Summary
This review focuses on mechanisms and emerging concepts that drive the science of stroke in a therapeutic direction. Once considered exclusively a disorder of blood vessels, growing evidence has led to the realization that the biological processes underlying stroke are driven by the interaction of neurons, glia, vascular cells and matrix components, which actively participate in mechanisms of tissue injury and repair. As new targets are identified, new opportunities emerge that build on an appreciation of acute cellular events acting in a broader context of ongoing destructive, protective and reparative processes. The burden of disease is great and its magnitude widens as a role for blood vessels and stroke in vascular and non-vascular dementias becomes more clearly established. This review then poses a number of fundamental questions, the answers to which may generate new directions for research and possibly new treatments that could reduce the impact of this enormous economic and societal burden.
Introduction
Few neurological conditions are as complex and devastating as stroke, the second leading cause of death worldwide. Also called a brain attack, victims may suddenly experience paralysis, impaired speech or loss of vision due to interruption of blood flow (ischemia) caused by thrombosis or embolism. Less frequently (<15%), strokes are caused by hemorrhage or cardiac arrest. On average, strokes in the USA strike once every 40 seconds and cause death every 4 minutes, with an estimated 41.6% death rate in 2007 (Lloyd-Jones et al., 2009). With an aging population, the absolute numbers are likely to rise. Among survivors, work capacity is compromised in 70% of victims, and 30% need assistance with self-care. Hence, the disease burden is great. The estimated cost for stroke is 73.7 billion dollars in 2010 (USA) and projected to be 1.52 trillion dollars in 2050 (in 2005 dollars)(Lloyd-Jones et al., 2009). No racial or ethnic groups are immune and the problem is global. For example, in the Russian Federation and China, the estimated death rates per 100,000 population are 5–10 times higher than in the USA (Lloyd-Jones et al., 2009). Hence, stroke is an affliction of mankind.
For the above considerations and more, there is a compelling need to accelerate efforts to interrogate the stroke process and to define the links that exist with other conditions such as vascular and neurodegenerative dementia. It is also crucial to expand the narrow repertoire of therapeutic opportunities for these devastating conditions. To accomplish this, novel approaches are required that expand upon our evolving mechanistic understanding of the fundamentals of cell survival and death processes as well as tissue repair. The future depends upon how successful we are in deciphering these mechanisms and bringing clarity to the complex interactions between the multiplicity of cell and tissue types within brain (Lo et al., 2003). Armed with this knowledge and its successful therapeutic application, the field of stroke could be transformed.
In this spirit then, this brief review addresses selected issues fundamental to the science of ischemic stroke and vascular dementia. It begins with posing questions about stroke risk factors followed by a discussion of key cell and tissue mechanisms that render brain susceptible as well as tolerant to ischemic injury, including those promoting tissue protection and repair. The review ends by highlighting promising treatment strategies, inspired by these endogenous mechanisms, which present the opportunity to open new avenues in stroke therapy.
Stroke risk factors and triggers
A stroke risk factor is a characteristic of an individual that increases the risk for stroke compared to someone without that characteristic (Hankey, 2006). Some risk factors cannot be modified, such as a family history of cerebrovascular diseases, older age, male sex and Hispanic or Black race (Allen and Bayraktutan, 2008) (Hankey, 2006),. Other risk factors are modifiable and their correction reduces the chance of having a stroke (Table 1). These factors, which often coexist, have been estimated to account for 60–80% of stroke risk in the general population (Allen and Bayraktutan, 2008) (Hankey, 2006). Genome-wide association studies are increasingly being employed to identify susceptibility genes for stroke (Hegele and Dichgans, 2010). Although several loci have been identified, the need for independent replication and the modest effect sizes have precluded the full assessment of the clinical relevance of these findings (Hegele and Dichgans, 2010).
Table 1.
Major Modifiable Risk Factors for Ischemic Stroke
| Classification | Risk factor | References |
|---|---|---|
| Causal1 | Hypertension | (Lawes et al., 2004a) |
| Diabetes | (Lawes et al., 2004b) | |
| Hypercholesterolemia | (Amarenco et al., 2006) | |
| Cigarette smoking | (Bonita et al., 1999) | |
| Atrial fibrillation | (Hart et al., 1999) | |
| Valvular heart disease | (Kizer et al., 2005) | |
| Ischemic cardiomyopathy | (Loh et al., 1997) | |
| Carotid stenosis | (Rothwell et al., 2003) | |
| Probable2 | Hyperhomocysteinemia | (Wald et al., 2002) |
| Increased inflammatory markers (WBC, CRP, infection) | (Di Napoli et al., 2005) | |
| Elevated plasma fibrinogen | (Rothwell et al., 2004) | |
| Dyslipidemia | (Holme et al., 2009) | |
| Patent foramen ovale | (Nedeltchev et al., 2008) | |
| Obstructive sleep apnea | (Culebras, 2009) | |
| Body mass index | (Hu et al., 2007) | |
| Lack of exercise | (Hu et al., 2005) | |
| Low fruit and vegetable diet | (Dauchet et al., 2005) | |
| Psychosocial stress | (Simons et al., 1998) |
Factors demonstrated in randomized clinical trials to reduce stroke risk if corrected, or for which a strong, consistent and independent association with stroke has been established (Hankey, 2006).
Factors for which the association with stroke is less firm and/or no causative role has been established.
WBC, white blood cells; CRP, C-reactive protein.
How do risk factors increase the propensity to stroke?
Risk factors have profound effects on the structure and function of blood vessels, and on their interface with circulating blood. Many of the established risk factors alter vascular structure by promoting atherosclerosis and stiffening of arteries, and by inducing narrowing, thickening and tortuosity of arterioles and capillaries (Allen and Bayraktutan, 2008) (Iadecola and Davisson, 2008). In brain, these morphological changes are often associated with reductions in resting cerebral blood flow (CBF) and marked alterations in CBF regulation. Thus, aging, hypertension, diabetes and hypercholesterolemia impair vital adaptive mechanisms that assure that the brain is adequately perfused (Arrick et al., 2007; Iadecola and Davisson, 2008; Iadecola et al., 2009; Kitayama et al., 2007). The ability of the endothelium to regulate microvascular flow is compromised, while the increase in blood flow evoked by neural activity is suppressed, resulting in a mismatch between the brain’s energy supply and demand (Arrick et al., 2007) (Iadecola and Davisson, 2008), (Iadecola et al., 2009) (Zou et al., 2004a)
Some risk factors, like hypertension and diabetes, impair protective vascular mechanisms that keep CBF stable during reductions in blood pressure (cerebrovascular autoregulation), facilitating the occurrence of ischemia if intravascular pressure drops (Immink et al., 2004) (Kim et al., 2008). These vascular alterations increase the brain’s vulnerability to ischemia after arterial occlusion because they compromise the development of collateral flow arising from adjacent non-ischemic vascular territories, which is vital to the survival of the ischemic perinfarct zone (see Parenchymal Failure section). In addition to their vascular effects, some risk factors, like aging and diabetes, may enhance the intrinsic susceptibility of brain cells to injury, amplifying the tissue damage produced by ischemia (Biessels et al., 2002), but the biological bases of this effect are not well understood. Little is known about the interaction among the different stroke risk factors and whether their vascular effects are additive or synergistic. Furthermore, the relative contribution of parenchymal and vascular factors to stroke risk remains to be determined.
How do risk factors alter cerebral blood vessels?
Many cardiovascular risk factors increase production of reactive oxygen species (ROS) and promote inflammation in systemic and cerebral blood vessels. The predominant vascular sources of ROS are the superoxide-producing enzyme NADPH oxidase, xanthine oxidase, mitochondrial enzymes and uncoupling of nitric oxide synthase (NOS), a state in which this enzyme generates superoxide instead of NO (Faraci, 2006) (fig. 1). Many of the damaging effects of oxidative stress on blood vessels are related to the biological inactivation of NO by the free radical superoxide, which reduces NO bioavailability and prevents its beneficial effects (Pacher et al., 2007) (Schulz et al., 2008). Thus, loss of the vasoregulatory effects of endothelial NO leads to vasoconstriction and reduced NO-dependent vascular responses, with a negative impact on the regulation of microvascular flow (Schulz et al., 2008). Loss of the antiaggregant, anti-proliferative, and anti-cell adhesion effects of NO promotes platelet aggregation, leukocyte adhesion to endothelial cells, and smooth muscle proliferation, key steps in vascular inflammation (Pepine, 2009) (Zou et al., 2004b). In addition, ROS can directly promote inflammation by increasing blood brain barrier (BBB) permeability through upregulation of vascular endothelial growth factor (VEGF), and by inducing the expression of cytokines, matrix remodeling enzymes, including metalloproteases, and proinflammatory genes through NF-kb activation (Marchesi et al., 2008). One such NFκb-dependent gene product, inducible nitric oxide synthase, produces large amounts of NO, which alter vascular structure and function through nitration and nitrosylation of critical proteins (Gunnett et al., 2005) (Guzik et al., 2003) (Lima et al., 2010). Peroxynitrite, a potent nitrating agent produced by the reaction of NO with superoxide, alters vasomotor reactivity by inactivating critical endothelial and smooth muscle enzymes, and by activating the DNA repair enzyme poly-ADP-ribose polymerase (PARP), which leads to ATP depletion and vascular ion channel dysfunction (Pacher et al., 2007). Whereas ROS may set the stage for inflammation, vascular inflammation, in turn, leads to ROS production creating a vicious circle that enhances vascular damage. Activation of the plasminogen system by oxidative stress and inflammation promotes matrix remodeling, smooth muscle cell migration, and intimal hyperplasia (Nicholl et al., 2006), factors that promote atherosclerosis and alterations in vascular structure. Thus, oxidative stress and vascular inflammation are major pathways through which risk factors exert their deleterious effects on blood vessels. However, it remains to be determined how individual risk factors trigger the activation of one or both of these processes. This is a critical question for targeting preventive strategies in patients with specific risk factors.
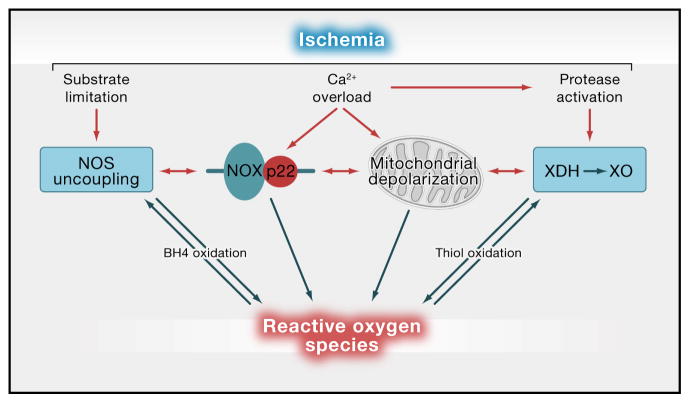
Figure 1. Major sources of ROS in brain and blood vessels.
