Abstract
S-nitrosothiols are nitric oxide (NO)-derived molecules found in biological systems. They have been variously discussed as both NO reservoirs and as major actors in NO-dependent, but cGMP-independent, signal transduction. Although S-nitrosation of specific cysteine residues has been suggested to represent a novel redox-based signaling mechanism, the exact mechanisms of S-nitrosothiol formation under (patho)physiological conditions and the determinants of signaling specificity have not yet been established. Here we examined the sensitivity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to inhibition by S-nitrosocysteine (CysNO) and NO both intracellularly and in isolation. Bovine aortic endothelial cells (BAECs) and purified GAPDH preparations were treated with CysNO or NO, and enzymatic activity was monitored. Intracellular GAPDH was irreversibly inhibited upon CysNO administration, whereas treatment with NO resulted in a DTT-reversible inhibition of the enzyme. Purified GAPDH was inhibited by both CysNO and NO, but the inhibition pattern was diametrically opposite to that observed in the cells; CysNO-dependent inhibition was reversed with DTT, whereas NO-dependent inhibition was not. In the presence of GSH, NO inhibited purified GAPDH in a DTT-reversible way. Our data suggest that in response to CysNO treatment, cellular GAPDH undergoes S-nitrosation, which results in an irreversible inhibition of the enzyme under turnover conditions. In contrast, NO inhibits the enzyme via oxidative mechanisms that do not involve S-nitrosation and are reversible. In summary, our data show that GAPDH is a target for CysNO- and NO-dependent inhibition; however, these two agents inhibit the enzyme via different mechanisms both inside the cell and in isolation. Additionally, the differences observed between the cellular system and purified protein strongly imply that the intracellular environment dictates the mechanism of inhibition.
Keywords: nitric oxide, S-nitrosation, S-nitrosocysteine
the observation that S-nitrosocysteine (CysNO) is transported into cells via amino acid transport system L and can modify intracellular protein thiols through a transnitrosation reaction, without the intermediacy of nitric oxide (NO), has provided an important tool that can be used to gain insight into the fundamental mechanisms of NO signaling (33). There is significant literature that has emphasized the role of protein S-nitrosation as the major posttranslational modification involved in cGMP-independent signaling by NO (9). Through relatively unclear mechanisms, NO is thought to S-nitrosate specific protein thiols, resulting in the initiation or the modulation of cellular signaling cascades. Some examples are the S-nitrosation of the Ras oncogene, which has been reported to lead to proliferative responses (14), and the modulation of inflammatory signaling by the inhibition of inhibitory κB kinase activity (23).
Although S-nitrosothiols have been discussed as important NO-dependent thiol modifications, these compounds have been shown to be relatively minor products in chemical systems consisting of NO, thiols, and oxygen, with thiol disulfides being by far the major product (11, 13). In a cellular environment, the level of S-nitrosothiol formed upon the exposure of cells to NO or upon the induction of inducible NO synthase is relatively modest, suggesting that S-nitrosation is a highly inefficient process in cells (32). Rigorous comparisons between the effects of NO and the effects of S-nitrosation on a specific cellular activity have rarely been accomplished, and NO-dependent effects are often ascribed to S-nitrosation with little to no quantitative confirmatory data. We have previously demonstrated clear differences between NO and CysNO in their ability to inhibit caspase 3-like activity in doxorubicin-treated bovine aortic endothelial cells (BEACs) (1). Whereas CysNO appeared to inhibit this enzyme through S-nitrosation, as has been previously proposed, we observed no inhibition upon the exposure of cells to NO. In this study we have extended these observations to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an enzyme that is directly inhibited by NO exposure, to compare mechanisms of NO-dependent and CysNO-dependent inhibition.
GAPDH is a glycolytic enzyme that catalyzes the oxidation and phosphorylation of d-glyceraldehyde-3-phosphate to 1,3-diphosphoglycerate with the conversion of NAD+ to NADH. In addition to this function, GAPDH is involved in gene transcription, RNA transport, and DNA replication (28) and has been shown to translocate to the nucleus during apoptosis (3, 6, 7, 26). GAPDH is a tetramer of four identical 37-kDa subunits and contains four cysteines per subunit. The active site cysteine-149 is characterized by a pKa of 5.5, lower than other cysteines, and has been shown to undergo S-nitrosation in vitro (2, 15, 18). This modification not only reversibly inhibits the enzymatic activity but also promotes irreversible enzyme modification. Although it was initially thought to be ADP ribosylated (4, 18), it was later concluded that the nitrosation of cysteine in the active site facilitated the covalent attachment of the enzyme-bound cofactor NADH, resulting in an irreversible inhibition of the protein activity (18). In addition, S-nitrosation of GAPDH has been proposed to serve as a signal to promote a subsequent translocation to the nucleus (6). When GAPDH undergoes S-nitrosation, its binding affinity toward Siah1 increases and the whole complex translocates to the nucleus. GAPDH increases the stability of Siah1, which is an E3 ubiquitin ligase, and Siah1 participates in the degradation of nuclear proteins and cell death.
We show here that both NO and CysNO inhibit GAPDH in cellular and noncellular environments but that the characteristics of inhibition differ. Specifically, NO inhibits GAPDH irreversibly in isolation but reversibly in cells, whereas CysNO inhibits GAPDH reversibly in isolation but irreversibly in cells. This pattern of inhibition can only be explained if NO-dependent inhibition of GAPDH is oxidative and not nitrosative.
MATERIALS AND METHODS
Materials.
Ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate fluorescence (SBD-F), β-actin antibody, l-cysteine, sodium nitrite, diethylenetriamine pentaacetic acid (DTPA), GSH, N-ethylmaleimide, iodine, mercuric chloride, and protease inhibitor cocktail were purchased from Sigma. (Z)-1-{N-(3-ammoniopropyl)-N-[4-(3-aminopropylammonio)butyl]-amino}diazen-1ium-1,2-diolate (Sper/NO) was from Cayman Chemical (Ann Arbor, MI). GAPDH antibody was purchased from Ambion. Sulfanilamide was supplied by Aldrich. BAECs were purchased from Cambrex. Dulbecco's modified Eagle's medium, streptomycin-penicillin, HEPES buffer solution, phosphate-buffered saline (PBS), trypsin EDTA, and Hanks' balanced salt solution were obtained from Gibco, and fetal bovine serum was obtained from HyClone. S-nitrosoglutathione (GSNO) was synthesized in house as previously described (8).
Cell culture and treatments.
BAECs were routinely cultured as previously described (34). For each experiment, cells were seeded into six-well plates and grown overnight to reach 70–80% confluence unless otherwise mentioned. The medium was removed by double wash with PBS before specific compounds were added to cells either in full medium or in Hanks' balanced salt solution (supplemented with 10 mM HEPES). Cells used in this study were between passages 5 and 12.
GAPDH activity assay.
BAECs were grown on 6-cm dishes; after treatment with specific compounds, cells were washed twice with PBS and scraped into 400 μl Tris·HCl (pH 7.5) containing protease inhibitor cocktail. The activity of GAPDH was examined in a coupled assay by following the decrease in absorbance of NADH at 340 nm. The reaction mixture (1 ml) consisted of 50 mM Tris·HCl (pH 7.5), 5 mM magnesium chloride, 1 mM DTPA, 1 mM ATP, 3 U/ml of 3-phosphoglyceric phosphokinase, and 1 mM 3-phosphoglyceric acid as described by Wilson et al. (31) with minor modifications. When mentioned, DTT (5 mM) was added to the assay buffer. Depending on the experiment, GAPDH activity present in either 50 μl of cell lysate or in 0.1–0.5 μg of purified enzyme was measured. One unit of enzyme is the amount that converts 1 μmol of 3-phosphoglycerate to glyceraldehyde 3-phosphate per minute in a coupled system with 3-phosphoglyceric phosphokinase at room temperature.
S-nitrosothiol determinations.
For the detection of S-nitrosothiols, the cells were washed twice with PBS, and 250 μl of lysis buffer containing 50 mM phosphate, 1 mM DTPA, and 50 mM N-ethylmaleimide (pH 7.4) were added to the cells. The cells were scraped and sonicated (550 Sonic Dismembrator from Fisher Scientific, power level 2 for 15 s), followed by centrifugation (12,000 g for 5 min). The supernatant was used for the measurement of S-nitrosothiols using the triiodide-dependent ozone-based chemiluminescence method as described previously (32, 33). Briefly, the reaction solution was made fresh daily from potassium iodide (28 mg) and I2 (18 mg) in glacial acetic acid (3.75 ml) and double-distilled H2O (1.25 ml). This solution was added into the reaction vessel together with antifoaming agent and maintained at 30°C. Samples were pretreated with 10% (vol/vol) of sulfanilamide (100 mM in 2 N HCl) to remove nitrite. Mercuric chloride (5 mM for 10 min) was used to verify the presence of S-nitrosothiols. A standard curve was generated using GSNO.
Thiol determination.
The HPLC-based SBD-F method was used to measure intracellular thiols (30). Briefly, BAECs were washed twice with PBS and scraped into 200 μl of buffer containing 50 mM phosphate and 1 mM DTPA (pH 7.4), followed by sonication and centrifugation. Sample (25 μl) was mixed with 25 μl of a chilled solution of 10% trichloroacetic acid and centrifuged to precipitate protein. The resulting supernatant (25 μl) was mixed with 75 μl of SBD-F (0.66 mg/ml in 2.5 M borate, pH 9.5) and incubated for 1 h at 60°C. The analysis was performed on a Kromasil C-18 column (5 μm, 250 mm × 4.6 mm ID, Alltech) using solvent A (0.1 M sodium acetate, pH 4, methanol; 98:2), solvent B (0.1 M sodium phosphate, pH 6, methanol; 95:5), and methanol. The chromatographic conditions were as follows: solvent A (100%) graded to solvent B (100%) between 0 and 20 min, methanol (100%, 20–30 min), and solvent A (100%, 30–35 min). The retention time for GSH was 10 min.
Determination of GAPDH protein levels.
The protein levels of GAPDH and β-actin were probed using Western blot analysis after reducing SDS-PAGE. Briefly, the cells were harvested in lysis buffer consisting of 50 mM Tris·HCl (pH 8.0), 150 mM NaCl, 0.5% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS, containing protease inhibitors, and 5 μg of cellular proteins were separated on a 10% SDS-PAGE gel. The levels of GAPDH and β-actin were detected using specific antibodies and visualized with enhanced chemiluminescence.
