Abstract
The prevalence of left ventricular hypertrophy (LVH) is frequent in patients with end-stage renal disease following chronic renal failure (CRF). We investigated the therapeutic efficacy of curcumin, the principal curcuminoid of the Indian curry spice turmeric, in attenuation of LVH and sought to delineate the associated signaling pathways in blunting the hypertrophic response in nephrectomized rats. Adult Sprague-Dawley rats underwent nephrectomy (Nx) by removal of 5/6 of the kidneys. Four groups were studied for 7 wk: 1) control (sham), 2) Nx, 3) Nx + curcumin (150 mg/kg bid), and 4) Nx + enalapril (15 mg/kg bid) as positive control. Subtotal nephrectomy caused renal dysfunction, as evidenced by a gradual increase in proteinuria and elevation in blood urea nitrogen and plasma creatinine. Nx rats showed a significant hypertrophic response and increased diameter of inferior vena cava at inspiration, which was inhibited by treatment with curcumin or enalapril. Moreover, the Nx rats demonstrated changes in the signaling molecules critically involved in the hypertrophic response. These include increased glycogen synthase kinase-3β phosphorylation, β-catenin expression, calcineurin, phosphorylated (p) nuclear factor of activated T cells, pERK, and p-cAMP-dependent kinase. Both curcumin and enalapril variably but effectively deactivated these pathways. Curcumin attenuates cardiac hypertrophy and remodeling in nephrectomized rats through deactivation of multiple hypertrophic signaling pathways. Considering the safety of curcumin, these studies should facilitate future clinical trials in suppressing hypertrophy in patients with CRF.
Keywords: remnant, uremia, five-sixths nephrectomy
cardiac hypertrophy is one of the most common cardiac abnormalities in uremic patients and represents a leading cause of death (38). Experimental studies have also shown similar cardiovascular abnormalities in nephrectomized rats, the animal model of chronic uremia (16). The classical hypertrophy inducers, including endothelin, catecholamines, and ANG II, are known to play a major role in clinical and experimental uremia (3, 16). We and others have previously shown that uremic rats with chronic renal failure develop significant cardiac hypertrophy (2, 16). Two important mechanisms of cardiac hypertrophy have been described, which include glycogen synthase kinase-3β (GSK-3β)/β-catenin and calcineurin/nuclear factor of activated T cell (NFAT) pathways (43, 46). It has been shown that hypertrophic stimuli promote phosphorylation (inactivation) of GSK-3β via cAMP-dependent kinase (Akt) (48) and ERK phosphorylation (14). Constitutively active GSK-3β remains dephosphorylated and prevents hypertrophic growth of cardiac myocytes by inhibiting transcriptional regulators, including β-catenin and NFAT (43). GSK-3β acts by both the noncanonical pathway, such as calcineurin/NFAT, and canonical Wnt signaling involving β-catenin (46). Calcineurin regulates pathological hypertrophy through dephosphorylation of NFAT, which results in its nuclear translocation and modulation of gene expression (7). GSK-3β is also capable of modulating NFAT phosphorylation and subsequent nuclear migration of the transcription factor (6, 33).
Curcumin (diferuloylmethane) is a yellow pigment in the spice turmeric (also called curry powder) that has been used for centuries as a treatment for inflammatory diseases. Extensive research within the past two decades has shown that curcumin exerts its anti-inflammatory effects through the downregulation of inflammatory transcription factors (e.g., NF-κB), enzymes (e.g., cyclooxygenase 2 and 5-lipoxygenase), and cytokines (e.g., tumor necrosis factor, interleukin-1, and interleukin-6) (4). Recent studies also show that curcumin blocks cardiac hypertrophy in Dahl salt-sensitive rats (32). Moreover, we demonstrated that curcumin ameliorated chronic renal failure, which was comparable to the angiotensin-converting enzyme inhibitor (ACEI) enalapril (17). One of the downstream targets of Akt is GSK-3β. In leukemic cells, curcumin has been shown to inhibit GSK-3β phosphorylation by blocking Akt (45). Moreover, β-catenin is regulated by GSK-3β, and curcumin has been shown to induce degradation of β-catenin (25). In addition, it has been demonstrated that cardioprotection with curcumin was mediated by interruption of p300-histone acetyltransferase activity-dependent signaling pathways, resulting in protection against the deleterious effects of cardiac hypertrophy, inflammation, and fibrosis (32).
In the present study, we hypothesized that chronic renal failure induced by partial nephrectomy causes cardiac hypertrophy through mechanisms involving deactivation of multiple signaling pathways. These include GSK-3β/catenin, calcineurin/NFAT, Akt, and ERK1/2. Because several of the downstream targets of Akt phosphorylation can be inhibited by curcumin (39, 50), we further conjectured that curcumin would be effective in blunting hypertrophy in this model. Because ANG II is a known mediator of cardiac hypertrophy and ACEIs are known to reduce cardiac hypertrophy (2, 9), we concurrently compared the effect of enalapril in attenuating hypertrophy and the associated signaling pathways.
MATERIALS AND METHODS
Chemicals.
Curcumin was purchased from Biomol. All antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-GSK-3β was purchased from Cell Signaling (Cell Signaling Technology, Danvers, MA).
Animals.
The animal procedures were approved by the Institutional Animal Care and Use Committees of the Virginia Commonwealth University.
Surgical procedures.
All surgical procedures were carried out using sterile conditions under isoflurane anesthesia. Chronic renal failure was induced in rats by performing five-sixths (5/6) nephrectomy as described previously by Ghosh et al. (16). Rats weighing between 150 and 200 g were anesthetized, and a left flank incision was made to expose the left kidney. The renal artery was temporarily occluded, and the upper and lower thirds of the kidney were ligated and excised, thereby leaving only 1/3 of the mass of the left kidney intact. Bleeding was controlled by compression until it stopped. The muscle and skin incisions were sutured with polypropylene suture. The animals were returned to the vivarium for recovery. Later (1 wk), a right flank incision was made, the renal vessels and ureter were tied, and the right kidney was excised. The animals were allowed to recover, and the drug treatment was initiated 7 days later.
Experimental groups.
