirmed by measuring the conductance of these T84 monolayers (Fig. 6B). As expected, the mean conductance values of the transfected T84 monolayers was about eightfold higher than those across “conventionally” grown monolayers. Since the magnitude of the vectorial ion transport response is related to the tightness of the monolayer, the rather low Isc response observed in our cells is likely due to the high conductance across the monolayers. Nevertheless, since these cells are obviously able to generate vectorial ion transport responses, albeit with lower magnitudes, our data represent valid physiological findings demonstrating that PTPN2 knockdown promotes EGF-induced inhibition of CCh-stimulated chloride secretion in intestinal epithelial cells. In subsequent studies, we demonstrated that the PI3K inhibitor, LY294002, significantly reduced the enhanced inhibitory effect of EGF on CCh-stimulated Isc in PTPN2-deficient cells (P < 0.01; n = 5; Fig. 6C), and this effect was more pronounced in PTPN2-deficient cells than control siRNA-transfected cells. This finding supports our biochemical data indicating elevated recruitment of PI3K signaling following EGF treatment of PTPN2-deficient cells (cf. Fig. 4). Moreover, we observed more robust ion transport responses to carbachol (ranging from 12–35 μA/cm2 depending on the treatment conditions) than in the experiment shown in Fig. 6A. Although the experiments (Fig. 6, A vs. C) showed quantitative differences in ion transport responsiveness, they were qualitatively similar with respect to the inhibitory effect of EGF on CCh-stimulated Isc.
Fig. 6.
PTPN2 knockdown potentiates inhibition of calcium-dependent chloride secretion across T84 monolayers via PI3K. T84 cells transfected with either control siRNA or PTPN2 siRNA and grown on permeable supports for 4 days, were pretreated with EGF (100 ng/ml, basolaterally) for 20 min and subsequently stimulated with carbachol (100 μM, basolaterally) and/or the PI3K inhibitor, LY294002 (20 μM, bilaterally). A: change (Δ) in short-circuit current (Isc) in response to carbachol. Data are shown as a percentage of the respective control and represent 8 similar experiments. B: conductance data for the monolayers used in A are expressed in mS/cm2. C: data are shown as the % inhibitory effect of EGF on the Isc response to carbachol with or without pretreatment with the PI3K inhibitor, LY294002 (20 μM, bilaterally). Five similar experiments were performed. D: transepithelial resistance (TER) across T84 monolayers after treatment with IFN-γ (100 ng/ml, basolaterally, 72 h), AG1478 (10 μM, bilaterally, 72 h), or a combination of both stimuli is demonstrated in Ω/cm2. Histogram represents data of 4 similar experiments. Significant difference vs. the respective control: *P < 0.05; ***P < 0.001. #P < 0.05; ##P < 0.01 vs. EGF treatment of T84 cells transfected with control siRNA in A or PTPN2/control siRNA in C.
Maintenance of an effective epithelial barrier is also a prerequisite for regulation of epithelial ion transport. Conflicting roles have been reported for EGFR in the regulation of epithelial barrier function (3, 39). Since we have previously determined that the proinflammatory cytokine IFN-γ induces phosphorylation of the EGFR and suppression of CCh-stimulated Cl− secretion by a mechanism that is insensitive to AG1478, and thus occurs independently of intrinsic EGFR kinase activity (49), we next wished to identify whether EGFR might be involved in the transduction of IFN-γ-induced effects on epithelial monolayer TER. Although an important pathophysiological feature of IFN-γ is its capacity to induce a deleterious effect on intestinal epithelial barrier function, the exact signaling mechanisms that mediate the IFN-γ-induced effects on barrier functions are still unclear. Therefore, we treated T84 monolayers for 72 h with either IFN-γ (1,000 U/ml), the pharmacological EGFR inhibitor AG1478 (10 μM), or both in combination. As shown in Fig. 6D, IFN-γ treatment significantly decreased the TER across T84 monolayers, whereas EGFR inhibition alone had no effect on TER (P < 0.001; n = 4). Interestingly, coincubation with AG1478 was not sufficient to prevent the IFN-γ-induced decrease in TER across T84 monolayers. These data demonstrate that increased EGFR activation is likely not involved in the IFN-γ-induced barrier defect in intestinal epithelial cells.
