Abstract
While most rats gain weight when placed on a high-fat diet (HFD), some strains resist HFD-induced weight gain. To maintain weight, obesity-resistant (OR) rats must either eat less than obesity-prone (OP) rats or increase total energy expenditure (TEE). To determine if changes in TEE predispose to or protect from weight gain, energy expenditure, energy intake, and weight gain were measured in male and female OP and OR rats consuming a low-fat diet (LFD) and for 5 days after switching to a HFD. After 5 days on a HFD, OP rats gained significantly more weight (male: 42.8 ± 6.9 g, female: 25.5 ± 3.0 g) than their OR counterparts (male: 24.0 ± 7.5 g, female: 13.7 ± 1.4 g). Both male and female rats significantly increased their energy intake when transitioned to the HFD, and TEE increased modestly in all groups. Compared with female OP rats, female OR rats had a significantly greater increase in TEE on the HFD. This was due to an increase in both resting and nonresting energy expenditure. In contrast, the effect of the HFD in males was minor. TEE was also measured in female rats consuming a HFD, pair fed to LFD calories. The increase in TEE of pair-fed female OR rats was substantially less than what was seen in the HFD ad libitum condition. Physical activity was also measured in female rats. There was no evidence that increases in physical activity were the cause of the increased TEE seen in female OR rats consuming a HFD. These results suggest that resistance to HFD-induced weight gain in female OR rats may be due in part to an increase in TEE and a greater reliance on lipid as an energy source. Changes in TEE appear to be triggered by overconsumption of the HFD and not simply the diet composition.
Keywords: obesity, thinness, energy expenditure, fat oxidation, weight gain, high-fat diet, calorimetry, animal models
despite a recent plateau in prevalence, obesity remains a serious public health problem in the United States and around the world (15, 40). It has been suggested that weight gain is a “normal” response to the modern environment and that the weight regulatory system is biased toward obesity (46). However, not everyone becomes obese when consuming a diet high in fat (HFD). Some individuals appear to be predisposed to weight gain on a HFD (obesity prone, OP) while others resist the development of obesity (obesity resistant, OR) (10). Resistance to HFD-induced obesity likely reflects the ability of the OR individuals to sense energy balance accurately and respond to the increased energy density with adaptive responses that counteract the tendency for weight gain. In contrast, OP individuals possess a weight regulatory system that either does not sense the energy value of a HFD accurately or has a blunted homeostatic response that impairs the ability to defend against weight gain (46).
A number of rodent models of predisposition to or resistance to HFD-induced obesity have been studied (20, 28). We have used a model of OP/OR rats developed by Levin to examine the early adaptive responses that occur following the introduction of a HFD. Although OP rats are physically larger, in the selectively bred Levin OP/OR model, rats of each phenotype have a similar body composition while consuming a low-fat diet (LFD) (28). Following the introduction of a HFD, OP rats become obese, developing many features of the metabolic syndrome, including insulin resistance, hyperlipidemia, and hypertension (29, 51). A number of other differences have also been shown in this model, including the findings that OP rats overexpress arcuate nucleus neuropeptide Y (NPY) mRNA and are characterized as having both reduced leptin and insulin signaling in the arcuate nucleus (8, 21). Given these differences, one reason OP rats are thought to become obese is because of a reduced anorectic and thermogenic response to leptin. The suppressive effect of leptin upon NPY would also be blunted, and the overexpression of NPY would tend to make OP rats more metabolically efficient, facilitating the onset of obesity by affecting food intake, energy expenditure (EE), and white adipose tissue storage (31).
To be effective in maintaining body weight, adaptive responses to a change in diet or energy balance likely need to occur quickly. In support of this idea, total energy expenditure (TEE) has been shown to rapidly increase with overfeeding, and differences in the ability of a subject to increase TEE in response to overfeeding may promote or protect against weight gain (5, 32). Furthermore, leptin and insulin resistance appear to develop in OP rats within 3–7 days of exposure to a high-energy diet (41, 43, 53). Finally, using a dietary fatty acid tracer, we have demonstrated that, within a few days following the introduction of a HFD, OP rats have a greater tendency to store dietary fat, whereas the OR rats exhibited greater dietary fat oxidation (22). However, the effect of introducing a HFD on TEE in OP and OR rats is unknown.
The purpose of this study was to examine EE and fuel utilization during the first 5 days of exposure to a HFD. We hypothesized that OR rats would increase TEE in response to the introduction of the HFD. A series of three studies was performed. First, TEE was measured in male and female OP and OR rats while they consumed a LFD and over the first 5 days following the introduction of the HFD. We found that, in response to the HFD, TEE increased significantly in OR females, whereas it remained unchanged in OP females. Because total energy intake was also increased on the HFD, we next sought to isolate the effects of diet composition on TEE, independent of energy intake on EE. To accomplish this, we measured EE in OP and OR rats consuming a HFD pair fed (PF) to the energy intake of rats consuming a LFD. Finally, to determine if the observed increase in TEE was due to increases in physical activity, physical activity was measured in female OP and OR rats following a transition from LFD to HFD.
METHODS
Animals.
A breeding colony of Sprague-Dawley OP and OR [Levin model (28)] rats was bred and maintained at the Surgical Research Facility at Denver Health Medical Center (DHMC). A total of 36 female (18 OP, 18 OR) and 12 male (6 OP, 6 OR) rats (total n = 48) were used for these studies. For all studies, individual animals were age (83–95 days old) and weight matched to those included in other experimental studies. Room temperature was maintained at 22–24°C (12:12-h light-dark cycle). All experiments were started at 0900. Protocols were approved by the Animal Care and Use Committees at the University of Colorado Denver and DHMC. The following three studies were performed: measures of EE during LFD feeding and following the introduction of a HFD consumed ad libitum, measures of EE during pair feeding of HFD, and measures of physical activity during the transition to a HFD.
Studies of TEE during ad libitum LFD/HFD feeding.
