Abstract
When the spinal cord is injured at or below thoracic level 5 (T5), cardiovascular control is markedly unbalanced as the heart and blood vessels innervated by upper thoracic segments remain under brain stem control, whereas the vasculature of the lower body is affected by unregulated spinal reflexes. Importantly, the regulation of heart rate and cardiac function is abnormal after spinal cord injury (SCI) at T5 because sympathetic outflow to the heart is increased. An increase in tonic sympathetic outflow may be attributable to multiple mechanisms, such as increases in cardiac sympathetic innervation density, altered morphology of stellate ganglia neurons, and/or structural neuroplasticity of cardiac sympathetic preganglionic neurons (SPNs). Furthermore, these neuroplastic changes associated with SCI may be mediated by nerve growth factor (NGF). NGF is a neurotrophin that supports the survival and differentiation of sympathetic neurons and enhances target innervation. Therefore, we tested the hypothesis that T5 spinal cord transection (T5X) is associated with an increased left ventricular (LV) NGF content, LV sympathetic innervation density, and cardiac SPN arborization. In intact and paraplegic (9 wk posttransection) rats, LV NGF content (ELISA), LV sympathetic innervation density (tyrosine hydroxylase immunohistochemistry), and cardiac SPN arborization (cholera toxin B immunohistochemistry and Sholl Analysis) were determined. Paraplegia, compared with intact, significantly increased LV NGF content, LV sympathetic innervation density, and cardiac SPN arborization. Thus, altered autonomic behavior following SCI is associated with structural neuroplastic modifications.
Keywords: cardiovascular risks, arrhythmia
since world war ii, there has been a dramatic reduction in the mortality rates during the acute period following a spinal cord injury (SCI) (81). However, mortality rates in the chronic period (>2 yr following injury) have changed very little, and people with SCI have a significantly reduced life expectancy (17, 28, 81). The reduced life expectancy is due, in part, to a higher prevalence and earlier onset of cardiovascular diseases (57). In fact, cardiovascular disease is now a leading cause of death and morbidity for individuals with SCI (17, 28, 81).
Excessive sympathetic activity is responsible for, and/or contributes to, the morbidity and mortality associated with cardiovascular disease. Accordingly, efforts to reduce sympathetic activity are the first-line therapy for most, if not all, cardiovascular diseases. Importantly, sympathetic activity is elevated above the lesion in individuals with midthoracic SCI, and the regulation of heart rate and cardiac function is abnormal because sympathetic outflow to the heart is increased (14). For example, we recently documented that thoracic level 5 (T5) spinal cord transection (T5X) increased cardiac sympathetic tonus (48), altered cardiac electrophysiology (14, 71), and increased the susceptiblity to ischemia/reperfusion-induced as well as ischemia-induced sustained ventricular tachycardia (48, 49). The increased susceptibility to the life-threatening arrhythmia was prevented with cardiac β1-adrenergic receptor blockade, documenting that increased sympathetic activity mediates, in part, the increased risk.
An increase in tonic sympathetic outflow may be attributable to multiple mechanisms, such as increases in cardiac sympathetic innervation density, altered morphology of stellate ganglia neurons, or alterations in cardiac sympathetic preganglionic neurons (SPNs). Furthermore, these neuroplastic changes associated with SCI may be mediated by nerve growth factor (NGF). NGF is a neurotrophin that supports the survival and differentiation of sympathetic neurons and enhances target innervation (40). Sympathetic neurons have a lifelong requirement for NGF. For example, cardiac NGF originates from the target tissue (e.g., myocardium) and is transported via sympathetic fibers back to cell bodies in the stellate ganglia and possibly to SPNs located in the intermediate zone of spinal cord thoracic segments T1–T5. In adult animals, NGF is critical in regulating the density of cardiac sympathetic innervation. A deficiency of NGF causes the loss of sympathetic fibers, whereas excessive amounts of NGF cause hyperinnervation (21, 26). Increases in NGF due to cardiac dyssynchrony (abnormal electrical conduction with discoordinate contraction), disease, or injury can produce adverse sympathetic remodeling (7). Importantly, myocardial damage and cardiac dyssynchrony, related to calcium overload, begins as early as 15 min after SCI in humans and animals (78). Thus, individuals with SCI may have increased cardiac NGF content and cardiac sympathetic hyperinnervation.