Stroke risk factors and ischemia lead to the activation of several ROS-generating enzymatic systems. In ischemia, the increase in cytosolic Ca2+ activates the superoxide-producing enzyme NADPH oxidase (NOX), through PKC and NO derived from neuronal NOS (Brennan et al., 2009b; Girouard et al., 2009). Mitochondria become depolarized by the Ca2+ overload (Nicholls, 2008) and, possibly, by ROS derived from NADPH located near mitochondria (Girouard et al., 2009) producing large amounts of superoxide. The Ca2+-induced activation of proteases and/or the pro-oxidant environment convert xanthine dehydrogenase (XDH) to xanthine oxidase (XO) (Abramov et al., 2007), a superoxide-generating enzyme involved in purine degradation. Finally, the substrate deprivation induced by ischemia and the oxidative inactivation of essential co-factors like tetrahydrobiopterin (BH4) uncouple L-arginine oxidation from NO formation by NOS, resulting in superoxide production (NOS uncoupling) (Forstermann, 2010). Other potential sources of ROS include enzymes for arachidonic acid or catecholamine metabolism, but their participation in stroke-induced oxidative stress remains in question (Adibhatla and Hatcher, 2010; Kunz et al., 2007a).
Why now? Role of stroke triggers
While vascular risk factors increase the likelihood of stroke in affected individuals, precipitating factors that act as “stroke triggers” must also be postulated. In some patients, the specific event responsible for the arterial occlusion can be identified, e.g., neck trauma leading to dissection and occlusion of the internal carotid artery (Kim and Schulman, 2009). But in most instances the factors precipitating the ischemic event cannot be established. Although systemic infections, pregnancy and puerperium, use of illicit drugs, and mental stress often trigger a stroke (Elkind, 2007), precisely how these factors exert their effect remains unclear. Exacerbation of vascular inflammation and activation of the coagulation cascade are likely to play a role (Welsh et al., 2009). For example, the added vascular dysfunction and blood clotting abnormalities, superimposed on those induced by stroke risk factors, could precipitate vascular occlusion or hemodynamic insufficiency. This view is supported by the fact that acute stroke often occurs in the setting of increased circulating leukocytes, and elevated plasma markers of systemic inflammation and vascular activation, which also predict a poor outcome (Elkind, 2007) (Welsh et al., 2009). Thus, our understanding of stroke triggers is limited, and mechanistic studies addressing these issues would be valuable in the identification of high-risk cases.
Parenchymal Failure: Why and how does the brain die during ischemia?
Because of its high intrinsic metabolic activity activity and large concentrations of the neurotransmitter-excitotoxin glutamate (Choi, 1992), the brain is especially vulnerable to ischemic insult. This can develop either as a consequence of thrombosis in situ or following embolic occlusion of a cerebral blood vessel, the latter usually arising from the heart or atherosclerotic plaques in the carotid artery and aortic arch. Although neurological dysfunction occurs within seconds to minutes of vessel occlusion, the evolution of ischemic injury and cell death continues in stages for minutes, hours and even days, depending, in part, upon the vulnerability of the particular brain region, its cellular constituents, and the extent of residual perfusion.
The responses of blood vessels and their perfusion are important to outcome as well. For example, a deficiency of vasodilating molecules such as endothelial NO enlarges stroke size (Huang et al., 1996). Early restoration of blood flow by clot lysis and reperfusion decreases ischemic injury, and this may be achievable by giving recombinant tissue plasminogen activator (tPA), the only FDA-approved treatment for reestablishing flow and salvaging brain tissue. However, tPA is used in <10% of patients and even less frequently after 3 hrs because of the risk of hemorrhage into ischemic tissue (NINDS TPA Study) (1995). Moreover, tPA may have other unintended risks (Kaur et a., 2002) such as injury to the blood brain barrier by activating matrix metalloproteinases (Wang et al., 2003), or excitotoxicity in experimental models (Nicole et al., 2001). Combination therapies that ameliorate these effects may extend tPA’s therapeutic window while mitigating the untoward effects of reperfusion and plasminogen activation (Liu et al., 2004) (Cheng et al., 2006) (Murata et al., 2008). Theoretically, that could prolong tissue viability for hours or even longer, depending upon the rapid delivery of drug to compromised brain (Ginsberg, 2008).
Whether or not vulnerable brain tissue can be rescued or protected after a protracted time period in the absence of partial or complete restoration of blood flow is an unanswered question. In all likelihood, areas of vulnerable brain do remain viable for hours after vessel occlusion, at least in some instances. For example, the hemiparesis that follows middle cerebral artery occlusion in the baboon was reversible in 73% of animals with reperfusion at 2–4 hours, compared to 33% and 17% in animals with reperfusion at 8 and 16–24 hours, respectively (Marcoux et al., 1982) (Crowell et al., 1981) (Jones et al., 1981). The challenge then is to restore adequate blood flow quickly after vessel occlusion and to protect viable tissue from unleashed mechanisms that lead to cell and tissue demise.
Salvageable vs Non-Salvageable Tissue: Salvageable tissue is the target for therapy
Tissue distal to an occluded blood vessel is often heterogeneous, meta-stable and differentially susceptible to ischemic injury. This meta-stable zone, termed the ischemic penumbra or perinfarct zone (Astrup et al., 1981), is characterized by significantly depressed tissue perfusion that is barely sufficient to support basal ATP levels and oxygen metabolism as well as normal ionic gradients in the presence of electrical silence and suppressed protein synthesis. The ischemic penumbra then denotes an “at risk” region that is functionally impaired, but potentially salvageable. However, unless perfusion is improved or cells made relatively more resistant to injury, the tissue at risk dies within a few hours (Lo, 2008a, b). The ischemic core by contrast, refers to the irreversibly damaged tissue distal to an occluded blood vessel characterized by <20% of baseline blood flow levels, depleted ATP stores and irreversible failure of energy metabolism (Lo, 2008b). With time, the infarct core expands into the ischemic penumbra and therapeutic opportunity is lost. Therefore, detecting a penumbra in patients can help to identify those who might benefit most from acute treatments that restore blood flow (thrombolysis or endovascular clot retrieval) or treatments for the future that render viable brain more resistant to ischemic injury. For this purpose, magnetic resonance imaging methods such as perfusion-weighted and diffusion-weighted imaging are most often used. Perfusion-weighted imaging measures the spatial extent of blood flow compromise whereas diffusion weighted imaging is thought to detect the region of attenuated diffusion of water, a putative surrogate marker for severely damaged brain tissue. The difference is thought to reflect the ischemic penumbra (fig. 2) (Ebinger et al., 2009). Although the predictive value of this method is still debated, perfusion-diffusion mismatch provides a useful first attempt to define the tissue at risk.
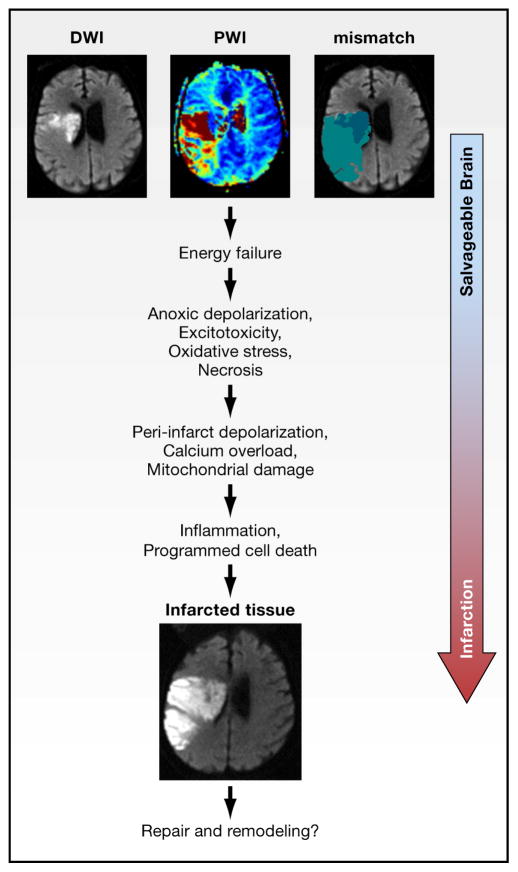
Figure 2. The Ischemic Penumbra.
The ischemic penumbra represents compromised but viable brain tissue lying distal to an occluded blood vessel. The top 3 MR images in this figure were taken in the same patient early after occlusion of the middle cerebral artery. The images are shown in the horizontal plane passing through the lateral ventricles. “Stunned but not dead tissue” is detected by magnetic resonance imaging (MRI) techniques that assess (i) the spatial extent of the perfusion or blood flow deficit that presents as red and yellow areas on perfusion weighted imaging (PWI, top left image), and (ii) severely damaged tissue in the ischemic core that presents as high-intensity areas on diffusion weighted imaging (DWI, top middle image) (Ebinger et al, 2009). In this patient, the perfusion deficit in this patient is larger than diffusion lesion. The top right image depicts the mismatch between core areas with a diffusion lesion (dark blue) and the larger areas with low perfusion (light blue). Over hours to days (see text), the core territory expands due to the complex cascades involving excitotoxicity, oxidative stress, programmed cell death mechanisms and inflammation, as the initial trigger of energy failure ultimately progresses to infarcted tissue The resulting infarct is shown in the image at the bottom of the figure and is about the size of the initial perfusion deficit in this untreated patient. In later stages, some patients may also partially recover in part, due to endogenous mechanisms of repair and remodeling (Chopp et al, 2007). MRI scans courtesy of G. Albers, Stanford.
What are the prominent mechanisms leading to cell and tissue demise?
Within the ischemic penumbra, multiple mechanisms have been identified over the past few decades that irreversibly damage brain tissue (fig 2). Understanding the contribution of each informs us about potential therapeutic opportunities and treatment targets. In the area of severely limited blood supply, ATP utilization continues in the presence of minimal synthesis and levels drop, leading to acidosis and loss of ionic homeostasis. As a consequence, cells swell and membranes rupture. However, ischemic cell death cannot be equated with limited ATP availability; rather, tissue death develops as a consequence of numerous ionic, biochemical and cellular events that impose overwhelming stresses upon already compromised tissue (see for review (Dirnagl et al., 1999) (Lo et al., 2003).
Excitotoxicity
Excitotoxicity and calcium overload are major factors contributing to the early stages of ischemic cell death. The canonical pathway asserts that glutamate, the most abundant neurotransmitter, accumulates into the extracellular space as a consequence of energy and ion pump failure, as well as failure of reuptake mechanisms (Choi and Rothman, 1990). The glutamate overload leads to prolonged stimulation of AMPA and NMDA ionotropic receptor subtypes to dramatically enhance the influx of calcium, sodium and water into neurons. Massive calcium influx activates catabolic processes mediated by proteases, lipases, and nucleases (Ankarcrona et al., 1995). In addition, activation of nNOS, PLA2 and other Ca2+ dependent enzymes, leads to production of NO, arachidonic acid metabolites and superoxide, which act as additional triggers of cell death (Dirnagl et al., 1999) (Lo et al., 2003). For these and other reasons, oxidative phosphorylation becomes uncoupled, leading to further ATP depletion, ROS production and release of stored Ca2+ from mitochondria, further accelerating a series of catastrophic events that lead to acute cell death.