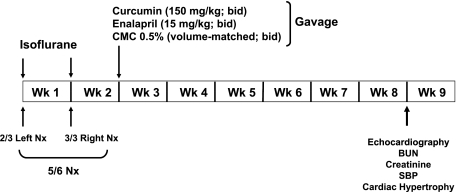
Rats were randomized into four groups (n = 6/group) as shown in Fig. 1. The control group underwent sham surgery, and the other three groups underwent 5/6 nephrectomy as described above. Before surgery, the animals were kept overnight in metabolic cages, and urine was collected for determining proteinuria. The animals were kept in the metabolic cages at the beginning of weeks 3, 5, and 7 for urine collection.
Fig. 1.
Experimental protocol. Arrows indicate time points for treatment, performance of surgical procedures, and measurement of various parameters. CMC, carboxymethylcellulose; Nx, nephrectomized; BUN, blood urea nitrogen; SBP, systolic blood pressure.
Treatment protocol.
Suspension of curcumin and enalapril was prepared in 0.5% carboxymethylcellulose (CMC). Because of the instability of curcumin in the aqueous solution, it was prepared fresh and was administered within 10 min of the preparation. As shown in Fig. 1, the nephrectomized (Nx) rats were randomized to receive the following treatments: 1) vehicle (0.5% CMC), which was administered by oral gavage; 2) curcumin at a dose of 150 mg/kg daily; or 3) enalpril (10 mg/kg, suspended in CMC). The control group consisted of sham-operated rats where the capsule was removed. On the ninth week, the animals were killed, and blood as well as heart tissue were collected for analyses.
Determination of cardiac mass.
Cardiac mass was assessed by measuring right and left ventricle wet weight (RVW and LVW, respectively). The RVW- or LVW-to-body weight ratio was calculated.
Echocardiography.
Echocardiography was performed using the Vevo770 imaging system (VisualSonics, Toronto, Canada) before surgery (baseline) and 8 wk after surgery before killing the animal. Pentobarbital (30 mg/kg ip) was used for anesthesia, and the procedure was carried out as previously described (37) to measure left ventricular (LV) end-diastolic diameter (LVEDD) and end-systolic diameter. Inferior vena cava (IVC) diameter (in mm) was measured at end-inspiration by echocardiography using a subcostal approach. This technique provides simple and useful measurements of IVC size and function in cardiac disease (36) and is reflective of right atrial pressure, which increases with heart failure.
Measurement of blood urea nitrogen and creatinine.
Blood urea nitrogen (BUN) and creatinine were measured by a NOVA16 autoanalyzer (NOVA Biomedical, Waltham, MA).
Longitudinal measurement of arterial pressure by tail plethysmography.
Arterial blood pressure (BP) was determined by tail plethysmography as stated before (17), using the CODA 2 system (Kent Scientific, Torrington, CT). CODA 2 utilizes volume pressure recording sensor technology to measure rat tail blood pressure. This is a computerized, noninvasive tail cuff acquisition system that can simultaneously measure systolic, diastolic, and mean arterial pressure without operator intervention. Before surgery, rats were trained for 3 days and were kept in a restraining holder for a 5- to 10-min period. On the fourth day, BP was recorded (week 0). During this period, 25 sequential readings were obtained. Values within a range of 10 mmHg were averaged. After the second surgery (2 wk), the animals were retrained, and BP was recorded (week 2). Similar BP recording was done on weeks 5 and 8 postsurgery.
Preparation of LV homogenate.
The LV was dissected, immediately frozen in liquid nitrogen, and stored at −70°C until use. The frozen ventricle was ground to a powder and distributed into three batches. One batch was used for RNA extraction (given below), and the other two batches were mixed in ice-cold HEPES buffer in the presence and absence of phosphatase inhibitors (10 mM HEPES, 0.2% Triton X-100, 50 mM NaCl, 0.5 mM sucrose, 0.1 mM EDTA, protease inhibitors, with or without phosphatase inhibitors) and homogenized with an ice-chilled dounce homogenizer at 4°C. Preparations with phosphatase inhibitors were used to measure phosphorylated proteins. An aliquot of the homogenate was stored, and the rest was used to prepare cytosolic and nuclear extracts. The homogenate was spun at 10,000 rpm for 10 min, and the supernatant was separated into aliquots and stored at −70°C as cytosolic extract. The pellet was suspended in ice-cold buffer (10 mM HEPES, 500 mM NaCl, 10% glycerol, 0.1 mM EDTA, 0.1 mM EGTA, 0.1% IGEPAL, and protease inhibitors, with or without phosphatase inhibitors), vortexed at 4°C for 15 min, and centrifuged for 10 min at 14,000 rpm. The resulting supernatant was separated into aliquots and stored as nuclear extract at −70°C. Absence of cross-reactivity with β-actin in Western blots confirmed the purity of nuclear extracts. A small aliquot of kidney homogenate, cytosol, and nuclear extract was used for protein estimation.
Immunoblotting.
The cytosol (75–100 μg total protein) and nuclear extracts (50 μg total protein) were separated on a 4–20% SDS-PAGE, and proteins were transferred to a polyvinylidene difluoride membrane as described previously (16). After being washed briefly in PBS containing 1% Tween 20 (PBS-T) and blocked in 5% nonfat dry milk, blots were incubated with appropriate antibodies in 5% nonfat dry milk overnight at 4°C. The membranes were washed three to five times in PBS-T and subsequently incubated with appropriate secondary antibody diluted in 5% nonfat dry milk for 1 h at room temperature. After three to five washes in TBS, blots were developed using Lightning Chemiluminescence Reagent Plus and exposed to X-ray film.
Quantitative real-time RT-PCR analysis.
Total RNA was extracted from the LV with the RNeasy Mini Kit and analyzed by real-time RT-PCR as described before (16). Briefly, 2 μg RNA was reverse transcribed with a Thermoscript RT-PCR System (Invitrogen), and first-strand cDNA was used to perform real-time PCR using a Stratagene Mx3000p real-time PCR system with TaqMan Gene Expression Assays for GSK-3β (Rn00583429), β-catenin (Rn00584431), calcineurin (Rn00820912), NFAT (Rn01426728), and β-actin obtained from Applied Biosystems (Foster City, CA). The amount of mRNA was calculated by the ΔΔCT method and normalized to β-actin.
