Abstract
TNF-α is a proinflammatory cytokine and is an important mediator of maternal endothelial dysfunction leading to preeclampsia. In this study, we tested whether IL-10 protects against TNF-α-induced endothelial dysfunction in murine aorta. In in vitro experiments, aortic rings of C57BL/6 female mice were incubated in Dulbecco's modified Eagle's medium in the presence of either vehicle (distilled H2O), TNF-α (4 nmol/l), or recombinant mouse IL-10 (300 ng/ml) or in the presence of both TNF-α and IL-10 for 22 h at 37°C. In in vivo experiments C57BL6/IL-10 knockout female mice were treated with saline or TNF-α (220 ng·kg−1·day−1) for 14 days. Aortic rings were isolated from in vitro and in vivo experiments and mounted in a wire myograph (Danish Myotech) and stretched to a tension of 5 mN. Endothelium-dependent relaxation was assessed by constructing cumulative concentration-response curves to acetylcholine (ACh, 0.001–10 μmol/l) during phenylephrine (10 μmol/l)-induced contraction. As a result, overnight exposure of aortic rings to TNF-α resulted in significant blunted maximal relaxing responses (Emax) to ACh compared with untreated rings (22 ± 4 vs. 82 ± 3%, respectively). IL-10 knockout mice treated with TNF-α showed significant impairment in ACh responses (Emax) compared with C57BL/6 mice treated with TNF-α (51 ± 3 vs. 72 ± 3%, respectively). Western blot analysis showed that endothelial nitric oxide synthase (eNOS) expression was reduced by TNF-α in in vitro and in vivo experiments, whereas IL-10 restored the eNOS expression. In conclusion, the anti-inflammatory cytokine IL-10 prevents impairment in endothelium-dependent vasorelaxation caused by TNF-α by protecting eNOS expression.
Keywords: tumor necrosis factor-α, nuclear factor-κB
tumour necrosis factor-α (TNF-α) is a proinflammatory cytokine responsible for maternal endothelial dysfunction leading to pathological state-like preeclampsia (43). Many markers have been reported in preeclamptic women, suggesting it as an endothelial cell disorder (54). Endothelial dysfunction in preeclampsia may disrupt the balance between proinflammatory (TNF-α, IL-1β, IL-2, and IL-6) and anti-inflammatory cytokines, which leads to vascular damage (21). TNF-α and IL-6 are elevated twofold in preeclampsia (65). The most common possible mechanism in preeclampsia would be that the factors derived from the placenta produce TNF-α that leads to endothelial disturbances (60a).
TNF-α is a cytokine with antitumoral activity, which has multiple cell functions regulating immune responses. In addition to its proinflammatory actions, there are several reports showing that increased levels of TNF-α are associated with impaired endothelial function (8, 57). This TNF-α-induced endothelial dysfunction is induced by the production of reactive oxygen species (ROS) (14). The pathways activated by TNF-α vary considerably in different conditions and treatments (35, 59). It causes cell death via apoptosis through the activation of nuclear factor-κB (NF-κB). The mechanism of ROS generation by TNF-α is mainly a proapoptotic or cytotoxic effect (25, 30). These studies have shown that mitochondria play an essential role in the TNF-α-induced ROS generation (58). An increased production of ROS leads to reduced nitric oxide (NO) bioavailability because of the scavenging of NO (42). NO production is mainly regulated by the endothelial NO synthase (eNOS) enzyme, which is in turn regulated by various other proteins (20). The pathways activated by TNF-α lead to NF-κB activation and ROS generation, causing an uncoupling of the eNOS enzyme (13, 20, 50). Hence, we hypothesize that TNF-α treatment can cause a downregulation of eNOS expression. NF-κB is activated when it is dissociated from a cytosolic inhibitory protein IκB (24). NF-κB then translocates to the nucleus where DNA binding occurs (7, 37, 70). This may lead to a decrease in the eNOS enzyme activity and/or expression, resulting in endothelial dysfunction (29). Thalidomide inhibits a lipopolysaccharide-induced production of TNF-α (12, 56). Previous studies have also shown that thalidomide blocks NF-κB activation through suppression of IκB kinase activity (40). Studies have reported that IL-10 inhibited the NF-κB activity in purified T lymphocytes (51, 55). Based on these studies, we hypothesized that IL-10 inhibits NF-κB activation and consequently restores the endothelium-dependent relaxation impaired by TNF-α.
In the present study, we investigated whether TNF-α causes an impairment of acetylcholine (ACh)-induced endothelium-dependent relaxation in murine aorta. Our next goal was to determine whether the impairment in endothelium-dependent relaxation caused by TNF-α could be restored by IL-10. To study the protective effects of IL-10, we used isolated aortic rings of wild-type (WT) mice that were treated with IL-10, as well as mice deficient in IL-10. The former studies are referred to as the in vitro effects of IL-10 (see materials and methods), whereas the latter are referred to as the in vivo effects of IL-10.
MATERIALS AND METHODS
Experimental strategy.
For in vitro experiments, aortas from WT mice were divided into four rings of 2 mm length. Each of the aortic rings was incubated in 2 ml DMEM containing either vehicle, TNF-α, recombinant mouse IL-10, or a combination of both TNF-α and IL-10 for 22 h at 37°C. Following incubation, these aortic segments were mounted in a wire myograph to analyze the ACh-induced endothelium-dependent relaxations during contraction with phenylephrine (PE). We hypothesized that IL-10 would restore the impaired ACh-induced relaxation caused by TNF-α by an inhibition of the NF-κB pathway.
For in vivo experiments, we placed osmotic minipumps filled with saline-TNF-α in both WT mice and mice deficient in IL-10. After 14 days, the aortic rings were isolated to perform the functional studies. We hypothesized that TNF-α would cause impairment in ACh-induced endothelium-dependent relaxation when infused in WT mice and IL-10-deficient mice but that this impairment in relaxation would be more in IL-10-deficient mice.
Animals.