Despite the validation of glutamate toxicity in the laboratory setting and its enormously positive impact on the quest for new therapeutics in neuroscience, numerous clinical trials targeting NMDA and AMPA receptors have failed to improve outcome in stroke patients (Ginsberg, 2009). Although replete with shortcomings in trial design and implementation, the failure to translate has reinforced the notion that the glutamate hypothesis may have oversimplified the complexity of the cell death process, and underestimated the diversity of expressing cell types as well as the heterogeneity of glutamate responses following receptor over stimulation in stroke. Moreover, it is likely that NMDA-AMPA pathways comprise only a subset of routines disrupting ionic imbalance following glutamate receptor activation. As originally conceived, the NMDA-AMPA model did not consider the delayed and invariably fatal glutamate-independent calcium influx following oxygen-glucose deprivation or the failure of glutamate receptor blockade to halt cell death after very intense oxygen-glucose deprivation (Aarts et al., 2003). Equally importantly, it could not account in an obvious way for the death of non-neuronal cell types such as, astrocytes, vascular cells or microglia in ischemic tissue. An alternative viewpoint is that the drugs tested in clinical trials were poorly chosen, because many of them block all receptor functions (Lipton, 2007a). As a result, the dosing may have been suboptimal, due to the development of untoward side effects that prevented dose escalation to neuroprotective levels. A more suitable approach would be to use therapeutic agents that selectively target excessive channel opening (e.g., uncompetitive inhibitors with a relatively fast off-rate, such as memantine (Orgogozo et al., 2002; Mobius and Stoffler, 2002; Lipton, 2006; Kaviragan and Schneider 2007) thereby retaining normal receptor functions (Lipton, 2007a).
To add to the complexity, it is now known that NMDA receptor location and subunit composition differentially regulate neuronal survival or death, although the strength of the calcium signal may specify the fate of neurons best following NMDA over activation (Stanika et al., 2009). With notable exceptions, selective enhancement of synaptic receptors (in which NR2A peptide subunits predominate over NR2B) promotes neuronal survival whereas activating extrasynaptic receptors (in which NR2B peptide subunits predominate over NR2A) promote cell death (Chen et al., 2007), in part, by activating death-associated protein kinase 1 (Tu et al., 2010). Turning CREB-dependent signaling and BDNF expression up or down is critical for cell fate determination during NMDA receptor activation. The MAP kinase family members P38 and ERK1/2 participate, most likely by phosphorylation-dependent regulation of submembrane scaffolding proteins and NR2A and NR2B receptor subunits (Hardingham et al., 2002). Under ischemic conditions, the vulnerability to cell death is reduced when postsynaptic scaffolding proteins are modified and PSD-95 binding is prevented (Cui et al., 2007). Moreover, successfully targeting the glutamate receptor complex early on would also diminish the opening of downstream channels, e.g., transient receptor potential channels (TRP) and acid sensing ion channels, as well as suppress the frequency of cortical spreading depolarizations (see below), ongoing within the ischemic penumbra. Hence, understanding and leveraging the repertoire of diverse responses and mediators at the receptor and second messenger level can only enhance possibilities for successfully targeting excitotoxicity for the treatment of ischemic injury.
Calcium dysregulation
It is also known that increased calcium influx from glutamate receptor overactivation combined with release of Ca2+ from mitochondria and other stores (e.g., endoplasmic reticulum) may not fully account for the irreversible buildup of intracellular Ca2+ after excitotoxic stimulation. Other channels and ion pumps activated during ischemia in addition to glutamate receptors have been implicated in the Ca2+ accumulation. These include the Na/Ca2+ exchanger (Bano et al., 2007) hemichannels (Contreras et al., 2004), acid sensing ion channels (Xiong et al., 2004), volume regulated anion channels (Kimelberg et al., 2006; Simard et al., 2007), and TRP channels (Aarts and Tymianski, 2005). In particular, ASIC1a, activated by ischemia-induced acidosis, is involved in the Ca2+ influx and its inhibition is neuroprotective (Simon, 2006). ASICs are stimulated within the pH range commonly found in ischemic brain tissue, thus explaining the well established link between acidosis and worsening of ischemic outcome in animals and humans. Failure of Ca2+ efflux mechanisms, especially the Na/Ca2+ exchanger also contributes to the Ca2+ accumulation. Prostaglandin E2 EP1 receptors have a role in the failure of the Na/Ca2+ exchanger during ischemia and their inhibition is markedly neuroprotective (Abe et al., 2009); (Kawano et al., 2006). Therefore, targeting Ca2+ influx-efflux mechanisms may be a valuable approach to counteract Ca2+ dysregulation and associated injury in conjunction with novel drugs targeting glutamate receptors.
Oxidative and Nitrosative Stress
Oxidative and nitrosative stress are also powerful mediators of ischemic injury. In one scenario, the redox environment of cells modulates signal transduction cascades that tip the balance between pro-death and pro-survival pathways (Crack and Taylor, 2005). In the second scenario, ROS and perhaps reactive nitrogen species, including peroxynitrite, act directly as executioners of cell death (Chan, 2001). The brain appears particularly vulnerable to radical-mediated attack because of its limited antioxidant defenses (Adibhatia and Hatcher, 2010). Until recently, it was accepted that during ischemia ROS were generated principally by mitochondria, a well-known ROS source during electron transport and oxidative phosphorylation. However, it was recently shown that NADPH oxidase generates the majority of superoxide anion produced in vivo and in vitro during NMDA receptor activation and ischemia (Brennan et al., 2009a; Girouard et al., 2009). Thus, the mitochondrion does not appear to be the major source of radicals following NMDA receptor activation. However, the close proximity of NADPH oxidase to neuronal mitochondria (Girouard et al., 2009) raises that possibility that this enzyme generates “kindling” ROS that promote mitochondrial uncoupling, triggering a secondary ROS surge from mitochondria (fig. 1). These observations point to NADPH oxidase as a potentially important therapeutic target in stroke, but also highlight the need for a better understanding of the interaction among the different ROS sources and the relative contribution of vascular vs. neuronal sources. In this regard, a surprising finding has been that activation of NMDA receptors increases ROS both in neurons and vascular cells (Girouard et al., 2006), suggesting a role of vascular ROS in excitotoxic brain damage. Although this observation emphasizes the close relationships between vessels and neurons (see Protecting the Neurovascular Unit), it has not been established how vascular oxidative stress may contribute to tissue outcome in excitotoxic brain injury.
NO and related oxidation products are key players in excitotoxicity. Reactive nitrogen species have important cellular effects, such as inhibition of key mitochondrial enzymes, facilitating mitochondrial transition pore formation, DNA damage, PARP activation, and activation of Ca2+ permeable TRPM7 channels (Aarts and Tymianski, 2005) (Pacher et al., 2007). As a potential second mechanism, NO modifies protein groups by covalently attaching to cysteine residues forming S-nitrosothiol derivatives. This posttranslational modification significantly impacts cell survival by altering for example, the function of critical regulatory proteins such as caspases, metalloproteases (Gu et al., 2002), and the glycolytic enzyme GAPDH (Nakamura and Lipton, 2009). As NO has the potential to target a number of specific cysteine residues to alter protein function, the impact of S-nitrosylation is [largely] underexplored in the ischemic process.
Taken together, the experimental evidence implicating oxidative and nitrosative stress in stroke is strong. But the translation of these fundamental concepts into clinical applications has proven to be challenging. Most recently, a nitrone-based radical spin trap failed in the final Phase 3 clinical trial for acute ischemic stroke (Shuaib et al., 2007). And more broadly, the history of anti-oxidant therapies in clinical trials for neurodegeneration has been littered with many disappointments (Kamat et al., 2008). Over-production of reactive nitrogen and oxygen species are surely damaging to brain cells. But at lower, homeostatic levels, radicals are critical signaling molecules participating in normal neuronal and vascular function (Faraci, 2006). Finding novel ways to scavenge or suppress deleterious radicals without interfering with endogenous signaling will be important to design effective therapies. As discussed above for excitotoxicity, a desirable strategy would be to develop drugs that only become activated by the pathological state intended for inhibition, i.e., during oxidative stress (Lipton, 2007b). Another approach would be to test antioxidant molecules with pleiotropic properties, such as activated protein-C (APC). APC blocks ROS generation, suppresses post-ischemic inflammation and blocks apoptosis in neurons and endothelium, thereby providing both parenchymal and vascular protection (Yamaji et al., 2005) (Cheng et al., 2006) (Liu et al., 2004). APC, as well as other pleiotropic agents with a broad spectrum of activity in experimental stroke, are undergoing clinical trial testing (Ginsberg, 2009).
Cortical Spreading Depolarizations
In addition to excitotoxic events at the molecular and cellular level, massive release of glutamate and ionic imbalance negatively impact the evolution of ischemic injury at the tissue level. Cortical spreading depolarization (CSD), a high energy consuming phenomenon akin to the cortical spreading depression of Leao, is triggered by high levels of extracellular glutamate and K+ and is characterized by slowly propagating massive depolarization of neurons and astrocytes along with drastic disruption of ionic gradients (Somjen, 2001). Electrophysiological and imaging data convincingly demonstrate CSD in patients with ischemic stroke (Dohmen et al., 2008). In experimental animals, a single cortical spreading depolarization expands the area of severe hypoperfusion by more than 20% (Shin et al., 2006). In fact, it appears that a greater frequency but more importantly, duration of DC shifts correspond to accelerated infarct maturation and core expansion (Mies et al., 1993) (Back et al., 1996). The infarct expansion is probably due to mismatch between high metabolic demand to support membrane repolarization and marginal tissue perfusion due to constrained penumbral blood flow (Back et al., 1996). Hence, suppressing CSD may prove worthwhile for reducing infarct maturation. Notably, the non-competitive NMDA receptor antagonist ketamine markedly suppressed CSD frequency in head injury patients (Sakowitz et al., 2009), but it is unclear whether it improved clinical outcome. By contrast, irreversible depolarizations resistant to NMDA receptor antagonists (anoxic depolarization) develop in the ischemic core territory (Nellgard and Wieloch, 1992) and are probably terminal events contributing to severe ionic imbalance and tissue failure.
Inflammation
Focal ischemia evokes a robust inflammatory response that begins within a few hrs of onset and typifies the secondary or delayed response to ischemia. It involves activation of microglia and astrocytes as well as influx of hematogenous cells recruited by cytokines, adhesion molecules and chemokines across the activated blood vessel wall. Stimulated brain cells and blood vessels then communicate with one another through a complex network of paracrine and autocrine signaling. The highly choreographed response evolves over many days, with multiple signals and targets triggering expression of novel proteins and secondary infarct expansion (Kleinig and Vink, 2009). Innate and adaptive immune systems participate as well. At least early on, inflammation amplifies the ischemic lesion, but, as discussed later, it may it may set the stage for tissue repair in the late post-ischemic period. A number of therapeutic trials designed to target the inflammatory response have failed to show clinical benefit (Sughrue et al., 2004), underscoring the need to clarify further the complexities of acute and delayed post-ischemic inflammatory signaling within brain.
As noted above, cerebral blood vessels are the first to be exposed to the ischemic insult and their reaction to injury sets the stage for the inflammatory response. Early on, production of cytokines, such as TNFα and IL1β, in vascular cells and perivascular microglia-macrophages upregulates adhesion molecule expression (e.g, ICAM-1, P and E-selectin) and, along with integrins, promote leukocyte rolling and sticking to the vessel surfaces (Zhang et al., 1998). Neutrophils are the first blood borne cells to be recruited into the brain, followed by monocytes and, starting on days 1–2, lymphocytes. As the brain’s primary immune cell, activated microglia transform into macrophages and accumulate at the border zone along with blood-borne macrophages to clear debris and dead cells, and produce proinflammatory mediators and toxic molecules (Schilling et al., 2003).
