Abstract
The sequence encoding the N-propeptide of collagen I is characterized by significant conservation of amino acids across species; however, the function of the N-propeptide remains poorly defined. Studies in vitro have suggested that one activity of this propeptide might be to act as a feedback inhibitor of collagen I synthesis. To determine whether the N-propeptide contributed to decreased collagen content in SPARC-null mice, mice carrying a deletion of exon 2, which encodes the globular domain of the N-propeptide of collagen I, were crossed to SPARC-null animals. Mice lacking SPARC and expressing collagen I without the globular domain of the N-propeptide were viable and fertile. However, a significant number of animals developed abdominal hernias within the first 2 months of life with an approximate 20% penetrance (~35% of males). The dermis of SPARC-null/exon 2-deleted mice was thinner and contained fewer large collagen fibers in comparison with wild-type or in either single transgenic animal. The average collagen fibril diameter of exon 2-deleted mice did not significantly differ from wild-type mice (WT: 87.9 nm versus exon 2-deleted: 88.2 nm), whereas SPARC-null/exon 2-deleted fibrils were smaller than that of SPARC-null dermis (SPARC-null: 60.2 nm, SPARC-null/exon 2-deleted: 40.8 nm). As measured by hydroxyproline analysis, double transgenic skin biopsies contained significantly less collagen than those of wild-type, those of exon 2-deleted, and those of SPARC-null biopsies. Acetic acid extraction of collagen from skin biopsies revealed an increase in the proportion of soluble collagen in the SPARC-null/exon 2-deleted mice. These results support a function of the N-propeptide of collagen I in facilitating incorporation and stabilization of collagen I into the insoluble ECM and argue against a primary function of the N-propeptide as a negative regulator of collagen synthesis.
Keywords: Fibrillar collagen, Matricellular, Skin, Transgenic, Extracellular Matrix, osteonectin, BM40
1. Introduction
Collagen I is a critical component of tissues throughout the body of vertebrate animals. Genetic diseases that lead to decreases in collagen deposition impair tissue function whereas fibrotic deposition of collagen frequently results in organ failure. Hence, the characterization of factors that control collagen deposition are of critical importance.
Collagen I is synthesized as procollagen with N- and C-terminal propeptides that are generally considered to undergo proteolytic cleavage prior to incorporation into insoluble fibrillar extracellular matrices (ECM) (Chen and Raghunath, 2009). Whereas the C-propeptide is implicated in chain recognition within the endoplasmic reticulum, the functional significance of the evolutionarily conserved N-propeptide is poorly understood (Bornstein, 2002; Holmes et al., 1996; McLaughlin and Bulleid, 1998). To address the role of the N-propeptide of collagen I in tissues, a transgenic mouse was generated that produced mutant procollagen I without the globular domain of the N-propeptide of the collagen α1(I) protein (collagen I is composed of two α1(I) chains and one α2(I) chain). These mice were found to exhibit no overt morphological or phenotypic differences from wild-type (WT) mice. Specifically, secretion, processing, and fibrillogenesis appeared normal and indistinguishable from WT counterparts (Bornstein et al., 2002).
SPARC (osteonectin/BM40) is a matricellular protein implicated in collagen I deposition and fibrillogenesis (Bradshaw, 2009). SPARC-null mice are reported to exhibit reductions in collagen content in skin, heart, fat, and in association with tumor formation (Bradshaw et al., 2009; Bradshaw et al., 2003a; Bradshaw et al., 2003b; Brekken et al., 2003). In addition, these mice failed to exhibit a robust fibrosis in response to a number of different stimuli in lung, kidney, skin, and heart (Bradshaw et al., 2009; Puolakkainen et al., 2003; Strandjord et al., 1999; Taneda et al., 2003). As significant differences in collagen I mRNA have not been found in SPARC-null mice, a proposed function of SPARC as a facilitator of collagen deposition in the extracellular milieu has been put forth (Bradshaw, 2009; Bradshaw et al., 2003a). A number of functions have been attributed to SPARC including modulation of cell adhesion, proliferation, growth factor efficacy, and matrix metalloproteinase expression (Clark and Sage, 2008). To date, the majority of phenotypic differences characteristic of SPARC-null mice reside in alterations in ECM deposition and/or assembly and include osteopenia, cataractogenesis, and curly tails (Delany et al., 2003; Yan et al., 2002).