Therefore, we tested the hypothesis that T5X is associated with an increased left ventricular (LV) NGF content, LV sympathetic innervation density, and cardiac SPN arborization. To test this hypothesis, cholera toxin B subunit (CTB) and tyrosine hydroxylase immunohistochemistry procedures were used to examine the dendritic branching pattern of cardiac projecting SPNs and LV sympathetic innervation density, respectively. In addition, Nissl staining procedures on stellate ganglia neurons was used to determine the number and size of cardiac projecting postganglionic sympathetic neurons. We hypothesized that T5X would induce structural neuroplastic changes within the spinal cord, stellate ganglia, and heart.
MATERIALS AND METHODS
Surgical Procedures
Experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of Wayne State University and complied with The American Physiological Society's Guiding Principles in the Care and Use of Animals. Eighteen adult Sprague-Dawley male rats [n = 7: T5X and n = 11: Intact (sham T5X)] were studied to determine LV NGF content, LV sympathetic innervation density, stellate neuron size and number, and cardiac SPN arborization.
All surgical procedures were performed using aseptic surgical techniques. Rats were anesthetized with pentobarbital sodium (50 mg/kg ip), atropinized (0.05 mg/kg ip), intubated, and prepared for aseptic surgery. Supplemental doses of pentobarbital sodium (10–20 mg/kg ip) were administered if the rat regained the blink reflex or responded during the surgical procedures.
First surgical procedure: spinal cord transection.
After anesthesia was induced, rats were intubated and positioned prone over a thoracic roll that slightly flexed the trunk. The fourth thoracic vertebra was exposed via a midline dorsal incision and the spinous process and laminae were removed. Two ligatures (6-0 silk) were tightened around the underlying spinal cord between the fifth and sixth thoracic segments, and the spinal cord was completely transected by cutting between the ligatures with scissors (48, 49). In this way, there was minimal bleeding. Identical procedures were used for the Intact rats; however, the spinal cord was not ligated or cut. Sympathetic innervation to the heart is derived from preganglionic fibers that exit the spinal cord at the first through fourth thoracic levels (80). Transection between the fifth and sixth thoracic levels of the spinal cord preserves supraspinal control of cardiac sympathetic activity. The completeness of the transection was confirmed by visual inspection of the lesion site. During the acute recovery period (∼10 days), all rats were handled at least six times daily. During these periods, visual inspections and physical manipulations were performed to detect and prevent pressure sores. In addition, the urinary bladder was voided by manual compression, and all animals were weighed. After this acute recovery period, rats required only daily inspection, and the bladders did not require manual compression. The diets of all rats were continuously supplemented with palatable, nutritious, and enrichment treats (Bio-Serv, Frenchtown, NJ). No other dietary interventions were necessary. At day 7 posttransection, the rats received a motor activity score using criteria described previously (84). The motor activity score was assessed by placing the animal on a paper-covered table and observing spontaneous motor activity for 1 min. Motor scores ranged from 0 to 5. A motor score of 5 indicates normal walking, whereas a score of 0 indicates no weight-bearing or spontaneous voluntary movement in the hind limbs. All rats had a motor score of 0, which indicates no weight bearing. All rats were allowed to recover for 9 wk. Upon completion of the studies, the site of the spinal transection was confirmed by autopsy.
Second surgical procedure: stellate ganglia injections.
T5X and Intact animals were anesthetized as described above, and the stellate ganglia were approached (one at a time) via a ventral thoracotomy through the first intercostal space (50, 51). Specifically, a 2-cm midline incision was made, and the left and, subsequently, right pectoral and intercostal muscles were partially dissected (∼5 mm) between the first and second rib to obtain access to the left and, subsequently, the right stellate ganglion, which are localized dorsal of the subclavian artery and vein. Four microliters of CTB (1 mg CTB dissolved in 200 μl of sterile water) were mixed with 1 μl of 3% Evans blue dye. The purpose of the Evans blue dye was to visualize the injectate (CTB is colorless), assuring localization within the ganglia. All injections were confined within the stellate ganglia. The CTB/Evans blue dye solution (∼5 μl total volume) were mineral oil pressure injected into the right and left stellate ganglia by using a glass micropipette (10–15 micron tip). The animals recovered for 5 days. A 3- to 7-day period is optimal for the retrograde transport of CTB and labeling of SPNs (45). It is important to note that stellate ganglia injections of CTB do not alter resting, reflex, or exercise-induced sympathetic activity (50).
Third (terminal) surgical procedure: perfusion.