RESULTS
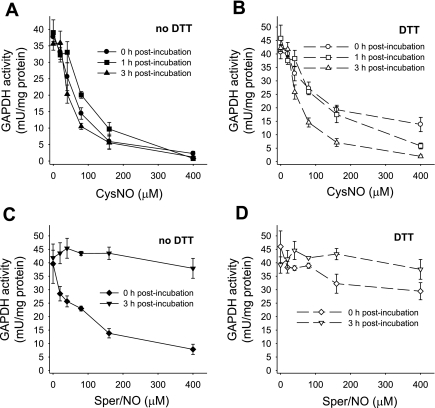
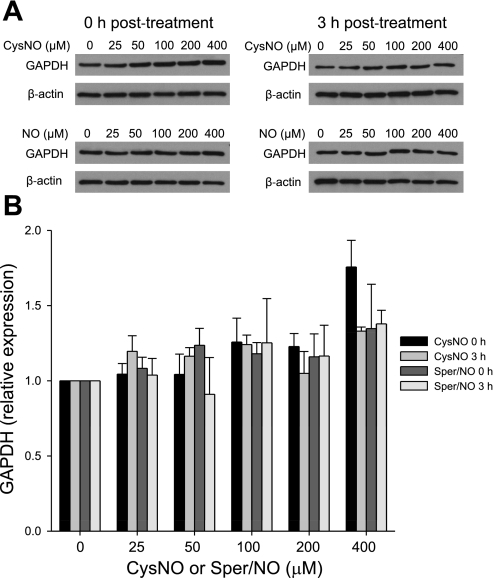
GAPDH has been previously shown to be inhibited in response to NO or S-nitrosothiol treatment (10, 17, 20, 21). Here we characterized the pattern of GAPDH inhibition and distinguished between the effects of S-nitrosothiol transport and NO by treating endothelial cells with either a transportable S-nitrosothiol, CysNO, or with Sper/NO, a NO donor. Sper/NO is a diazenium diolate-class NO donor that released two molecules of NO per parent molecule with a half-life of 39 min at pH 7.4 and 37°C (12). In this series of experiments, we treated the cells with increasing concentrations of CysNO and Sper/NO and examined the activity of GAPDH immediately after the treatment and after 1 and 3 h postincubation in cell culture medium (Fig. 1). When BAECs were treated with CysNO, a dose-dependent inhibition of GAPDH was observed. At 400 μM CysNO, less than 5% of the original activity remained. This was not reversible with time (Fig. 1A), because when cells were washed after 1 h and further incubated in medium for up to 3 h, the activity was not restored. The inhibition of GAPDH was only partially restored by DTT immediately after the treatment (Fig. 1B), indicating that a large component of the inhibition was not due to a DTT-reversible modification of the active site thiol. We additionally observed that the ability of DTT to partially reverse the loss of GAPDH activity was lost in a time-dependent manner (Fig. 1B). When BAECs were treated with Sper/NO, the activity of GAPDH decreased in a dose-dependent manner, but this was completely reversed within 3 h after the Sper/NO removal (Fig. 1C). In this case the inhibition of the enzyme was DTT reversible, and incubation with this reducing agent brought the activity of GAPDH back to almost control levels (Fig. 1D). Our results show that both CysNO and NO are able to inhibit the enzyme in the intracellular environment, but only in the case of NO is this process recoverable with time and DTT reversible. There are two possible explanations for the observed incomplete reversibility of GAPDH activity in CysNO-treated cells. The first is that the protein may be degraded as a result of the modification, and the second is that the modification is more complex than S-nitrosation or S-thiolation and is insensitive to reduction with DTT. To exclude protein degradation, we measured GAPDH protein levels by Western blot analysis following CysNO or Sper/NO administration either immediately after the treatment or after 3 h of postincubation (Fig. 2A). Figure 2B shows a densitometric analysis of GAPDH protein after normalization to β-actin levels. We did not observe any significant decrease in GAPDH protein levels under any of the tested conditions, indicating that the loss of enzyme activity could not be attributed to the reduction in protein content. Interestingly, GAPDH protein levels were elevated after 1 h incubation with 400 μM CysNO, which may be due to the inhibition of protein degradation at these very high levels of CysNO, and under these conditions the enzyme was almost completely inhibited. These data suggest that a CysNO-dependent loss of GAPDH activity is not due to changes in protein steady state within our experimental timescale but is due to the inhibition of the enzyme.
Fig. 1.
GAPDH activity after S-nitrosocysteine (CySNO) and (Z)-1-{N-(3-ammoniopropyl)-N-[4-(3-aminopropylammonio)butyl]-amino}diazen-1ium-1,2-diolate (Sper/NO) treatment assayed immediately and after 1 and 3 h postincubation. Bovine aortic endothelial cells (BAECs) were incubated for 1 h with CysNO or Sper/NO in HBSS. Cells were washed and incubated further for up to 3 h in 2% FBS medium (1 and 3 h postincubation). GAPDH activity was determined in cell lysate following the consumption of NADH at 340 nm in the presence and absence of DTT. For the assay with DTT, cell lysate was pretreated with DTT for 30 min. Data were normalized to protein concentration and represent means ± SE (n = 3 experiments). A: CysNO treatment, assayed in the absence of DTT. B: CysNO treatment, assayed in the presence of DTT. C: Sper/NO treatment, assayed in the absence of DTT. D: Sper/NO treatment, assayed in the presence of DTT.
Fig. 2.
GAPDH protein levels. BAECs were incubated for 1 h with CysNO or Sper/NO in HBSS. Cells were washed and either harvested immediately (0 h posttreatment) or incubated further for 3 h in 2% FBS medium (3 h posttreatment). GAPDH levels were assessed by Western blot analysis. A: representative Western blot of GAPDH. NO, nitric oxide, represents the concentration of Sper/NO after nitric oxide. B: densitometry analysis of GAPDH levels normalized to β-actin. Data represent means ± SE (n = 3 experiments).
