Abstract
Neutrophils and their chemoattractants, the CXC-ELR chemokines keratinocyte cytokine (KC) and macrophage inflammatory protein-2 (MIP-2), play a critical role in pancreatitis. While acute pancreatitis is initiated in acinar cells, it is unclear if these are a source of CXC-ELR chemokines. KC and MIP-2 have NF-κB, activator protein-1 (AP-1) sites in their promoter regions. However, previous studies have shown increased basal and reduced caerulein-induced AP-1 activation in harvested pancreatic tissue in vitro, which limits interpreting the caerulein-induced response. Moreover, recent studies suggest that NF-κB silencing in acinar cells alone may not be sufficient to reduce inflammation in acute pancreatitis. Thus the aim of this study was to determine whether acinar cells are a source of KC and MIP-2 and to understand their transcriptional regulation. Primary overnight-cultured murine pancreatic acini were used after confirming their ability to replicate physiological and pathological acinar cell responses. Upstream signaling resulting in KC, MIP-2 upregulation was studied along with activation of the transcription factors NF-κB and AP-1. Cultured acini replicated critical responses to physiological and pathological caerulein concentrations. KC and MIP-2 mRNA levels increased in response to supramaximal but not to physiological caerulein doses. This upregulation was calcium and protein kinase C (PKC), but not cAMP, dependent. NF-κB inhibition completely prevented upregulation of KC but not MIP-2. Complete suppression of MIP-2 upregulation required dual inhibition of NF-κB and AP-1. Acinar cells are a likely source of KC and MIP-2 upregulation during pancreatitis. This upregulation is dependent on calcium and PKC. MIP-2 upregulation requires both NF-κB and AP-1 in these cells. Thus dual inhibition of NF-κB and AP-1 may be a more successful strategy to reduce inflammation in pancreatitis than targeting NF-κB alone.
Keywords: keratinocyte cytokine, macrophage inflammatory protein-2, activator protein-1, nuclear factor-κB, acinar
acute inflammation is a hallmark of acute pancreatitis (2, 15, 49, 54, 55, 57, 85). Neutrophil recruitment into the pancreas as a part of the inflammatory response worsens pancreatic injury (63). Serum levels of potent neutrophil chemoattractants, the CXC-ELR chemokines, are increased in severe human pancreatitis (68) and rodent pancreatitis (24, 27, 53, 77, 88). In addition, neutralizing antibodies to the CXC-ELR chemokines, cytokine-induced neutrophil chemoattractant (CINC), the rat homologue of keratinocyte cytokine (KC) (CXCL1) or IL-8 (5, 9), and macrophage inflammatory protein-2 (MIP-2) (CXCL2) (56), or their receptor CXCR2 (10), ameliorate the inflammatory response, resulting in decreased local (10, 56) and systemic injury (9, 10, 56) during pancreatitis.
The dependence of the CXC chemokine mob-1 (25, 30, 31) and CC chemokine monocyte chemotactic protein-1 (MCP-1) (8, 25) on NF-κB in acinar cells has been demonstrated. Although NF-κB has been proposed as a therapeutic target in pancreatitis (15) and its activation in acinar cells triggers pancreatitis (6), caerulein-induced pancreatitis in mice with selective inducible deletion of Rela/p65 in the exocrine pancreas was associated with more severe pancreatic neutrophil infiltration, necrosis, and systemic inflammation (2) than in wild-type mice. Therefore, it is important to explore potential NF-κB-independent regulation of neutrophil chemoattractants. Interestingly, CXC-ELR chemokines have both NF-κB and activator protein-1 (AP-1) binding sites in their promoter regions (60, 67, 81). Additionally, MIP-2 regulation depends on cyclic adenosine 5′-monophosphate (cAMP) in other systems (40). These observations, along with the fact that the relative contribution of different transcription factors in regulating these important players in pancreatitis (9, 10, 27, 53, 56, 77, 88) is cell specific (22, 51), persuaded us to study the transcriptional regulation of these chemokines in pancreatic acinar cells.
Whereas acute pancreatitis is thought to be initiated in acinar cells (76, 80), which express tumor necrosis factor-α (26), mob-1, and MCP-1 (8, 25), other cells shown to upregulate CXC-ELR chemokines include periacinar myofibroblasts (3), their precursor stellate cells (79), and later in pancreatitis, inflammatory cells (75). Factors limiting analysis of AP-1 signaling in fresh acinar cells include basal activation of upstream signaling and AP-1 itself (11, 74). This has been thought to be due to the harvesting protocol (11). Consequent basal upregulation of chemokine mRNAs results in decreased responsiveness to further stimulation (11, 25). Therefore, our first aim was to develop a system to study the signaling involved in CXC-ELR expression in acinar cells in vitro. Our second aim was to determine the differential role of NF-κB and AP-1 in the regulation of CXC-ELR chemokines. To explore this, we used acini from TRE-luc mice [these mice have the luciferase gene under the control of the 12-O-tetradecanoylphorbol-3-acetate response element (TRE)] to study AP-1 (16, 38, 59, 89) activation. We preferred this as a measure of transcriptional activation, since several proteins that differ considerably in their ability to activate transcription of target genes can form complexes that bind to AP-1 sites (33, 44) and may be present in nuclear protein extracts analyzed by enzyme-linked immunosorbent assay or electrophoretic mobility shift assays (EMSAs). Moreover, phosphorylation of specific sites on proteins enhances their AP-1 transactivating potential without affecting their DNA-binding activities (19, 33). Interestingly, we show in this study that NF-κB inhibition completely prevented KC upregulation, but MIP-2 upregulation was much less affected. On the other hand, dual inhibition of AP-1 and NF-κB with 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2) prevented the upregulation of both KC and MIP-2. Therefore, dual inhibition of NF-κB and AP-1 may be a more successful strategy than inhibiting NF-κB alone in reducing the inflammatory response of pancreatitis.
MATERIALS AND METHODS
C57bl/6 mice were purchased from Charles River Laboratories (Wilmington, MA). AP-1 luciferase (TRE-luc) mice were a kind gift from Dr. Mercedes Rincon, University of Vermont. Mice were housed with a 12:12-h light-dark cycle at temperatures from 21 to 25°C, were fed standard laboratory chow, and allowed to drink ad libitum. Caerulein was purchased from Research Plus (Bayonne, NJ). RNA was extracted using Trizol (Invitrogen, Carlsbad, CA). Universal 18S Internal Standards containing primers/Competimers were purchased from Applied Biosystems (Foster City, CA). These primers yield a 315-bp band. Cyber Gold, Platinum PCR Supermix, and KC, MIP-2 primers (see sequences in Table 1) were purchased from Invitrogen. The Gel Doc system was from Bio-Rad (Hercules, CA). All other reagents and chemicals were purchased from Sigma (St. Louis, MO). All experimental protocols were approved by the Institutional Animal Use Committee of the Beth Israel Deaconess Medical Center (Boston, MA) and the University of Pittsburgh (Pittsburgh, PA).
Table 1.
Primer sequences used for semiqunatitative PCR
| mRNA | Forward Primer | Reverse Primer | Size, bases | Accession No. |
|---|---|---|---|---|
| KC | 5′-GACGAGACCAGGAGAAACAGGGTT | 5′-AACGGAGAAAGAAGACAGACTGCT | 533 | J04596 |
| MIP-2 | 5′-TGGGTGGGATGTAGCTAGTTCC | 5′-AGTTTGCCTTGACCCTGAAGCC | 466 | X53798 |
The primer sequences are shown from the 5′−3′ end, along with the base pair size expected from the mRNA sequence and the GeneBank accession no. KC, keratinocyte cytokine; MIP-2, macrophage inflammatory protein-2.
Selection of Mice
AP-1-luciferase reporter transgenic mice with a firefly luciferase gene driven by two copies of the 2XTRE (human collagenase TRE) and generated as previously reported (59) were crossed with C57bl/6 mice. Offspring (4 wk old) were tested for the AP-1 luciferase reporter gene as described previously (38). Mice transgenic for the AP-1 luc were used at 3–4 mo of age.
