Abstract
The production of 20-hydroxyeicosatetraenoic acid (20-HETE) is increased during ischemia-reperfusion, and inhibition of 20-HETE production has been shown to reduce infarct size caused by ischemia. This study was aimed to discover the molecular mechanism underlying the action of 20-HETE in cardiac myocytes. The effect of 20-HETE on L-type Ca2+ currents (ICa,L) was examined in rat isolated cardiomyocytes by patch-clamp recording in the whole cell mode. Superfusion of cardiomyocytes with 20-HETE (10–100 nM) resulted in a concentration-dependent increase in ICa,L, and this action of 20-HETE was attenuated by a specific NADPH oxidase inhibitor, gp91ds-tat (5 μM), or a superoxide scavenger, polyethylene glycol-superoxide dismutase (25 U/ml), suggesting that NADPH-oxidase-derived superoxide is involved in the stimulatory action of 20-HETE on ICa,L. Treatment of cardiomyocytes with 20-HETE (100 nM) increased both NADPH oxidase activity and superoxide production by approximately twofold. To study the molecular mechanism mediating the 20-HETE-induced increase in NADPH oxidase activity, PKC activity was measured in cardiomyocytes. Incubation of the cells with 20-HETE (100 nM) significantly increased PKC activity, and pretreatment of cardiomyocytes with a selective PKC inhibitor, GF-109203 (1 μM), attenuated the 20-HETE-induced increases in ICa,L and in NADPH oxidase activity. In summary, 20-HETE stimulates NADPH oxidase-derived superoxide production, which activates L-type Ca2+ channels via a PKC-dependent mechanism in cardiomyocytes. 20-HETE and 20-HETE-producing enzymes could be novel targets for the treatment of cardiac ischemic diseases.
Keywords: 20-hydroxyeicosatetraenoic acid, L-type calcium channel, protein kinase C, cardiac myocytes, reactive oxygen species
20-hydroxyeicosatetraenoic acid (20-HETE) is a lipid metabolite of arachidonic acid that is produced by ω-hydroxylase enzymes of the cytochrome P-450 (CYP)4A and CYP4F families, which are relatively abundant and exert regulatory functions dependent on the tissue (18). For example, in the kidney, 20-HETE regulates renal functions, such as renal vascular tone, tubuloglomerular feedback, autoregulation of renal blood flow, tubular transport, and mitogenesis (20). In blood vessels, 20-HETE is a potent vasoconstrictor that activates L-type Ca2+ channels and inhibits Ca2+-sensitive K+ channels in vascular smooth muscle cells (28, 29). In pulmonary arteries, 20-HETE enhances NADPH oxidase-dependent production of ROS in endothelial cells (21). Thus, it was proposed that 20-HETE plays an important role in the control of apoptosis and angiogensis in vascular endothelial cells in the pulmonary microcirculation (13). Recently, 20-HETE and CYP ω-hydroxylase were also identified in hearts from the rat and dog (13, 26). In isolated Langendorff-perfused rat hearts, the administration of nonspecific CYP enzyme inhibitors, such as chloramphenicol, cimetidine, and sulfaphenazole, prevented ischemia-reperfusion-induced myocardial damage, an effect that was associated with reduced ROS production (12). These results were confirmed in an ischemia-reperfusion injury rat model evoked by coronary artery ligation (17). More interestingly, production of 20-HETE is enhanced during ischemia-reperfusion injury (26). Specific CYP ω-hydroxylase inhibitors, such as 17-octadecanoic acid (17-ODYA) and N-methylsulfonyl-12,12-dibromododec-11-enamide (DDMS), markedly inhibit 20-HETE production and produce a profound reduction in myocardial infarct size (13, 26). Conversely, exogenous 20-HETE administration significantly increases infarct size (26). Thus, it has been proposed that 20-HETE production is linked to myocardial injury induced by ischemia-reperfusion. However, the exact molecular mechanisms underlying the action of 20-HETE in ischemia-reperfusion injury and its actions on cardiomyocytes are not yet fully understood.
Alterations in intracellular ROS levels occur during hypoxia and oxidative stress, and ROS are well recognized as important mediators of cardiovascular pathologies, such as myocardial infarction-related injury, cardiac hypertrophy, myocardial remodeling, and heart failure (1). In cardiomyocytes, both mitochondria and NADPH oxidase are capable of producing superoxide (1). Recent studies have suggested a role for NADPH oxidase-derived ROS in cardiac pathology. Mice lacking gp91dsphox, a subunit of NADPH oxidase, do not develop ANG II-induced cardiac hypertrophy (2). On the other hand, excessive intracellular Ca2+ accumulation is another factor mediating ischemia-induced cardiac injury. Elevated intracellular Ca2+ alters myocyte electrophysiological properties, leading to cardiac arrhythmias as well as to inhibition of Na+-K+ATPase activity and damage to mitochondrial function (15). In addition, excessive Ca2+ accumulation in cardiomyocytes causes abnormal contractile function and altered gene expression, leading to heart failure and cardiac remodeling (15). The L-type Ca2+ channel is the main route for Ca2+ influx into cardiac myocytes and is an important determinant of Ca2+ homeostasis. Both L-type Ca2+ current (ICa,L)- and ROS-mediated signaling pathways cross-talk with each other in cardiac myocytes. For example, there is considerable evidence that the function of the L-type Ca2+ channel is influenced by the redox state of the cell, leading to intracellular Ca2+ overload (15, 23). Conversely, Ca2+ overload damages mitochondrial function and results in an increase in intracellular ROS production (24). However, it is still not clear whether NADPH oxidase-derived ROS and L-type Ca2+ channels are involved in the action of 20-HETE in cardiac myocytes.
In the present study, we examined the effect of 20-HETE on L-type Ca2+ channel activity and intracellular ROS production in cardiomyocytes and further identified the intracellular signaling pathways involved in the cardiac actions of 20-HETE.
MATERIALS AND METHODS
Animals and drugs.
Experiments were performed on male Sprague-Dawley rats (220–250 g) purchased from the Medical School of Jilin University or Charles River Farms (Wilmington, MA). Rats were housed under controlled conditions with a 12:12-h light-dark cycle. Food and water were available to the animals ad libitum. All protocols were approved by the North Dakota State University and Northeast Normal University Institutional Animal Care and Use Committees.