Molecules released from injured or dying brain cells also contribute to the inflammatory response. In particular, products from cells undergoing oxidative stress and necrosis, for example lipopeptides, advanced glycation end-products (AGE), modified lipids, heat shock proteins, hyaluronic acid, and the nuclear protein HMGB1, lead to activation of the innate immune system by engaging toll like receptors (TLRs). TLRs, are expressed in multiple cells and, in conjunction with CD36, receptors for AGE (RAGE) and other scavenger receptors, may serve to detect “danger signals” triggering NFκb-dependent inflammatory signaling (Oppenheim and Yang, 2005) (Wang et al., 2007). PARP activity appears to function as an NFκb co-activator (Chiarugi and Moskowitz, 2003), with therapeutic potential for delayed administration of PARP-1 inhibitors (Kauppinen et al., 2009). Beside NF-kb, other transcriptional activators including activator protein-1, STAT-3, interferon regulatory factor 1, and C/EBP beta, exert a proinflammatory effect (Kapadia et al., 2006) (Iadecola et al., 1999), while PPARgamma acts as an a negative modulator of post-ischemic inflammation (Kapadia et al., 2008). In addition to neutrophils and monocytes, T cell subsets have recently been implicated as modulators of secondary infarct progression. Treg cells express the antinflammatory cytokines IL10 and TGFβ, and normally dampen the immune response by suppressing T cell effector activity (Liesz et al., 2009). Secondary infarct progression is impacted by a second subset of invading T lymphocytes, gamma-delta T cells, a source of the proinflammatory cytokine IL-17. After deletion of IL-17, infarct size decreases (Shichita et al., 2009).
These findings support the notion that taming post-ischemic inflammation may block secondary events that extend brain injury. However, as noted below, during the later stages in the injury process, inflammation promotes critical events necessary for tissue repair. Therefore, therapeutic interventions targeting post-ischemic inflammation should be mindful of temporal considerations by minimizing the destructive potential of inflammation in the acute phase while enhancing its beneficial contributions to tissue repair in the late stages of cerebral ischemia.
Necrosis, necroptosis and autophagy: The execution
Necrosis and apoptosis are the principal mechanisms of cell death after ischemic injury, and the details of both have been reviewed (Yuan, 2006, 2009). Depending in part upon the magnitude, type of stimulus, and cell type, a number of cell death triggers promote necrosis or apoptosis, although cellular features of apoptosis and necrosis are sometimes found together in dying ischemic cells. Probably due to its ATP dependence, apoptotic cell death is prominent after milder injury and is more delayed (Bonfoco et al., 1995), although severe ischemia is associated with early-onset caspase cleavage, enzyme activation and acute cleavage of substrate proteins (Namura et al., 1998). Apoptosis and necrosis can be induced by multiple triggers such as Ca2+ overload or oxidative stress. Mitochondria play an essential role in both (Schinzel et al., 2005), serving as a reservoir for pro-apoptotic and anti-apoptotic proteins and cytochrome C. One such protein apoptosis inducing factor (AIF) is released from the outer membrane of mitochondria in response to activation of the nuclear protein PARP and generation of a PAR polymer, its toxic product (Yu et al., 2006). AIF then translocates to the nucleus to promote a caspase independent form of cell death in part by promoting DNA fragmentation and chromatin condensation. Release of mitochondrial proteins sets in motion (or suppresses) multiple caspase-dependent and independent processes to dismantle critical homeostatic and repair mechanisms. Despite the expression of executioner pro-caspases, activated caspases and caspase cleavage products, classic apoptotic morphology is rarely found in humans within adult ischemic brain. However, it does appear more prominently in the ischemic brains of neonates (Ferriero, 2004).
Until now, necrotic cell death was defined as unregulated, unprogrammed and lacking cell signaling events. It is the predominant form of ischemic cell death. It was recently shown that certain cells including those within brain possess a molecular switch that determines whether cells die by necrosis or apoptosis in a TNF-dependent way (Holler et al., 2000). In this cell death variation, necrosis is triggered by two kinases, RIP 1 and RIP 3, reciprocally interacting, often in the presence of an apoptosis inhibitor (Cho et al., 2009). Activated RIP3 is capable of stimulating enzymes such as the glycogen degrading enzyme, glycogen phosphorylase to steer TNF-induced apoptosis towards necrosis in part, through bursting energy metabolism and ROS generation (Zhang et al., 2009). Nec-1, a small molecule inhibitor of RIP-1, decreases ischemic cell injury in models of ischemia-reperfusion and improves neurological outcome, suggesting RIP-1 as a potential therapeutic target (Degterev et al., 2005).
Autophagy, an energy-dependent process implicated as a cell death mechanism, is used by eukaryotes to degrade and recycle subcellular organelles. Its precise role in the death process is controversial because autophagic activity maybe cytoprotective, at least in some disease models, e.g., polyglutamine expansions or in aging models (Levine and Kroemer, 2009). In other disease models such as in neonatal hypoxia-ischemia, autophagy reportedly promotes cell death (Puyal et al., 2009). Precisely how this is accomplished remains for further study.
Stroke and dementia
The major mechanisms of tissue dysfunction and death reviewed above develop as a consequence of acute vessel occlusion and the attendant activation of endogenous mechanisms of cell injury and tissue demise. More subtle and less well understood mechanisms are beginning to define a new research frontier linking cerebrovascular dysfunction and small strokes to the development of vascular cognitive impairment (VCI) and Alzheimer’s disease (AD), the most common causes of dementia (Fotuhi et al., 2009). AD and “vascular” dementia have traditionally been considered distinct entities (Iadecola, 2004). Thus, AD, the most prevalent of the two, is characterized pathologically by deposition of the amyloid beta peptide (Abeta) in the brain parenchyma (amyloid plaques) and blood vessels (amyloid angiopathy), and by neurofibrillary tangles (Querfurth and LaFerla, 2010). On the other hand, cerebrovascular diseases can lead to cognitive impairment in different ways. For example, a single stroke can cause dementia by damaging brain regions critical for cognition (strategic infarct dementia), while multiple strokes can cause stepwise cognitive deterioration through cumulative brain damage (multi infarct dementia) (Leys et al., 2005) (Pinkston et al., 2009). Most often VCI is due to small white matter lesions (leukoaraiosis) that are thought to interrupt neural pathways involved in cognition (Pinkston et al., 2009). A recent realization has been that AD and cerebrovascular diseases coexist in up to 60% of cases (Leys et al., 2005) (Pinkston et al., 2009). Thus, although cases of “pure” AD and vascular dementia can exist, in most cases the cognitive decline can be attributed to overlapping cerebrovascular and AD pathologies (Querfurth and LaFerla, 2010). The causes of leukoaraiosis and the overlap between AD and cerebrovascular diseases are discussed in the next sections.
What causes the white matter damage underlying VCI?
In the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL syndrome), a monogenic disease associated with dementia, extensive white matter damage is caused by notch-3 mutations in vascular smooth muscle leading to vascular insufficiency (Chabriat et al., 2009). In common forms of leukoaraiosis, however, the mechanism of the white matter damage remains unclear, although family history, hypertension, diabetes, and chronic smoking are well-established risk factors (Selnes and Vinters, 2006). The white matter lesions consist of demyelination, axonal loss, enlarged perivascular spaces, astrogliosis and microglial activation (Fernando et al., 2006) (Pantoni and Garcia, 1995). The wall of arterioles is thickened by accumulation of hyaline material (lipoialinosis) or completely disrupted (fibrinoid necrosis) leading to microhemorrhages (Fisher, 1968) (Pantoni and Garcia, 1995). Capillary density, resting CBF and cerebrovascular reactivity are reduced both in normal and affected white matter (Brown et al., 2007) (Mandell et al., 2008) (O’Sullivan et al., 2002).
Disruption of the arteriolar wall by amyloid, as observed in genetic or sporadic forms of cerebral amyloid angiopathy and in AD, is also associated with white matter lesions (Weller et al., 2009). Hypoxia-inducible genes and markers of endothelial activation are upregulated, suggesting hypoxia-ischemia (Fernando et al., 2006). A causative role for hypoxia-ischemia is also suggested by the fact that the blood supply to the subcortical and basal ganglia white matter is provided by terminal arterioles located at the watershed between two vascular territories: perforating arteries from the neocortex and penetrating branches arising from larger arteries at the base of the brain (Iadecola et al., 2009). Therefore, these areas may be particularly susceptible to vascular insufficiency, especially if the structure of the arterioles and their vasomotor function are altered. On the other hand, energy and blood flow requirements of white matter are much lower than those of gray matter, rendering the tissue, in theory, less vulnerable to anoxia-ischemia (Hertz, 2008). Therefore, alternative mechanisms leading to an increased intrinsic vulnerability to injury may also play a role. For example, risk factors for dementia could enhance white matter susceptibility to anoxia, as aging has been described to do (Baltan et al., 2008). Disruption of the BBB, an early event in leukoaraiosis, could lead to injury by producing perivascular edema, micro-hemorrhages and inflammation, contributing to demyelination (Farrall and Wardlaw, 2009). Demylinated axons may be more susceptible to ischemic injury due to their heightened metabolic requirements caused by loss of energy-efficient saltatory conduction and leaky sodium channels (Trapp and Stys, 2009). Furthermore, dysfunction within the endothelium may compromise the trophic effect that these cells exert on neurons and glia, contributing to brain dysfunction and damage (see section on repair mechanisms). Aging, inflammation, hypoxia and, possibly, other risk factors can also reduce the repair potential of the damaged white matter by impairing oligodendrocyte progenitors recruitment and differentiation (Back et al., 2002) (Pang et al., 2010) (Sim et al., 2002). Therefore, the mechanisms underlying leukoaraiosis might be distinct from those of ischemic cell death and are a fertile ground for new investigations.
Why does stroke increase the risk of dementia?
