Abstract
Aberrant interactions between heat shock protein (Hsp)90 and its client proteins could contribute to pulmonary hypertension. We tested the hypotheses that 1) the interaction between Hsp90 and its known client protein, endothelial nitric oxide synthase (eNOS), is impaired in pulmonary resistance arteries (PRAs) from piglets with pulmonary hypertension caused by exposure to 3 or 10 days of hypoxia and 2) Hsp90 interacts with the prostanoid pathway proteins prostacyclin synthase (PGIS) and/or thromboxane synthase (TXAS). We also determined whether Hsp90 antagonism with geldanamycin alters the agonist-induced synthesis of prostacyclin and thromboxane or alters PRA responses to these prostaglandin metabolites. Compared with normoxic piglets, less eNOS coimmunoprecipitated with Hsp90 in PRAs from hypoxic piglets. Despite reduced Hsp90-eNOS interactions, dilation to ACh was enhanced in geldanamycin-treated PRAs from hypoxic, but not normoxic, piglets. In PRAs from all groups of piglets, PGIS and TXAS coimmunoprecipitated with Hsp90. Geldanamycin reduced the ACh-induced synthesis of prostacyclin and thromboxane and altered responses to the thromboxane mimetic U-46619 in PRAs from all groups. Although geldanamycin enhanced responses to prostacyclin in PRAs from both groups of hypoxic piglets, geldanamycin had no effect on prostacyclin responses in PRAs from either group of normoxic piglets. Our findings indicate that Hsp90 influences both prostanoid and eNOS signaling in the pulmonary circulation of newborn piglets and that the impact of pharmacological inhibition of Hsp90 on these signaling pathways is altered during exposure to chronic hypoxia.
Keywords: endothelial nitric oxide synthase, prostacyclin synthase, thromboxane synthase, prostanoids, arachidonic acid metabolites
heat shock protein (Hsp)90 is a molecular chaperone with essential roles in stress tolerance, protein folding, signal transduction, and cell cycle control (19, 28, 38). In some cases, the physical interaction of a client protein with Hsp90 has been shown to influence the activity of the binding partner (6, 18, 40, 41). Via interactions with its client proteins, Hsp90 is involved in the regulation of tone and reactivity in a number of vascular beds (4, 5, 17, 20). For example, we and others (4, 33) have shown that Hsp90 associates with endothelial nitric oxide (NO) synthase (eNOS) and that the interaction between these two proteins influences the production of NO in the pulmonary circulation (4, 33). There is an increasing appreciation that aberrant interactions between Hsp90 and its client proteins, including eNOS, play a role in abnormal cell signaling and thereby contribute to a number of vascular diseases, including pulmonary hypertension (23, 26, 31, 33).
We (5) have previously shown that interactions between Hsp90 and eNOS mature over the first weeks of life in pulmonary resistance arteries (PRAs) of newborn piglets, likely contributing to the postnatal fall in pulmonary vascular resistance and changes in agonist-induced pulmonary vascular responses characteristic of the early neonatal period. We (9, 11) have also previously reported that piglets develop pulmonary hypertension and exhibit aberrant responses to the agonist ACh when exposed to chronic hypoxia for either 3 or 10 days. We designed the present study to test the hypotheses that 1) the interaction between Hsp90 and eNOS is disrupted in PRAs of piglets exposed to chronic hypoxia and 2) the impaired interaction between Hsp90 and eNOS has a functional impact on ACh-induced responses in PRAs from chronically hypoxic piglets. Our findings led us to test the additional hypotheses that Hsp90 interacts with client proteins other than eNOS and influences the production of vasoactive mediators, other than NO, that regulate vascular tone and reactivity in the neonatal pulmonary circulation, particularly under conditions of chronic hypoxia and diminished NOS signaling.
METHODS
Animals and tissue preparation.
For hypoxic piglets, newborn pigs (2 days old) were placed in a hypoxic normobaric chamber for 3 or 10 days. Normobaric hypoxia was produced by delivering compressed air and N2 to a Plexiglas chamber. O2 content was regulated at 8–10% O2 (Po2: 60–72 Torr), and CO2 was maintained at 3–6 Torr by absorption with soda lime. The chamber was opened twice daily for cleaning and to weigh the piglets. Animals were fed ad libitum with an artificial sow milk replacer from a feeding device attached to the chamber. We (10) have previously found no differences in vascular responses between piglets raised in a room air chamber environment for 3 or 10 days and piglets raised on a farm. Therefore, for this study, the age-matched normoxic control animals were piglets studied on the day of arrival from the farm at 5–7 or 10–12 days of age, i.e., the same postnatal ages as the hypoxic piglets on the day of study. All piglets were preanesthetized with ketamine (30 mg/kg im) and acepromazine (2 mg/kg im) and then anesthetized with pentobarbital (10 mg/kg iv). All animals were given heparin (1,000 IU/kg iv) and then exsanguinated. The thorax was opened, and the lungs were removed and placed in cold (4°C) physiological saline solution (PSS). All experimental protocols were performed in adherence with National Institutes of Health guidelines for the use of experimental animals and were approved by the Animal Care and Use Committee of Vanderbilt University Medical Center. The animal resource facility is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Coimmunoprecipitation assays.
One series of experiments was performed to determine whether the interaction between Hsp90 and eNOS is disrupted in piglets exposed to chronic hypoxia. Additional experiments were performed to assess whether Hsp90 coimmunoprecipitates with prostacyclin synthase (PGIS) or thromboxane synthase (TXAS), proteins of the prostanoid pathway of arachidonic acid metabolism. For these experiments, pulmonary arteries (PRAs; ≤300-μm diameter) were dissected from the lungs of both groups of control piglets and both groups of hypoxic piglets and stored at −80°C until use in coimmunoprecipitation assays. Another series of experiments was performed to assess the effect of ACh stimulation and Hsp90 antagonism on the interaction between Hsp90 and either eNOS, PGIS, or TXAS. For these experiments, dissected PRAs were incubated in HEPES at 37°C for 20 min in the presence of vehicle (DMSO) or geldanamycin (GA; 1 μM) before the addition of ACh (10 μM) or HEPES buffer for an additional 1 min. Supernatants were discarded, and the vessels were homogenized in lysis buffer [20 mM Tris·HCl (pH 7.4), 2.5 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 100 mM NaCl, 1.0 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml pepstatin]. Vessel homogenates were stored at −80°C until used in coimmunoprecipitation assays. Vessel homogenates were thawed, and protein concentrations of the cell lysates were determined by protein assay (Bradford). To reduce nonspecific binding of proteins, 500 μg of detergent soluble protein were precleared with pansorbin and then incubated for 6 h at 4°C with an antibody either against eNOS (1 μg/mg total cell protein, BD Transduction Laboratories), Hsp90 (1 μg/mg total cell protein, BD Transduction Laboratories), PGIS (1 μg/mg total cell protein, Cayman Chemical), TXAS (1 μg/mg total cell protein, provided by S. L. Pfister's laboratory), or an isotype-specific IgG (to serve as a control for each antibody). Immune complexes were precipitated by an overnight incubation at 4°C with protein G-Sepharose. The next morning, beads were washed in lysis buffer and pelleted in a microcentrifuge to remove all unbound protein. Immunoprecipitated samples were heated at 80°C for 15 min in Laemmli loading buffer, and proteins were resolved by SDS-PAGE on a 4–20% Tris-glycine gel. Proteins were electroblotted onto nitrocellulose membranes. Membranes were blocked in 0.1% Tween-PBS with 7.5% nonfat milk and then incubated with primary antibodies against eNOS (1:1,000), Hsp90 (1:000), PGIS (1:500), or TXAS (1:2,000). Western blots were probed with a horseradish peroxidise-conjugated secondary antibody diluted in carrier buffer (1:2,500 goat anti-mouse for eNOS, 1:2,500 goat anti-mouse for Hsp90, 1:2,500 goat anti-rabbit for PGIS, and 1:2,500 goat anti-rabbit for TXAS). To visualize the antibodies, membranes were developed using enhanced chemiluminescence reagents (Amersham), and the chemiluminescent signal was captured on X-ray film (ECL Hyperfilm, Kodak). Bands for each protein were quantified using densitometry.
Cannulated artery protocols.
The system used to study cannulated arteries has been previously described in detail (5, 9). Briefly, it consists of a water-jacketed plastic chamber in which proximal (inflow) and distal (outflow) cannulas are mounted. PRAs (80–300 μm diameter) were threaded onto the cannulas and adjusted so that the slack was taken out of the artery. The exterior of the artery was suffused with PSS from a reservoir at 37°C and aerated with a gas mixture containing 21% O2-5% CO2-balance N2 to maintain a pH of 7.37–7.40. The arterial lumen was filled from a syringe containing PSS, aerated with the same gas mixture as the reservoir, and connected to the cannula with polyethylene tubing. The inflow pressure of the artery was adjusted by changing the height of the infusion syringe. The diameter of the artery was observed continuously with a video system containing a color camera (model VCC-151, Hitachi) and a television monitor. Vessel diameters were measured with a video scaler (model IV 550 FOR-A, Gainesville, FL). The video scaler was calibrated with a micrometer scale.