PTPN2 does not modulate IFN-γ-induced EGFR transactivation.
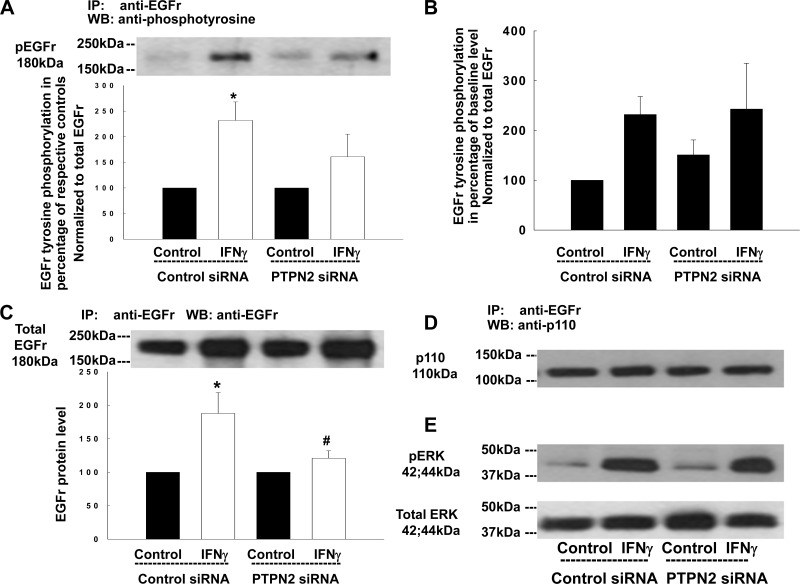
In addition to direct activation by exogenous EGF, EGFR can be transactivated by a number of other ligand-receptor systems, including following activation of the IFN-γ receptor by IFN-γ (11, 49). We have recently shown that PTPN2 plays a major role in regulating epithelial barrier responses to IFN-γ (36). We therefore wished to investigate whether PTPN2 modified EGFR transactivation and downstream signaling induced by IFN-γ. IFN-γ treatment for 24 h exerts an inhibitory effect on Ca2+-dependent Cl− secretion in response to CCh in intestinal epithelial cells (IECs) (9, 49) and increases phosphorylation of ERK1/2 via an EGFR-dependent pathway (11). To test whether PTPN2 regulates EGFR activation in response to IFN-γ, we performed PTPN2 knockdown studies and then treated T84 cells with IFN-γ for 24 h. As demonstrated previously, PTPN2 siRNA caused a clear reduction of PTPN2 protein in these experiments (36). Using a previously validated concentration of IFN-γ (1,000 U/ml) (49), IFN-γ increased phosphorylation of EGFR in cells transfected with control siRNA (Fig. 7A). However, phospho-EGFR levels of IFN-γ-treated PTPN2-deficient cells did not differ significantly from the respective controls or from the level of IFN-γ-induced EGFR phosphorylation relative to total EGFR in PTPN2-competent cells (Fig. 7A). In accordance with our previous results (Fig. 1C), baseline EGFR phosphorylation levels did not significantly differ under these conditions (Fig. 7B). Additionally, in an as yet unpublished manuscript, we demonstrate that IFN-γ treatment increases EGFR mRNA and protein in T84 monolayers (G. Paul, unpublished observations). These findings are in line with previous data in other epithelial cell systems (6, 21). As expected, and shown in Fig. 7C, IFN-γ treatment increased EGFR protein in immunoprecipitates from cells transfected with control siRNA (P < 0.05; n = 3). Interestingly, PTPN2 knockdown significantly diminished the cytokine-induced rise in EGFR protein (Fig. 7C; P < 0.05; n = 3). Additionally, IFN-γ did not increase association of EGFR with the p110 subunit of PI3K in either control siRNA-transfected or in PTPN2 siRNA-transfected cells (Fig. 7D). Similarly, loss of PTPN2 had no effect on IFN-γ-induced activation of ERK1/2 by 24 h treatment (Fig. 7E). These data demonstrate that PTPN2 regulates EGFR expression but not EGFR activation or signaling in response to IFN-γ.