Male and female OP and OR rats (n = 6/group, total n = 24) were caged individually in metabolic chambers. Rats were allowed to acclimate to the metabolic chambers for 4–5 days while feeding ad libitum on a purified ingredient LFD [no. D11724, 21% of energy as protein, 12% of energy as fat, 68% of energy as carbohydrate (CHO), 3.9 kcal/g; Research Diets]. Following this period of acclimation, EE was measured by indirect calorimetry as described below while rats continued to consume the LFD ad libitum for an additional 5-day period. Next, the LFD was replaced with a HFD [21% protein, 39% CHO, and 40% fat (33% saturated and mono- and polyunsaturated), no. D12147, 4.62 kcal/g; Research Diets], and EE was measured for an additional 5 days.
Studies of TEE during pair feeding.
To determine the effects of diet composition alone without the additional effects of increased intake observed following the introduction of the HFD, a separate cohort of female OP and OR rats (n = 6/group, total n = 12) was separated and caged individually and provided ad libitum access to the LFD in the metabolic chambers, as described above for a baseline period. Intake of the diet was monitored daily, and a baseline intake of LFD calories was determined for each rat. Rats were then PF an equicaloric ration of the 40% HFD (PF) for an additional 5 days while EE was measured continuously. Only female rats were used in this study because the first study only showed a significant effect of phenotype (OP vs. OR) on 24-h EE in female rats.
Body composition, feeding efficiency.
Body composition analyses were performed on frozen carcasses of rats subjected to the LFD, HFD, and PF HFD diets. Briefly, entire carcasses were homogenized with water, and aliquots of the homogenate were then analyzed for lipid content as described previously (16). Feeding efficiency for the 5-day LFD, HFD, and PF HFD feeding protocols was calculated as weight gain relative to the amount of energy consumed [weight gained (g·rat−1·5 days−1)/food intake (kcal·rat−1·5 days−1)].
Metabolic monitoring.
The studies described above were performed in a custom metabolic monitoring system developed by the Energy Balance Core Laboratory at the University of Colorado Nutrition Obesity Research Center that has been described previously (35). This system is composed of a four-chamber indirect calorimeter designed for the continuous monitoring of up to four rats simultaneously. Air flow rate requirements of males and females differ because of differences in mass and EE, and metabolic monitoring was performed on males and females separately. Within a given experimental run, a cohort of two OP and two OR male or female rats was run together to offset any bias. No statistical differences were noted between cohorts, and data were pooled for each group. V̇o2 and V̇co2 were measured from each chamber every 6 min. While in the chambers, rats were monitored continuously for 23 h daily. During the remaining 1 h (occurring in the middle of the light cycle), chambers were cleaned. Chambers were equipped for the collection of urine, feces, and food spillage. During the daily cleaning, urine was collected, and a portion of it was saved for determination of urinary nitrogen excretion (ThermoDMA, Louisville, CO). Daily intake of the study diets was measured and corrected for measured spillage.
Metabolic rate (MR) was calculated with the Weir equation (MR = 3.941 × V̇o2 + 1.106 × V̇co2 − 2.17 × N) throughout 24 h to estimate TEE (54). Estimates of whole body substrate oxidation were calculated from V̇o2, V̇co2, and from measurements of urinary nitrogen (N), using derivations of Weir's equation: CHO disappearance = (4.57 × V̇co2) − (3.23 × V̇o2) − (2.6 × N); lipid disappearance = (1.69 × V̇o2) − (1.69 × V̇co2) − (2.03 × N); protein disappearance = 6.25 × N. The data were also used to determine; 1) resting metabolic rate (RMR), which was extrapolated to a 24-h period to estimate resting energy expenditure (REE); 2) nonresting energy expenditure (NREE), as the difference between TEE and REE; and 3) 24-h nonprotein respiratory quotient (NPRQ). RMR was estimated as an average MR over a 1-h period occurring in the latter part of the light cycle, as we have previously reported (35). The 1-h period was selected during a time in which MR and respiratory quotient indicated minimal physical activity and food intake for the previous 3 h. Daily energy balance was calculated from the difference between energy intake and TEE.
Studies of physical activity levels.
To determine if the effects of the HFD on TEE were due to changes in physical activity, a separate cohort of animals (n = 12, 6 OP and 6 OR females) was housed (12 days) in a round Plexiglas cage placed within the confines of a series of infrared photocell sensors (Opto-Varimex, Columbus Instruments, on loan from J. A. Levine, Mayo Clinic and Mayo Foundation, Rochester, MN). A single physical activity monitoring device was used for these studies. Female OP and OR rats were run alternately in 12 cohorts of n = 1. Each experimental animal was age and weight matched to those used in the calorimetry and pair-feeding studies. Beam breaks were counted from the activity photosensors and recorded every 10 s for each 24-h period. Photocell sensor arrays that surrounded the cage measured both horizontal and vertical movement. Subtraction of ambulatory counts from horizontal counts provides an indication of stereotypic activities such as grooming, scratching, and other nonambulatory activities. Physical activity was monitored continuously for each rat for a period of 12 days. Following a 2-day acclimation period, physical activity was measured while rats consumed a LFD ad libitum for 5 days. The diet was then switched to the HFD, and physical activity was monitored for an additional 5 days. Pre- and postdiet intervention body weights were recorded, and food intake corrected for spillage was recorded on a daily basis.
Plasma hormone and metabolite concentrations.
Following the experimental protocols, rats were killed at 9:00 A.M. following an overnight feed. A blood sample was obtained from the inferior vena cava, and serum was stored at −80°C until analyses of insulin, triglyceride, glucose, and leptin were performed. Serum was assayed for insulin and leptin with commercially available ELISAs (ALPCO Diagnostics, Windam, NJ). Glucose and triglycerides were measured via colorimetric analysis (34).
Calculations and statistics.
All data are presented as means ± SE. To examine the effect of diet on EE, fuel utilization, and physical activity, differences in phenotype (OP vs. OR) and diet (LFD, HFD) were analyzed using a two-way repeated-measures ANOVA (Sigma Stat; SPSS). The effects of gender on experimental variables were not analyzed. Where significant differences were found, multiple-comparison procedures were performed by Tukey post hoc analysis. Statistical significance was accepted at P < 0.05.
