Abstract
Hepatic steatosis results from several processes. To assess their relative roles, hepatocellular long-chain fatty acid (LCFA) uptake was assayed in hepatocytes from C57BL/6J control mice, mice with steatosis from a high-fat diet (HFD) or 10%, 14%, or 18% ethanol (EtOH) in drinking water [functioning leptin-signaling groups (FLSGs)], and ob/ob and db/db mice. Vmax for uptake was increased vs. controls (P < 0.001) and correlated significantly with liver weight and triglycerides (TGs) in all FLSG mice but was minimally or not increased in ob/ob and db/db mice, in which liver weights and TGs greatly exceeded projections from regressions in FLSG animals. Coefficients of determination (R2) for these FLSG regressions suggest that increased LCFA uptake accounts for ∼80% of the increase in hepatic TGs within these groups, but increased lipogenic gene expression data suggest that enhanced LCFA synthesis is the major contributor in ob/ob and db/db. Got2, Cd36, Slc27a2, and Slc27a5 gene expression ratios were significantly upregulated in the EtOH groups, correlating with sterol regulatory element binding protein 1c (SREBP1c) and Vmax, but only Cd36 expression was increased in HFD, ob/ob, and db/db mice. Comparison of Vmax with serum insulin and leptin suggests that both hormones contribute to upregulation of uptake in the FLSG animals. Thus, increased LCFA uptake, reflecting SREBP1c-mediated upregulation of four distinct transporters, is the dominant cause of steatosis in EtOH-fed mice. In ob/ob and db/db mice, increased LCFA synthesis appears more important. In FLSG animals, insulin upregulates hepatocellular LCFA uptake. Leptin appears to upregulate LCFA uptake or to be essential for full expression of upregulation by insulin.
Keywords: ethanol, fatty acid transport, fatty acid synthesis, gene expression, obesity
current concepts divide fatty liver into two principal categories: alcoholic liver disease, which is related to ethanol (EtOH), and nonalcoholic fatty liver disease (NAFLD). Each category consists of a spectrum. In both, the earliest recognizable form is simple hepatic steatosis, which is typically reversible with removal of the inciting condition: EtOH abuse in the former or, most often, obesity in the latter. In both settings, however, simple hepatic steatosis may progress to steatohepatitis, either alcoholic or nonalcoholic (NASH), which in turn may result in hepatic fibrosis, cirrhosis, and end-stage liver disease. Since the transition to steatohepatitis is a critical point in the pathogenesis of severe liver disease, research interest in alcoholic liver disease and NAFLD has focused heavily on the pathogenesis of alcoholic hepatitis and NASH. Nevertheless, simple hepatic steatosis remains the essential first step in disease progression.
Simple hepatic steatosis reflects increased accumulation of lipid, principally triglyceride (TG), in hepatocytes. This may be imported intact or can be assembled from long-chain fatty acids (LCFAs) and glycerol in a process in which LCFA availability is usually rate-limiting. Simple hepatic steatosis can, in theory, result from any process that leads to increased TG input to or decreased TG output from the intrahepatocellular TG mass (13, 27). These include increased LCFA uptake or synthesis, esterification of LCFA to TG, and uptake of preformed lipoprotein TG or decreased LCFA oxidation, synthesis of apolipoproteins, TG mobilization and insertion into VLDL, or VLDL secretion. Virtually all these processes have been reported to play a role in one or another model of hepatic steatosis (19, 22, 35, 44), but the data are not always conclusive, and few studies have assessed several of these processes simultaneously. Therefore, their relative contributions remain largely unclear.
The aim of the present studies was to characterize the processes contributing to simple hepatic steatosis in various mouse models of fatty liver. We studied three different models of obesity-associated simple hepatic steatosis and simple hepatic steatosis in mice consuming three different doses of EtOH. Simple hepatic steatosis was assessed semiquantitatively in histological sections and by biochemical measurements of tissue TG; hepatocellular LCFA uptake was quantitated from studies of [3H]oleic acid (OA) uptake kinetics, and the expression of multiple genes involved in, e.g., LCFA uptake, synthesis, esterification to triglyceride, oxidation, and assembly and secretion of VLDL was assessed by quantitative real-time PCR (qRT-PCR), in many cases supported by Western immunoblotting. Results demonstrated important pathophysiological similarities among all these models but also significant differences, the most striking of which depend on the functional status of leptin signaling.
MATERIALS AND METHODS
Chemicals and Reagents
9,10-[3H]OA (2.6 Ci/mmol) was purchased from PerkinElmer (Waltham, MA), nonradioactive oleate from Sigma Chemical (St. Louis, MO), BSA (fatty acid-free) from Boehringer Mannheim (Indianapolis, IN), and collagenase type I from Worthington (Lakewood, NJ). Protein concentrations were determined by the bicinchoninic acid (BCA) assay kit (Thermo Scientific, Rockford, IL).
Mice and Diets
Male C57BL/6J, ob/ob, and db/db mice were purchased from Jackson Laboratories (Bar Harbor, ME) at 6 wk of age. Upon receipt, mice were housed in group cages in a temperature-controlled facility with a 12:12-h light-dark cycle, with free access to water and a standard chow diet (3.07 kcal/g; LabDiet 5001, PMI, St. Louis, MO). Starting at 8 wk of age, groups of at least six ob/ob, db/db, and control C57BL/6J mice received the standard laboratory chow diet and water. Additional C57BL/6J mice received the chow diet and water containing 10% EtOH. Yet another group of C57BL/6J mice were fed a high-fat diet (HFD) containing 35% lard (5.45 kcal/g, 55% of calories from fat; Bio-Serv, Frenchtown, NJ) and water. In some of the EtOH groups, 10% EtOH was replaced with 14% EtOH after 4 wk (14% EtOH group), and in some of these, 14% EtOH was replaced with 18% EtOH after a further 4 wk (18% EtOH group). All EtOH-fed mice were killed after a total of 12 ± 1 wk of EtOH treatment. Thus, groups designated as receiving 10% EtOH received that dose for the full 12 wk, those designated as 14% EtOH received that dose for 8 wk, and those designated as 18% EtOH received that dose for 4 wk. This incremental increase in the concentration of EtOH was necessary, because the C56BL/6J mice will freely consume 10% EtOH in water but will only consistently consume 14% and 18% EtOH after a period of conditioning at lower concentrations. Weights were recorded weekly, as was consumption of water or water-EtOH and food, until the final week before death, when weights were measured daily. Mice were euthanized at 20 ± 1 wk of age after an overnight (12-h) fast. All applicable institutional and governmental regulations concerning ethical use of animals were followed. The protocol was approved by the Institutional Animal Care and Use Committee of Columbia University Medical Center.
Euthanasia and Tissue Harvesting
Euthanasia was accomplished with intraperitoneal injections of ketamine (0.1 mg/g) and xylazine (0.01 mg/g). Upon their death, the mice were randomly assigned to one of two protocols.
Protocol 1.
Abdomens were opened, and after perfusion of the portal vein with HBSS, livers were removed and used for cell isolation (see below).
Protocol 2.
Livers were removed without perfusion and weighed. A portion of each liver was placed in neutral buffered formalin for subsequent paraffin embedding, sectioning, and staining with hematoxylin-eosin and Masson's trichrome. An additional portion was embedded in optimal cutting temperature compound (Tissue-Tek, Sakura Finetek, Torrance, CA), frozen on dry ice, and stored at −80°C for future sectioning. In the latter case, serial 7-μm-thick sections were collected on poly-d-lysine-coated slides and stained with oil red O (ORO) and hematoxylin. A final portion was frozen for subsequent biochemical analyses.
Blood and Serum Analysis
A glucose meter (One-Touch, LifeScan, Milpitas, CA) was used to measure whole blood glucose by the glucose oxidase reaction in tail vein samples obtained just prior to induction of anesthesia. Additional blood was collected from the inferior vena cava upon the animal's death and promptly separated by centrifugation. Serum was stored at −20°C for subsequent analysis. The following serum measurements were performed in our laboratory with use of commercially available kits: albumin (Pointe Scientific, Canton, MI), free fatty acids (Wako Chemicals, Richmond, VA), TG (L-Type TG H, Wako Chemicals), total cholesterol (Cholesterol E, Wako Pure Chemical Industries, Osaka, Japan), and aspartate aminotransferase and alanine aminotransferase (AST and ALT; Stanbio, Boerne, TX). Serum leptin, insulin, and adiponectin concentrations were determined by immunoassay at the Hormone Research Core Laboratories of Vanderbilt University. The unbound oleate concentration in plasma was calculated from the oleate-to-albumin molar ratio (ν) (50, 56, 62) using the LCFA-albumin binding constants of Spector et al. (50). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin concentrations as previously described (2).
Determination of Hepatic Tissue TG and Cholesterol
Livers were homogenized in PBS. The total protein content was determined with the BCA protein analysis kit. Hepatic TG and cholesterol contents were determined, after Folch extraction (23) with the Cholesterol E and L-Type TG H kits described above, according to the manufacturer's instructions.