Preparation and the Use of Acini
The procedure was carried out with sterile precautions, solutions, and apparatus. Acini were prepared as previously described (70) in RPMI 1640 complemented with HEPES, 10% FCS, and penicillin streptomycin antibiotics. The acinar cells were sterile filtered through a 70-μm mesh and poured in six-well plates. The cells were in suspension culture with 5% CO2 overnight (i.e., ∼16 h). After overnight incubation, viability of cells was checked with trypan blue exclusion, and cells were stimulated for variable periods after preincubation with various inhibitors for 90 min (details of the treatments are provided in the legends for Figs. 1–6). Samples that were not immediately processed were stored in RNAlater (Applied Biosystems). Amylase, trypsin, or lactate dehydrogenase (LDH) leakage were measured in cells washed and resuspend in HEPES Ringer buffer (to reduce the interference from the FCS) as described previously (72).
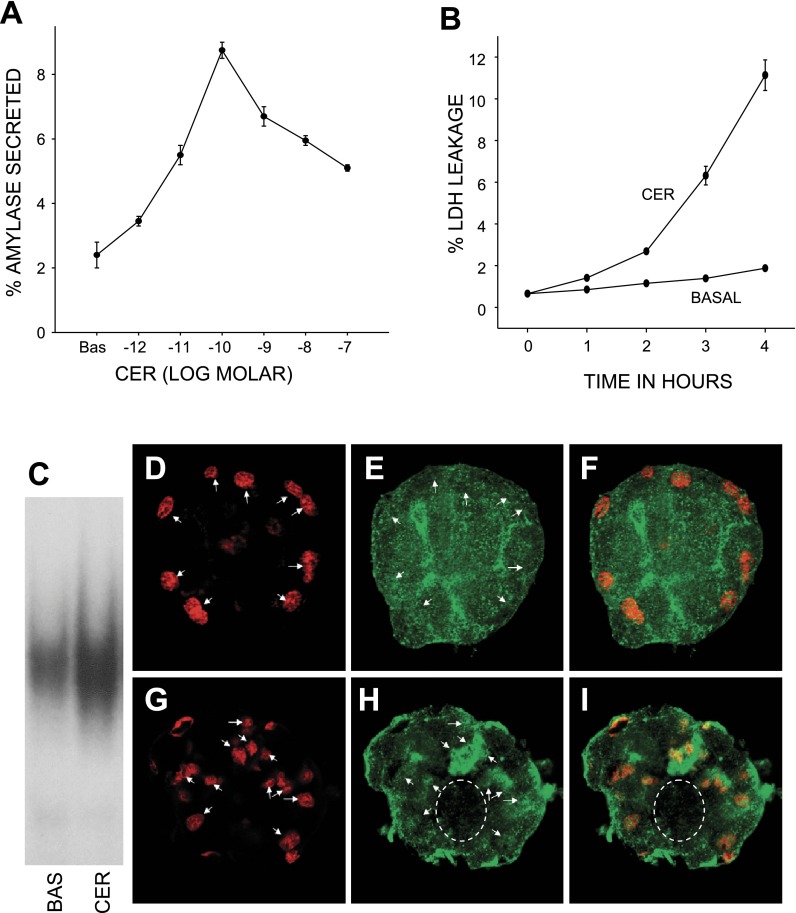
Fig. 1.
Acinar cells after overnight culture display dose-dependent physiological and pathological responses to caerulein. Amylase secretion (A) measured over 30 min in response to logarithmic doses of caerulein (shown on the x-axis), and expressed as a percentage of total amylase, peaks at 100 pM caerulein. Cultured acinar cells treated with 100 nM caerulein leak lactate dehydrogenase (LDH) (B) in the medium compared with control acini [basal (BAS)]. LDH leakage in the medium was measured in aliquots taken every hour over 4 h, expressed as a percentage of the total LDH content, and plotted against time (x-axis). C: comparison of 100 nM caerulein (CER)-mediated NF-kB activation with basal levels as measured by EMSA on nuclear protein extracts of cultured acinar cells. D–I: confocal immunofluorescence images of cultured acinar cells under basal conditions (D–F) and 45 min after stimulation with 100 nM caerulein (G–I), showing lack of nuclear enrichment (D and E, arrows) of p65 under basal conditions (F). Caerulein causes p65 (H) to enrich in the nucleus (arrows in G and H) with a decrease in cytoplasmic staining (dashed oval).
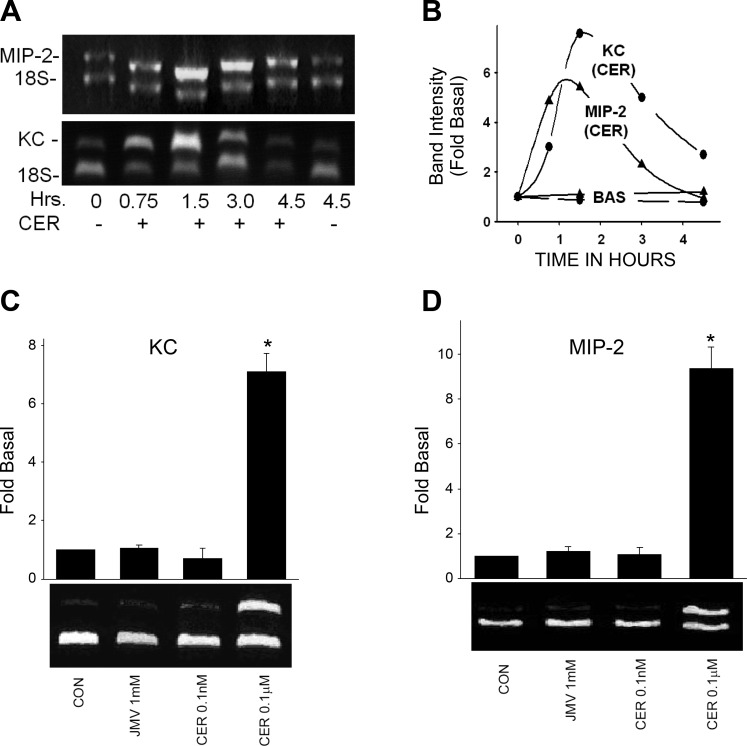
Fig. 2.
Supraphysiological but not physiological caerulein increases keratinocyte cytokine (KC) and macrophage inflammatory protein-2 (MIP-2) mRNA levels. A: representative images, and corresponding quantification (B), of changes in levels of MIP-2 (upper band, top) and KC (upper band, bottom) compared with 18S (lower bands) in response to 100 nM caerulein over 4.5 h in cultured acini. There is no change in basal levels (A and B). C and D: supraphysiological (0.1 μM), but not physiological, doses of caerulein (0.1 nM) or the high-affinity receptor agonist JMV (1 mM) cause an increase in KC. *P value <0.01 over basal. Representative images are below the graphs showing the results from 3 experiments.
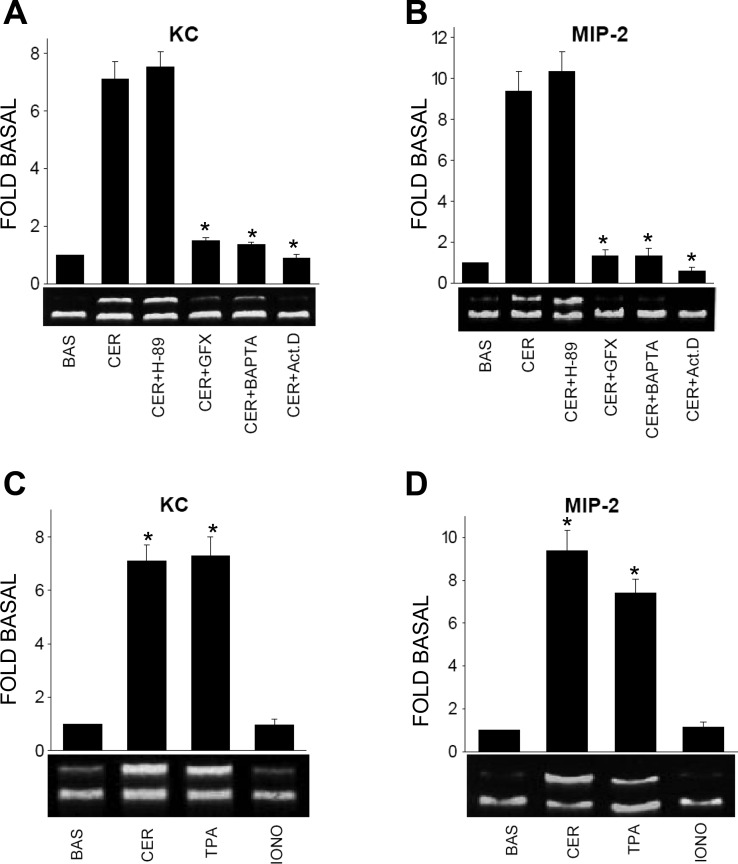
Fig. 3.