Fig. 7.
IFN-γ-induced phosphorylation of the EGFr and consequent downstream signaling is not affected by PTPN2 knockdown. T84 cells transfected with either control siRNA or PTPN2 siRNA were treated with IFN-γ (1,000 U/ml, bilaterally) for 24 h. Whole cell lysates were immunoprecipitated with an anti-EGFr antibody, blotted for phosphotyrosine (A), stripped, and reblotted for EGFr (B) and p110 (C). A: representative Western blot and densitometric analysis represent EGFr tyrosine phosphorylation. Data are expressed as a percentage of the respective controls (n = 3). B: secondary densitometric analysis demonstrates the magnitude of induction of EGFr tyrosine phosphorylation from baseline level (analysis was performed as described in Fig. 1C) in percentage of untreated cells transfected with control siRNA. C: representative Western blot and densitometric analysis show EGFr protein level. Data are expressed as a percentage of the respective controls (n = 3). D: representative Western blot and densitometric analysis show p110 protein level. E: representative Western blots of 3 similar experiments demonstrate the phosphorylation of ERK1/2 and the expression of total ERK1/2 in response to IFN-γ. Significant difference vs. the respective control: *P < 0.05. #P < 0.05 vs. IFN-γ treatment of T84 cells transfected with control siRNA.
DISCUSSION
In this study, we demonstrate that the CD candidate gene, PTPN2, regulates EGFR-mediated downstream signaling in response to EGF. Loss of PTPN2 directs EGF-induced EGFR activity toward the PI3K pathway and thereby potentiates EGF-induced inhibition of intestinal epithelial Ca2+-dependent Cl− secretion.
Furthermore, PTPN2 knockdown enhanced EGF-induced EGFR tyrosine phosphorylation in IECs. Specifically, PTPN2 knockdown increased the EGF-induced phosphorylation of Tyr-992 and Tyr-1068, and, in agreement with our earlier findings and those in other systems, phosphorylation of these residues likely contributes to increased PI3K activity and Akt phosphorylation (29, 45). Interestingly, we found that PTPN2 knockdown alone caused a noticeable but nonsignificant increase in phosphorylation of Tyr-992, whereas neither the phosphorylation status of the other studied EGFR tyrosine residues, namely Tyr-1068, Tyr-1148, and Tyr-1173, nor the overall phosphorylation status of the EGFR was markedly affected in PTPN2-deficient cells. Therefore, the elevated basal phosphorylation level of Tyr-992 is likely to be of negligible physiological relevance. We also saw no elevation in phosphorylation of Tyr-1148 or Tyr-1173 or in phosphorylation of ERK1/2 in EGF-treated PTPN2-deficient cells. This indicates that these latter two EGFR tyrosine residues are likely not involved in PTPN2 regulation of the PI3K/Akt pathway in this system. However, this does not exclude their possible involvement in EGF activation of the MAPK-ERK pathway in T84 cells. These findings are in accord with our previous work (29) and provide further insight into the mechanisms of EGFR-mediated signal transduction in intestinal epithelial cells. In particular, we have shown that PTPN2 is a key regulator of EGF-induced recruitment of signaling pathways of the EGFR that mediate regulation of ion transport in intestinal epithelial cells.