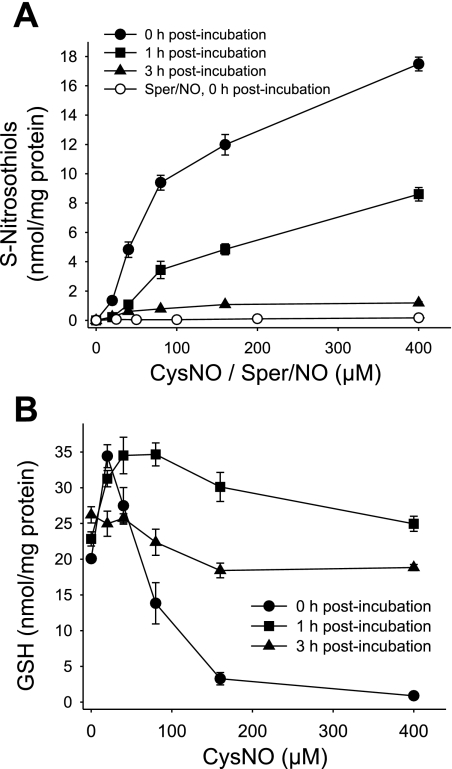
We previously reported that CysNO causes a dose-dependent increase in intracellular S-nitrosothiols in BAECs (1). We also showed that BAEC lysate possesses an activity that metabolizes GSNO (1). Here we examined the stability of S-nitrosothiols formed inside the cell after CysNO treatment. In this set of experiments, the cells were either analyzed immediately after exposure to CysNO (0 h posttreatment) or incubated further for up to 3 h (1 and 3 h posttreatment time points). An incubation with CysNO caused a dose-dependent increase in intracellular S-nitrosothiols, reaching values up to 18 nmol/mg protein. The cells were able to effectively metabolize S-nitrosothiols, as after 1 h the levels decreased by half and after 3 h only about 10% of intracellular S-nitrosothiols remained (Fig. 3A). The treatment with Sper/NO increased the S-nitrosothiol levels in a concentration-dependent manner, with 400 μM Sper/NO resulting in two orders of magnitude lower levels of intracellular S-nitrosothiols (0.17 nmol/mg protein). This is in agreement with previous data that NO is an inefficient nitrosating agent compared with transportable S-nitrosothiol (33).
Fig. 3.
Intracellular S-nitrosothiols and GSH levels 1 and 3 h after CysNO removal. BAECs were incubated for 1 h with CysNO or Sper/NO in HBSS. Cells were washed and incubated further for up to 3 h in 2% FBS medium (0–3 h postincubation). A: intracellular S-nitrosothiol levels were measured by chemiluminescence. B: the GSH levels were determined in cell lysate by HPLC. Data were normalized to protein concentration and represent means ± SE (n = 3 experiments).
GSH is a low-molecular weight thiol present in the cell at high concentrations, generally in the millimolar range depending on the cell type (22), and is a major component of antioxidant defense. Previously, we reported that CysNO could rapidly deplete GSH levels in BAECs (1). Figure 3B shows that immediately after CysNO treatment (0 h recovery), the GSH levels were increased at low micromolar levels of CysNO, but above 40 μM CysNO, GSH was substantially depleted in a concentration-dependent manner. Surprisingly, when CysNO was washed out and the cells were further incubated in the medium, the GSH levels exceeded the control levels after 1 h and then returned to control levels within 3 h. Taken together, we show that within 3 h of CysNO removal, S-nitrosothiols are largely metabolized and GSH returns to control levels; however, the activity of GAPDH is not restored.
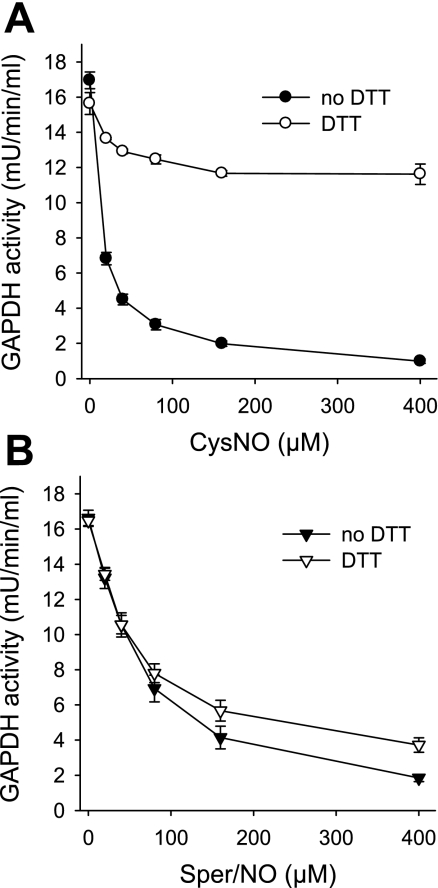
To get a better insight into the mechanism of GAPDH inhibition by CysNO and Sper/NO, we examined the effects of S-nitrosothiols and NO on purified enzyme. The incubation of GAPDH with CysNO resulted in a dose-dependent inhibition that was largely recovered with DTT (Fig. 4A). The incubation of GAPDH with 400 μM CysNO for 30 min (the highest concentration in Fig. 4A) resulted in the formation of an S-nitrosated protein with 0.68 ± 0.05 S-nitrosothiol per monomer (mean ± SE, n = 3 experiments) as assessed by chemiluminescence. A treatment with NO also inhibited GAPDH, but in this case the activity could not be recovered by an incubation with DTT (Fig. 4B). The administration of 400 μM Sper/NO, which inhibited more than 90% of GAPDH activity, only generated 0.10 ± 0.01 S-nitrosothiol per monomer (mean ± SE, n = 3 experiments), indicating that the major component of GAPDH inhibition was not due to thiol nitrosation. Startlingly, the effects of CysNO and NO on purified GAPDH were diametrically opposed to the behavior we observed in the cellular environment, where CysNO administration caused mainly irreversible modification and where NO-dependent inhibition of GAPDH was largely reversed by DTT.
Fig. 4.
Effects of CysNO and NO on purified GAPDH enzyme. GAPDH from chicken muscle was incubated for 30 min with indicated concentration of CysNO (A) or Sper/NO (B) in Tris buffer (pH 7.4). GAPDH activity was determined following the consumption of NADH at 340 nm in the presence and absence of DTT. For the assay with DTT, samples were pretreated with DTT for 30 min. Data represent means ± SE (n = 3 experiments).