Activation of NFAT.
NFAT DNA-binding activity was assessed with a TransAM NFAT transcription factor assay kit (Active Motif) using nuclear extracts from the kidney. Briefly, nuclear extracts were added to each well of a 96-stripwell plate to which the consensus NFAT binding site oligonucleotide had been immobilized. A primary antibody specific for an epitope on the bound and active form of the transcription factor was then added, followed by subsequent incubation with secondary antibody and developing solution. Intensity of the developed color was quantified and is reported as a measure of activated and DNA-bound NFAT in the tested nuclear extract.
Statistical analysis.
Statistical comparisons among groups were performed using ANOVA followed by Tukey's Multiple Comparison Test. Groups were considered to be significantly different with a P value ≤0.05.
RESULTS
Effect of curcumin and enalapril on systolic blood pressure, proteinuria, plasma creatinine, and BUN.
As shown in Table 1, the systolic blood pressure (SBP) of Nx animals measured at 3, 5, and 7 wk was significantly higher than control rats (P < 0.01). Longitudinal measurement of SBP showed that enalapril treatment effectively curtailed the increase in SBP (P < 0.01). Curcumin had no significant effect on SBP until the seventh week. Subtotal nephrectomy resulted in renal dysfunction, as evidenced by a gradual increase in proteinuria (Table 1) and elevation in BUN and plasma creatinine measured at 8 wk (Table 2). Moreover, Nx animals demonstrated progressive increase in proteinuria. Both curcumin and enalapril treatment significantly reduced proteinuria by 40–60%. The proteinuria in curcumin and enalapril cohorts was not significantly different. BUN and plasma creatinine were almost fourfold higher in Nx animals than the vehicle control at 8 wk. Curcumin was as effective as enalapril in reducing both the BUN and creatinine levels (P < 0.01). These data suggest that curcumin is as effective as enalapril in attenuating renal dysfunction in NX animals.
Table 1.
Changes in systolic blood pressure and proteinuria in sham-operated control rats, 5/6 Nx, curcumin-treated 5/6 Nx, and enalapril-treated 5/6 Nx rats
| Week | Control | Nx | Curcumin | Enalapril |
|---|---|---|---|---|
| Systolic blood pressure, mmHg | ||||
| 0 | 119 ± 5.7 | 120 ± 3.1 | 123 ± 3.8 | 121 ± 3.5 |
| 3 | 117 ± 4.4 | 146 ± 6.3a | 135 ± 15.1 | 121 ± 2.5b |
| 5 | 122 ± 3.9 | 151 ± 7.9a | 145 ± 11.2 | 125 ± 7.2b |
| 7 | 124 ± 7.8 | 157 ± 7.2a | 145 ± 4.5c | 130 ± 3.7b |
| Proteinuria, mg/24 h | ||||
| 0 | 25.1 ± 5.1 | 23.4 ± 4.2 | 27.3 ± 5.1 | 23.7 ± 7.1 |
| 3 | 23.5 ± 6.8 | 139.7 ± 60.1a | 66.8 ± 14.5b | 60.4 ± 16.8b |
| 5 | 34.3 ± 12.6 | 316.1 ± 88.4a | 176.7 ± 81.5b | 200.8 ± 38.6c |
| 7 | 33.1 ± 13.7 | 543.6 ± 109.7a | 213.0 ± 79.5b | 220.4 ± 44.6b |
The values are means ± SD of 6–8 animals/group. Nx, nephrectomized.
P < 0.001 compared with control.
P < 0.01 compared with Nx.
P < 0.05 compared with Nx.
Table 2.
Changes in physiological and biochemical parameters in sham-operated control rats, 5/6 Nx, curcumin-treated 5/6 Nx, and enalapril-treated 5/6 Nx rats
| Cont | Nx | Curc | Enap | |
|---|---|---|---|---|
| Body wt, g | 409.6 ± 29.6 | 318.3 ± 13.8a | 339.8 ± 25.4a | 324.8 ± 26.5a |
| LV, g | 1.13 ± 0.14 | 1.34 ± 0.24d | 0.83 ± 0.10c | 0.89 ± 0.09c |
| LV/body wt | 0.0028 ± 0.0004 | 0.0042 ± 0.0009b | 0.0025 ± 0.0004c | 0.0028 ± 0.0003c |
| RV, g | 0.15 ± 0.04 | 0.20 ± 0.04 | 0.13 ± 0.03 | 0.14 ± 0.03 |
| RV/body wt | 0.00037 ± 0.00010 | 0.00064 ± 0.00014b | 0.00040 ± 0.00010d | 0.00042 ± 0.00014d |
| Creatinine, mg/dl | 0.49 ± 0.13 | 1.92 ± 0.18a | 0.95 ± 0.14c | 0.88 ± 0.1c |
| BUN, mg/dl | 17.6 ± 1.13 | 66.6 ± 15.6a | 39.8 ± 4.7c | 42.5 ± 7c |
| Left kidney wt, g | 1.2 ± 0.09 | 1.5 ± 0.25 | 1.3 ± 0.42 | 1.3 ± 0.22 |
The values are means ± SD of 6–8 animals/group. Cont, control; Curc, curcumin-treated 5/6 Nx; Enap, enalapril-treated 5/6 Nx; LV, left ventricular weight; RV, right ventricular weight; BUN, blood urea nitrogen.
P < 0.001 compared with control.
P < 0.01 compared with control.
P < 0.01 compared with Nx.
P < 0.05 compared with Nx.
Effect of nephrectomy on kidney weight.
Nephrectomized animals had their right kidney removed, therefore, the left kidney of the control was compared with the 2/3 excised left kidney of the nephrectomized animals. As shown in Table 2, there was no significant difference in kidney weight between the groups. However, it is to be noted that the control animals had intact kidney, and the partially ablated left kidney of the Nx group and treated animals (whose left kidney was also partially ablated) had hypertrophied to compensate for the nephron loss. Therefore, there was no significant difference in the weight.
Effect of curcumin and enalapril on cardiac remodeling.