Dihydroethidium (DHE) was purchased from Molecular Probes (Carlsbad, CA). DMEM was obtained from GIBCO (Carlsbad, CA). The selective NADPH oxidase inhibitor gp91ds-tat (H-RKKRRQRRR-CSTRIRRQL-NH3), and its control, scrambled gp91ds-tat (H-RKKRRQRRR-CLRITRQSR-NH3), were synthesized by Tufts University Core Facility (Medford, MA). 20-HETE was purchased from Cayman Chemical (Ann Arbor, MI). The selective PKC inhibitor GF-109203 was obtained from BioMol (Plymouth Meeting, PA). Monoclonal antibodies against phosphoserine were purchased from BD Transduction Laboratories, and rabbit antibodies against p47phox were obtained from Upstate (Lake Placid, NY). Polyethylene glycol (PEG)-SOD, ATP, GTP, HEPES, and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Isolation of adult rat ventricular myocytes.
Male Sprague-Dawley rats were deeply anesthetized with urethane (10%, 100 mg/kg), and hearts were rapidly excised, kept in normal Tyrode solution containing (in mmol/l) 100 NaCl, 10 KCl, 5 MgSO4, 1.2 Na2HPO4, 1.8 CaCl2, 10 MOPS, 10 taurine, and 20 glucose (pH 7.2, adjusted with NaOH), and put on ice (4°C). Hearts were perfused retrogradely through the aorta on a Langendorff perfusion apparatus at 37°C. Retrograde perfusion was started with Tyrode solution for 2 min to wash out any remaining blood followed by perfusion with nominally Ca2+-free normal Tyrode solution until beating stopped completely. Hearts were then perfused with Ca2+-free normal Tyrode solution containing 0.16 mg/ml collagenase type II and 0.16 mg/ml BSA until the myocardial tissue was soft (∼35–40 min). Ventricles were then removed and minced into small pieces. Cells were dispersed and suspended in a solution containing (in mmol/l) 70 KOH, 50 l-glutamic acid, 40 KCl, 20 taurine, 20 KH2PO4, 3 MgCl2, 10 glucose, 10 HEPES, and 0.5 EGTA (pH 7.4, adjusted with KOH). Isolated cells were stored in this solution at 4°C until they were used for patch-clamp or bioassay experiments.
Electrophysiological recordings.
Using the whole cell voltage-clamp configuration, ICa,L was recorded in isolated adult cardiomyocytes bathed in a solution (9) containing (in mmol/l) 136 tetraethylammonium Cl, 5.4 CsCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose (pH 7.4, adjusted with CsOH). Recording pipettes were made of capillaries pulled by a flaming micropipette puller (P-97, Sutter Instruments) and heat polished before use. Pipette resistance was in the range of 2–5 MΩ when filled with a pipette solution containing (in mmol/l) 110 CsOH, 110 aspartate, 20 CsCl, 1 MgCl2, 10 EGTA, 10 HEPES, and 5 Mg-ATP (pH 7.2, adjusted with CsOH). All experiments were performed at room temperature (22–25°C). An EPC-10 patch-clamp amplifier and Pulse/Pulse Fit software (HEKA Elektronik, Lambrecht, Germany) were used for data collection and analysis. ICa,L was measured from a holding potential of −50 mV using a square wave pulse from −50 to +50 mV in 10-mV voltage steps.
Culture of neonatal rat cardiomyocytes.
Primary cultured cardiomyocytes were prepared from neonatal rats according to previously published procedures with minor modifications (14). In brief, ventricles from 1-day-old Sprague-Dawley rats were digested with collagenase in Hanks' solution at 37°C, and isolated cardiomyocytes were suspended in culture medium composed of 8% FBS, DMEM, and 0.2% penicillin-streptomycin solution. The cell suspension was placed in 35-mm diameter tissue culture dishes at a cell density of 4 × 106 cells/dish and incubated at 37°C in a humidified atmosphere with 95% O2 and 5% CO2 until used in the NADPH oxidase assay or for ROS measurements.
Measurement of intracellular ROS levels.
ROS generation was measured using the oxidant-sensitive fluorogenic probe DHE as previously described with a minor modification (16). In brief, primary cultured cardiomyocytes were loaded with 100 nM DHE for 30 min at 37°C. Cells were then incubated with 20-HETE (100 nM, 5 min), 20-HETE plus the specific NADPH oxidase inhibitor gp91ds-tat (5 μM, 5 min), or 20-HETE plus the PKC inhibitor GF-109203 (1 μM, 5 min). Ethidium fluorescence within cardiomyocytes was detected by a fluorescence microscope (Nikon), and its intensity in individual cells was analyzed using Quantity One software (Bio-Rad, Hercules, CA).
Measurement of NADPH oxidase activity.
20-HETE-induced NADPH oxidase activity was measured by lucigenin-derived chemiluminescence. Cardiomyocytes were prepared as described above and treated with 20-HETE (100 nM, 5 min), 20-HETE plus the specific NADPH oxidase inhibitor gp91ds-tat (5 μM, 5 min), or 20-HETE plus the PKC inhibitor GF-109203 (1 μM, 5 min). Cells were collected and sonicated for 1 s. Ten minutes before luminescence was recorded, NADPH (100 μM) and lucigenin (5 μM) were added, and light emission was recorded during the next 10 s by a Wallac 1450 Micro-Beta JET Luminometer (PerkinElmer Life and Analytical Sciences, Waltham, MA). Protein concentrations were determined using a Bio-Rad protein assay kit with BSA as the standard. Data are presented as counts per minute per milligram of protein.
Assessment of phosphorylated p47phox.
Intracellular phosphorylated p47phox was measured by immunoprecipitation with anti-p47phox antibody followed by regular Western blots using an anti-phosphoserine antibody. Before coimmunoprecipitation assays, isolated cardiomycytes were incubated with control (PBS), 20-HETE (100 nM, 5 min), 20-HETE plus GF-109203 (1 μM, 5 min), or GF-109203 alone. Cells were suspended in the lysis buffer and sonicated briefly. p47phox was immunoprecipitated using an anti-p47phox antibody. The immunoprecipitates were resolved by gel electrophoresis, proteins were transferred to nitrocellulose membranes, and membranes were probed with an anti-phosphoserine antibody (1:1,000) using regular Western blot analysis as previously described (32).