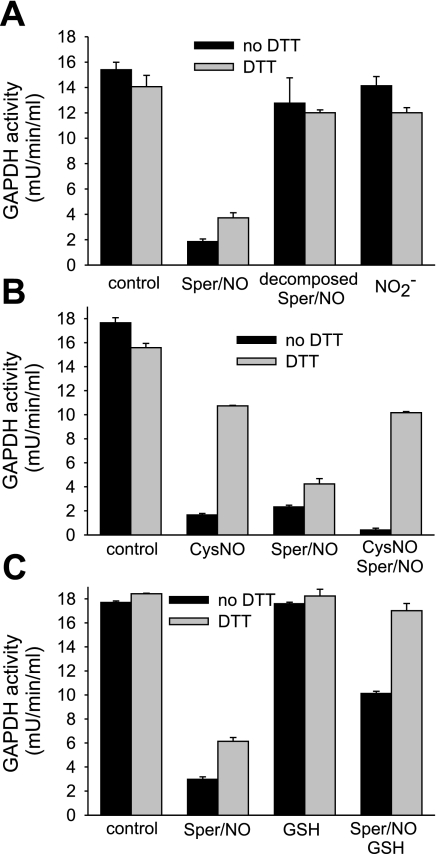
To further characterize the differences in the modes of inhibition of purified GAPDH, we tested decomposed Sper/NO and nitrite in their ability to inhibit GAPDH. Sper/NO releases two molecules of NO, leaving behind the spermine group; NO in turn can oxidize to nitrite in aqueous solution. The incubation of GAPDH with either decomposed Sper/NO or nitrite did not inhibit GAPDH (Fig. 5A), showing that these compounds do not account for the differences observed between an enzyme in isolation and in a cellular environment.
Fig. 5.
DTT-dependent reversibility of inhibition of purified GAPDH protein. GAPDH from chicken muscle was incubated for 30 min with indicated compounds in Tris buffer (pH 7.4). A: Sper/NO (400 μM), decomposed Sper/NO (400 μM), and nitrite (NO2−, 600 μM). Decomposed Sper/NO was obtained by incubating Sper/NO at neutral pH overnight. Nitrite concentration matched the nitrite concentration that was measured in the decomposed Sper/NO sample. B: CysNO (400 μM) and Sper/NO (400 μM). For the sample that was incubated with CysNO and Sper/NO, 30-min incubation with CysNO was followed by 30-min incubation with Sper/NO. C: Sper/NO (400 μM) and GSH (800 μM). For the sample that was incubated with Sper/NO and GSH, both compounds were added at the same time. The GAPDH activity was determined following the consumption of NADH at 340 nm in the presence and absence of DTT. For the assay with DTT, samples were pretreated with DTT for 30 min. Data represent means ± SE (n = 3 experiments).
To investigate whether NO and CysNO inhibit GAPDH at the same site, we tested whether the preincubation of GAPDH with CysNO can protect the enzyme from irreversible modification caused by NO. GAPDH was treated with either CysNO or Sper/NO alone, and with CysNO followed by Sper/NO. A sequential administration of CysNO and NO resulted in a type of inhibition that resembled that of CysNO alone (Fig. 5B) in that it was largely DTT reversible. This observation suggests that CysNO modifies the active site in such a way that it becomes insensitive to NO-mediated irreversible inhibition. This indicates that CysNO treatment leads to S-nitrosation or S-thiolation of the active site cysteine, whereas NO treatment largely leads to an alternative inhibitory modification.
To examine whether a NO-dependent inhibition of GAPDH proceeded via an intermediate that could be repaired by cellular reducing agents, Sper/NO was incubated with purified GAPDH in the presence and absence of GSH. If NO causes the formation of a reactive thiolic intermediate (for example a thiyl radical) in the active site that leads to an irreversible loss of activity, GSH should be able to react with the radical forming a DTT-sensitive mixed disulfide. Our data support this hypothesis since the presence of GSH during the treatment with Sper/NO results in a completely DTT-reversible loss of activity (Fig. 5C). Additionally, the presence of GSH during the treatment with NO protects the enzyme from inhibition (83% inhibition for Sper/NO alone compared with 43% inhibition in the presence of GSH). In total, the addition of GSH to purified enzyme alters its response to NO to mirror more closely that observed in a cellular environment (Fig. 1C).
To gain a better insight into the mechanism of NO-dependent inhibition of GAPDH, we exposed the cells to Sper/NO under anaerobic conditions and assayed the enzymatic activity (Fig. S1; note: supplemental material may be found posted with the online version of this article). If an oxidative process is involved in the inhibition of GAPDH by NO, this effect should be diminished by the removal of oxygen. Indeed, we observed that at the highest Sper/NO levels (400 μM), cellular GAPDH was inhibited by 31.9 ± 7.2% in the absence of oxygen compared with 63.2 ± 1.7% at ambient oxygen at the same temperature.