T5X and Intact rats were deeply anesthetized with pentobarbital sodium (100 mg/kg), injected with heparin (1,000 IU), and flushed transcardially with 500 ml oxygenated tissue culture medium (cat. no. D-8900; Sigma, St. Louis, MO). Subsequently, a small full-width, cross section of the LV free wall (∼1 mm2) was carefully dissected from the left ventricle, flash frozen in liquid nitrogen, and stored at −80°C until analysis for LV NGF content (48) (see below). Extreme care was taken, by the same investigator during all perfusions, to obtain the sample from approximately the same area from all hearts (midway between the base and apex just lateral to the interventricular septum). Subsequently, the rats were perfused transcardially with 1 liter of 4% formaldehyde in 0.1 M phosphate buffer, pH 7.4. The remaining LV free wall was removed, cryoprotected in increasing concentrations of sucrose (10, 20, and 30%), and embedded in blocks with Tissue-Tek optimal cutting temperature compound. Spinal cords and stellate ganglia were removed and postfixed intact for 3 days at room temperature on a shaker in the same fixative solution as for the perfusion. After postfixation, the thoracic spinal cord (T1–T4) was dissected out as a single block. The rostral edge of the dorsal root entry zone was taken as the rostral boundary for the block. Blocks of thoracic segments T1–T4 were obtained because SPNs projecting to the stellate ganglia are concentrated in these segments (80). Spinal cords and ganglia were cryoprotected in increasing concentrations of sucrose (10%, 20%, and 30%) and embedded in Tissue-Tek optimal cutting temperature. All tissues were stored at −80°C until analysis.
Tissue Processing, Analysis, and Immunohistochemistry
Stellate ganglia.
The entire stellate ganglia from seven T5X and six Intact rats were sectioned at 10-μm intervals, thaw mounted on Superfrost Plus slides (Fisher Scientific), and stained with cresyl violet. The cardiac sympathetic postganglionic cell bodies, on every 10th section, were counted and measured with the aid of a ×20 objective and MicroBrightField Neurolucida software interfaced with a BH-2 Olympus microscope (50, 51). Only neurons with distinct, prominent nucleoli were counted. Based on a study by Jones (29), no correction for split nucleoli is necessary in 10-μm sections.
Spinal cord.
The spinal cord blocks (T1–T4) from seven T5X and six Intact rats were sectioned horizontally at 40-μm intervals. Tissue sections were washed in 10 mM Tris, 0.9% NaCl, 0.05% thimerosal in 10 mM phosphate buffer, pH 7.4 (TPBS) containing 0.3% Triton X-100 for 3× 10 min and then incubated in 10% heat-inactivated normal horse serum (Invitrogen) in TPBS-Triton for at least 1 h. The sections were then incubated in goat anti-CTB antiserum (1:400,000 List Biologicals) in TPBS-Triton containing 10% normal horse serum for 3 days at room temperature. After rinsing (in TPBS, 3× 10 min each), sections were incubated with biotinylated donkey anti-goat immunoglobulin (1:500; Jackson Laboratories) in TPBS-Triton with 1% normal horse serum overnight at room temperature. Sections were rinsed again (TPBS, 3× 10 min each) and incubated 4–6 h in 1:1,500 ExtrAvidin-Perixodase in TPBS-Triton (cat. no. E-2886; Sigma). Immunoreactive axons were revealed with the nickel-intensified diaminobenzidine reaction [Fig. 1 (44)].
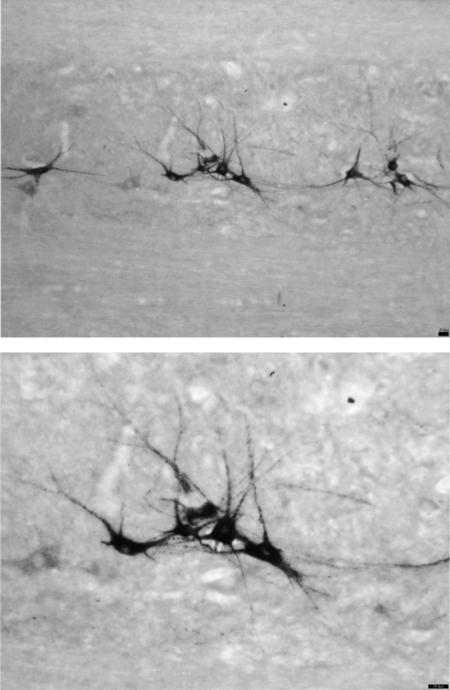
Fig. 1.
