Abstract
Chronic ethanol consumption increases mitochondrial oxidative stress and sensitivity to form the mitochondrial permeability transition pore (MPTP). The mechanism responsible for increased MPTP sensitivity in ethanol-exposed mitochondria and its relation to mitochondrial Ca2+ handling is unknown. Herein, we investigated whether increased sensitivity to MPTP induction in liver mitochondria from ethanol-fed rats compared with controls is related to an ethanol-dependent change in mitochondrial Ca2+ accumulation. Liver mitochondria were isolated from control and ethanol-fed rats, and Ca2+-mediated induction of the MPTP and mitochondrial Ca2+ retention capacity were measured. Levels of proposed MPTP proteins as well as select pro- and antiapoptotic proteins were measured along with gene expression. We observed increased steatosis and TUNEL-stained nuclei in liver of ethanol-fed rats compared with controls. Liver mitochondria from ethanol-fed rats had increased levels of proapoptotic Bax protein and reduced Ca2+ retention capacity compared with control mitochondria. We observed increased cyclophilin D (Cyp D) gene expression in liver and protein in mitochondria from ethanol-fed animals compared with controls, whereas there was no change in the adenine nucleotide translocase and voltage-dependent anion channel. Together, these results suggest that enhanced sensitivity to Ca2+-mediated MPTP induction may be due, in part, to higher Cyp D levels in liver mitochondria from ethanol-fed rats. Therefore, therapeutic strategies aimed at normalizing Cyp D levels may be beneficial in preventing ethanol-dependent mitochondrial dysfunction and liver injury.
Keywords: mitochondria, alcohol, steatosis, calcium, bioenergetics
prolonged, heavy consumption of alcohol is the third leading cause of preventable death in the US, with alcoholic liver disease specifically continuing to be a significant cause of morbidity and mortality. It is estimated that ∼12,000 deaths occur each year from alcohol-related chronic liver disease and cirrhosis in the US (58). Chronic ethanol consumption causes liver disease by a complex interaction of multiple metabolic disturbances, including oxidative and nitrative stress, redox imbalance (i.e., increased NADH/NAD+), inflammation, and bioenergetic defects (59). Some of the earliest pathophysiological changes induced by chronic ethanol consumption in the liver occur at the level of the mitochondrion (26, 27). Studies show that the oxidative metabolism of alcohol is a key causative factor in liver injury through increased reactive oxygen (ROS) and nitrogen species (RNS) production within the organelle (7, 9). Damage to mtDNA, mitochondrial protein synthesis inhibition, and enhanced susceptibility of hepatocytes to hypoxic and apoptotic stimuli implicate the mitochondrion in the pathobiology of alcoholic liver disease.
In addition to its role in cellular energy conservation, the mitochondrion has attracted much attention with regard to impacts on redox signaling pathways (44, 51) and as a regulator of cellular calcium (Ca2+) homeostasis. Specifically, mitochondria can control cytosolic Ca2+ levels by their ability to sequester and retain large amounts of Ca2+ (>3 μmol Ca2+/mg protein) via uptake of Ca2+ through the uniporter (37) and by regulated release of Ca2+ by either Na+-dependent or Na+-independent mechanisms (35, 68). Importantly, dysregulation of Ca2+ homeostasis is implicated in cell death mechanisms (71). Additionally, a link between ROS and mitochondrial Ca2+ exists (21, 46, 67), since increased ROS may cause Ca2+ overload in the cell (78) and increased Ca2+ can stimulate ROS production (20). Oxidants can disrupt Ca2+ transport systems, leading to increased cytosolic and mitochondrial Ca2+ levels (21). Although the exact mechanism for Ca2+-induced mitochondrial ROS generation is not fully understood, Brookes et al. (20) proposed that Ca2+ could enhance ROS production by stimulating the tricarboxylic acid cycle and oxidative phosphorylation, thereby making mitochondria work “faster,” which would increase O2 consumption and presumably stimulate ROS production. Others propose that high mitochondrial Ca2+ triggers the mitochondrial permeability transition pore (MPTP), which may be followed by increased ROS production (50, 80). Together, these findings suggest that dysregulation of Ca2+ metabolism may contribute to oxidative injury to the mitochondrion.
Under conditions where there is increased ROS and mitochondrial Ca2+ storage capacity is exceeded, Ca2+ release from mitochondria occurs rapidly through the MPTP. This pore is a high-conductance Ca2+-activated channel that can be regulated by a wide variety of molecules (39, 80). MPTP formation leads to the free passage of molecules <1.5 kDa into and out of the mitochondrion. These events can lead to mitochondrial swelling, the inability to maintain the mitochondrial membrane potential, decreased ATP production, and possible release of mitochondrial proteins involved in the initiation of apoptotic and/or necrotic cell death mechanisms. Although many laboratories have studied the MPTP, the exact components of the pore and their functional roles remain elusive (39). One widely accepted paradigm suggests that the MPTP is composed of three main protein components: the voltage-dependent anion channel (VDAC), the adenine nucleotide translocase (ANT), and cyclophilin D (Cyp D) (16, 41, 80). VDAC is located in the outer mitochondrial membrane and allows low-molecular-weight solutes to gain access to the inner membrane transport systems (13), whereas the ANT is located in the inner mitochondrial membrane and functions to import ADP into the matrix and export ATP to the cytosol (28). Cyp D is a mitochondrial matrix protein that has cis-trans peptidyl-prolyl isomerase activity, which allows it to behave like a chaperone protein (3, 32). Early experiments performed using in vitro systems provided some evidence to support the concept that oxidative stress may promote the translocation of Cyp D to the inner mitochondrial membrane, where it participates in MPTP induction (12, 25). Previously, Pastorino et al. (65) showed that mitochondria isolated from rats chronically fed ethanol had increased sensitivity to MPTP induction. The impact of chronic ethanol ingestion on the components that comprise and/or regulate the pore is not known.
Taken together, these results support the hypothesis that increased sensitivity for MPTP induction may contribute, in part, to chronic ethanol-dependent mitochondrial dysfunction and liver injury. In the present study, we tested this hypothesis by feeding male rats a control or ethanol-containing liquid diet for 5 wk and examined liver mitochondrial bioenergetics, mitochondrial Ca2+ retention capacity, and Ca2+-mediated induction of the MPTP. These functional measurements were complemented by an assessment of ethanol-dependent changes in protein composition of the MPTP and select apoptotic proteins associated with mitochondria. Results from this study indicate that enhanced vulnerability to mitochondrial Ca2+ overload and increased Cyp D could predispose liver mitochondria to undergo MPTP formation and opening in response to chronic ethanol exposure.
MATERIALS AND METHODS
Materials.
All chemicals were of the highest analytical grade and purchased from Sigma (St. Louis, MO) unless otherwise noted. Lieber-DeCarli control and ethanol liquid diets were purchased from Bio-Serv (Frenchtown, NJ). The pyruvate dehydrogenase antibody was a gift provided by Dr. Kirill Popov, Department of Biochemistry, University of Alabama at Birmingham.
Ethanol feeding protocol.
Male Sprague-Dawley rats were individually housed and maintained under a 12–12:h light-dark cycle for the duration of the experiment. Animals were fed a standard rat chow diet for ∼1 wk after procurement and weighed ∼200 g at the start of the feeding protocol. Lieber-DeCarli control and ethanol-containing liquid diets were formulated by Bio-Serv. The nutritionally adequate ethanol diet contains 36% of the total daily calories as ethanol, 35% as fat, 11% as carbohydrate, and 18% as protein. The control diet is an identical formulation with ethanol calories substituted by carbohydrate (i.e., dextrin maltose). Control animals were pair-fed to their ethanol counterparts so that each pair was isocaloric. Animals were maintained on the feeding protocols for at least 31 days before experiments. All animal protocols were approved by the Institutional Animal Care and Use Committee of University of Alabama at Birmingham, and animals received humane care in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23).
Mitochondria isolation and respiration measurements.
Liver mitochondria were isolated by differential centrifugation techniques (74). Oxygen consumption of isolated liver mitochondria was monitored using a Clark-type oxygen electrode (Hansatech Instruments, Amesbury, MA). Respiratory capacity was assessed by measuring state 3 (i.e., ADP-dependent) and state 4 (i.e., ADP-independent) respiration, using succinate as the oxidizable substrate in the presence of rotenone (1 μM) to inhibit complex I-mediated respiration. The respiratory control ratio (RCR) was calculated as the ratio of state 3 and state 4 respiration rates (i.e., state 3 divided by state 4 respiration).
Liver histology and biochemical measurements.
Liver from control- and ethanol-fed rats was fixed in 10% formalin, sectioned, and stained with hematoxylin-eosin for visualization of steatosis. Serum samples were assayed for alanine aminotransferase activity and alcohol content using appropriate reagent sets from Pointe Scientific (Canton, MI). Triglyceride levels were measured in serum and cytosolic liver fractions using an enzymatic-coupled assay that measures glycerol released from triglyceride (Pointe Scientific).
Mitochondria Ca2+ retention capacity assessment.