Experiments were conducted in 12-wk-old female IL-10 knockout (KO) mice and their WT control C57BL/6 mice (JAX mice and services, Bar Harbor, ME). All procedures were approved by the Institutional Animal Care Committee.
Isolation of aortic rings for in vitro studies.
Mice were euthanized with pentobarbital sodium (50 mg/kg iv, Abbott, Abbott Park, IL), after which the abdomino-thoracic aorta was excised, placed in ice-cold physiological saline solution (PSS), and cleaned of adhering connective and adipose tissue. Aorta from each mouse was divided into four rings of 2 mm length. Each of the aortic rings was incubated in 2 ml DMEM containing 120 U/ml penicillin and 120 μg/ml streptomycin. The incubation medium contained either vehicle (distilled H2O), TNF-α (4 nmol/l), recombinant mouse IL-10 (300 ng/ml), or a combination of both TNF-α and IL-10 for 22 h at 37°C. Supraphysiological levels of IL-10 (300 ng/ml) were selected for this study, although previous studies suggest that plasma levels of IL-10 are about 2.4 ± 2.1 pg/ml (19). Previous studies have reported that higher plasma levels of IL-10 (505 ± 22.3 ng/ml) had no adverse effects in the subjects (22). Following incubation, the aortic rings were removed and immediately placed in oxygenated PSS with the following ionic composition: (in mmol/l) 130 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.18 MgSO4·7H2O, 14.9 NaHCO3, 5.6 dextrose, 1.56 CaCl2·2H2O, and 0.026 EDTA. Aortic rings were mounted in a wire myograph (Danish Myotech) filled with 5 ml PSS maintained at 37°C and continuously gassed with a mixture of 95% O2-5% CO2. A resting tension of 5 mN was applied to the aortic rings, and they were allowed to equilibrate for at least 30 min. Endothelium-dependent relaxation was performed on PE-contracted (10 μmol/l) rings followed by a cumulative concentration response curve to ACh (0.001–10 μmol/l). Endothelium-independent relaxation was tested with sodium nitroprusside (SNP; 0.001–100 μmol/l) during contraction with PE (10 μmol/l).
In vivo experimental protocol and isolation of aortic rings.
Mice were anesthetized with ketamine-xylazine, and an osmotic pump containing saline or TNF-α (220 ng·kg−1·day−1) was inserted in the neck of WT mice and IL-10 KO mice as previously described (27). Mice were treated with saline or TNF-α for 14 consecutive days. Mice were divided into four groups: WT mice infused with saline (WT), WT mice infused with TNF-α (WT + TNF), IL-10 KO mice treated with saline (KO), and IL-10 KO mice treated with TNF-α (KO + TNF). Following treatment for 14 days, the mice were euthanized with pentobarbital sodium (50 mg/kg iv, Abbott), after which the abdomino-thoracic aorta was excised. The aorta was either used for performing Western blot analysis or functional studies on the wire myograph.
Blood pressure recordings and treatment.
At the end of treatment (14 days), the mice were anesthetized with ketamine-xylazine anesthesia. Following anesthesia, a sterile catheter was inserted into the carotid artery. The incision was closed with a sterile 6-0 Ethicon Ophthalmic suture. The catheter was secured on the back of the mouse to avoid any biting of the tube by the mouse. All surgeries were conducted under aseptic and sterile conditions to avoid any chances of infection. Once the mouse had recovered from anesthesia, the catheter was connected to a transducer to record mean arterial pressure. Recording measurements of mean arterial pressure were made for 3 to 4 h. Subsequently, the mice were euthanized and the aorta was isolated for functional studies or Western blot analysis.
Western blot analysis.
Four aortae were isolated from four mice from the in vitro or in vivo groups as described in In vivo experimental protocol and isolation of aortic rings. Following an overnight incubation or 14 days of treatment, aortae were used for Western blot analysis. Aortic tissue was lysed by radioimmunoprecipitation assay lysis buffer in the presence of 1 mmol/l PMSF, 1 mmol/l sodium orthovanadate, 1 mmol/l sodium fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin for 20 min on ice. Whole tissue lysates were centrifuged at 10,000 g for 20 min at 4°C, and the supernatants were collected. The protein concentrations were determined by the Bio-Rad protein assay. Samples of 50 μg were separated by 10% SDS polyacrylamide gel electrophoresis and transferred by electroblotting onto a nitrocellulose membrane (Hybond; Amersham Biosciences, NJ). For the immunoassay, the membranes were blocked in 5% (wt/vol) nonfat dry milk in 1 × PBS-0.2% Tween 20 for 1 h at 4°C with primary antibodies. Polyclonal rabbit anti-phosphorylated eNOS, anti-eNOS, anti-phosphorylated IκB, anti-IκB, anti-phosphorylated NF-κB, and anti-NF-κB antibody (Cell Signaling) and monoclonal anti-β-actin (Sigma-Aldrich) were used. Immunocomplexes were detected through horseradish peroxidase-conjugated goat anti-mouse antisera (Amersham Biosciences), followed by enhanced chemiluminescence reaction (ECL, Pierce Biotechnology).
Drugs.
ACh, human recombinant TNF-α, PE, SNP, and thalidomide were purchased from Sigma Chemical (St. Louis, MO). Mouse Recombinant IL-10 was purchased from R&D Laboratories (Minneapolis, MN).
Statistical analysis.
Results are presented as means ± SE. Experimental values were calculated relative to the maximal changes from the contraction produced by PE in each segment, which was taken as 0.0%. The baseline tension before the addition of PE was considered as 100%. The pEC50 values for PE, ACh, and SNP were expressed as −log of the molar concentration to produce 50% of the maximal response. Statistical analysis was performed using two-way analysis of variance to compare the concentration-responses curves between the groups. A standard nonparametric t-test was performed to compare the two groups in Figs. 2, 3, and 4. The analyses were performed using the GraphPad Prism software. Values of P < 0.05 were considered a statistically significant difference.