Photomicrograph of a 40-μm horizontal section through the intermediolateral cell column of thoracic segments T1–T4 processed for cholera toxin B subunit (CTB)-immunoreactivity (IR). CTB was injected into the right and left stellate ganglia to retrogradely label sympathetic preganglonic neurons (SPNs) projecting mainly to the heart. CTB-IR was revealed with nickel-intensified diaminobenzidine (black). Top: note the clusters of SPNs (magnification, ×10). Bottom: same photomicrograph at a higher magnification (×20). Scale bar = 25 μm.
structural analysis.
Morphological features of cardiac SPNs in the Intact and injured spinal cords were analyzed as described by Nelson and colleagues (58–60). Specifically, each neuron was analyzed using the Sholl analysis [Fig. 2, (79)] of dendritic branching, which assumes that dendritic arborization is an indirect measure of available postsynaptic space. A series of concentric rings calibrated at 10-μm intervals was superimposed on each neuron and centered on the cell body. Intersections between dendrites and each concentric ring were then counted. The location and number of intersections were plotted (Fig. 3) and used for statistical comparisons. In each animal, two to three horizontal sections through the intermediolateral column were examined, and nine to ten neurons located rostral (T2–T4) to the injury were measured using Neurolucida software (MBF Bioscience, Williston, VT). Each cell's morphometric features were measured within one 40-μm-thick horizontal section. Only cells with clearly distinguishable perikarya and dendritic trees were assessed. Specifically, to be selected for analysis, CTB-labeled neurons satisfied the following criteria: 1) dark and consistent staining in the entire dendritic tree, 2) lack of truncated dendrites, and 3) relative separation from nearby stained neurons to avoid overlapping dendrites. These selection criteria were similar to previous studies examining dendritic arborization in other regions (59, 85). The examined neurons also fulfilled the criteria of lying within the nucleus intermediolateralis, pars principalis, and pars funicularis and within two segments from the injury site and were chosen randomly from both left and right intermediolateral columns. No differences were observed between the left and right sides. Three morphological features were recorded: 1) area of soma, 2) overall length of all visible processes, and 3) number of primary dendrites. The morphological features were compared between Intact and T5X animals. Specifically, multiplanar photomicrographs were taken, and the images were stacked using MicroBrightfield Neurolucida software. Stacked images of CTB-labeled neurons identified as cardiac SPNs, were examined on an Olympus BH-2 microscope outfitted with a motorized stage, Neurolucida imaging software, and high-resolution digital camera (model CX9000; MBF Bioscience). Cell bodies and dendrites were reconstructed using the neuron tracing feature on the Neurolucida system and dendritic branching was assessed in the NeuroExplorer 3D visualization and morphometric analysis program included with the Neurolucida system.
Fig. 2.
Example of the Sholl analysis of dendritic branching. Multiplanar photomicrographs of CTB-labeled SPNs in spinal cord blocks (T1–T4) were taken at ×20 magnification. The images were stacked using the Neurolucida software. Cell bodies and dendrites were digitally reconstructed using the Neuron Tracing feature. A series of concentric rings calibrated at 10-μm intervals were superimposed on each neuron and centered on the cell body. The number of dendritic intersections within each concentric ring was counted, and the dendritic length was measured. Scale bar = 10 μm.
Fig. 3.
Morphological features of cardiac SPNs in 6 Intact and 7 T5X spinal cords were analyzed using the Sholl analysis of dendritic branching. A series of concentric rings calibrated at 10-μm intervals were superimposed on each neuron and centered on the cell body. A: the number of dendritic intersections within each concentric ring was counted, and the dendritic length was measured. The mean number of dendritic intersections [Intact group and T5 spinal cord-transected group (SCI)] was significantly increased in the SCI group. B: photomicrograph of a representative CTB immunoreactive SPN projecting to the stellate ganglia from 1 Intact and 1 SCI rat. C: same neurons after digital neuron tracing. Scale bar = 25 μm; *P < 0.05 Intact vs. SCI.
Left ventricle.
As stated above, a small full-width, cross section of the LV free wall, ∼1 mm2 was carefully dissected from the left ventricle and used for the analysis for LV NGF content (48). The remaining LV free wall was removed, cryoprotected, and embedded in blocks in O.C.T. compound. The LV blocks from four T5X and four Intact hearts were cut in transverse cross sections, at 10-μm intervals, serially thaw mounted on 25 Superfrost Plus slides (4 sections/slide), and stored at −20°C until analysis. The sequence of processing was planned so that each adjacent section was separated by 150 microns.