Histological Examination and Estimation of Tissue Neutral Lipids
Hematoxylin-eosin-, Masson's trichrome-, and ORO-stained sections of liver were examined. Semiquantitative estimates of neutral lipids in the ORO-stained hepatic sections were determined in a blind study by two observers (F. Ge and P. D. Berk) who were unaware of the treatments of the individual mice and resulting biochemical measurements of tissue lipid content. The degree of steatosis was visually scored on a scale of 0–3 in five noncontiguous high-power fields in three slides from each mouse on the basis of published criteria (32). Results of the two observers were averaged to calculate a steatosis score for each animal. Histological images were observed at ×250 magnification with a Nikon Eclipse 80i microscope and captured with a Nikon digital camera (model DXM 1200C) using a standard exposure for all photographs.
Isolation of Hepatocytes for Kinetic Studies
Hepatocytes were isolated as previously reported (48, 53, 54, 57). All preparations were checked for viability by trypan blue staining, and only preparations in which ≥90% of hepatocytes excluded trypan blue were used. Cells in suspension were counted under a microscope, and cell concentrations were adjusted to 2–4 × 106 cells/ml for use in OA uptake assays.
Cellular Uptake of OA
The rapid filtration methods well established in our laboratory were used to determine the initial uptake velocity of OA into hepatocytes (53, 54) at 37°C from medium containing 500 μM BSA in triplicate over 15 s at five different unbound OA concentrations. Uptake by these cell types is linear over this time frame, and we have established that, under these conditions, measured uptake principally reflects transmembrane LCFA transport relatively independently of unstirred water layer effects or subsequent intracellular binding or metabolism (47, 53).
Computations and Data Fitting
On the basis of multiple prior studies in rodents and humans (4, 5, 40, 56, 57), values for initial OA uptake velocity at the five studied concentrations of unbound OA were fitted by computer to Eq. 1 with use of SAAM II software as modified for implementation on a laptop computer (3, 9). Equation 1 indicates that LCFA uptake is the sum of a saturable plus a nonsaturable function of the unbound LCFA concentration in plasma. Thus
| (1) |
where Uptake (OAu) is the rate of uptake of labeled OA (pmol·s−·5 × 104 cells−1) at unbound OA concentration OAu (nM), Vmax (pmol·s−1·5 × 104 cells−1) and Km (nM) are the maximal uptake rate of the saturable uptake component and the unbound OA concentration at half-maximal uptake velocity (nM), respectively, and kn (ml·s−1 × 5 × 104 cells−1) is the rate constant for nonsaturable OA uptake (4, 5, 37, 56, 57). Computed values for physiological variables are expressed as means ± SE.
Analysis of Hepatic Gene and Protein Expression
qRT-PCR.
For qRT-PCR, QIAamp RNA mini kits (Qiagen, Valencia, CA) were used, according to the manufacturer's protocol, to extract total RNA from tissue samples. First-strand cDNAs were synthesized using TaqMan reverse transcription reagent kits (Applied Biosystems, Foster City, CA). Random hexamers were used as primers. PCRs were performed in a total volume of 50 μl containing 500 ng of cDNA on the 7300 real-time PCR system using SYBR Green PCR Master Mix (Applied Biosystems) as described in the manufacturer's guidelines. We used the mouse geNorm kit (β2-microglobulin), GAPDH, and ubiquitin C (PrimerDesign, Southhampton, UK) for housekeeping gene analysis. Primer sequences were selected with the use of Primer 3 software [S. Rozen and H. Skaletsky (http://frodo.wi.mit.edu/primer3/)]. All primers were synthesized by IDT (San Jose, CA). The genes studied, the primers employed, and the commonly used names of the encoded proteins are listed in Table 1. We use the gene designation to describe RNA expression studies (e.g., qRT-PCR) and their results and protein names for studies of protein expression (e.g., Western blots).
Table 1.
PCR primers
| Sequences |
||||
|---|---|---|---|---|
| Gene Name | Protein Name | Forward (5′-3′) | Reverse (5′-3′) | Product Size, bp |
| Got2 | Aspartate aminotransferase, mitochondrial (mAspAT) or (FABPpm) | AAGCGGCCGGTTCGTCACTG | GGGGTTGTGAGCGCAGGCAT | 283 |
| Cav1 | Caveolin 1 (CAV1) | ACATTACAGCTCTGCCCTTG | GGTAGCAGGTTGGTAAAGTGTC | 134 |
| Slc27a1 | Fatty acid transport proteins (FATP1) | AGCAGCACCTGTGAGAGTAGG | TGCTGGGTAGGGAGAAAAGAG | 125 |
| Slc27a2 | Fatty acid transport proteins (FATP2) | TTTCCGGTGGAAAGGAGA | AGGTACTCCGCGATGTGTTG | 198 |
| Slc27a5 | Fatty acid transport proteins (FATP5) | AGGACCAGCTGCATCCTTC | TCTCCTACGCGTCGTACATTC | 227 |
| Cd36 | FAT/CD36 | TCCCTCTCTGGAGTTCTTGG | TTGCAGCTGAGCAGAAAGAG | 232 |
| SREBP1c | Sterol regulatory element binding protein 1c (SREBP1c) | GTGGTCTTCCAGAGGGTGAG | AGGTGCCTACAGAGCAAGAG | 119 |
| Fasn | Fatty acid synthase (FASN) | GAGCCTTTTCTACCGTGTGG | GAGCAGGGACAGGACAAGAC | 214 |
| Scd1 | Stearoyl-CoA desaturase 1 (SCD1) | GGGCAGTTCTGAGGTGATTAG | TTCATGGCAGTGGGTAGGTAG | 189 |
| Dgat2 | Diacylglycerol acyltransferase 2 (DGAT2) | TCCTCACCCTAGCCCTCTTC | AAGAGAAGCCCTTCCTCACAC | 215 |
| Dgat1 | Diacylglycerol acyltransferase 1 (DGAT1) | GTGGTGCATCAGACACTTCTAC | CAGCTGCATTGCCATAGTTC | 221 |
| Lpl | Lipoprotein lipase (LPL) | GGGCTCTGCCTGAGTTGTAG | CCATCCTCAGTCCCAGAAAA | 167 |
| Ldlr | LDL receptor (LDLR) | CCAAATAGGCTGTCCCAGAA | CGAGTCACTTTCCTGGCTTC | 235 |
| ApoB | Apolipoprotein B (ApoB) | CAGCTGCTGATTTTGCTTCC | GGCAGTGACCCAGCTTTTC | 167 |
| Mttp | Microsomal triglyceride transfer protein (MTTP) | CCACATACAGCCTTGACATCC | CAAGGTTCTCCTCTCCTTCATC | 174 |
| Dci | Dodecenoyl-CoA δ-isomerase (DCI) | CCCTCAGCTTGGAGTGTCTC | GAGTCTCAGCCACAGCTCCT | 200 |
| Cyp2e1 | Cytochrome P-450, family 2, subfamily E polypeptide 1 (CYP2E1) | TCAACCTCGTCCCTTCCAAC | AGGCCTTCTCCAACACACAC | 244 |
| Cyp4a | Cytochrome P-450, family 4, subfamily A (CYP4A) | TGAGGGAGAGCTGGAAAAGA | CTGTTGGTGATCAGGGTGTG | 208 |
| Acc1 | Acetyl-CoA carboxylase (ACC1) | GGCACAGTGAAGGCTTACGTCTGG | TCTGCTCGCTGGGTGGGTGA | 229 |
Western blots.
For detection of fatty acid synthase (FASN), steroyl-CoA desaturase (SCD1), acetyl-CoA carboxylase (ACC1), CD36, fatty acid transport protein 2 (FATP2), and plasma membrane-associated fatty acid-binding protein (FABPpm), total protein extracts were prepared from murine liver with use of cell lysis buffer (Cell Signaling Technology). Particulate material was removed by centrifugation, and protein concentration was determined using the BCA protein assay kit. Equal amounts of total protein (50 μg/sample) were subjected to electrophoresis in NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA) and then electrophoretically transferred to a nitrocellulose membrane. Nonspecific binding was blocked by incubation of membranes with nonfat dry milk (5%) for 1 h at room temperature. The blots were incubated with the following primary antibodies: rabbit anti-mouse FASN, ACC1, FABPpm, FATP2, and β-actin or goat anti-mouse SCD1 and CD36. All primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), except anti-FABPpm, which was prepared in our laboratory, anti-FATP2 (a gift from Dr. Andreas Stahl), and anti-CD36 (R & D Systems, Minneapolis, MN). Each primary antibody was incubated for 2 h or overnight at a dilution of 1:1,000, except for β-actin, which was diluted at 1:10,000. Goat anti-rabbit and donkey anti-goat IgG secondary antibodies (1:4,000 dilution; Santa Cruz Biotechnology) were used to identify primary antibody binding sites. All the Western blot results were analyzed by scanning densitometry and densitometric image analyzer software [Image J, National Institutes of Health, Bethesda, MD (rsb.info.nih.gov/ij/download/)].
Statistical Analysis
All results are expressed as means ± SE. Significance of differences between groups for the various parameters studied was assessed using Student's t-tests. Statistical significance was set at P < 0.05.