The body weights of curcumin- and enalapril-treated rats were lower by 17 and 21%, respectively, compared with the corresponding vehicle control group (P < 0.001, Table 2). LV weights of control and Nx rats were not different, i.e., 1.13 ± 0.14 vs. 1.34 ± 0.24 g, P > 0.05. LV weight in rats treated with curcumin and enalapril were lower compared with Nx (P < 0.01). The LV-to-body weight ratios for curcumin- and enalapril-treated animals were not different from control, but they were significantly lower than Nx animals (P < 0.001). The average RV-to-body weight ratio of Nx animals was 1.7-fold higher than the control (P < 0.01). Both curcumin and enalapril treatment significantly improved the RV-to-body weight ratio (P < 0.05).
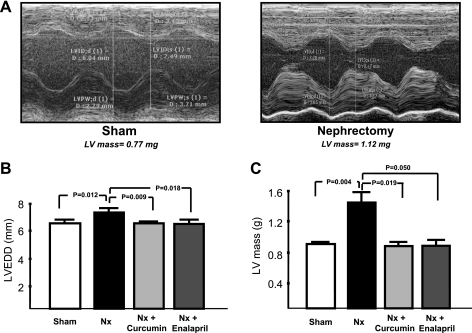
Figure 2A shows representative M-mode images from sham-operated and Nx rats at 8 wk. These images clearly show more dilatation and wall thickening in Nx rats compared with sham control. The average data (in mm) showed a significant increase in LV mass in Nx rats (1.47 ± 0.19) compared with controls (0.98 ± 0.04, P < 0.01, Fig. 2B). Curcumin- and enalapril-treated rats had LV mass of 0.95 ± 0.1 (P < 0.01) and 0.93 ± 0.3 (P < 0.05), respectively. LVEDD (in mm) was higher in Nx rats (7.2 ± 0.2) compared with controls (6.4 ± 0.2, P = 0.01). Curcumin and enalapril attenuated LV dilatation as shown by EDD of 6.3 ± 0.5 and 6.2 ± 0.5, respectively (P = 0.01, Fig. 2C).
Fig. 2.
Doppler echocardiography showing left ventricular (LV) function in rats. A: representative M-mode images of LV from sham-operated and 5/6 Nx rats at 8 wk. Note that there is more LV dilatation and wall thickening in Nx rats compared with sham control. B: average data showing left ventricular end-diastolic diameter (LVEED, m). C: LV mass. Note that there is a significant increase in LVEDD and LV mass in Nx rats compared with sham control. Curcumin- and enalapril-treated rats had significantly lower LV mass.
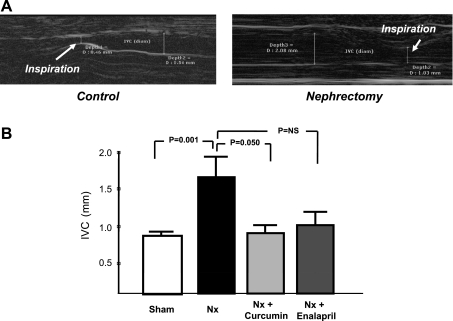
Using a subcostal approach, we also measured changes in IVC diameter at end-inspiration, which is a diagnostic tool for identification of increased right atrial pressure and congestive heart failure. As shown in Fig. 3A, IVC diameter decreased with inspiration in both the control and Nx rats. However, the IVC diameter at end-inspiration was significantly higher in the Nx rats compared with the sham control. Furthermore, rats treated with curcumin or enalapril demonstrated significantly smaller IVC diameter compared with the untreated Nx controls (Fig. 3B).
Fig. 3.
Changes of diameter of the inferior vena cava (IVC) at end-inspiration, as measured by echocardiography using a subcostal approach. A: representative images of sham control and Nx rats. B: average IVC diameter data. Note that end-inspiration IVC diameter is significantly higher in Nx rats compared with the sham control. Treatment with curcumin or enalapril significantly attenuated the increase in end-inspiration IVC diameter, reflective of lower right atrial pressure. NS, not significant.
Effect of curcumin and enalapril on GSK-3β.
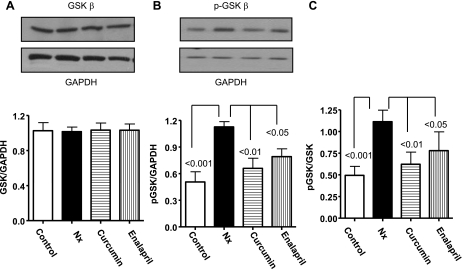
GSK-3β is among the serine/threonine kinases. It is inactivated by hypertrophic stimuli through phosphorylation, and inactive GSK-3β is responsible for cardiac hypertrophy (29). Our results show no significant changes in GSK-3β mRNA (data not shown) and nonphosphorylated GSK-3β (total) protein between the groups (Fig. 4A). However, pGSK-3β in Nx hearts was almost twofold higher compared with the controls (P < 0.001, Fig. 4B). Moreover, the phosphorylated (p) GSK-3β-to-GSK-3β ratio was 2.2-fold higher in Nx (P < 0.001) compared with controls, demonstrating inactivation of the protein (Fig. 4C). A significantly lower pGSK-3β was observed in curcumin (44%, P < 0.01)- and enalapril (30%, P < 0.05)-treated animals.
Fig. 4.
Western blot showing glycogen synthase kinase-3β (GSK-3β) expression in LV from the experimental groups. Note the enhancement in GSK-3β phosphorylation with 5/6 Nx and its attenuation by treatment with curcumin and enalapril. The summary results are shown as the ratio of total GSK-3β/glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphorylated (p) GSK-3β/GAPDH, and pGSK-3β/GSK-3β.
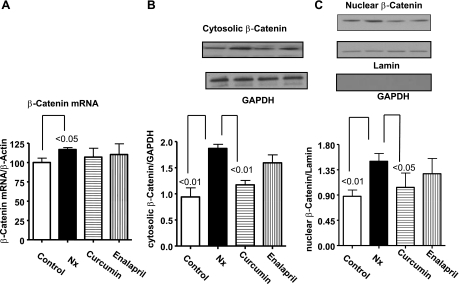
Effect of curcumin and enalapril on β-catenin expression.