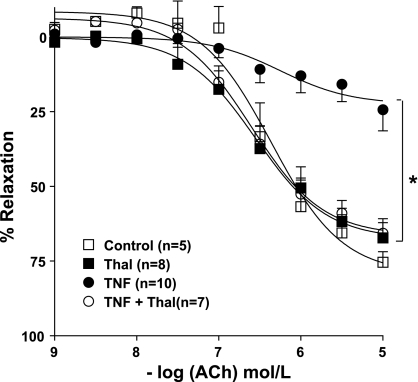
Fig. 2.
Cumulative concentration-response curves to ACh (0.001–10 μmol/l) in PE (10 μmol/l)-contracted aortic rings treated with thalidomide (Thal) or TNF-α or both overnight (control, Thal, TNF, and TNF + Thal). Vasorelaxation was expressed as percentage of the contraction induced by PE. Values are means ± SE (n = 5–10; *P < 0.05).
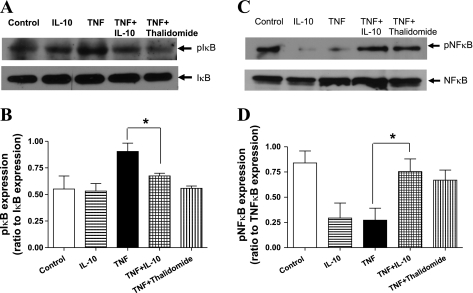
Fig. 3.
Representative Western blot images and corresponding bar graphs showing expression of phosphorylated (p)IκB (A and B) and pNF-κB (C and D) in untreated (control), IL-10-treated (300 ng/ml), TNF-α-treated (4 nmol/l), TNF-α + IL-10-treated, and TNF-α + Thal-treated aortic rings in A and C. Densitometric analysis were performed on untreated (control), IL-10-treated (300 ng/ml), TNF-α-treated (TNF; 4 nmol/l), the TNF-α + IL-10-treated, and TNF-α + thalidomide-treated aortic rings as shown in B and D. Values are expressed as normalized ratios of the intensities of pIκB to IκB and pNF-κB to NF-κB (n = 4; *P < 0.05) for B and D, respectively.
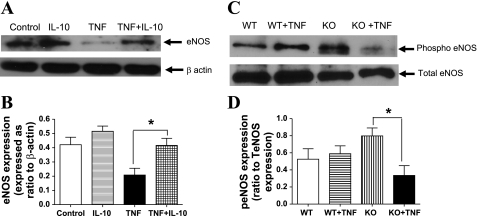
Fig. 4.
A and B: representative Western blot showing expression of the endothelial nitric oxide synthase (eNOS) in untreated (control), IL-10-treated (300 ng/ml), TNF-α-treated (4 nmol/l), and TNF-α + IL-10-treated aortic rings in A. Densitometric analysis were performed on untreated (control), IL-10-treated (300 ng/ml), TNF-α-treated (4 nmol/l), and TNF-α + IL-10-treated aortic rings as shown in B. Values are expressed as normalized ratios of the intensities of eNOS to β-actin (n = 5 to 6; *P < 0.05) for B. C and D: representative Western blot showing expression of the peNOS in 14 days of treatment with saline and TNF-α in WT and IL-10 KO mice. The 4 groups are WT mice treated with saline, WT mice treated with TNF-α, IL-10 KO mice treated with saline, and IL-10 KO mice treated with TNF-α as shown in C. Densitometric analysis were performed on WT, WT + TNF, KO, KO + TNF as shown in D. Values are expressed as normalized ratios of the intensities of peNOS to eNOS (n = 5; *P < 0.05) for D.
RESULTS
PE-induced contractions and ACh-induced relaxation in aortic rings treated overnight with recombinant TNF-α: in vitro effects.
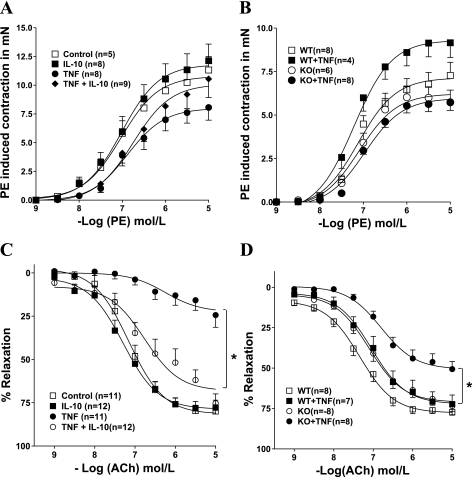
There was no significant change in the sensitivity (pEC50) to PE in vessels treated overnight with vehicle (7.03 ± 0.14) or recombinant TNF-α (4 nmol/l) (6.89 ± 0.15) or IL-10 (300 ng/ml) (7.02 ± 0.15) or a combined treatment of TNF-α and IL-10 (6.75 ± 0.16). The maximal tension (Emax) in response to PE in vessels treated overnight with recombinant TNF-α (4 nmol/l) (8.00 ± 0.51 mN) was significantly decreased compared with vessels treated with vehicle (10.78 ± 0.57 mN) or IL-10 (300 ng/ml) (11.80 ± 0.67 mN). Emax in response to PE in vessels subjected to combined treatment of TNF-α and IL-10 (10.09 ± 0.69 mN) was similar to other groups (Fig. 1A). The sensitivity to ACh and maximal relaxation with 10 μmol/l ACh after overnight treatment in control-treated aortic rings were 7.16 ± 0.09 and 82 ± 3%, respectively (Fig. 1C). Overnight exposure to TNF-α (4 nmol/l) resulted in an impairment of ACh-induced relaxation (pEC50, 6.25 ± 0.35; Emax, 23 ± 4%; P < 0.05; Fig. 1C). This effect of TNF-α appears to be endothelium dependent since relaxation to the NO donor SNP was similar in aortic rings treated with vehicle or TNF-α (data not shown).
Fig. 1.