RESULTS
Age, Caloric Intake, Body Weight, and Serum Biochemical Tests
Age and caloric intake.
The mean age of the mice at death was 19.8 ± 0.1 wk and was virtually identical in all the groups. Total caloric intake in the week prior to death is presented in Table 2. It was marginally increased in the HFD group and significantly increased in the ob/ob and db/db groups. Among EtOH-fed mice, as EtOH intake, which was nil in the control animals, increased from 3.0 ± 0.1 to 3.9 ± 0.2 to 4.8 ± 0.6 kcal·day−1·mouse−1 in the 10%, 14%, and 18% EtOH groups, respectively, food intake decreased to 9.8 ± 0.6, 9.5 ± 0.6, and 10.4 ± 1.2 kcal·day−1·mouse−1. Overall, there was a small, dose-related upward trend in total caloric intake in the EtOH groups, but in none was total caloric intake significantly greater than in controls. Total caloric intakes in the HFD and 18% EtOH groups were not significantly different (Table 2).
Table 2.
Basic physical and biochemical data
| Group | Caloric Intake, kcal·day−1·mouse−1 | Weight, g | Glucose, mg/dl | Cholesterol, mg/dl | TG, mg/dl | AST, U/l | ALT, U/l | Insulin, ng/ml | Leptin, ng/ml | Adiponectin, μg/ml | HOMA-IR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 12.28 ± 1.54 | 26.7 ± 0.3 | 137 ± 15 | 69.6 ± 6.2 | 51.8 ± 3.1 | 37.8 ± 2.4 | 8.8 ± 1.8 | 0.087 ± 0.011 | 0.86 ± 0.17 | 9.94 ± 0.733 | 0.28 ± 0.04 |
| EtOH | |||||||||||
| 10% | 12.83 ± 0.63 | 26.4 ± 0.5 | 112 ± 13 | 83.1 ± 5.7 | 67.4 ± 9.6 | 43.0 ± 8.4 | 14.6 ± 3.9 | 0.11 ± 0.020 | 2.42 ± 0.19f | 9.77 ± 1.217 | 0.36 ± 0.07 |
| 14% | 13.44 ± 0.65 | 24.3 ± 0.5b | 143 ± 9 | 68.6 ± 4.2 | 64.7 ± 6.0 | 48.8 ± 10.5 | 12.5 ± 2.4 | 0.13 ± 0.010c | 1.84 ± 0.38b | 13.39 ± 0.735b | 0.45 ± 0.05d |
| 18% | 15.24 ± 1.36 | 25.5 ± 0.5 | 108 ± 7 | 64.0 ± 5.2 | 86.0 ± 9.4b | 46.6 ± 10.9 | 10.0 ± 2.0 | 0.17 ± 0.010f | 3.66 ± 0.98c | 13.06 ± 0.976c | 0.56 ± 0.05f |
| HFD | 18.53 ± 2.73a | 36.6 ± 1.2d | 220 ± 10d | 104 ± 7.3e | 77 ± 9.2c | 41.4 ± 4.8 | 7.8 ± 1.2 | 0.13 ± 0.02f | 49.6 ± 11.3d | 12.06 ± 0.863a | 0.50 ± 0.08c |
| db/db | 18.11 ± 1.04c | 51.4 ± 0.8d | 120 ± 10 | 182 ± 6d | 59 ± 16 | 57.2 ± 7.4b | 102 ± 22d | 2.41 ± 0.69d | 1145 ± 486b | 8.32 ± 0.930 | 7.35 ± 2.19e |
| ob/ob | 20.57 ± 1.54d | 61.8 ± 0.6d | 155 ± 9 | 194 ± 7d | 83 ± 8d | 54.0 ± 4.4e | 99 ± 7d | 1.48 ± 0.46b | 0.44 ± 0.09 | 11.77 ± 0.875 | 4.95 ± 1.57d |
Values are means ± SE. EtOH, ethanol; TG, triglyceride; HFD, high-fat diet; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HOMA-IR, homeostasis model assessment of insulin resistance.
0.1 > P > 0.05,
P < 0.05,
P < 0.025,
P < 0.01,
P < 0.005,
P < 0.001 vs. control.
Body weights and relevant serum biochemical parameters.
Data at death are summarized in Table 2. The 14% and 18% EtOH groups were slightly lighter than the chow-fed controls (P < 0.05), whereas the HFD, ob/ob, and db/db mice were significantly heavier. Among mice receiving EtOH, serum TG, AST, insulin, and leptin tended to be at least modestly increased, with values in the 18% EtOH group (TG), 14% and 18% EtOH groups (insulin), or 10%, 14%, and 18% EtOH groups (leptin) that were significantly increased compared with controls. EtOH had no consistent effects on blood glucose or serum cholesterol. By contrast, HFD animals were uniformly hyperglycemic, hypertriglyceridemic, and hypercholesterolemic, in addition to exhibiting elevated insulin and leptin concentrations. The highest glucose, cholesterol, AST, ALT, and insulin concentrations occurred in the ob/ob and db/db mice. Computed insulin resistance (HOMA-IR) values were increased compared with controls in all experimental groups, the increases being statistically significant in all but the 10% EtOH animals. Highest values were found in the ob/ob and db/db groups, which were not significantly different from each other. Values in the 18% EtOH and HFD mice were also equivalently increased.
Fatty acid concentrations.
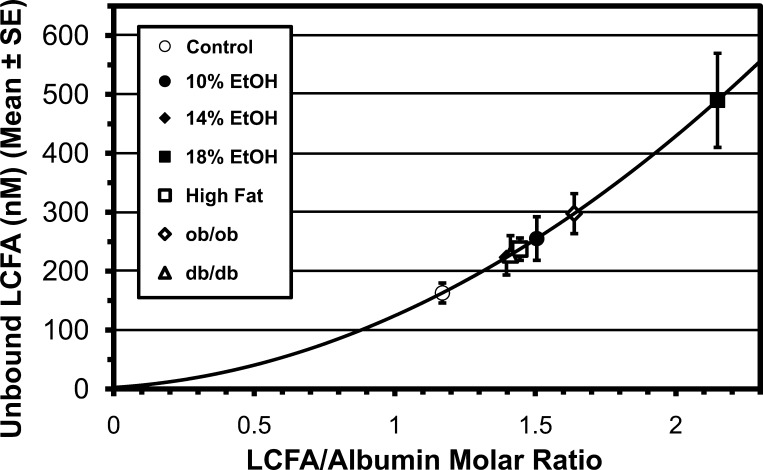
Serum total LCFA concentration rose a maximum of 1.8-fold, from 0.67 ± 0.07 mM in controls to their highest value, 1.22 ± 0.30 mM, in 18% EtOH mice (0.1 > P > 0.05). While the mean total LCFA concentration was greater in all experimental groups than in controls, in none of the groups was the increase statistically significant. However, it is the unbound LCFA level (LCFAu), not the total LCFA level, that drives nonsaturable and saturable LCFA uptake. Because of the complex binding relationship between LCFA and the multiple classes of LCFA binding sites on albumin, LCFAu increases as a quasiexponential function of ν (4, 48, 50, 56). EtOH produced a threefold increase in LCFAu, from 163 ± 17 nM in controls to 490 ± 123 nM in 18% EtOH mice (Fig. 1). In contrast to total LCFA, the increase in LCFAu was statistically significant in all experimental groups. The underappreciated relationship of LCFAu to ν is of high clinical relevance in lipotoxic diseases.
Fig. 1.
Unbound long-chain fatty acid (LCFA) concentrations in serum calculated for each experimental group from molar ratios of LCFA to albumin. EtOH, ethanol. Results in all experimental groups are significantly different from control group (P < 0.05 or better).
Liver Weights and Lipid Content
Liver weight and hepatic TG were significantly increased in all experimental groups (Table 3). Hepatic cholesterol content was also increased, although this was not statistically significant in the 10% EtOH and HFD groups.
Table 3.
Liver weight and lipid content
| Group | Liver Weight, g | Liver Triglycerides, mg | Liver Cholesterol, mg |
|---|---|---|---|
| Control | 1.16 ± 0.02 | 29.7 ± 2.2 | 6.92 ± 0.65 |
| EtOH | |||
| 10% | 1.28 ± 0.03d | 44.2 ± 5.5b | 11.56 ± 2.55 |
| 14% | 1.21 ± 0.04 | 43.4 ± 5.8b | 14.24 ± 3.04b |
| 18% | 1.27 ± 0.03d | 42.7 ± 3.1d | 14.01 ± 3.14b |
| HFD | 1.38 ± 0.06f | 64.35 ± 6.81f | 15.49 ± 6.05 |
| db/db | 3.36 ± 0.25f | 101.8 ± 1.53d | 65.7 ± 11.1d |
| ob/ob | 4.22 ± 0.15f | 284.71 ± 24.02d | 46.5 ± 3.5d |
Values are means ± SE.
P < 0.05,
P < 0.01,
P < 0.001 vs. control.