Measurement of PKC activity.
The effect of 20-HETE on PKC activity was measured with a PepTag assay kit (Promega) according to the manufacturer's instructions. In brief, isolated cardiomyocytes were incubated in Kraftbrane solution containing the vehicle control, 20-HETE (100 nM, 5 min), 20-HETE plus GF-109203 (1 μM, 5 min), or GF-109203 alone at 37°C. Cells were then suspended in lysis buffer containing 25 mM Tris·HCl (pH 7.4), 0.5 mM EGTA, 0.5 mM EDTA, 0.05% Triton X-100, 10 mM β-mercaptoethanol, and 0.5 mM PMSF. Samples were sonicated and centrifuged at 14,000 g for 5 min at 4°C. The supernatant was incubated with a fluorescent PKC substrate for 30 min at 30°C. The phosphorylated product and nonphosphorylated substrate were separated on a 0.8% agarose gel at 100 mV for 30 min. The phosphorylated bands were excised, heated at 95°C to melt, and transferred to a tube containing gel solubilization solution and glacial acetic acid. The absorbance at 570 nm was read with a 96-well plate reader. The total protein concentration in each sample was determined with the Bradford assay. The results are expressed as nanomoles per minute per milligram of protein.
Statistics.
All data are expressed as means ± SE. Differences between groups were assessed using Student's t-test or one-way ANOVA for multiple comparisons. P values of <0.05 were taken as significant, and significance levels are given in the text.
RESULTS
20-HETE activates L-type Ca2+ channels.
Previous studies (6, 11) have demonstrated that 20-HETE constricts vascular and airway smooth muscle cells by activating L-type Ca2+ channels. In the present study, we examined the action of 20-HETE on ICa,L in isolated adult rat cardiac myocytes. Figure 1A shows the concentration-dependent effects of externally applied 20-HETE (10–100 nM) on ICa,L recorded from freshly dissociated rat cardiomyocytes. 20-HETE (10–100 nM) caused a significant increase in the magnitude of peak ICa,L recorded during step depolarizations between −50 and +50 mV in 10-mV increments from a holding potential of −50 mV compared with the control (Fig. 1B). Thus, 20-HETE increased peak ICa,L density at +10 mV at concentrations from 10 to 100 nM (Fig. 1A). Peak ICa,L was increased at +10 mV by 37% (from 200 ± 19 to 271 ± 15 pA, n = 13) at 10 nM and by 101% (from 216 ± 24 to 418 ± 16 pA) at 100 nM (Fig. 1C). However, the 20-HETE-induced increase in ICa,L did not shift the peak current-voltage relationship (Fig. 1B). The effect of 20-HETE on ICa,L was readily reversible on washout (Fig. 1A). Bath application of 0.1% ethanol, the vehicle for 20-HETE, had no significant effect on peak ICa,L (before ethanol: 4.8 ± 0.5 pA/pF and after ethanol: 5.0 ± 0.4 pA/pF). In addition, the superfusion of cells with nifedipine (2 μM), an L-type Ca2+ channel blocker, attenuated ICa,L by 89% (Fig. 1D).
Fig. 1.
Effects of 20-hydroxyeicosatrienoic acid (20-HETE) on L-type Ca2+ current (ICa,L) in rat isolated cardiomyocytes. A: concentration-dependent response of peak ICa,L to 20-HETE (from 10 to 100 nM) in rat isolated cardiomyocytes. Wash, washout. Results are means ± SE; n = 13. *P < 0.05 compared with vehicle (Veh) control (Con). B: representative current-voltage curve recorded before and after the administration of 20-HETE (100 nM). C: representative examples of ICa,L recorded from isolated cardiomyocytes elicited by 300-ms depolarizing pulses from a holding potential of −50 mV to test potentials of +50 mV before and after the administration of 20-HETE (10 nM). D: representative current-voltage curve recorded before and after the administration of nifedipine (Nife; 2 μM). E: bar graph summarizing the effects of Nife (2 μM) on 20-HETE-induced activation of ICa,L in isolated cardiomyocytes. Results are means ± SE; n = 10. *P < 0.001 compared with Con. F: time-dependent response of peak ICa,L to 20-HETE in rat isolated cardiomyocytes. ICa,L was recorded in adult rat cardiomyocytes treated with 20-HETE (100 nM) at the time points indicated in the graph. Results are means ± SE; n = 9. *P < 0.001 compared with Con.
To confirm that 20-HETE activates ICa through voltage-dependent L-type Ca2+ channels, we examined the effect of 20-HETE in the presence of nifedipine (2 μM). Pretreatment with nifedipine significantly attenuated ICa (Fig. 1D, 1E), and the subsequent addition of 20-HETE (100 nM) had no stimulatory effect on the amplitude of the residual current (nifedipine alone: 0.4 ± 0.1 pA/pF and nifedipine plus 20-HETE: 0.5 ± 0.1 pA/pF, n = 5, P > 0.05). In addition, the time dependence of the 20-HETE-induced stimulatory effect on ICa,L was examined in adult rat cardiomyocytes. The results are shown in Fig. 1F and indicate that 20-HETE increased ICa in 5 min, reached a peak within 10 min, and lasted >20 min. In summary, these results indicate that 20-HETE increases ICa through activating L-type Ca2+ channel activity in cardiomyocytes.
20-HETE induced increases in ICa,L through NADPH oxidase-derived superoxide.
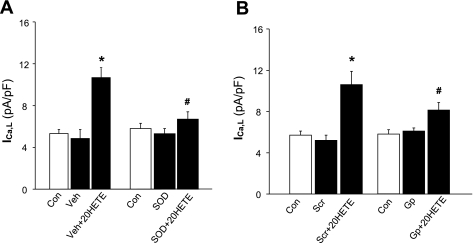
It has been reported that the function of L-type Ca2+ channels can be modified during changes in the cellular redox state (15). Thus, we next examined the role of superoxide on the 20-HETE-induced increase in ICa,L using a cell-permeable superoxide scavenger, PEG-SOD (25 U/ml), in isolated adult rat cardiac myocytes. The results are shown in Fig. 2A and demonstrate that pretreatment of cardiomyocytes with PEG-SOD (25 U/ml) significantly attenuated 20-HETE-induced ICa,L activation and that PEG-SOD alone did not alter basal ICa,L activity. In addition, in the presence of PEG alone, 20-HETE still increased ICa,L density from 5.4 ± 0.4 to 10.1 ± 0.7 pA/pF (n = 7 cells, P < 0.05), indicating that PEG itself had no effect on the action of 20-HETE in cardiac myocytes.