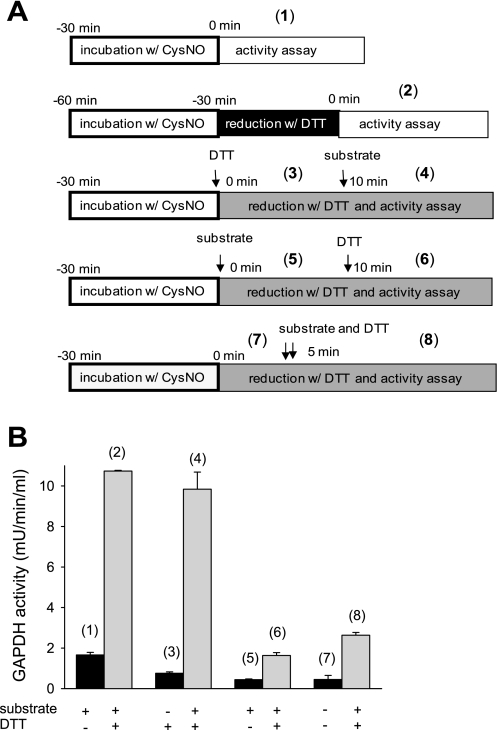
The mechanism of irreversible GAPDH inhibition described in the literature proposes that S-nitrosation of the cysteine in the active site inhibits the enzyme in a reversible way and at the same time facilitates the covalent attachment of the NADH cofactor causing a modification that can no longer be reversed (4, 15, 18). There is a striking difference in CysNO-dependent inhibition of GAPDH inside the cells compared with the purified enzyme preparation in terms of reversibility. The biggest distinction between these two experimental situations is that inside the cell, when the enzyme undergoes modification, all the cofactors required for the catalytic reaction are present, including NADH, whereas these compounds are absent in the isolated enzyme system until we assay activity. In the above experiments, when we tested DTT reversibility, the cell lysates or purified enzyme preparations were pretreated with DTT for 30 min before assaying. Thus the CysNO-dependent modification of isolated GAPDH was subjected to reduction before the enzyme was incubated with the substrate and cofactors. In contrast, S-nitrosated cellular GAPDH was exposed to substrate and cofactors, and this could lead to covalent NADH attachment before incubation with DTT. To test whether catalytic turnover of S-nitrosated GAPDH leads to irreversible modification, we measured the activity of purified CysNO-treated GAPDH under turnover conditions and examined DTT reversibility depending on the sequence of substrate and DTT addition. Figure 6 presents the schemes of treatment (Fig. 6A) and the activity of GAPDH measured under given conditions (Fig. 6B). Schemes 1 and 2 represent the activity assays performed in the same way as for all previous experiments, with 30 min DTT pretreatment followed by activity assay. In this case, CysNO inhibits GAPDH in a DTT-reversible way (rates 1 and 2). The inhibition of GAPDH is also reversible with DTT when CysNO-treated enzyme is incubated with DTT in addition to all of the components of the activity assay, including NADH, before addition of 3-phosphoglycerate (rates 3 and 4). However, GAPDH inhibition is not reversible when the CysNO-treated enzyme is incubated with substrate and cofactor before the administration of DTT (rates 5 and 6). The addition of the substrate and DTT at the same time also results in an irreversible inhibition (rates 7 and 8), suggesting that the turnover and the irreversible inhibition occur faster than DTT reduction. These data indicate that when isolated S-nitrosated GAPDH is under turnover conditions, i.e., exposed to substrate and cofactors, it undergoes irreversible inhibition. This inhibition is most likely to be NADH attachment as previously reported (18). This type of inhibition resembles the behavior of GAPDH in CysNO-treated cells.
Fig. 6.
Inhibition of CysNO-treated GAPDH under turnover conditions. A: schemes of the treatment and the activity assay of GAPDH from chicken muscle. Arrows indicate in which order DTT and substrate were added. Numbers in parenthesis correspond to the activity of GAPDH measured under given conditions and presented in B. For the simplicity reasons, substrate addition on the scheme represents the addition of 3-phosphoglyceric phosphokinase as the GAPDH activity was measured in the coupled assay and 3-phosphoglyceric phosphokinase supplied the substrate for GAPDH. B: GAPDH from chicken muscle was incubated for 30 min with CysNO (400 μM) in Tris buffer (pH 7.4). DTT (5 mM) and substrate (3-phosphoglyceric phosphokinase) were added as indicated. Data represent means ± SE (n = 3 experiments).
DISCUSSION
We treated BAECs with either CysNO or NO and observed that GAPDH activity was sensitive to both S-nitrosothiol transport and NO. We compared the results obtained for BAECs with a purified GAPDH preparation. Using the reduction with DTT as an index of thiol-dependent inhibition of activity, we differentiated between reversible and irreversible modifications. We observed that GAPDH was inhibited after the treatment of the cells with the transportable S-nitrosothiol, CysNO, and this inhibition could not be recovered when CysNO was removed, nor was it restored by a reduction with DTT. At the same time, the administration of CysNO dramatically increased intracellular S-nitrosothiol levels and depleted cellular GSH in agreement with our previous studies (1). Interestingly, within 3 h after the removal of the nitrosating agent, cellular S-nitrosothiols were effectively metabolized and GSH returned to the original levels but GAPDH remained inactive. In contrast, when BAECs were treated with NO, GAPDH activity was inhibited in a dose-dependent manner and the enzymatic activity was restored with reducing agent or the removal of NO followed by further incubation. Unlike CysNO, NO was a relatively inefficient nitrosating agent since it led to the formation of cellular S-nitrosothiols at levels two orders of magnitude lower than did CysNO.