Cumulative concentration-response curves to phenylephrine (PE; 0.001–10 μmol/l) in aortic rings treated with IL-10 or TNF-α overnight (A) and in aortic rings from mice treated for 14 days with TNF-α (B). Cumulative concentration-response curves to acetylcholine (ACh; 0.001–10 μmol/l) in PE (10 μmol/l)-contracted aortic rings treated with IL-10 or TNF-α overnight (C) and in aortic rings from mice treated for 14 days with TNF-α (D) (see materials and methods). A and C: overnight treated groups: control, IL-10, TNF, and TNF + IL-10. B and D: in vivo treated groups: wild-type (WT), WT + TNF, knockout (KO), and KO + TNF. Contraction was expressed as force induced in mN. Vasorelaxation was expressed as percentage of the contraction induced by PE. Values are means ± SE (n = 4–12; *P < 0.05).
We also examined the effect of exogenously added recombinant IL-10 on ACh-induced relaxations in aortic rings treated with/without TNF-α. Figure 1C shows that IL-10 completely restored relaxation responses to ACh (pEC50, 6.77 ± 0.16; and Emax, 68 ± 4%) in aortic rings treated with TNF-α, whereas those treated with IL-10 alone showed responses similar to control vessels (pEC50, 7.29 ± 0.10; and Emax, 79 ± 3%). Relaxation to SNP in aortic rings treated with IL-10 or TNF-α in combination with IL-10 was not different from control values (data not shown).
PE-induced contractions and ACh-induced relaxation in aortic rings from mice treated for 14 days with recombinant TNF-α: in vivo effects.
Sensitivity to PE (pEC50) in the in vivo groups was relatively similar (Fig. 1B; WT, 7.12 ± 0.11; WT + TNF, 7.22 ± 0.11; KO, 7.09 ± 0.13; and KO + TNF, 6.97 ± 0.19). Emax generated by PE in WT mice infused with TNF-α was increased compared with WT mice treated with saline (WT, 7.14 ± 0.30; and WT + TNF, 9.30 ± 0.37 mN). There was no significant difference in Emax between KO mice infused with either saline or TNF-α (KO, 6.23 ± 0.30; and KO + TNF, 5.97 ± 0.19 mN) (Fig. 1B).
In WT mice infused with either saline or TNF-α, pEC50 to ACh and Emax with 10 μmol/l ACh did not significantly differ (7.05 ± 0.14 and 72 ± 3% vs. 7.34 ± 0.08 and 78 ± 2%, respectively; Fig. 1D). However, TNF-α infusion in IL-10 KO mice caused an impairment in the ACh-induced relaxation compared with saline-infused IL-10 KO mice (pEC50, 6.80 ± 0.12; and Emax, 51 ± 3% vs. pEC50, 7.12 ± 0.09; and Emax, 71 ± 2%; Fig. 1D).
ACh-induced relaxation in aortic rings treated overnight with recombinant TNF-α: in vitro effects.
We examined the effect of added thalidomide on ACh-induced relaxations in aortic rings treated with/without TNF-α. Figure 2 shows that thalidomide completely restored relaxing responses to ACh (pEC50, 6.6 ± 0.09; and Emax, 66 ± 3%) in aortic rings treated with TNF-α, whereas those treated with thalidomide alone showed similar responses to control vessels (pEC50, 6.36 ± 0.14; and Emax, 79 ± 6%).
In vitro treatment with TNF-α decreases endothelium-dependent relaxation by potentiating IκB phosphorylation in murine aorta.
Representative Western blotting images for phosphorylated IκB (pIκB) in samples from aortic rings treated with vehicle, TNF-α, IL-10, a combination of TNF-α and IL-10, or a combination of TNF-α and thalidomide (inhibitor of TNF-α induced NF-κB activation) are shown in Fig. 3A. The protein expression of pIκB quantified via densitometric analysis was significantly increased in aortic rings treated with TNF-α (4 nmol/l) compared with aortic rings treated with vehicle (0.9 ± 0.1 vs. 0.6 ± 0.1; ratio of pIκB to IκB; Fig. 3B). Treatment with IL-10 (300 ng/ml) in the presence of TNF-α normalized/reduced the pIκB protein expression levels to that of aortic rings treated with vehicle alone (0.7 ± 0.1; Fig. 3B). IL-10 treatment alone had no effect on the expression level of pIκB compared with control vessels (0.5 ± 0.1; Fig. 3B). Thalidomide also reduced the pIκB expression in aortic segments treated with TNF-α (0.6 ± 0.1; Fig. 3, A and B).
In vitro treatment with TNF-α decreases endothelium-dependent relaxation by reducing cytosolic phosphorylated NF-κB expression in murine aorta.
Phosphorylated NF-κB (pNF-κB) expression was measured to indicate the amount of NF-κB activated by TNF-α and to elucidate the potential role of IL-10 to inhibit this activation. Representative Western blotting images of pNF-κB expression in samples from aortic rings incubated with vehicle, TNF-α, IL-10, a combination of TNF-α and IL-10, or a combination of TNF-α and thalidomide are shown in Fig. 3C. Protein levels of pNF-κB quantified via densitometric analysis were significantly decreased in aortic rings incubated with TNF-α compared with aortic rings incubated with vehicle (0.3 ± 0.1 vs. 0.8 ± 0.1; Fig. 3D). Incubation with IL-10 in the presence of TNF-α normalized pNF-kB protein expression levels to values observed in aortic rings incubated with vehicle alone (0.8 ± 0.1; Fig. 3D). IL-10 incubation alone decreased the expression level of pNF-kB (0.3 ± 0.1; Fig. 3D). Thalidomide incubation along with TNF-α also restored pNF-kB expression compared with TNF-α-incubated aortic rings (0.7 ± 0.1; Fig. 3, C and D).
eNOS and phosphorylated eNOS expression is reduced by in vitro or in vivo TNF-α treatment.