Caerulein-induced KC and MIP-2 mRNA increase is dependent on transcription, calcium, and protein kinase C (PKC): H-89 (30 μM) (CER + H-89), 1 μM GF-109203X (CER + GFX), 20 μM BAPTA-AM (CER + BAPTA), or 5 μM actinomycin D (CER + Act.D) were added to the cultured acinar suspension 90 min before stimulation with 0.1 μM caerulein. These were then stimulated for 90 min, and the mRNA levels of KC (A) or MIP-2 (B) were measured by semiquantitative RT-PCR, with 18S as an internal standard. All but H-89 completely prevented the increase in chemokine mRNAs. C and D: cultured acini were stimulated with 0.1 μM caerulein, 1 μM 12-O-tetradecanoylphorbol-3-acetate (TPA), or 1 μM ionomycin (IONO) for 90 min, and the mRNA levels of KC (C) or MIP-2 (D) were measured by semiquantitative RT-PCR, with 18S as an internal standard. Representative images are below the graphs showing the results from 3 experiments. *P < 0.01 compared with basal.
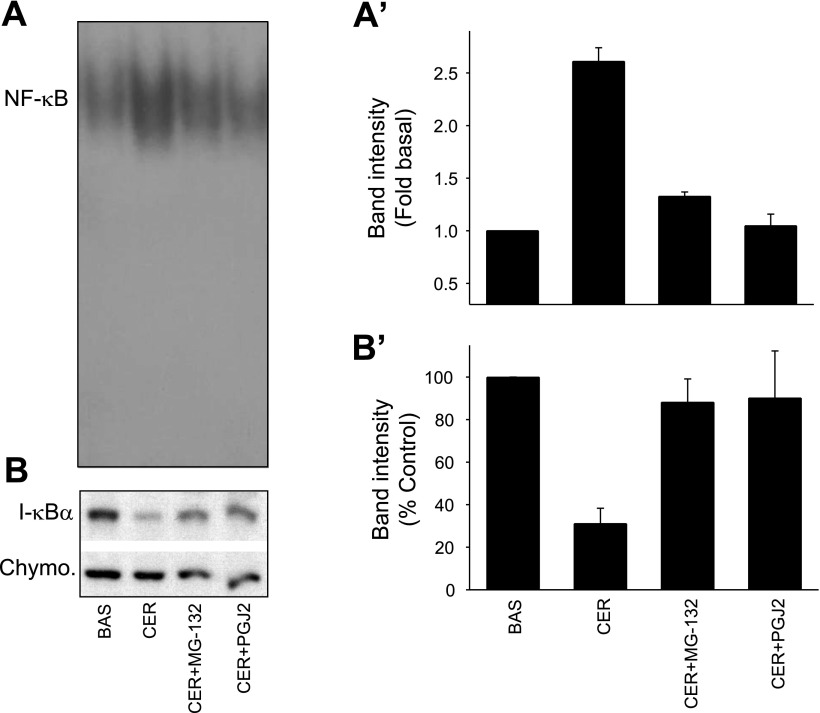
Fig. 4.
MG-132 and 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2) prevent caerulein-induced NF-κB activation, inhibitory κB (IκB) degradation in the cultured acini: The cultured acini were preincubated with or without 20 μM MG-132 (CER + MG-132), or 20 μM PGJ2 (CER + PGJ2) for 90 min and then stimulated with 0.1 μM caerulein for 45 min. Electrophoretic mobility shift assay (EMSA) for NF-κB was run on the nuclear protein. A: bands from a representative gel; A′: densitometry; B: IκB-α degradation induced by 100 nM caerulein on Western blotting using chymotrypsin (Chymo) as a loading control, and its prevention by MG-132 and PGJ2. Quantization of this can be seen in B′. *P < 0.05 compared with other values.
Fig. 5.
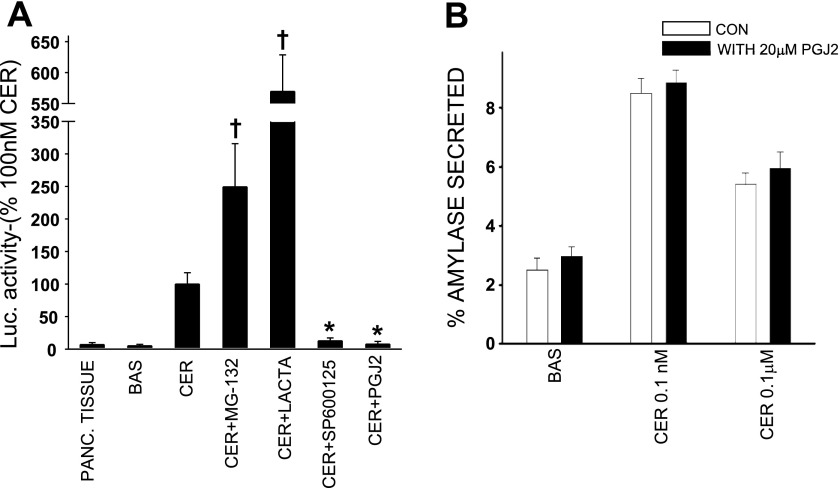
PGJ2 inhibits activator protein-1 (AP-1) activity but does not affect stimulation secretion coupling in acini. A: cultured acini were either left unstimulated (BASAL) or were stimulated with 0.1 μM caerulein after a 90-min pretreatment with 20 μM MG-132 (CER + MG-132), 10 μM lactacystin (CER + Lacta), 25 μM SP-600125, or 20 μM PGJ2 (CER + PGJ2) for a total duration of 24 h. Luciferase activity (arbitrary units/mg protein) was measured in each sample, and results were plotted as a percentage of caerulein. The luciferase activity in freshly ground pancreatic tissue was also measured (PANC. TISSUE). †Significant (P < 0.05) increase compared with 100 nM caerulein. *Significant (P < 0.05) decrease compared with caerulein. Cultured acini were washed and suspended in HEPES buffer to measure amylase release (B). Some of these were then preincubated with 20 μM PGJ2 for 15 min (filled bars), and the amylase release was measured over 30 min from acini that were either unstimulated (BAS) or in response to 0.1 nM and 0.1 μM caerulein. Open bars: amylase from acini untreated with PGJ2. Secretion was expressed as a percentage of total amylase content of the acini.
Fig. 6.
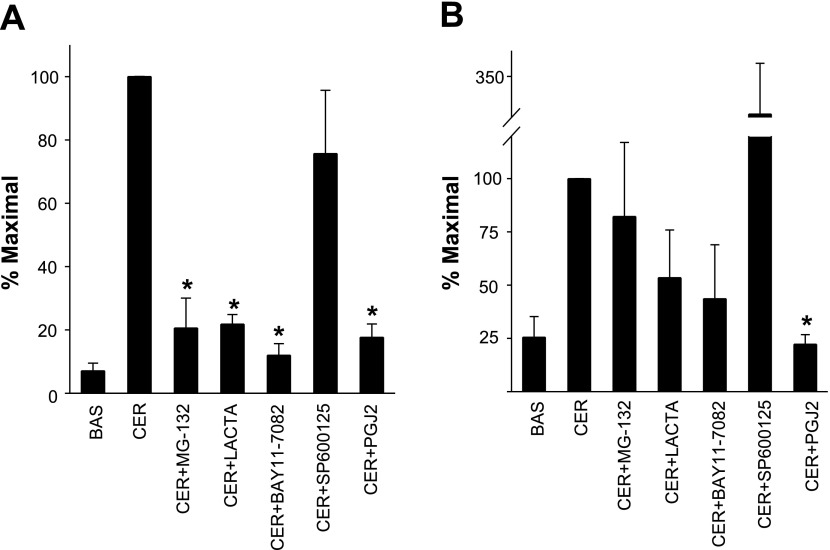
KC upregulation is NF-κB dependent, but that of MIP-2 requires both NF-κB and AP-1. MG-132 (20 μM) (CER + MG-132), 10 μM lactacystin (CER + Lacta), 25 μM SP-600125, or 20 μM PGJ2 (CER + PGJ2) were added to the acinar culture before stimulating these with 0.1 μM caerulein for 90 min. mRNA levels of KC (A) or MIP-2 (B) were measured by real-time PCR. The graphs show the means ± SE from 3 separate experiments. *P < 0.01 compared with CER.