Mitochondrial Ca2+ retention capacity, (i.e., the threshold load of Ca2+ required to induce the MPTP) was measured using the cell-impermeable fluorescent dye Calcium Green 5N (CaG5N; Invitrogen, Carlsbad, CA). Freshly isolated mitochondria were washed and resuspended in 0.25 M sucrose buffer without EDTA. Experiments were performed using 0.2 mg/ml of isolated liver mitochondria incubated in respiration buffer (130 mM KCl, 2 mM KH2PO4, 3 mM HEPES, and 2 mM MgCl2), 8 mM succinate, 1 μM rotenone, 0.2 mM ADP, 1 μg/ml oligomycin, and 0.2 μM CaG5N. The volume of the incubation mixture in the cuvette was 2.0 ml. Experiments were performed at 37°C in a PerkinElmer LS 55 spectrofluorometer, monitoring fluorescence with the excitation and emission wavelengths set at 506 and 532 nm, respectively. Single injections of Ca2+ (20 nmol) were added to the reaction mixture in the cuvette sequentially until induction of the MPTP. In select experimental runs, cyclosporin A (CsA; 1 μM) was used to demonstrate involvement of the MPTP, since CsA is an inhibitor of the MPTP (19). Inclusion of CsA allows for increased Ca2+ retention capacity before induction of the MPTP and subsequent release of Ca2+ from mitochondria (14).
Mitochondrial swelling assay.
Mitochondria were resuspended in Ca2+ depletion buffer (1 mM EGTA, 10 mM NaCl, and 5 mM succinate) and gently stirred at room temperature for 10 min, followed by additional stirring on ice for 5 min. Mitochondria were centrifuged for 10 min at 10,000 g at 4°C. The mitochondrial protein pellet was then resuspended in buffer containing 195 mM mannitol, 25 mM sucrose, and 40 mM HEPES, washed two times, and centrifuged for 10 min at 10,000 g at 4°C (53). The pellet was resuspended in 2–3 ml of buffer, and the protein concentration was determined by the Bradford protein assay (18). Isolated mitochondria (0.25 mg/ml) were incubated in a KCl-based buffer (150 mM KCl, 25 mM NaHCO3, 1 mM MgCl2, 1 mM KH2PO4, and 20 mM HEPES, pH 7.4) and energized with the oxidizable substrate succinate (5 mM) in the presence of rotenone (10 μM). Ca2+ (200 nmol) was added to cuvettes, and swelling was monitored by recording the decrease in absorbance for 20 min using a Beckman Coulter DU 640 spectrophotometer at 540 nm and 30°C. CsA (1 μM) was added to select samples prior to the addition of Ca2+, and swelling was monitored as described.
Western blotting.
Immunoblots were performed by loading equal amounts of mitochondrial or cytosolic protein onto 10 or 12% SDS-PAGE gels. Note that the levels of mitochondrial proteins were measured from whole mitochondrial extracts. Levels of Cyp D were detected using a 1:10,000 dilution of antibody (Calbiochem, Gibbstown, NJ). Levels of ANT were detected using a 1:500 dilution of antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Levels of VDAC were detected using 1:20,000 dilution of antibody (Calbiochem). Levels of Bax and Bcl-2 were detected using 1:1,000 dilution of antibody (Cell Signaling Technology, Beverly, MA). Levels of cytochrome c were detected using a 1:1,000 dilution of antibody (BD Pharmingen, San Diego, CA). After incubation of membranes with appropriate horseradish peroxidase-conjugated secondary antibodies (Sigma), proteins were visualized using chemiluminescence. Membranes were then stripped and incubated with the appropriate loading control antibody. Levels of pyruvate dehydrogenase were detected using a 1:5,000 dilution as a loading control for mitochondrial protein. Levels of β-actin (Sigma) were detected using a 1:5,000 dilution as a loading control for cytosolic proteins. Detection methods and the intensity of immunoreactive protein bands were quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA), as described in Ref. 10.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling.
Depariffinized and rehydrated liver sections from control and ethanol-fed rats were subjected to antigen retrieval in 0.1 M citrate buffer, pH 6.0. Tissues were blocked for 1 h at room temperature with 0.1 M Tris·HCl, pH 7.5, containing 5% (wt/vol) BSA. Fifty microliters of the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) reaction reagent (Roche, Indianapolis, IN) was added, and liver sections were incubated for 60 min in the dark at 37°C in a humidified atmosphere. After sections were washed in phosphate-buffered saline (PBS), 50 μl of converter-alkaline phosphatase was added, and liver sections were incubated in a humidified dark chamber for 30 min at 37°C. Following this, sections were washed again in PBS, and 100 μl of nitro-blue tetrazolium chloride-5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt solution was added and incubated for 10 min at 25°C in the dark. Sections were then washed with PBS and mounted in PBS-glycerol and visualized under light microscopy. For analysis by light microscopy, the number of TUNEL-positive cells per liver sample was counted from 20 random high-power fields (i.e., ×40 magnification).
Gene expression.
Total RNA was isolated from liver tissue using TRIzol (Invitrogen, Carlsbad, CA), following the manufacturer's directions. Reverse transcription of 1 μg of total RNA was performed using RT2 FirstStrand Kit (SABiosciences, Frederick, MD). Real-Time PCR was performed using an Applied Biosystems 7300 instrument with verified, gene-specific primers purchased from SABiosciences (RT2 qPCR SYBR Green-based primers). Relative expression changes were determined by normalizing the relative amount of gene-specific mRNA comparative threshold (Ct) to the Gapdh (housekeeping gene) Ct using the comparative cycle threshold (ΔΔCT) method. The following rat-specific RT2 qPCR primer assays were used: Vdac (PPR50827A), Ant (PPR54853A), Cyp D (PPR59729A), Bax (PPR06496A), Bcl-2 (PPR06577A), cytochrome c (PPR42696A), and Gapdh (PPR06557A).
Statistical analysis.
Data represent the mean ± SE for six pairs of animals per group. Significant differences between groups were obtained using the Student's paired t-test. For real-time PCR, significant differences between groups were obtained using the Wilcoxon Mann-Whitney nonparametric test. The level of statistical significance was set at P < 0.05.
RESULTS
Animals and liver histology.
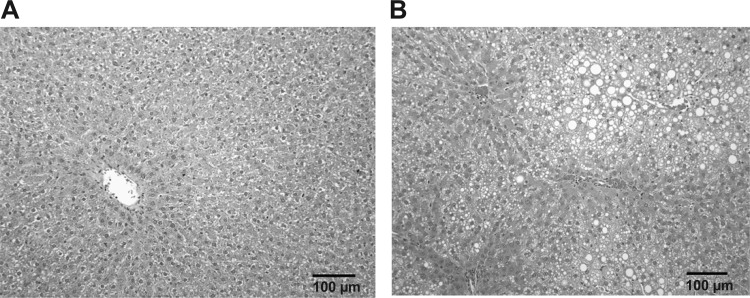
For this study, rats were pair-fed a control or an ethanol-containing liquid diet for 5 wk, a diet and exposure protocol that are known to induce steatosis and mitochondrial dysfunction (10). There was no significant difference in body weight gain, liver weight, or the liver-to-body weight ratio between groups (Table 1). Consumption of ethanol increased serum triglyceride levels compared with control (Table 1); however, this difference was not statistically significant. In addition, there was a significant increase in liver triglyceride levels in the ethanol group compared with the control (Table 1). These data are in accordance with the increase in steatosis observed in livers of ethanol-fed animals compared with control-fed animals (Fig. 1B). Controls showed no overt pathology or steatosis (Fig. 1A). There was also a significant increase in serum alanine aminotransferase levels in the ethanol group compared with controls indicating mild hepatocellular injury (Table 1). These results demonstrate that this ethanol-feeding regimen caused the early stage of alcoholic liver disease, steatosis.
Table 1.
Effect of chronic ethanol consumption on various liver and serum measurements
| Control | Ethanol | |
|---|---|---|
| Body weight gain, g | 100.5 ± 9.3 | 95.3 ± 10.6 |
| Liver weight, g | 10.8 ± 0.6 | 11.9 ± 0.8 |
| Liver/body weight ratio, % | 3.1 ± 0.05 | 3.5 ± 0.15 |
| Blood alcohol, mg/dl | 178 ± 35* | |
| Serum ALT, U/l | 43.2 ± 5.0 | 57.5 ± 3.0† |
| Serum triglycerides, mg/dl | 104.6 ± 12.5 | 162.1 ± 35 |
| Liver triglycerides, mg/mg protein | 9.8 ± 1.8 | 41.1 ± 7.5* |
Data are means ± SE. ALT, alanine aminotransferase.
P < 0.05,
P < 0.005 compared with control.
Fig. 1.
Chronic ethanol consumption causes liver steatosis. Light microscopy images from representative livers of control- and ethanol-fed rats. A: representative image of hematoxylin-eosin-stained liver section from a control-fed rat. B: representative image of hematoxylin-eosin-stained liver section from ethanol-fed rat. Note that these images are from the same control and ethanol pair and are representative of 6 pairs of control- and ethanol-fed rats. Magnification is ×20.
Chronic ethanol consumption decreases mitochondrial respiratory function.