We have reported that SPARC-null dermal fibroblasts exhibited increased association of collagen I with cell surfaces in comparison with WT cells (Rentz et al., 2007). In addition, a higher proportion of the collagen I on SPARC-null cell surfaces was found in the form of fully processed collagen I with both propeptides removed. Hence, a function of SPARC in the regulation of the removal of procollagen propeptides was suggested (Rentz et al., 2007). Evidence from studies carried out in vitro has suggested that the N-propeptide might serve to function as a feed-back inhibitor of collagen production (Fouser et al., 1991). In the absence of SPARC, increases in N-propeptide generation might contribute to reductions in collagen content in SPARC-null tissues. In this case, the phenotypic reductions in collagen in SPARC-null mice would be predicted to diminish in mice that produced mutant procollagen I without the globular domain of the N-propeptide.
In this study, SPARC-null mice were crossed with mice carrying the exon 2- deleted mutant form of procollagen I to determine whether effects attributable to the N-propeptide contributed to the SPARC-null phenotype. Surprisingly, the mice carrying both transgenic deletions exhibited a collagen phenotype that was more severe than that of mice deficient only in SPARC. These data support the conclusion that SPARC is a critical regulator of collagen assembly and that the N-propeptide can effect the incorporation of collagen I into fibrillar ECM.
2. Results
2.1 Phenotypic Alterations in SPARC-null/Exon 2-deleted Mice
Generation of mice that produce mutant procollagen α1(I) without the globular domain of the N-propeptide of α1(I) (exon 2-deleted) and that did not express SPARC resulted in live offspring that followed predicted Mendelian ratios. In addition, mice carrying both deletions were fertile and capable of reproduction. However, a striking phenotype, characterized by abdominal protrusions, emerged in the litters during the first 2 months of life (Fig. 1). Although the protrusions were initially believed to reflect tumor growth, upon autopsy it was clear that abdominal herniations were the source of the protrusions. The hernias developed in approximately 20% of the SPARC-null/exon 2-deleted animals with a higher incidence in male mice (~35%). Hernias only occurred in young mice and were never observed in mice beyond 3 months of age, even in pregnant females. Hernias were never observed in mice of any other genotype.
Figure 1.
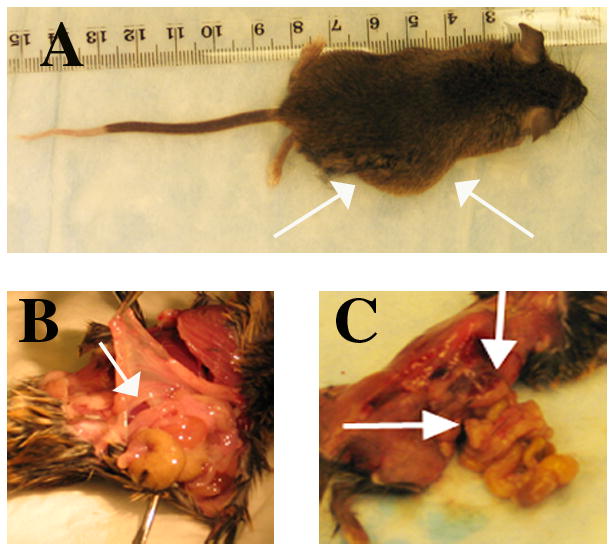
A fraction of SPARC-null/exon 2-deleted mice develop hernias within the first two months of age. White arrows denote an abdominal protrusion in A and sites of herniation in B and C.
In addition, mice that carried both SPARC and collagen I exon 2 genomic deletions exhibited a higher mortality rate than either deletion alone or in WT mice. The greatest loss of life occurred in animals 6–12 months of age at which time 21.4% of SPARC-null/exon 2-deleted mice had died whereas less than 5% of mice of either single deletion or WT genotype were found deceased at this age (a minimum of 85 animals in each colony contributed to this analysis). In most cases, the higher incidence of death in SPARC-null/exon 2-deleted mice was not preceded by signs of illness and was therefore attributed to sudden death.
2.2 Histological Analysis of Skin from SPARC-null/Exon 2-Deleted Mice
Previously, we had reported a decrease in the level of fibrillar collagen in the skin of SPARC-null mice whereas no detectable differences in the level of collagen were found in exon 2-deleted animals. To determine whether the absence of the N-propeptide of collagen I on a SPARC-null background further influenced dermal morphology, sections were taken from each genotype and stained with H&E. As shown in Fig. 2, the width of the dermis was substantially thinner in SPARC-null/exon 2-deleted animals versus SPARC-null mice (Fig. 2, D versus C). WT and exon 2-deleted mice demonstrated similar skin morphology, although sections from exon 2-deleted mice tended to have more dermal fat than WT (Fig. 2, A and B). The increase in subdermal adipose tissue was further increased in SPARC-null and SPARC-null/exon 2-deleted sections likely due to an enhanced subdermal adipose accumulation previously reported in SPARC-null mice (Bradshaw et al., 2003a).