Assays
Semiquantitative RT-PCR with 18S as an internal standard for KC, MIP-2.
RNA was extracted as per the manufacturer's protocol, and quality was checked on a 1% Tris-borate EDTA agarose gel with ethidium bromide and quantified by measuring the absorbance at 260 nm. Nondegraded RNA (5 μg) was used for the reverse transcriptase reaction done using random primers and Superscript (Invitrogen) as per the manufacturer's instructions. For PCR, the primers used were gene-specific intron-spanning primers as described in Table 1. Samples were initially denatured at 94°C for 4 min, followed by cyclical denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 60 s followed by a final extension phase at 72°C for 10 min at the end of the reaction. This yielded a single band corresponding to the base-pair size expected from the primer sequence. The total number of cycles for the semiquantitative PCR using 18S as an internal standard was decided according to the manufacturer's instructions using the following protocol: a sample was removed after every two cycles starting at 22 cycles, and going up to 34 cycles. Each of these was then run in a separate lane on a 1.0% Tirs-acetate EDTA (TAE) gel, and the bands were stained with Cyber Gold. The density of these bands was quantified using the GEL-DOC and plotted (y-axis) against the number of cycles (x-axis), yielding a sigmoid curve. A vertical line was drawn on the x-axis from the center of the linear phase, and the number of cycles chosen (28) corresponded to this number. The 18S primer-to-competimer ratio was found to be optimal at 1:3, and 1 μl of the mix was added to each PCR tube with 1 μl of cDNA. The TAE gels were run, and band density was measured as described above. The ratio of the chemokine/18S band was measured in each case and converted to the fold amount of the basal band ratio. The system was validated by comparing the results with real-time PCR as described below.
Real-time PCR.
Real-time PCR was performed on an ABI Prism 7000 Sequence Detection System (Applied Biosystems) using proprietary Taqman Gene Expression assays (Applied Biosystems) as described previously (7) (KC: CXC11, Mm00433859_m1 and MIP-2: CXC12, Mm00436450_m1). The relative expression levels were calculated after normalization to β-actin (catalog no. 4352341E; Applied Biosystems) using the ΔΔCt method recommended by Applied Biosystems (User Bulletin no. 2, 1997).
EMSA.
For evaluation of NF-κB activation, 10 μg nuclear protein extracts prepared as described by Dyer and Herzog (21) were incubated with 1 × 106 cpm of the [γ-32P]ATP end-labeled κB oligonucleotide probe (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) for 20 min at room temperature. DNA-protein complexes were resolved on a 6% nondenaturing polyacrylamide gel. Gels were dried and exposed to Kodak Bio Max MR films at −70°C. NF-κB bands from films were quantified by an HP Scanjet 4100 scanner and a Scion image analysis program.
AP-1 Luciferase Activity
Acini cultured overnight were preincubated with the inhibitors at the indicated concentration, stimulated with 100 nM caerulein. The reaction was then stopped on ice, and cells were washed and lysed in the 5× lysis buffer accompanying the Luciferase Assay Kit (Promega, Madison, WI) and read on a luminometer (TD-20/20; Turner Biosystems, San Francisco, CA). Results were calculated as relative light units per microgram protein. Preliminary results revealed a 2.5-fold upregulation at 8 h and 14.3-fold upregulation at 24 h with 100 nM caerulein. We chose the 24-h time point for further studies.
SDS-PAGE and Western Blotting
These assays were performed as previously described (52). Protein levels of inhibitory κB (IκB) and chymotrypsin were assessed using polyclonal anti-IκB-α antibody from Santa Cruz (Santa Cruz, CA) and monoclonal anti-chymotrypsin antibody from Millipore (Temecula, CA). Rabbit polyclonal and mouse monoclonal horseradish peroxidase-conjugated secondary antibodies were from Millipore. Secondary antibodies were detected using ECL reagents form Calbiochem (Darmstadt, Germany). All procedures were carried out according to the manufacturers' instructions.
Immunofluorescence Studies
Acini were fixed with 2% paraformaldehyde, permeabilized, blocked with 5% normal goat serum, and exposed to NF-κB p65 polyclonal antibody (Santa Cruz Biotechnology) (1:50) overnight at 4°C. After three washes, goat anti-rabbit Alexa 488 (Invitrogen) and DRAQ5 (1:1,000) for nuclear staining were added for 1 h. After being washed, slides were mounted (fluormount; Sigma) and imaged on a Zeiss Meta (LSM510) confocal microscope using a ×63 lens using 1-μm-thick sections. Images were processed as described previously (71).
Analysis of Data
The results represent means ± SE from three or more separate experiments. In Figs. 1–6, vertical bars denote SE values. Statistical evaluation of data was accomplished by using a Student's t-test to compare two samples or ANOVA for more than two samples, and P values <0.05 were considered significant. All EMSA and RT-PCR gels shown are representative of at least three such gels prepared from independent experiments.
RESULTS
Validation of the Experimental Setup
Before studying the signaling involved in KC and MIP-2 upregulation, we optimized the experimental setup to replicate in vivo acinar responses. Although fresh acini do replicate this in vivo injury and inflammatory response with caerulein (61), they have basal activation of transcription factors AP-1 and NF-κB along with upstream signaling events (11, 74), with consequent upregulation of chemokine mRNAs and decreased responsiveness to further stimulation (11, 25). A 3-h pacification of these was ineffective in our studies [Supplemental Fig. 3 (Supplemental data for this article may be found on the American Journal of Physiology: Gastrointestinal and Liver Physiology website.)]. Optimization involved culturing the acini overnight. We therefore confirmed viability, secretory responsiveness, and injury response to supraphysiological caerulein. These cells displayed a typical biphasic pattern of amylase secretion (Fig. 1A) that peaked at 0.1 nM as in fresh acini (86). They also displayed an injury response to supramaximal caerulein with blebbing (data not shown) and LDH leakage (Fig. 1B). Trypsinogen activation at 30 min in response to 100 nM caerulein was significantly (P < 0.05) less in cultured acini (1.2-fold basal vs. 2.3-fold in fresh acini). However, this was not a concern since strong evidence supports the independence of transcription factor activation from trypsinogen activation (29, 34, 41). Thus they showed several of the physiological and injury responses of fresh acinar cells and pancreatic exocrine tissue in vivo. The viability of the cultured acini typically exceeded 95%.
To verify that transcription factor activation was indeed in acinar cells and not in contaminating cells (e.g., stellate or duct cells), we studied p65 translocation to the nucleus by immunostaining. As shown in Fig. 1, E and F, p65 is uniformly distributed all over the acinar cell, with some enrichment in the apical area compared with the basal nuclear area. Stimulation with 100 nM caerulein results in p65 translocating to the nuclear area (Fig. 1, H and I), with depletion in the cytoplasmic area (Fig. 1, H and I). This was verified on EMSA (Fig. 1C), which showed increased NF-kB activation with caerulein.
We also validated the semiquantitative RT-PCR, comparing it with real-time PCR. The semiquantitative method yielded single bands of the appropriate size (Supplemental Fig. 1). Comparison of real-time and semiquantitative PCR methods showed a similar 8.4-fold increase (Supplemental Fig. 2) in MIP-2 mRNA after 90 min of stimulation with 100 nM caerulein compared with basal amounts at the same time in unstimulated cells. We thus chose the semiquantitative method for further studies and verified critical conclusions with real-time PCR.