Each artery was allowed to equilibrate for 30 min to establish basal tone. Control arteries were equilibrated at a transmural pressure of 15 cmH2O, and hypoxic arteries were equilibrated at a transmural pressure of 25 cmH2O. These pressures were used as they represent in vivo pressure (11). We (14) have previously shown no effect from these transmural pressures on pulmonary arterial responses to ACh. After the establishment of basal tone, all arteries were tested for viability by contraction to the thromboxane mimetic U-46619 (10−8 M). To check for a functional endothelium in control arteries, responses to ACh (10−6 M) were evaluated. We (14) have previously found that hypoxic arteries constrict to ACh but dilate to another endothelium-dependent agent, the Ca2+ ionophore A-23187. Therefore, responses to A-23187 were used to check for a functional endothelium in hypoxic arteries.
One series of experiments was performed to determine whether the interaction between Hsp90 and eNOS has a functional impact on ACh-induced responses in PRAs. To do this, we evaluated the effect of the Hsp90 antagonist GA (1 μM) on ACh responses (10−9–10−5 M) in PRAs from both groups of control and hypoxic piglets. The potential contribution of both O2·− and H2O2 in GA-treated PRAs was also assessed. For these latter experiments, ACh responses (10−8–10−5 M) were measured in some PRAs after the addition of GA (1 μM) and both a cell-permeable SOD mimetic, M-40403 (3 μg/ml), which dismutates O2·− to H2O2, and a H2O2-decomposing enzyme, polyethylene glycol (PEG)-catalase (CAT) (250 U/ml), which converts H2O2 to H2O to the reservoir. We (8, 15) have previously shown the abolition of PRA O2·− production using this concentration of M-40403 and abolition of PRA H2O2 production using this concentration of PEG-CAT. Another series of experiments was performed to assess the potential contribution from prostanoids in GA-treated vessels. For these latter experiments, ACh responses were measured in PRAs after the addition of GA (1 μM) plus the prostanoid inhibitor indomethacin (10−5 M) to the reservoir. For all these experiments, responses to ACh were measured in PRAs with tone elevated by the addition of endothelin (10−10–10−9 M) or U-46619 (10−9–10−8 M) to the reservoir in increasing doses until the arterial diameter had decreased by 40–50%. In the case of GA-treated vessels, GA was added and the vessels were allowed to achieve a new baseline diameter before the addition of endothelin or U-46619 to constrict the vessels. This methodology ensured that the degree of preconstriction was similar in all vessels.
In another series of experiments, we evaluated the effect of the Hsp90 antagonist GA on PRA responses to either the thromboxane mimetic U-46619 (10−9–5 × 10−7 M) or exogenous prostacyclin (PGI2; 10−9–10−6 M). PGI2 experiments were performed in vessels with tone elevated as described above. U-46619 experiments were performed with vessels at basal tone.
In an additional series of experiments, we evaluated the effect of the Hsp90 antagonist GA on PRA responses to arachidonic acid. For these experiments, responses to arachidonic acid (10−8–10−5 M) were measured in both GA-treated and untreated PRAs from both groups of normoxic and hypoxic piglets. An additional series of experiments was performed to assess the potential contribution from both O2·− and H2O2 in GA-treated PRAs. For these latter experiments, arachidonic acid responses (10−8–10−5 M) were measured in PRAs after the addition of GA (1 μM) and both M-40403 (3 μg/ml) and PEG-CAT (250 U/ml) to the reservoir. These experiments were performed in vessels with elevated tone as described for the ACh experiments.
For all of the above experiments, vessel viability was tested at the completion of the study by the addition of U-46619. In addition, vessel responses to the vehicles used for solubilization of each agent were evaluated.
Radioimmunoassay of the stable metabolite of thromboxane, thromboxane B2, and enzyme immunoassay of the stable metabolite of prostacyclin, 6-keto-PGF1α.
These experiments were performed to assess whether the Hsp90 antagonist GA inhibits either ACh-induced or arachidonic acid-stimulated production of PGI2 or thromboxane A2 (TXA2). To do this, dissected PRAs (≤300-μm diameter) from all groups of piglets were first incubated for 15 min at 37°C in HEPES buffer containing vehicle (DMSO) or GA (1 μM). The supernatant was discarded and replaced with new HEPES buffer containing vehicle or GA. Either ACh (10 μM), arachidonic acid (10 μM), or additional vehicle was then added, and vessels were incubated for a second time period (1 min for ACh experiments and 5 min for arachidonic acid experiments). After the second incubation period, the supernatant was collected and stored at −20°C until the time of assay for specific metabolites. Vessels were dried for at least 72 h. The synthesis of thromboxane B2 (TXB2) was measured using the methods of Campbell and Ojeda (6a). The antibody for TXB2 was from S. L. Pfister's laboratory. The sensitivity of the assay was 1 pg/0.3 ml for TXB2. The synthesis of 6-keto-PGF1α was measured by enzyme immunoassay following standard methods and using kits from Cayman Chemicals. The sensitivity of the assay was 11 pg/ml. Radioimmunoassay determinations of TXB2 and enzyme immunoassay determinations of 6-keto-PGF1α were normalized to vessel dry weight.
Drugs.
ACh, PEG-CAT, indomethacin, and arachidonic acid were from Sigma Chemical and were solubilized respectively in saline, distilled H2O, or ethanol previously sparged with nitrogen. PGI2 was from Cayman Chemical and was solubilized in saline. U-46619 and GA were from BioMol and were solubilized in ethanol or DMSO, respectively. M-40403 was a generous gift from Activbiotics (Lexington, MA) and was solubilized in 26 mM NaHCO3.
Statistics.
Data are presented as means ± SE. Coimmunoprecipitation experiments comparing hypoxic versus comparable-age control groups were analyzed by unpaired t-tests (Figs. 1, A–D, 6, A and B, and 9, A and B). Coimmunoprecipitation experiments comparing ACh-induced changes in vehicle or GA-treated PRAs were analyzed by paired t-tests (Figs. 2, A–D, 7, A–D, and 10, A–D). For the cannulated artery experiments, we used a linear mixed effects model to examine the percent dilation or constriction to all doses of ACh, PGI2, U-46619, and arachidonic acid in untreated and inhibitor-treated vessels from each group of control and hypoxic piglets (24). Findings in arteries studied at elevated tone were similar regardless of the agent, endothelin or U-46619, used for preconstriction; results with both agents were combined for statistical analysis. Each artery was exposed to multiple concentrations of ACh, PGI2, U-46619, or arachidonic acid, so we included a random intercept or random intercept and slope to account for the correlation arising from taking repeated observations on the same vessel. Model fit was evaluated using the Akaike information criterion (1). Results from the final models with the lowest Akaike information criterion are presented. A paired t-test was used to compare TXB2 or 6-keto-PGF1α production under basal versus stimulated (either ACh or arachidonic) conditions for vehicle- and GA-treated PRAs from each group of piglets. P < 0.05 was considered significant.
Fig. 1.
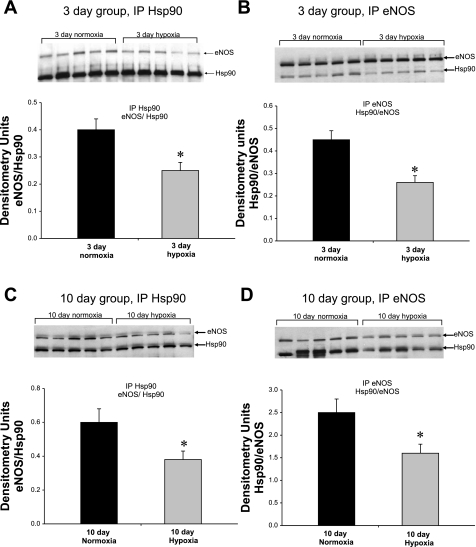
Coimmunoprecipitation of endothelial nitric oxide synthase (eNOS) with heat shock protein (Hsp)90 for pulmonary resistance arteries (PRAs) from piglets raised in normoxia or hypoxia for 3 days (A and B) or 10 days (C and D). PRA protein homogenates (n = 5 different piglets in each group) were immunoprecipitated (IP) with either anti-Hsp90 (A and C) or anti-eNOS (B and D). The resultant immunoprecipitates were subjected to immunoblot (IB) analysis using antibodies against eNOS and Hsp90. *Significantly different from the comparable-age normoxic group (P < 0.05 by unpaired t-test).
Fig. 6.
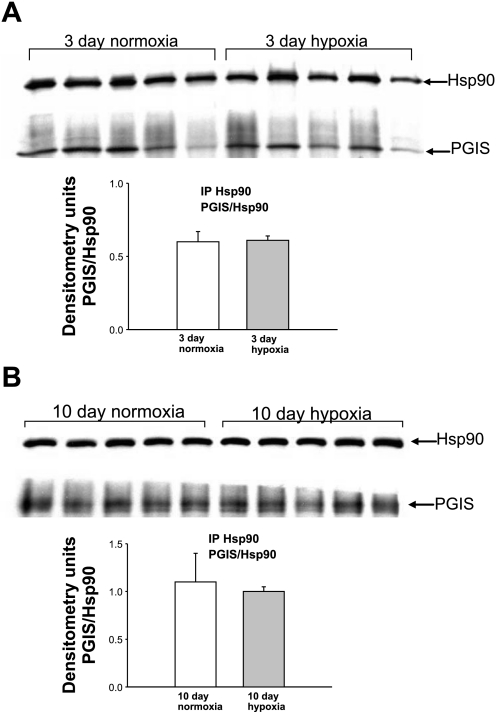
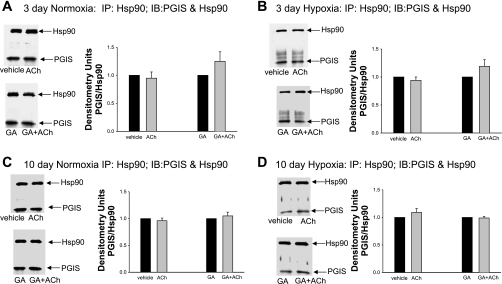
Coimmunoprecipitation of Hsp90 with PGIS for PRAs from piglets raised in normoxia or hypoxia for 3 days (A) or 10 days (B). PRA protein homogenates (n = 5 different piglets in each group) were immunoprecipitated with anti-Hsp90, and the resultant immunoprecipitates were subjected to immunoblot analysis using antibodies against PGIS and Hsp90. Summary densitometric data show no differences in PGIS/Hsp90 coimmunoprecipitation for PRAs from hypoxic versus comparable-age control groups.