Representative Western blotting images for aortic rings incubated with vehicle, TNF-α, IL-10, or a combination of TNF-α and IL-10 are shown in Fig. 4A. The protein levels of total eNOS quantified via densitometric analysis were significantly decreased in aortic rings incubated with TNF-α compared with aortic rings incubated with vehicle (0.2 ± 0.1 vs. 0.4 ± 0.1; Fig. 4B). An incubation with IL-10 in the presence of TNF-α normalized the eNOS protein expression levels to that of aortic rings incubated with vehicle alone (0.4 ± 0.1; Fig. 4B). IL-10 incubation alone had no effect on the expression level of eNOS compared with control vessels (0.5 ± 0.1; Fig. 4, A and B).
Representative Western blotting images of eNOS expression in aortic rings isolated from four groups, WT, WT + TNF, IL-10 KO, and IL-10 KO + TNF, are shown in Fig. 4C. Since the total eNOS expression levels were similar, we speculated that there might be a change in phosphorylated eNOS. Hence Western blot analysis to address phospho-eNOS expression levels was performed. The protein levels of phosphorylated eNOS quantified via densitometric analysis were significantly decreased in IL-10 KO mice infused with TNF-α (220 ng·kg−1·day−1) compared with IL-10 KO mice infused with the vehicle (0.8 ± 0.1 vs. 0.3 ± 0.1; Fig. 4D). In TNF-α-infused WT mice, no change in phosphorylated eNOS protein expression level was observed compared with saline-infused WT mice (0.6 ± 0.1 vs. 0.5 ± 0.1; Fig. 4D).
DISCUSSION
TNF-α levels are elevated in various vascular diseases like type 2 diabetes, ischemic heart disease, and preeclampsia (34, 52, 53). TNF-α contributes to vascular pathology in these diseases mostly by inducing injury to endothelial cells (61, 68). Injury to endothelial cells (endothelial dysfunction) can be elicited by apoptosis of the cells or by an impairment in the endothelium-dependent relaxation (5, 47). An impairment in the endothelium-dependent relaxation is mainly caused by a decrease in the bioavailability of NO (31, 49). An uncoupling of eNOS enzyme leads to a decrease in NO production and an increase in ROS generation (20). ROS such as superoxide anions can convert NO into peroxynitrite, leading to an impairment in the eNOS-dependent relaxation (6). Studies conducted on human umbilical vein endothelial cells show that TNF-α causes the downregulation of eNOS enzyme (69). On the other hand, an anti-inflammatory cytokine like IL-10 restores eNOS expression impaired by endothelin-1 (72). IL-10 plays an essential role to suppress the production of proinflammatory cytokines like TNF-α (10). Limited studies have been conducted to show the protective effects of IL-10 against vascular damage induced by TNF-α (18, 41, 65a). IL-10-deficient mice develop chronic enterocolitis and show increased levels of TNF-α (60). Based on this correlation between TNF-α and IL-10, we hypothesized that IL-10 restores endothelium-dependent relaxation impaired by TNF-α. We performed in vivo and in vitro experiments to test our hypothesis. Differences in the effects of TNF-α in in vitro and in vivo conditions may be due to various factors. First, in the in vitro assay the phenomenon of IL-10 release from circulating lymphocytes is lacking and supraphysiological concentrations of recombinant IL-10 is delivered exogenously, which is expected to bind to its receptors located on endothelial (11) and smooth muscle cells (45). Second, in in vivo studies endogenously produced IL-10 from various immune cells are the only available source to observe its effects on vascular pathology (48). We performed in vivo studies to confirm our findings in in vitro studies to show that the effects of IL-10 are also observed at physiological concentrations. In addition, very few studies have performed vascular studies in mice deficient in IL-10, which were used for in vivo studies (32, 33).
We performed TNF-α infusion using osmotic minipumps for 14 days in KO mice and WT mice. Studies have reported that endothelial dysfunction is associated with various forms of vascular and renal disease, including hypertension, coronary artery disease, chronic heart failure, chronic renal failure, and type 2 diabetes (44). We observed that TNF-α infusion did not alter blood pressure (BP) in WT and KO mice, suggesting its association with diseases other than hypertension (data not shown). This is in contrast to our previous study where we found elevated BP in IL-10 KO male mice infused with TNF-α compared with the IL-10 KO male mice infused with saline (ΔBP, 15 ± 4 mmHg) (27). This variation in BP observed in IL-10 KO male and female mice by TNF-α infusion suggests that female mice are protected from a BP rise caused by TNF-α, probably because of enhanced EDHF produced in resistance vessels (23, 38). We found that there was a significant decrease in the PE-induced contraction in vessels incubated with TNF-α compared with vessels incubated with vehicle or IL-10 (Fig. 1A). It could be due to injury to endothelial cells because of apoptosis (15). In vivo studies performed on the KO and WT mice also showed no significant difference in the PE-induced contraction (Fig. 1B). Supporting our studies performed on female virgin mice, a study showed that PE induced contraction was enhanced only in the pregnant rats infused with TNF-α (10–1,000 pg/ml), whereas in virgin rats the responses were normal (28).