Supraphysiological Amounts of Caerulein Increase mRNAs of KC and MIP-2 in Acinar Cells
To study the regulation of CXC-ELR chemokines in pancreatic acinar cells, we first determined the time course of changes in their mRNA levels in response to supramaximal caerulein (100 nM). The increase was rapid and detectable within 45 min. The mRNA levels peaked at 90 min (KC: 6.6 ± 1.4-fold basal, MIP-2: 4.8 ± 1.5-fold basal), followed by a slower decay until 4.5 h (Fig. 2, A and B). This nature of upregulation for KC/CXCL1 and MIP-2/CXCL2 has been seen in rat β-cells in response to extracellular matrix (58).
To study whether the upregulation was in response to a physiological or pathological stimulus, we stimulated the acinar cells with different concentrations of caerulein. No upregulation of either chemokine could be detected in response to physiological doses (up to 0.1 nM caerulein, Fig. 2, C and D). As cholecystokinin (CCK) is known to have two states of the CCKA receptors on the acinar cells, i.e., the low-affinity and the high-affinity state (64), we stimulated the cells with JMV-180 (73), a well-known selective ligand for the high-affinity state and antagonist for the low-affinity state (62). However, there was no upregulation of KC or MIP-2 mRNA in response to JMV (Fig. 2, C and D). Only supraphysiological doses (0.1 μM) known to be equivalent to those causing pancreatitis (37, 61) increased the levels of the chemokines (KC: 7.1 ± 0.6-fold basal, P < 0.01 and MIP-2: 9.3 ± 0.9-fold basal, P < 0.01).
KC and MIP-2 mRNA Increase is Transcriptionally Mediated and is Dependent on Calcium and PKC, but not cAMP
We used two approaches to study the intracellular signaling involved in the upregulation of KC and MIP-2 in response to supramaximal caerulein. In the first approach, we stimulated acinar cells after pretreating them with various inhibitors, at concentrations commonly used in acinar cells (30, 36). BAPTA-AM, a calcium chelator, and GF-109203X, a PKC inhibitor, prevented caerulein-induced KC (1.5 ± 0.1- and 1.4 ± 0.1-fold basal, respectively, P < 0.01) and MIP-2 (1.3 ± 0.2- and 1.3 ± 0.4-fold basal, respectively, P < 0.01) upregulation. On the other hand, H-89, a protein kinase A inhibitor, had no effect (Fig. 3, A and B) on these. Therefore, the upregulation of KC, MIP-2 mRNA requires calcium and protein kinase C (PKC), with no involvement of cAMP.
The second approach involved using non-receptor-mediated activation of secondary mediators. We stimulated the acini with the PKC activator 12-O-tetradecanoylphorbol-3-acetate (TPA) and the calcium ionophore ionomycin, which causes extracellular calcium entry. TPA caused upregulation of both chemokine genes (KC: 7.3 ± 0.7-fold and MIP-2: 7.4 ± 0.6-fold, P < 0.01, Fig. 3, C and D). However, ionomycin did not affect their expression (Fig. 3, C and D). Thus calcium is essential but not sufficient for the upregulation of either KC or MIP-2. Actinomycin D at a commonly used concentration (47) prevented the upregulation of both RNAs (Fig. 3, A and B). Thus the upregulation of KC and MIP-2 is transcriptionally mediated and is not due to increased RNA stability.
KC mRNA Upregulation is Dependent on NF-κB While MIP-2 is Transcriptionally Regulated by Both AP-1 and NF-κB
Pretreatment of the acinar cells with the proteasomal inhibitor MG-132 reduced the 100 nM caerulein-mediated increase in NF-κB activation at 45 min from 2.61 ± 0.13-fold over basal to 1.32 ± 0.04-fold, (P < 0.01) (Fig. 4, A and A′). Lactacystin (10 μM), another proteasomal inhibitor, had a similar effect (results not shown). PGJ2, at concentrations known to prevent NF-κB activation in other systems (20 μM) (12), also prevented NF-κB activation (1.04 ± 0.11-fold basal, P < 0.01) (Fig. 4, A and A′). Reciprocal changes were noted when we studied IκB-α degradation (Fig. 4, B and B′), with caerulein decreasing IκB-α levels to 31.0 ± 5.0% of controls. This was effectively prevented by MG-132 and PGJ2 (88.1 ± 7.7 and 90.1 ± 15.7%, respectively). Chymotrypsin, used as a loading control, was unaffected by the treatments.
We then studied the effect of these agents on AP-1 activation. Acini were pretreated with MG-132, lactacystin, SP-600125 (25 μM), and PGJ2, and the luciferase activity after stimulation with 100 nM caerulein under these conditions was compared with basal and 100 nM caerulein-stimulated levels. Caerulein at 100 nM increased luciferase activity 14.3 ± 3.2-fold (P < 0.01) over basal levels. This increase was prevented by treatment with SP-600125 and PGJ2 (12.8 ± 4.6 and 7.8 ± 4.2% of 100 nM caerulein, respectively, P < 0.05), but not by MG-132 or lactacystin (249.4 ± 66.2 and 569 ± 59% of 100 nM caerulein, P < 0.05), showing that SP-600125 and PGJ2 inhibited AP-1 activity under the TRE promoter (Fig. 5A). PGJ2 did not affect the viability of the acini as shown by the unaltered amylase secretion curve (Fig. 5B) and trypan blue uptake (results not shown).
Having determined the effect of MG-132, lactacystin, SP-600125, and PGJ2 on transcription factor activation, we studied the transcriptional regulation of KC and MIP-2 by stimulating acinar cells with 0.1 μM caerulein after pretreating them with MG-132, lactacystin, BAY11–7082 (10 μM, another NF-κB inhibitor), SP-600125, and PGJ2. Real-time PCR showed that all agents, which inhibit NF-κB, significantly reduced KC levels (MG-132: 20.6 ± 9.4%, P < 0.01, lactacystin: 21.8 ± 3.0%, P < 0.01, BAY11–7082: 12.0 ± 3.6% and PGJ2: 7.6 ± 4.1%, P < 0.01 vs. 0.1 μM caerulein alone, Fig. 6A). AP-1 inhibition with SP-600125 did not significantly reduce KC upregulation (75.6 ± 19%, P = 0.29). On the contrary, MIP-2 mRNA levels were not significantly reduced by inhibiting NF-κB (MG-132: 82.2 ± 34.8%, P = 0.63; lactacystin: 53.4 ± 22.4%, P = 0.10; BAY11–7082: 43.6 ± 25.3%, P = 0.09 of 0.1 μM caerulein) or AP-1 alone with SP-600125 (326 ± 98%, P = 0.08). In contrast, PGJ2, which dually inhibits AP-1 and NF-κB, completely prevented the upregulation of MIP-2 (22.2 ± 4.3%, of 0.1 μM caerulein, P < 0.01, Fig. 6B).
DISCUSSION
In this study, we developed a system to study the regulation of CXC-ELR chemokine synthesis in pancreatic acinar cells and show that both KC and MIP-2 are upregulated by supraphysiological doses (sufficient to induce pancreatitis in vivo) but not by physiological doses of caerulein or JMV-180 (62). This upregulation of chemokine genes was dependent on calcium and PKC, but not cAMP. Interestingly, while KC is transcriptionally regulated by NF-κB, MIP-2 requires both AP-1 and NF-κB.
There are several potential endogenous sources of chemokines apart from acinar cells in the normal pancreas. These sources include duct cells, islet cells, stellate cells, periacinar myofibroblasts, resident macrophages, and endothelial cells, which could be activated in a paracrine manner [e.g., trypsin released from acinar cells cleaving protease-activated receptors on these (50), unpublished observations] during pancreatitis. The multiple potential sources of pancreatic chemokines, in addition to infiltration of inflammatory cells during pancreatitis, limit our ability to study the signaling involved in transcriptional regulation in a clean manner in vivo. Our study involved generating an in vitro system in which well-characterized in vivo responses of acinar cells are replicated.