Fig. 2.
Role of NADPH oxidase and superoxide in the action of 20-HETE on ICa,L of cardiac myocytes. A: role of superoxide. ICa,L was recorded in isolated cardiomyocytes using the whole cell patch-clamp recording approach under the following conditions: Con, Veh, and Veh plus 20-HETE (100 nM) or Con, superfusion with polyethylene glycol (PEG)-SOD (SOD; 25 U/ml), and PEG-SOD plus 20-HETE (100 nM). Data are means ± SE of ICa,L density in cardiomyocytes; n = 11. *P < 0.05 compared with Con; #P < 0.05 vs. treatment with Veh plus 20-HETE. B: role of NADPH oxidase. ICa,L was recorded in isolated cardiomyocytes using whole cell patch-clamp recording techniques under the following conditions: Con, superfusion with scrambled gp91ds-tat (Scr; 5 μM), and Scr plus 20-HETE (100 nM) or Con, superfusion with gp91ds-tat (Gp; 5 μM), and Gp plus 20-HETE. Data are means ± SE of ICa,L density in cardiomyocytes; n = 9–11. *P < 0.05 compared with Con; #P < 0.05 vs. treatment with Scr plus 20-HETE.
In the next experiment, the NADPH oxidase inhibitor gp91ds-tat was used to confirm the involvement of superoxide and further to identify the source of superoxide in the action of 20-HETE. Pretreatment of cardiomyocytes with gp91ds-tat (5 μM) significantly attenuated the 20-HETE-induced increase in ICa,L (P < 0.05 compared with 20-HETE alone; Fig. 2B). In contrast, scrambled gp91ds-tat (5 μM) did not alter the 20-HETE-induced activation of ICa,L under the same treatment conditions (Fig. 2B). Neither gp91da-tat nor scrambled gp91ds-tat alone had any significant effect on basal ICa,L in cardiac myocytes. Together, these results suggest that 20-HETE stimulates ICa,L in cardiac myocytes through an NADPH oxidase-derived superoxide pathway in cardiac myocytes.
20-HETE increases superoxide production in cardiomyocytes.
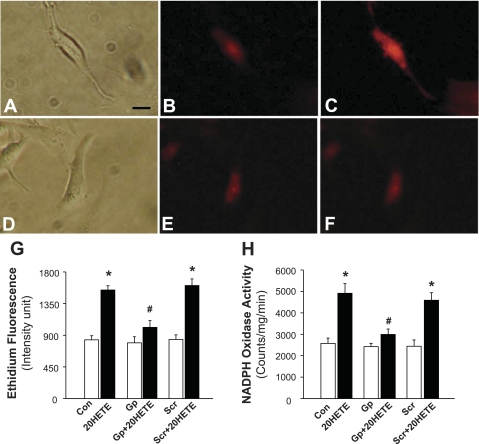
The effect of 20-HETE on superoxide production in cardiomycytes primary cultured from neonatal rats was assessed using the fluorogenic probe DHE. Under control conditions, ethidium fluorescence was low in PBS-treated cardiomyocytes (Fig. 3B); however, treatment of the same cells with 20-HETE (100 nM) resulted in a significant increase (85%) in the density of ethidium fluorescence within cardiomyocytes (Fig. 3, C and G). In addition, the time-dependent response to 20-HETE (100 nM) on superoxide levels was also examined in cultured neonatal rat cardiomyocytes. Ethidium fluorescence density was 841 ± 59, 1,582 ± 67, 1,707 ± 95, 1,792 ± 101, and 1,758 ± 83 intensity units in cardiomyocytes treated with the control vehicle and at 5, 10, 15, and 20 min, respectively, after 20-HETE treatment. The 20-HETE-induced increases in ethidium fluorescence were significantly attenuated by 5 μM gp91ds-tat (793 ± 85 and 1,012 ± 96 ethidium fluorescence intensity units in cells treated with gp91ds-tat and gp91ds-tat + 20-HETE, respectively, n = 25, P > 0.05; Fig. 3G). However, the scrambled gp91ds-tat control did not alter the stimulatory action of 20-HETE on superoxide production (840 ± 67 and 1,607 ± 94 ethidium fluorescence intensity units in cells treated with scrambled gp91ds-tat and scrambled gp91ds-tat + 20-HETE, respectively, n = 21, P < 0.01). These results suggest that 20-HETE increases superoxide production, an effect that is blocked by the inhibition of NADPH oxidase.
Fig. 3.
Effects of 20-HETE on superoxide production and NADPH oxidase activity in cardiomyocytes. Superoxide levels were detected using the fluorogenic probe dihydroethidium (DHE) in primary cultured cardiomyocytes. A–C: representative cardiomyocytes treated under the following conditions. A: a cardiomyocyte in the normal optical phase. B: fluorescence micrograph of a cardiomyocyte loaded with DHE and treated with Veh. C: fluorescence micrograph of the same cardiomyocyte shown in B after treatment with 20-HETE (100 nM). D–F: cardiomyocytes treated under the following conditions. D: a cardiomyocyte in the normal optical phase. E: fluorescence micrograph of cardiomyocytes loaded with DHE after treatment with Gp (5 μM) for 5 min. F: fluorescence micrograph of the same cardiomyocytes shown in E after treatment with 20-HETE (100 nM). Bar = 20 μm. G: bar graph summarizing ethidium fluorescence intensity before and after treatment with 20-HETE in the presence of the Veh (PBS) Con, Gp (5 μM), or Scr (5 μM). In each treatment group, 21–25 cardiomyocytes were used for the quantification of fluorescence. They were derived from three experiments and at least three dishes in each experiment. Data are means ± SE. *P < 0.01, significant difference from the respective Con; #P < 0.05, significant difference vs. treatment with Scr plus 20-HETE. H: primary cultured cardiomyocytes were pretreated under the following conditions: Veh (PBS) Con, Gp (5 μM), or Scr (5 μM) for 5 min before treatment with 100 nM 20-HETE for 5 min. Cells were collected, and NADPH activity was measured and expressed as mean light emission (counts·mg protein−1·min−1). Data are means ± SE; n = 14. *P < 0.05, significantly different from the respective Con; #P < 0.05, significantly different from Scr plus 20-HETE treatment.