Hepatic Histology
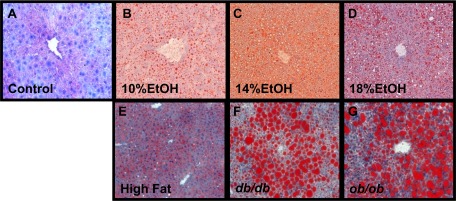
Occasional, small inflammatory foci of predominantly neutrophilic inflammation were seen in all groups including controls in hematoxylin-eosin-stained sections but were not appreciated in ORO-stained sections. Ballooning, Mallory bodies, and fibrosis were not observed in any group in this study. In ORO-stained sections (Fig. 2), a limited number of small lipid droplets was seen in the centrilobular regions of normal livers. ORO-stainable droplets were visually more prominent in the EtOH groups as the EtOH dose increased. Coalescence into even larger lipid droplets occurred in HFD, db/db, and ob/ob mice. Hepatic steatosis scores ranged from 0.59 ± 0.2 in controls to 3.0 ± 0.0 in the ob/ob and db/db groups and were significantly increased (from 1.33 ± 0.2 to 1.7 ± 0.2, P < 0.001) in all the EtOH groups. The score in the 18% EtOH group was not significantly different from the score in HFD mice (1.7 ± 0.2 and 2.33 ± 0.2, respectively). Hepatic steatosis scores were significantly correlated with log10(hepatic TG content) (r = 0.8, P < 0.05).
Fig. 2.
Hepatic histology. A–G: representative oil red O (ORO)-stained sections. While ORO is not ideal for fine detail, collectively, sections clearly illustrate increases in ORO-stainable lipid between controls and experimental groups. Hematoxylin-eosin- and Masson's trichrome-stained sections were also examined and revealed no significant inflammation and no ballooning, Mallory-Denk bodies, or fibrosis in any of the groups. Original magnifications ×250.
LCFA Uptake in Hepatocytes
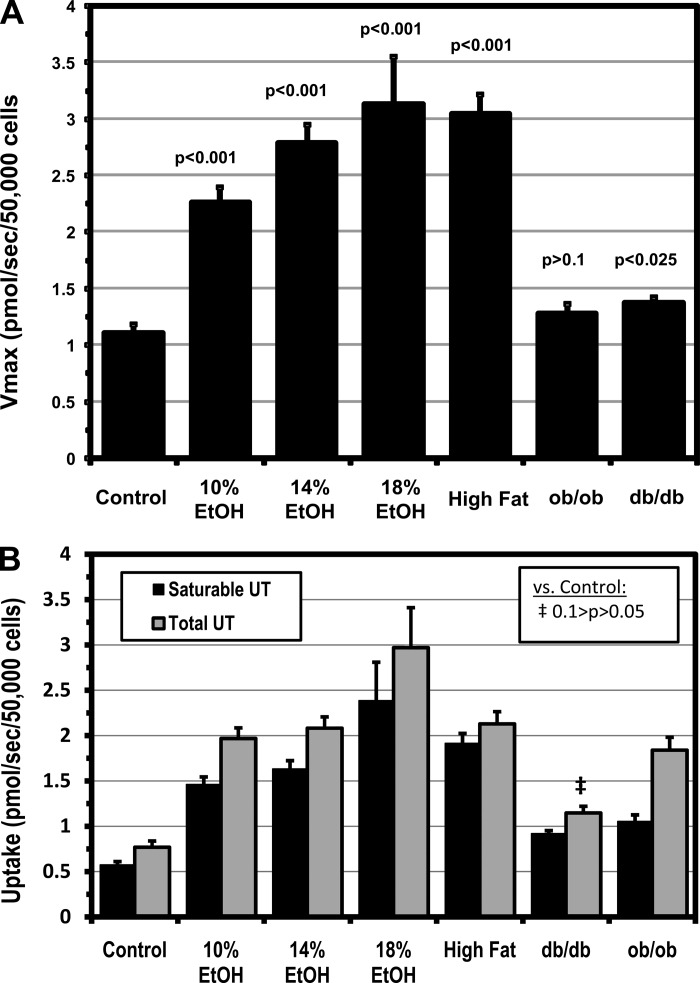
Vmax for hepatocellular LCFA uptake increased in a dose-dependent fashion in all three EtOH groups and in HFD mice (P < 0.001 vs. control in all; Fig. 3A) and correlated with liver weight (r = 0.8, P < 0.01) and TG content (r = 0.7, P < 0.05), suggesting that increased saturable LCFA uptake is a key contributor to steatosis in FLSG animals. By contrast, Vmax for hepatocellular LCFA uptake was not increased in ob/ob, and only marginally increased in db/db, mice, although these two strains had the heaviest, most steatotic livers. While it is possible that the marked hepatic steatosis in ob/ob and db/db livers inhibited further increases in LCFA uptake, no such inhibition was seen in the HFD group, which also had a highly significant increase in hepatic TG. Absolute rates of LCFA uptake in each group (Fig. 3B) were calculated from the kinetic constants and the values for LCFAu. The absolute rates of saturable uptake generally paralleled the pattern exhibited by the Vmax data but were significantly increased in all experimental groups, reflecting increases in Vmax and LCFAu in the FLSG mice and increases in LCFAu in the ob/ob and db/db animals. As with Vmax, the smallest increases in saturable uptake were found in ob/ob and db/db mice. Total LCFA uptake, reflecting increases in saturable and nonsaturable uptake, was significantly increased in all experimental groups except db/db mice. Saturable LCFA uptake was increased 2.5- to 4.2-fold and total LCFA uptake 2.6- to 3.9-fold in the EtOH and HFD groups. Despite the small changes in Vmax, saturable LCFA uptake was, nonetheless, increased 1.6- and 1.8-fold in the db/db and ob/ob mice, respectively, principally driven by the increase in LCFAu. Total uptake in these groups was increased 1.5- and 2.4-fold, again principally reflecting the changes in LCFAu.
Fig. 3.
A: Vmax for saturable hepatocellular LCFA uptake. Values are means ± SE. B: absolute rates of saturable and total hepatocellular LCFA uptake (UT) calculated from computed kinetic constants and unbound LCFA concentrations. Values are means ± SE. Except where noted, all values are significantly greater than control (P < 0.01).
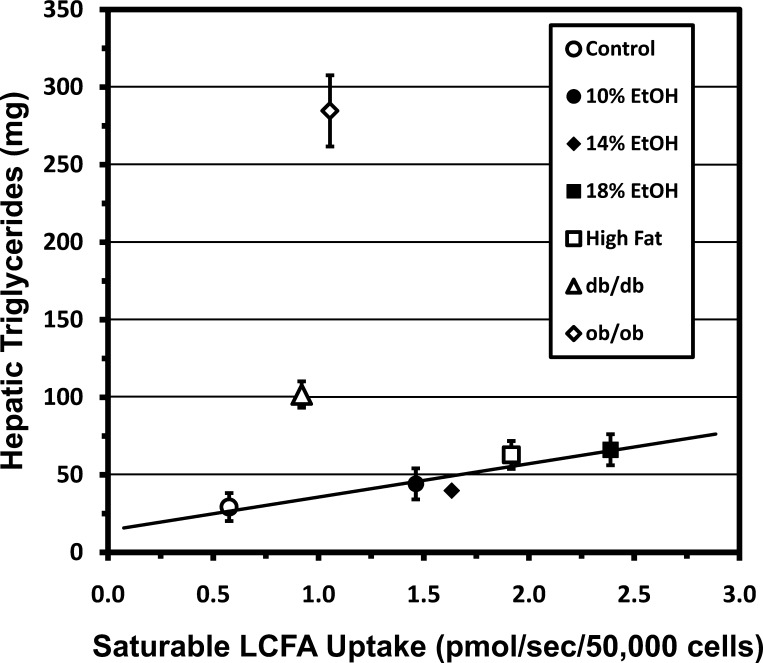
In FLSG mice, saturable and total LCFA uptake were significantly correlated with hepatic TG content (Fig. 4) and liver weight (not shown). The coefficients of determination (R2) for these regressions suggest, e.g., that increased saturable LCFA uptake accounts for ∼80% of the increase in hepatic TG within these groups. Liver TG content and organ weights in ob/ob and db/db mice greatly exceeded values projected as functions of Vmax for saturable or total LCFA uptake from regressions in FLSG mice, implying that processes besides LCFA uptake contributed appreciably to hepatic steatosis in these strains (Fig. 4).
Fig. 4.
Hepatic triglyceride (TG) content plotted as a function of saturable hepatocellular LCFA uptake. Values are group means ± SE. Regression line for groups with functional leptin signaling (control; 10%, 14%, and 18% EtOH; and high fat) indicates that LCFA uptake is highly correlated with hepatic TG content in these groups (R2 = 0.8414, r = 0.92, P < 0.05). Hepatic TG is much greater than predicted by this regression in ob/ob and db/db mice.
Gene Expression Studies
The expression of 19 genes considered relevant to the pathogenesis of hepatic steatosis was quantitated by qRT-PCR (Table 1). The expression of six of these, three enzymes central to the de novo synthesis of fatty acids and three plasma membrane fatty acid transporters, was also studied by Western blotting. In all these cases, the direction of change from that observed in control livers was identical as measured by both methods, and expression ratios measured by qRT-PCR and Western blotting were highly correlated.