Elevated mRNA levels of KC and MIP-2 have been previously shown in pancreatitis (24, 27, 53, 77, 88). KC, a rapidly upregulated gene (13, 14), is a murine equivalent of human Gro-α, and rat CINC (83). It is a potent neutrophil chemoattractant and upregulates Mac-1 on the surface of neutrophils (13). MIP-2, while being a chemoattractant, elicits significantly greater release of elastase (14). KC and MIP-2 have been shown to be upregulated in murine models of peritonitis (14), meningitis (20), glomerulonephritis (23), and endotoxemia-induced lung injury (66). These act synergistically to induce increased leukocyte rolling, adhesion, and tissue extravasation dependent on P-selectin (90, 91). Their principal receptor CXCR2 is expressed on neutrophils (39, 42) and mast cells (46). Neutralizing antibodies to CINC, MIP-2, and CXCR2 reduced local and systemic injury during pancreatitis (9, 10, 56).
MIP-2 (67, 81) and IL-8 (60) both have AP-1 and NF-κB binding sites in their promoter region. MIP-2 is solely dependent on AP-1 in the mouse liver (22) and dually dependent on NF-κB and AP-1 activation, like in our studies in a model of myocardial ischemia and reperfusion (51). These along with cAMP response element-binding protein (CREB) regulate MIP-2 in murine macrophages (40). However, CREB seems to be an unlikely mediator in our system since the protein kinase A inhibitor H-89 had no effect on the caerulein-stimulated increase in either chemokine.
Dual requirement of AP-1 and NF-κB in the transcriptional regulation of MIP-2 suggests that, while NF-kB does have a prominent role in pancreatitis (1, 27, 74, 84), its silencing may not be sufficient to prevent neutrophil infiltration. MG-132, lactacystin, and BAY11–7082, which inhibit NF-κB, did not significantly inhibit the caerulein-induced MIP-2 increase. This is in agreement with the study by Algul et al. (2) in which selective conditional deletion of Rela/p65 in the exocrine pancreas resulted in worse neutrophil infiltration and local, systemic injury during pancreatitis. The finding that dual inhibition of NF-κB and AP-1 with curcumin (28) resulted in reduced neutrophil infiltration in two different models of pancreatitis resonates well with our finding, since inhibiting AP-1 alone did not reduce caerulein-mediated upregulation of KC or MIP-2. Constitutive expression of peroxisome proliferator-activated receptor (PPAR) γ has previously been shown to decrease AP-1 and NF-κB activation (82). PGJ2, which is a PPARγ ligand [PPARγ forms hetrodimers with the retinoid X receptor (65)], has previously been shown to prevent both NF-κB and AP-1 activation (12). PGJ2's mechanisms may involve covalent adduct formation with thiol residues on specific proteins altering redox-sensitive cell pathways and inhibiting IκB kinase (43, 65). Our findings are in agreement with its dual role in AP-1, NF-κB inhibition. Hashimoto et al. (32) have shown PGJ2 to reduce intercellular adhesion molecule-1 expression and decrease NF-κB activation, inflammation, and local injury during pancreatitis (32).
TPA activates NF-κB in acini (30). It also results in AP-1 binding to the TPA response element (TRE) (4). TRE corresponds to the human collagenase promoter and is the consensus site for the binding of the Fos/Jun and Jun/Jun dimers that comprise AP-1 (4, 33, 59). Their activity is regulated by transcription, protein amount, nuclear translocation, and phosphorylation (48). AP-1 EMSAs, which we plan to do in future studies, would help to determine which of these is activated by specific stimuli. AP-1 also transcriptionally regulates proinflammatory genes like MCP-1 (69), IL-2 (17), IL-6 (18), iNOS (45), and cyclooxygenase-2 (78).
The proteasome inhibitors MG-132 and lactacystin did not inhibit, but rather increased, AP-1 luciferase activity in response to caerulein. This is seen with proteasome inhibitors in other systems (35, 87) and probably results from decreased proteasomal degradation of luciferase protein. Whether this holds true for inflammatory proteins is unknown.
In summary, we have shown that inhibiting NF-κB alone does not completely prevent the upregulation of MIP-2 in response to caerulein. Although KC is regulated by NF-κB, dual inhibition of NF-κB and AP-1 is essential for preventing the upregulation of MIP-2. Therefore, dual AP-1, NF-κB inhibition may be a more successful strategy in decreasing pancreatic inflammation during pancreatitis than silencing NF-κB alone (2). Future experiments will be directed toward determining whether deletion of both AP-1 and p65 can prevent the worsening of neutrophil infiltration and injury caused by deleting Rela/p65 alone during pancreatitis.
GRANTS
This work was funded by University of Pittsburgh Department of Medicine Startup Funds (V. P. Singh) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-058694 (A. K. Saluja).
DISCLOSURES
No conflicts of interest exist.
Supplementary Material
REFERENCES
- 1. Algul H, Tando Y, Beil M, Weber CK, Von Weyhern C, Schneider G, Adler G, Schmid RM. Different modes of NF-κB/Rel activation in pancreatic lobules. Am J Physiol Gastrointest Liver Physiol 283: G270–G281, 2002. [DOI] [PubMed] [Google Scholar]
- 2. Algul H, Treiber M, Lesina M, Nakhai H, Saur D, Geisler F, Pfeifer A, Paxian S, Schmid RM. Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J Clin Invest 117: 1490–1501, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andoh A, Takaya H, Saotome T, Shimada M, Hata K, Araki Y, Nakamura F, Shintani Y, Fujiyama Y, Bamba T. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology 119: 211–219, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49: 729–739, 1987. [DOI] [PubMed] [Google Scholar]
- 5. Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Ann Rev Immunol 15: 675–705, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Baumann B, Wagner M, Aleksic T, von Wichert G, Weber CK, Adler G, Wirth T. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J Clin Invest 117: 1502–1513, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, Apte U, Wu T, Evans R, Monga SP.Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol 176: 744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhatia M, Brady M, Kang YK, Costello E, Newton DJ, Christmas SE, Neoptolemos JP, Slavin J. MCP-1 but not CINC synthesis is increased in rat pancreatic acini in response to cerulein hyperstimulation. Am J Physiol Gastrointest Liver Physiol 282: G77–G85, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Bhatia M, Brady M, Zagorski J, Christmas SE, Campbell F, Neoptolemos JP, Slavin J. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut 47: 838–844, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhatia M, Hegde A. Treatment with antileukinate, a CXCR2 chemokine receptor antagonist, protects mice against acute pancreatitis and associated lung injury. Regul Pept 138: 40–48, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Blinman TA, Gukovsky I, Mouria M, Zaninovic V, Livingston E, Pandol SJ, Gukovskaya AS. Activation of pancreatic acinar cells on isolation from tissue: cytokine upregulation via p38 MAP kinase. Am J Physiol Cell Physiol 279: C1993–C2003, 2000. [DOI] [PubMed] [Google Scholar]
- 12. Boyault S, Simonin MA, Bianchi A, Compe E, Liagre B, Mainard D, Becuwe P, Dauca M, Netter P, Terlain B, Bordji K. 15-Deoxy-delta12,14-PGJ2, but not troglitazone, modulates IL-1beta effects in human chondrocytes by inhibiting NF-kappaB and AP-1 activation pathways. FEBS Lett 501: 24–30, 2001. [DOI] [PubMed] [Google Scholar]
- 13. Bozic CR, Kolakowski LF, Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol 154: 6048–6057, 1995. [PubMed] [Google Scholar]
- 14. Call DR, Nemzek JA, Ebong SJ, Bolgos GR, Newcomb DE, Wollenberg GK, Remick DG. Differential local and systemic regulation of the murine chemokines KC and MIP2. Shock 15: 278–284, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology 122: 448–457, 2002. [DOI] [PubMed] [Google Scholar]