Fig. 9.
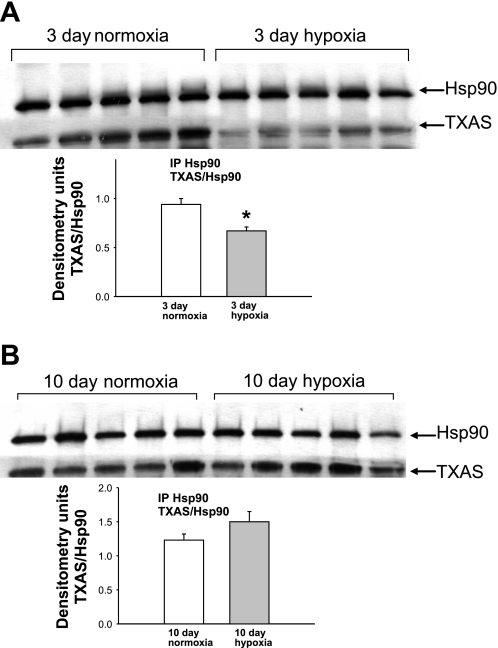
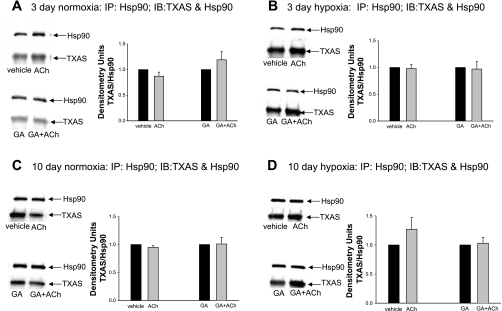
Coimmunoprecipitation of Hsp90 with TXAS for PRAs from piglets raised in normoxia or hypoxia for 3 days (A) or 10 days (B). PRA protein homogenates (n = 5 different piglets in each group) were immunoprecipitated with anti-Hsp90, and the resultant immunoprecipitates were subjected to immunoblot analysis using antibodies against TXAS and Hsp90. Summary densitometric data show less TXAS/Hsp90 coimmunoprecipitation for PRAs from piglets raised in hypoxia versus normoxia for 3 days (A; *P < 0.05 by unpaired t-test) but no differences in TXAS/Hsp90 coimmunoprecipitation for PRAs from piglets raised in hypoxia versus normoxia for 10 days (B).
Fig. 2.
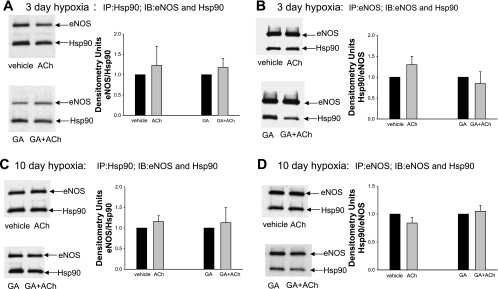
Effect of ACh stimulation on coimmunoprecipitation of eNOS with Hsp90 under untreated (vehicle) or geldanamycin (GA)-treated conditions for PRAs from piglets raised in hypoxia for either 3 days (A and B) or 10 days (C and D). PRA protein homogenates were immunoprecipitated with either anti-Hsp90 (A, 3-day hypoxic piglets, n = 7 vehicle-treated or untreated piglets and 7 GA-treated piglets; and C, 10-day hypoxic piglets, n = 7 vehicle-treated or untreated piglets and 7 GA-treated piglets) or anti-eNOS (B, 3-day hypoxic piglets, n = 5 vehicle-treated or untreated piglets and 5 GA-treated piglets; and D, 10-day hypoxic piglets, n = 7 vehicle-treated or untreated piglets and 7 GA-treated piglets). The resultant immunoprecipitates were subjected to immunoblot analysis using antibodies against eNOS and Hsp90. The summary densitometric data shows that ACh stimulation had no effect on the coimmunoprecipitation of eNOS with Hsp90 for untreated and GA-treated PRAs from either group of hypoxic piglets.
Fig. 7.
Effect of ACh stimulation on coimmunoprecipitation of PGIS with Hsp90 under untreated (vehicle) or GA-treated conditions for PRAs from piglets raised in normoxia (A and C) or hypoxia (B and D) for either 3 days (A and B) or 10 days (C and D). PRA protein homogenates were immunoprecipitated with anti-Hsp90 (A, 3-day normoxic piglets, n = 8 vehicle-treated piglets and 8 GA-treated piglets; B, 3-day hypoxic piglets, n = 4 vehicle-treated piglets and 4 GA-treated piglets; C, 10-day normoxic piglets, n = 4 vehicle-treated piglets and 4 GA-treated piglets; and D, 10-day hypoxic piglets, n = 4 vehicle-treated piglets and 4 GA-treated piglets). The resultant immunoprecipitates were subjected to immunoblot analysis using antibodies against PGIS and Hsp90. The summary densitometric data show that ACh stimulation had no effect on the coimmunoprecipitation of PGIS with Hsp90 for untreated and GA-treated PRAs from any group of piglets.
Fig. 10.
Effect of ACh stimulation on the coimmunoprecipitation of TXAS with Hsp90 under untreated (vehicle) or GA-treated conditions for PRAs from piglets raised in normoxia (A and C) or hypoxia (B and D) for either 3 days (A and B) or 10 days (C and D). PRA protein homogenates were immunoprecipitated with anti-Hsp90 (A, 3-day normoxic piglets, n = 7 vehicle-treated piglets and 7 GA-treated piglets; B, 3-day hypoxic piglets, n = 6 vehicle-treated piglets and 6 GA-treated piglets; C, 10-day normoxic piglets, n = 7 vehicle-treated piglets and 7 GA-treated piglets; and D, 10-day hypoxic piglets, n = 8 vehicle-treated piglets and 8 GA-treated piglets). The resultant immunoprecipitates were subjected to immunoblot analysis using antibodies against TXAS and Hsp90. The summary densitometric data show that ACh stimulation had no effect on the coimmunoprecipitation of TXAS with Hsp90 for untreated and GA-treated PRAs from any group of piglets.
RESULTS
As shown in Fig. 1, A–D, we found markedly less coimmunoprecipitation between Hsp90 and eNOS in PRAs from piglets raised in hypoxia for either 3 or 10 days compared with PRAs from comparable-age (normoxic) control piglets. Reduced Hsp90/eNOS coimmunoprecipitation in hypoxic piglets was observed regardless of whether the vessels were immunoprecipitated with antibody against Hsp90 (Fig. 1, A and C) or eNOS (Fig. 1, B and D). Moreover, ACh treatment failed to enhance the coimmunoprecipitation of eNOS and Hsp90 in PRAs from either group of chronically hypoxic piglets (Fig. 2, A–D). These findings contrast with our previously reported findings in comparable-age control piglets of enhanced Hsp90/eNOS coimmunoprecipitation in ACh-stimulated PRAs (4, 5). In ACh-stimulated PRAs from control piglets, we (4, 5) have previously shown that the enhanced Hsp90/eNOS coimmunoprecipitation was abolished by the Hsp90 antagonist GA. In contrast, in this study with PRAs from chronically hypoxic piglets, GA had no effect on Hsp90/eNOS coimmunoprecipitation in the presence or absence of ACh. As shown in Fig. 2, A–D, similar results were obtained when vessels from hypoxic piglets were immunoprecipitated with anti-Hsp90 or with anti-eNOS.
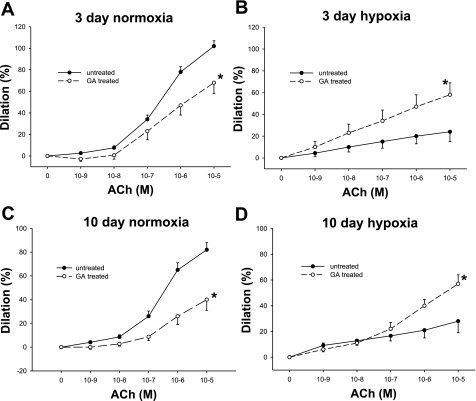
To evaluate the functional importance of Hsp90 chaperone function, we assessed the effect of Hsp90 antagonism on vascular responses to ACh. As we (4, 5, 9) have previously shown, Hsp90 antagonism with GA diminished dilation responses to ACh in PRAs from both age groups of (normoxic) control piglets (Fig. 3, A and C). There was minimal dilation in response to ACh in PRAs from both groups of chronically hypoxic piglets (Fig. 3, B and D). Interestingly, in PRAs from both groups of chronically hypoxic piglets, Hsp90 antagonism with GA enhanced dilation to ACh (Fig. 3, B and D).