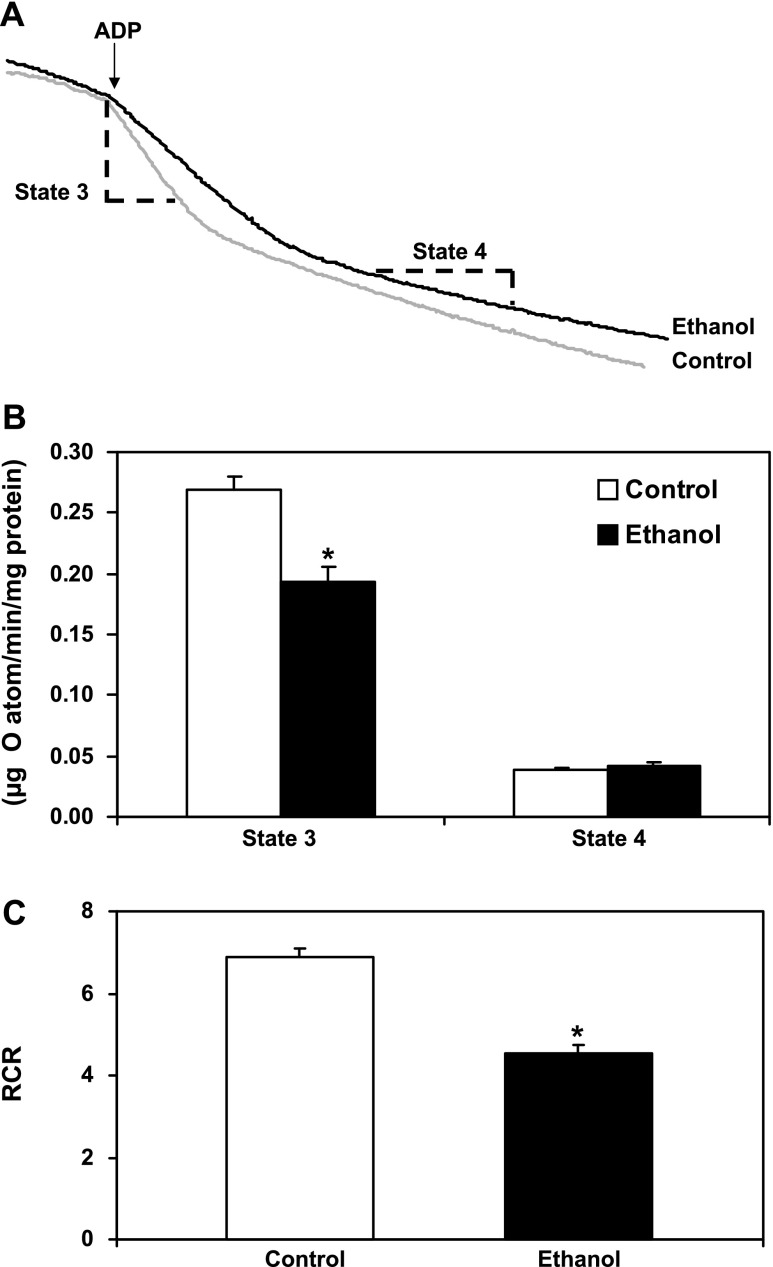
Mitochondrial oxygen consumption in the presence of succinate and ADP (i.e., state 3 respiration) was measured in freshly isolated liver mitochondria from control and ethanol-fed animals. Note that the terms control mitochondria and ethanol mitochondria are used in this article and refer to mitochondria isolated from livers of control-fed (i.e., ethanol-naïve) and ethanol-fed rats, respectively. Figure 2A shows typical respiration results from mitochondria isolated from an ethanol-fed rat and its pair-fed control. As shown in Fig. 2B, state 3 respiration was decreased in mitochondria isolated from the livers of ethanol-fed rats compared with controls. A decrease in state 3 respiration is notable because it indicates that chronic alcohol consumption decreases electron transport and subsequently the rate at which liver mitochondria may synthesize ATP. In contrast, state 4 respiration was unaffected by ethanol consumption (Fig. 2B), suggesting that uncoupling does not occur during the early stage of the disease process and does not contribute to defects in mitochondrial bioenergetics at this stage. In addition, the respiratory control ratio (i.e., RCR = state 3/state 4 respiration) was determined for each pair of ethanol- and control-fed rats. A higher RCR indicates mitochondria that are more tightly coupled with better function (i.e., better quality of mitochondria), whereas a lower RCR indicates that mitochondria are more loosely coupled with poorer function (i.e., damaged mitochondria) in response to treatment. The RCR was significantly lower in the ethanol group compared with the control group (Fig. 2C). These data demonstrate that the bioenergetics of liver mitochondria are compromised in the early steatosis phase of alcoholic liver disease and are consistent with earlier studies done in our laboratory (10).
Fig. 2.
Chronic ethanol consumption decreases mitochondrial respiration. A: representative results from oxygen consumption studies from control (gray line) and ethanol (black line) mitochondria. Oxygen consumption was determined using succinate as the oxidizable substrate. ADP was added at the arrow to initiate state 3 respiration (dashed line marking). After all ADP is converted to ATP, mitochondria enter state 4 respiration (lower rate of respiration), which is marked on the trace (dashed line marking). B: state 3 respiration was significantly lower in ethanol mitochondria compared with controls, whereas there was no difference in state 4 respiration between groups. C: the respiratory control ratio (RCR; state 3/state 4 respiration) was significantly lower in ethanol mitochondria compared with controls. Data are expressed as the mean ± SE for 6 pairs of control- and ethanol-fed rats. *P < 0.05 compared with control.
Mitochondria isolated from ethanol-fed rats are more sensitive to Ca2+-induced swelling.
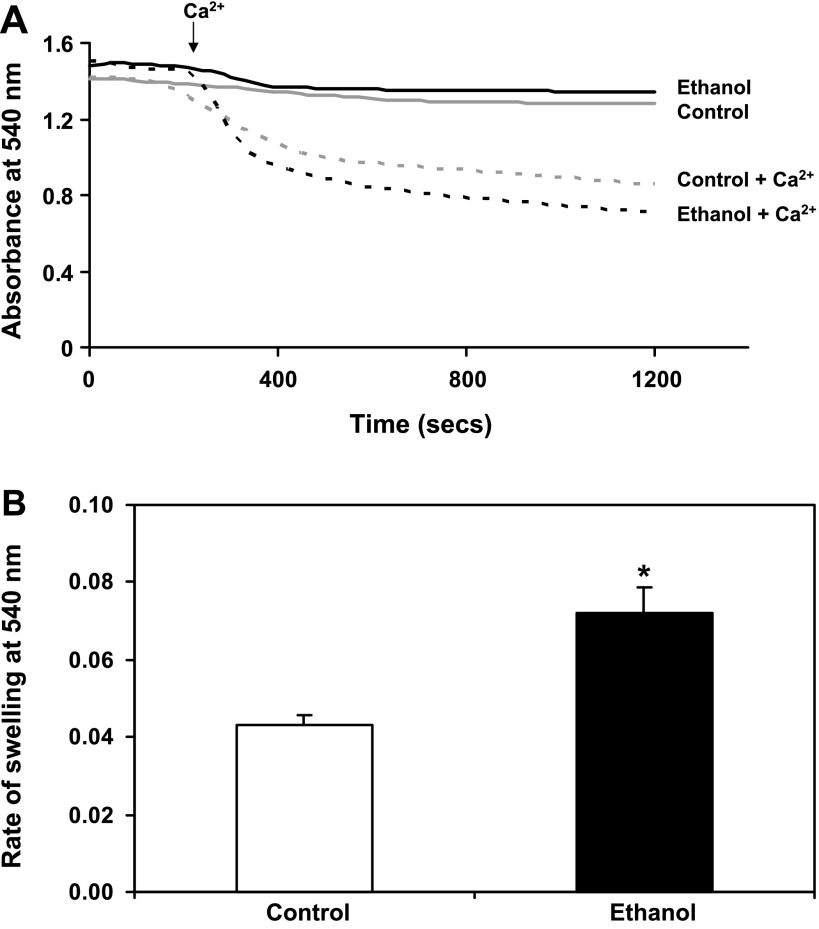
We measured mitochondrial swelling in response to Ca2+, a known inducer of the MPTP, to determine whether chronic ethanol consumption changes the sensitivity of MPTP induction. Figure 3A shows representative results of freshly isolated liver mitochondria from control- and ethanol-fed rats incubated with 200 nmol of Ca2+ to induce mitochondrial swelling. Both control and ethanol mitochondria were sensitive to Ca2+-dependent swelling. However, mitochondria from ethanol-fed animals were more sensitive to Ca2+-triggered swelling as shown by the faster rate of swelling (i.e., steeper slope) immediately following the addition of Ca2+ (Fig. 3A, arrow). The rate of swelling was taken over the same time period, 180–330 s, for each sample (Fig. 3B). There was a 71% increase in the rate of Ca2+-dependent swelling in ethanol mitochondria compared with control over this time frame. Pretreatment with CsA, a known inhibitor of the MPTP, blocked Ca2+-induced swelling in both control and ethanol mitochondria, indicating that this is a MPTP-dependent process (data not shown).
Fig. 3.
Chronic ethanol consumption increases sensitivity to Ca2+-mediated mitochondrial swelling. Isolated mitochondria (0.25 mg/ml) were incubated in a KCl-based buffer containing 150 mM KCl, 25 mM NaHCO3, 1 mM MgCl2, 1 mM KH2PO4, and 20 mM HEPES, pH 7.4. A: representative results of mitochondrial swelling using 0 (solid lines) and 200 nmol Ca2+ (dashed lines) at an absorbance of 540 nm. B: the decrease in absorbance was followed for 20 min, and the rate of swelling was calculated from the initial slope of the decrease in absorbance (i.e., from 180–330 s). Mitochondria from ethanol-treated animals (black dashed line) were significantly more sensitive to Ca2+-mediated mitochondrial swelling than mitochondria from control animals (gray dashed line). Data are expressed as the mean ± SE for 6 pairs of control- and ethanol-fed rats. *P < 0.05 compared with control.
Chronic ethanol consumption induces cell death in the liver.