Figure 2.
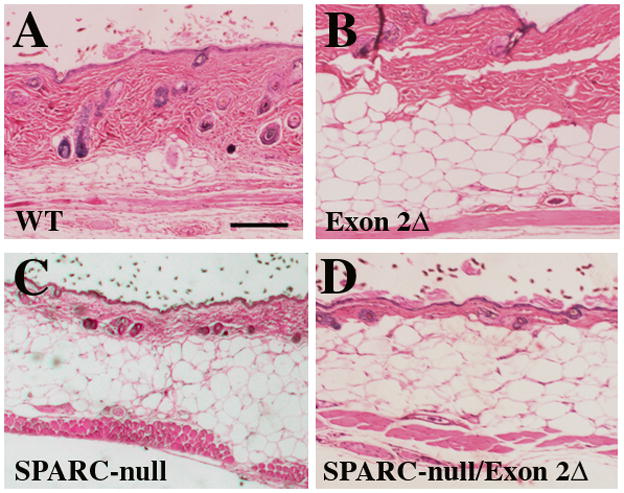
Representative sections of skin from age-matched mice at 3 months stained with hematoxylin and eosin: A) WT, B) Exon 2-deleted, C) SPARC-null, and D) SPARC-null/exon 2-deleted mice (D). Scale bar in A equals 1.0 mm.
Picrosirius red (PSR) stained sections were viewed under polarized light to evaluate fibrillar dermal collagen in the dermis of the four genotypes as shown in Fig. 3. Whereas the dermis of WT (Fig. 3A) and exon 2-deleted (Fig. 3B) were populated primarily with larger, red collagen fibers, SPARC-null and SPARC-null/exon 2-deleted sections had fewer large, red-stained fibers and an increase in the population of smaller, green-stained fibers. These results indicate that in the absence of SPARC expression combined with the absence of the N-propeptide of collagen I, the amount of dermal collagen in the ECM is decreased and that the collagen fiber quality in SPARC-null/exon-2 deleted differs from that of the three other genotypes.
Figure 3.
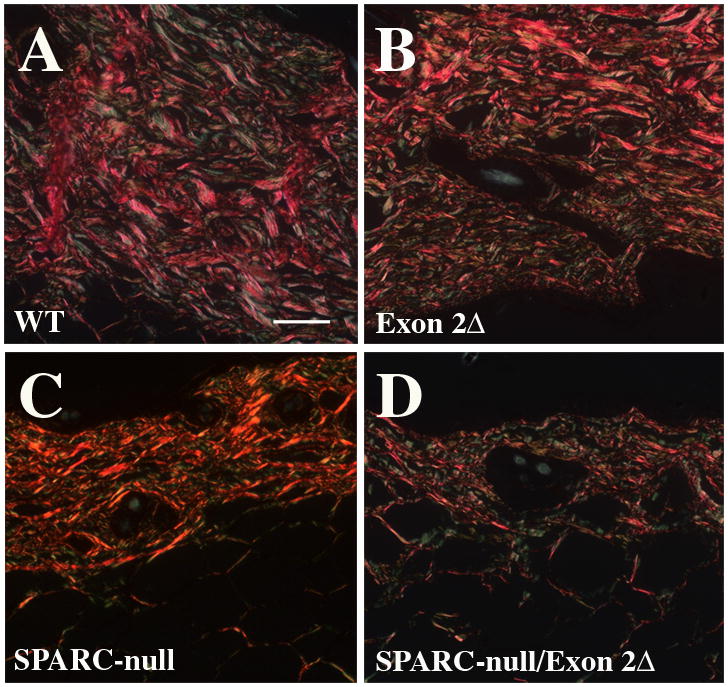
Representative sections of skin stained with PSR and viewed under polarized light. A) WT, B) Exon 2-deleted, C) SPARC-null, and D) SPARC-null/exon 2-deleted. Scale bar in A equals 50 μm.
2.3 Collagen Fibril Diameter
Transmission electron microscopy of dermal tissue from exon 2-deleted and SPARC-null/exon 2-deleted mice was carried out to assess differences in collagen fibril morphology and diameter in these mice. The morphology and distribution of collagen fibril diameters in the skin of Exon 2-deleted mice ~six months of age did not differ significantly from that of wild-type (Figure 4, A and C) (Bradshaw et al., 2003b). However, collagen fibrils that composed the dermis of SPARC-null/exon 2-deleted mice were sparse and not as densely packed as the other genotypes (Figure 4, B and D). Quantification of collagen fibril diameter demonstrated a smaller average diameter than that of the other three genotypes including previously published values for SPARC-null dermal fibrils (WT: 87.9 nm ± 31.6 (SD); Exon 2-deleted: 88.2 nm ± 33.5; SPARC-null: 60.2 nm ± 14.3; SPARC-null/exon 2-deleted: 40.8 nm ± 12.3). The distribution of collagen fibrils in mice without SPARC lacking the N-propeptide of collagen α1(I) demonstrated a leftward shift from that of the previously characterized SPARC-null fibrils and that of exon 2-deleted and wild-type histograms (Figure 4E) (Bradshaw et al., 2003b).