- 16. Conze D, Krahl T, Kennedy N, Weiss L, Lumsden J, Hess P, Flavell RA, Le Gros G, Davis RJ, Rincon M. c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 have distinct roles in CD8(+) T cell activation. J Exp Med 195: 811–823, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science 243: 355–361, 1989. [DOI] [PubMed] [Google Scholar]
- 18. Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem 274: 32048–32054, 1999. [DOI] [PubMed] [Google Scholar]
- 19. Deng T, Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature 371: 171–175, 1994. [DOI] [PubMed] [Google Scholar]
- 20. Diab A, Abdalla H, Li HL, Shi FD, Zhu J, Hojberg B, Lindquist L, Wretlind B, Bakhiet M, Link H. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1alpha attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect Immun 67: 2590–2601, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dyer RB, Herzog NK. Isolation of intact nuclei for nuclear extract preparation from a fragile B-lymphocyte cell line. Biotechniques 19: 192–195, 1995. [PubMed] [Google Scholar]
- 22. Faouzi S, Burckhardt BE, Hanson JC, Campe CB, Schrum LW, Rippe RA, Maher JJ. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem 276: 49077–49082, 2001. [DOI] [PubMed] [Google Scholar]
- 23. Feng L, Xia Y, Yoshimura T, Wilson CB. Modulation of neutrophil influx in glomerulonephritis in the rat with anti-macrophage inflammatory protein-2 (MIP-2) antibody. J Clin Invest 95: 1009–1017, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frossard JL, Saluja AK, Mach N, Lee HS, Bhagat L, Hadenque A, Rubbia-Brandt L, Dranoff G, Steer ML. In vivo evidence for the role of GM-CSF as a mediator in acute pancreatitis-associated lung injury. Am J Physiol Lung Cell Mol Physiol 283: L541–L548, 2002. [DOI] [PubMed] [Google Scholar]
- 25. Grady T, Liang P, Ernst SA, Logsdon CD. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology 113: 1966–1975, 1997. [DOI] [PubMed] [Google Scholar]
- 26. Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest 100: 1853–1862, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 275: G1402–G1414, 1998. [DOI] [PubMed] [Google Scholar]
- 28. Gukovsky I, Reyes CN, Vaquero EC, Gukovskaya AS, Pandol SJ. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol 284: G85–G95, 2003. [DOI] [PubMed] [Google Scholar]
- 29. Han B, Ji B, Logsdon CD. CCK independently activates intracellular trypsinogen and NF-κB in rat pancreatic acinar cells. Am J Physiol Cell Physiol 280: C465–C472, 2001. [DOI] [PubMed] [Google Scholar]
- 30. Han B, Logsdon CD. CCK stimulates mob-1 expression and NF-kappaB activation via protein kinase C and intracellular Ca2+. Am J Physiol Cell Physiol 278: C344–C351, 2000. [DOI] [PubMed] [Google Scholar]
- 31. Han B, Logsdon CD. Cholecystokinin induction of mob-1 chemokine expression in pancreatic acinar cells requires NF-κB activation. Am J Physiol Cell Physiol 277: C74–C82, 1999. [DOI] [PubMed] [Google Scholar]
- 32. Hashimoto K, Ethridge RT, Saito H, Rajaraman S, Evers BM. The PPARgamma ligand, 15d-PGJ2, attenuates the severity of cerulein-induced acute pancreatitis. Pancreas 27: 58–66, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117: 5965–5973, 2004. [DOI] [PubMed] [Google Scholar]
- 34. Hietaranta AJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Relationship between NF-kappaB and trypsinogen activation in rat pancreas after supramaximal caerulein stimulation. Biochem Biophys Res Commun 280: 388–395, 2001. [DOI] [PubMed] [Google Scholar]
- 35. Hipp MS, Urbich C, Mayer P, Wischhusen J, Weller M, Kracht M, Spyridopoulos I. Proteasome inhibition leads to NF-kappaB-independent IL-8 transactivation in human endothelial cells through induction of AP-1. Eur J Immunol 32: 2208–2217, 2002. [DOI] [PubMed] [Google Scholar]
- 36. Hirohata Y, Fujii M, Okabayashi Y, Nagashio Y, Tashiro M, Imoto I, Akiyama T, Otsuki M. Stimulatory effects of bilirubin on amylase release from isolated rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 282: G249–G256, 2002. [DOI] [PubMed] [Google Scholar]
- 37. Hofbauer B, Saluja AK, Lerch MM, Bhagat L, Bhatia M, Lee HS, Frossard JL, Adler G, Steer ML. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol 275: G352–G362, 1998. [DOI] [PubMed] [Google Scholar]
- 38. Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, Dong Z. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci USA 94: 5826–5830, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huber AR, Kunkel SL, Todd RF, 3rd, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 254: 99–102, 1991. [DOI] [PubMed] [Google Scholar]
- 40. Jaramillo M, Olivier M. Hydrogen peroxide induces murine macrophage chemokine gene transcription via extracellular signal-regulated kinase- and cyclic adenosine 5′-monophosphate (cAMP)-dependent pathways: involvement of NF-kappa B, activator protein 1, and cAMP response element binding protein. J Immunol 169: 7026–7038, 2002. [DOI] [PubMed] [Google Scholar]
- 41. Ji B, Gaiser S, Chen X, Ernst SA, Logsdon CD. Intracellular trypsin induces pancreatic acinar cell death but not NF-kappaB activation. J Biol Chem 284: 17488–17498, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Chemokine antagonists that discriminate between interleukin-8 receptors. Selective blockers of CXCR2. J Biol Chem 272: 16166–16169, 1997. [DOI] [PubMed] [Google Scholar]
- 43. Kansanen E, Kivela AM, Levonen AL. Regulation of Nrf2-dependent gene expression by 15-deoxy-Delta12,14-prostaglandin J2. Free Radic Biol Med 47: 1310–1317, 2009. [DOI] [PubMed] [Google Scholar]
- 44. Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270: 16483–16486, 1995. [DOI] [PubMed] [Google Scholar]
- 45. Li M, Pascual G, Glass CK. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol 20: 4699–4707, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lippert U, Artuc M, Grutzkau A, Moller A, Kenderessy-Szabo A, Schadendorf D, Norgauer J, Hartmann K, Schweitzer-Stenner R, Zuberbier T, Henz BM, Kruger-Krasagakes S. Expression and functional activity of the IL-8 receptor type CXCR1 and CXCR2 on human mast cells. J Immunol 161: 2600–2608, 1998. [PubMed] [Google Scholar]
- 47. Liu H, Palmer D, Jimmo SL, Tilley DG, Dunkerley HA, Pang SC, Maurice DH. Expression of phosphodiesterase 4D (PDE4D) is regulated by both the cyclic AMP-dependent protein kinase and mitogen-activated protein kinase signaling pathways. A potential mechanism allowing for the coordinated regulation of PDE4D activity and expression in cells. J Biol Chem 275: 26615–26624, 2000. [DOI] [PubMed] [Google Scholar]
- 48. Mueller JM, Pahl HL. Assaying NF-kappa B and AP-1 DNA-binding and transcriptional activity. Methods Mol Biol 99: 205–216, 2000. [DOI] [PubMed] [Google Scholar]
- 49. Nagar AB, Gorelick FS. Acute pancreatitis. Curr Opin Gastroenterol 20: 439–443, 2004. [DOI] [PubMed] [Google Scholar]
- 50. Nguyen TD, Moody MW, Steinhoff M, Okolo C, Koh DS, Bunnett NW. Trypsin activates pancreatic duct epithelial cell ion channels through proteinase-activated receptor-2. J Clin Invest 103: 261–269, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nossuli TO, Frangogiannis NG, Knuefermann P, Lakshminarayanan V, Dewald O, Evans AJ, Peschon J, Mann DL, Michael LH, Entman ML. Brief murine myocardial I/R induces chemokines in a TNF-alpha-independent manner: role of oxygen radicals. Am J Physiol Heart Circ Physiol 281: H2549–H2558, 2001. [DOI] [PubMed] [Google Scholar]
- 52. Orlichenko L, Huang B, Krueger E, McNiven MA. Epithelial growth factor-induced phosphorylation of caveolin 1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J Biol Chem 281: 4570–4579, 2006. [DOI] [PubMed] [Google Scholar]