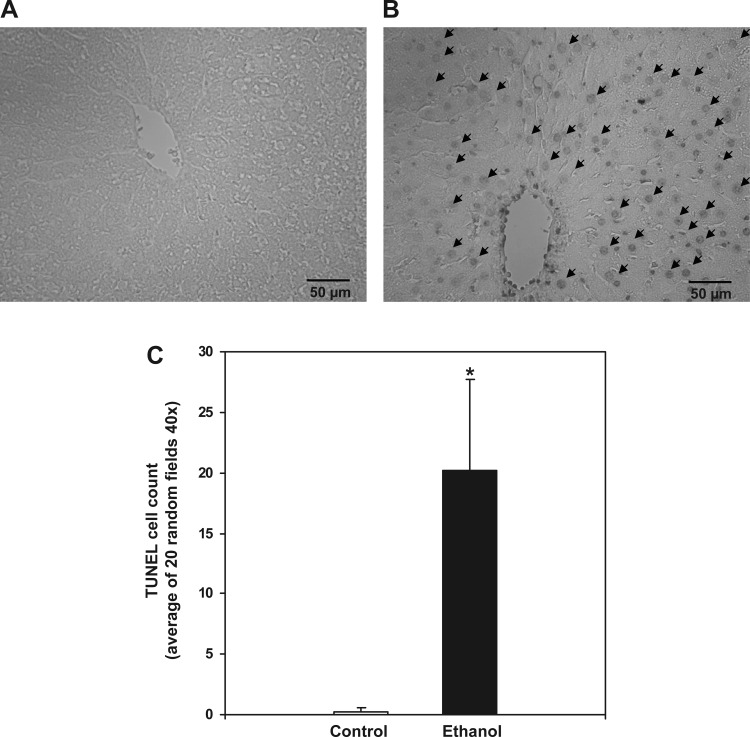
Chronic ethanol-dependent oxidative stress is thought to contribute to hepatocyte injury and lead to apoptosis and/or necrosis. To determine whether cell death was increased by chronic ethanol consumption, TUNEL staining was performed in liver sections prepared from control- and ethanol-fed rats. This method is used for detecting DNA fragmentation, an indicator of cell death, by labeling the terminal end of nucleic acids. We observed a significant increase in TUNEL-positive cells in livers of ethanol-fed rats compared with controls (Fig. 4, A–C). It is important to note that DNA fragmentation, as evidenced by the TUNEL reaction, is not solely exclusive for apoptosis because DNA fragmentation may occur during necrosis or DNA repair processes (43, 64), although both of these alternative interpretations support the occurrence of ethanol-dependent cell injury and death in this model of early alcoholic liver disease.
Fig. 4.
Chronic ethanol consumption increases hepatic terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining. TUNEL-positive nuclei were visualized in formalin-fixed liver sections for control- (A) and ethanol-fed rats (B). Images are representative of at least 6 rats/treatment group taken at ×40 magnification. C: for quantification, the number of TUNEL-positive cells per liver sample was counted from 20 random high-powered fields (×40 magnification). Data are expressed as the mean ± SE for 6 pairs of control- and ethanol-fed rats. *P < 0.05 compared with control.
Effect of chronic ethanol consumption on select pro- and antiapoptotic proteins.
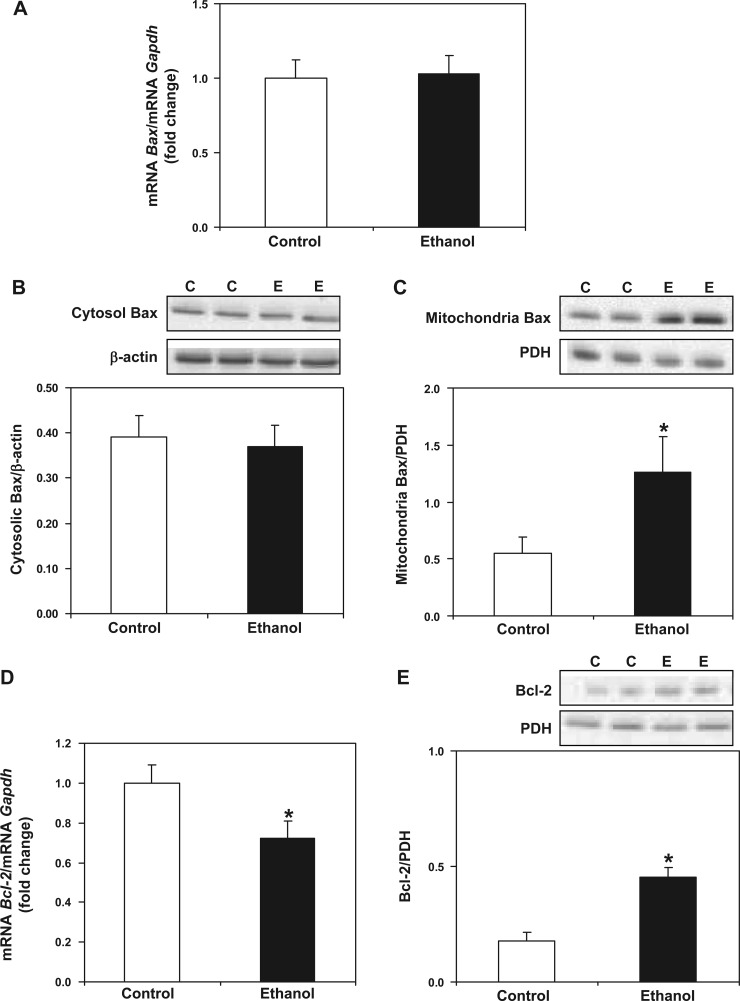
Increased susceptibility to cell death may occur from an interaction between proapoptotic proteins and components of the MPTP or from these proteins inducing mitochondrial outer membrane permeability (MOMP) independent of the classic MPTP process (1, 65). Therefore, we investigated the impact of chronic alcohol consumption to alter the levels of three classic pro- and antiapoptotic proteins, cytochrome c, Bax, and Bcl-2, at the level of transcript and protein. Alterations in the levels of proapoptotic Bax and antiapoptotic Bcl-2 in the mitochondrion may be critical in determining the response to chronic ethanol exposure and whether MPTP induction will occur. First, chronic alcohol consumption had no effect on cytochrome c gene expression between control and ethanol groups (Fig. 5A). Similarly, we observed no difference in mitochondrial cytochrome c protein levels between control and ethanol groups (Fig. 5B), and we were unable to detect monomeric cytochrome c in cytosol (data not shown). Like cytochrome c, we observed no difference in Bax gene expression between control and ethanol groups (Fig. 6A). There was also no difference in the cytosolic levels of Bax protein between control and ethanol groups (Fig. 6B). However, we observed a significant increase in mitochondrial Bax protein levels in ethanol compared with control mitochondria (Fig. 6C). Last, we found a significant decrease in Bcl-2 gene expression in ethanol compared with control liver (Fig. 6D), whereas the levels of Bcl-2 protein were increased in ethanol compared with control mitochondria (Fig. 6E).
Fig. 5.
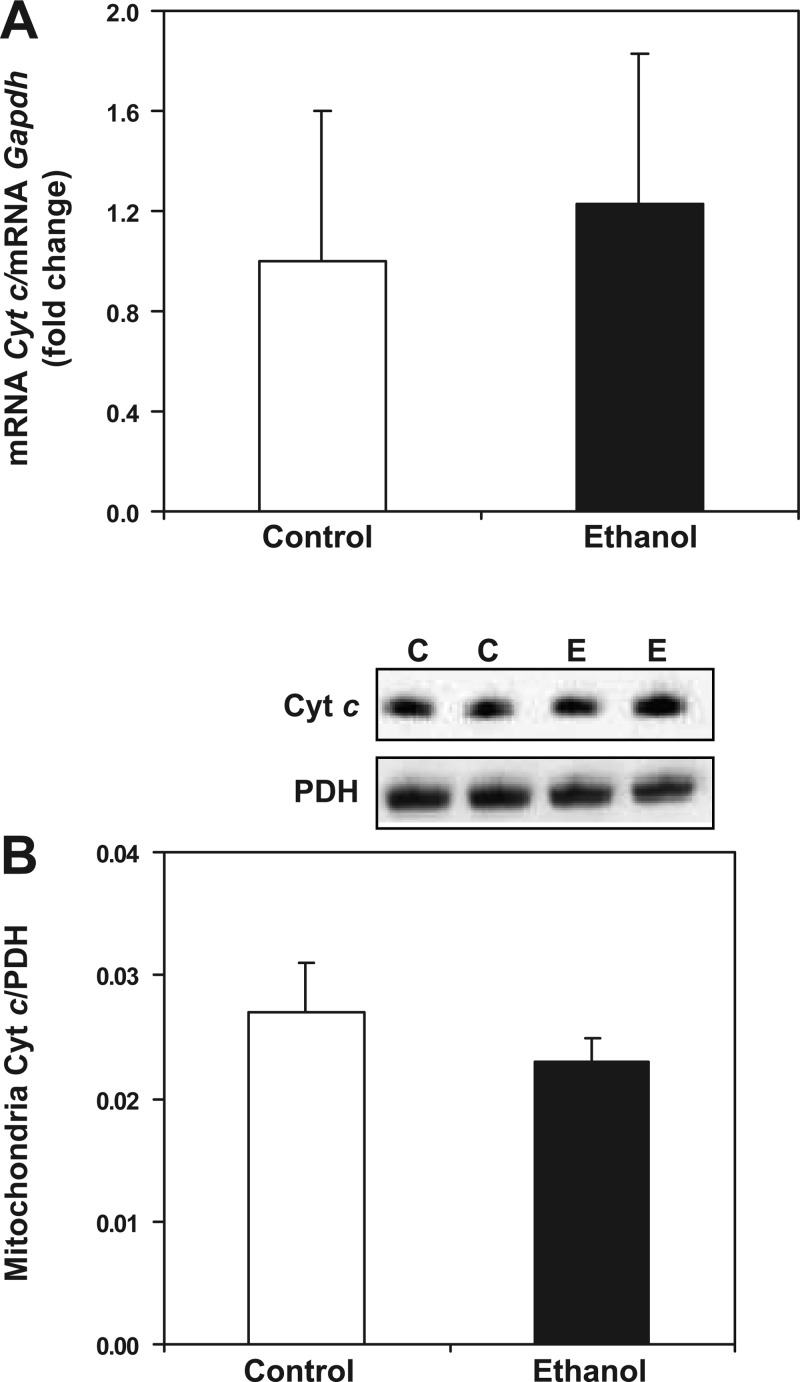
Effect of chronic ethanol consumption on cytochrome c (Cyt c) transcript and protein levels. For gene expression analyses, total RNA was isolated and measured by real-time PCR. The relative amount of mRNA was determined using the comparative threshold (Ct) method by normalizing target cDNA levels to Gapdh. Protein was measured by Western blotting technique and normalized to pyruvate dehydrogenase (PDH). A: there was no difference in Cyt c gene expression between control- (C) and ethanol-fed (E) animals. B: there was no difference in mitochondrial Cyt c protein between control and ethanol groups. Data are expressed as the mean ± SE for 6 pairs of control- and ethanol-fed rats.