EGFR plays an important role in the regulation of the intestinal epithelial chloride secretion (8, 23, 24, 28, 47) and inhibits calcium-dependent chloride secretion via two distinct mechanisms. The muscarinic agonist carbachol stimulates chloride secretion utilizing intracellular calcium as second messenger. However, carbachol also exerts an inhibitory effect on intestinal chloride secretion via transactivation of the EGFR and the subsequent activation of ERK1/2 (24). On the other hand, direct activation of EGFR by EGF and subsequent recruitment of PI3K signaling constitutes the second mechanism whereby EGFR inhibits Cl− secretion (48). In this study, we show that knockdown of PTPN2 results in increased activity of the EGFR and subsequently PI3K in response to EGF treatment. Likewise, PTPN2 knockdown potentiates the inhibitory effect of EGF on carbachol-induced chloride secretion in T84 cells via increased recruitment of PI3K signaling. These findings not only correlate with previous studies showing a role for the EGFR and PI3K in the inhibition of intestinal chloride secretion (48), they also imply that PTPN2 critically limits the ability of EGF to regulate ion transport in intestinal epithelial cells. The identification of this functional role for PTPN2 in the regulation of EGFR function in IECs may be important in the setting of chronic inflammation. This would extend not only to ion transport, but also, perhaps, the involvement of EGFR in epithelial restitution, wound repair, and barrier function, all of which are dysregulated in IBD (20, 31, 37, 40, 55). This possibility is of even greater significance following the identification of PTPN2 as an IBD candidate gene (17, 51a). Although the secretion of ions and water into the intestinal lumen helps to preserve epithelial integrity by flushing the crypts and subsequently removing cell detritus and luminal pathogens, hyposecretory conditions in the intestine promote and sustain intestinal inflammation (1, 2). Therefore, any PTPN2 mutation that results in a loss of protein function could result in increased or sustained EGFR phosphorylation with many consequences for epithelial function. For example, in addition to well-described changes in epithelial transport protein expression due to inflammation (20, 41), prolonged EGFR activation may contribute to a hyposecretory state by further suppressing the function of existing ion transporters, thus exacerbating overall dysregulation of fluid homeostasis in the gut.
The proinflammatory cytokine IFN-γ inhibits chloride secretion (9, 41) and also activates EGFR in IECs. However, the inhibitory effect on Cl− secretion could not be reversed by inhibition of EGFR kinase activity (49). Interestingly, and in contrast to EGF-induced EGFR phosphorylation, PTPN2 knockdown did not affect IFN-γ-induced phosphorylation of EGFR in our studies. Since IFN-γ did not increase p110 association with EGFR, and this was also unaltered in PTPN2-deficient cells, we conclude that the PI3K/Akt pathway is likely not activated by IFN-γ at the time points tested, which is in agreement with observations by other groups (10, 30). In addition, although IFN-γ treatment induced phosphorylation of ERK1/2, this was not altered by PTPN2 knockdown. These data suggest that PTPN2 regulates only EGFR-mediated PI3K, but not ERK1/2 signaling, and is likely not recruited to the EGFR in response to IFN-γ. However, PTPN2 does appear to regulate the expression of EGFR in response to the cytokine. Intriguingly, PTPN2 knockdown had the opposite to anticipated effect in that it reduced rather than enhanced the effect of IFN-γ on EGFR expression. Although the molecular mechanisms responsible for this have still to be determined, this finding could possibly be of significance for EGFR signaling outcomes in the joint setting of reduced PTPN2 expression or activity and active inflammation. These questions are being addressed in ongoing investigations by our group. In agreement with our previous work showing EGFR kinase-independent effects of IFN-γ on EGFR activation and inhibition of Cl− secretion, we also found no involvement of EGFR kinase activity in the IFN-γ-induced decrease of intestinal epithelial barrier function. These data support our hypothesis that PTPN2 plays a more involved role in regulating EGFR signaling, and consequent outcomes, in response to EGF activation rather than IFN-γ induced EGFR activation.
In summary, our data establish a role for PTPN2 in the regulation of EGFR signaling in intestinal epithelial cells in response to EGF but not IFN-γ. Loss of PTPN2 promotes PI3K activation and potentiates the suppression of epithelial chloride secretory responses. In addition to changes in ion transporter expression caused by inflammation, our findings conform with hyposecretory states seen in chronic intestinal inflammation. Moreover, given the genetic association of PTPN2 with IBD, it will be important to determine whether PTPN2 plays a greater, or lesser, role in regulating EGF-stimulated EGFR signaling in the setting of inflammation. Thus our findings highlight the role of PTPN2 in regulating EGFR signaling events in intestinal epithelial cells and suggest that PTPN2 dysfunction could contribute to ion transport suppression in IBD.