20-HETE stimulates NADPH oxidase in cardiomyocytes.
We next determined the effect of 20-HETE on NADPH oxidase activity. Treatment of cardiomyocytes cultured from neonatal rats with 20-HETE (100 nM) resulted in a significant increase in NADPH oxidase activity (2,574 ± 245 and 4,914 ± 453 counts·mg−1·min−1 in cells treated with vehicle and 20-HETE, respectively, n = 14, P < 0.01; Fig. 3H). Pretreatment of cardiomyocytes with gp91ds-tat (5 μM) significantly attenuated the 20-HETE-induced activation of NADPH oxidase (2,426 ± 256 and 2,944 ± 256 counts·mg−1·min−1 in cells treated with gp91ds-tat and gp91ds-tat + 20-HETE, respectively, n = 14, P > 0.05). However, pretreatment of cardiomyocytes with scrambled gp91ds-tat (5 μM) did not alter the 20-HETE-induced increases in NADPH oxidase activity (Fig. 3H). In addition, treatment of cells with either gp91ds-tat or scrambled gp91ds-tat alone had no significant effect on the basal NADPH oxidase activity in culture cardiomyocytes.
PKC is involved in 20-HETE-induced increases in NADPH oxidase and in superoxide production.
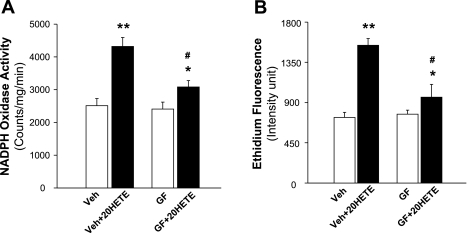
PKC has been reported to be involved in the action of 20-HETE in cerebral arteries (19, 27). Thus, we next examined the effect of a PKC inhibitor, GF-109203, on the 20-HETE-induced activation of NADPH oxidase in primary cultured cardiomyocytes. As expected, incubation of cells with 20-HETE (100 nM) resulted in a significant increase in NADPH oxidase (Fig. 4A). However, pretreatment of cells with GF-109203 (1 μM) significantly attenuated the 20-HETE-induced increases in NADPH oxidase activity in cardiomyocytes by 60%. In addition, GF-109203 alone did not alter basal NADPH oxidase activity. Similarly, pretreatment of cardiomyocytes with GF-109203 (1 μM) also significantly diminished 20-HETE-induced ethidium fluorescence intensity (Fig. 4B), suggesting that blockade of PKC attenuated the stimulatory action of 20-HETE on superoxide production. GF-109203 itself had no effect on the basal levels of superoxide within cardiomyocytes.
Fig. 4.
Effects of PKC inhibition on 20-HETE-induced increases in NADPH oxidase activity and in superoxide production in cardiomyocytes. A: effect of PKC inhibition on 20-HETE-induced increases in NADPH oxidase. Primary cultured cardiomyocytes were pretreated under the following condtions: Veh Con or with the PKC inhibitor GF-109203 (GF; 1 μM) for 5 min. This was followed by an incubation with 100 nM 20-HETE for an additional 5 min. Cells were then collected, and NADPH activity was measured and expressed as mean light emission (counts·mg protein−1·min−1). Data ares mean ± SE; n = 10. *P < 0.05, significantly different from the respective Con; **P < 0.01, significantly different from the respective Con; #P < 0.05, significantly different from the treatment of Veh plus 20-HETE. B: effect of PKC inhibition on 20-HETE-induced increases in superoxide generation in cardiomyocytes. Superoxide levels were measured using the fluorogenic probe DHE in primary cultured cardiomyocytes. The ethidium fluorescence intensity was measured before and after treatment with 20-HETE (100 nM) in the presence of GF (1 μM) or Veh Con. In each treatment group, 20–25 cardiomyocytes were used for the quantification of fluorescence. They were derived from three experiments and at least three dishes in each experiment. Data are means ± SE. *P < 0.05, significantly different from treatment with GF; **P < 0.01, significantly different from treatment with Veh Con; #P < 0.05, significantly different from treatment with Veh plus 20-HETE.
20-HETE increases PKC activity and augments the phosphorylation of NADPH oxidase.
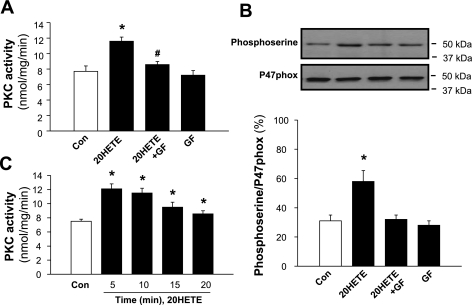
To determine the effect of 20-HETE on PKC, PKC activity was measured in isolated adult rat cardiomyocytes treated with the vehicle control or 20-HETE (100 nM) for 5 min. Incubation of cells with 20-HETE significantly increased PKC activity by 40% (n = 8, P < 0.01; Fig. 5A). The stimulatory effect of 20-HETE on PKC activity peaked at 5 min, followed by a plateau response, and lasted up to 20 min (Fig. 5C). In another set of cells, isolated cardiomyocytes were pretreated with the PKC inhibitor GF-109203 (1 μM) for 5 min followed by the addition of 20-HETE (100 nM) or the vehicle for an additional 5 min. Treatment of cells with GF-109203 completely abolished the 20-HETE-induced increase in PKC activity (Fig. 5A). GF-109203 alone did not alter basal PKC activity in cardiomyocytes. The results from these experiments demonstrate that 20-HETE stimulates PKC activity and that this effect of 20-HETE is completely blocked by GF-109203 in isolated adult rat cardiomyocytes. To detect whether 20-HETE also stimulates PKC in cardiomyocytes cultured from neonatal rats, we repeated the same protocol as described above to detect the effects of 20-HETE on PKC activity in cardiomyocytes cultured from neonatal rats. Treatment of cardiomyocytes with 20-HETE significantly increased PKC activity by 42% (from 9.2 ± .08 to 13.1 ± 0.9 nmol·mg−1·min−1, n = 6, P < 0.01), indicating that 20-HETE also stimulates PKC activity in cultured neonatal rat cardiomyocytes.