Fig. 6.
Effect of chronic ethanol consumption on transcript and protein levels of key pro- and antiapoptotic mediators. For gene expression analyses, total RNA was isolated and measured by real-time PCR. The relative amount of mRNA was determined using the Ct method by normalizing target cDNA levels to Gapdh. Protein was measured by Western blotting technique and normalized to either β-actin or PDH. A: there was no difference in Bax gene expression between control- and ethanol-fed animals. The proapoptotic protein Bax was measured in cytosolic (B) and mitochondrial fractions (C). There was no difference in cytosolic Bax protein between control- and ethanol-fed rats. There was a significant increase in mitochondrial Bax protein in ethanol compared with control rats. D: there was a significant decrease in Bcl-2 gene expression in ethanol compared with control animals. E: Bcl-2 protein was increased significantly in liver mitochondria from ethanol-fed rats compared with controls. Data are expressed as means ± SE for 6 pairs of control- and ethanol-fed rats. *P < 0.05 compared with control.
Mitochondria from ethanol-fed rats have lower Ca2+ retention capacity than control mitochondria.
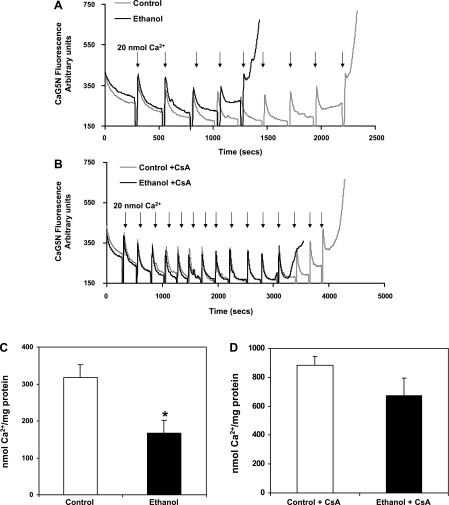
Mitochondria are recognized as an important cellular Ca2+ store (2, 36), with mitochondrial Ca2+ able to stimulate electron transport, increase ROS production, and induce the MPTP (21). Freshly isolated mitochondria were incubated with the Ca2+-sensitive fluorescent dye CaG5N (22, 72) to determine the effect of chronic ethanol consumption on mitochondrial Ca2+ retention capacity. In this experiment, bolus additions of Ca2+ (20 nmol) were added sequentially to energized mitochondria until MPTP induction, which results in release of intramitochondrial Ca2+ (as indicated by a rapid rise in CaG5N fluorescence at the end of the experimental run). As shown in Fig. 7A, initial aliquots of Ca2+ led to a transient rise in extramitochondrial Ca2+ followed by a rapid return to baseline fluorescence, as Ca2+ is taken up and sequestered by mitochondria. Note that Ca2+ retention was slower in mitochondria from ethanol-fed animals compared with control, and upon further additions of Ca2+, mitochondria from ethanol-fed animals were unable to retain as much Ca2+ before undergoing induction of the MPTP (Fig. 7A). Indeed, there was a significant decrease in the amount of Ca2+ stored prior to the induction of the MPTP in mitochondria from ethanol-fed animals (167 ± 21 nmol Ca2+/mg protein) compared with mitochondria from control animals (317 ± 36 nmol Ca2+/mg protein) (Fig. 7C). When the Ca2+ retention capacity experiment was performed in the presence of CsA, both mitochondria from control- and ethanol-fed animals stored more Ca2+ before induction of the MPTP (Fig. 7, B and D). For example, control plus CsA- and ethanol plus CsA-treated mitochondria stored 883 ± 60 and 675 ± 120 nmol Ca2+/mg protein, respectively. Although mitochondria from ethanol-fed animals treated with CsA stored slightly less Ca2+ before pore induction, this difference was not statistically significant compared with control mitochondria treated with CsA (P = 0.17). Together, these findings are noteworthy because they demonstrate that chronic ethanol ingestion increases the susceptibility of mitochondria to Ca2+ overload, which presumably increases sensitivity to the MPTP.
Fig. 7.
Effect of chronic ethanol consumption on liver mitochondria Ca2+ retention capacity. Liver mitochondria (0.2 mg/ml) were incubated in respiration buffer (130 mM KCl, 2 mM KH2PO4, 3 mM HEPES, and 2 mM MgCl2) with 8 mM succinate, 1 μM rotenone, 0.2 mM ADP, 1 μg/ml oligomycin, and 0.2 μM Calcium Green 5N (CaG5N). A: representative results of Ca2+ uptake in control (gray line) and ethanol (black line) mitochondria. Ca2+ was added as 20-nmol additions every 240 s (i.e., 20 nmol at each arrow). Mitochondrial Ca2+ retention capacity was lower in ethanol compared with control mitochondria before MPTP induction. B: representative results of Ca2+ uptake in control (gray line) and ethanol (black line) mitochondria pretreated with cyclosporin A (CsA). Ca2+ was added as 20-nmol additions every 240 s (i.e., 20 nmol at each arrow). Note that in the presence of CsA, mitochondrial Ca2+ retention capacity was increased for both control and ethanol mitochondria. C: quantification of mitochondrial Ca2+ retention capacity in ethanol and control mitochondria. D: quantification of mitochondrial Ca2+ retention capacity in ethanol and control mitochondria pretreated with CsA. Data are expressed as the mean ± SE for 6 pairs of control- and ethanol-fed rats. *P < 0.005 compared with control.
Chronic ethanol consumption causes an increase in Cyp D.
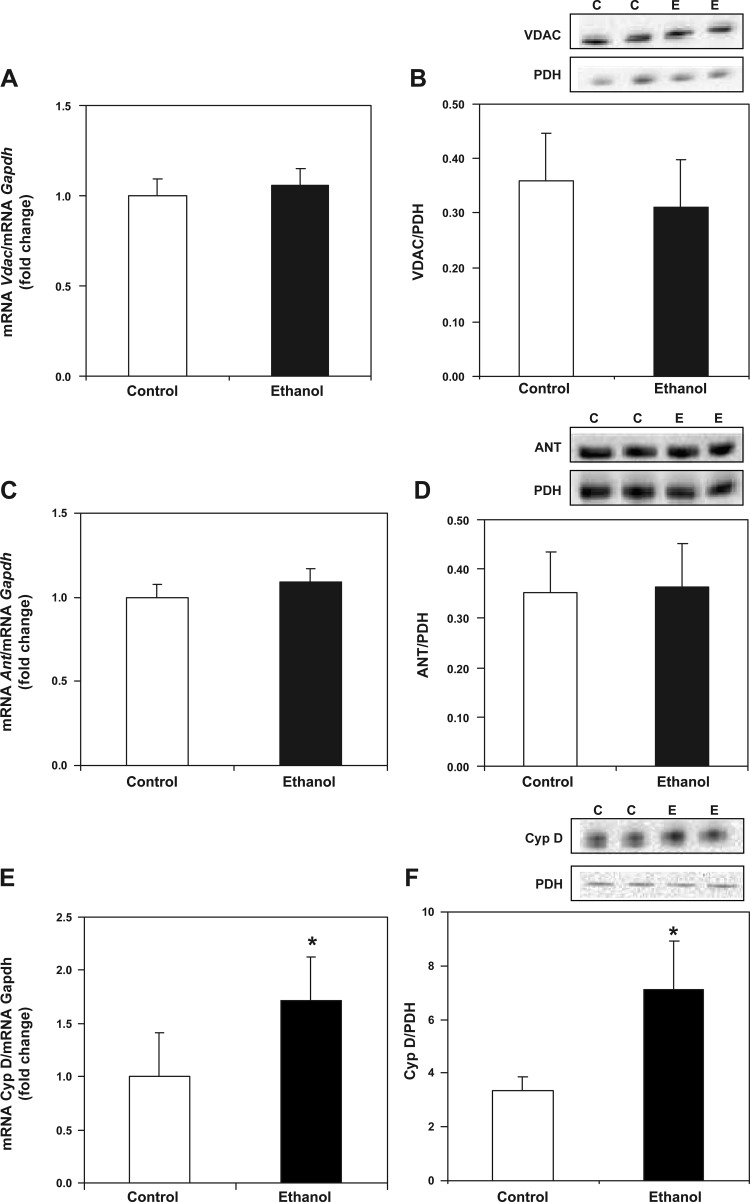
The exact composition of the pore is unknown; however, the mitochondrial permeability transition (MPT) is proposed to occur in response to the opening of a pore (i.e., the MPTP) involving the inner and outer membrane proteins ANT and VDAC, the matrix protein Cyp D, and other accessory proteins (80). To investigate the mechanisms that may contribute to increased mitochondrial swelling and reduced Ca2+ retention capacity in response to chronic ethanol exposure, components of the MPTP were examined. Real-time PCR and Western blot analysis were used to determine the effect of chronic ethanol consumption on components of the MPTP. As shown in Fig. 8, A and B, chronic ethanol consumption had no effect on Vdac gene expression or VDAC protein levels between control and ethanol groups. Similarly, chronic ethanol consumption had no effect on Ant gene expression or ANT protein levels between control and ethanol groups (Figs. 8, C and D). However, we observed a significant increase in Cyp D gene expression in ethanol compared with control liver (Fig. 8E) along with a significant increase in Cyp D protein in ethanol compared with control mitochondria (Fig. 8F).