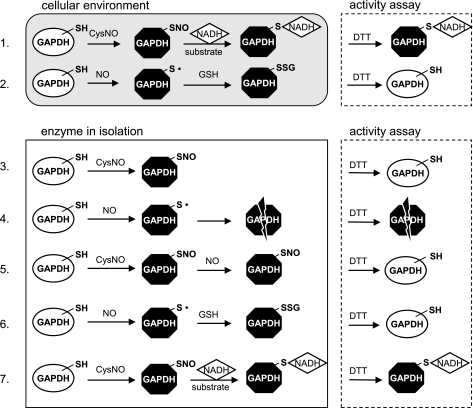
In contrast to previous studies (21), we observed striking differences in the inhibition of GAPDH inside the cell and in the isolated enzyme preparation. In the case of purified enzyme, both CysNO and NO inhibited the activity but only CysNO-dependent inhibition was reversible with DTT. In Fig. 7, we present our current model for the mechanism of CysNO- and NO-dependent inhibition of GAPDH. In all cases, both CysNO and NO substantially inhibited the activity of GAPDH. However, the enzyme behaved completely differently inside the cell than in isolation in terms of reversibility with DTT (Fig. 7). The key to understanding the above discrepancies in the types of inhibition lies in the fact that substrate, cofactors, and antioxidants are present inside the cell but are absent in the enzyme preparation. The partial DTT-dependent recovery of the cellular GAPDH activity after treatment with CysNO suggests that one pool of the enzyme was oxidatively modified and the other, larger pool underwent irreversible modification, most probably via NADH attachment (Fig. 7, no. 1). The partial reversal of CysNO-dependent inhibition with DTT observed immediately after the treatment may be attributed to the NO that is released from CysNO inside the cell. We have previously shown that CysNO is able to generate NO once taken up into the cells (24). GAPDH monomer contains four cysteine residues, cysteine-149 in the active site is the best candidate for modification, since it is the most reactive (pKa of 5.5), and the treatment of purified GAPDH with 400 μM CysNO resulted on average in 0.68 ± 0.05 S-nitrosothiol per monomer and a complete inhibition of the enzyme. The active site cysteine has been shown to be the most reactive and readily modified after an exposure to peroxynitrite (29), SNAP (10), sodium nitroprusside (25), and the NO-donor (±)-E-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamide (NOR-3) (19).
Fig. 7.
Model for a mechanism of GAPDH inhibition. 1: Inside the cell, S-nitrosated GAPDH undergoes irreversible modification, most probably via NADH attachment. 2: NO inhibits cellular GAPDH in a DTT-reversible way, and the inhibition recovers with time inside the cell, consistent with GSH repairing the cysteine in the active site (reaction between thiyl radical and GSH is simplified). 3: CysNO causes S-nitrosation of isolated GAPDH and the modification is reversible with DTT. 4: In the isolated enzyme situation, NO causes DTT-irreversible modifications of GAPDH, most probably because of secondary reactions that cannot be repaired by DTT. 5: CysNO treatment of purified GAPDH leads to S-nitrosation of the active site cysteine, thus preventing from irreversible NO-dependent inhibition of the enzyme. 6: GSH protects the isolated enzyme from irreversible NO-dependent inhibition (reaction between thiyl radical and GSH is simplified). 7: Purified S-nitrosated GAPDH undergoes irreversible inhibition under turnover conditions. Note: GAPDH monomer contains 4 cysteine residues, but for simplicity only the most reactive active site cysteine is represented. SH, protein thiol group; SNO, protein S-nitrosothiol group; S·, protein thiyl radical; SSG, glutathionylated protein.
Our data suggest that NO inhibits GAPDH via an oxidative mechanism that does not involve S-nitrosation; the inhibition reverses with time inside the cell, consistent with GSH repairing the cysteine in the active site, and is also DTT recoverable (Fig. 7, no. 2). This is further substantiated by data obtained under anaerobic conditions since the inhibitory effect of Sper/NO on GAPDH was diminished in the absence of oxygen (Fig. S1). In the isolated enzyme situation, GSH is absent and NO causes DTT-irreversible modifications of GAPDH, most probably because of secondary reactions that cannot be repaired by DTT (Fig. 7, no. 4). Recently, Schöneich (27) proposed that protein cysteine thiyl radical may lead to the oxidation of amino acids adjacent to the site of radical formation. The idea of thiyl radical as a precursor for irreversible GAPDH inhibition would explain our observation in the isolated enzyme system and imply that NO is rather an oxidative than a nitrosative agent in this situation. Indeed, the formation of the thiyl radical intermediate has been previously reported after the exposure of GAPDH to NO gas, S-nitroso-N-acetyl-D,l-penicillamine (SNAP), and 3-morpholinosydnonimine hydrochloride (SIN-1) (16). The lack of DTT reversibility in the NO-dependent inhibition of purified enzyme was completely abolished when the enzyme was either pretreated with CysNO (Fig. 7, no. 5) or when Sper/NO was administered in the presence of GSH (Fig. 7, no. 6). This data suggest that the blockade of the active site thiol by S-nitrosation prevents an NO-dependent oxidation of the same thiol. In addition, GSH is able to protect GAPDH from NO-dependent irreversible damage, presumably by scavenging a reactive protein-based intermediate such as a thiyl radical. By incubating purified S-nitrosated enzyme in the presence of substrate and NADH, we were able to convert the inhibition from DTT-reversible to DTT-irreversible (Fig. 7, no. 7), imitating the behavior of cellular GAPDH in response to CysNO exposure.
GAPDH is a multifunctional enzyme, and the modification of this protein has been correlated with multiple pathological states. GAPDH inhibition and glutathionylation have been observed in cardiac tissue upon ischemia-repefusion (5). The formation of amyloid-like aggregates of GAPDH via aberrant disulfide bonds of the active site cysteine was detected in response to oxidative stress and correlated with cell death (19). Recently, it was discovered that S-nitrosation of GAPDH augments its binding to E3 ubiquitin ligase, Siah1, leading to the nuclear translocation and degradation of nuclear proteins followed by cell death (6). Understanding the mechanisms of GAPDH inhibition by NO is clearly important in many biological processes in addition to glycolysis.