β-Catenin is a downstream target of GSK-3β, and its overexpression is known to induce cardiomyocyte growth in vivo and in vitro (20). β-Catenin mRNA, measured by real-time PCR, was increased in Nx animals compared with controls (P < 0.05). Curcumin and enalapril treatment had no effect on the mRNA levels (Fig. 5A). The cytosolic β-catenin was significantly higher in Nx animals compared with controls (P < 0.01) and was reduced by curcumin treatment (P < 0.05, Fig. 5B). Similarly, β-catenin was significantly higher in the nuclear fractions compared with controls (P < 0.01, Fig. 5C) and was decreased by curcumin. In the Nx animals, the β-catenin levels in total homogenates were 1.4-fold higher than the controls, but this was not statistically significant (data not shown). Enalapril treatment had no significant effect on β-catenin protein expression.
Fig. 5.
The effect of curcumin and enalapril on changes in β-catenin in LV from sham and 5/6 Nx rats. mRNA (A), cytosolic (B), and nuclear (C) levels, normalized with lamin. Note that 5/6 Nx caused significant increase in mRNA and cytosolic as well as nuclear levels of β-catenin. Curcumin significantly reduced β-catenin in the cytosol and nuclear fraction without having any effect on the mRNA. Enalapril had no effect on β-catenin. The nuclear fraction was devoid of GAPDH signal, suggesting that the nuclear extracts were free from cytosolic contamination.
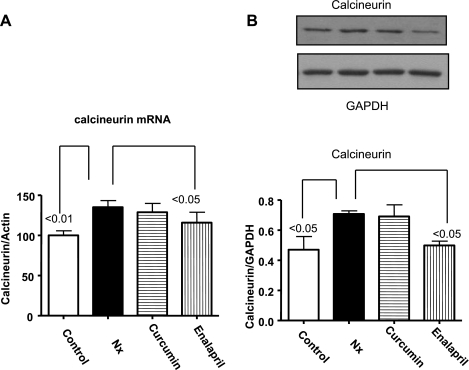
Effect of curcumin and enalapril on calcineurin.
The calcium-activated protein phosphatase calcineurin is a serine- and threonine-specific phosphatase that plays a central role in the development of pathological cardiac hypertrophy (31). ANG II has been shown to activate calcineurin in various settings (40, 41). Calcineurin mRNA (measured by real-time PCR) and protein expression were increased in Nx animals (Fig. 6, A and B) and decreased following treatment with enalapril. However, curcumin had no effect on calcineurin mRNA or protein expression.
Fig. 6.
The effect of curcumin and enalapril on changes in calcineurin in LV from sham and 5/6 Nx rats. mRNA (A), cytosolic (B), and nuclear (C) levels, normalized with lamin. Note that 5/6 Nx caused significant increase in mRNA and cytosolic levels of calcineurin that were significantly reduced by enalapril but not curcumin.
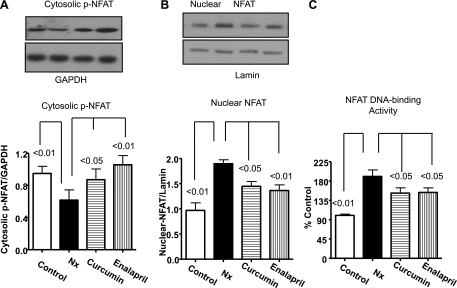
Effect of curcumin and enalapril on NFAT.
Dephosphorylation of NFAT facilitates its nuclear migration and enhanced DNA binding, leading to hypertrophy. Both calcineurin and GSK-3β are known to regulate NFAT phosphorylation and hypertrophy (34, 47). In the present study, we did not observe a significant difference in nonphosphorylated cytosolic NFAT between the groups (data not shown). However, cytosolic pNFAT was significantly decreased in Nx animals compared with control (Fig. 7A) and was significantly attenuated by enalapril and curcumin (P < 0.05). The NFAT in the nuclear fraction of Nx animals was significantly higher compared with the control (P < 0.01, Fig. 7B) and was decreased by curcumin (P < 0.05) and enalapril (P < 0.01). There was no significant difference in the cytosolic and nuclear NFAT between curcumin and enalapril. We also confirmed these results by using NFAT-DNA binding assay (TransAm transcription factor ELISA). As shown in Fig. 7C, NFAT-DNA binding was nearly twofold higher than the control (P < 0.01), and both curcumin and enalapril significantly decreased it (P < 0.05).
Fig. 7.
The effect of curcumin and enalapril on changes in p-nuclear factor of activated T cells (NFAT) and nuclear NFAT in the cytosolic and nuclear fractions of LV from sham and 5/6 Nx rats. Cytosolic (A) and nuclear (B) levels, normalized with lamin. C: DNA binding of NFAT determined by TransAm Assay. Note that 5/6 Nx caused significantly reduced cytosolic levels of pNFAT and increased nuclear NFAT translocation and activity determined by Western blots and DNA-binding assay, respectively. Both curcumin and enalapril reversed this trend.
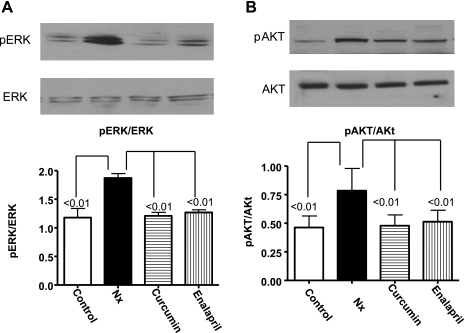
Both Akt and ERK modulate the phosphorylation of GSK-3β and activation of the calcineurin-NFAT pathway (30, 43). We investigated the role of these signaling molecules in the Nx hearts to see if curcumin and enalapril can modify their phosphorylation state. The ratios of pERK/ERK (Fig. 8A) and pAkt/Akt were significantly higher in the Nx animals compared with the controls (P < 0.01, Fig. 8B). Curcumin and enalapril significantly reduced the pERK and pAkt (P < 0.01) without affecting the nonphosphorylated proteins (Fig. 8).
Fig. 8.
The effect of curcumin and enalapril on changes in pERK (A) and p-cAMP-dependent kinase (Akt) (B) from LV of sham and 5/6 Nx rats. The summary results are shown as the ratio of pERK/ERK and pAkt/Akt. Compared with controls, the phosphorylation of both ERK and Akt were significantly higher in Nx animals. Curcumin and enalapril treatment blunted the increase in phosphorylation.