A study showed that TNF-α infusion in vivo depresses the ACh-induced endothelium-dependent relaxation (66). When we analyzed the ACh-induced relaxation in the in vitro-treated aortic rings, there was a significant decrease in the relaxation in TNF-α-treated rings compared with control rings (Fig. 1C). This observation was supported by studies performed on cat carotid arteries, which showed that TNF-α blunted the ACh-induced relaxation. TNF-α contributes to the release of proteins like extracellular-regulated kinase, mitogen-activated protein kinase, and NF-κB translocation that activates the ceramide pathway. This leads to an injury to endothelial cells, thus inhibiting the release of vasodilators like NO or prostacyclin from the endothelium (4, 46, 73). The impaired relaxation was significantly restored when TNF-α was treated in combination with IL-10 (Fig. 1C). This suggests that IL-10 inhibits the actions of TNF-α. In vivo studies performed in IL-10 KO mice have shown that IL-10 protects the eNOS-mediated relaxation of carotid arteries by attenuating increases in superoxide production (33). IL-10 inhibits the production of proinflammatory cytokines like TNF-α and IL-6 that stimulate the production of ROS (48). Indeed, our in vivo studies supported our findings in the in vitro studies. KO mice treated with TNF-α showed significant impairment in ACh-induced relaxation compared with the other three groups (WT, WT + TNF-α, and KO; Fig. 1D). This suggests that IL-10 is essential to protect the vascular endothelium from the effects of proinflammatory cytokines like TNF-α. Lipopolysaccharide is a proinflammatory antigen, and IL-10 protects the endothelium after lipopolysaccharide treatment, supporting our findings (32). We found similar results when TNF-α was infused through osmotic pump in KO mice for 14 days. To detect the proteins involved in the TNF-α-induced impairment in relaxation, we performed Western blot analysis in the samples from the in vitro and in vivo studies. We observed that eNOS expression was reduced in the in vitro samples treated with TNF-α compared with those samples in the control groups. The eNOS expression was restored when TNF-α was treated in combination with IL-10. IL-10 by itself had no effect on eNOS expression (Fig. 4A). In in vivo studies, eNOS expression was not altered in the four groups, whereas phosphorylated eNOS expression was reduced in the KO mice treated with TNF-α compared with the other three groups. This explains the discrepancy we observed in in vitro and in vivo studies. The possible explanation would be that at physiological concentrations, IL-10 would be affecting only the phosphorylated levels of eNOS. Phosphorylated eNOS at Ser1177 shows that eNOS was activated in the three groups except the TNF-α-treated KO mice (17). Thus these results so far show that IL-10 is essential to maintain the eNOS-mediated endothelium-dependent relaxation in aortic rings. In addition, IL-10 restores eNOS expression, which was downregulated by TNF-α.
TNF-α leads to the activation of the NF-κB signal transduction pathway. In unstimulated cells, IκB proteins localize NF-κB dimers in the cytoplasm by masking the nuclear localization sequence of NF-κB. The activation of NF-κB through the receptor activation of TNFR1, IL-1R1, and various Toll-like receptors initiates signal transduction cascades, ultimately leading to the activation of the IκB kinase complex (16, 71). The activation of IκB kinase leads to the phosphorylation of IκB-α and IκB-β at specific serine residues, which targets IκB for ubiquination and subsequent degradation by a proteasome-dependent pathway (2). The degradation of IκB leads to the unmasking of the nuclear localization sequence of NF-κB, allowing nuclear accumulation, DNA binding, and transcriptional activation of target genes (26). Based on this background, we performed protein expression for pIκB and pNF-κB in the in vitro aortic rings. We observed that pIκB expression was increased in TNF-α-treated aortic rings compared with the other groups (Fig. 3A; control, IL-10, TNF-α + IL-10, and TNF-α + thalidomide). The increase in pIκB expression indicates the release of NF-κB and thus its translocation to the nucleus as shown in previous studies (1, 26). Studies have shown that IL-10 inhibited proinflammatory cytokine (IL-1)-induced IκB expression (1). Indeed, in our study, when TNF-α was treated in combination with IL-10 or thalidomide, the phosphorylation of IκB was decreased. Drugs like glimepiride and thalidomide cause an increase in the eNOS activity by the inhibition of TNF-α-induced NF-κB activation (39a, 56). Thalidomide suppresses the NF-κB activation induced by TNF-α and hydrogen peroxide; hence, we used it in in vitro studies as a NF-κB inhibitor (40). We observed that thalidomide prevented the decrease in ACh-induced relaxation caused by TNF-α (Fig. 2). This suggests a functional effect of IL-10 similar to thalidomide in restoring endothelium-dependent relaxation, primarily by the inhibition of NF-κB activity. Additionally, pNF-κB was decreased in the TNF-α-treated aortic rings compared with the other three groups (except IL-10; Fig. 3C). The main reason for the decrease in the pNF-κB is due to the nuclear translocation of the phosphorylated protein. Studies conducted on human monocytes have also shown that IL-10 inhibits NF-κB activation (67). Hence, in our studies, we found that when TNF-α was incubated along with IL-10 and thalidomide, the phosphorylation of NF-κB was increased. The decrease in the pNF-κB expression seen in IL-10-treated aortic rings may be due to its inhibitory effects on the phosphorylation of NF-κB, besides its action to inhibit the phosphorylation of IκB. Previous studies have shown that activation of NF-κB and the degradation of IκB lead to endothelial damage, supporting our studies (36). Overall in this study, we emphasize the potential role of IL-10 in preventing endothelial dysfunction induced by proinflammatory cytokines like TNF-α by inhibiting the NF-κB pathway and stimulating eNOS expression. As mentioned previously, elevated levels of TNF-α are observed in various vascular diseases (34, 52, 53). Conditions like preeclampsia show elevated levels of TNF-α, primarily associated with endothelial dysfunction (65). IL-10 on the other hand protects the endothelium against any injury by TNF-α. IL-10 levels are shown to be decreased in preeclamptic patients, whereas higher levels of TNF-α are noted (60a). This would lead us to consider the potential use of IL-10 as a therapeutic drug in the treatment of Crohn's disease, rheumatoid arthritis, and ischemia-reperfusion injury for thoraco-abdominal aortic aneurysm repair (14a, 39, 62).
In conclusion, our studies demonstrate that TNF-α causes an impairment in the endothelium-dependent relaxation, whereas IL-10 plays an important role to suppress this effect by inhibiting the TNF-α-induced NF-κB activation and restoring the impaired eNOS expression.