- 53. Pandol SJ, Periskic S, Gukovsky I, Zaninovic V, Jung Y, Zong Y, Solomon TE, Gukovskaya AS, Tsukamoto H. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology 117: 706–716, 1999. [DOI] [PubMed] [Google Scholar]
- 54. Pandol SJ, Raraty M. Pathobiology of alcoholic pancreatitis. Pancreatology 7: 105–114, 2007. [DOI] [PubMed] [Google Scholar]
- 55. Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 133: e1051–e1056, 2007. [DOI] [PubMed] [Google Scholar]
- 56. Pastor CM, Rubbia-Brandt L, Hadengue A, Jordan M, Morel P, Frossard JL. Role of macrophage inflammatory peptide-2 in cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. Lab Invest 83: 471–478, 2003. [DOI] [PubMed] [Google Scholar]
- 57. Rao SS, Watt IA, Donaldson LA, Crocket A, Joffe SN. A serial histologic study of the development and progression of acute pancreatitis in the rat. Am J Pathol 103: 39–46, 1981. [PMC free article] [PubMed] [Google Scholar]
- 58. Ribaux P, Ehses JA, Lin-Marq N, Carrozzino F, Boni-Schnetzler M, Hammar E, Irminger JC, Donath MY, Halban PA. Induction of CXCL1 by extracellular matrix and autocrine enhancement by interleukin-1 in rat pancreatic beta-cells. Endocrinology 148: 5582–5590, 2007. [DOI] [PubMed] [Google Scholar]
- 59. Rincon M, Flavell RA. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J 13: 4370–4381, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roebuck KA, Carpenter LR, Lakshminarayanan V, Page SM, Moy JN, Thomas LL. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-kappaB. J Leukoc Biol 65: 291–298, 1999. [DOI] [PubMed] [Google Scholar]
- 61. Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer ML. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 276: G835–G842, 1999. [DOI] [PubMed] [Google Scholar]
- 62. Saluja AK, Saluja M, Printz H, Zavertnik A, Sengupta A, Steer ML. Experimental pancreatitis is mediated by low-affinity cholecystokinin receptors that inhibit digestive enzyme secretion. Proc Natl Acad Sci USA 86: 8968–8971, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sandoval D, Gukovskaya A, Reavey P, Gukovsky S, Sisk A, Braquet P, Pandol SJ, Poucell-Hatton S. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology 111: 1081–1091, 1996. [DOI] [PubMed] [Google Scholar]
- 64. Sankaran H, Goldfine ID, Bailey A, Licko V, Williams JA. Relationship of cholecystokinin receptor binding to regulation of biological functions in pancreatic acini. Am J Physiol Gastrointest Liver Physiol 242: G250–G257, 1982. [DOI] [PubMed] [Google Scholar]
- 65. Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med 57: 703–708, 2009. [DOI] [PubMed] [Google Scholar]
- 66. Schmal H, Shanley TP, Jones ML, Friedl HP, Ward PA. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol 156: 1963–1972, 1996. [PubMed] [Google Scholar]
- 67. Shi MM, Chong I, Godleski JJ, Paulauskis JD. Regulation of macrophage inflammatory protein-2 gene expression by oxidative stress in rat alveolar macrophages. Immunology 97: 309–315, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shokuhi S, Bhatia M, Christmas S, Sutton R, Neoptolemos JP, Slavin J. Levels of the chemokines growth-related oncogene alpha and epithelial neutrophil-activating protein 78 are raised in patients with severe acute pancreatitis. Br J Surg 89: 566–572, 2002. [DOI] [PubMed] [Google Scholar]
- 69. Shyy JY, Lin MC, Han J, Lu Y, Petrime M, Chien S. The cis-acting phorbol ester “12-O-tetradecanoylphorbol 13-acetate”-responsive element is involved in shear stress-induced monocyte chemotactic protein 1 gene expression. Proc Natl Acad Sci USA 92: 8069–8073, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Singh VP, Bhagat L, Navina S, Sharif R, Dawra RK, Saluja AK. Protease-activated receptor-2 protects against pancreatitis by stimulating exocrine secretion. Gut 56: 958–964, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Singh VP, McNiven MA. Src-mediated cortactin phosphorylation regulates actin localization and injurious blebbing in acinar cells. Mol Biol Cell 19: 2339–2347, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Singh VP, Saluja AK, Bhagat L, Hietaranta AJ, Song A, Mykoniatis A, Van Acker GJ, Steer ML. Serine protease inhibitor causes F-actin redistribution and inhibition of calcium-mediated secretion in pancreatic acini. Gastroenterology 120: 1818–1827, 2001. [DOI] [PubMed] [Google Scholar]
- 73. Stark HA, Sharp CM, Sutliff VE, Martinez J, Jensen RT, Gardner JD. CCK-JMV-180: a peptide that distinguishes high-affinity cholecystokinin receptors from low-affinity cholecystokinin receptors. Biochim Biophys Acta 1010: 145–150, 1989. [DOI] [PubMed] [Google Scholar]
- 74. Steinle AU, Weidenbach H, Wagner M, Adler G, Schmid RM. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology 116: 420–430, 1999. [DOI] [PubMed] [Google Scholar]
- 75. Sun J, Bhatia M. Blockade of neurokinin-1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 292: G143–G153, 2007. [DOI] [PubMed] [Google Scholar]
- 76. Thrower E, Husain S, Gorelick F. Molecular basis for pancreatitis. Curr Opin Gastroenterol 24: 580–585, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 280: G1197–G1208, 2001. [DOI] [PubMed] [Google Scholar]
- 78. von Knethen A, Callsen D, Brune B. Superoxide attenuates macrophage apoptosis by NF-kappa B and AP-1 activation that promotes cyclooxygenase-2 expression. J Immunol 163: 2858–2866, 1999. [PubMed] [Google Scholar]
- 79. Vonlaufen A, Apte MV, Imhof BA, Frossard JL. The role of inflammatory and parenchymal cells in acute pancreatitis. J Pathol 213: 239–248, 2007. [DOI] [PubMed] [Google Scholar]
- 80. Vonlaufen A, Wilson JS, Apte MV. Molecular mechanisms of pancreatitis: current opinion. J Gastroenterol Hepatol 23: 1339–1348, 2008. [DOI] [PubMed] [Google Scholar]
- 81. Walpen S, Beck KF, Schaefer L, Raslik I, Eberhardt W, Schaefer RM, Pfeilschifter J. Nitric oxide induces MIP-2 transcription in rat renal mesangial cells and in a rat model of glomerulonephritis. FASEB J 15: 571–573, 2001. [DOI] [PubMed] [Google Scholar]
- 82. Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, Stemerman MB. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem 277: 34176–34181, 2002. [DOI] [PubMed] [Google Scholar]
- 83. Watanabe K, Konishi K, Fujioka M, Kinoshita S, Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem 264: 19559–19563, 1989. [PubMed] [Google Scholar]
- 84. Weber CK, Adler G. From acinar cell damage to systemic inflammatory response: current concepts in pancreatitis. Pancreatology 1: 356–362, 2001. [DOI] [PubMed] [Google Scholar]
- 85. Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med 354: 2142–2150, 2006. [DOI] [PubMed] [Google Scholar]
- 86. Williams JA, Groblewski GE, Ohnishi H, Yule DI. Stimulus-secretion coupling of pancreatic digestive enzyme secretion. Digestion 58, Suppl 1: 42–45, 1997. [DOI] [PubMed] [Google Scholar]
- 87. Wu HM, Chi KH, Lin WW. Proteasome inhibitors stimulate activator protein-1 pathway via reactive oxygen species production. FEBS Lett 526: 101–105, 2002. [DOI] [PubMed] [Google Scholar]
- 88. Xie MJ, Motoo Y, Su SB, Mouri H, Sawabu N. Induction of chemokines in rat pancreatic acinar cell injury. Pancreas 24: 198–204, 2002. [DOI] [PubMed] [Google Scholar]
- 89. Young MR, Li JJ, Rincon M, Flavell RA, Sathyanarayana BK, Hunziker R, Colburn N. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci USA 96: 9827–9832, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang XW, Liu Q, Wang Y, Thorlacius H. CXC chemokines, MIP-2 and KC, induce P-selectin-dependent neutrophil rolling and extravascular migration in vivo. Br J Pharmacol 133: 413–421, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang XW, Wang Y, Liu Q, Thorlacius H. Redundant function of macrophage inflammatory protein-2 and KC in tumor necrosis factor-alpha-induced extravasation of neutrophils in vivo. Eur J Pharmacol 427: 277–283, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.