Figure 4.
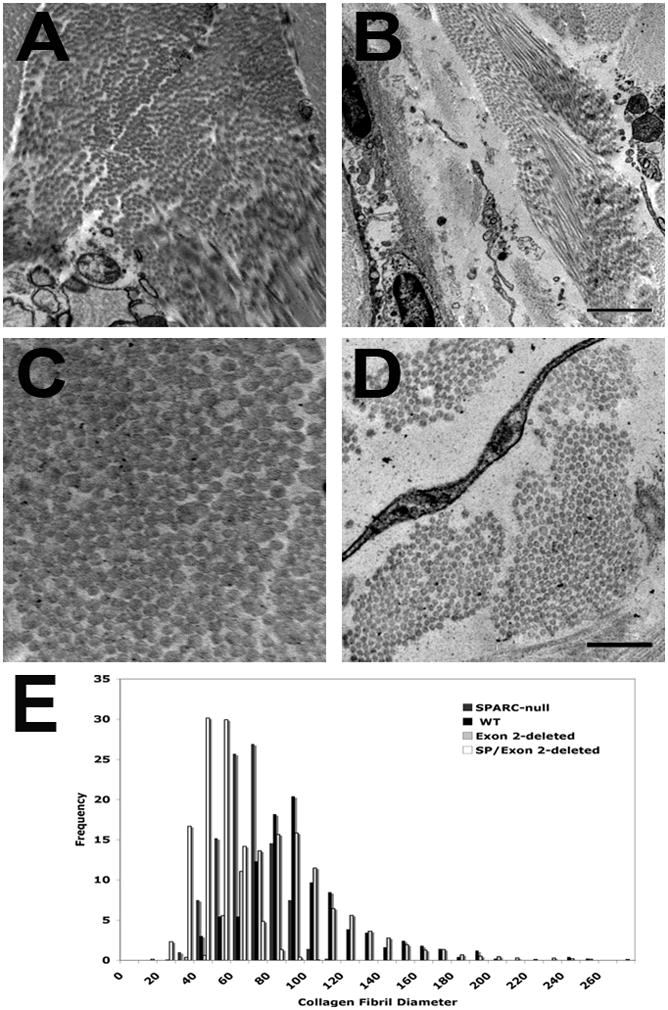
Representative EM images from Exon 2-deleted (A and C) and SPARC-null/Exon 2-deleted dermal sections. Bar in B equals 2 μm for panels A and B. Bar in D equals 500 nm for panels C and D. E) Collagen fibril diameter distribution for the four genotypes. WT (black bars), Exon 2-deleted (light gray bars), SPARC-null (dark gray bars), SPARC-null/exon 2-deleted (double deleted, white bars).
2.4 Biochemical Analysis of Dermal ECM Collagen
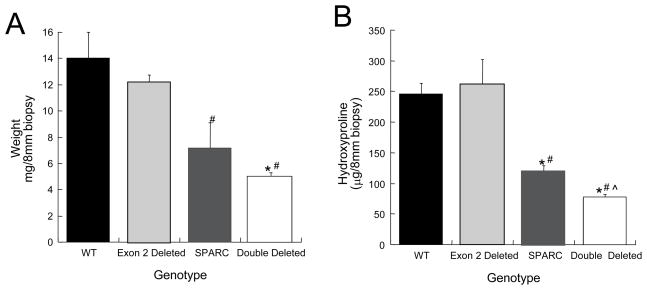
To quantify differences in levels of collagen in the skin of SPARC-null/exon 2-deleted mice versus the other relevant genotypes, hydroxyproline analysis of 8 mm biopsies was performed on samples taken from at least 4 mice per genotype. The dry weight of each biopsy is shown in Fig. 5A and revealed the extent to which the lack of collagen in the dermis influenced the overall weight of each sample. The biopsies from the double mutant mice weighed significantly less than WT and exon 2-deleted samples and were less than SPARC-null samples although this difference did not reach statistical significance. Hydroxyproline content of the biopsies revealed a similar trend with the exception that hydroxyproline levels in SPARC-null/exon 2-deleted samples were significantly less than all genotypes including that of SPARC-null. The SPARC-null biopsies contained significantly less hydroxyproline than WT or exon 2-deleted samples as expected from prior studies (Bradshaw et al., 2003b). Notably, exon 2-deleted biopsies were not significantly different than WT in either weight or hydroxyproline content, a finding that is consistent with previous analyses (Bornstein et al., 2002).