In summary, our data show that cellular GAPDH is a target for CysNO-dependent inhibition; it is also sensitive to NO-mediated oxidative damage. We show that these two agents inhibit GAPDH via separate mechanisms both inside the cell and in isolation. Our data strongly imply that low molecular weight S-nitrosothiols are not simply NO-donating agents and should not be used as such in experimental models. As illustrated here, even when they affect the same cellular targets as NO, they may act through separate mechanisms. Additionally, discrepancies observed between the cellular system and purified GAPDH strongly imply that the results obtained in the simple system do not mimic the situation inside the cell.
GRANTS
This research was supported by National Institute of General Medical Sciences Grant R01-GM-55792.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Broniowska KA, Zhang Y, Hogg N. Requirement of transmembrane transport for S-nitrosocysteine-dependent modification of intracellular thiols. J Biol Chem 281: 33835–33841, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brune B, Lapetina EG. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem 264: 8455–8458, 1989 [PubMed] [Google Scholar]
- 3.Chuang DM, Hough C, Senatorov VV. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu Rev Pharmacol Toxicol 45: 269–290, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Dimmeler S, Lottspeich F, Brune B. Nitric oxide causes ADP-ribosylation and inhibition of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem 267: 16771–16774, 1992 [PubMed] [Google Scholar]
- 5.Eaton P, Wright N, Hearse DJ, Shattock MJ. Glyceraldehyde phosphate dehydrogenase oxidation during cardiac ischemia and reperfusion. J Mol Cell Cardiol 34: 1549–1560, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol 7: 665–674, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Hara MR, Cascio MB, Sawa A. GAPDH as a sensor of NO stress. Biochim Biophys Acta 1762: 502–509, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Hart T. Some observations concerning the S-nitroso and S-phenylsulphonyl derivatives of l-cysteine and glutathione. Tetrahedron Lett 26: 2013–2026, 1985 [Google Scholar]
- 9.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Ishii T, Sunami O, Nakajima H, Nishio H, Takeuchi T, Hata F. Critical role of sulfenic acid formation of thiols in the inactivation of glyceraldehyde-3-phosphate dehydrogenase by nitric oxide. Biochem Pharmacol 58: 133–143, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Jourd'heuil D, Jourd'heuil FL, Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J Biol Chem 278: 15720–15726, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol 268: 281–293, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Keszler A, Zhang Y, Hogg N. Reaction among nitric oxide, glutathione, and oxygen in the presence and absence of protein: How are S-nitrosothiols formed? Free Radic Biol Med 48: 55–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lander HM, Sehajpal PK, Novogrodsky A. Nitric oxide signaling: a possible role for G proteins. J Immunol 151: 7182–7187, 1993 [PubMed] [Google Scholar]
- 15.McDonald LJ, Moss J. Stimulation by nitric oxide of an NAD linkage to glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci USA 90: 6238–6241, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minetti M, Pietraforte D, Di Stasi AM, Mallozzi C. Nitric oxide-dependent NAD linkage to glyceraldehyde-3-phosphate dehydrogenase: possible involvement of a cysteine thiyl radical intermediate. Biochem J 319: 369–375, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohr S, Hallak H, de BA, Lapetina EG, Brune B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem 274: 9427–9430, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Mohr S, Stamler JS, Brune B. Posttranslational modification of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosylation and subsequent NADH attachment. J Biol Chem 271: 4209–4214, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Nakajima H, Amano W, Fujita A, Fukuhara A, Azuma YT, Hata F, Inui T, Takeuchi T. The active site cysteine of the proapoptotic protein glyceraldehyde-3-phosphate dehydrogenase is essential in oxidative stress-induced aggregation and cell death. J Biol Chem 282: 26562–26574, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Padgett CM, Whorton AR. S-nitrosoglutathione reversibly inhibits GAPDH by S-nitrosylation. Am J Physiol Cell Physiol 269: C739–C749, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Padgett CM, Whorton AR. Glutathione redox cycle regulates nitric oxide-mediated glyceraldehyde-3-phosphate dehydrogenase inhibition. Am J Physiol Cell Physiol 272: C99–C108, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Reed DJ, Fariss MW. Glutathione depletion and susceptibility. Pharmacol Rev 36: 25S–33S, 1984 [PubMed] [Google Scholar]
- 23.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet, Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci USA 101: 8945–8950, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riego JA, Broniowska KA, Kettenhofen NJ, Hogg N. Activation and inhibition of soluble guanylyl cyclase by S-nitrosocysteine: involvement of amino acid transport system L. Free Radic Biol Med 47: 269–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera-Nieves J, Thompson WC, Levine RL, Moss J. Thiols mediate superoxide-dependent NADH modification of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem 274: 19525–19531, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Sawa A, Khan AA, Hester LD, Snyder SH. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci USA 94: 11669–11674, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schöneich C. Mechanisms of protein damage induced by cysteine thiyl radical formation. Chem Res Toxicol 21: 1175–1179, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Sirover MA. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta 1432: 159–184, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Souza JM, Radi R. Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Arch Biochem Biophys 360: 187–194, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Toyo'oka T, Imai K. High-performance liquid chromatography and fluorometric detection of biologically important thiols, derivatized with ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate (SBD-F). J Chromatogr A 282: 495–500, 1983 [DOI] [PubMed] [Google Scholar]
- 31.Wilson JE, Reid S, Masters CJ. A comparative study of the binding of aldolase and glyceraldehyde-3-phosphate dehydrogenase to the human erythrocyte membrane. Arch Biochem Biophys 215: 610–620, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Hogg N. Formation and stability of S-nitrosothiols in RAW 264.7 cells. Am J Physiol Lung Cell Mol Physiol 287: L467–L474, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci USA 101: 7891–7896, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Keszler A, Broniowska KA, Hogg N. Characterization and application of the biotin-switch assay for the identification of S-nitrosated proteins. Free Radic Biol Med 38: 874–881, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.