GRANTS
This research was funded by a Crohn's and Colitis Foundation of America Senior Research Award and a Jon I. Isenberg Endowed Fellowship Award to D. F. McCole, by a scholarship from the German Research Foundation (Deutsche Forschungsgemeinschaft) to M. Scharl, and by the UCSD Digestive Diseases Research Development Center, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK080506.
DISCLOSURES
The authors declare that they do not have any competing financial interests.
ACKNOWLEDGMENTS
We are grateful to Cheryl Stork for assistance with cell culture and transfection assays and to Kim E. Barrett, University of California, San Diego (UCSD), for helpful discussions.
REFERENCES
- 1. Asfaha S, Bell CJ, Wallace JL, MacNaughton WK. Prolonged colonic epithelial hyporesponsiveness after colitis: role of inducible nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol 276: G703–G710, 1999. [DOI] [PubMed] [Google Scholar]
- 2. Asfaha S, MacNaughton WK, Appleyard CB, Chadee K, Wallace JL. Persistent epithelial dysfunction and bacterial translocation after resolution of intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 281: G635–G644, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Banan A, Fields JZ, Zhang Y, Keshavarzian A. Key role of PKC and Ca2+ in EGF protection of microtubules and intestinal barrier against oxidants. Am J Physiol Gastrointest Liver Physiol 280: G828–G843, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Batzer AG, Rotin D, Urena JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol 14: 5192–5201, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beerli RR, Hynes NE. Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. J Biol Chem 271: 6071–6076, 1996. [DOI] [PubMed] [Google Scholar]
- 6. Bernstein W, Zou ZQ, Black RJ, Pirollo KF, Chang EH. Association of interferon-gamma induced growth inhibition and modulation of epidermal growth factor receptor gene expression in squamous cell carcinoma cell lines. J Biol Regul Homeost Agents 2: 186–192, 1988. [PubMed] [Google Scholar]
- 7. Berschneider HM, Knowles MR, Azizkhan RG, Boucher RC, Tobey NA, Orlando RC, Powell DW. Altered intestinal chloride transport in cystic fibrosis. FASEB J 2: 2625–2629, 1988. [DOI] [PubMed] [Google Scholar]
- 8. Bertelsen LS, Barrett KE, Keely SJ. Gs protein-coupled receptor agonists induce transactivation of the epidermal growth factor receptor in T84 cells: implications for epithelial secretory responses. J Biol Chem 279: 6271–6279, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Bertelsen LS, Eckmann L, Barrett KE. Prolonged interferon-gamma exposure decreases ion transport, NKCC1, and Na+-K+-ATPase expression in human intestinal xenografts in vivo. Am J Physiol Gastrointest Liver Physiol 286: G157–G165, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Boivin MA, Roy PK, Bradley A, Kennedy JC, Rihani T, Ma TY. Mechanism of interferon-gamma-induced increase in T84 intestinal epithelial tight junction. J Interferon Cytokine Res 29: 45–54, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burova E, Vassilenko K, Dorosh V, Gonchar I, Nikolsky N. Interferon gamma-dependent transactivation of epidermal growth factor receptor. FEBS Lett 581: 1475–1480, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Carpenter CL, Auger KR, Chanudhuri M, Yoakim M, Schaffhausen B, Shoelson S, Cantley LC. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J Biol Chem 268: 9478–9483, 1993. [PubMed] [Google Scholar]
- 13. Cartwright CA, McRoberts JA, Mandel KG, Dharmsathaphorn K. Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest 76: 1837–1842, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Datta K, Bellacosa A, Chan TO, Tsichlis PN. Akt is a direct target of the phosphatidylinositol 3-kinase. Activation by growth factors, v-src and v-Ha-ras, in Sf9 and mammalian cells. J Biol Chem 271: 30835–30839, 1996. [DOI] [PubMed] [Google Scholar]
- 15. Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 379: 557–560, 1996. [DOI] [PubMed] [Google Scholar]