Figure 5.
A) Dry weight of skin biopsies and B) hydroxyproline analysis of 8 mm punch biopsies. Four animals per genotype and 2 biopsies per animal contributed to the analysis. WT (black bars), Exon 2-deleted (light gray bars), SPARC-null (dark gray bars), SPARC-null/exon 2-deleted (double deleted, white bars). Error bars represent standard error of the mean. # p<0.05 versus WT, * p<0.05 versus Exon 2-deleted, ^ p<0.05 versus SPARC-null.
Skin samples were treated with 1M NaCl followed by acetic acid to evaluate whether collagen I in the dermis of double mutant mice was extracted differently in comparison with that of the other genotypes. A representative experiment is shown in Fig. 6, in which acetic acid extracts separated by SDS-PAGE gel and stained with Coomassie blue are shown in the top panel and an immunoblot analysis using anti-murine collagen I antibodies of acetic acid extracts is shown in the bottom panel. Although consistent differences in the levels of collagen I soluble in 1M NaCl were not demonstrated between genotypes, greater amounts of collagen I were reproducibly detected in acetic-acid extracts from SPARC-null/exon 2-deleted samples versus all other genotypes. Given the lower levels of total collagen in the double mutant as determined by hydroxyproline analysis, the greater levels of extracted collagen I in these mice were substantial and indicated a deficiency in the incorporation of collagen I into stable insoluble ECM.
Figure 6.
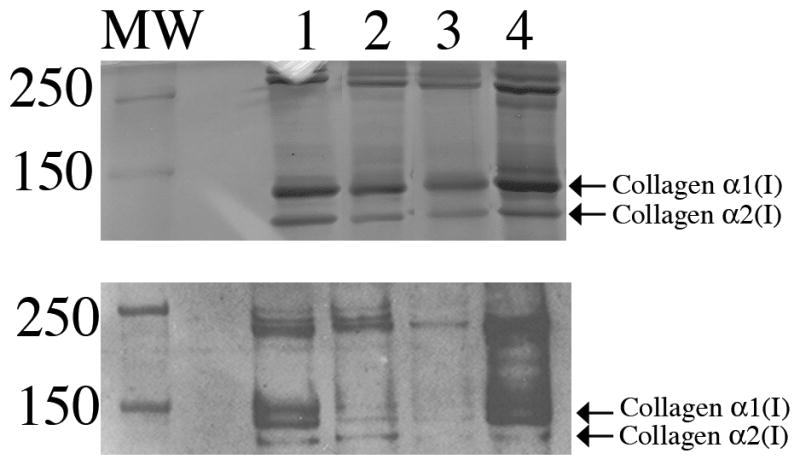
Acetic acid extraction of collagen from skin. Top panel: Coomassie-blue stained SDS-PAGE of collagen extracted in acetic acid in equal weight per volume. Equal volumes of acetic extracts were loaded. Bottom panel: samples shown in top panel analyzed by immunoblot analysis using anti-murine collagen I antibody. Lane 1: WT; Lane 2: exon 2-deleted, Lane 3: SPARC-null, Lane 4: SPARC-null/exon 2-deleted. MW: molecular weight markers.
3. Discussion
In this report, SPARC-null/exon 2-deleted collagen α1(I) mice were shown to demonstrate altered dermal collagen fiber assembly and deposition as compared with that of either individual transgenic mutation alone. Whereas mice expressing mutant procollagen I that lacks the globular domain of the N-propeptide exhibited no overt defects in collagen content or fibrillogenesis, expression of this mutant procollagen in the absence of SPARC expression resulted in a striking reduction in levels of dermal collagen content that were below that of SPARC-null mice. In addition, collagen fibril diameters in double mutant mice were smaller than that of SPARC-null dermis.
The development of abdominal herniations in the double mutant mice is, to our knowledge, a unique phenotype that has not been reported previously in transgenic mice at this age. Bone morphogenic protein 1-null transgenic mice exhibited delayed closure of the abdominal wall in new born pups that might underscore a common mechanism to that of the double mutants (Suzuki et al., 1996). However, BMP 1-null collagen fibrils exhibited abnormal surfaces indicative of retained C-propeptide, whereas SPARC-null/exon 2-deleted collagen fibrils were smaller but did not demonstrate aberrant surface morphology (Fig. 4). In the more than 10 years of maintaining SPARC-null mice on at least 2 different genetic backgrounds, herniations have never been observed. These observations also indicate that the herniations were clearly dependent upon the absence of SPARC in association with the absence of the N-propeptide. These results also suggest a function of the globular domain of the N-propeptide to promote collagen fiber assembly and stabilization of insoluble ECM, a finding that was previously undetected in mice with a WT background.