Stroke doubles the risk of developing dementia (Leys et al., 2005). Although, as discussed above, location, size, and number of ischemic lesions, as well as leukoaraiosis can account for the cognitive impairment in some cases, epidemiological and pathological evidence indicates that stroke also increases the risk for AD. Alzheimer pathology can facilitate the development of dementia in patients with ischemic injury and, vice-versa, ischemic injury can aggravate the cognitive deficits induced by AD (Fotuhi et al., 2009). These findings have suggested a previously unrecognized interaction between amyloid pathology and ischemic injury. This is not surprising considering that Abeta has powerful cerebrovascular effects. Abeta constricts cerebral vessels, suppresses vascular reactivity and impairs autoregulation, leading to vascular insufficiency and increased susceptibility to ischemic injury (Iadecola, 2004) (Zhang et al., 1997). Conversely, ischemia and hypoxia can induce Abeta accumulation by promoting its cleavage from APP (Tesco et al., 2007) and by downregulating the lipoprotein related receptor protein-1, a critical receptor for the vascular clearance of Abeta (Bell et al., 2009) (Tesco et al., 2007) (Wu et al., 2005). Therefore, in a vicious cycle, the vascular effects of Abeta aggravate ischemia, and, in turn, brain ischemia enhances Abeta accumulation (Iadecola, 2004). Recent studies have also linked plasma Abeta to the white matter damage underlying leukoaraiosis (Gomis et al., 2009) (Gurol et al., 2006). The significance of this finding and its underlying mechanisms remain to be defined and placed in context with other findings showing that Abeta could promote axonal injury directly through its cytotoxic effects (Querfurth and LaFerla, 2010) or indirectly by inducing vasoconstriction and ischemia (Iadecola, 2004). Therefore, the vascular dysfunction induced by circulating and parenchymal Abeta may act in concert with the structural and functional vascular changes induced by risk factors to amplify the vascular insufficiency in small white matter arterioles leading to white matter injury. However, factors other than Abeta could also contribute to the interplay between AD pathology and cerebrovascular diseases. For example, cerebral arterioles of AD patients exhibit a tendency to constrict due to increased activity of transcription factors (serum response factor/myocardin) that control the expression of contractile proteins in smooth muscle cells (Chow et al., 2007). This finding may play a role in the increased vasoconstrictor tone and reduced CBF observed in AD (Iadecola, 2004). It remains to be established whether these changes in gene expression are related to Abeta or to upstream molecular events independent of it.
How does the brain repair itself after stroke?
A well-known fact in clinical stroke is that most patients show some degree of recovery over time. As the brain recovers, considerable remapping of cortical areas take place. Functional MRI studies demonstrate that peri-infarct areas are highly dynamic (Dijkhuizen et al., 2003) (Chopp et al., 2007). Representational areas shift as latent networks are unmasked and parallel circuits are recruited adjacent to damaged regions (Murphy and Corbett, 2009). More recently, advanced optical imaging techniques combined with electrophysiology demonstrated that this remodeling at a circuit level can be correlated with specific processes of dendritic and synaptic plasticity at a cellular level (Li and Murphy, 2008). Imaging of mitochondrial function (Liu and Murphy, 2009) and vascular dynamics (Schaffer et al., 2006) have provided new insight into how the brain responds to microvascular perturbations following ischemia. These powerful tools may eventually allow one to interrogate mechanisms of stroke recovery at a molecular and cellular level while testing pro-recovery therapies and strategies (Murphy and Corbett, 2009; Zhang and Chopp, 2009).
Repair and remodeling processes after stroke
A major advance within this past decade has been the realization that adult mammalian brains also exhibit focal areas of ongoing neurogenesis in the subventricular zone and subgranular zone. Under normal conditions, these newborn neurons migrate to olfactory regions and hippocampus. But after cerebral ischemia or hemorrhage, the birth of new cells seems to increase and neuroblasts re-route towards damaged brain tissue (Arvidsson et al., 2002); (Parent et al., 2002), perhaps as part of an endogenous attempt by the brain to repair itself. However, whether these new neurons significantly contribute to functional recovery remains unclear. Neurogenic responses may persist for surprisingly long periods of time, but the majority of these newborn cells survives only for a few days (Arvidsson et al., 2002). Whether these cells develop normally, express neurotransmitters or even integrate into existing neural networks remain to be fully elucidated. Future attempts to promote endogenous neurogenesis for therapeutic purposes will have to address ways to modify parenchymal micro-environments to enhance neuronal survival and integrate newly born cells into viable circuitry.
In concert with neurogenesis and neural plasticity, the brain recovering from stroke exhibits complex patterns of vascular remodeling. Angiogenesis and vasculogenesis in peri-infarct regions have been detected in rodent models of cerebral ischemia (Ding et al., 2008) as well as in human stroke (Krupinski et al., 1996). Indeed, it is now recognized that angiogenic and neurogenic responses are tightly co-regulated after stroke and brain injury (Arai et al., 2009). This might not be surprising since molecular mechanisms of neurogenesis and angiogenesis have been evolutionarily conserved so that similar mediators and pathways are involved in both phenomena (Carmeliet and Tessier-Lavigne, 2005). In the normal brain, the neurovascular niche defines these complex mechanisms of cell-cell signaling between cerebral endothelium and neural precursors in the subventricular and subgranular zones of ongoing neurogenesis. In the context of post-stroke recovery, these close relationships between neurogenesis and angiogenesis are maintained. Neuroblasts migrate along perivascular routes (Thored et al., 2007). Promotion of neurogenesis enhances vascular regrowth and conversely, angiogenic stimulation enhances neurogenesis (Ohab et al., 2006; Taguchi et al., 2004). Hence, it seems likely that brain recovery after stroke comprises inter-dependent neurovascular plasticity and remodeling processes that recruit multiple common mediators and signals (Snapyan et al., 2009). The new frontier of neurovascular repair may require a detailed exploration of how these multi-cell and multi-signal phenomena are regulated in the context of stroke. Recent experimental studies have suggested that pharmacologic interventions such as EPO, statins, activated protein C, and phosphodiesterase inhibitors may promote functional recovery after cerebral ischemia (Wang et al., 2004) (Zhang et al., 2006) (Thiyagarajan et al., 2008) (Chen et al., 2009). Ultimately, therapies that can augment these endogenous signals and substrates of neurovascular remodeling may have long therapeutic time windows in stroke (Zhang and Chopp, 2009).
Moving forward: charting a course toward new stroke therapies
Tremendous progress has been made in the understanding of fundmental mechanisms of neuronal cell death. However, the translation of these powerful molecular and cellular principles into clinically effective neuroprotective therapies in stroke has been challenging. More recently, emerging data from both experimental and clinical studies now suggest that all cell types in the brain participate in the complex pathophysiology of brain injury following stroke. Consequently, therapeutic approaches should target multiple cell types in an attempt to protect their structural and functional integrity, and their reciprocal interactions.
Protecting the Neurovascular Unit
The so-called “neurovascular unit” provides a conceptual framework that integrates responses in all cell types, including neuronal, glial and vascular elements (del Zoppo, 2009) (Iadecola, 2004) (Zacchigna et al., 2008) (fig. 3). Cell-cell interactions in the neurovascular unit form the basis for brain function. Dysfunctional signaling in the neurovascular unit underlies the basis for disease. Whereas the intricate molecular pathways of cell death in the neuron have been dissected in detail, the mechanisms of how the entire neurovascular unit responds to stroke are not completely understood. As discussed in the previous sections, cerebral ischemia can rapidly induce perturbations in cell-cell signaling and function within the neurovascular unit. How each cell type succumbs or adapts to injury is dependent on responses in other cells. For example, genomic and proteomic responses in neurons subjected to metabolic stress have been fairly well described. But how each cell type might alter its response to vascular risk factors and ischemia in the context of other elements in the neurovascular unit remains to be elucidated. As the nexus of cell-cell interactions grows, a systems biology approach may eventually be required to rigorously define mechanisms as well as targets for complete neurovascular protection. The importance of cell-cell signaling in the neurovascular unit has been underscored by many studies (Arai and Lo, 2009b) (Dugas et al., 2008) (Eroglu et al., 2009), (Guo et al., 2008). Hence, under normal conditions, trophic coupling within the neurovascular unit helps maintain homeostasis. In contrast, under diseased conditions, it is likely that loss of coupling would lead to pathophysiology (Grammas et al., 1999) (Nagai et al., 2007). Loss of trophic coupling is also likely to play a role in the way vascular risk factors render neurons and glia more vulnerable, such as in the link between leukoaraiosis and brain dysfunction underlying cognitive impairment. Recently, BDNF has been implicated as a candidate factor that mediates trophic coupling between vascular and neuronal compartments (Arai and Lo, 2009a) (Guo et al., 2008). Insofar as BDNF polymorphisms have been implicated in modulating synaptic plasticity (Kleim et al., 2006), these neurovascular trophic signals may also influence mechanisms of recovery after stroke.
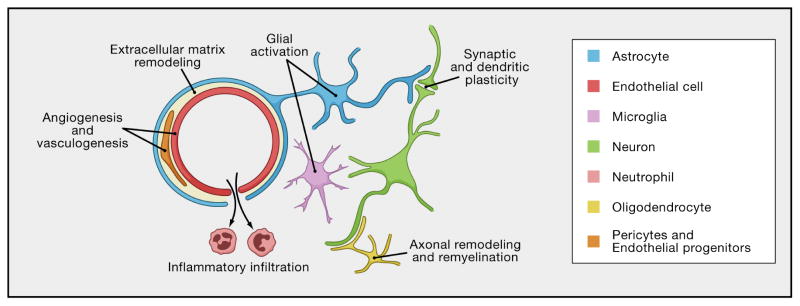
Figure 3. The Neurovascular Unit.
This schematic depicts a cerebral blood vessel and surrounding brain parenchyma that comprise the neurovascular unit. The neurovascular unit encompasses cell-cell and cell-matrix interactions between component vascular, glial and neuronal cells. Homeostatic signaling in the neurovascular unit sustains normal brain function. Dysfunctional neurovascular signaling mediates injury after stroke. During the recovery phase after stroke, neurovascular signaling may also be critically important with repair mechanisms that may involve angiogenesis, vasculogenesis and endothelial precursor mechanisms. As the blood-brain barrier heals, inflammation may convert from a deleterious to beneficial role. Reactive glia (astrocytes and pericytes) may secrete trophic factors. Synaptic and dendritic plasticity as well as axonal remyelination may provide parenchymal substrates for functional recovery. Emerging data now suggest that these phenomenon do not occur in isolation. All components of the NVU are likely to remodel in synchrony (Arai et al, 2009; Murphy and Corbett, 2009; Zhang and Chopp, 2009).
The concept of the neurovascular unit may provide an integrated framework for investigating mechanisms and therapies. Salvaging neurons alone may not be sufficient. One has to protect glial and vascular elements as well. Future investigations might be better served by pursuing targets that are expressed on multiple cell types in brain. Furthermore, prevention of cell death alone may also not be sufficient. In order for therapies to be clinically meaningful, cellular function must be preserved and/or restored. Neurons that are alive but lack proper connectivity or do not express the correct array of transmitter release-reuptake and synaptic activity may not be functional. Astrocytes that survive but cannot provide hemodynamic coupling would be unable to link neuronal activation with the required vascular responses. Oligodendrocytes that are respiring but metabolically impaired might not provide the necessary myelination for axonal conduction. Endothelial cells that survive but do not maintain blood-brain barrier properties would facilitate damage to adjacent parenchyma. Ultimately, any stroke therapy must include both prevention of cell death as well as rescue of integrated neurovascular function. Ischemic tolerance and post-ischemic hypothermia are some of the most potent protective strategies for ischemic brain injury and represent examples of multimodal therapies targeting the neurovascular unit as a whole. These are described in the next sections.