De novo lipogenesis.
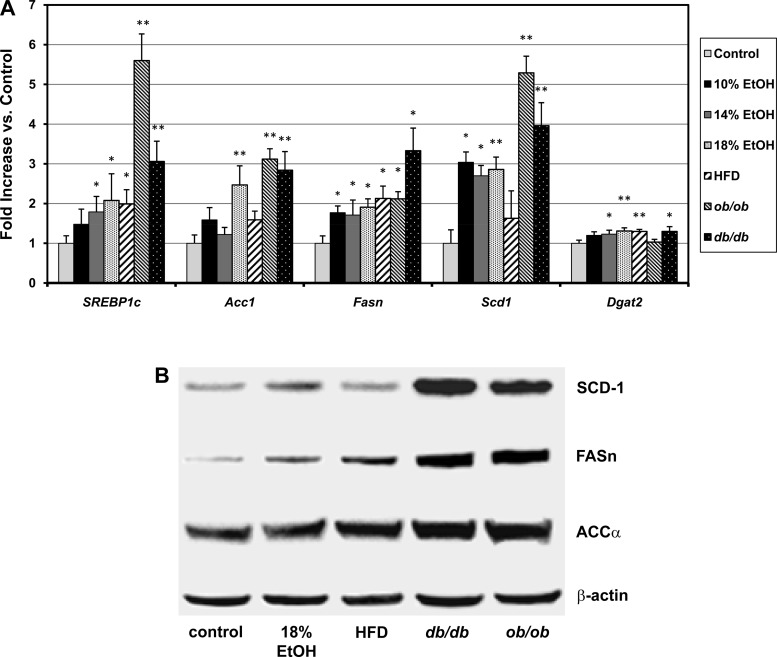
Statistically significant increases vs. control were found in the qRT-PCR gene expression ratios of the hepatic transcription factor sterol regulatory element binding protein 1c (SREBP1c) and of three key enzymes downstream of SREBP1c that regulate LCFA synthesis, acetyl-CoA carboxylase 1 (Acc1), fatty acid synthase (Fasn), and stearoyl-CoA desaturase 1 (Scd1), in most of the models studied, with the greatest increases typically occurring in the ob/ob and db/db groups (Fig. 5A). qRT-PCR expression ratios for SREBP1c were significantly correlated with those of Acc1 (r = 0.85, P < 0.01), Fasn (r = 0.62, P < 0.02), and Scd1 (r = 0.96, P < 0.01), consistent with prior reports that SREBP1c regulates each of these enzymes (30). qRT-PCR expression ratios for diacylglycerol acyltransferase 2 (Dgat2) were also statistically significantly increased. These data suggest that increased LCFA synthesis and, possibly, increased conversion of LCFA to TG, are processes contributing to the development of simple hepatic steatosis from many causes. The data further suggest that upregulation of de novo lipogenesis may be appreciably greater in the ob/ob and db/db than FLSG mice (Fig. 5A). In contrast to Dgat2, we found no statistically significant upregulation of Dgat1 in any experimental group (data not shown). Hepatic lipoprotein lipase (Lpl), which may accelerate LCFA uptake by increasing the local lipolysis of lipopotein TG to LCFA, was significantly upregulated only in ob/ob and db/db mice, and the LDL receptor (Ldlr), which may increase the uptake of preformed TG within lipoproteins, was upregulated only in the HFD group (data not shown). Representative Western blots of the expression of ACC1, FASN, and SCD1 are shown in Fig. 5B. The Western blot expression ratios in the experimental groups tended to be increased to a greater extent than the corresponding qRT-PCR data, but the two data sets were highly significantly correlated (r = 0.688, degrees of freedom = 13, P < 0.01).
Fig. 5.
A: hepatic quantitative real-time PCR gene expression ratios in control mice, EtOH-fed mice, mice fed a high-fat diet (HFD), and ob/ob and db/db mice for sterol regulatory element binding protein 1C (SREBP1c), 3 enzymes regulated by SREBP1c in the LCFA synthetic pathway [acetyl CoA carboxylase 1 (Acc1), fatty acid synthase (Fasn), and stearoyl CoA desaturase 1 (Scd1)], and diacylglycerol acyltransferase 2 (Dgat2). Values are means ± SE. *P < 0.05; **P < 0.01. B: representative Western blots of expression of ACC1, FASN, and SCD1 in control, 18% EtOH, HFD, ob/ob, and db/db mice. β-Actin was used as internal control.
LCFA transporters.
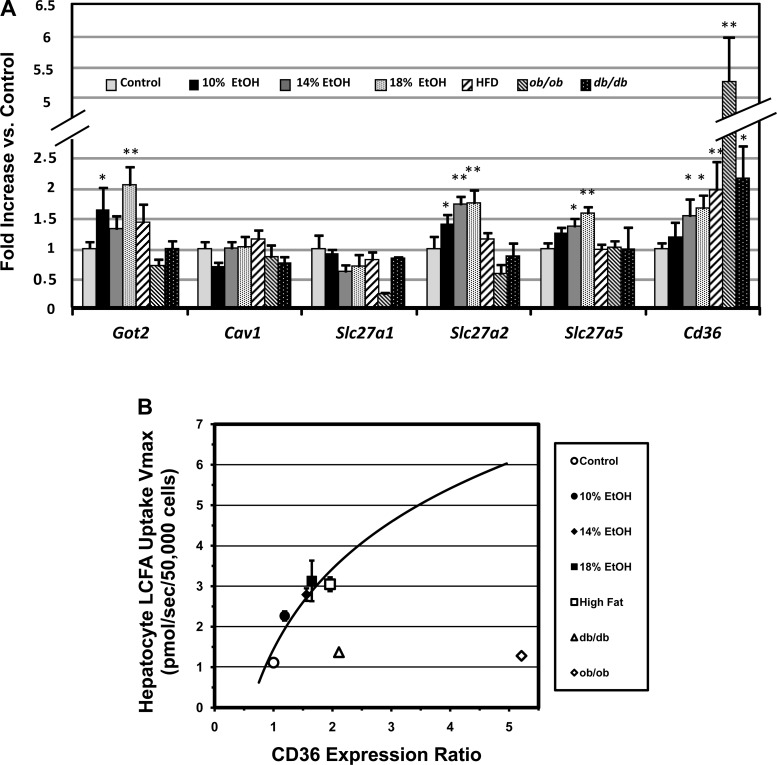
When assessed by qRT-PCR, no statistically significant upregulation was observed in the hepatocellular expression of caveolin 1 (Cav1) or Slc27a1 in any experimental group (Fig. 6A). Got2, Slc27a2, and Slc27a5 gene expression ratios were significantly upregulated in a dose-dependent fashion in the EtOH groups, correlating roughly with Vmax, but were not significantly increased in any of the obesity groups (HFD, ob/ob, or db/db), whether or not they had functional leptin signaling. Cd36 was upregulated most widely, including the 14% and 18% EtOH groups (a small increase in the 10% EtOH group was not statistically significant) and HFD, ob/ob, and db/db groups. Thus, upregulation of Cd36 gene expression was found in the presence and absence of functional leptin signaling. While the Cd36 upregulation also correlated with the Vmax for LCFA uptake (r = 0.95, P < 0.01) in FLSG mice, this relationship broke down in the ob/ob and db/db strains, which had the greatest degrees of Cd36 upregulation but exhibited little or no increase in Vmax (Fig. 6B). Western blot studies for FABPpm, FATP2, and FAT/CD36 protein expression were performed in the control, 18% EtOH, HFD, ob/ob, and db/db groups. Western blot expression ratios were significantly correlated (P ≤ 0.05) with the qRT-PCR results for FABPpm (r = 0.75), FATP2 (r = 0.88), and CD36 (r = 0.89).
Fig. 6.
A: hepatic quantitative real-time PCR gene expression ratios for LCFA transporter genes in controls and 6 experimental groups with hepatic steatosis. No regulation was seen for Cav1, and no upregulation for Slc27a1 was observed in any of the experimental groups. Got2, Slc27a2, and Slc27a5 were significantly upregulated in the EtOH groups, but not in HFD mice. Cd36 was upregulated in all experimental groups. *P < 0.05; **P < 0.01. B: relationship of hepatocyte Cd36 gene expression ratio to Vmax for saturable hepatocellular LCFA uptake. A similar relationship was found for total LCFA uptake (not shown). Cd36 expression shows a tight logarithmic correlation with LCFA uptake in groups with functional leptin signaling (r = 0.95). However, uptake is far less than predicted from this regression and Cd36 expression ratio in ob/ob and db/db mice. Values are group means ± SE.
Additional hepatic gene expression data.
We found no statistically significant regulation of the hepatic genes for apolipoprotein B (ApoB) or microsomal TG transfer protein (Mttp) and, therefore, no support for a significant decrease in LCFA and TG excretion from the liver in VLDL, in any of the models. Similarly, variations in hepatic expression of genes for carnitine palmitoyl transferase 1 (Cpt1), which imports LCFA into mitochondria for oxidation, and in dodecenoyl-CoA δ-isomerase (Dci), a mitochondrial enzyme involved in β-oxidation of unsaturated LCFA, were not statistically significant in these models. Furthermore, genes for cytochrome P-4502E1 (Cyp2e1) and cytochrome P-4504A (Cyp4a), both of which have been implicated in lipid peroxidation and generation of reactive oxygen species in alcoholic liver disease and NAFLD (20, 28, 33, 49), showed no significant changes in expression in any of the groups in the present experiments.