Fig. 5.
Effects of 20-HETE on PKC activity and on the phosphorylation of p47phox. A: effect of 20-HETE on PKC activity in isolated cardiomyocytes. PKC activity was measured in isolated cardiomyocytes treated under the following conditions: Veh Con, 20-HETE (100 nM), 20-HETE plus GF (1 μM), or GF alone. Data are means ± SE of 8 independent experiments. *P < 0.01 compared with Con; #P < 0.05 compared with 20-HETE. B: effect of 20-HETE on the phosphorylation of p47phox in isolated cardiomyocytes. Bottom, phosphorylation of p47phox was measured using immunoprecipitation with an anti-p47phox antibody followed by immunoblotting with an anti-phosphoserine antibody in isolated cardiomyocytes treated under the following conditions: Con, 20-HETE (100 nM), 20-HETE plus GF (1 μM), or GF alone. Top, representative blots showing the levels of phosphorylated p47phox and total p47phox in isolated cardiomyocytes under each treatment condition. Data are means ± SE; n = 4 experiments. *P < 0.05 compared with Con. C: time-dependent response of PKC activity to 20-HETE in isolated adult rat cardiomyocytes. PKC activity was measured in cardiomyocytes treated with 20-HETE (100 nM) at the time points indicated in the graph.
The phosphorylation of p47phox is critical in the activation of NADPH oxidase (4). Thus, we determined whether PKC-dependent phosphorylation of p47phox is involved in the 20-HETE-induced increases in NADPH oxidase activity in cardiomyocytes isolated from adult rats. Isolated cardiomyocytes were treated with 20-HETE (100 nM) or the vehicle control for 5 min. 20-HETE treatment caused a 95% increase (n = 4, P < 0.05) in phosphorylated p47phox (Fig. 5B). Pretreatment of cells with the PKC inhibitor GF-109203 (1 μM) completely inhibited 20-HETE-induced phosphorylation. GF-109203 alone did not significantly alter the basal phosphorylation of p47phox.
20-HETE-induced increases in ICa,L are mediated by a PKC-dependent mechanism.
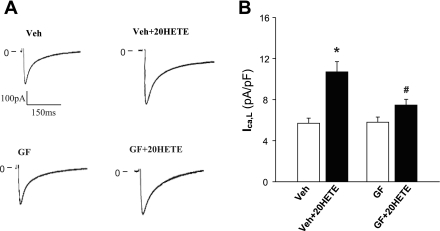
The role of PKC in the stimulatory action of 20-HETE on ICa,L in isolated cardiac myocytes was examined using whole cell patch-clamp recording. Superfusion of cardiac myocytes with 20-HETE (100 nM) resulted in a 96% increase in ICa,L (n = 9 cells, P < 0.01; Fig. 6, A and B). In another set of isolated cardiomyocytes, cells were superfused with GF-109203 (1 μM) followed by the addition of 20-HETE (100 nM). Pretreatment of cells with GF-1091230 significantly attenuated the 20-HETE-induced increase in ICa,L by 61% (n = 11, P < 0.05). GF-109203 alone had no effect on basal ICa,L (Fig. 6B). These results indicate that the PKC signaling pathway is involved in the 20-HETE-induced increase in ICa,L in cardiac myocytes.
Fig. 6.
Effect of PKC inhibition on 20-HETE-induced increases in L-type Ca2+ channel activity. A: representative original recording of ICa,L in isolated cardiac myocytes treated with Veh Con or GF (1 μM) before and after the addition of 20-HETE (100 nM). B: bar graph summarizing the action of 20-HETE (100 nM) on ICa,L of isolated cardiomyocytes pretreated with GF (1 μM) or Veh Con. Data are means ± SE of ICa,L density in isolated cardiomyocytes; n = 9 and 11. *P < 0.01 compared with Con; #P < 0.05 vs. treatment with Veh plus 20-HETE.
DISCUSSION
The current study presents the first evidence that 20-HETE stimulates NADPH oxdiase-derived ROS production and increases L-type Ca2+ channel activity via a PKC-dependent mechanism in cardiac myocytes. This conclusion is supported by the following observations: 1) superfusion of cardiomyocytes with 20-HETE resulted in a concentration-dependent increase in ICa,L; 2) the stimulatory action of 20-HETE on ICa,L was attenuated by both an NADPH oxidase inhibitor and a superoxide scavenger; 3) treatment of cardiomyocytes with 20-HETE increased NADPH oxidase activity and elevated intracellular ROS production, effects that were blocked by the PKC inhibitor GF-109203; and 4) 20-HETE treatment increased PKC activity and blockade of PKC attenuated the 20-HETE-induced increase in ICa,L in cardiomyocytes. These results indicate that 20-HETE stimulates PKC, which increases the phosphorylation of NADPH oxidase and results in NADPH oxidase-dependent ROS production, subsequently leading to an increase in ICa,L in cardiomyocytes. These actions of 20-HETE may contribute to 20-HETE-induced myocardial injury during ischemia-reperfusion.