- 16. Franke A, Balschun T, Karlsen TH, Hedderich J, May S, Lu T, Schuldt D, Nikolaus S, Rosenstiel P, Krawczak M, Schreiber S. Replication of signals from recent studies of Crohn's disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet 40: 713–715, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81: 727–736, 1995. [DOI] [PubMed] [Google Scholar]
- 18. Galic S, Klingler-Hoffmann M, Fodero-Tavoletti MT, Puryer MA, Meng TC, Tonks NK, Tiganis T. Regulation of insulin receptor signaling by the protein tyrosine phosphatase TCPTP. Mol Cell Biol 23: 2096–2108, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J 16: 1647–1655, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greig ER, Boot-Handford RP, Mani V, Sandle GI. Decreased expression of apical Na+ channels and basolateral Na+, K+-ATPase in ulcerative colitis. J Pathol 204: 84–92, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Hamburger AW, Pinnamaneni GD. Increased epidermal growth factor receptor gene expression by gamma-interferon in a human breast carcinoma cell line. Br J Cancer 64: 64–68, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu P, Margolis B, Skolnik EY, Lammers R, Ullrich A, Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol Cell Biol 12: 981–990, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keely SJ, Barrett KE. p38 mitogen-activated protein kinase inhibits calcium-dependent chloride secretion in T84 colonic epithelial cells. Am J Physiol Cell Physiol 284: C339–C348, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Keely SJ, Uribe JM, Barrett KE. Carbachol stimulates transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T84 cells. Implications for carbachol-stimulated chloride secretion. J Biol Chem 273: 27111–27117, 1998. [DOI] [PubMed] [Google Scholar]
- 25. Madara JL, Dharmsathaphorn K. Occluding junction structure-function relationships in a cultured epithelial monolayer. J Cell Biol 101: 2124–2133, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Margolis BL, Lax I, Kris R, Dombalagian M, Honegger AM, Howk R, Givol D, Ullrich A, Schlessinger J. All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. Identification of a novel site in EGF receptor. J Biol Chem 264: 10667–10671, 1989. [PubMed] [Google Scholar]
- 27. Mattila E, Pellinen T, Nevo J, Vuoriluoto K, Arjonen A, Ivaska J. Negative regulation of EGFR signalling through integrin-alpha1beta1-mediated activation of protein tyrosine phosphatase TCPTP. Nat Cell Biol 7: 78–85, 2005. [DOI] [PubMed] [Google Scholar]
- 28. McCole DF, Keely SJ, Coffey RJ, Barrett KE. Transactivation of the epidermal growth factor receptor in colonic epithelial cells by carbachol requires extracellular release of transforming growth factor-alpha. J Biol Chem 277: 42603–42612, 2002. [DOI] [PubMed] [Google Scholar]
- 29. McCole DF, Truong A, Bunz M, Barrett KE. Consequences of direct versus indirect activation of epidermal growth factor receptor in intestinal epithelial cells are dictated by protein-tyrosine phosphatase 1B. J Biol Chem 282: 13303–13315, 2007. [DOI] [PubMed] [Google Scholar]
- 30. McKay DM, Watson JL, Wang A, Caldwell J, Prescott D, Ceponis PM, Di Leo V, Lu J. Phosphatidylinositol 3′-kinase is a critical mediator of interferon-gamma-induced increases in enteric epithelial permeability. J Pharmacol Exp Ther 320: 1013–1022, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Okamoto R, Watanabe M. Cellular and molecular mechanisms of the epithelial repair in IBD. Dig Dis Sci 50, Suppl 1: S34–S38, 2005. [DOI] [PubMed] [Google Scholar]
- 32. Olayioye MA, Graus-Porta D, Beerli RR, Rohrer J, Gay B, Hynes NE. ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol Cell Biol 18: 5042–5051, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perez-Navarro R, Martinez-Augustin O, Ballester I, Zarzuelo A, Sanchez de Medina F. Experimental inflammation of the rat distal colon inhibits ion secretion in the proximal colon by affecting the enteric nervous system. Naunyn Schmiedebergs Arch Pharmacol 371: 114–121, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Rotin D, Margolis B, Mohammadi M, Daly RJ, Daum G, Li N, Fischer EH, Burgess WH, Ullrich A, Schlessinger J. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J 11: 559–567, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandle GI, Higgs N, Crowe P, Marsh MN, Venkatesan S, Peters TJ. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology 99: 97–105, 1990. [DOI] [PubMed] [Google Scholar]