Fig. 3.
ACh-induced dilation in untreated arteries and arteries treated with the Hsp90 antagonist GA from piglets raised in normoxia for 3 days (A; n = 13 untreated arteries and 7 treated arteries), hypoxia for 3 days (B; n = 9 untreated arteries and 9 treated arteries), normoxia for 10 days (C; n = 19 untreated arteries and 12 treated arteries), and hypoxia for 10 days (D; n = 14 untreated arteries and 14 treated arteries). Data are expressed as percent dilation of contraction elicited by either U-46619 or endothelin. All values are means ± SE. *Significantly different from the concentration-response curve in untreated arteries (P < 0.05 by the linear mixed effects model).
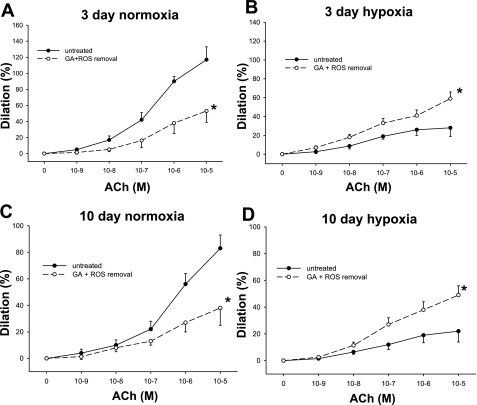
Given the reported redox-cycling properties of GA, we considered the possibility that the effect of Hsp90 antagonism on ACh responses could be due, at least in part, to influences from ROS. To evaluate this possibility, vessels were treated with a combination of agents to remove ROS, including the cell-permeable SOD mimetic M-40403, which dismutates O2·− to H2O2, and the H2O2-decomposing enzyme PEG-CAT, which converts H2O2 to H2O (Fig. 4, A–D). In vessels from control piglets of both age groups, GA inhibited ACh-stimulated dilation to a similar extent in the presence (Fig. 4, A and C) and absence (Fig. 3, A and C) of agents to remove ROS. Likewise, the GA-mediated enhancement of ACh-induced dilation in PRAs from both groups of chronically hypoxic piglets persisted in the presence of M-40403 and PEG-CAT (Fig. 4, B and D) and was similar to the results in the presence of GA alone (Fig. 3, B and D).
Fig. 4.
ACh-induced dilation in untreated arteries and arteries treated with a combination of the Hsp90 antagonist GA, the SOD mimetic M-40403, and the H2O2-decomposing agent polyethylene glycol-catalase (PEG-CAT) from piglets raised in normoxia for 3 days (A; n = 6 untreated arteries and 6 treated arteries), hypoxia for 3 days (B; n = 8 untreated arteries and 8 treated arteries), normoxia for 10 days (C; n = 6 untreated arteries and 6 treated arteries), and hypoxia for 10 days (D; n = 17 untreated arteries and 16 treated arteries). Data are expressed as percent dilation of contraction elicited by either U-46619 or endothelin. All values are means ± SE. *Significantly different from the concentration-response curve in untreated arteries (P < 0.05 by the linear mixed effects model).
We reasoned that the ability of the Hsp90 inhibitor GA to enhance ACh-induced dilation in PRAs of chronically hypoxic piglets could be due to an augmented production of dilators or impaired production of constrictors. We (9, 12, 14) have previously shown that prostanoids are involved in aberrant ACh-mediated responses in PRAs from hypoxic piglets. We therefore investigated whether Hsp90 forms a complex with the prostanoid synthases PGIS and/or TXAS and evaluated whether these complexes are regulated in an agonist-dependent manner. As shown in Fig. 5, A and B, we found that Hsp90 and PGIS coimmunoprecipitated in PRAs. The amount of PGIS bound to Hsp90 was unchanged with exposure to either 3 or 10 days of hypoxia compared with age-matched controls (Fig. 6, A and B). The presence or absence of the Hsp90 antagonist GA did not influence the amount of PGIS that coimmunoprecipitated with Hsp90 in reponse to ACh stimulation in PRAs from any of the four groups of piglets (Fig. 7, A–D).
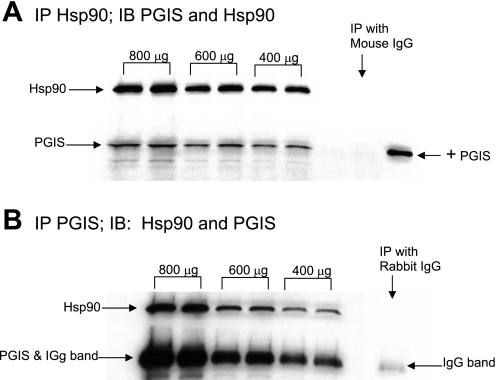
Fig. 5.
Coimmunoprecipitation of Hsp90 with prostacyclin synthase (PGIS) for PRA homogenates from a piglet raised in normoxia for 3 days. Different amounts of total protein were immunoprecipitated with anti-Hsp90 or an isotype-specific IgG (A) or with anti-PGIS or an isotype-specific IgG (B). The resultant immunoprecipitates were subjected to immunoblot analysis using antibodies against PGIS and Hsp90. +PGIS obtained from Cayman Chemicals, which was used as a positive control.
As shown in Fig. 8, A and B, we also found that Hsp90 and TXAS coimmunoprecipitated in piglet PRAs. In PRAs from piglets exposed to 3 days of hypoxia, there was significantly less coimmunoprecipitation between Hsp90 and TXAS than was observed in comparable-age control piglets (Fig. 9A); this effect of hypoxia on Hsp90 binding to TXAS was not seen when hypoxia was extended to 10 days (Fig. 9B). Similar to our findings with PGIS, ACh had no effect on Hsp90-TXAS coimmunoprecipitation in the presence or absence of GA in any of the four groups of piglets (Fig. 10).
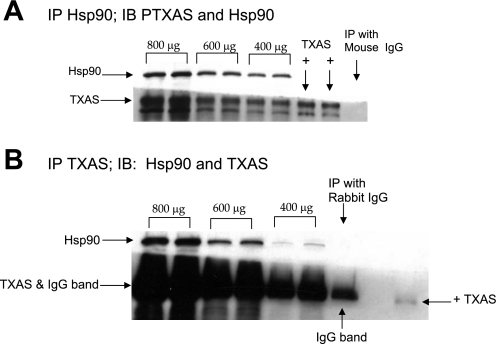
Fig. 8.
Coimmunoprecipitation of Hsp90 with thromboxane synthase (TXAS) for PRA homogenates from a piglet raised in normoxia for 3 days. Different amounts of total protein were immunoprecipitated with anti-Hsp90 or an isotype-specific IgG (A) or with anti-TXAS or an isotype-specific IgG (B). The resultant immunoprecipitates were subjected to immunoblot analysis using antibodies against TXAS and Hsp90. +Platelet lysate used as a positive control for TXAS.
Consistent with current understanding of Hsp90 function (19, 41), we reasoned the Hsp90 may modulate PGIS and TXAS function without altering the amount of enzyme bound to Hsp90. Therefore, we evaluated whether Hsp90 antagonism alters the ACh-induced production of PGI2 and/or TXA2. In all four groups of piglets, PGI2 production was greater in ACh-stimulated PRAs than in vehicle-treated PRAs (Table 1). The greatest increase in PGI2 was seen in ACh-stimulated PRAs from the older control group. Notably, treatment with GA inhibited the ACh-induced increase in PGI2 in PRAs from all four groups of piglets (Table 1). With the exception of the older group of control piglets, TXA2 production was also greater in ACh-stimulated PRAs than in vehicle-treated PRAs (Table 1). Likewise, the ACh-induced increase in TXA2 production was inhibited by treatment with GA (Table 1).
Table 1.
6-Keto-PGF1α and TXB2 production as well as the ratio of 6-keto-PGF1α to TXB2 production in vehicle- and GA-treated PRAs under basal and ACh-stimulated conditions for piglets exposed to control and hypoxic conditions for 3 and 10 days
| 3-Day Control | 3-Day Hypoxia | 10-Day Control | 10-Day Hypoxia | |
|---|---|---|---|---|
| 6-keto-PGF1α production, pg/ml | ||||
| Vehicle-treated PRAs | ||||
| Basal | 7,286 ± 771 | 2,918 ± 303 | 6,283 ± 1,177 | 2,946 ± 403 |
| ACh stimulation | 8,793 ± 947* | 3,394 ± 294* | 12,697 + 2,641 | 3,908 ± 556* |
| GA-treated PRAs | ||||
| Basal | 9,177 ± 1,315 | 3,381 ± 287 | 8,449 ± 1,369 | 4,404 ± 601 |
| ACh stimulation | 9,528 ± 1,815 | 3,246 ± 267 | 9,224 ± 1,685 | 3,908 ± 457 |
| TXB2 production, pg/mg | ||||
| Vehicle-treated PRAs | ||||
| Basal | 3.3 ± 0.9 | 5.0 ± 0.4 | 2.5 ± 0.7 | 1.9 ± 0.6 |
| ACh stimulation | 6.0 ± 1.9* | 6.2 ± 0.5 | 2.3 ± 0.6 | 2.9 ± 0.7* |
| GA-treated PRAs | ||||
| Basal | 5.0 ± 1.0 | 5.2 ± 0.5 | 2.0 ± 0.5 | 2.6 ± 0.8 |
| ACh stimulation | 4.0 ± 1.0 | 4.8 ± 1.4 | 2.0 ± 0.5 | 3.2 ± 0.7 |
| Ratio of 6-keto-PGFαto TXB2production | ||||
| Vehicle-treated PRAs | ||||
| Basal | 3,307:1 ± 841:1 | 625:1 ± 60:1 | 4,772:1 ± 1,634:1 | 2,596:1 ± 706:1 |
| ACh stimulation | 2,293:1 ± 422:1* | 547:1 ± 43:1 | 7,149:1 ± 1,269:1* | 2,442:1 ± 347:1 |
| GA-treated PRAs | ||||
| Basal | 3,318:1 ± 940:1 | 750:1 ± 107:1 | 5,502:1 ± 973:1 | 2,496:1 ± 583;1 |
| ACh stimulation | 5,127:1 ± 1,242:1 | 1,322:1 ± 352:1* | 6,488:1 ± 1,613:1 | 1,910:1 ± 535:1 |
Values are means ± SD; n = 8 pulmonary resistance arteries (PRAs)/group. TXB2, thromboxane B2; GA, geldanamycin.