RESULTS
Anthropomorphic data.
Baseline body weights and the effects of the LFD, HFD, and PF HFD feeding protocols on weight gain are shown in Table 1. In general, both male and female OP rats were slightly larger than OR rats before the introduction of the HFD (P < 0.05). To adjust for the variability due to differences in initial body weights, weight gain, rather than final body weight, is listed as the primary outcome for each diet (Table 1). Compared with the LFD condition, the ad libitum HFD condition produced greater weight gain in all rats in all groups (P < 0.05), but weight gain was greater in the OP rats than in OR rats (P < 0.05). Weight gain in OP and OR females, however, did not differ under the LFD and PF HFD conditions.
Table 1.
Anthropomorphic data: Effect of 5 days of a LFD, HFD or pair-fed HFD on weight gain, feed efficiency, and %body fat in female and male OR and OP rats
| Female |
Male |
|||
|---|---|---|---|---|
| OR | OP | OR | OP | |
| Baseline wt | 261 ± 9* | 299 ± 13* | 307 ± 9* | 345 ± 8* |
| Weight gain, g | ||||
| Ad libitum LFD | 2.5 ± 1.1† | 3.4 ± 1.6† | 30.8 ± 4.7† | 31.2 ± 4.3 |
| Ad libitum HFD | 13.7 ± 1.4†‡ | 25.5 ± 3.0*†‡ | 24.0 ± 7.5*† | 42.8 ± 6.9* |
| Pair-fed HFD | 3.5 ± 1.4‡ | −2.8 ± 0.5‡ | ||
| Feed efficiency (× 10−5) | ||||
| Ad libitum LFD | 5.5 ± 0.9 | 5.6 ± 0.9 | 8.0 ± 0.6 | 7.7 ± 0.9 |
| Ad libitum HFD | 3.4 ± 0.8 | 4.0 ± 0.6 | 5.8 ± 0.9 | 6.9 ± 0.3 |
| Pair-fed HFD | 5.6 ± 0.6 | 5.2 ± 0.7 | ||
| Body fat, % | ||||
| Ad libitum LFD | 6.3 ± 0.5§ | 7.3 ± 0.7†§ | 6.0 ± 0.2† | 6.8 ± 0.0† |
| Ad libitum HFD | 7.3 ± 0.6* | 12.3 ± 0.8*† | 8.9 ± 0.5*† | 11.5 ± 0.4*† |
| Pair-fed HFD | 10.1 ± 1.6§ | 12.5 ± 1.8§ | ||
Data are means ± SE. LFD, low-fat diet; HFD, high-fat diet; Pair-fed HFD, pair feeding of the HFD isocaloric to LFD daily caloric intake. In females, weight gain on LFD did not differ across the calorimetry, pair feeding, and physical activity experiments, and the data were pooled. Within a given gender, like symbols (*, †, ‡, §) are used to denote a significant difference across either phenotype or feeding protocol.
Within-gender phenotype difference.
Within phenotype LFD ≠ HFD.
Within phenotype HFD ≠ pair-fed HFD.
Within phenotype LFD ≠ pair-fed HFD. Across-gender analyses were not performed. Pair feeding was not performed in males. No phenotype or within-phenotype diet effects were present for feed efficiency.
Feeding efficiency for the 5-day LFD, HFD, and PF HFD feeding protocols was calculated as weight gain relative to the amount of energy consumed [weight gained (g·rat−1·5 days−1)/food intake (kcal·rat−1·5 days−1)] (Table 1). Compared with LFD, feeding efficiency decreased in both males and females on the ad libitum HFD (males, P = 0.036, females P = 0.025). In contrast, pair feeding of the HFD did not result in a change in feed efficiency and was unchanged from LFD values. As a result, PF HFD feed efficiency was greater than ad libitum HFD values (P = 0.036). Whole body carcass lipid content was determined following the LFD, HFD, and PF dietary interventions (Table 1). Although rats of the OP phenotype were physically larger than the OR phenotype, within a given gender, there were no differences in percent body fat on the LFD. Following HFD feeding, both male and female OP rats increased percent body fat by roughly 1.7-fold, whereas OR males and females increased 1.5- and 1.1-fold, respectively. Five days of ad libitum HFD feeding resulted in marked increases in body fat in OP females relative to the LFD condition (P < 0.001), whereas the HFD produced minimal changes in percent body fat in OR females. Similarly, although percent body fat was significantly increased in OR males relative to LFD conditions, this increase was smaller than what was observed in OP males. The group that maintained body composition most consistently following the introduction of the HFD was the OR female group. In contrast to ad libitum feeding of the HFD, isocaloric pair feeding of the HFD resulted in significant increases in percent body fat in both OP and OR females (P = 0.019, P < 0.001). Despite consuming fewer total calories, PF OR females exhibited increased body fat relative to the ad libitum HFD condition (P = 0.029).
Energy intake.
Total energy intake for the rats for the 5-day feeding period is shown in Table 2. OP and OR rats consumed the same amount of LFD, and all rats consumed more calories on the HFD (P < 0.001). Male rats consumed considerably more than female rats (P < 0.001). Both male and female OP rats had a higher total energy intake on the HFD compared with their OR counterparts (P < 0.01). By design, the PF HFD was isoenergetic, with the ad libitum LFD and total caloric intake not differing.
Table 2.