- 36. Scharl M, Paul G, Weber A, Jung BC, Docherty MJ, Hausmann M, Rogler G, Barrett KE, McCole DF. Protection of epithelial barrier function by the Crohn's disease associated gene protein tyrosine phosphatase n2. Gastroenterology 137: 2030–2040, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 116: 301–309, 1999. [DOI] [PubMed] [Google Scholar]
- 38. Seger R, Krebs EG. The MAPK signaling cascade. FASEB J 9: 726–735, 1995. [PubMed] [Google Scholar]
- 39. Singh AB, Sugimoto K, Dhawan P, Harris RC. Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol 293: C1660–C1668, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol 14: 348–353, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sugi K, Musch MW, Field M, Chang EB. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology 120: 1393–1403, 2001. [DOI] [PubMed] [Google Scholar]
- 42. Ten Hoeve J, Ibarra-Sanchez JM, Fu Y, Zhu W, Tremblay M, David M, Shuai K. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol Cell Biol 22: 5662–5668, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tiganis T, Bennett AM, Ravichandran KS, Tonks NK. Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol Cell Biol 18: 1622–1634, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tiganis T, Kemp BE, Tonks NK. The protein-tyrosine phosphatase TCPTP regulates epidermal growth factor receptor-mediated and phosphatidylinositol 3-kinase-dependent signaling. J Biol Chem 274: 27768–27775, 1999. [DOI] [PubMed] [Google Scholar]
- 46. Tonks NK, Neel BG. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol 13: 182–195, 2001. [DOI] [PubMed] [Google Scholar]
- 47. Uribe JM, Gelbmann CM, Traynor-Kaplan AE, Barrett KE. Epidermal growth factor inhibits Ca2+-dependent Cl− transport in T84 human colonic epithelial cells. Am J Physiol Cell Physiol 271: C914–C922, 1996. [DOI] [PubMed] [Google Scholar]
- 48. Uribe JM, Keely SJ, Traynor-Kaplan AE, Barrett KE. Phosphatidylinositol 3-kinase mediates the inhibitory effect of epidermal growth factor on calcium-dependent chloride secretion. J Biol Chem 271: 26588–26595, 1996. [DOI] [PubMed] [Google Scholar]
- 49. Uribe JM, McCole DF, Barrett KE. Interferon-gamma activates EGF receptor and increases TGF-alpha in T84 cells: implications for chloride secretion. Am J Physiol Gastrointest Liver Physiol 283: G923–G931, 2002. [DOI] [PubMed] [Google Scholar]
- 50. Vega QC, Cochet C, Filhol O, Chang CP, Rhee SG, Gill GN. A site of tyrosine phosphorylation in the C terminus of the epidermal growth factor receptor is required to activate phospholipase C. Mol Cell Biol 12: 128–135, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walker J, Jijon HB, Churchill T, Kulka M, Madsen KL. Activation of AMP-activated protein kinase reduces cAMP-mediated epithelial chloride secretion. Am J Physiol Gastrointest Liver Physiol 285: G850–G860, 2003. [DOI] [PubMed] [Google Scholar]
- 51a. Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447: 661–678, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamamoto T, Sekine Y, Kashima K, Kubota A, Sato N, Aoki N, Matsuda T. The nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylation. Biochem Biophys Res Commun 297: 811–817, 2002. [DOI] [PubMed] [Google Scholar]
- 53. Yu J, Wjasow C, Backer JM. Regulation of the p85/p110alpha phosphatidylinositol 3′-kinase. Distinct roles for the n-terminal and c-terminal SH2 domains. J Biol Chem 273: 30199–30203, 1998. [DOI] [PubMed] [Google Scholar]
- 54. Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell Biol 18: 1379–1387, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56: 61–72, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125: 1137–1149, 2006. [DOI] [PubMed] [Google Scholar]
- 57. Zhu W, Mustelin T, David M. Arginine methylation of STAT1 regulates its dephosphorylation by T cell protein tyrosine phosphatase. J Biol Chem 277: 35787–35790, 2002. [DOI] [PubMed] [Google Scholar]