How does the brain protect itself? Lessons from ischemic tolerance
Some of the very same mechanisms implicated in the tissue damaging responses after stroke can also be engaged to protect the brain after threatened injury and to induce ischemic tolerance (IT). IT is a concerted organ response to protect itself against tissue injury and has been widely studied over the past 3 decades in an effort to identify endogenous mechanisms of protection and to exploit them for therapeutic purposes (Obrenovitch, 2008). In its delayed form, it is induced after challenge by a subthreshold noxious stimulus, developing within 1–3 days and lasting for several additional days. Experimentally it can be induced by exposure to preconditioning stimuli such as neurotoxins (e.g., glutamate) substrate deprivation (e.g., ischemia, hypoxia), or inflammation (e.g., cytokines and immune responses) and engages mechanisms that detect, transduce and promote a complex tissue reaction that reflects a fundamental genomic reprogramming after threatened injury (Dirnagl et al., 2009) (Gidday, 2006) (Stenzel-Poore et al., 2004). In this altered physiological state, tissue damage is mitigated when challenged by exposure to a wide range of deleterious stimuli, even if unrelated to the initial challenge.
IT is mediated by highly redundant cellular, molecular and physiological mechanisms involving neurons, astrocytes and vascular cells (Gidday, 2006). Upstream sensors have been implicated including adenosine and its A1 receptor, the NR2A NMDA receptor subunit, and the transcriptional regulator hypoxia inducible factor (HIF). HIF is an attractive candidate because it regulates a constellation of genes including those whose protein products facilitate oxygen transport (erythropoietin), regulate glycolytic metabolism (e.g., glucose transporters), and promote angiogenesis (e.g., VEGF), by way of example. Unexpectedly, genetic deletion of HIF1α improves infarct volume after middle cerebral artery occlusion, implicating as yet unknown pathogenic mechanisms activated by ischemia (Helton et al., 2005). Erythropoietin, one of the gene products of HIF1α, promotes PI3 kinase/AKT activity and the expression of NFkB-dependent protective genes (Digicaylioglu and Lipton, 2001). Erythropoietin also downregulates hypoxia-induced pro-apoptotic mechanism (Noguchi et al., 2007) (Prass et al., 2003) (Ruscher et al., 2002), and erythropoietin-based therapies have been investigated in the clinical arena (Ehrenreich et al., 2009). Heat shock protein family members are upregulated in ischemic tolerance and, based on their multiple cytoprotective actions, may promote survival. Preconditioning stimuli preserve connexin 43 hemichannels in astrocytes facilitating ATP released from these cells and increasing the extracellular concentration of the beneficial nucleoside adenosine (Lin et al., 2008). At the same time, preconditioning safeguards the function of cerebral blood vessels from the deleterious effects of cerebral ischemia resulting in improved penumbral flow and reduced brain damage (Kunz et al., 2007b). These astrocytic and vascular changes are coupled with mechanisms that reduce energy demands, lessen inflammatory responses, and ion channel activity, and decrease cascades promoting blood clotting and apoptotic cell death (Dirnagl et al., 2009) (Obrenovitch, 2008) (Gidday, 2006). Factors involving mitochondria as well as its uncoupling proteins have also been suggested and include improved efficiency of ATP synthesis, preservation of the mitochondrial membrane potential and delayed opening of mitochondrial transition pore formation, as well as opening of mitochondrial ATP-dependent K+ channels (Dirnagl and Meisel, 2008). One of the key questions defying explanation at the moment is how and why diverse preconditioning stimuli targeting single genes or key steps in seemingly unrelated cascades all impact infarct injury in genetically engineered and wild type mice. It may be telling us that the mechanisms through which the brain protects itself involve a highly integrated network response that emphasizes multiple cells within the neurovascular unit. If true, comparable multicellular approaches are needed that capitalize on this integrated response in the design of successful treatments (see next section).
Therapeutic Hypothermia: Engaging Pleiotropic Mechanisms in Multiple Cell Types
The therapeutic hypothermia field has advanced significantly during the past decade, inspired by positive outcomes from clinical trials in cardiac arrest victims (Bernard et al., 2002). Satisfactory neurological recovery was achieved in about half of the treated patients when treatment was begun within 2 hrs of cardiac arrest. Promising results were reported in small feasibility studies in neonatal ischemia (Shankaran et al., 2005). Current efforts are directed towards translating these successes in cardiac arrest and neonatal ischemia to patients with ischemic stroke, but there remain unresolved technical issues surrounding the timing and method for inducing hypothermia, and the deleterious effect of re-warming (Wagner and Zuccarello, 2005). It is now well accepted that hypothermia reduces ischemia in animal models of stroke, and so far, preclinical results have helped guide the design of clinical trials in human subjects. Combining hypothermia with thrombolysis is one example. Experimental evidence suggests that hypothermia protects the brain through pleiotropic effects acting on multiple cell types within the neurovascular unit. Hypothermia reduces brain metabolism, thereby significantly decreasing oxygen consumption and the work load of the entire organ (Sakoh and Gjedde, 2003). Hypothermia also reduces glutamate release, ROS production, and peri-infarct depolarizations. Inflammatory markers decrease, as well as microglial activation, leucocyte infiltration, MMP activation and BBB disruption. In addition, hypothermia suppresses apoptotic cell death, decreases mitochondrial release of cytochrome C and apoptosis inducing factor (see for review (Tang and Yenari, 2009)). Cell survival pathways are upregulated and appear to contribute to the multiplicity of neuroprotective mechanisms. To what extent each of these promotes tissue protection or interacts synergistically to enhance tissue survival is not known and remains for further investigation. Nevertheless, therapeutic hypothermia does illustrate the potential power of approaching stroke therapy using combinations of modalities that engage multiple cell types to enhance protection and recovery.
Additional strategies for repair and recovery: trophic factors and cell based therapies
No matter how successful acute neuroprotective strategies become, many stroke patients may still fall outside of clinical time windows for effective treatment. Hence, approaches that promote repair and recovery will be essential for an integrated stroke armamentarium. Logically, these approaches can be divided into two categories – trophic factor treatments that seek to amplify and augment endogenous processes of neurovascular plasticity and recovery, and cell-based therapies that seek to replace damaged or lost brain cells. Many approaches that augment endogenous recovery have been explored. Growth factors such as NGF, FGF, GDNF and BDNF have all met with variable degrees of success in animal models (Semkova and Krieglstein, 1999). But a limiting factor in many of these studies is their relatively large molecular weight, making transport into brain difficult. Some alternatives may yet exist such as smaller peptides, transnasal delivery of growth factors to bypass the blood brain barrier (Fletcher et al., 2009), peptidomimetics and compounds that can upregulate endogenous trophic factor production but do not require direct infusion into brain (Zhang and Chopp, 2009). Additionally, one may also target inhibitory molecules that accumulate in the area surrounding the injury and block neuronal repair, such as chondroitin sulfate proteoglycans, myelin associated proteins, NOGO and semaphorins (Silver and Miller, 2004).
Although stroke-damaged brain usually exhibits varying degrees of spontaneous recovery, there will always be significant areas where endogenous repair is insufficient. In this regard, cell-based therapies may represent a new frontier. To date, a variety of cell sources have been tested including neural stem cells, bone marrow-derived mesenchymal cells as well endothelial progenitor cells (Koch et al., 2009). Treatments have typically involved direct transplantations into brain parenchyma, catheter infusions into cerebral ventricles, as well as systemic injections into circulating blood. The latter approach is especially intriguing, as complex mechanisms of cell targeting seem to be operational in brain. Infarcted brain tissue upregulates and releases various chemokines such as SDF-1, to establish gradients that broadly serve to attract stem and precursor cell populations (Madri, 2009). Induced pluripotent stem cells may also provide an alternative cell source to embryonic stem cells (Takahashi and Yamanaka, 2006). One advantage is the theoretical possibility that cell repair can be custom-designed to suit specific diseases or individual needs, as described in a flurry of recent studies showing that neurons can be successfully derived from pluripotent cells taken from patients with Huntingtons disease, ALS and spinal muscular atrophy (Dimos et al., 2008) (Ebert et al., 2009) (Park et al., 2008). Whether similar methods can be applied to stroke victims remains to be tested.
Overall, cell-based therapies in models of experimental stroke suggest that delayed repair is a viable treatment approach. But more work is needed to translate these promising results into effective stroke therapies. Many questions remain and the precise mechanisms of cell-induced repair are still unclear. For example, do the reported benefits occur because cells are being explicitly replaced or because transplanted cells serve as a source of trophic factor production? How well do transplanted cells survive? Will adjunctive therapies to decrease apoptotic dropout be necessary? If cells survive, how well do they integrate into existing neural or vascular networks? What is the optimum timing for such therapy? What types of cells should be used? And finally, what subtypes of stroke patients should be selected for robust clinical trial results?
As the basic mechanisms of trophic and cell-based repair continue to be defined, an interesting underlying theme has emerged that links the survival of newborn or transplanted cells to the host environment. As discussed in the previous sections, activated vascular cells and microglia may be detrimental to cell survival in the early stages of cerebral ischemia, due to production of cytokines, ROS and toxic amount of NO. In the recovery phase, however, chronically activated microglia may now secrete beneficial factors such as BDNF and GDNF. Hence, prolonged inflammation can serve biphasic roles – dampening cellular recovery acutely but then enhancing tissue repair later on. In fact, this principle of biphasic mediators may be broadly applicable in stroke pathophysiology. There are many examples. Over-activation of NMDA receptors induces acute excitotoxicity (Besancon et al., 2008), but without NMDA signaling, chronic neuronal remodeling cannot take place (Young et al., 1999). Matrix metalloproteinases degrade and damage neurovascular substrates in the acute phase (Rosenberg, 2009), but the same proteases are critically important for neurovascular remodeling during the recovery phase (Zhao et al., 2006) (Lee et al., 2006). The intracellular mediator HMGB1 promotes necrosis and expands the core infarct during acute cerebral ischemia (Qiu et al., 2008) but during the repair phase, HMGB1 release from reactive astrocytes promotes angiogenesis and synaptic plasticity (Hayakawa et al., 2010a) (Hayakawa et al., 2010b) Altogether, the boundaries between cell death and cell repair may be blurred (Lo, 2008b). Future studies that dissect these biphasic mechanisms should prove fruitful if we are to develop clinically meaningful strategies for both neurovascular protection as well as repair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Because of the vastness of this research area, we have chosen to cite reviews whenever possible and apologize to our colleagues whose important original contributions were not acknowledged.