DISCUSSION
Cardiac hypertrophy is an adaptive response that occurs as a result of increased workload. Although the initial hypertrophic response is considered to be beneficial, sustained cardiac hypertrophy often leads to heart failure (22, 24, 28). LV hypertrophy is seen in up to 72% of adults and children with chronic renal failure and end-stage renal disease (18). Cardiac hypertrophy is frequently observed in 5/6 nephrectomy, an animal model for chronic renal failure (16, 27). The present investigation was designed to show the effect of curcumin on attenuation of hypertrophy and to delineate the associated signaling pathways in blunting the hypertrophic response in Nx rats. Our results show that subtotal nephrectomy resulted in renal dysfunction, as evidenced by a gradual increase in proteinuria and elevation in BUN and plasma creatinine. Nx rats showed a significant hypertrophic response and increased end-inspiration IVC diameter, which were inhibited by treatment with curcumin and enalapril. Moreover, the Nx rats demonstrated changes in the signaling molecules that are critically involved in the hypertrophic response. These included increased GSK-3β phosphorylation, β-catenin expression, calcineurin, pNFAT, pERK, and pAkt. Both curcumin and enalapril effectively deactivated most of these pathways triggered by nephrectomy in the heart. Taken together, these results demonstrate excellent protective effect of curcumin, similar to enalapril, in attenuating cardiac hypertrophy in rats with chronic renal failure.
In the present study, we utilized 150 mg·kg−1·day−1 curcumin, which effectively reduced the biochemical markers of renal dysfunction in the Nx group, similar to enalapril treatment. However, curcumin did not have a significant effect on lowering SBP, although both gravimetric and echo analyses demonstrated a significant decrease in cardiac hypertrophy. These data suggest that curcumin modulated cardiac hypertrophy without lowering SBP in Nx mice. Although pressure overload plays an important role in inducing hypertrophy, there can be pressure-independent enhancement of cardiac hypertrophy as well (1, 26), which is clearly ameliorated by curcumin in the uremic rats in this study.
Our results provide strong evidence that curcumin blunted the action of several key signaling molecules implicated in the hypertrophic response. GSK-3β is unique among serine/threonine kinases in that it exists in its active form even in unstimulated cells and gets inactivated by phosphorylation. Active GSK-3β is an endogenous negative regulator of cardiac hypertrophy (21). Increased inactive GSK-3β has been observed in the myocardium of diabetic mice with cardiac hypertrophy (19). Nonphosphorylated GSK-3β controls positive mediators of hypertrophy, including β-catenin (20) and NFAT (6), thereby imposing a negative constraint on the prohypertrophic mechanisms. Our results show a significant increase in the ratio of pGSK-3β/GSK-3β in the LV of untreated Nx animals, indicating an abundance of the inactive form of this protein. The pGSK-3β was reduced by curcumin and enalapril, suggesting an important role of GSK-3β in regulating hypertrophy with these drugs. It has been shown that the ANG II receptor antagonist losartan abrogates hypertrophy by antagonizing GSK-3β inactivation (19). Curcumin or enalapril treatment did not affect GSK-3β mRNA, which suggests that these drugs are affecting hypertrophy by posttranscriptional modification of GSK-3β. Moreover, curcumin has been shown to prevent phosphorylation of GSK-3β by Akt (45).
The role of Akt and ERK in the development of cardiac hypertrophy has been established with the use of transgenic mice overexpressing Akt and ERK (10, 13). Both Akt and ERK regulate cardiac hypertrophy by modulating phosphorylation of downstream proteins, including GSK-3β, β-catenin, and NFAT (23). A significant increase in the phosphorylation of both Akt and ERK in the Nx animals was observed, which was effectively blocked by curcumin and enalapril. Hypertrophic stimuli stabilize β-catenin in cardiomyocytes, and overexpression of β-catenin induced hypertrophic growth both in vitro and in vivo (20). Moreover, GSK-3β inhibition via phosphorylation of Ser-9 by Akt appears to be the mechanism by which β-catenin is stabilized (20). The cytoplasmic stabilized β-catenin enters the nucleus and regulates hypertrophic gene expression (8, 42). It has been shown that targeted deletion of β-catenin in the heart leads to a blunted hypertrophic response to pathological stress-induced growth (11). Increased cytosolic β-catenin is also observed in renal tubular epithelial cells following injury initiated by unilateral ureteral obstruction (44). In the present study, increased GSK-3β in the Nx animals was associated with augmentation in cytosolic β-catenin, suggesting stabilization of the protein. This was followed by increased nuclear translocation of β-catenin in Nx animals. Because curcumin did not affect β-catenin mRNA levels, it probably decreased the accumulation of cytosolic β-catenin and its subsequent migration to the nucleus by blunting phosphorylation of GSK-3β. Furthermore, curcumin-mediated breakdown of β-catenin (25) might also contribute to the reduced level of this protein in the curcumin-treated animals. Enalapril did not have any effect on β-catenin although it reduced GSK-3β phosphorylation. The exact reason is not clear, but it has been shown that ANG II was able to induce cardiac hypertrophy when inducible cardiomyocyte specific deletion of the β-catenin gene was used. Accordingly, it was suggested that β-catenin is not required for induction of cardiac hypertrophy induced by ANG II (5). Moreover, it has been proposed that the effect of β-catenin on cardiac hypertrophy might be dependent on the hypertrophic stimulus (7).
We also observed a significant increase in calcineurin mRNA and protein expression in the LV of Nx animals, which was inhibited by enalapril. Calcineurin is a protein phosphatase that regulates gene expression associated with pathological hypertrophy. Inhibitors of calcineurin have been reported to prevent cardiac hypertrophy in several experimental models (31). ANG II binds to the angiotensin type 1 receptor and increases the intracellular calcium content. Elevated intracellular calcium concentration, in turn, activates the cytoplasmic calcineurin, which dephosphorylates NFAT (30, 35). In the present study, there was a significant increase in both calcineurin mRNA and protein expression in the Nx animals which was reduced by enalapril but not curcumin. ANG II is known to induce de novo synthesis of calcineurin in neutrophils (15). Moreover, modulation of renal failure by enalpril or ANG II receptor blocker has been shown to decrease cardiac hypertrophy (2, 49). Therefore, it is likely that blunting of ANG II formation by enalapril reduces ANG II-stimulated calcineurin synthesis as well.