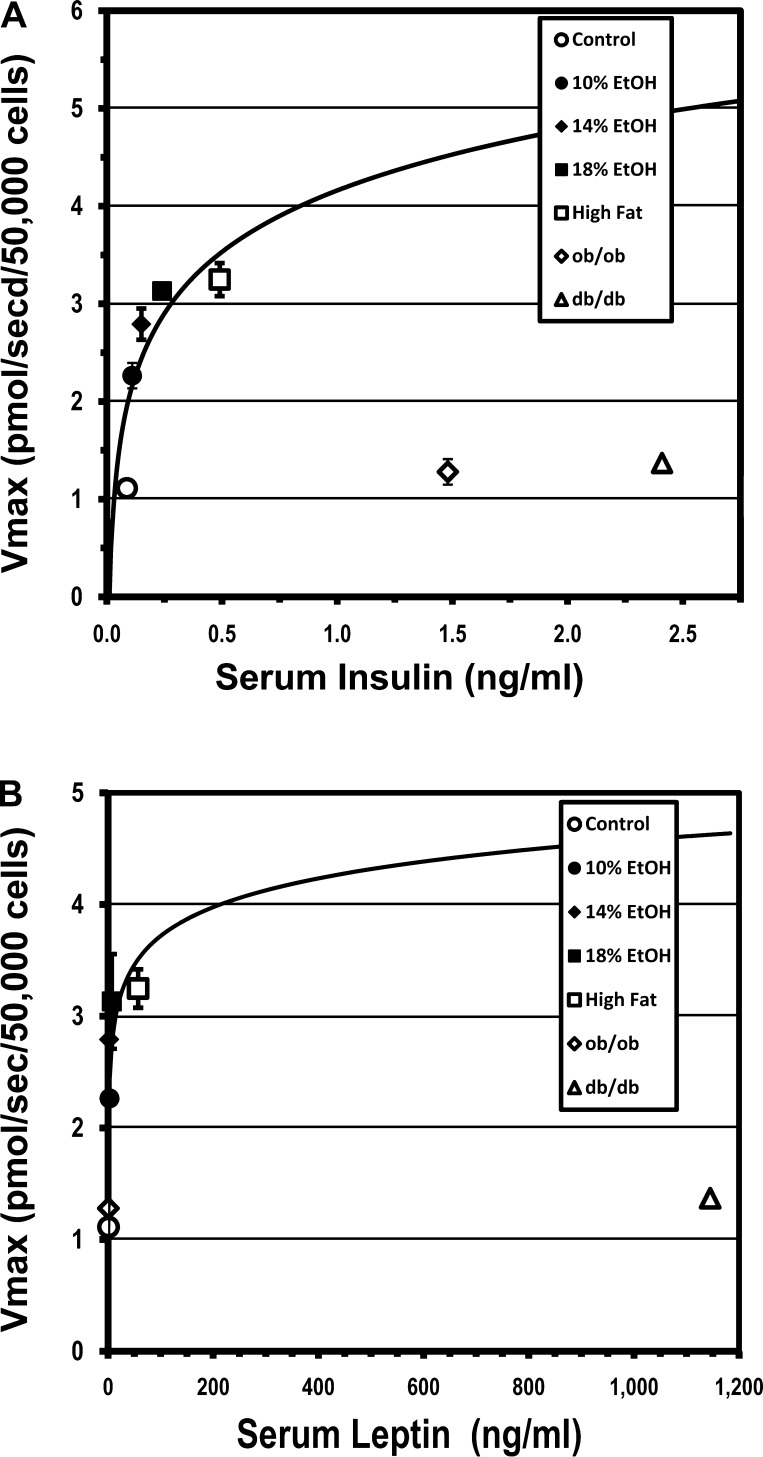
Influence of Insulin and Leptin on Hepatocellular LCFA Uptake
Our studies found an increase in serum levels of insulin and leptin in all the FLSG mice. The increase in leptin in the EtOH groups, in particular, while reported previously (38), does not appear to be widely appreciated. The increase in the Vmax for hepatocellular LCFA uptake that characterized all the other models with hepatic steatosis was absent in ob/ob and db/db mice (Fig. 3). These data amplify observations reported earlier in leptin receptor-deficient Zucker fatty (fa/fa) and Wistar control rats (7). One possible explanation for these data is that leptin may be necessary for upregulation of hepatocellular LCFA uptake. An alternative is that the failure of Vmax to increase in parallel with the increased insulin concentrations observed in ob/ob and db/db mice reflects the marked insulin resistance also present in these strains (Table 2), rather than the absence of leptin signaling per se. To further explore the effects of insulin and leptin on saturable hepatocyte LCFA uptake, the respective Vmax data were plotted as functions of the values for serum insulin and leptin concentrations in the various experimental groups. In FLSG hepatocytes, Vmax for LCFA uptake appeared to be a saturable function of insulin and leptin concentrations (Fig. 7). On the insulin curve (Fig. 7A), the ob/ob and db/db Vmax at the Vmax-insulin data points was less than one-third of that predicted from the FLSG Vmax-insulin regression. On the leptin curve (Fig. 7B), the ob/ob Vmax at the Vmax-leptin data point was similar to that in controls and fell virtually on the FLSG Vmax-leptin regression curve, whereas in db/db animals the corresponding Vmax-leptin data point was, again, less than one-third of the value predicted by the FLSG regression. Thus, leptin and insulin appear to contribute to upregulation of hepatocyte LCFA uptake. The data are, again, consistent with either of two interpretations: 1) leptin is required for expression of the upregulatory effects of insulin on hepatic parenchymal cells, or 2) the failure of ob/ob and db/db mice to upregulate their saturable LCFA uptake processes reflects the marked insulin resistance in these strains. Further studies are required to clarify this issue.
Fig. 7.
Relationship of Vmax for hepatocyte LCFA uptake to serum insulin (A) and leptin (B) concentrations. Values are group means ± SE. Vmax for hepatocyte LCFA uptake in functioning leptin-signaling groups (FLSGs) was an apparent saturable function of serum insulin or leptin concentration, respectively. Deviations from these curves by ob/ob and db/db groups were consistent with a role for insulin in upregulating LCFA uptake. Leptin appears to be necessary for expression of upregulating effects of insulin in hepatocytes.
DISCUSSION
The spectrum of NAFLD is the most prevalent form of liver disease in the Western world. The proportion of patients who traverse the NAFLD spectrum, from simple hepatic steatosis to end-stage liver failure requiring transplantation, is small. However, since the absolute number of NAFLD patients is so large, the number requiring liver transplantation is also large, growing, and already projected to replace chronic hepatitis C as the dominant indication for liver transplantation in the not too distant future (18). Alcoholism is the third most prevalent preventable cause of death in the United States, and end-stage alcoholic liver disease is responsible for many of these deaths (16, 36). Similar to NAFLD, alcoholic liver disease also comprises a spectrum of which the earliest component is simple hepatic steatosis. While this term has been somewhat abused (14), we use it here to describe a histological picture dominated by intracellular large-droplet fat in the absence of major evidence of inflammation, ballooning, Mallory-Denk bodies, other manifestations of cellular degeneration, or fibrosis. Although a greater research effort is devoted to the more advanced and potentially progressive entities, namely, NASH and alcoholic steatohepatitis, we have focused our attention on the pathogenesis and pathophysiology of simple hepatic steatosis because of its position at the start of the slippery slope that can lead to serious alcoholic liver disease and NAFLD.
Our goal was to investigate the pathogenetic mechanisms leading to the development of simple hepatic steatosis in mouse models of obesity and chronic EtOH consumption. We sought to conduct the studies in primarily natural, widely available, minimally invasive models in which, for example, gene knockouts did not interfere with the normal regulatory and counterregulatory responses of intermediary metabolism. In view of a recent study demonstrating that the body's initial responses to nutritional perturbations such as the introduction of an HFD may be quite transitory (39), we also sought to work in models in which steady-state responses to such perturbations had been achieved. Finally, between 8 and 20 wk of age, mice continue to grow and undergo various other maturational changes. Therefore, to eliminate differences in age-related changes in body and organ weights from our final intergroup comparisons, our biostatistical consultant urged us to study all our mice at the same age (20 wk), at which preliminary studies showed clear-cut differences in the major parameters of interest between the three EtOH groups at death. The models in which studies were conducted 3 mo after the introduction of EtOH or an HFD meet these stipulations. While the final requirement regarding normal regulatory responses is not met by congenic strains such as ob/ob and db/db mice, because of the wealth of general information accumulated in these strains coupled with the lack of information about issues of particular interest to our laboratory, such as hepatic LCFA uptake processes, it was very appealing to include them in the study.