Fig. 8.
Chronic ethanol consumption increases cyclophilin D (Cyp D) transcript and protein levels. For gene expression analyses, total RNA was isolated and measured by real-time PCR. The relative amount of mRNA was determined using the Ct method by normalizing target cDNA levels to Gapdh. Protein was measured by Western blotting technique and normalized to PDH. A: there was no difference in voltage-dependent anion channel (Vdac) gene expression between control and ethanol groups. B: there was no difference in VDAC protein between control and ethanol mitochondria. C: there was no difference in adenine nucleotide translocase (Ant) gene expression between control and ethanol groups. D: There was no difference in ANT protein between control and ethanol mitochondria. E: there was a significant increase in Cyp D gene expression in liver from ethanol-fed compared with control animals. F: there was a significant increase in Cyp D protein in ethanol compared with control mitochondria. Data are expressed as the mean ± SE for 6 pairs of control- and ethanol-fed rats. *P < 0.05 compared with control.
DISCUSSION
Previous studies from our laboratory and others have shown that chronic ethanol consumption causes mitochondrial dysfunction due to mtDNA and ribosomal damage, depressed oxidative phosphorylation, and increased ROS generation (59). The negative impact of chronic ethanol consumption on liver mitochondria bioenergetics is attributed largely to losses in both mitochondrial and nuclear-encoded proteins that comprise several of the oxidative phosphorylation complexes (4, 24, 74). The alcohol-dependent decrease in these subunits results in reduced respiratory complex activities and depressed rates of ADP-dependent respiration (i.e., state 3 respiration) (10). It is these alcohol-mediated changes at the molecular level that contribute largely to decreased ATP synthesis and levels in liver mitochondria following chronic alcohol consumption (8, 26). In addition to these changes, it is possible that increased liver fat following chronic ethanol consumption (Table 1 and Fig. 1) contributes, in part, to increased mitochondrial ROS production and subsequent oxidative damage. For example, in steatotic liver there will be increased delivery of free fatty acids to mitochondria for β-oxidation, leading to increased transfer of reducing equivalents to the respiratory chain. This event, coupled with a damaged respiratory chain, will amplify electron “leak” to molecular oxygen, thereby increasing the generation of superoxide anions and other ROS (7). Increased ROS/RNS are proposed to damage the respiratory complexes by posttranslational oxidative modifications (75). Thus, ROS-dependent damage is predicted to occur in response to chronic alcohol ingestion and cause oxidative damage to the respiratory chain machinery, resulting in impaired bioenergetic capacity and other key mitochondrial functions.
One critical mitochondrial function involves control of cellular Ca2+ levels to ensure proper functioning of numerous signal transduction and cell cycle systems (78). Ca2+ is also a key regulator of internal mitochondrial functions and acts at several levels within the organelle to control ATP synthesis and ROS production (20). Dysregulation of mitochondrial Ca2+ homeostasis is now recognized to play a key role in several pathologies, including liver disease (55). It is known that mitochondria have the ability to rapidly sequester large amounts of Ca2+ in response to increased cytosolic Ca2+ levels (30, 38); however, the ability to store and retain Ca2+ may be impaired when mitochondrial function is negatively impacted during pathological states. Recently, Yan et al. (77) reported that chronic ethanol consumption increased intracellular Ca2+ levels in isolated hepatocytes compared with levels measured in ethanol-naïve hepatocytes. The data presented in the current study show that liver mitochondria from animals fed ethanol chronically are more susceptible to Ca2+ overload than control mitochondria. Isolated mitochondria in the presence of phosphate will take up and sequester Ca2+ until buffering is reached (i.e., Ca2+ retention capacity). However, when mitochondria become overloaded with Ca2+, they will undergo the MPTP, allowing Ca2+ to flood out of the matrix compartment back into the cytosol. Mitochondria isolated from livers of ethanol-fed animals have a lower threshold for Ca2+-mediated induction of the MPTP and, therefore, have a reduced Ca2+ retention capacity compared with control mitochondria (Fig. 7). Accordingly, we investigated whether alterations in the proposed components that compromise and/or regulate the MPTP might be involved in the increased sensitivity of mitochondria to undergo the MPT following chronic ethanol exposure.
The MPTP is a nonselective pore that spans the inner and outer membranes of the mitochondrion and allows for the passage of small molecular solutes (16, 80). Although controversial, studies suggest that the MPTP is composed of VDAC, ANT, and Cyp D (16, 41, 80), with the opening of the pore enhanced by adenine nucleotide depletion, ROS, and Ca2+ (6, 46, 50). One consequence of pore opening is that the inner mitochondrial membrane becomes more permeable to H+ and other ions, leading to dissipation of the proton-motive force and decreased ATP generation. Similarly, opening of the MPTP will allow small molecular solutes to equilibrate across the inner membrane, resulting in swelling of the mitochondrion. As the matrix compartment expands, it exerts pressure on both the inner and outer membranes, causing the outer membrane to rupture (i.e., MOMP). This can result in the release of mitochondrial proteins from the inner membrane space, which then engage other proteins in the cytosol and nucleus, initiating cell death pathways. Although the mechanisms responsible for MPTP and MOMP induction remain poorly defined (39), several theories have been proposed and include changes in mitochondria membrane potential, Ca2+ overload, increased ROS production, and posttranslational modification of MPTP components (34, 57, 69).
With regard to alcohol-dependent changes in MPTP components, we observed no change in VDAC or ANT gene expression or protein levels. The lack of effect on ANT was predicted, since previous studies showed no effect on ANT protein following chronic ethanol consumption (23). However, we cannot exclude the possibility that chronic ethanol treatment may have altered VDAC functionality via posttranslational modification, e.g., phosphorylation (29). In contrast, whole liver mitochondria isolated from chronic ethanol-fed animals exhibited a higher content of Cyp D protein compared with control mitochondria (Fig. 8F). This finding is mirrored at the gene level, since higher transcript levels of Cyp D are also observed in liver from ethanol-fed rats compared with controls (Fig. 8E). Cyp D is a member of the CsA-binding cyclophilin family of proteins that catalyzes cis-trans isomerization of peptidyl-prolyl bonds (3, 34). This activity participates in the correct folding, assembly, and transport of newly synthesized proteins (32). In addition to its chaperone function in mitochondria, Cyp D is believed to serve as a regulator or “sensitizer” of the MPTP (11, 34). The role of Cyp D in the MPTP was originally proposed by Halestrap and Davidson (40) and based on studies showing that opening of the MPTP was inhibited by CsA after binding to Cyp D. These early studies also suggested that ROS promoted MPTP formation through oxidation of critical ANT thiols, which presumably increased the affinity of ANT for Cyp D and sensitivity to undergo the MPTP (46, 60, 80). On the basis of this, it is possible that the chronic ethanol-mediated increase in oxidative stress (7) and Cyp D may contribute to increased MPTP sensitivity and impaired mitochondrial Ca2+ handling through increased interactions among MPTP components. Previous studies have shown that tissues with high Cyp D content like synaptic mitochondria also have reduced mitochondrial Ca2+ retention capacity compared with tissues (i.e., nonsynaptic mitochondria) that contain lower levels of Cyp D (62). Likewise, we observed a similar response of reduced Ca2+ retention capacity (Fig. 7, A and C) and increased Cyp D protein levels in liver mitochondria isolated from chronic ethanol-fed animals compared with ethanol-naïve controls (Fig. 8E). Therefore, higher levels of Cyp D in response to chronic ethanol ingestion may contribute, in part, to reduce the mitochondrial Ca2+ threshold that initiates the MPTP (Figs. 3 and 7).
More recently, the role of Cyp D in regulation of the MPTP has been supported in studies using Cyp D-null mice. In these studies, genetic ablation of Cyp D increased mitochondrial Ca2+ buffering capacity to levels measured in CsA-treated mitochondria from wild-type controls, with no effect of CsA observed in Cyp D-null mitochondria (12, 15). Although this work supports the hypothesis that Cyp D regulates the MPTP, it is important to point out that new reservations have been raised concerning the significance of the VDAC-ANT-Cyp D interaction in MPTP regulation (13, 17, 34, 39). For example, the ANT-Cyp D interaction has only been shown using in vitro systems with detergent extracts of mitochondria and following ANT purification (76). Moreover, ANT-null mitochondria display CsA-sensitive MPTP (49), and VDAC may be not required for MPTP opening (13). Also, studies show that the absence of Cyp D does not exclude Ca2+-mediated MPTP induction but simply increases the amount of Ca2+ needed to open the pore (49). Our data show that although CsA treatment increases mitochondria Ca2+ retention capacity, it does not block pore formation but merely delays the onset of MPTP in both control and ethanol mitochondria (Fig. 7, B and D). Recent studies also suggest additional regulatory interactions of Cyp D with the inorganic phosphate carrier (54), a heat shock protein 90 tumor necrosis factor receptor-associated protein-1 complex (45), and a GSK-3-ERK complex (70) in mitochondria, with all of these interactions having possible implications for MPTP regulation. Last, some models even suggest that it is the isomerase activity of Cyp D facilitating a conformational change to an unknown inner membrane protein that is responsible for sensitizing pore opening; however, this protein has not been identified (39). Although these studies do not exclude the involvement of Cyp D in the MPTP, they do highlight an emerging complexity of the mitochondrial effects of Cyp D, which are independent of the MPTP. Thus, the role of Cyp D in mitochondrial Ca2+ metabolism and MPTP regulation should be assumed to go beyond the classic VDAC-ANT-Cyp D interaction model.