Energy intake and energy expenditure during the 5-day periods of ad libitum LFD, HFD, or pair-fed HFD
| Female |
Male |
|||
|---|---|---|---|---|
| OR | OP | OR | OP | |
| Energy intake, kcal | ||||
| LFD | 306.5 ± 9.3† | 318.5 ± 10.8† | 423.3 ± 26.4† | 446.4 ± 12.0† |
| HFD | 486.2 ± 11.7*†‡ | 522.1 ± 9.0*†‡ | 509.0 ± 19.1*† | 583.1 ± 28.4*† |
| PF | 298.0 ± 5.0‡ | 313.2 ± 3.4‡ | ||
| Energy expenditure, kcal | ||||
| LFD | 284.8 ± 9.5† | 271.0 ± 5.1 | 307.5 ± 7.2 | 316.2 ± 6.4 |
| HFD | 331.3 ± 14.1*† | 281.9 ± 10.1 | 318.9 ± 4.5 | 322.7 ± 7.8 |
| PF | 305.1 ± 6.2 | 302.6 ± 11.1 | ||
| Energy imbalance, kcal | ||||
| LFD | 18.5 ± 3.7† | 20.6 ± 2.7† | 121.5 ± 6.18 | 128.1 ± 5.7 |
| HFD | 145.9 ± 17.3*† | 218.9 ± 16.8*† | 198.7 ± 15.7 | 262.3 ± 20.8 |
| PF | −7.8 ± 5.0 | 3.3 ± 5.1 | ||
Data are means ± SE. PF, pair fed. Within a given gender, like symbols (*, †, ‡) are used to denote a significant difference across either phenotype or feeding protocol.
Within-gender phenotype difference.
Within phenotype LFD ≠ HFD.
Within phenotype HFD ≠ PF HFD. Across-gender analyses were not performed. Pair feeding was not performed in males.
Energy expenditure.
The total 5-day EE (5-day TEE) is shown in Table 2. The 5-day TEE did not differ between phenotypes under LFD feeding, nor did it differ for the PF HFD condition. Despite marked changes in intake and body weight following the introduction of ad libitum HFD, 5-day TEE did not differ from LFD levels in males, nor did it differ in OP females. In contrast, the ad libitum HFD 5-day TEE was markedly increased in OR females relative to LFD (P < 0.05) and was significantly greater than that of OP females (P < .05). Pair feeding of the HFD had no effect on 5-day TEE.
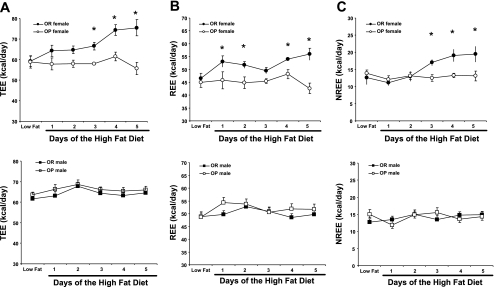
Daily TEE during the transition from a LFD to a HFD is shown in Fig. 1A. In females (Fig. 1A, top), this transition had little impact in OP rats, but it induced a gradual increase (∼30% by day 4, P < 0.05) in TEE over the 5-day monitoring period in OR rats. TEE in OR females was significantly greater than that of OP females on days 3, 4, and 5 of HFD (P < 0.05, all). In contrast, both OP and OR male rats exhibited a more modest increase in TEE in response to HFD feeding that was statistically significant by day 2 (P < 0.01). No other differences were noted in males.
Fig. 1.
Effects of a change from a low-fat diet (LFD) to a high-fat diet (HFD) on total energy expenditure (TEE, A), resting energy expenditure (REE, B), and nonresting energy expenditure (NREE, C) in female (top) and male (bottom) obesity-prone (OP) and obesity-resistant (OR) rats. *P < 0.05, within-day phenotypic differences (OP vs. OR). The first point in each graph represents the mean of 5 days of measurement while rats consumed the LFD ad libitum. Additional day-to-day differences are discussed in the text.
An analysis of the metabolic chamber data was performed to determine which component of daily EE increased in OR female rats in response to the HFD. Daily measures of REE and NREE (= energy expended in physical activity + thermic effect of food) are shown in Fig. 1, B and C. In female rats, changes in REE paralleled changes in TEE such that the 2nd day of HFD was significantly greater than days 3, 4, and 5 of HFD (Fig. 1B, top, P < 0.01, all). While consuming the ad libitum HFD, REE of OR females was significantly greater than that of OP females on days 1, 2, 4, and 5 of HFD (P < 0.01), indicating that the observed increase in TEE seen in this group may be partially explained by increased basal energy requirements. No phenotypic differences were present in male rats; however, REE did increase slightly over time following the introduction of the HFD. NREE did not change as a function of diet in either gender of the OP phenotype, nor did it vary in OR males (Fig. 1C). NREE increased in OR females on day 3 of the HFD and remained significantly elevated for the remainder of the 5-day HFD feeding (P < 0.01).
Compared with LFD feeding, pair feeding the HFD did not significantly affect either TEE or REE in any group although NREE trended lower in both OP and OR females for the first few days of pair feeding the HFD and then rebounded back to LFD levels by the final day (data not shown).
Energy imbalance.
The energy imbalance over the 5-day feeding period is shown in Table 2. Males generally experienced a larger imbalance than females on both the LFD and the HFD (P < 0.05). Regardless of sex, OP rats had a greater imbalance than OR rats (P < 0.001). The PF HFD feeding in female rats resulted in rats of both phenotypes being in a state of energy balance.
Substrate utilization.
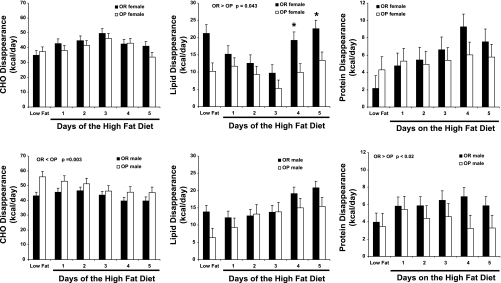
Using the estimated NPRQ values, disappearance of fat, CHO, and protein (kcal/day) was calculated for the LFD and HFD conditions (Fig. 2). There are admittedly limitations to this approach. While in energy balance, calculations of substrate disappearance are a good reflection of substrate oxidation. However, in the present study, both OP and OR phenotypes were in positive energy balance during ad libitum HFD feeding. Under these conditions, other metabolic pathways such as lipogenesis may affect the relationship between substrate disappearance and macronutrient-specific oxidation. It is important to note though that even though animals ate more food on the HFD, all groups consumed less total CHO than they did on the LFD. It was, therefore, surprising that females exhibited an increase in CHO disappearance during the first 3 days of HFD feeding. CHO disappearance in females was greater on day 3 of HFD compared with LFD and days 1, 2, 4, and 5 of HFD (P < 0.01). This was accompanied by a reduction in lipid disappearance, which reached a nadir on day 3 of the HFD, a lower level than that seen on days 1, 4, and 5 of HFD (P < 0.05). Estimated net fat disappearance on day 3 of the HFD was 40% lower than that seen during LFD feeding (P < 0.001). In contrast to OP females, OR rats showed an increase in lipid disappearance on days 4 and 5 of HFD (P < 0.01). Protein disappearance increased over the 5-day HFD feeding period such that protein oxidation on day 5 of the HFD was significantly greater than it was during the LFD (P < 0.05).