On the day of analysis (66), the slides were air dried, washed 3× 10 min in PBS (10 mM phosphate buffer containing 0.9% NaCl, and 0.1% sodium azide). The slides were then treated with 10 mg/ml sodium borohydride (3× 10 min) to reduce autoflourescence followed by one wash of PBS. The slides were incubated for 1.5 h at room temperature in PBS containing 2% BSA-fraction 5 (dilution buffer) followed by rabbit antityrosine hydroxylase (cat. no. AB152, 1:300 in dilution buffer; Millipore) overnight at room temperature. After being rinsed (PBS, 3× 10 min each) slides were incubated with Alexa Fluor 488 goat anti-rabbit immunoglobulin (1:300 in PBS containing 5% goat serum) for 1.5 h at room temperature. After being rinsed, slides were treated with 10 mM CuSO4/50 mM NH4C2H3O2 for 30 min, rinsed, and coverslipped with buffered glycerol.
LV INNERVATION DENSITY ANALYSIS.
Identical areas of the heart were analyzed from four Intact and four T5X rats. Photos were obtained, using consistent camera settings, from slides that had been processed together. Subsequently, four sections at least 150 μm apart from each heart were analyzed. Specifically, four regions of each LV free wall was quantified by threshold discrimination with the aid of a ×20 objective using the MicroBrightField Neurolucida software interfaced with a BH-2 Olympus microscope (51).
All photomicrographs were treated in an identical manner. Black and white photos were opened in the Neurolucida software program, and the brightness/contrast tool was used to adjust each image. The threshold tool was then used to identify nerve fibers. The automated function set a beginning threshold level, but this did not reliably include all sympathetic fibers, while excluding all nonneuronal tissue. The threshold was then manually adjusted to ensure that only specific tyrosine hydroxylase staining was identified. Innervation density (i.e., tyrosine hydroxylase-positive fibers) was expressed as the percent area that was above the threshold. Specifically, the area of the tyrosine hydroxylase-positive fibers was divided by the area of the section. The data were averaged to obtain one data point per animal. Heart tissue obtained from Intact and T5X animals revealed many tyrosine hydroxylase immunopositive sympathetic terminals (Fig. 4).
Fig. 4.
Tyrosine hydroxylase (TH) immunohistochemistry was used to determine sympathetic innervation density on the left ventricular (LV) free wall in 4 Intact and 4 T5 SCI rats. TH-positive fibers were identified using the Neurolucida software. Innervation density was expressed as the area of TH-positive fibers divided by the area of the section. The data from each animal were averaged to obtain 1 data point per animal. Left ventricular sympathetic nerve fiber density (A) and number of LV sympathetic nerve fibers (B) were significantly higher in the SCI group compared with the Intact group. C: these results are consistent with photomicrographs (×20) of TH immunoreactive sympathetic fibers in 1 SCI (right) and 1 Intact rat (left). Many more TH immunoreactive sympathetic fibers are seen in the photomicrograph from the SCI rat. Scale bar = 25 μm; *P < 0.05 Intact vs. SCI.
ELISA for Left Ventricular NGF Content
The LV free wall from six Intact and six T5X animals was pulverized into fine powder using a liquid nitrogen-cooled mortar and pestle. Approximately 70 mg of tissue was suspended in ∼100 μl of lysis buffer [137 mM NaCl, 20 mM Tris·HCl (pH = 8.0), 1% NP40, 10% glycerol, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 0.5 mM sodium vanadate]. The samples were homogenized with Kontes Pellet Pestle Micro Grinders (∼30 s), vortexed, and centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant was diluted 1:20 in DPBS buffer (0.2 g KCl, 8.0 g NaCl, 0.2 g KH2PO4, 1.15 g Na2HPO4, 133 mg CaCl2·2H2O, and 100 mg MgCl2·6H2O per 1 liter ddH2O). All samples were stored at −80° C.
The concentration of NGF was determined using Promega's NGF Emax ImmunoAssay System (Promega, Madison, WI). Subsequently, the samples and standards were assayed in duplicate according the manufacturer instructions. MaxiSorp Plates (cat. no. 439454; Nunc) were incubated with carbonate coating buffer containing polyclonal anti-NGF overnight at 4°C. The next day, the plates were washed one time, blocked with one-time block and sample buffer for 1 h at room temperature, and washed again. Next, 100 μl of either sample or standard was added to each well and incubated for 6 h at room temperature. The wells were then incubated with a secondary monoclonal anti-NGF overnight at 4°C. Subsequently, the wells were incubated with anti-rat IgG conjugated to horseradish peroxidase for 2.5 h at room temperature. Between incubations, the plates were washed five times (unless otherwise stated). A TMB One solution was used to develop color in the wells for 10 min at room temperature. The reaction was stopped with the addition of 100 μl/well 1 N HCl. The absorbance was read at A450 within 30 min in a Molecular Devices ThermoMax microplate reader with SOFTmax PRO v3.1 software (Sunnyvale, CA).