Significantly different from vehicle-treated or GA-treated PRAs under basal conditions (P < 0.05 by paired t-test).
As the ratio of dilator to constrictor prostanoids can impact vascular responses, we compared the ratio of 6-keto-PGF1α to TXB2 in vehicle- and GA-treated PRAs (Table 1). In vehicle-treated PRAs from the 3-day control group, the 6-keto-PGF1α-to-TXB2 ratio decreased after ACh stimulation, i.e., there was an ACh-induced shift away from the dilator PGI2. In PRAs from the 10-day control group, the 6-keto-PGF1α-to-TXB2 ratio increased after ACh stimulation in vehicle-treated PRAs, i.e., there was an ACh-induced shift toward the dilator PGI2. This is due to the failure of ACh stimulation to increase TXB2 production combined with the dramatic increase in PGI2 production in PRAs from this group of piglets (Tables 1). Notably, in both groups of control piglets, the ratio of 6-keto-PGF1α to TXB2 did not change with ACh stimulation in GA-treated PRAs (Table 1). Thus, after GA treatment, the ACh-induced shift toward the dilator PGI2 in the 10-day normoxic group was inhibited. In vehicle-treated PRAs from hypoxic piglets, the ratio of 6-keto-PGF1α to TXB2 was similar before and after ACh stimulation. In GA-treated PRAs from the 3-day but not the 10-day hypoxic piglets, the ratio of 6-keto-PGF1α to TXB2 increased with ACh stimulation, favoring the dilator PGI2. Thus, the effect of GA on ACh-induced prostanoid production could contribute to the blunted dilator response to ACh found in the 10-day group of normoxic piglets (Fig. 3C) and to the enhanced dilator response to ACh found in the 3-day group of hypoxic piglets (Fig. 3B).
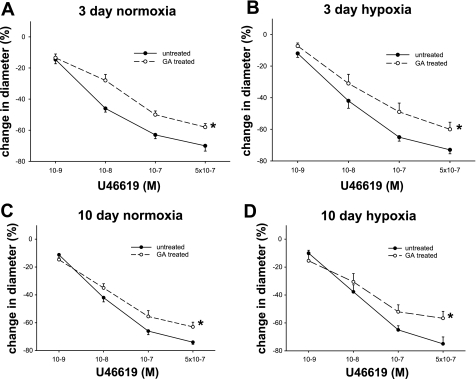
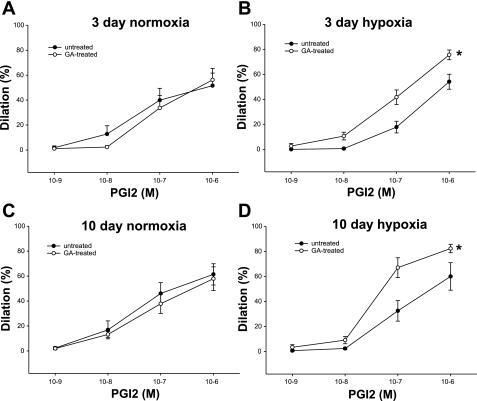
Because the impact on prostanoid production did not explain the effect of GA on ACh responses in all groups of piglets, we next explored that possibility that GA might alter PRA functional responses to PGI2 and/or TXA2. As shown in Fig. 11, A–D, constrictor responses to exogenous administration of the thromboxane mimetic U-46619 were diminished by treatment with GA in PRAs from all groups of piglets. Moreover, although dilation to exogenous PGI2 was not altered in PRAs from either group of control piglets (Fig. 12, A and C), dilation to PGI2 was augmented by treatment with GA in PRAs from both groups of hypoxic piglets (Fig. 12, B and D). Thus, altered responsiveness to endogenous PGI2 and TXA2 could contribute to the enhanced ACh-induced dilator responses found in GA-treated PRAs in both groups of chronically hypoxic piglets.
Fig. 11.
Change in diameter to cumulative concentrations of the thromboxane mimetic U-46619 for untreated and GA-treated arteries from piglets raised in normoxia for 3 days (A; n = 5 untreated arteries and 5 treated arteries), hypoxia for 3 days (B; n = 7 untreated arteries and 7 treated arteries), normoxia for 10 days (C; n = 6 untreated arteries and 6 treated arteries), and hypoxia for 10 days (D; n = 7 untreated arteries and 6 treated arteries). All values are means ± SE. *Significantly different from the concentration-response curve in untreated arteries (P < 0.05 by the linear mixed effects model).
Fig. 12.
Change in diameter to cumulative concentrations of prostacyclin (PGI2) for untreated and GA-treated arteries from piglets raised in normoxia for 3 days (A; n = 8 untreated arteries and 8 treated arteries), hypoxia for 3 days (B; n = 6 untreated arteries and 6 treated arteries), normoxia for 10 days (C; n = 6 untreated arteries and 6 treated arteries), and hypoxia for 10 days (D; n = 6 untreated arteries and 6 treated arteries). Data are expressed as percent dilation of contraction elicited by either U-46619 or endothelin. All values are means ± SE. *Significantly different from the concentration-response curve in untreated arteries (P < 0.05 by the linear mixed effects model).
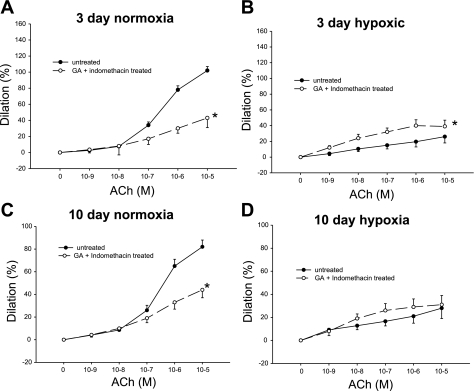
We performed an additional series of experiments to further evaluate whether prostanoids contribute to the effect of the Hsp90 inhibitor GA on ACh responses. We reasoned that the enhanced ACh-induced dilation in GA-treated PRAs from chronically hypoxic piglets and the reduced ACh-induced dilation in GA-treated PRAs from normoxic control piglets (Fig. 3) would be lost in the presence of indomethacin if prostanoids contributed significantly to these effects. As shown in Fig. 13, A and C, in both groups of normoxic piglets, diminished responses to ACh persisted in GA-treated vessels in the presence of indomethacin. In the 3-day group of hypoxic piglets, responses to ACh were only slightly augmented in GA-treated vessels in the presence of indomethacin (Fig. 13B); in the 10-day group of hypoxic piglets, indomethacin abolished the GA-mediated augmentation of ACh-induced dilation (Fig. 13D). These findings support a prostanoid contribution to the enhanced dilator response to ACh found in GA-treated PRAs of chronically hypoxic piglets.
Fig. 13.
ACh-induced dilation in untreated arteries and arteries treated with a combination of the Hsp90 antagonist GA and the prostanoid inhibitor indomethacin from piglets raised in normoxia for 3 days (A; n = 13 untreated arteries and 6 treated arteries), hypoxia for 3 days (B; n = 12 untreated arteries and 14 treated arteries), normoxia for 10 days (C; n = 19 untreated arteries and 20 treated arteries), and hypoxia for 10 days (D; n = 14 untreated arteries and 14 treated arteries). Data are expressed as percent dilation of contraction elicited by either U-46619 or endothelin. All values are means ± SE. *Significantly different from the concentration-response curve in untreated arteries (P < 0.05 by the linear mixed effects model).