Because results in a number of lines of our investigation have diverged substantially between mice with functional leptin signaling (e.g., EtOH- and HFD-fed mice) and those with defective leptin signaling, we have used the term “FLSGs” as a convenient, descriptive shorthand for the former. While the data unequivocally indicate significant differences in some parameters between FLSG and ob/ob and db/db mice, this shorthand should not be interpreted to indicate that these differences are the direct consequences of differences in leptin signaling. As already noted, some of these differences are also interpretable in terms of marked differences in insulin resistance and, consequently, insulin signaling.
In the present study, upregulation of saturable, facilitated hepatocellular LCFA uptake, as reflected, for example, in an increase in the Vmax for total uptake, was found to account for the majority of the increase in hepatic TG content in mice consuming EtOH or an HFD (R2 = 0.73–0.84). In contrast to these results in mice with functioning leptin-signaling systems, the Vmax for saturable LCFA uptake was not increased in ob/ob animals and only modestly increased in db/db animals. Nevertheless, because of an increase in LCFAu in all strains studied, ob/ob and db/db mice also had smaller, but statistically significant, increases in total LCFA uptake that were driven entirely by the increase in LCFAu. Liver weight and hepatic TG content in the ob/ob and db/db strains were increased to a far greater extent than would be predicted from the relationships between these parameters and Vmax or absolute hepatic uptake defined in the FLSG mice. This suggests that another process is a major, if not the major, cause of hepatic steatosis in mice with these mutations. Gene expression studies in these strains suggest that an especially prominent increase in de novo hepatic LCFA synthesis is likely to be that process. Isotope infusion/dilution techniques can be used for direct measurement of de novo hepatic LCFA synthesis in mouse livers in vivo or in isolated mouse hepatocytes in vitro (24–26, 31, 51) and will be employed in our models in future studies. An additional factor that might contribute to the large discrepancy between LCFA uptake and hepatic TG content in the ob/ob and db/db groups is suggested by the upregulation of hepatic Lpl expression, which was observed only in these groups. If hepatic Lpl enzymatic activity is indeed increased in these strains, the resulting increase in local lipolysis of lipoprotein-TG might lead to a local increase in intrasinusoidal LCFA and LCFAu concentrations that are not detected by measurements of these parameters in peripheral blood. This would be unlikely to alter our measurements of the kinetic constants for LCFA uptake, but it is conceivable that, under these circumstances, calculations of absolute rates of LCFA uptake might underestimate the actual fluxes. It is unlikely that the underestimates would be sufficient to account for the large discrepancies between our estimates of LCFA uptake and hepatic TG content.
The small, but statistically significant, increases in Dgat2 found in all strains studied suggest that the enzymatic machinery required to convert observed increases in LCFA uptake and/or synthesis to TG was also increased in all strains studied. Our studies found no evidence that significant decreases in LCFA oxidation or in LCFA and TG excretion in lipoproteins represent a major contributor to hepatic steatosis in our models.
Given the marked increases in saturable hepatocellular LCFA uptake in all models with functional leptin signaling, it was of particular interest to examine the expression of genes for putative LCFA transporters. FABPpm (12, 55), believed to be identical to mitochondrial AST (6, 58), the lipid scavenger receptor CD36 (1), fatty acid transporting protein isoforms 2 and 5 (FATP2 and FATP5) (29, 45, 52), and caveolin-1 (41, 60) have been reported to be transporters for hepatocellular LCFA uptake. Among >3,500 articles about hepatic steatosis published in the past 5 years, there is an extensive literature on the possible roles of each of these proteins in LCFA transport, summarized in multiple reviews (4, 10, 13, 59). All have been found to be upregulated in one or more cell types in a variety of models and systems. However, no prior study of which we are aware examines their roles simultaneously in multiple models. The studies reported here clearly document significant upregulation of saturable hepatocellular LCFA uptake in the setting of hepatic steatosis associated with consumption of an HFD or EtOH, but not in the setting of steatosis due to deficient leptin signaling (ob/ob and db/db mice). In the groups with increased saturable LCFA uptake due to EtOH feeding, there were appreciable increases in Got2, Cd36, Slc27a2, and Slc27a5 gene expression, all of which were highly correlated with increased expression of SREBP1c. Expression of Cd36, but not the others, was also upregulated in the HFD mice. Indeed, Cd36 expression was significantly correlated with the Vmax for hepatocellular LCFA uptake across all the FLSG mice (r = 0.89, P < 0.05). However, the relationship between transporter gene expression and saturable LCFA uptake breaks down in leptin-signaling-deficient strains. The strongest upregulation of Cd36 was found in ob/ob and db/db mice, which had the smallest increases in saturable LCFA uptake. The reasons for this interesting discrepancy remain to be determined. The nonsignificant upregulation of Got2 and absence of upregulation of Slc27a2 and Slc27a5 in HFD mice, which were hyperleptinemic, as well as in the ob/ob and db/db animals, suggest that overall regulation of these multiple transporters may well be multifactorial, with leptin, insulin, and EtOH playing a role. Hepatic expression of Slc27a1 and Cav1 was not found to be upregulated in any of the experimental groups.
The concept that facilitated LCFA transport is mediated by a single transporter in any given cell type is looking increasingly naïve (59). Indeed, evidence is growing that multiple different transporters, possibly responding to common and specific regulatory signals, may play a role in cellular LCFA uptake in a given cell type. This concept is most strongly supported in the heart, where several studies have presented evidence that FABPpm and CD36 may work cooperatively in facilitated LCFA uptake (11, 17, 46). Our study suggests parallel regulation of these two transporters, as well as FATP2 and FATP5, in hepatocytes.
The present studies provide strong functional evidence that regulated LCFA uptake processes play a dominant role in the accumulation of TG in the liver in hepatic steatosis associated with common causes such as obesity and EtOH. The gene expression studies also indicate that several transporters participate in the increased saturable LCFA uptake observed at least in EtOH-fed mice. Head-to-head comparisons of the hepatocellular expression of these multiple LCFA transporters have not been previously reported. However, as summarized in several cited reviews (15, 21, 34, 42, 43), gene expression data from these and other studies suggest that increased de novo synthesis of LCFA is a second, important contributing factor in simple hepatic steatosis from many causes and, perhaps, the dominant process in the absence of leptin signaling. By sequestering increased amounts of TG in lipotoxicity-resistant obese adipocytes, increased facilitated LCFA uptake, demonstrated functionally in rodents (8, 61) and humans (40), and decreased LCFA oxidation, suggested by gene expression results for Dci in mice and for multiple proteins involved in LCFA oxidation in humans (61), may play an important, protective role against lipotoxicity in tissues such as liver, heart, and pancreas.
Other results suggested by the present study, such as the roles of insulin and leptin in regulating facilitated LCFA uptake and the roles and regulation of specific LCFA transporters in promoting simple hepatic steatosis under various circumstances, represent interesting suggestions that should attract future efforts at more concrete experimental validation.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-52401 and DK-72526 and the Columbia Liver Disease Research Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank the Histology Service of the Herbert Irving Comprehensive Cancer Center for preparing the frozen sections and oil red O staining.