The N-propeptide of collagen I contains a highly conserved domain characterized by cysteine-rich repeats (CRR) (Bornstein, 2002). Rodents, specifically mice and rats, appear to have the most divergent sequence encoding the N-propeptide in comparison with other known organisms (Bornstein, 2002). Generation of the transgenic mouse line with deletion of the second exon encoding the globular domain of the propeptide yielded results that indicated a subtle consequence of the missing N-propeptide on collagen fibril morphology as viewed by electron microscopy (Bornstein et al., 2002). EM analysis presented here demonstrated no significant differences in diameters of exon 2-deleted collagen fibrils in comparison with those of WT mice. Likewise, differences from WT mice, as judged by overall collagen content and acetic acid extraction of dermis were not found in these or in the initial characterization. In contrast, SPARC-null mice have smaller collagen fibrils and reduced levels of dermal collagen in comparison with WT mice (Bradshaw et al., 2003b). SPARC-null dermal collagen did not, however, demonstrate significant differences in collagen extraction in acetic acid in comparison with collagen from WT skin. Mice lacking SPARC and expressing exon 2-deleted collagen I had a collagen content that was less than that of SPARC-null mice and an increase in acetic-acid extracted collagen I. These results suggest that lack of the N-propeptide decreases cross-linking of collagen I, but that an absence of SPARC expression is required for detection of the functional consequences of this activity.
A function of the N-propeptide in collagen fibril assembly has been suggested by results of previous studies. For example, electron microscopy revealed a preferential immunoreactivity for N-propeptide that was associated with smaller collagen fibrils in skin (Fleischmajer et al., 1981). In bovine dermatosparaxis and Ehlers Danlos type VII C, inefficient cleavage of the N-propeptide of collagen I results in irregular collagen fibrils and fragile skin (Colige et al., 1999). Clearly, efficient removal of the N-propeptide is required for normal collagen fibril assembly; however, the concept that the N-propeptide might facilitate fibril fusion during maturation has also been suggested (Watson et al., 1998). The phenotype of the SPARC-null/exon 2-deleted mouse is consistent with the postulate that a consequence of a lack of the N-propeptide leads to a reduction of fibril fusion, as fewer large collagen fibers were detected in double mutant skin. However, the reason that this effect on collagen fiber assembly is apparent in mice that do not express SPARC and not in WT animals will require further investigation.
An intracellular activity of the N-propeptide has also been suggested by studies carried out using COS-7 cells and C2C12 skeletal myoblasts (Oganesian et al., 2006). Whereas endogenously acting trimeric N-propeptide expression led to an inhibition of the TGF-β downstream signaling pathway and a reduction in collagen synthesis, exogenously added recombinant N-propeptide did not have these effects. The conclusion from these studies was that an interaction of N-propeptide with intracellular receptors was responsible for the decrease in collagen synthesis and effects observed on cell adhesion (Oganesian et al., 2006). The phenotype of the SPARC-null/exon 2-deleted mouse, characterized by herniations and significant reductions in dermal fibrillar collagen, argues against the view that the N-propeptide acts primarily as a feedback inhibitor of collagen I synthesis in vivo. In summary, the elusive functional significance of the conserved N-propeptide of collagen I, the most abundant of the animal proteins, remains to be fully elucidated. Further characterization of SPARC-null/exon 2-deleted mice can be expected to shed more light on this provocative topic.
4. Experimental Procedures
4.1 Animals
SPARC-null mice and mice carrying the exon 2-deleted procollagen α1(I) gene were generated and described previously (Bornstein et al., 2002; Norose et al., 1998). Each genotype was maintained on a mixed C57BL/6/129SV background. SPARC-null mice were mated to exon 2-deleted homozygous mice to generate mice heterozygous for both transgenic insertions. Heterozygous mice were then mated to generate mice with a targeted deletion of SPARC and a procollagen α1(I) exon 2-deleted gene used in these studies. In addition, littermates generated from initial crosses of heterozygous animals that were WT, or deficient in SPARC only, and those that were homozygous for the exon 2 deletion only were maintained to provide control mice with identical genetic backgrounds. Experiments were conducted under a protocol approved by the Institutional Animal Case and Use Committee (IACUC) of the Medical University of South Carolina and the Veterans Affairs Medical Center, Charleston, South Carolina, USA.