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Aarts MM, Tymianski M. TRPMs and neuronal cell death. Pflugers Arch. 2005;451:243–249. doi: 10.1007/s00424-005-1439-x. [DOI] [PubMed] [Google Scholar]
- Abe T, Kunz A, Shimamura M, Zhou P, Anrather J, Iadecola C. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab. 2009;29:66–72. doi: 10.1038/jcbfm.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- Allen CL, Bayraktutan U. Risk factors for ischaemic stroke. Int J Stroke. 2008;3:105–116. doi: 10.1111/j.1747-4949.2008.00187.x. [DOI] [PubMed] [Google Scholar]
- Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. Febs J. 2009;276:4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Lo EH. Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. Journal of neuroscience research. 2009a;88:758–763. doi: 10.1002/jnr.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009b;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrick DM, Sharpe GM, Sun H, Mayhan WG. nNOS-dependent reactivity of cerebral arterioles in Type 1 diabetes. Brain Res. 2007;1184:365–371. doi: 10.1016/j.brainres.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature medicine. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke; a journal of cerebral circulation. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back T, Ginsberg MD, Dietrich WD, Watson BD. Induction of spreading depression in the ischemic hemisphere following experimental middle cerebral artery occlusion: effect on infarct morphology. J Cereb Blood Flow Metab. 1996;16:202–213. doi: 10.1097/00004647-199603000-00004. [DOI] [PubMed] [Google Scholar]
- Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR. White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. J Neurosci. 2008;28:1479–1489. doi: 10.1523/JNEUROSCI.5137-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano D, Munarriz E, Chen HL, Ziviani E, Lippi G, Young KW, Nicotera P. The plasma membrane Na+/Ca2+ exchanger is cleaved by distinct protease families in neuronal cell death. Annals of the New York Academy of Sciences. 2007;1099:451–455. doi: 10.1196/annals.1387.006. [DOI] [PubMed] [Google Scholar]
- Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, et al. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. The New England journal of medicine. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends in pharmacological sciences. 2008;29:268–275. doi: 10.1016/j.tips.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol. 2002;441:1–14. doi: 10.1016/s0014-2999(02)01486-3. [DOI] [PubMed] [Google Scholar]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonita R, Duncan J, Truelsen T, Jackson RT, Beaglehole R. Passive smoking as well as active smoking increases the risk of acute stroke. Tob Control. 1999;8:156–160. doi: 10.1136/tc.8.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Won Suh S, Joon Won S, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009b;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257:62–66. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Roberts C, Chopp M. eNOS mediates TO90317 treatment-induced angiogenesis and functional outcome after stroke in mice. Stroke; a journal of cerebral circulation. 2009;40:2532–2538. doi: 10.1161/STROKEAHA.108.545095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH, Xiong ZQ. Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci. 2007;27:542–552. doi: 10.1523/JNEUROSCI.3607-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Moskowitz MA. Poly(ADP-ribose) polymerase-1 activity promotes NF-kappaB-driven transcription and microglial activation: implication for neurodegenerative disorders. Journal of neurochemistry. 2003;85:306–317. doi: 10.1046/j.1471-4159.2003.01684.x. [DOI] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. Journal of neurobiology. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annual review of neuroscience. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke; a journal of cerebral circulation. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci U S A. 2007;104:823–828. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Veliz LP, Bukauskas FF, Bennett MV, Saez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev. 2004;47:290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free radical biology & medicine. 2005;38:1433–1444. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Crowell RM, Marcoux FW, DeGirolami U. Variability and reversibility of focal cerebral ischemia in unanesthetized monkeys. Neurology. 1981;31:1295–1302. doi: 10.1212/wnl.31.10.1295. [DOI] [PubMed] [Google Scholar]
- Cui H, Hayashi A, Sun HS, Belmares MP, Cobey C, Phan T, Schweizer J, Salter MW, Wang YT, Tasker RA, et al. PDZ protein interactions underlying NMDA receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. J Neurosci. 2007;27:9901–9915. doi: 10.1523/JNEUROSCI.1464-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culebras A. Sleep and stroke. Semin Neurol. 2009;29:438–445. doi: 10.1055/s-0029-1237121. [DOI] [PubMed] [Google Scholar]
- Dauchet L, Amouyel P, Dallongeville J. Fruit and vegetable consumption and risk of stroke: a meta-analysis of cohort studies. Neurology. 2005;65:1193–1197. doi: 10.1212/01.wnl.0000180600.09719.53. [DOI] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature chemical biology. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–982. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, Emsley HC, Forconi S, Hopkins SJ, Masotti L, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36:1316–1329. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, Rosen BR, Lo EH. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003;23:510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science (New York, NY) 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, Gollapalli L, Panda S, Li Q, Ewing JR, Chopp M. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2*WI. Stroke; a journal of cerebral circulation. 2008;39:1563–1568. doi: 10.1161/STROKEAHA.107.502146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet neurology. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends in neurosciences. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, Brinker G, Dreier JP, Woitzik J, Strong AJ, Graf R. Spreading depolarizations occur in human ischemic stroke with high incidence. Annals of neurology. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Mandemakers W, Rogers M, Ibrahim A, Daneman R, Barres BA. A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons. J Neurosci. 2008;28:8294–8305. doi: 10.1523/JNEUROSCI.2010-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinger M, De Silva DA, Christensen S, Parsons MW, Markus R, Donnan GA, Davis SM. Imaging the penumbra - strategies to detect tissue at risk after ischemic stroke. J Clin Neurosci. 2009;16:178–187. doi: 10.1016/j.jocn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke; a journal of cerebral circulation. 2009;40:e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- Elkind MS. Why now? Moving from stroke risk factors to stroke triggers. Curr Opin Neurol. 2007;20:51–57. doi: 10.1097/WCO.0b013e328012da75. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. The New England journal of medicine. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1968;12:1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- Fletcher L, Kohli S, Sprague SM, Scranton RA, Lipton SA, Parra A, Jimenez DF, Digicaylioglu M. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. Journal of neurosurgery. 2009;111:164–170. doi: 10.3171/2009.2.JNS081199. [DOI] [PubMed] [Google Scholar]
- Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010 doi: 10.1007/s00424-010-0808-2. in press. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nature Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke; a journal of cerebral circulation. 2009;40:S111–114. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, Pickel VM, Iadecola C. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci. 2009;29:2545–2552. doi: 10.1523/JNEUROSCI.0133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis M, Sobrino T, Ois A, Millan M, Rodriguez-Campello A, Perez de la Ossa N, Rodriguez-Gonzalez R, Jimenez-Conde J, Cuadrado-Godia E, Roquer J, Davalos A. Plasma beta-amyloid 1–40 is associated with the diffuse small vessel disease subtype. Stroke. 2009;40:3197–3201. doi: 10.1161/STROKEAHA.109.559641. [DOI] [PubMed] [Google Scholar]
- Grammas P, Moore P, Weigel PH. Microvessels from Alzheimer’s disease brains kill neurons in vitro. The American journal of pathology. 1999;154:337–342. doi: 10.1016/S0002-9440(10)65280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- Gunnett CA, Lund DD, McDowell AK, Faraci FM, Heistad DD. Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:1617–1622. doi: 10.1161/01.ATV.0000172626.00296.ba. [DOI] [PubMed] [Google Scholar]
- Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, Rosand J, Growdon JH, Greenberg SM. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- Hankey GJ. Potential new risk factors for ischemic stroke: what is their potential? Stroke. 2006;37:2181–2188. doi: 10.1161/01.STR.0000229883.72010.e4. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature neuroscience. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Arai K, Lo EH. Role of ERK map kinase and CRM1 in IL-1beta-stimulated release of HMGB1 from cortical astrocytes. Glia. 2010a;58:1007–1015. doi: 10.1002/glia.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Nakano T, Irie K, Higuchi S, Fujioka M, Orito K, Iwasaki K, Jin G, Lo EH, Mishima K, Fujiwara M. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2010b;30:871–82. doi: 10.1038/jcbfm.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele RA, Dichgans M. Advances in stroke 2009: update on the genetics of stroke and cerebrovascular disease 2009. Stroke. 2010;41:e63–66. doi: 10.1161/STROKEAHA.109.571034. [DOI] [PubMed] [Google Scholar]
- Helton R, Cui J, Scheel JR, Ellison JA, Ames C, Gibson C, Blouw B, Ouyang L, Dragatsis I, Zeitlin S, et al. Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci. 2005;25:4099–4107. doi: 10.1523/JNEUROSCI.4555-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology. 2008;55:289–309. doi: 10.1016/j.neuropharm.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature immunology. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Relationships between lipoprotein components and risk of ischaemic and haemorrhagic stroke in the Apolipoprotein MOrtality RISk study (AMORIS) J Intern Med. 2009;265:275–287. doi: 10.1111/j.1365-2796.2008.02016.x. [DOI] [PubMed] [Google Scholar]
- Hu G, Sarti C, Jousilahti P, Silventoinen K, Barengo NC, Tuomilehto J. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke. 2005;36:1994–1999. doi: 10.1161/01.STR.0000177868.89946.0c. [DOI] [PubMed] [Google Scholar]
- Hu G, Tuomilehto J, Silventoinen K, Sarti C, Mannisto S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med. 2007;167:1420–1427. doi: 10.1001/archinte.167.13.1420. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell metabolism. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke; a journal of cerebral circulation. 2009;40:S40–44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Salkowski CA, Zhang F, Aber T, Nagayama M, Vogel SN, Ross ME. The Transcription Factor Interferon Regulatory Factor 1 Is Expressed after Cerebral Ischemia and Contributes to Ischemic Brain Injury. J Exp Med. 1999;189:719–727. doi: 10.1084/jem.189.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immink RV, van den Born BJ, van Montfrans GA, Koopmans RP, Karemaker JM, van Lieshout JJ. Impaired cerebral autoregulation in patients with malignant hypertension. Circulation. 2004;110:2241–2245. doi: 10.1161/01.CIR.0000144472.08647.40. [DOI] [PubMed] [Google Scholar]
- Jones TH, Morawetz RB, Crowell RM, Marcoux FW, FitzGibbon SJ, DeGirolami U, Ojemann RG. Thresholds of focal cerebral ischemia in awake monkeys. Journal of neurosurgery. 1981;54:773–782. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia R, Tureyen K, Bowen KK, Kalluri H, Johnson PF, Vemuganti R. Decreased brain damage and curtailed inflammation in transcription factor CCAAT/enhancer binding protein beta knockout mice following transient focal cerebral ischemia. Journal of neurochemistry. 2006;98:1718–1731. doi: 10.1111/j.1471-4159.2006.04056.x. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen TM, Suh SW, Berman AE, Hamby AM, Swanson RA. Inhibition of poly(ADP-ribose) polymerase suppresses inflammation and promotes recovery after ischemic injury. J Cereb Blood Flow Metab. 2009;29:820–829. doi: 10.1038/jcbfm.2009.9. [DOI] [PubMed] [Google Scholar]
- Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomized controlled trials. Lancet Neurol. 2007;6:782–292. doi: 10.1016/S1474-4422(07)70195-3. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nature medicine. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Kim YK, Schulman S. Cervical artery dissection: pathology, epidemiology and management. Thromb Res. 2009;123:810–821. doi: 10.1016/j.thromres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Kim YS, Immink RV, Stok WJ, Karemaker JM, Secher NH, van Lieshout JJ. Dynamic cerebral autoregulatory capacity is affected early in Type 2 diabetes. Clin Sci (Lond) 2008;115:255–262. doi: 10.1042/CS20070458. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Macvicar BA, Sontheimer H. Anion channels in astrocytes: biophysics, pharmacology, and function. Glia. 2006;54:747–757. doi: 10.1002/glia.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007;38:2136–2141. doi: 10.1161/STROKEAHA.107.481879. [DOI] [PubMed] [Google Scholar]
- Kizer JR, Wiebers DO, Whisnant JP, Galloway JM, Welty TK, Lee ET, Best LG, Resnick HE, Roman MJ, Devereux RB. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the Strong Heart Study. Stroke. 2005;36:2533–2537. doi: 10.1161/01.STR.0000190005.09442.ad. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature neuroscience. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Current opinion in neurology. 2009;22:294–301. doi: 10.1097/wco.0b013e32832b4db3. [DOI] [PubMed] [Google Scholar]