GRANTS
This study was supported by National Institutes of Health Grants DK-83685 and HL-74157.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Al-Ashy R, Chakroun I, El-Sabban ME, Homaidan FR. The role of NF-κB in mediating the anti-inflammatory effects of IL-10 in intestinal epithelial cells. Cytokine 36: 1–8, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 92: 10599–10603, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki N, Siegfried M, Lefer AM. Anti-EDRF effect of tumor necrosis factor in isolated, perfused cat carotid arteries. Am J Physiol Heart Circ Physiol 256: H1509–H1512, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, Natividad F, Bishop SP, Vatner SF. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol 20: 1493–1499, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS, Koppenol WH. Nitric oxide, superoxide, peroxynitrite: the good, the bad, ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Beg AA, Ruben SM, Scheinman RI, Haskill S, Rosen CA, Baldwin AS. I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev 6: 1899–1913, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Bilsborough W, Keen H, Taylor A, O'Driscoll G, Arnolda L, Green D. Anti-tumour necrosis factor-alpha therapy over conventional therapy improves endothelial function in adults with rheumatoid arthritis. Rheumatol Int 26: 1125–1131, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med 178: 2207–2211, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattaruzza M, Slodowski W, Stojakovic M, Krzesz R, Hecker M. Interleukin-10 induction of nitric-oxide synthase expression attenuates CD40-mediated interleukin-12 synthesis in human endothelial cells. J Biol Chem 278: 37874–37880, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Chen CS, Perng WC, Chen CW, Huang KL, Wu CP, Yen MH. Thalidomide reduces lipopolysaccharide/zymosan-induced acute lung injury in rats. J Biomed Sci 11: 591–598, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Chen JW, Chen YH, Lin FY, Chen YL, Lin SJ. Ginkgo biloba extract inhibits tumor necrosis factor-α-induced reactive oxygen species generation, transcription factor activation, and cell adhesion molecule expression in human aortic endothelial cells. Arterioscler Thromb Vasc Biol 23: 1559–1566, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol 24: 762–768, 2001 [DOI] [PubMed] [Google Scholar]
- 14a.de Villiers S. Crohn's disease and IL-10 therapy: promise regained. Inflamm Bowel Dis 9: 210–211, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Deshpande SS, Angkeow P, Huang J, Ozaki M, Irani K. Rac1 inhibits TNF-α-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J 14: 1705–1714, 2000 [DOI] [PubMed] [Google Scholar]
- 16.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388: 548–554, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Jacobs F, Chaussabel D, Truyens C, Leclerq V, Carlier Y, Goldman M, Vray B. IL-10 up-regulates nitric oxide (NO) synthesis by lipopolysaccharide (LPS)-activated macrophages: improved control of Trypanosoma cruzi infection. Clin Exp Immunol 113: 59–64, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fichtlscherer S, Breuer S, Heeschen C, Dimmeler S, Zeiher AM. Interleukin-10 serum levels and systemic endothelial vasoreactivity in patients with coronary artery disease. J Am Coll Cardiol 44: 44–49, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 44: 708–714, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Fuchs AC, Granowitz EV, Shapiro L, Vannier E, Lonnemann G, Angel JB, Kennedy JS, Rabson AR, Radwanski E, Affrime MB, Cutler DL, Grint PC, Dinarello CA. Clinical, hematologic, and immunologic effects of interleukin-10 in humans. J Clin Immunol 16: 291–303, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Fujiki T, Shimokawa H, Morikawa K, Kubota H, Hatanaka M, Talukder MA, Matoba T, Takeshita A, Sunagawa K. Endothelium-derived hydrogen peroxide accounts for the enhancing effect of an angiotensin-converting enzyme inhibitor on endothelium-derived hyperpolarizing factor-mediated responses in mice. Arterioscler Thromb Vasc Biol 25: 766–771, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Ganchi PA, Sun SC, Greene WC, Ballard DW. I kappa B/MAD-3 masks the nuclear localization signal of NF-kappa B p65 and requires the transactivation domain to inhibit NF-kappa B p65 DNA binding. Mol Biol Cell 3: 1339–1352, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species: role of mitochondrial glutathione. J Biol Chem 272: 11369–11377, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell 109: S81–S96, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Giachini FR, Zemse SM, Carneiro FS, Lima VV, Carneiro ZN, Callera GE, Ergul A, Webb RC, Tostes RC. Interleukin-10 attenuates vascular responses to endothelin-1 via effects on ERK1/2-dependent pathway. Am J Physiol Heart Circ Physiol 296: H489–H496, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giardina JB, Green GM, Cockrell KL, Granger JP, Khalil RA. TNF-α enhances contraction and inhibits endothelial NO-cGMP relaxation in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol 283: R130–R143, 2002 [DOI] [PubMed] [Google Scholar]
- 29.González-Fernández F, Jiménez A, López-Blaya A, Velasco S, Arriero MM, Celdrán Á, Rico L, Farré J, Casado S, López-Farré A. Cerivastatin prevents tumor necrosis factor-α-induced downregulation of endothelial nitric oxide synthase: role of endothelial cytosolic proteins. Atherosclerosis 155: 61–70, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA 92: 8115–8119, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986 [DOI] [PubMed] [Google Scholar]
- 32.Gunnett CA, Heistad DD, Berg DJ, Faraci FM. IL-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am J Physiol Heart Circ Physiol 279: H1555–H1562, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Gunnett CA, Heistad DD, Faraci FM. Interleukin-10 protects nitric oxide-dependent relaxation during diabetes: role of superoxide. Diabetes 51: 1931–1937, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Haddy N, Sass C, Droesch S, Zaiou M, Siest G, Ponthieux A, Lambert D, Visvikis S. IL-6, TNF-α and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis 170: 277–283, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem 269: 3125–3128, 1994 [PubMed] [Google Scholar]
- 36.Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell specific NF-κB suppression attenuates hypertension-induced renal damage. Circ Res 101: 268–276, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Henkel T, Zabel U, van Zee K, Müller JM, Fanning E, Baeuerle PA. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-κB subunit. Cell 68: 1121–1133, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol 280: H2462–H2469, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Huber TS, Gaines GC, Welborn MB, 3rd, Rosenberg JJ, Seeger JM, Moldawer LL. Anticytokine therapies for acute inflammation and the systemic inflammatory response syndrome: IL-10 and ischemia/reperfusion injury as a new paradigm. Shock 13: 425–434, 2000 [DOI] [PubMed] [Google Scholar]