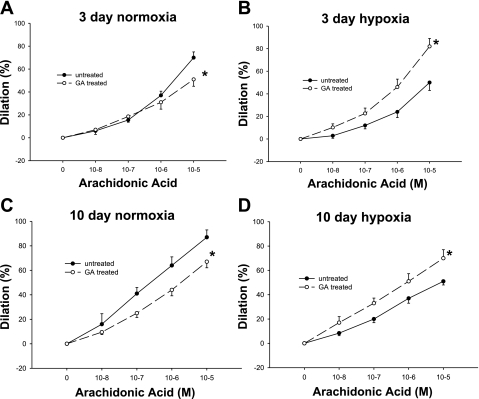
Since prostanoids are arachidonic acid metabolites, we also evaluated the possibility that Hsp90 inhibition modulates responses to arachidonic acid. We found that Hsp90 antagonism with GA altered responses to arachidonic acid in vessels from all groups of piglets (Fig. 14, A–D). In PRAs from both groups of control piglets (Fig. 14, A and C), treatment with the Hsp90 antagonist diminished dilation responses to arachidonic acid. In PRAs from both groups of chronically hypoxic piglets, Hsp90 antagonism with GA enhanced dilation to arachidonic acid (Fig. 14, B and D). Hence, similar to findings with ACh (Fig. 3, A–D), the influence of Hsp90 antagonism with GA on arachidonic acid responses differs between normotensive and hypertensive vessels.
Fig. 14.
Arachidonic acid-induced dilation in untreated arteries and arteries treated with the Hsp90 antagonist GA from piglets raised in normoxia for 3 days (A; n = 10 untreated arteries and 10 treated arteries), hypoxia for 3 days (B; n = 9 untreated arteries and 9 treated arteries), normoxia for 10 days (C; n = 13 untreated arteries and 6 treated arteries), and hypoxia for 10 days (D; n = 14 untreated arteries and 10 treated arteries). Data are expressed as percent dilation of contraction elicited by either U-46619 or endothelin. All values are means ± SE. *Significantly different from the concentration-response curve in untreated arteries (P < 0.05 by the linear mixed effects model).
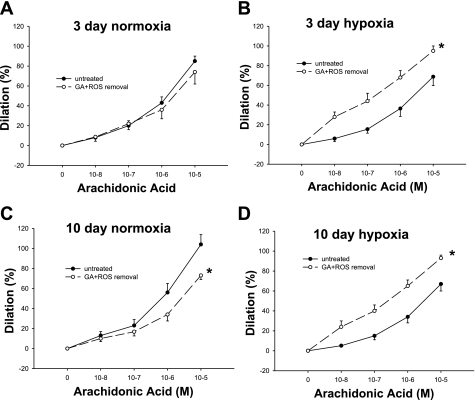
We also evaluated a potential contribution from ROS to the GA-mediated effects on arachidonic acid responses. For these experiments, vessels were treated with a combination of M-40403 and PEG-CAT (Fig. 15, A–D). Compared with untreated vessels, the response to arachidonic acid was diminished in the 10-day group of control arteries treated with a combination of GA, M-40403, and PEG-CAT, whereas the response to arachidonic acid was augmented in both groups of hypoxic arteries treated with GA, M-40403, and PEG-CAT.
Fig. 15.
Arachidonic acid-induced dilation in untreated arteries and arteries treated with a combination of the Hsp90 antagonist GA, the SOD mimetic M-40403, and the H2O2-decomposing agent PEG-CAT from piglets raised in normoxia for 3 days (A; n = 12 untreated arteries and 9 treated arteries), hypoxia for 3 days (B; n = 10 untreated arteries and 10 treated arteries), normoxia for 10 days (C; n = 10 untreated arteries and 10 treated arteries), and hypoxia for 10 days (D; n = 8 untreated arteries and 8 treated arteries). Data are expressed as percent dilation of contraction elicited by either U-46619 or endothelin. All values are means ± SE. *Significantly different from the concentration-response curve in untreated arteries (P < 0.05 by the linear mixed effects model).
We next determined whether the altered responses to arachidonic acid in GA-treated vessels were due to an effect on arachidonic acid-induced production of prostacyclin and/or thromboxane. As expected, the substrate arachidonic acid markedly increased the production of both prostacyclin and thromboxane (Table 2) in PRAs from all groups of piglets. However, unlike the agonist ACh, inhibition of Hsp90 with GA had no effect on arachidonic acid-stimulated production of either prostacyclin or thromboxane in PRAs from any group of piglets (Table 2). Thus, the impact of GA treatment on arachidonic acid responses cannot be attributed to altered production of prostacyclin and thromboxane in any group of piglets. However, altered responsiveness to endogenous PGI2 and TXA2 (Figs. 11, B and D, and 12, B and D) likely contributes to the altered arachidonic acid-induced responses found in GA-treated PRAs in both groups of hypoxic piglets.
Table 2.
6-Keto-PGF1α and TXB2 production in vehicle- and GA-treated PRAs under basal and AA-stimulated conditions for piglets exposed to control and hypoxic conditions for 3 and 10 days
| 3-Day Control | 3-Day Hypoxia | 10-Day Control | 10-Day Hypoxia | |
|---|---|---|---|---|
| 6-keto-PGFαproduction, pg/ml | ||||
| Number of PRAs/group | 7 | 7 | 8 | 6 |
| Vehicle-treated PRAs | ||||
| Basal | 30,963 ± 5,000 | 9,134 ± 1,531 | 6,396 ± 896 | 4,449 ± 896 |
| AA stimulation | 39,617 ± 7,120* | 17,766 ± 22,619* | 13,771 ± 1,193* | 10,295 ± 483* |
| GA-treated PRAs | ||||
| Basal | 29,922 ± 7,078 | 11,373 ± 1,854 | 7,480 ± 666 | 6,396 ± 896 |
| AA stimulation | 56,726 ± 13,315† | 20,971 ± 3,203† | 13,610 ± 1,456† | 13,771 ± 1,193† |
| TXB2production, pg/ml | ||||
| Number of PRAs/group | 7 | 7 | 8 | 6 |
| Vehicle-treated PRAs | ||||
| Basal | 3.7 ± 1.0 | 8.2 ± 2.8 | 1.0 ± 0.5 | 2.4 ± 0.8 |
| AA stimulation | 7.7 ± 1.6* | 12.0 ± 2.6* | 6.1 ± 1.9* | 8.3 ± 2.1* |
| GA-treated PRAs | ||||
| Basal | 3.6 ± 0.5 | 5.2 ± 1.0 | 1.3 ± 0.7 | 2.0 ± 1.0 |
| AA stimulation | 8.0 ± 1.6† | 12.0 ± 2.3† | 5.8 ± 2.2† | 7.5 ± 1.5† |
Values are means ± SD. AA, arachidonic acid.
Significantly different from vehicle-treated PRAs under basal conditions (P < 0.05 by paired t-test);
different from GA-treated PRAs under basal conditions (P < 0.05 by paired t-test).
DISCUSSION
In this investigation, we report a number of novel findings elucidating the role of protein complex interactions with the chaperone protein Hsp90 in the newborn pulmonary circulation. In addition to expanding on our knowledge of Hsp90-eNOS interactions in a model of postnatal chronic hypoxia-induced pulmonary hypertension, we report, for the first time, coimmunoprecipitation of Hsp90 with PGIS and TXAS and identify several potential sites in the arachidonic acid-prostanoid signaling pathway that could be targeted by inhibitors of Hsp90.
Hsp90 interactions with eNOS.
The interaction between Hsp90 and eNOS has been shown by us and other investigators (4, 16–18, 27, 29, 30, 32) to regulate eNOS activation and NO generation in a number of vascular beds, including the pulmonary circulation. In this study, we report, for the first time, that both basal and ACh-mediated interactions between Hsp90 and its client protein, eNOS, are impaired in PRAs of newborn piglets with pulmonary hypertension caused by either 3 or 10 days of exposure to chronic hypoxia. In studies in control piglets, we (4, 5) previously showed that GA, an Hsp90 antagonist that binds to the Hsp90 ATP-binding site, inhibits ACh-induced increases in Hsp90-eNOS association and impairs PRA dilation to ACh. The present study confirms these findings, once again highlighting the functional relevance of Hsp90-eNOS interactions in the normoxic pulmonary circulation in the first few weeks of postnatal life.
The contributions of impaired Hsp90-eNOS interactions to aberrant regulation of pulmonary vascular tone is receiving increasing attention. Basal Hsp90-eNOS interactions were diminished in peripheral lung tissue of lambs with shunt-induced (increased blood flow) pulmonary hypertension (31, 33). Both basal and agonist-stimulated increases in Hsp90-eNOS interactions were reduced in pulmonary arteries of fetal lambs with pulmonary hypertension caused by in utero ligation of the ductus arteriosus (22, 23) and in pulmonary arteries of adult rats with pulmonary hypertension induced by 1–3 wk of exposure to chronic hypoxia (26). Also of interest, the coimmunoprecipitation of Hsp90 and eNOS was diminished in pulmonary artery endothelial cells from adult pigs cultured under hypoxic conditions (32). In addition to these findings by others, we now report that both basal and ACh-induced interactions between Hsp90 and eNOS are impaired in PRAs of piglets with pulmonary hypertension due to either 3 or 10 days of exposure to hypoxia.
Our new data shed light on some of our previous findings. In particular, we (9, 10, 13) have previously shown that lungs and PRAs from newborn piglets exposed to 10 days of hypoxia demonstrate impaired basal and ACh-induced NO signaling. These alterations in pulmonary vascular NO signaling could not be attributed to reduced expression of either eNOS or Hsp90 (9). Our new findings suggest that posttranslational impairments in the protein-protein interaction between eNOS and Hsp90 underlie, at least in part, the reduced basal and agonist-induced NO production and function in the hypertensive pulmonary circulation of piglets exposed to 10 days of hypoxia.