Fig. 2.
The effects of a change from a LFD to a HFD on substrate disappearance. Carbohydrate (CHO), lipid, and protein disappearance in female (top) and male (bottom) OP and OR rats. *P < 0.05, within-day phenotypic (OP vs. OR) differences. Additional day-to-day differences are provided in the text.
As would be predicted from the composition of the diet, the PF HFD condition was associated with a dramatic decrease in the absolute amount of CHO consumed, no difference in protein, and a marked increase in the intake of fat compared with the LFD condition. However, in contrast to what was seen with overfeeding of the HFD, NPRQ in PF rats decreased in both phenotypes on day 1, and, relative to LFD, NPRQ was lower on all days of pair feeding (P < 0.001), reflecting a more rapid transition to utilizing fat as a primary fuel for energy production (data not shown).
Compared with OP, OR males exhibited a lower daily CHO disappearance rate throughout the ad libitum HFD experiment (P = 0.003). In contrast to the rise and fall in CHO disappearance seen in females, male rats demonstrated a steady decrease in CHO disappearance and a concomitant increase in lipid disappearance. CHO disappearance on days 3, 4, and 5 of HFD was lower than was seen during the baseline LFD period (P < 0.001). Conversely, lipid disappearance increased in both OP and OR males, being greater on days 4 and 5 of HFD compared with LFD (P < 0.001). Similar to OR females, OR males tended to exhibit greater lipid disappearance compared with OP males during the later days of ad libitum HFD feeding; however, this did not reach statistical significance (P = 0.08). Protein disappearance was greater in OP males compared with OR (P = 0.02). A tendency for protein disappearance to rise was seen in both phenotypes of male rats consuming the ad libitum HFD, but this also failed to reach statistical significance.
Serum hormone and substrate concentrations.
Mean values of serum glucose, insulin, leptin, and triglyceride from fed rats are presented in Table 3. Ad libitum HFD feeding increased serum glucose in both males and females (P < 0.05), and serum glucose was consistently lower in OR males relative to OP males regardless of the dietary condition (P = 0.01). Insulin levels were significantly lower in female OR rats compared with OP regardless of diet (P < 0.001). Insulin levels increased in all groups following HFD, although this change was only significant in females (P = 0.021). Pair feeding did not alter either insulin or glucose levels compared with levels seen with the LFD.
Table 3.
Serum hormone and substrate concentrations following LFD, HFD, and pair feeding of the HFD isocaloric to LFD calories
| LFD |
HFD |
Pair-Fed HFD |
||||
|---|---|---|---|---|---|---|
| OP | OR | OP | OR | OP | OR | |
| Glucose, mM | ||||||
| Female* | 13.6 ± 0.6 | 12.3 ± 0.7 | 15.1 ± 0.9 | 16.3 ± 1.1 | 11.2 ± 0.2 | 9.7 ± 0.5 |
| Male*† | 16.5 ± 0.8 | 13.3 ± 0.6 | 19.0 ± 1.6 | 15.0 ± 1.0 | ||
| Insulin, ng/ml | ||||||
| Female*† | 4.2 ± 0.6 | 2.0 ± 0.5 | 7.6 ± 0.8 | 3.6 ± 0.9 | 4.8 ± 0.5 | 3.7 ± 0.6 |
| Male | 4.4 ± 1.1 | 3.5 ± 0.6 | 5.2 ± 0.9 | 5.3 ± 0.9 | ||
| Leptin, ng/ml | ||||||
| Female*† | 3.3 ± 1.1 | 1.2 ± 0.2 | 7.8 ± 0.9 | 3.4 ± 1.0 | 6.5 ± 1.4 | 1.6 ± 0.2 |
| Male*† | 5.5 ± 1.0 | 2.3 ± 0.3 | 13.4 ± 1.9 | 13.4 ± 1.9 | ||
| Triglyceride, mg/dl | ||||||
| Female† | 38.9 ± 17.0 | 64.8 ± 15.5 | 117.6 ± 11.9 | 86.1 ± 12.7 | 49.0 ± 10.3 | 27.7 ± 2.2 |
| Male*† | 257.4 ± 40.2 | 125.1 ± 13.7 | 368.1 ± 37.9 | 214.5 ± 17.9 | ||
Values presented are means ± SE; n = 8 rats/phenotype. Within-gender main effects are provided in the first column
Phenotype effect {obesity prone (OP) vs. obesity resistant (OR)}.
Diet effect (LFD vs. HFD), P < 0.05. No across- or within-phenotype differences were present for any of the parameters. Across-gender analyses were not performed. Pair feeding was not performed in male rats.
In general, serum leptin levels were greater in OP females compared with OR females regardless of diet (P < 0.001), and both HFD and PF resulted in increased leptin (P = 0.001 and P < 0.001). In OR females, leptin levels during the ad libitum HFD were threefold greater than those seen on the LFD (P = 0.002). Similarly, HFD leptin levels were higher in OP females relative to those seen on the LFD (P = 0.024). PF leptin levels were also higher than LFD levels (P < 0.001). In males, leptin was greater in OP rats compared with OR (P < 0.001), and the HFD resulted in increases in serum leptin levels in both OP and OR rats (P = 0.002 and P = 0.003, respectively). HFD feeding increased triglyceride levels in both male and female rats (P < 0.001, both). In males, an effect of phenotype was seen, with triglyceride levels being higher in OP rats regardless of diet (P < 0.001).
Physical activity.