- Koch P, Kokaia Z, Lindvall O, Brustle O. Emerging concepts in neural stem cell research: autologous repair and cell-based disease modelling. Lancet neurology. 2009;8:819–829. doi: 10.1016/S1474-4422(09)70202-9. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke; a journal of cerebral circulation. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- Kunz A, Anrather J, Zhou P, Orio M, Iadecola C. Cyclooxygenase-2 does not contribute to postischemic production of reactive oxygen species. J Cereb Blood Flow Metab. 2007a;27:545–551. doi: 10.1038/sj.jcbfm.9600369. [DOI] [PubMed] [Google Scholar]
- Kunz A, Park L, Abe T, Gallo EF, Anrather J, Zhou P, Iadecola C. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci. 2007b;27:7083–7093. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2004a;35:776–785. doi: 10.1161/01.STR.0000116869.64771.5A. [DOI] [PubMed] [Google Scholar]
- Lawes CM, Parag V, Bennett DA, Suh I, Lam TH, Whitlock G, Barzi F, Woodward M. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004b;27:2836–2842. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in aging, disease and death: the true identity of a cell death impostor. Cell death and differentiation. 2009;16:1–2. doi: 10.1038/cdd.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- Li P, Murphy TH. Two-photon imaging during prolonged middle cerebral artery occlusion in mice reveals recovery of dendritic structure after reperfusion. J Neurosci. 2008;28:11970–11979. doi: 10.1523/JNEUROSCI.3724-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature medicine. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, et al. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Current drug targets. 2007a;8:621–632. doi: 10.2174/138945007780618472. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Pathologically activated therapeutics for neuroprotection. Nature reviews. 2007b;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- Liu RR, Murphy TH. Reversible cyclosporin A-sensitive mitochondrial depolarization occurs within minutes of stroke onset in mouse somatosensory cortex in vivo: a two-photon imaging study. The Journal of biological chemistry. 2009;284:36109–36117. doi: 10.1074/jbc.M109.055301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Lo EH. Experimental models, neurovascular mechanisms and translational issues in stroke research. British journal of pharmacology. 2008a;153(Suppl 1):S396–405. doi: 10.1038/sj.bjp.0707626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008b;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Loh E, Sutton MS, Wun CC, Rouleau JL, Flaker GC, Gottlieb SS, Lamas GA, Moye LA, Goldhaber SZ, Pfeffer MA. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med. 1997;336:251–257. doi: 10.1056/NEJM199701233360403. [DOI] [PubMed] [Google Scholar]
- Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60(Suppl 4):95–104. [PubMed] [Google Scholar]
- Mandell DM, Han JS, Poublanc J, Crawley AP, Kassner A, Fisher JA, Mikulis DJ. Selective reduction of blood flow to white matter during hypercapnia corresponds with leukoaraiosis. Stroke. 2008;39:1993–1998. doi: 10.1161/STROKEAHA.107.501692. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29:367–374. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Marcoux FW, Morawetz RB, Crowell RM, DeGirolami U, Halsey JH., Jr Differential regional vulnerability in transient focal cerebral ischemia. Stroke; a journal of cerebral circulation. 1982;13:339–346. doi: 10.1161/01.str.13.3.339. [DOI] [PubMed] [Google Scholar]
- Mies G, Iijima T, Hossmann KA. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport. 1993;4:709–711. doi: 10.1097/00001756-199306000-00027. [DOI] [PubMed] [Google Scholar]
- Mobius HJ, Stoffler A. New approaches to clinical trials in vascular dementia: memantine in small vessel disease. Cerebrovasc Dis. 2002;13(Suppl 2):61–66. doi: 10.1159/000049153. [DOI] [PubMed] [Google Scholar]
- Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke; a journal of cerebral circulation. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nature reviews. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nature neuroscience. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Lipton SA. According to GOSPEL: filling in the GAP(DH) of NO-mediated neurotoxicity. Neuron. 2009;63:3–6. doi: 10.1016/j.neuron.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeltchev K, Wiedmer S, Schwerzmann M, Windecker S, Haefeli T, Meier B, Mattle HP, Arnold M. Sex differences in cryptogenic stroke with patent foramen ovale. Am Heart J. 2008;156:461–465. doi: 10.1016/j.ahj.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Nellgard B, Wieloch T. NMDA-receptor blockers but not NBQX, an AMPA-receptor antagonist, inhibit spreading depression in the rat brain. Acta physiologica Scandinavica. 1992;146:497–503. doi: 10.1111/j.1748-1716.1992.tb09451.x. [DOI] [PubMed] [Google Scholar]
- Nicholl SM, Roztocil E, Davies MG. Plasminogen activator system and vascular disease. Curr Vasc Pharmacol. 2006;4:101–116. doi: 10.2174/157016106776359880. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Oxidative stress and energy crises in neuronal dysfunction. Ann N Y Acad Sci. 2008;1147:53–60. doi: 10.1196/annals.1427.002. [DOI] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- NINDS tPA Study Group. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. The New England journal of medicine. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Critical reviews in oncology/hematology. 2007;64:159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, Markus HS. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiological reviews. 2008;88:211–247. doi: 10.1152/physrev.00039.2006. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Current opinion in immunology. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Rigaud AS, Stoffler A, Mobius HJ, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial. Stroke. 2002;33:1834–1839. doi: 10.1161/01.str.0000020094.08790.49. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Campbell L, Zheng B, Fan L, Cai Z, Rhodes P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. 2010;166:464–475. doi: 10.1016/j.neuroscience.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. The significance of cerebral white matter abnormalities 100 years after Binswanger’s report. A review. Stroke. 1995;26:1293–1301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Annals of neurology. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepine CJ. The impact of nitric oxide in cardiovascular medicine: untapped potential utility. Am J Med. 2009;122:S10–15. doi: 10.1016/j.amjmed.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Pinkston JB, Alekseeva N, Gonzalez Toledo E. Stroke and dementia. Neurol Res. 2009;31:824–831. doi: 10.1179/016164109X12445505689643. [DOI] [PubMed] [Google Scholar]
- Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke; a journal of cerebral circulation. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Annals of neurology. 2009;66:378–389. doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet neurology. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, Warlow CP, Barnett HJ. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116. doi: 10.1016/s0140-6736(03)12228-3. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Howard SC, Power DA, Gutnikov SA, Algra A, van Gijn J, Clark TG, Murphy MF, Warlow CP. Fibrinogen concentration and risk of ischemic stroke and acute coronary events in 5113 patients with transient ischemic attack and minor ischemic stroke. Stroke. 2004;35:2300–2305. doi: 10.1161/01.STR.0000141701.36371.d1. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoh M, Gjedde A. Neuroprotection in hypothermia linked to redistribution of oxygen in brain. American journal of physiology. 2003;285:H17–25. doi: 10.1152/ajpheart.01112.2002. [DOI] [PubMed] [Google Scholar]
- Sakowitz OW, Kiening KL, Krajewski KL, Sarrafzadeh AS, Fabricius M, Strong AJ, Unterberg AW, Dreier JP. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke; a journal of cerebral circulation. 2009;40:e519–522. doi: 10.1161/STROKEAHA.109.549303. [DOI] [PubMed] [Google Scholar]
- Schaffer CB, Friedman B, Nishimura N, Schroeder LF, Tsai PS, Ebner FF, Lyden PD, Kleinfeld D. Two-photon imaging of cortical surface microvessels reveals a robust redistribution in blood flow after vascular occlusion. PLoS Biol. 2006;4:e22. doi: 10.1371/journal.pbio.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Experimental neurology. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neurol. 2006;2:538–547. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- Semkova I, Krieglstein J. Neuroprotection mediated via neurotrophic factors and induction of neurotrophic factors. Brain Res Brain Res Rev. 1999;30:176–188. doi: 10.1016/s0165-0173(99)00013-2. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England journal of medicine. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nature medicine. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T, Wasiewski WW, Emeribe U. NXY-059 for the treatment of acute ischemic stroke. The New England journal of medicine. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nature Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon RP. Acidotoxicity trumps excitotoxicity in ischemic brain. Archives of neurology. 2006;63:1368–1371. doi: 10.1001/archneur.63.10.1368. [DOI] [PubMed] [Google Scholar]
- Simons LA, McCallum J, Friedlander Y, Simons J. Risk factors for ischemic stroke: Dubbo Study of the elderly. Stroke. 1998;29:1341–1346. doi: 10.1161/01.str.29.7.1341. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiological reviews. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9854–9859. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Simon RP. Genomics of preconditioning. Stroke; a journal of cerebral circulation. 2004;35:2683–2686. doi: 10.1161/01.STR.0000143735.89281.bb. [DOI] [PubMed] [Google Scholar]
- Sughrue ME, Mehra A, Connolly ES, Jr, D’Ambrosio AL. Anti-adhesion molecule strategies as potential neuroprotective agents in cerebral ischemia: a critical review of the literature. Inflamm Res. 2004;53:497–508. doi: 10.1007/s00011-004-1282-0. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. The Journal of clinical investigation. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tang XN, Yenari MA. Hypothermia as a cytoprotective strategy in ischemic tissue injury. Ageing research reviews. 2009;9:61–68. doi: 10.1016/j.arr.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiyagarajan M, Fernandez JA, Lane SM, Griffin JH, Zlokovic BV. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke; a journal of cerebral circulation. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–291. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM, Balel C, Wang M, Jia N, Zhang W, et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140:222–234. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KR, Zuccarello M. Local brain hypothermia for neuroprotection in stroke treatment and aneurysm repair. Neurological research. 2005;27:238–245. doi: 10.1179/016164105X25261. [DOI] [PubMed] [Google Scholar]
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Bmj. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke; a journal of cerebral circulation. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. Journal of neuroimmunology. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nature medicine. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- Weller RO, Boche D, Nicoll JA. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer’s disease and their potential impact on therapy. Acta Neuropathol. 2009;118:87–102. doi: 10.1007/s00401-009-0498-z. [DOI] [PubMed] [Google Scholar]
- Welsh P, Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc Dis. 2009;27:247–253. doi: 10.1159/000196823. [DOI] [PubMed] [Google Scholar]
- Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia; blocking calcium-permeable Acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Yamaji K, Wang Y, Liu Y, Abeyama K, Hashiguchi T, Uchimura T, Krishna Biswas K, Iwamoto H, Maruyama I. Activated protein C, a natural anticoagulant protein, has antioxidant properties and inhibits lipid peroxidation and advanced glycation end products formation. Thrombosis research. 2005;115:319–325. doi: 10.1016/j.thromres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nature medicine. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Molecular cell. 2006;23:1–12. doi: 10.1016/j.molcel.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14:469–477. doi: 10.1007/s10495-008-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nature reviews. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science (New York, NY) 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhang F, Eckman C, Younkin S, Hsiao KK, Iadecola C. Increased susceptibility to ischemic brain damage in transgenic mice overexpressing the amyloid precursor protein. J Neurosci. 1997;17:7655–7661. doi: 10.1523/JNEUROSCI.17-20-07655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zhang Z, Jiang N, Powers C. The expression of P- and E-selectins in three models of middle cerebral artery occlusion. Brain research. 1998;785:207–214. doi: 10.1016/s0006-8993(97)01343-7. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. Journal of neuroscience research. 2006;83:1213–1219. doi: 10.1002/jnr.20813. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet neurology. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nature medicine. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004a;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004b;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]