We have previously reported that impairments in pulmonary vascular NO signaling differ depending on the length of in vivo exposure to chronic hypoxia. Specifically, unlike piglets exposed to 10 days of hypoxia, we (9, 10, 37) previously found no evidence that basal pulmonary vascular NO function is impaired in piglets exposed to hypoxia for only 3 days. Thus, our finding of reduced basal Hsp90-eNOS interactions in PRAs of piglets exposed to 3 days of hypoxia was unanticipated. It is possible that the shorter duration of in vivo hypoxia caused a degree of NO signaling impairment that was too small to be detected by the methodology used in our previous studies (9, 10, 37). However, it is also possible that despite impaired basal Hsp90-eNOS interactions, basal pulmonary vascular NO signaling is preserved after this shorter duration of hypoxic exposure. A mechanism for this latter possibility is provided by the recent finding that high biopterin (BH4) levels could offset the impact of diminished Hsp90-eNOS interactions on eNOS activity in a study (39) in cultured bovine aortic endothelial cells. Thus, it is possible that PRAs of piglets exposed to 3 days of hypoxia have sufficient BH4 to sustain basal NO production in the face of reduced Hsp90-eNOS interactions. This possibility has yet to be explored in an in vivo model of pulmonary hypertension.
In both this and previous studies, we have shown that pulmonary vascular responses to the NO-dependent agonist ACh are impaired in piglets exposed to 3 days of hypoxia. Moreover, in contrast to our previously reported findings in comparable-age control piglets, ACh failed to increase eNOS-Hsp90 interactions in PRAs from both groups of chronically hypoxic piglets. It seems likely that the lack of agonist-induced enhancement of eNOS-Hsp90 interactions could contribute to impaired pulmonary vascular responses to NO-dependent agonists during exposure to 3 or 10 days of hypoxia.
Hsp90 and the prostanoid signaling pathway.
Another important finding in this study was that GA treatment augmented dilation to ACh in preconstricted PRAs of both groups of chronically hypoxic piglets. We (9) have previously reported that two Hsp90 antagonists, GA and the macrocyclic antifungal radicicol, diminished the dilator response to ACh in PRAs from normoxic control piglets and abolished the constriction to ACh in PRAs from chronically hypoxic piglets when studied at basal tone. Findings in this study performed at elevated tone confirmed a differential impact of Hsp90 antagonism on vascular reactivity in vessels from normoxic and hypoxic piglets.
To our knowledge, we are the first to evaluate the effect of an Hsp90 antagonist on functional responses in the pulmonary circulation of animals with pulmonary hypertension. Our findings in both this and our previous study were surprising because Hsp90 anatagonism should reduce agonist-induced NO production, leading to diminished, not augmented, dilation to ACh. Because of possible redox-cycling properties of GA, it was important to assess the possible contribution from ROS generated by this compound. Mitigating this possibility, we (8, 15) previously found that removal of superoxide and H2O2 enhanced dilation to ACh in hypoxic arteries. Therefore, any superoxide or H2O2 generated by GA would be expected to enhance constriction, not augment dilation, to ACh. Moreover, in this study, we found that the ability of GA to enhance dilation to ACh in hypoxic arteries persisted despite treatment with compounds that remove superoxide and H2O2. It must be acknowledged that peroxynitrite formation may not be prevented by these ROS-removing compounds and that peroxynitrite could cause differential effects in normoxic and hypoxic vessels. However, we (9) also previously found that treatment with radicicol, an Hsp90 antagonist that does not redox cycle, had the same effect on ACh responses as GA. Thus, it is highly unlikely that ROS, including peroxynitrite, explain the GA-enhanced dilation to ACh found in both groups of hypoxic vessels.
We reasoned that increased dilation to ACh could occur if Hsp90 antagonism influenced the synthesis or responses to vasoactive substances other than NO, particularly in a circulatory bed in which NO signaling was already impaired. Given the recognized role of the prostanoid pathway in pulmonary vascular responses and because of our previous evidence implicating these arachidonic acid metabolites in chronic hypoxia-induced pulmonary hypertension (12, 14), we examined the effect of Hsp90 antagonism on the ACh-stimulated synthesis of PGI2 and TXA2.
We found that ACh increases the production of PGI2 in all groups of piglets but most potently in the 10-day control group. ACh also stimulated TXA2 production in all but the 10-day control group of piglets. It thus appears that for the older group of control piglets, in addition to augmenting NO production, ACh stimulates an increase in the ratio of dilator to constrictor prostanoids that could contribute to the robust dilation to ACh in vehicle-treated PRAs in this age group. A greater reliance on the NO signaling pathway than the prostanoid pathway could explain why PRAs from the younger group of control piglets exhibited robust dilation to ACh despite a reduction in the ratio of dilator to constrictor prostanoid production. In the case of both groups of hypoxic piglets, the failure of ACh to alter the dilator-to-constrictor prostanoid ratio, in the face of impaired NO signaling, provides an explanation for the extremely poor dilator response to ACh in vehicle-treated PRAs.
An important new finding in this study is that GA treatment reduced the ACh-induced production of PGI2 in PRAs from all groups of piglets and simultaneously also reduced TXA2 production in all but the 10-day control group. To our knowledge, we are the first to report that agonist-induced PGI2 and TXA2 production are inhibited by an Hsp90 antagonist. However, we are not the first to evaluate the possibility that Hsp90 regulates the production of an arachidonic acid metabolite. Tanoika et al. (36) reported that cytosolic PGE2 synthase (cPGES) was identical to p23, a known binding partner of Hsp90. Hence, their finding that cPGES associates with Hsp90 in cultured rat fibroblasts was not surprising (21, 35). The same investigators also found that the association of cPGES and Hsp90 increased with agonist stimulation; the agonist-induced increase in cPGES-Hsp90 association was inhibited by Hsp90 antagonists (35), and changes in the production of PGE2 mirrored the changes in cPGES-Hsp90 association (21, 35).
In the present, we add to Tanioka et al.'s findings and show, for the first time, that Hsp90 coimmunoprecipitates with two other prostanoid enzymes, PGIS and TXAS. Hsp90 functions as a part of a multichaperone complex via association with a variety of cochaperones (including p23) to influence the activity and stability of numerous proteins (19, 41). Our findings demonstrate that PGIS and TXAS are part of a multiprotein complex that includes Hsp90; we further show that agonist-induced prostanoid synthesis is altered in the presence of an Hsp90 antagonist that interferes with its ATP conformational state. However, unlike PGES (35), agonist-mediated regulation of PGIS and TXAS enzymatic activity was not associated with changes in the amount of complex formation with Hsp90. Assessment of the amount of complex formation between Hsp90 and a protein, however, does not evaluate whether the protein is being held in an ATP or ADP conformation, which influences protein folding and chaperone function. Thus, despite no change in the amount of Hsp90 that complexes with PGIS and TXAS, Hsp90 may still be modulating the function of these prostanoid synthases. Investigations into the regulatory and structural relationships between Hsp90 and these prostaglandin synthases should prove to be a fruitful area for further study.
In both groups of control vessels, the Hsp90 antagonist GA reduced dilator responses to ACh and inhibited agonist-induced PGI2 production. Moreover, by preventing an increase in the ratio of dilator to constrictor prostanoid production, GA contributed to the blunted dilator responses to ACh found in the 10-day group of control piglets. In both groups of hypoxic vessels, GA enhanced dilator responses to ACh. This could be explained, at least in part, by a shift in the ratio of dilator to constrictor prostanoid production in vessels from the 3-day hypoxic piglets. However, a GA-mediated effect on the ratio of dilator to constrictor prostanoid production does not explain findings in either the 3-day group of control piglets or the 10-day group of hypoxic piglets. Therefore, we explored other sites in the arachidonic acid-prostanoid signaling pathway that might help explain these findings.
Another important finding in this study is that GA decreases vascular responses to exogenous thromboxane in arteries from both control and hypoxic piglets. In control arteries, where GA inhibits ACh-mediated dilator responses, the impact from inhibiting NO production appears to predominate over the diminished constrictor response to TXA2. The persistence of diminished ACh-induced dilator responses in GA-treated control arteries in the presence of indomethacin also suggests that loss of NO production from Hsp90 antagonism predominates over any influence on prostanoid signaling in normoxic conditions. In hypoxic arteries, GA both inhibits constriction to thromboxane and increases dilator responses to exogenous prostacyclin. These effects on vasoreactivity provide a mechanistic explanation for the enhanced dilation response in GA-treated hypoxic arteries to the agonist ACh. Moreover, the finding in the 10-day hypoxic group that the enhanced dilation to ACh in GA-treated hypoxic arteries was abolished by indomethacin also supports the role of prostanoids as a target for Hsp90 antagonism in hypertensive vessels.
Additional support that arachidonic acid metabolites are a target for Hsp90-mediated modulation of vascular signaling is provided by our findings that GA alters arachidonic acid responses in arteries from all groups of piglets. The sites in the arachidonic acid signaling pathway that are modulated by Hsp90 are not yet known. Our findings point to several possibilities that will require further investigation. For example, the failure of GA to alter arachidonic acid-induced PGI2 and TXA2 production suggests that a site upstream from arachidonic acid release is a potential target of action for the Hsp90 inhibitor. This upstream inhibition of arachidonic acid release would explain the ability of GA to inhibit the ACh-induced production of both PGI2 and TXA2. Consistent with this possibility, other investigators (34) recently reported that GA inhibits the ability of human peripheral mononuclear cells to release arachidonic acid in response to stimulation with either PMA or the combination of PMA and the Ca2+ ionophore A-23187.