It is well known that persistent increases in intracellular Ca2+ are important to the pathogenesis of myocardial ischemic diseases, and the L-type Ca2+ channel is the major source of Ca2+ influx in cardiomyocytes (15, 24). Thus, we first examined the effect of 20-HETE on ICa,L of cardiac myocytes, and the results from this study show that 20-HETE reversibly increases ICa,L (Fig. 1). This observation is consistent with a report (11) in cerebral arterial smooth muscle cells showing that 20-HETE induces vasoconstriction via the stimulation of ICa,L. In cardiac myocytes, ICa,L is the main route for Ca2+ influx, which shapes the long plateau phase of the ventricular action potential and the upstroke of the atrial pacemaker action potential. In pathological ischemic conditions, L-type Ca2+ channel activity and sensitivity are enhanced, which results in the prolongation of phase 2 or phase 3 of the cardiac action potential, causing early afterdepolarizations and arrhythmias (15). In addition, intracellular Ca2+ also plays a central role in cellular excitability, contraction, and gene regulation, leading to heart failure and cardiac remodeling. Thus, an unanswered question is whether 20-HETE is involved in the cardiac damage caused by ischemia. This notion is supported by a previous study (13) demonstrating that 20-HETE production was significantly enhanced in ischemia-reperfusion injury in canine hearts. CYP ω-hydroxylase inhibitors (including 17-ODYA and DDMS) markedly inhibit 20-HETE production and produce a profound reduction in myocardial infarct size in hearts subjected to ischemia-reperfusion (13, 26). Conversely, exogenously administered 20-HETE significantly increases infarct size, indicating that 20-HETE is involved in ischemia-reperfusion injury in the heart. The involvement of 20-HETE in the cardiac response to ischemia is also supported by the present study, showing that incubation of cardiomyocytes with 20-HETE results in increases in both ROS production and NADPH oxidase activity. This result is also confirmed by a study (5) in pulmonary artery endothelial cells, which shows that 20-HETE increases superoxide production, an effect that is blocked by an NADPH oxidase inhibitor and by PEG-SOD. In the heart, NADPH oxidase-derived ROS have been shown to play an important role in the pathophysiological response to ischemia, including cardiac hypertrophy, apoptosis, contractile dysfunction, and interstitial fibrosis (1). However, the role of 20-HETE-induced NADPH oxidase-derived ROS production and increases in L-type Ca2+ channel activity in the pathogenesis of cardiac ischemic diseases still remain to be further investigated in vivo.
The present study demonstrates that the 20-HETE-induced increase in ICa,L in cardiomyocytes is attenuated by a ROS scavenger (PEG-SOS) or an NADPH oxidase inhibitor (gp91ds-tat), indicating that NADPH oxidase-dependent ROS production is involved in the stimulatory action of 20-HETE on L-type Ca2+ channel activity in cardiac myocytes. The ROS-mediated acute Ca2+ channel regulation may be responsible for the rapid electrophysiological changes, prolongation of the action potential, and induction of arrhythmias. Chronic alteration in the redox state may cause persistent activation of L-type Ca2+ channels, and the altered Ca2+ homeostasis may be responsible for the induction of hypertrophic growth (3). However, the nature of the intracellular signaling mechanism by which ROS increases ICa,L in cardiac myocytes remains to be clarified. One possibility may be a direct redox-dependent mechanism, since the pore-forming α1C-subunit of the cardiac L-type Ca2+ channel contains >10 cysteine residues (20) that can potentially undergo redox modification. Indeed, oxidization of Src homology groups causes the activation of ICaL, whereas GSH and DTT, which reduce disulfide bonds, inhibit this current in ferret ventricular myocytes (5).
20-HETE is produced in several tissues, but the biological functions of 20-HETE are tissue specific. For example, in pulmonary artery endothelial cells, 20-HETE has been reported to prevent apoptosis induced by serum deprivation, LPS, and hypoxia-reperfusion (7). In contrast to the salutary effects in pulmonary artery endothelial cells, in cerebral vascular smooth muscle cells of spontaneously hypertensive rats, 20-HETE contributes to the severity of oxidative stress and stroke. Inhibition of 20-HETE production greatly reduces infarct size after ischemia-reperfusion injury in the brain and heart (8, 13). Two possible mechanisms could contribute to 20-HETE-induced reperfusion injury. One possibility is that 20-HETE may induce arterial vasoconstriction (29), decreasing blood flow to the affected area. Another possibility is that 20-HETE may directly damage ischemic tissue by increasing the formation of ROS. The present study demonstrated that 20-HETE increases Ca2+ channel activity and intracellular ROS levels, important factors in causing apoptosis and other detrimental effects in ischemic-reperfusion injury. However, the effect of 20-HETE on apoptosis of cardiomyocytes remains to be investigated in future studies.
In this investigation, we demonstrated that PKC activity appears to be essential for the 20-HETE-induced increase in NADPH oxidase activity. This is supported by the following lines of evidence obtained in the present study: 1) incubation of cardiomyocytes with a PKC inhibitor, GF-109203, significantly attenuated the stimulatory action of 20-HETE on NADPH oxidase activity; 2) treatment of cardiomyocytes with 20-HETE resulted in a significant increase in PKC activity; 3) blockade of PKC also abolished 20-HETE-induced increases in intracellular ROS levels and ICa,L activation in cardiomyocytes; and 4) 20-HETE increased the phosphorylation of p47phox, the NADPH oxidase regulatory subunit. Several studies from other research groups have demonstrated that PKC is involved in the regulation of NADPH oxidase activity via phosphorylation of the p47phox subunit. For example, Bey et al. (4) reported that PKC plays a pivotal role in stimulating NADPH oxidase activity via the phosphorylation of p47phox. Moreover, Fontayne et al. (10) identified several serine residues in p47phox as targets for PKC. The phosphorylation of p47phox subunits induces their translocation from the cytosolic compartment to the cellular membrane, where they combine with other NADPH subunits to assemble the fully activated NADPH oxidase complexes that generate superoxide. Moreover, PKC-dependent activation of NADPH oxidase is reportedly involved in early ischemic preconditioning in the heart (2) and also in the action of ANG II in the regulation of neuronal activity in the brain (30). Several isoforms of PKC have been identified that phosphorylate the regulatory p47phox subunits of NADPH oxidase to augment the production of superoxide radicals. These include PKC-α, PKC-βII, PKC-δ, and PKC-ζ isoforms, as idenfied in neutrophils (10). Although it is clear that the p47phox subunit is regulated by PKC in the action of 20-HETE, the specific PKC isoform(s) involved in the 20-HETE-induced activation of NADPH oxidase is still not clear. A recent study (31) demonstrated that NADPH oxidase-derived pathways are also involved in ANG II-induced inhibition of the Na+-K+ pump in cardiac myocytes and that ANG II-induced NADPH oxidase activation is completely abolished by a myristolated peptide inhibitor of PKC-ε, suggesting that PKC-ε is involved in the stimulatory action of ANG II on NADPH oxidase activity in cardiac myocytes. However, whether PKC-ε or other PKC isoforms are involved in the stimulatory action of 20-HETE on NADPH oxidase and L-type Ca2+ channels requires further investigation.