REFERENCES
- 1. Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 268: 17665–17668, 1993. [PubMed] [Google Scholar]
- 2. Akagiri S, Naito Y, Ichikawa H, Mizushima K, Takagi T, Handa O, Kokura S, Yoshikawa T. A mouse model of metabolic syndrome: increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat diet-fed male KK/Ta mice. J Clin Biochem Nutr 42: 150–157, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anonymous. SAAM II Users' Guide. Seattle, WA: University of Washington, 1998. [Google Scholar]
- 4. Berk PD. Regulatable fatty acid transport mechanisms are central to the pathophysiology of obesity, fatty liver, and metabolic syndrome. Hepatology 48: 1362–1376, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berk PD, Stump DD. Mechanisms of cellular uptake of long chain free fatty acids. Mol Cell Biochem 192: 17–31, 1999. [PubMed] [Google Scholar]
- 6. Berk PD, Wada H, Horio Y, Potter BJ, Sorrentino D, Zhou SL, Isola LM, Stump D, Kiang CL, Thung S. Plasma membrane fatty acid-binding protein and mitochondrial glutamic-oxaloacetic transaminase of rat liver are related. Proc Natl Acad Sci USA 87: 3484–3488, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berk PD, Zhou S, Bradbury MW. Increased hepatocellular uptake of long chain fatty acids occurs by different mechanisms in fatty livers due to obesity or excess ethanol use, contributing to development of steatohepatitis in both settings. Trans Am Clin Climatol Assoc 116: 335–345, 2005. [PMC free article] [PubMed] [Google Scholar]
- 8. Berk PD, Zhou S, Kiang C, Stump DD, Fan X, Bradbury MW. Selective up-regulation of fatty acid uptake by adipocytes characterizes both genetic and diet-induced obesity in rodents. J Biol Chem 274: 28626–28631, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Berman M, Weiss MF. User's Manual for SAAM. Washington, DC: Department of Health and Human Services, 1967. (US Public Health Service Publication 1703.) [Google Scholar]
- 10. Bonen A, Chabowski A, Luiken JJ, Glatz JF. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical and physiological evidence. Physiology 22: 15–29, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Bonen A, Luiken JJ, Glatz JF. Regulation of fatty acid transport and membrane transporters in health and disease. Mol Cell Biochem 239: 181–192, 2002. [PubMed] [Google Scholar]
- 12. Bradbury M, Berk PD. Cellular uptake of long chain free fatty acids: the structure and function of plasma membrane fatty acid binding protein. Adv Mol Cell Biol 33: 47–81, 2004. [Google Scholar]
- 13. Bradbury MW, Berk PD. Lipid metabolism in hepatic steatosis. Clin Liver Dis 8: 639–671, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Brunt EM. What's in a NAme? Hepatology 50: 663–667, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Byrne CD, Olufadi R, Bruce KD, Cagampang FR, Ahmed MH. Metabolic disturbances in non-alcoholic fatty liver disease. Clin Sci (Lond) 116: 539–564, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control. Alcohol-attributable deaths and years of potential life lost—United States. MMWR Morb Mortal Wkly Rep 53: 866–970, 2001. [PubMed] [Google Scholar]
- 17. Chabowski A, Gorski J, Luiken JJ, Glatz JF, Bonen A. Evidence for concerted action of FAT/CD36 and FABPpm to increase fatty acid transport across the plasma membrane. Prostaglandins Leukot Essent Fatty Acids 77: 345–353, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol 2: 1048–1058, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Chirieac DV, Chirieac LR, Corsetti JP, Cianci J, Sparks CE, Sparks JD. Glucose-stimulated insulin secretion suppresses hepatic triglyceride-rich lipoprotein and apoB production. Am J Physiol Endocrinol Metab 279: E1003–E1011, 2000. [DOI] [PubMed] [Google Scholar]
- 20. Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis 21: 27–41, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr Opin Lipidol 19: 295–300, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Diehl AM. Lessons from animal models of NASH. Hepatol Res 33: 138–144, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 24. Giudetti AM, Leo M, Geelen MJ, Gnoni GV. Short-term stimulation of lipogenesis by 3,5-l-diiodothyronine in cultured rat hepatocytes. Endocrinology 146: 3959–3966, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Gnoni GV, Geelen MJ, Bijleveld C, Quagliariello E, van den Bergh SG. Short-term stimulation of lipogenesis by triiodothyronine in maintenance cultures of rat hepatocytes. Biochem Biophys Res Commun 128: 525–530, 1985. [DOI] [PubMed] [Google Scholar]
- 26. Gnoni GV, Paglialonga G, Siculella L. Quercetin inhibits fatty acid and triacylglycerol synthesis in rat-liver cells. Eur J Clin Invest 39: 761–768, 2009. [DOI] [PubMed] [Google Scholar]
- 27. Goldberg IJ, Ginsberg HN. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology 130: 1343–1346, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Gomez-Lechon MJ, Jover R, Donato MT. Cytochrome P450 steatosis. Curr Drug Metab 10: 692–699, 2009. [DOI] [PubMed] [Google Scholar]
- 29. Hirsch D, Stahl A, Lodish HF. A family of fatty acid transporters conserved from Mycobacterium to man. Proc Natl Acad Sci USA 95: 8625–8629, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horton JG, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109: 1125–1131, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeske DJ, Dietschy JM. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J Lipid Res 21: 364–376, 1980. [PubMed] [Google Scholar]
- 32. Kleiner D, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Nonalcoholic Steateohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005. [DOI] [PubMed] [Google Scholar]
- 33. Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest 105: 1067–1075, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malaguarnera M, Di Rosa M, Nicoletti F, Malaguarnera L. Molecular mechanisms involved in NAFLD progression. J Mol Med 87: 679–695, 2009. [DOI] [PubMed] [Google Scholar]
- 35. Minehira K, Young SG, Villanueva CJ, Yetukuri L, Oresic M, Hellerstein MK, Farese RV, Jr, Horton JD, Preitner F, Thorens B, Tappy L. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J Lipid Res 49: 2038–2044, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 291: 1238–1245, 2004. [DOI] [PubMed] [Google Scholar]
- 37. Nunes R, Sorrentino D, Berk PD. Oleate uptake by isolated hepatocytes consists of two components, each driven by the unbound oleate concentration. In: Proceedings of the Third International Congress of Mathematical Modelling of the Liver Excretory Process. Tokyo: Juntendo University Press, 1990, p. 312–316. [Google Scholar]
- 38. Obradovic T, Meadows GG. Chronic ethanol consumption increases plasma leptin levels and alters leptin receptors in the hypothalamus and the perigonadal fat of C57BL/6 mice. Alcohol Clin Exp Res 26: 255–262, 2002. [PubMed] [Google Scholar]
- 39. Petrescu O, Cheema AF, Fan X, Bradbury MW, Berk PD. Differences in adipocyte long chain fatty acid uptake in Osborne-Mendel and S5B/Pl rats in response to high-fat diets. Int J Obes (Lond) 32: 853–862, 2008. [DOI] [PubMed] [Google Scholar]
- 40. Petrescu O, Fan X, Gentileschi P, Hossain S, Bradbury M, Gagner M, Berk PD. Long-chain fatty acid uptake is upregulated in omental adipocytes from patients undergoing bariatric surgery for obesity. Int J Obes (Lond) 29: 196–203, 2005. [DOI] [PubMed] [Google Scholar]
- 41. Pohl J, Ring A, Stremmel W. Uptake of long-chain fatty acids in HepG2 cells involves caveolae: analysis of a novel pathway. J Lipid Res 43: 1390–1399, 2002. [DOI] [PubMed] [Google Scholar]
- 42. Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 118: 829–838, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Postic C, Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab 34: 643–648, 2008. [DOI] [PubMed] [Google Scholar]
- 44. Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res 49: 1068–1076, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79: 427–436, 1994. [DOI] [PubMed] [Google Scholar]
- 46. Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res 79: p. 249–258, 2008. [DOI] [PubMed] [Google Scholar]
- 47. Sorrentino D, Kiang CL, Berk PD. At physiologic albumin/oleate concentrations oleate uptake by isolated hepatocytes, cardiac myocytes and adipocytes is a saturable function of the unbound oleate concentration. Uptake kinetics are consistent with the conventional theory. J Clin Invest 84: 1325–1333, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sorrentino D, Van Ness K, Berk PD. Preparation and characterization of isolated hepatocytes for transport studies. In: Methods in Biliary Research, edited by Muraca M. Boca Raton, FL: CRC, 1995, p. 255–264. [Google Scholar]
- 49. Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab 295: E10–E16, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Spector AA, Fletcher JE, Ashbrook JD. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry 10: 3229–3232, 1971. [DOI] [PubMed] [Google Scholar]
- 51. Stacpoole PW, Harwood HJ, Jr, Varnado CE. Regulation of rat liver hydroxymethylglutaryl coenzyme A reductase by a new class of noncompetitive inhibitors. Effects of dichloroacetate and related carboxylic acids on enzyme activity. J Clin Invest 72: 1575–1585, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stahl A, Gimeno RE, Tartaglia LA, Lodish HF. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol Metab 12: 266–273, 2001. [DOI] [PubMed] [Google Scholar]
- 53. Stremmel W, Berk PD. Hepatocellular influx of [14C]oleate reflects membrane transport rather than intracellular metabolism or binding. Proc Natl Acad Sci USA 83: 3086–3090, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stremmel W, Strohmeyer G, Berk PD. Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc Natl Acad Sci USA 83: 3584–3588, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stremmel W, Strohmeyer G, Borchard F, Kochwa S, Berk PD. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci USA 82: 4–8, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stump DD, Fan X, Berk PD. Oleic acid uptake and binding by rat adipocytes define dual pathways for cellular fatty acid uptake. J Lipid Res 42: 509–520, 2001. [PubMed] [Google Scholar]
- 57. Stump DD, Nunes RM, Sorrentino D, Isola LM, Berk PD. Characteristics of oleate binding to liver plasma membranes and its uptake by isolated hepatocytes. J Hepatol 16: 304–315, 1992. [DOI] [PubMed] [Google Scholar]
- 58. Stump DD, Zhou SL, Berk PD. Comparison of plasma membrane FABP and mitochondrial isoform of aspartate aminotransferase from rat liver. Am J Physiol Gastrointest Liver Physiol 265: G894–G902, 1993. [DOI] [PubMed] [Google Scholar]
- 59. Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab 20: 72–77, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trigatti BL, Anderson RG, Gerber GE. Identification of caveolin-1 as a fatty acid binding protein. Biochem Biophys Res Commun 255: 34–39, 1999. [DOI] [PubMed] [Google Scholar]
- 61. Walewski J, Ge F, Gagner M, Inabnet WB, Pomp A, Branch AD, Berk PD. Adipocyte accumulation of long chain fatty acids in obesity is multifactorial, resulting from increased fatty acid uptake and decreased activity of genes involved in fat utilization. Obes Surg 20: 93–107, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wosilait WD, Nagy P. A method of computing drug distribution in plasma using stepwise association constants: clofibrate acid as an illustrative example. Comput Programs Biomed 6: 142–148, 1976. [DOI] [PubMed] [Google Scholar]