This intricacy in mitochondrial physiology is highlighted further by studies showing that Cyp D may also play a role as a redox sensor in the mitochondrion. Linard et al. (56) showed that oxidation of human Cyp D influences its conformation and activity. Using site-directed mutagenesis, it was shown that Cys157 and Cys203 influence the redox conformation of Cyp D through the formation of an intramolecular disulfide bridge. Whereas the reduced enzyme functions as a chaperone, i.e., refolding proteins after import into the mitochondrion, the oxidized enzyme may participate in the activation of cell death pathways through MPTP induction. Whether these redox sensor characteristics of Cyp D contribute to the increased vulnerability to undergo MPT in response to chronic ethanol consumption is not known and will be the focus of future studies.
In considering these alcohol-dependent changes in the MPTP machinery, it is important to also evaluate whether chronic alcohol consumption affects levels of other proteins involved in controlling mitochondrial function and cell death/survival pathways. For example, proapoptotic proteins, including Bax, are responsible for the permeabilization of the mitochondrial outer membrane (i.e., MOMP), whereas antiapoptotic proteins, including Bcl-2 and Bcl-xL, preserve mitochondrial integrity and prevent release of cytochrome c (5). The proapoptotic protein Bax is a cytosolic protein that translocates to the mitochondrion during apoptosis (5). After activation, Bax inserts into the outer mitochondrial membrane and forms larger oligomeric structures that may potentiate the MPTP (5, 61). Whereas we observed no change in total levels of Bax protein in the cytosol, we did see a significant increase in Bax in the mitochondrial compartment from ethanol-fed animals compared with controls. It is predicted that the alcohol-dependent increase in mitochondrial Bax may contribute to the defect in Ca2+ handling through interactions with the MPTP and/or other membrane components. In addition, Bax may directly affect respiratory chain function (66, 73). For example, activation of the Bax pathway alters intracellular pH, causing matrix alkalinization and cytosolic acidification, which could disrupt mitochondrial respiration or ATP synthase activity (5). Moreover, when Bax was expressed in respiration-competent yeast strains, mitochondrial oxygen consumption was decreased, suggesting that Bax may directly inhibit respiratory complex activities (42). Our data presented herein also show decreased respiration in isolated liver mitochondria from ethanol-fed animals that contain increased Bax protein in the mitochondrial compartment. Although preliminary, these results suggest that, in addition to aiding in membrane permeabilization and disruption in mitochondrial Ca2+ handling, the mitochondrial localization of Bax may have direct effects on the individual respiratory complexes, interfering with respiration and/or ATP production in mitochondria from chronically ethanol-exposed animals.
Important to this is also the concept that the balance between pro- and antiapoptotic proteins can determine cell fate. In contrast to Bax, the Bcl-2 family proteins Bcl-2 and Bcl-xL inhibit cell death depending upon the intracellular location of these proteins. For example, Bcl-2 is located on the cytoplasmic face of the outer mitochondrial membrane, where it is proposed to prevent the release of proapoptotic proteins (52, 79). Studies show that Bcl-2 overexpression can affect mitochondrial-mediated cell death by preventing oxidative stress and inhibiting the release of proapoptotic mitochondrial inner membrane proteins (52). One protein regulated by the Bcl-2 family proteins is cytochrome c, a peripheral protein of the inner mitochondrial membrane that functions as an electron shuttle between complexes III and IV of the electron transport chain. Upon an apoptotic stimulus, cytochrome c can be released from mitochondria into the cytosol, where it mediates activation and oligomerization of the adaptor molecule apoptosis protease-activating factor 1 (Apaf-1), forming the apoptosome and initiating apoptosis (33). Consistent with the central role of the mitochondrion in regulating apoptosis, several studies report that Bcl-2 prevents cytochrome c release (48, 79). In the current study, we observed increased mitochondrial Bcl-2 protein in mitochondria from ethanol-treated animals compared with controls. This increase may reflect a compensatory response to lessen apoptosis induced by chronic ethanol consumption. In addition, cells that overexpress Bcl-xL do not accumulate cytosolic cytochrome c during apoptosis because cytochrome c can be sequestered by binding to Bcl-xL (47). Interestingly, Cyp D overexpression may also decrease cytochrome c release from mitochondria via interaction with Bcl-2, a function of Cyp D independent of its presumed role in the MPTP (31). Together, these data are important because they provide a possible explanation for why we observed no overt loss in mitochondrial cytochrome c in ethanol-fed groups and our inability to detect the monomeric (12–15 kDa) cytochrome c in cytosol from both ethanol and control groups. These data also support the idea that increased cell death (as measured by TUNEL staining) observed in livers of ethanol-fed animals may be independent of the cytochrome c-Apaf-1 pathway and more likely reflect necrotic cell death due to the inability to maintain adequate ATP (8). Interestingly, studies suggest that Cyp D-dependent MPTP regulates necrotic but not apoptotic cell death (63). Together, these findings support a model in which release of cytochrome c from mitochondria during ethanol-mediated stress may be limited by interaction with antiapoptotic proteins like Bcl-2 and/or Bcl-xL or in response to increased Cyp D in mitochondria from ethanol-fed rats.
In conclusion, we show higher Cyp D levels in liver mitochondria following chronic ethanol consumption. One possible consequence of increased Cyp D may be dysregulation of mitochondrial Ca2+ handling capability and increased sensitivity for MPTP induction in response to chronic ethanol ingestion. However, as highlighted in previous sections, our understanding of the complex biological functions of Cyp D in mitochondria is rapidly evolving and extends beyond the classic idea that Cyp D functions only via interactions with the MPTP. Thus, experimental findings involving Cyp D and its presumed role in the MPTP should be interpreted with caution, as recommended in Refs. 17, 34, and 39. In the current study, we observed a significant reduction in the ability of mitochondria to retain Ca2+ that was matched by increased Ca2+-induced swelling in liver mitochondria from chronic ethanol-fed animals compared with control groups. Moreover, we show decreased mitochondrial respiration and increased Bax protein in mitochondria in response to chronic ethanol ingestion. In total, these results are significant because they provide a more comprehensive understanding of the molecular changes that contribute to chronic ethanol-induced mitochondrial dysfunction and damage. By more easily undergoing the MPT, chronic ethanol-exposed mitochondria may instigate hepatocyte death, leading to liver disease. Although the precise mechanisms responsible for the Ca2+ handling differences between liver mitochondria from control and ethanol groups remain to be determined, the results provided herein begin to provide a more complete explanation for the increased vulnerability of mitochondria from chronic ethanol exposure. These findings also suggest that normalization of Cyp D levels may be an effective therapeutic strategy to treat diseases in which mitochondrial dysfunction is linked to pathogenesis, like that seen in alcoholic fatty liver disease.
GRANTS
This work was supported in part by National Institute on Alcohol Abuse and Alcoholism Grants AA-15172 and AA-18841 to S. M. Bailey. A. L. King is supported by a Research Supplement to Promote Diversity in Health-Related Research linked to Grant AA-15172.
DISCLOSURES
The authors have no conflicts of interest and no disclosures.