4.2 Histology and Electron Microscopy
Skin taken from age-matched mice of each genotype was fixed in formalin and embedded in paraffin. Tissue sections were generated, subsequently deparaffinized, and stained with hematoxylin and eosin (H and E) or picrosirius red (PSR) to visualize cell bodies and fibrillar collagen in the ECM. PSR sections were visualized and captured using polarized light microscopy. Digital images were captured using an iSPOT camera and software on a Leica inverted microscope. Transmission electron microscopy of dermal sections from exon 2 deleted and SPARC-null/exon 2 deleted mice at ~6 months of age was performed as described (Rentz et al., 2007)). Measurements were taken of 4078 fibrils from three exon 2-deleted mice and 3771 fibrils from three SPARC/exon 2 deleted mice. Values used for WT and SPARC-null dermal fibril diameters were taken from previously published results (Bradshaw et al., 2003b).
4.3 Hydroxyproline Analysis
A minimum of four mice from each genotype contributed to each measurement. Two 8 mm in diameter biopsies were collected from the dorsum of each animal. Skin pieces uniform in color were chosen for analysis to ensure similar stages of hair follicle morphogenesis between samples (Muller-Rover et al., 2001; Yamamoto and Yamauchi, 1999). Samples were lyophilized and dry weight determined. Hydroxyproline analysis was carried out according to Woessner (Woessner, 1961), as described (Bradshaw et al., 2009).
4.4 Characterization of acetic acid-soluble collagen
Uniform circular skin biopsies (8mm in diameter) from the dorsa of mice representing the four genotypes were weighed, minced, and placed in 10X volumes (weight per volume) of 1M NaCl with protease inhibitors (Roche, protease inhibitor cocktail). Skin biopsies were tumbled overnight at 4o C. Samples were centrifuged at 1,000 x g for 5 minutes and the soluble component was collected and stored at −80o C. The skin pieces were then centrifuged at 10,000 × g for 15 minutes. Excess NaCl solution was removed and skin pieces were resuspended in 10× volumes of ice-cold 0.5M acetic acid. The samples were tumbled overnight at 4o C. Soluble collagen in acetic acid was separated from insoluble collagen by centrifugation at 10,000 × g for 15 minutes. Aliquots of acetic acid-soluble collagen (supernatant) were stored at −80o C. Protein concentrations of NaCl extracts were determined by micro BCA protein assay (Thermo Scientific). Equal volumes of proteins extracted in acetic acid from each genotype were neutralized with 1M tris-base, boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and resolved by SDS-PAGE on 7.5% acrylamide gels. The acrylamide gels were stained with Coomassie blue or transferred to a nitrocellulose membrane for western blot analysis. Primary incubation with rabbit polyclonal anti-murine collagen I antibodies (MD Biosciences) followed by incubation with secondary antibodies conjugated to horseradish peroxidase were used. Skin extraction analyses were carried out in 5 independent experiments.
Acknowledgments
This research was supported by the NIH, NIDCR through P20RR017696, NIHLB through 094517, and a Merit Award from the Veteran’s Administration to ADB.
Abbreviations
- SPARC
Secreted Protein Acidic and Rich in Cysteine
- PSR
picrosirius red
- ECM
extracellular matrix
- WT
wild-type
Footnotes
Author Contributions: Lauren Card, Nikki Henderson and Yuhua Zhang each contributed technical assistance with the experiments described in the manuscript. The exon 2-deleted mice were generated in the laboratory of Dr. Paul Bornstein. The experiments were carried out under the supervision of Amy D. Bradshaw who authored the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bornstein P. The NH(2)-terminal propeptides of fibrillar collagens: highly conserved domains with poorly understood functions. Matrix Biol. 2002;21:217–226. doi: 10.1016/s0945-053x(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Walsh V, Tullis J, Stainbrook E, Bateman JF, Hormuzdi SG. The globular domain of the proalpha 1(I) N-propeptide is not required for secretion, processing by procollagen N-proteinase, or fibrillogenesis of type I collagen in mice. J Biol Chem. 2002;277:2605–2613. doi: 10.1074/jbc.M106181200. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal. 2009 doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–280. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Graves DC, Motamed K, Sage EH. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci U S A. 2003a;100:6045–6050. doi: 10.1073/pnas.1030790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Sage EH. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol. 2003b;120:949–955. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH. Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest. 2003;111:487–495. doi: 10.1172/JCI16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CZ, Raghunath M. Focus on collagen: in vitro systems to study fibrogenesis and antifibrosis state of the art. Fibrogenesis Tissue Repair. 2009;2:7. doi: 10.1186/1755-1536-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CJ, Sage EH. A prototypic matricellular protein in the tumor microenvironment--where there’s SPARC, there’s fire. J Cell Biochem. 2008;104:721–732. doi: 10.1002/jcb.21688. [DOI] [PubMed] [Google Scholar]
- Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, Wilcox W, Krakow D, Cohn DH, Reardon W, Byers PH, Lapiere CM, Prockop DJ, Nusgens BV. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet. 1999;65:308–317. doi: 10.1086/302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany AM, Kalajzic I, Bradshaw AD, Sage EH, Canalis E. Osteonectin-null mutation compromises osteoblast formation, maturation, and survival. Endocrinology. 2003;144:2588–2596. doi: 10.1210/en.2002-221044. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R, Timpl R, Tuderman L, Raisher L, Wiestner M, Perlish JS, Graves PN. Ultrastructural identification of extension aminopropeptides of type I and III collagens in human skin. Proc Natl Acad Sci U S A. 1981;78:7360–7364. doi: 10.1073/pnas.78.12.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouser L, Sage EH, Clark J, Bornstein P. Feedback regulation of collagen gene expression: a Trojan horse approach. Proc Natl Acad Sci U S A. 1991;88:10158–10162. doi: 10.1073/pnas.88.22.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DF, Watson RB, Chapman JA, Kadler KE. Enzymic control of collagen fibril shape. J Mol Biol. 1996;261:93–97. doi: 10.1006/jmbi.1996.0443. [DOI] [PubMed] [Google Scholar]
- McLaughlin SH, Bulleid NJ. Molecular recognition in procollagen chain assembly. Matrix Biol. 1998;16:369–377. doi: 10.1016/s0945-053x(98)90010-5. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Norose K, Clark JI, Syed NA, Basu A, Heber-Katz E, Sage EH, Howe CC. SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci. 1998;39:2674–2680. [PubMed] [Google Scholar]
- Oganesian A, Au S, Horst JA, Holzhausen LC, Macy AJ, Pace JM, Bornstein P. The NH2-terminal Propeptide of Type I Procollagen Acts Intracellularly to Modulate Cell Function. J Biol Chem. 2006;281:38507–38518. doi: 10.1074/jbc.M607536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puolakkainen P, Bradshaw AD, Kyriakides TR, Reed M, Brekken R, Wight T, Bornstein P, Ratner B, Sage EH. Compromised production of extracellular matrix in mice lacking secreted protein, acidic and rich in cysteine (SPARC) leads to a reduced foreign body reaction to implanted biomaterials. Am J Pathol. 2003;162:627–635. doi: 10.1016/S0002-9440(10)63856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem. 2007;282:22062–22071. doi: 10.1074/jbc.M700167200. [DOI] [PubMed] [Google Scholar]
- Strandjord TP, Madtes DK, Weiss DJ, Sage EH. Collagen accumulation is decreased in SPARC-null mice with bleomycin-induced pulmonary fibrosis. Am J Physiol. 1999;277:L628–635. doi: 10.1152/ajplung.1999.277.3.L628. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, Takahara K, Peters DM, Greenspan DS, Hogan BL. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development. 1996;122:3587–3595. doi: 10.1242/dev.122.11.3587. [DOI] [PubMed] [Google Scholar]
- Taneda S, Pippin JW, Sage EH, Hudkins KL, Takeuchi Y, Couser WG, Alpers CE. Amelioration of diabetic nephropathy in SPARC-null mice. J Am Soc Nephrol. 2003;14:968–980. doi: 10.1097/01.asn.0000054498.83125.90. [DOI] [PubMed] [Google Scholar]
- Watson RB, Holmes DF, Graham HK, Nusgens BV, Kadler KE. Surface located procollagen N-propeptides on dermatosparactic collagen fibrils are not cleaved by procollagen N-proteinase and do not inhibit binding of decorin to the fibril surface. J Mol Biol. 1998;278:195–204. doi: 10.1006/jmbi.1998.1680. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yamauchi M. Characterization of dermal type I collagen of C3H mouse at different stages of the hair cycle. Br J Dermatol. 1999;141:667–675. doi: 10.1046/j.1365-2133.1999.03105.x. [DOI] [PubMed] [Google Scholar]
- Yan Q, Clark JI, Wight TN, Sage EH. Alterations in the lens capsule contribute to cataractogenesis in SPARC-null mice. J Cell Sci. 2002;115:2747–2756. doi: 10.1242/jcs.115.13.2747. [DOI] [PubMed] [Google Scholar]