Previous studies have demonstrated that inhibition of 20-HETE production induced a protective effect in the heart in response to ischemic injury. Here, we provide significant evidence that 20-HETE acts directly on cardiomyocytes and increases NADPH oxidase-derived ROS production via a PKC-dependent mechanism. This signaling pathway is involved in the 20-HETE-induced regulation of ICa,L in cardiac myocytes. Thus, 20-HETE may contribute to the cardiac pathological response to ischemic injury, such as apoptosis, interstitial fibrosis, hypertrophy, contractive dysfunction, and heart failure.
GRANTS
This work was supported by American Heart Association Grant 0635050N and by National Natural Science Foundation of China Grant 30870910. Funding for the North Dakota State University Core Biology Facility used in this study was made possible by National Center for Research Resources Grant 2P20-RR-015566.
DISCLAIMER
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol 47: 15–22, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Bell RM, Cave AC, Johar S, Hearse DJ, Shah AM, Shattock MJ. Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditioning. FASEB J 19: 2037–2039, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ. Remodelling Ca2+ signalling systems and cardiac hypertrophy. Biochem Soc Trans 34: 228–231, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase Cδ is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol 173: 5730–5738, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol 108: 277–293, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloutier M, Campbell S, Basora N, Proteau S, Payet MD, Rousseau E. 20-HETE inotropic effects involve the activation of a nonselective cationic current in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 285: L560–L568, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Dhanasekaran A, Bodiga S, Gruenloh S, Gao Y, Dunn L, Falck JR, Buonaccorsi JN, Medhora M, Jacobs ER. 20-HETE increases survival and decreases apoptosis in pulmonary arteries and pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol 296: H777–H786, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295: H2455–H2465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich JR, Cha TJ, Zhang L, Chartier D, Melnyk P, Hohnloser SH, Nattel S. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol 551: 801–813, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC α, βII, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41: 7743–7750, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol 507: 771–781, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granville DJ, Tashakkor B, Takeuchi C, Gustafsson AB, Huang C, Sayen MR, Wentworth P, Jr, Yeager M, Gottlieb RA. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci USA 101: 1321–1326, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross ER, Nithipatikom K, Hsu AK, Peart JN, Falck JR, Campbell WB, Gross GJ. Cytochrome P450 ω-hydroxylase inhibition reduces infarct size during reperfusion via the sarcolemmal KATP channel. J Mol Cell Cardiol 37: 1245–1249, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Gruh I, Beilner J, Blomer U, Schmiedl A, Schmidt-richter I, Kruse ML, Haverich A, Martin U. No evidence of transdifferentiation of human endothelial progenitor cells into cardiomyocytes after coculture with neonatal rat cardiomyocytes. Circulation 113: 1326–1334, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hool LC. The L-type Ca2+ channel as a potential mediator of pathology during alterations in cellular redox state. Heart Lung Circ 18: 3–10, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Hool LC, Di Maria CA, Viola HM, Arthur PG. Role of NAD(P)H oxidase in the regulation of cardiac L-type Ca2+ channel function during acute hypoxia. Cardiovasc Res 67: 624–635, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Ishihara Y, Sekine M, Nakazawa M, Shimamoto N. Suppression of myocardial ischemia-reperfusion injury by inhibitors of cytochrome P450 in rats. Eur J Pharmacol 611: 64–71, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Kroetz DL, Zeldin DC. Cytochrome P450 pathways of arachidonic acid metabolism. Curr Opin Lipidol 13: 273–283, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem 272: 27345–27352, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Maier KG, Roman RJ. Cytochrome P450 metabolites of arachidonic acid in the control of renal function. Curr Opin Nephrol Hypertens 10: 81–87, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Medhora M, Chen Y, Gruenloh S, Harland D, Bodiga S, Zielonka J, Gebremedhin D, Gao Y, Falck JR, Anjaiah S, Jacobs ER. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 294: L902–L911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature 340: 230–233, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Miura D, Kusano KF, Fujimoto Y, Sumita-Yoshikawa W, Fuke S, Nishii N, Nagase S, Hata Y, Morita H, Matsubara H, Ohe T, Ito H. 4-Hydroxy-2-nonenal induces calcium overload via the generation of reactive oxygen species in isolated rat cardiac myocytes. J Card Fail 15: 709–716, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 117: 2431–2444, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilakantan V, Maenpaa C, Jia G, Roman RJ, Park F. 20-HETE-mediated cytotoxicity and apoptosis in ischemic kidney epithelial cells. Am J Physiol Renal Physiol 294: F562–F570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nithipatikom K, Gross ER, Endsley MP, Moore JM, Isbell MA, Falck JR, Campbell WB, Gross GJ. Inhibition of cytochrome P450 ω-hydroxylase: a novel endogenous cardioprotective pathway. Circ Res 95: e65–e71, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Obara K, Koide M, Nakayama K. 20-Hydroxyeicosatetraenoic acid potentiates stretch-induced contraction of canine basilar artery via PKCα-mediated inhibition of KCa channel. Br J Pharmacol 137: 1362–1370, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Sun CW, Alonso-Galicia M, Taheri MR, Falck JR, Harder DR, Roman RJ. Nitric oxide-20-hydroxyeicosatetraenoic acid interaction in the regulation of K+ channel activity and vascular tone in renal arterioles. Circ Res 83: 1069–1079, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Sun C, Zubcevic J, Polson JW, Potts JT, Diez-Freire C, Zhang Q, Paton JF, Raizada MK. Shift to an involvement of phosphatidylinositol 3-kinase in angiotensin II actions on nucleus tractus solitarii neurons of the spontaneously hypertensive rat. Circ Res 105: 1248–1255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White CN, Figtree GA, Liu CC, Garcia A, Hamilton EJ, Chia KK, Rasmussen HH. Angiotensin II inhibits the Na+-K+ pump via PKC-dependent activation of NADPH oxidase. Circ Res 105: 185–193, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Yao F, Sumners C, O'Rourke ST, Sun C. Angiotensin II increases GABAB receptor expression in nucleus tractus solitarii of rats. Am J Physiol Heart Circ Physiol 294: H2712–H2720, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]