To examine the role that changes in physical activity might have played in the observed increase in TEE seen in female OR rats consuming a HFD ad libitum, physical activity was measured in a separate cohort of female OR rats consuming a LFD and over the first 5 days of exposure to a HFD. The estrous cycle can affect physical activity and was not monitored in this study. To minimize this effect, we have presented the vertical, horizontal, ambulatory, and total activity counts summed over the 5-day period of LFD and HFD feeding, and thus day-to-day variability due to differences in the estrous cycle should be minimized (Table 4). Although physical activity declined slightly in both OP and OR rats by the 5th day of ad libitum HFD feeding, no single measure of physical activity (horizontal, vertical, or ambulatory activity) or the total combined activity differed between phenotypes. These results suggest that the observed differences in TEE and NREE seen between OP and OR females were not due to alterations in physical activity as measured by this method.
Table 4.
Physical activity in female OP and OR rats
| OR |
OP |
|||
|---|---|---|---|---|
| LFD | HFD | LFD | HFD | |
| Vertical | 116 ± 27 | 114 ± 14 | 124 ± 24 | 122 ± 9 |
| Horizontal | 223 ± 11 | 210 ± 13 | 203 ± 22 | 199 ± 14 |
| Ambulatory | 104 ± 7 | 101 ± 4 | 100 ± 7 | 96 ± 8 |
| Total | 478 ± 47 | 453 ± 16 | 485 ± 30 | 446 ± 16 |
Depicted are the sum total 5-day activity counts (× 103) for vertical, horizontal, ambulatory, and total physical activity under the LFD and HFD feeding conditions. Nos. represent beam breaks in either a horizontal or vertical direction detected by photocells arrayed around a round Plexiglas cage. Rats (n = 6/phenotype) were studied individually in a single monitoring cage. No phenotype or diet effects were observed.
DISCUSSION
The novel observations from this study are that obesity resistance in female rats is associated with an increased TEE and a greater reliance on lipid as an energy source when challenged with a HFD. In contrast, obesity resistance in male rats is associated with better control of food intake such that it more closely matched EE. These physiological adaptations that attempt to maintain energy balance occur within a few days following the introduction of a HFD. The results from the PF HFD study support the notion that the observed increase in TEE seen in female rats is not a response to diet composition per se but rather represents a response to overfeeding of the HFD. Taken together, these observations suggest that OR rats acutely sense and adapt to HFD overfeeding.
The results of these studies are also consistent with and extend our previous observations that describe the metabolic characteristics of animals that are resistant to obesity. In earlier studies, dietary fat oxidation was shown to be reduced in obese Zucker and more so in reduced-obese Zucker rats compared with lean (3). However, these studies could not help answer the question of whether differences in fat oxidation are involved in promoting weight gain or are the result of weight gain. In more recent studies conducted in the Levin model of OP/OR rats, the metabolic fate of a dietary fat tracer was determined before and 5 days after the introduction of a HFD (22). This study demonstrated a decline in the oxidation of dietary fat in OP rats that was not observed in OR rats. Our present findings support the previous observation that male OR rats attempt to normalize the energy imbalance by reducing food intake while female OR rats respond primarily by increasing EE and increasing their reliance on fat as a fuel.
HFDs lead to overfeeding and are thought to contribute the growing prevalence of obesity (6, 19). If the protein content of the diet is constant, a transition from a high-CHO diet to a HFD results in both an increase in ingested lipid and a reduction in dietary CHO. When subjects are maintained in energy balance, variations in both fat and CHO intake lead to changes in their relative rates of oxidation (52). We found this to be true in the current study using a pair-feeding design where we observed a reduction in CHO, an increase in fat, and no change in protein disappearance following pair feeding of the HFD. The overall effect of these changes resulted in a reduction in fat-free mass while fat mass increased. Although OP and OR rats were in “energy balance,” it may well be that PF rats perceive an inadequate level of nutrition, resulting in a change in meal pattern or circulating hormone levels that shift fuel utilization toward an increased dependence on lipid.
In contrast, when subjects are overfed fat, the ability to adjust fat oxidation to fat intake is less precise than it is for CHO or protein (2, 14, 45). Net CHO oxidation is highly related to CHO intake, and CHO overfeeding results not only in increased CHO oxidation but also a decrease in fat oxidation (36). In contrast, most studies find that fat intake has little effect on the rate of CHO or fat oxidation. What is unique in the current study is the observation that OR rats are able to maintain or increase their dependence/reliance on fat oxidation in the face of overfeeding of a HFD.
In both humans and animals, overfeeding typically results in a modest increase in TEE (11, 24, 50). This was true in the current study where overfeeding was associated with a very modest increase in TEE in all groups. However, the mechanism(s) by which overfeeding leads to alterations in TEE and the role that differences in TEE following overfeeding play in a predisposition or protection from weight gain are controversial (12). Data from the present study support the idea that the ability to increase both lipid oxidation and EE may be part of an adaptive response that protects against weight gain.
The observed increase in TEE seen in OR female rats was in part due to an increase in REE which, although not measured directly, may have been due to an increase in the activity of the sympathetic nervous system (SNS). Overfeeding has been shown to stimulate the SNS (47), with several studies demonstrating an increase in SNS activity in liver, pancreas (55), and brown adipose tissue within 3–7 days of HFD feeding (29, 48). In contrast to OR rats, OP rats have been characterized by reduced levels of organ-specific norepinephrine turnover in response to a number of acute (nondietary) metabolic stressors (30). While increased SNS activity in OR rats following HFD overfeeding could underlie the observed increase in TEE, it is troubling that TEE continued to increase in OR rats despite a reduction in intake of the HFD by day 5. Alternatively, the increase in EE in female OR rats may be partially attributed to adaptive thermogenesis, which may in part be due to differences in the response to leptin. Leptin has been shown to increase sympathetic nerve activity to brown adipose tissue (9, 17), and leptin may also directly stimulate thermogenesis in skeletal muscle (13). Central leptin sensitivity is reduced in OP rats relative to OR rats (26, 27), and it is intriguing that, for a given increase in fat mass, leptin is disproportionately increased in OR rats relative to OP rats. Thus a relatively greater increase in serum leptin in OR rats in response to a 5-day HFD feeding could lead to an enhanced centrally mediated sympathetic response, as well as an enhanced peripherally mediated response.