NFAT usually resides in the cytosol and is constitutively phosphorylated by active GSK-3β. Dephosphorylation of NFAT by calcineurin results in the nuclear translocation and activation of hypertrophic response (12). Phosphorylation of GSK-3β by Akt and or ERK can inhibit GSK-3β. This reduction in GSK-3β activity allows NFAT to remain in the nucleus for a longer period of time, thereby promoting increased activation of hypertrophic genes (12, 47). A significant decrease of pNFAT in the cytosol with corresponding increase in nuclear NFAT in Nx animals was observed. The increase of dephosphorylated cytosolic NFAT in Nx animals appears to be due to increased calcineurin and pGSK-3β seen in this group. Curcumin and enalapril increased the levels of pNFAT in the cytosol and decreased its nuclear migration. It is to be noted that enalapril blocked both calcineurin and phosphorylation of GSK-3β, whereas curcumin only affected GSK-3β. Consequently, we anticipated that the levels of pNFAT in the enalapril group would be higher than the curcumin-treated group. Although the pNFAT in the cytosol of enalapril animals was 21% higher than the curcumin, it was statistically insignificant. Moreover, there was no significant change in nuclear translocation of NFAT between the groups. Curcumin and enalapril neither affected the mRNA nor the nonphosphorylated GSK-3β and NFAT, suggesting that these compounds might affect cardiac hypertrophy by posttranslational modification of these proteins. As summarized in Fig. 8, curcumin and enalapril both blunt phosphorylation of Akt and ERK, which in turn keeps GSK-3β activated, thereby increasing pNFAT in the cytosol and preventing its nuclear translocation. Enalapril also increases cytosolic pNFAT by blocking the phosphatase calcineurin. However, curcumin but not enalapril can prevent β-catenin-induced hypertrophy.
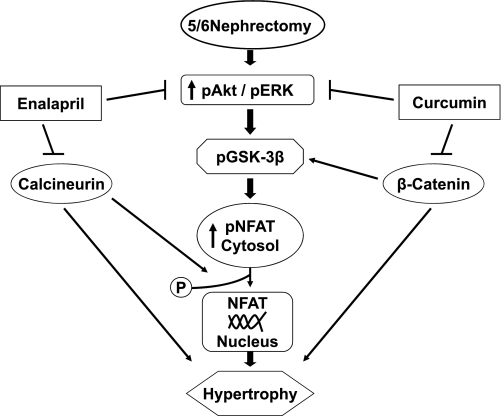
In summary, for the first time, we have demonstrated that curcumin attenuates cardiac hypertrophy and remodeling in nephrectomized rats independent of SBP reduction. Moreover, our results show that curcumin deactivates multiple hypertrophic signaling pathways, including the GSK-3β/catenin, calcineurin/NFAT, Akt, and ERK1/2, as summarized in Fig. 9. Our findings are of tremendous clinical interest given the high prevalence of ventricular hypertrophy in adults and children with chronic renal failure and end-stage renal disease. Because of the safety of curcumin, we believe that these studies would facilitate future clinical trials with this compound in the treatment of hypertrophy in patients with chronic renal failure.
Fig. 9.
Summary of signaling pathways by which curcumin and enalapril attenuate LV hypertrophy 5/6 Nx rats.
GRANTS
This study was supported, in part, by National Heart, Lung, and Blood Institute Grants HL-51045, HL-59469, and HL-79424 to R. C. Kukreja.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Ainscough JF, Drinkhill MJ, Sedo A, Turner NA, Brooke DA, Balmforth AJ, Ball SG. Angiotensin II type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovasc Res 81: 592–600, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Amann K, Gassmann P, Buzello M, Orth SR, Tornig J, Gross ML, Magener A, Mall G, Ritz E. Effects of ACE inhibition and bradykinin antagonism on cardiovascular changes in uremic rats. Kidney Int 58: 153–161, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Amann K, Wanner C, Ritz E. Cross-talk between the kidney and the cardiovascular system. J Am Soc Nephrol 17: 2112–2119, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett 267: 133–164, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, Busjahn A, Huelsken J, Taketo MM, Birchmeier W, Dietz R, Bergmann MW. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res 100: 1353–1362, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275: 1930–1934, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Blankesteijn WM, van de Schans VA, ter Horst P, Smits JF. The Wnt/frizzled/GSK-3 beta pathway: a novel therapeutic target for cardiac hypertrophy. Trends Pharmacol Sci 29: 175–180, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Brade T, Manner J, Kuhl M. The role of Wnt signalling in cardiac development and tissue remodelling in the mature heart. Cardiovasc Res 72: 198–209, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bro S, Bollano E, Bruel A, Olgaard K, Nielsen LB. Cardiac structure and function in a mouse model of uraemia without hypertension. Scand J Clin Lab Invest 68: 660–666, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19: 6341–6350, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Shevtsov SP, Hsich E, Cui L, Haq S, Aronovitz M, Kerkela R, Molkentin JD, Liao R, Salomon RN, Patten R, Force T. The beta-catenin/T-cell factor/lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Mol Cell Biol 26: 4462–4473, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clerk A, Cullingford TE, Fuller SJ, Giraldo A, Markou T, Pikkarainen S, Sugden PH. Signaling pathways mediating cardiac myocyte gene expression in physiological and stress responses. J Cell Physiol 212: 311–322, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA 99: 12333–12338, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 296: H1236–H1243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Bekay R, Alvarez M, Monteseirin J, Alba G, Chacon P, Vega A, Martin-Nieto J, Jimenez J, Pintado E, Bedoya FJ, Sobrino F. Oxidative stress is a critical mediator of the angiotensin II signal in human neutrophils: involvement of mitogen-activated protein kinase, calcineurin, and the transcription factor NF-kappaB. Blood 102: 662–671, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ghosh SS, Krieg RJ, Sica DA, Wang R, Fakhry I, Gehr T. Cardiac hypertrophy in neonatal nephrectomized rats: the role of the sympathetic nervous system. Pediatr Nephrol 24: 367–377, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Ghosh SS, Massey HD, Krieg R, Fazelbhoy ZA, Ghosh S, Sica DA, Fakhry I, Gehr TW. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: role of inflammation. Am J Physiol Renal Physiol 296: F1146–F1157, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Gross ML, Ritz E. Hypertrophy and fibrosis in the cardiomyopathy of uremia–beyond coronary heart disease. Semin Dial 21: 308–318, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Gurusamy N, Watanabe K, Ma M, Prakash P, Hirabayashi K, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Glycogen synthase kinase 3beta together with 14–3-3 protein regulates diabetic cardiomyopathy: effect of losartan and tempol. FEBS Lett 580: 1932–1940, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, Woodgett J, Kilter H, Force T. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci USA 100: 4610–4615, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardt SE, Sadoshima J. Negative regulators of cardiac hypertrophy. Cardiovasc Res 63: 500–509, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Harris DM, Chen X, Pesant S, Cohn HI, MacDonnell SM, Boucher M, Vinge LE, Raake P, Moraca SR, Li D, Most P, Houser SR, Koch WJ, Eckhart AD. Inhibition of angiotensin II Gq signaling augments beta-adrenergic receptor mediated effects in a renal artery stenosis model of high blood pressure. J Mol Cell Cardiol 46: 100–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Izzo JL, Jr, Gradman AH. Mechanisms and management of hypertensive heart disease: from left ventricular hypertrophy to heart failure. Med Clin North Am 88: 1257–1271, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 21: 8414–8427, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Knowles JW, Esposito G, Mao L, Hagaman JR, Fox JE, Smithies O, Rockman HA, Maeda N. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest 107: 975–984, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koleganova N, Piecha G, Ritz E, Gross ML. Calcitriol ameliorates capillary deficit and fibrosis of the heart in subtotally nephrectomized rats. Nephrol Dial Transplant 24: 778–787, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med 110: 101–107, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, Goto K, Takagi H, Tamamori-Adachi M, Kitajima S, Sadoshima J. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci USA 105: 20900–20905, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res 63: 467–475, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Molkentin JD. Calcineurin and beyond: cardiac hypertrophic signaling. Circ Res 87: 731–738, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T, Hasegawa K. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest 118: 868–878, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller MR, Sasaki Y, Stevanovic I, Lamperti ED, Ghosh S, Sharma S, Gelinas C, Rossi DJ, Pipkin ME, Rajewsky K, Hogan PG, Rao A. Requirement for balanced Ca/NFAT signaling in hematopoietic and embryonic development. Proc Natl Acad Sci USA 106: 7034–7039, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neal JW, Clipstone NA. Glycogen synthase kinase-3 inhibits the DNA binding activity of NFATc. J Biol Chem 276: 3666–3673, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Rana OR, Saygili E, Meyer C, Gemein C, Kruttgen A, Andrzejewski MG, Ludwig A, Schotten U, Schwinger RH, Weber C, Weis J, Mischke K, Rassaf T, Kelm M, Schauerte P. Regulation of nerve growth factor in the heart: the role of the calcineurin-NFAT pathway. J Mol Cell Cardiol 46: 568–578, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Rein AJ, Lewis N, Forst L, Gotsman MS, Lewis BS. Echocardiography of the inferior vena cava in healthy subjects and in patients with cardiac disease. Isr J Med Sci 18: 581–585, 1982 [PubMed] [Google Scholar]
- 37.Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, Kukreja RC. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 294: H1398–H1406, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Salvetti M, Muiesan ML, Paini A, Monteduro C, Bonzi B, Galbassini G, Belotti E, Movilli E, Cancarini G, Agabiti-Rosei E. Myocardial ultrasound tissue characterization in patients with chronic renal failure. J Am Soc Nephrol 18: 1953–1958, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol Med 43: 568–580, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saygili E, Rana OR, Meyer C, Gemein C, Andrzejewski MG, Ludwig A, Weber C, Schotten U, Kruttgen A, Weis J, Schwinger RH, Mischke K, Rassaf T, Kelm M, Schauerte P. The angiotensin-calcineurin-NFAT pathway mediates stretch-induced up-regulation of matrix metalloproteinases-2/-9 in atrial myocytes. Basic Res Cardiol 104: 435–448, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Sheng H, Zhu J, Wu X, Yang D, Zhang J. Angiotensin-converting enzyme inhibitor suppresses activation of calcineurin in renovascular hypertensive rats. Hypertens Res 30: 1247–1254, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Shevtsov SP, Haq S, Force T. Activation of beta-catenin signaling pathways by classical G-protein-coupled receptors: mechanisms and consequences in cycling and non-cycling cells. Cell Cycle 5: 2295–2300, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br J Pharmacol 153, Suppl 1: S137–S153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Tomita M, Matsuda T, Kawakami H, Uchihara JN, Okudaira T, Masuda M, Ohshiro K, Mori N. Curcumin targets Akt cell survival signaling pathway in HTLV-I-infected T-cell lines. Cancer Sci 97: 322–327, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.van de Schans VA, Smits JF, Blankesteijn WM. The Wnt/frizzled pathway in cardiovascular development and disease: friend or foe? Eur J Pharmacol 585: 338–345, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Vyas DR, Spangenburg EE, Abraha TW, Childs TE, Booth FW. GSK-3beta negatively regulates skeletal myotube hypertrophy. Am J Physiol Cell Physiol 283: C545–C551, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Yoeli-Lerner M, Chin YR, Hansen CK, Toker A. Akt/protein kinase b and glycogen synthase kinase-3beta signaling pathway regulates cell migration through the NFAT1 transcription factor. Mol Cancer Res 7: 425–432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida K, Xu HL, Kawamura T, Ji L, Kohzuki M. Chronic angiotensin-converting enzyme inhibition and angiotensin II antagonism in rats with chronic renal failure. J Cardiovasc Pharmacol 40: 533–542, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Yu S, Shen G, Khor TO, Kim JH, Kong AN. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther 7: 2609–2620, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]