Our findings that GA altered dilation to exogenous prostacyclin and constriction to exogenous thromboxane suggests that yet other sites of arachidonic acid signaling could be modulated by Hsp90. For example, thromboxane causes constriction, at least in part, via the activation of Rho kinase (7, 25). It is not currently known whether Hsp90 modulates the activity of any of the proteins in this Rho kinase signaling pathway in pulmonary vascular smooth muscle cells. However, findings of others (2, 3) with other cell lines are supportive of this possibility.
Conclusions.
In summary, we found that Hsp90-eNOS binding was disrupted in PRAs from chronically hypoxic piglets. This impaired interaction between Hsp90 and eNOS could contribute to the NO signaling abnormalities that we have previously identified in the pulmonary circulation of chronically hypoxic newborn piglets. Our findings continue to support the notion that when Hsp90 and eNOS interactions are intact, i.e., in PRAs from control piglets, Hsp90 antagonism will interfere with NO-dependent, agonist-induced responses, limiting dilation. Of importance, we also show that the effect of an Hsp90 antagonist on NO-dependent, agonist-induced responses differs between normoxic and hypoxic vessels. Moreover, arachidonic acid signaling pathways are also impacted differently by pharmacological antagonism of Hsp90 in hypoxic and normoxic vessels. Thus, our findings suggest that the impact of pharmacological antagonism of Hsp90 on at least two signaling pathways is altered during chronic hypoxia. These findings raise the possibility that manipulating Hsp90 function could have very different effects in the normal, normoxic situation than in conditions of hypoxia-related pulmonary hypertension. It is important to note that our findings may not be relevant to hypoxia during the fetal period or to the failure of the normal postnatal decline in pulmonary vascular resistance that occurs during the first few hours of life. Rather, our findings have physiological relevance for the postnatal development of pulmonary hypertension that occurs in newborns who suffer from chronic conditions associated with exposure to alveolar hypoxia, such as occurs in underventilated lung regions of infants with chronic lung disease. Our findings point to the possibility that augmenting Hsp90 function would exacerbate, not ameliorate, pulmonary vasoconstriction in the pulmonary circulation of newborns with established pulmonary hypertension. Thus, further investigation is warranted before attempts to enhance Hsp90 function, as has been suggested (33), is used as a therapeutic maneuver to treat established postnatally acquired pulmonary hypertension in newborns suffering from conditions associated with postnatal chronic hypoxia.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-68572 (to C. D. Fike) and RO1-HL-75511 (to J. L. Aschner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Akaike H. Likelihood and Bayes Procedure. Valencia, Spain: University, 1980 [Google Scholar]
- 2.Amiri A, Noei F, Feroz T, Lee JM. Geldanamycin anisimycins activate Rho and stimulate Rho- and ROCK-dependent actin stress fiber formation. Mol Cancer Res 5: 933–942, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Antonov A, Snead C, Gorshkov B, Antonova GN, Verin AD, Catravas JD. Heat shock protein 90 inhibitors protect and restore pulmonary endothelial barrier function. Am J Respir Cell Mol Biol 39: 551–559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aschner JL, Foster SL, Kaplowitz M, Zhang Y, Zeng H, Fike CD. Heat shock protein 90 modulates endothelial nitric oxide synthase activity and vascular reactivity in the newborn piglet pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 292: L1515–L1525, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Aschner JL, Zeng H, Kaplowitz MR, Zhang Y, Slaughter JC, Fike CD. Heat shock protein 90-eNOS interactions mature with postnatal age in the pulmonary circulation of the piglet. Am J Physiol Lung Cell Mol Physiol 296: L555–L564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Averna M, Stifanese R, Tullio RD, Passalcqua M, Salamino F, Pontremoli S, Melloni E. Functional role of Hsp90 complexes with endothelial nitric-oxide synthase and calpain on nitric oxide generation in endothelial cells. J Biol Chem 283: 29069–29076, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Cambell WB, Ojeda SR. Measurement of prostaglandin by radioimmunoassay. Methods Enzymol 141: 323–341, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Cogolludo A, MOreno L, lodi F, Tamarag J, Perez-Vizcaino F. Postnatal maturational shift from PKC and voltage-gated K+ channels to RhoA/Rho kinase in pulmonary vasoconstriction. Cardiovasc Res 66: 84–93, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fike CD, Aschner JL, Zhang Y, Kaplowitz MR. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn piglets. Am J Physiol Lung Cell Mol Physiol 286: L1244–L1254, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Fike CD, Kaplowitz MR. Chronic hypoxia alters nitric oxide dependent pulmonary vascular responses in lungs of newborn pigs. J Appl Physiol 81: 2078–2087, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Fike CD, Kaplowitz MR. Effect of chronic hypoxia on pulmonary vascular pressures in isolated lungs of newborn pigs. J Appl Physiol 77: 2853–2862, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Fike CD, Kaplowitz MR, Pfister SL. Arachidonic acid metabolites and an early stage of pulmonary hypertension in chronically hypoxic newborn pigs. Am J Physiol Lung Cell Mol Physiol 284: L316–L323, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fike CD, Kaplowitz MR, Thomas CJ, Nelin LD. Chronic hypoxia decreases nitric oxide production and endothelial nitric oxide synthase in newborn pig lungs. Am J Physiol Lung Cell Mol Physiol 274: L517–L526, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Fike CD, Pfister SL, Kaplowitz MR, Madden JA. Cyclooxygenase contracting factors and altered pulmonary vascular responses in chronically hypoxic newborn pigs. J Appl Physiol 92: 67–74, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L881–L888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the m domain of hsp90 serves as a molecular scaffold to regulate akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res 90: 866–873, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapeteropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392: 821–824, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin-stimulated displacement of eNOS from caveolin-1. J Biol Chem 275: 22268–22272, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 3: 213–217, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Khurana VG, Feterik K, Springett MJ, Eguchi D, Shah V, Katusic ZS. Functional interdependence and colocalization of endothelial nitric oxide shynthase and heat shock protein 90 in cerebral arteries. J Cereb Blood Flow Metab 20: 1563–1570, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Nakatani Y, Tanioka T, Tsujimoto M, Nakajo S, Nakaya K, Murakami M, Kudo I. Regulation of cytosolic prostaglandin E synthase by phosphorylation. Biochem J 381: 59–69, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konduri GG, Bakhutashvili I, Eis A, Pritchard K. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 292: H1812–H1820, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Konduri GG, Ou J, Shi Y, Pritchard KA. Decreased association of Hsp90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 285: H204–H211, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Laird N, Ware J. Random-effects models for longitudinal data. Biometrics 38: 963–974, 1982 [PubMed] [Google Scholar]
- 25.McKenzie C, MacDonald A, Shaw AM. Mechanisms of U46619-induced contraction of rat pulmonary arteries in the presence and absence of the endothelium. Br J Pharmacol 157: 581–596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem 277: 44085–44092, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Ou J, Ou Z, Ackerman AW, Oldham KT, Pritchard KA. Inhibition of heat shock protein 90 (HSP90) in porliferating endothelial cells uncouples endothelial nitric oxide synthase activity. Free Radic Biol Med 34: 269–276, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 228: 111–133, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Pritchard KA, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem 276: 17621–17624, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Russell KS, Haynes MP, Caulin-Glaser T, Rosneck J, Sessa WC, Bender JR. Estrogen stimulates heat shock protein 90 binding to endothelial nitric oxide synthase in human vascular endothelial cells: effects on calcium sensitivity and NO release. J Biol Chem 275: 5026–5030, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, Rau T, Wilham J, Harman C, Oishi P, Fineman JR, Black SM. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 294: L46–L56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su Y, Block ER. Role of calpain in hypoxic inhibition of nitric oxide synthase activity in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 278: L1204–L1212, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from enodothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol 293: L1444–L1453, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Szanto S, Csermely P, Kovacs I, Csongor J, Illes A, Bako G, Szegedi G, Sipka S. Inhibition of arachidonic acid release from peripheral mononuclear cells by heat shock treatment and geldanamycin. Immunol Lett 83: 181–185, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Tanioka T, Nakatani Y, Kobayashi T, Tsujimoto M, Oh-ishi S, Murakami M, Kudo I. Regulation of cytosolic prostaglandin E2 synthase by 90-kDa heat shock protein. Biochem Biophys Res Commun 303: 1018–1023, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem 275: 32775–32782, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Turley JE, Nelin LD, Kaplowitz MR, Zhang Y, Fike CD. Exhaled nitric oxide is decreased at an early stage of hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 284: L489–L500, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem 283: 18473–18477, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Whitsett J, Martasek P, Zhao H, Schauer DW, Hatakeyama K, Kalyanaraman B, Vasquez-Vivar J. Endothelial cell superoxide anion radical generation is not dependent on endothelial nitric oxide synthase-serine 1179 phosphorylation and endothelial nitric oxide synthase dimer/monomer distribution. Free Radic Biol Med 40: 2056–2068, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Shi Y, Wang J, Jones D, Weilrauch D, Ying R, Wakim B, Pritchard KA. A heat shock protein binding domain in endothelial nitric-oxide synthase influences enzyme function. J Biol Chem 282: 37567–37574, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med 82: 488–499, 2004 [DOI] [PubMed] [Google Scholar]