The increase in TEE was apparently also due in part to an increase in NREE. NREE is comprised of the thermic effect of food and energy expended in physical activity. A number of studies suggest that changes in either physical activity or the efficiency of movement (39) may be important in the adaptive response to overfeeding in OR humans (32, 33) and rats (23, 49), and others suggest that low physical activity may be a major contributor to obesity in mice (4). Other studies have failed to support this idea (7, 25). There are likely a number of reasons for these discrepancies. The most likely is that changes in physical activity in response to diet or positive energy balance are both strain and species specific. Adaptations in physical activity may also be gender dependent. In fact, in an alternate OP/OR model using the Wistar rat strain, we have observed modest differences in physical activity in older mature OP and OR females, yet we do not see differences in the Wistar OP and OR males. Regardless, similar to the current findings, a previous study in Levin model rats demonstrates no differences in physical activity between OP and OR rats while on LFD, whereas differences were observed following HFD feeding (38). Interestingly, physical activity measurements were conducted 29 days after the introduction of the HFD, a time when marked weight differences had already developed between the two phenotypes. In contrast, the current study did not show differences in physical activity at a time when there were marked group differences in TEE and less substantial differences in body weight. Similarly, using telemetry, Levin's group has not observed differences in physical activity in OP and OR rats under free living conditions (unpublished data). In following, the current study does not support the idea that increases in physical activity play an important role in protection against weight gain in OR rats in the first few days following the introduction of the HFD.
There are several limitations to this study. First, when provided ad libitum access to the HFD, all rats overeat considerably. As a result, throughout the course of the HFD feeding experiment, the OP and OR animals were in different states of energy balance, with OP rats being in marked positive energy balance for much of the HFD period. Although this makes interpretation of nutrient oxidation data problematic, it does not alter the measures of TEE of the HFD. As the second study shows, controlling for energy intake (placing rats in energy balance) eliminates the increase in TEE seen in the ad libitum condition. Nevertheless, since rats were in a state of positive energy balance, we need to be cautious in making conclusions about nutrient disposition. To reflect this limitation we refer to “substrate disappearance” rather than “substrate oxidation.” Specifically, the NPRQ estimate is not only affected by the relative oxidation of CHO and lipid, but it is also affected by the interconversion of substrates via gluconeogenesis and de novo lipogenesis. The higher NPRQ observed in female OR rats after several days on the HFD may reflect lower fat oxidation, a reduced rate of de novo lipogenesis, or both. Without a correction for lipogenesis, if present, nutrient disappearance estimates would overestimate CHO oxidation and underestimate fat oxidation. Because rats were in positive energy balance, a state where de novo lipogenesis might occur, our finding of an increase in fat oxidation in the OR phenotype may actually be an underestimate rather than an overestimate. Although plausible, this would be more likely to occur with a positive CHO balance rather than with a positive balance of fat. In fact, several studies demonstrate a decrease in lipogenic capacity in response to a HFD (18, 37, 44). In following, although the lack of a measure of lipogenesis and our inability to take this pathway into account is a potential limitation of the current study, given the fact that CHO intake is lower in the HFD condition compared with the LFD condition, the magnitude of this limitation is likely small.
Using the equations of Pullar and Webster (42), our measurements of expenditure with the accumulation of lean and fat mass in both female and male rats account for the energy consumed within ∼11%. The remaining calories may partially reflect the lack of our accounting for fecal energy lost. An alternative explanation is that maintenance energy costs are likely to have changed over the week-long overfeeding challenge. Because the error in caloric accounting tended to be positive in the OP and negative in the OR rats, we suspect that the maintenance energy cost in the OR rats increased, whereas the maintenance energy cost of OP rats decreased.
A final limitation is that no attempt was made to control the state of the reproductive cycle in females, and it is plausible that differences in the OP and OR females may have been due to differences in the estrous cycle. Although we did not examine how the cycle may affect TEE in the current study, Anantharaman-Barr and Decombaz (1) have shown a modest effect of the reproductive cycle on TEE, demonstrating that the four phases of the estrous cycle did not differ >5% from the mean 24-h EE. Similarly, we have made observations in an alternate model of older sedentary Wistar female OP and OR rats (E. Giles, unpublished observations), indicating that, while fluctuations in TEE do indeed occur, fluctuations are modest, accounting for <4% of total TEE. In following, it is unlikely that the marked differences in TEE observed in female rats in the current investigation are the result of estrous cycle effects.