REFERENCES
- 1. Adachi M, Higuchi H, Miura S, Azuma T, Inokuchi S, Saito H, Kato S, Ishii H. Bax interacts with the voltage-dependent anion channel and mediates ethanol-induced apoptosis in rat hepatocytes. Am J Physiol Gastrointest Liver Physiol 287: G695–G705, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc 65: 278–290, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Andreeva L, Heads R, Green CJ. Cyclophilins and their possible role in the stress response. Int J Exp Pathol 80: 305–315, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andringa KK, King AL, Eccleston HB, Mantena SK, Landar A, Jhala NC, Dickinson DA, Squadrito GL, Bailey SM. Analysis of the liver mitochondrial proteome in response to ethanol and S-adenosylmethionine treatments: novel molecular targets of disease and hepatoprotection. Am J Physiol Gastrointest Liver Physiol 298: G732–G745, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonsson B. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell Tissue Res 306: 347–361, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong JS. The role of the mitochondrial permeability transition in cell death. Mitochondrion 6: 225–234, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology 28: 1318–1326, 1998. [DOI] [PubMed] [Google Scholar]
- 8. Bailey SM, Cunningham CC. Effect of dietary fat on chronic ethanol-induced oxidative stress in hepatocytes. Alcohol Clin Exp Res 23: 1210–1218, 1999. [PubMed] [Google Scholar]
- 9. Bailey SM, Pietsch EC, Cunningham CC. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic Biol Med 27: 891–900, 1999. [DOI] [PubMed] [Google Scholar]
- 10. Bailey SM, Robinson G, Pinner A, Chamlee L, Ulasova E, Pompilius M, Page GP, Chhieng D, Jhala N, Landar A, Kharbanda KK, Ballinger S, Darley-Usmar V. S-adenosylmethionine prevents chronic alcohol-induced mitochondrial dysfunction in the rat liver. Am J Physiol Gastrointest Liver Physiol 291: G857–G867, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol 46: 850–857, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9: 550–555, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bambrick LL, Chandrasekaran K, Mehrabian Z, Wright C, Krueger BK, Fiskum G. Cyclosporin A increases mitochondrial calcium uptake capacity in cortical astrocytes but not cerebellar granule neurons. J Bioenerg Biomembr 38: 43–47, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem 280: 18558–18561, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Bernardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronilli V, Scorrano L. The mitochondrial permeability transition. Biofactors 8: 273–281, 1998. [DOI] [PubMed] [Google Scholar]
- 17. Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J 273: 2077–2099, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 19. Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem 264: 7826–7830, 1989. [PubMed] [Google Scholar]
- 20. Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol 291: C1082–C1088, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem 278: 19062–19070, 2003. [DOI] [PubMed] [Google Scholar]
- 23. Coleman WB, Cahill A, Ivester P, Cunningham CC. Differential effects of ethanol consumption on synthesis of cytoplasmic and mitochondrial encoded subunits of the ATP synthase. Alcohol Clin Exp Res 18: 947–950, 1994. [DOI] [PubMed] [Google Scholar]
- 24. Coleman WB, Cunningham CC. Effects of chronic ethanol consumption on the synthesis of polypeptides encoded by the hepatic mitochondrial genome. Biochim Biophys Acta 1019: 142–150, 1990. [DOI] [PubMed] [Google Scholar]
- 25. Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem 258: 729–735, 1998. [DOI] [PubMed] [Google Scholar]
- 26. Cunningham CC, Coleman WB, Spach PI. The effects of chronic ethanol consumption on hepatic mitochondrial energy metabolism. Alcohol Alcohol 25: 127–136, 1990. [DOI] [PubMed] [Google Scholar]
- 27. Cunningham CC, Spach PI. The effect of chronic ethanol consumption on the lipids in liver mitochondria. Ann NY Acad Sci 492: 181–192, 1987. [DOI] [PubMed] [Google Scholar]
- 28. Dahout-Gonzalez C, Nury H, Trézéguet V, Lauquin GJ, Pebay-Peyroula E, Brandolin G. Molecular, functional, and pathological aspects of the mitochondrial ADP/ATP carrier. Physiology (Bethesda) 21: 242–249, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res 103: 983–991, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duchen MR. Mitochondria and Ca(2+) in cell physiology and pathophysiology. Cell Calcium 28: 339–348, 2000. [DOI] [PubMed] [Google Scholar]
- 31. Eliseev RA, Malecki J, Lester T, Zhang Y, Humphrey J, Gunter TE. Cyclophilin D interacts with Bcl2 and exerts an anti-apoptotic effect. J Biol Chem 284: 9692–9699, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem 216: 689–707, 1993. [DOI] [PubMed] [Google Scholar]
- 33. Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 13: 1423–1433, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Giorgio V, Soriano ME, Basso E, Bisetto E, Lippe G, Forte MA, Bernardi P. Cyclophilin D in mitochondrial pathophysiology. Biochim Biophys Acta 1797: 1113–1118, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graier WF, Frieden M, Malli R. Mitochondria and Ca(2+) signaling: old guests, new functions. Pflugers Arch 455: 375–396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium 28: 285–296, 2000. [DOI] [PubMed] [Google Scholar]
- 37. Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol Cell Physiol 258: C755–C786, 1990. [DOI] [PubMed] [Google Scholar]
- 38. Hajnóczky G, Csordás G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 40: 553–560, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46: 821–831, 2009. [DOI] [PubMed] [Google Scholar]
- 40. Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 268: 153–160, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie 84: 153–166, 2002. [DOI] [PubMed] [Google Scholar]
- 42. Harris MH, Vander Heiden MG, Kron SJ, Thompson CB. Role of oxidative phosphorylation in Bax toxicity. Mol Cell Biol 20: 3590–3596, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hewitson TD, Bisucci T, Darby IA. Histochemical localization of apoptosis with in situ labeling of fragmented DNA. Methods Mol Biol 326: 227–234, 2006. [DOI] [PubMed] [Google Scholar]
- 44. Jacobson J, Duchen MR. Interplay between mitochondria and cellular calcium signalling. Mol Cell Biochem 256–257: 209–218, 2004. [DOI] [PubMed] [Google Scholar]
- 45. Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell 131: 257–270, 2007. [DOI] [PubMed] [Google Scholar]
- 46. Kanno T, Sato EE, Muranaka S, Fujita H, Fujiwara T, Utsumi T, Inoue M, Utsumi K. Oxidative stress underlies the mechanism for Ca(2+)-induced permeability transition of mitochondria. Free Radic Res 38: 27–35, 2004. [DOI] [PubMed] [Google Scholar]
- 47. Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan ZM, Saxena S, Weichselbaum R, Nalin C, Kufe D. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis. Proc Natl Acad Sci USA 94: 6939–6942, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136, 1997. [DOI] [PubMed] [Google Scholar]
- 49. Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427: 461–465, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett 495: 12–15, 2001. [DOI] [PubMed] [Google Scholar]
- 51. Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med 47: 333–343, 2009. [DOI] [PubMed] [Google Scholar]
- 52. Kowaltowski AJ, Fenton RG, Fiskum G. Bcl-2 family proteins regulate mitochondrial reactive oxygen production and protect against oxidative stress. Free Radic Biol Med 37: 1845–1853, 2004. [DOI] [PubMed] [Google Scholar]
- 53. Landar A, Shiva S, Levonen AL, Oh JY, Zaragoza C, Johnson MS, Darley-Usmar VM. Induction of the permeability transition and cytochrome c release by 15-deoxy-Delta12,14-prostaglandin J2 in mitochondria. Biochem J 394: 185–195, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem 283: 26312–26323, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li Y, Boehning DF, Qian T, Popov VL, Weinman SA. Hepatitis C virus core protein increases mitochondrial ROS production by stimulation of Ca2+ uniporter activity. FASEB J 21: 2474–2485, 2007. [DOI] [PubMed] [Google Scholar]
- 56. Linard D, Kandlbinder A, Degand H, Morsomme P, Dietz KJ, Knoops B. Redox characterization of human cyclophilin D: identification of a new mammalian mitochondrial redox sensor? Arch Biochem Biophys 491: 39–45, 2009. [DOI] [PubMed] [Google Scholar]
- 57. Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential [deltapsi(m)] in apoptosis; an update. Apoptosis 8: 115–128, 2003. [DOI] [PubMed] [Google Scholar]
- 58. Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health 27: 209–219, 2003. [PMC free article] [PubMed] [Google Scholar]
- 59. Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med 44: 1259–1272, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem J 367: 541–548, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mizuta T, Shimizu S, Matsuoka Y, Nakagawa T, Tsujimoto Y. A Bax/Bak-independent mechanism of cytochrome c release. J Biol Chem 282: 16623–16630, 2007. [DOI] [PubMed] [Google Scholar]
- 62. Naga KK, Sullivan PG, Geddes JW. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci 27: 7469–7475, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005. [DOI] [PubMed] [Google Scholar]
- 64. Pang Z, Geddes JW. Mechanisms of cell death induced by the mitochondrial toxin 3-nitropropionic acid: acute excitotoxic necrosis and delayed apoptosis. J Neurosci 17: 3064–3073, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pastorino JG, Marcineviciute A, Cahill A, Hoek JB. Potentiation by chronic ethanol treatment of the mitochondrial permeability transition. Biochem Biophys Res Commun 265: 405–409, 1999. [DOI] [PubMed] [Google Scholar]
- 66. Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V, Martinuzzi A, Hirano M, Przedborski S, Vila M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci USA 102: 19126–19131, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J Biol Chem 279: 53103–53108, 2004. [DOI] [PubMed] [Google Scholar]
- 68. Pfeiffer DR, Gunter TE, Eliseev R, Broekemeier KM, Gunter KK. Release of Ca2+ from mitochondria via the saturable mechanisms and the permeability transition. IUBMB Life 52: 205–212, 2001. [DOI] [PubMed] [Google Scholar]
- 69. Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 12: 815–833, 2007. [DOI] [PubMed] [Google Scholar]
- 70. Rasola A, Sciacovelli M, Chiara F, Pantic B, Brusilow WS, Bernardi P. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc Natl Acad Sci USA 107: 726–731, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rimessi A, Giorgi C, Pinton P, Rizzuto R. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta 1777: 808–816, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev 79: 1089–1125, 1999. [DOI] [PubMed] [Google Scholar]
- 73. Teles AV, Ureshino RP, Dorta DJ, Lopes GS, Hsu YT, Smaili SS. Bcl-x(L) inhibits Bax-induced alterations in mitochondrial respiration and calcium release. Neurosci Lett 442: 96–99, 2008. [DOI] [PubMed] [Google Scholar]
- 74. Venkatraman A, Landar A, Davis AJ, Chamlee L, Sanderson T, Kim H, Page G, Pompilius M, Ballinger S, Darley-Usmar V, Bailey SM. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. J Biol Chem 279: 22092–22101, 2004. [DOI] [PubMed] [Google Scholar]
- 75. Venkatraman A, Landar A, Davis AJ, Ulasova E, Page G, Murphy MP, Darley-Usmar V, Bailey SM. Oxidative modification of hepatic mitochondria protein thiols: effect of chronic alcohol consumption. Am J Physiol Gastrointest Liver Physiol 286: G521–G527, 2004. [DOI] [PubMed] [Google Scholar]
- 76. Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J 336: 287–290, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yan M, Zhu P, Liu HM, Zhang HT, Liu L. Ethanol induced mitochondria injury and permeability transition pore opening: role of mitochondria in alcoholic liver disease. World J Gastroenterol 13: 2352–2356, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin 27: 821–826, 2006. [DOI] [PubMed] [Google Scholar]
- 79. Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275: 1129–1132, 1997. [DOI] [PubMed] [Google Scholar]
- 80. Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta 1241: 139–176, 1995. [DOI] [PubMed] [Google Scholar]