- 39a.Jojima T, Suzuki K, Hirama N, Uchida K, Hattori Y. Glimepiride upregulates eNOS activity and inhibits cytokine-induced NF-κB activation through a phosphoinoside 3-kinase-Akt-dependent pathway. Diabetes Obes Metab 11: 143–149, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS., Jr Inhibition of NF-kappa B activity by thalidomide through suppression of Ikappa B kinase activity. J Biol Chem 276: 22382–22387, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Koch W, Kastrati A, Böttiger C, Mehilli J, von Beckerath N, Schömig A. Interleukin-10 and tumor necrosis factor gene polymorphisms and risk of coronary artery disease and myocardial infarction. Atherosclerosis 159: 137–144, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 43: 562–571, 1999 [DOI] [PubMed] [Google Scholar]
- 43.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 46: 82–86, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Libby P, Bakris G, Dunlap M, Mason P. Effect of endothelial dysfunction in cardiovascular disease. Cardiol Rev 23: 136–141, 2006 [Google Scholar]
- 45.Mazighi M, Pelle A, Gonzalez W, Mtairag EM, Philippe M, Henin D, Michel JB, Feldman LJ. IL-10 inhibits vascular smooth muscle cell activation in vitro and in vivo. Am J Physiol Heart Circ Physiol 287: H866–H871, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Modur V, Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Cermaide-dependent and independent mitogen activated protein kinase. J Biol Chem 271: 13094–13102, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Mombouli JV, Vanhoutte PM. Endothelial dysfunction: from physiology to therapy. J Mol Cell Cardiol 31: 61–74, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Omar HA, Cherry PD, Mortelliti MP, Burke-Wolin T, Wolin MS. Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation. Circ Res 69: 601–608, 1991 [DOI] [PubMed] [Google Scholar]
- 50.Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 18: 6853–6866, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Park SH, Kim KE, Hwang HY, Kim TY. Regulatory effect of SOCS on NF-κB activity in murine monocytes/macrophages. DNA Cell Biol 22: 131–139, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-α and increased risk of recurrent coronary events after myocardial infarction. Circulation 101: 2149–2153, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101: 1767–1772, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens 4: 700–708, 1991 [DOI] [PubMed] [Google Scholar]
- 55.Romano MF, Lamberti A, Petrella A, Bisogni R, Tassone PF, Formisano S, Venuta S, Turco MC. IL-10 inhibits nuclear factor-kappa B/Rel nuclear activity in CD3-stimulated human peripheral T lymphocytes. J Immunol 156: 2119–2123, 1996 [PubMed] [Google Scholar]
- 56.Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med 173: 699–703, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schinzari F, Armuzzi A, De Pascalis B, Mores N, Tesauro M, Melina D, Cardillo C. Tumor necrosis factor-α antagonism improves endothelial dysfunction in patients with Crohn's disease. Clin Pharmacol Ther 83: 70–76, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Schulze-Osthoff K, Beyaert R, Vandevoorde V, Haegeman G, Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF-α. EMBO J 12: 3095–3104, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schutze S, Berkovic D, Tomsing O, Unger C, Kronke M. Tumor necrosis factor induces rapid production of 1′2′diacylglycerol by a phosphatidylcholine-specific phospholipase C. J Exp Med 174: 975–988, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sewnath ME, Olszyna DP, Birjmohun R, ten Kate FJW, Gouma DJ, van der Poll T. IL-10-deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. J Immunol 166: 6323–6331, 2001 [DOI] [PubMed] [Google Scholar]
- 60a.Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-α, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol 58: 21–30, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Spyridopoulos I, Principe N, Krasinski KL, Xu Sh, Kearney M, Magner M, Isner JM, Losordo DW. Restoration of E2F expression rescues vascular endothelial cells from tumor necrosis factor-α-induced apoptosis. Circulation 98: 2883–2890, 1998 [DOI] [PubMed] [Google Scholar]
- 62.St Clair EW. Interleukin 10 treatment for rheumatoid arthritis. Ann Rheum Dis 58: I99–I102, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol 102: 20–25, 1995 [DOI] [PubMed] [Google Scholar]
- 65a.Waehre T, Halvorsen B, Damås JK, Yndestad A, Brosstad F, Gullestad L, Kjekshus J, Frøland SS, Aukrust P. Inflammatory imbalance between IL-10 and TNF-α in unstable angina potential plaque stabilizing effects of IL-10. Eur J Clin Invest 32: 803–810, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Wang P, Ba ZF, Chaudry IH. Administration of tumor necrosis factor-α in vivo depresses endothelium-dependent relaxation. Am J Physiol Heart Circ Physiol 266: H2535–H2541, 1994 [DOI] [PubMed] [Google Scholar]
- 67.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor-κB (NF-κB) activation in human monocytes. J Biol Chem 270: 9558–9563, 1995 [DOI] [PubMed] [Google Scholar]
- 68.Yamaoka J, Kabashima K, Kawanishi M, Toda KI, Miyachi Y. Cytotoxicity of IFN-γ and TNF-α for vascular endothelial cell is mediated by nitric oxide. Biochem Biophys Res Commun 291: 780–786, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Yan G, You B, Chen SP, Liao JK, Sun J. Tumor necrosis factor-α downregulates endothelial nitric oxide synthase mRNA stability via translation elongation factor 1-α 1. Circ Res 103: 591–597, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zabel U. HT, Silva MS, Baeuerle PA. Nuclear uptake control of NF-kappa B by MAD-3, an I kappa B protein present in the nucleus. EMBO J 12: 201–211, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91: 243–252, 1997 [DOI] [PubMed] [Google Scholar]
- 72.Zemse SM, Hilgers RH, Simkins GB, Rudic RD, Webb RC. Restoration of endothelin-1-induced impairment in endothelium-dependent relaxation by interleukin-10 in murine aortic rings. Can J Physiol Pharmacol 86: 557–565, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang DX, Yi FX, Zou AP, Li PL. Role of ceramide in TNF-α-induced impairment of endothelium-dependent vasorelaxation in coronary arteries. Am J Physiol Heart Circ Physiol 283: H1785–H1794, 2002 [DOI] [PubMed] [Google Scholar]