Perspectives and Significance
Collectively, these findings suggest that, following the introduction of a HFD, the ability to increase TEE may contribute to the obesity resistance phenotype. This increase in TEE appears to be greatest in female OR rats and is a response to the overfeeding of the HFD not just the composition of the diet. The increase in TEE is associated with a reduction in food intake, suggesting that, compared with the OP phenotype, OR rats have an enhanced capacity to sense and respond to a state of positive energy balance invoked by the HFD. Finally, the increase in TEE appears to coincide with an increased reliance on lipid oxidation and does not appear to be the result of an increase in physical activity. The current studies suggest that differences in fat oxidation and TEE occur quickly and as a result are an integral part of the processes promoting weight gain or weight maintenance following the introduction of a HFD.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-02935, DK-048520, DK-038088, DK-047311, and DK-010109.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Anantharaman-Barr HG, Decombaz J. The effect of wheel running and the estrous cycle on energy expenditure in female rats. Physiol Behav 46: 259–263, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Bennett C, Reed GW, Peters JC, Abumrad NN, Sun M, Hill JO. Short-term effects of dietary-fat ingestion on energy expenditure and nutrient balance. Am J Clin Nutr 55: 1071–1077, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Bessesen DH, Rupp CL, Eckel RH. Dietary fat is shunted away from oxidation, toward storage in obese Zucker rats. Obes Res 3: 179–189, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Bjursell M, Gerdin AK, Lelliott CJ, Egecioglu E, Elmgren A, Tornell J, Oscarsson J, Bohlooly YM. Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am J Physiol Endocrinol Metab 294: E251–E260, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins. N Engl J Med 322: 1477–1482, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr 68: 1157–1173, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Brownlow BS, Petro A, Feinglos MN, Surwit RS. The role of motor activity in diet-induced obesity in C57BL/6J mice. Physiol Behav 60: 37–41, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation (Abstract). Nature 380: 677, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One 4: e6310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deriaz O, Fournier G, Tremblay A, Despres JP, Bouchard C. Lean-body-mass composition and resting energy expenditure before and after long-term overfeeding. Am J Clin Nutr 56: 840–847, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Dirlewanger M, di Vetta V, Guenat E, Battilana P, Seematter G, Schneiter P, Jequier E, Tappy L. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int J Obes Relat Metab Disord 24: 1413–1418, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Dulloo AG, Stock MJ, Solinas G, Boss O, Montani JP, Seydoux J. Leptin directly stimulates thermogenesis in skeletal muscle. FEBS Lett 515: 109–113, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Flatt JP, Ravussin E, Acheson KJ, Jequier E. Effects of dietary fat on postprandial substrate oxidation and on carbohydrate and fat balances. J Clin Invest 76: 1019–1024, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flegal KMCM, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. J Am Med Assoc 303: 235–241, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 17.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100: 270–278, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzberg GR, Janmohamed N. Regulation of hepatic lipogenesis by dietary maize oil or tripalmitin in the meal-fed mouse. Br J Nutr 43: 571–579, 1980 [DOI] [PubMed] [Google Scholar]
- 19.Hill JO. Long-term weight control with meal replacements (Abstract). Nutrition 16: 385, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Hill JO, Fried SK, DiGirolamo M. Effects of a high-fat diet on energy intake and expenditure in rats. Life Sci 33: 141–149, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Irani BG, Dunn-Meynell AA, Levin BE. Altered hypothalamic leptin, insulin, and melanocortin binding associated with moderate-fat diet and predisposition to obesity. Endocrinology 148: 310–316, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Jackman MR, Kramer RE, MacLean PS, Bessesen DH. Trafficking of dietary fat in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab 291: E1083–E1091, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, Levine JA. Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience 142: 29–36, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Levin BE. Spontaneous motor activity during the development and maintenance of diet-induced obesity in the rat. Physiol Behav 50: 573–581, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283: R941–R948, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 286: R143–R150, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol 274: R412–R419, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Levin BE, Triscari J, Sullivan AC. Altered sympathetic activity during development of diet-induced obesity in rat. Am J Physiol Regul Integr Comp Physiol 244: R347–R355, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Levin BE, Triscari J, Sullivan AC. Relationship between sympathetic activity and diet-induced obesity in two rat strains. Am J Physiol Regul Integr Comp Physiol 245: R364–R371, 1983 [DOI] [PubMed] [Google Scholar]
- 31.Levine AS, Jewett DC, Cleary JP, Kotz CM, Billington CJ. Our journey with neuropeptide Y: effects on ingestive behaviors and energy expenditure. Peptides 25: 505–510, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283: 212–214, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of nonexercise activity. Am J Clin Nutr 72: 1451–1454, 2000 [DOI] [PubMed] [Google Scholar]
- 34.MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, Melanson EL, Hill JO. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 290: R1577–R1588, 2006 [DOI] [PubMed] [Google Scholar]
- 35.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R1306–R1315, 2004 [DOI] [PubMed] [Google Scholar]
- 36.McDevitt RM, Poppitt SD, Murgatroyd PR, Prentice AM. Macronutrient disposal during controlled overfeeding with glucose, fructose, sucrose, or fat in lean and obese women. Am J Clin Nutr 72: 369–377, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Muto Y, Gibson DM. Selective dampening of lipogenic enzymes of liver by exogenous polyunsaturated fatty acids. Biochem Biophys Res Commun 38: 9–15, 1970 [DOI] [PubMed] [Google Scholar]
- 38.Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am J Physiol Endocrinol Metab 290: E396–E403, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Novak CM, Levine JA. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J Neuroendocrinol 19: 923–940, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology 132: 2087–2102, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54: 3182–3189, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Pullar JD, Webster AJ. The energy cost of fat and protein deposition in the rat. Br J Nutr 37: 355–363, 1977 [DOI] [PubMed] [Google Scholar]
- 43.Ricci MR, Levin BE. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 285: R610–R618, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Sabine JR, McGrath H, Abraham S. Dietary fat and the inhibition of hepatic lipogenesis in the mouse. J Nutr 98: 312–318, 1969 [DOI] [PubMed] [Google Scholar]
- 45.Schutz Y, Flatt JP, Jequier E. Failure of dietary fat intake to promote fat oxidation: a factor favoring the development of obesity. Am J Clin Nutr 50: 307–314, 1989 [DOI] [PubMed] [Google Scholar]
- 46.Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes 52: 232–238, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Seematter G, Dirlewanger M, Rey V, Schneiter P, Tappy L. Metabolic effects of mental stress during over- and underfeeding in healthy women. Obes Res 10: 49–55, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Stock MJ, Rothwell NJ. Sympathetic control of brown adipose tissue in the regulation of body weight. Biochem Soc Trans 9: 525–527, 1981 [DOI] [PubMed] [Google Scholar]
- 49.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol 291: R889–R899, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Tremblay A, Despres JP, Theriault G, Fournier G, Bouchard C. Overfeeding and energy expenditure in humans. Am J Clin Nutr 56: 857–862, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Triscari J, Nauss-Karol C, Levin BE, Sullivan AC. Changes in lipid metabolism in diet-induced obesity. Metabolism 34: 580–587, 1985 [DOI] [PubMed] [Google Scholar]
- 52.Verboeket-van de Venne WP, Westerterp KR, ten Hoor F. Substrate utilization in man: effects of dietary fat and carbohydrate. Metabolism 43: 152–156, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes 50: 2786–2791, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young JB, Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in pancreas and liver. Am J Physiol Endocrinol Metab Gastrointest Physiol 236: E524–E533, 1979 [DOI] [PubMed] [Google Scholar]