Data Analysis
All data are reported as means ± SE. Using a Student's unpaired t-test, we compared LV NGF content (Fig. 5), LV sympathetic nerve fiber density and number (Fig. 4, A and B), SPN soma size, maximum dendritic length, and number of intersections/animal (which represents the total dendritic field; Fig. 6, A, B, and C) as well as stellate neuron number and size (Fig. 7, A and B) between Intact and T5 spinal cord transected rats.
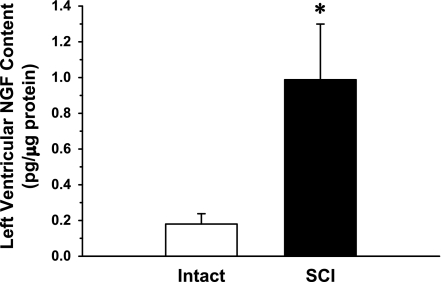
Fig. 5.
Left ventricular nerve growth factor (NGF) content, determined by ELISA, from a sample of the LV free wall from 6 Intact and 6 T5 SCI rats. The samples were individually tested for NGF. Left ventricular NGF content was significantly higher (∼5.4-fold) in SCI rats. *P < 0.05 Intact vs. T5X.
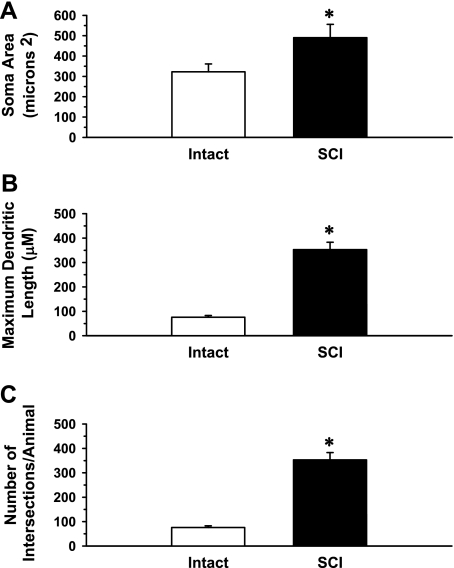
Fig. 6.
Morphological features of cardiac SPNs in 6 Intact and 7 T5X spinal cords were analyzed using the Neurolucida software. Shown are the soma area (A), maximum dendritic length (B), and number of intersections/animal (C) of SPNs projecting to the stellate ganglia in 2 groups of rats, Intact and T5 SCI. Morphological features showed a significant increase in SPN soma size, dendritic length, and the total dendritic field in the SCI group compared with Intact group. *P < 0.05 Intact vs. SCI.
Fig. 7.
Entire stellate ganglia from 7 T5 SCI and 6 Intact rats were sectioned at 10-μm intervals, thaw mounted on slides, and stained with cresyl violet. The cardiac sympathetic postganglionic cell bodies on every 10th section were counted and measured using the Neurolucida software. Fig. 7 presents stellate ganglia neuronal number (A) and soma area (B) from Intact and SCI rats. There were no differences in the neuronal number or soma area between the two groups. These results were consistent with that shown in C, of many stellate neurons in both the Intact and SCI groups. The counts reported in this figure are similar to counts reported in 2 recent publications (6, 21). Scale bar (C) = 25 μm.
The Sholl analysis was evaluated by the use of a two-way repeated-measures ANOVA applied to the dendritic intersections found at each concentric ring. Specifically, a two-way repeated-measures ANOVA [group (Intact or T5X) × branching order] was applied to the numbers of dendrites according to their order of branching (Fig. 3). Post hoc Tukey analysis was used to document a significant difference between the two groups for every point on Fig. 3A. Significance was set at P < 0.05.
RESULTS
Determination of Left Ventricular NGF Content
An ELISA was used on a small, ∼1-mm2, full-width, cross section of the LV free wall from six Intact and six T5X animals to determine NGF content. Fig. 5 presents LV NGF content for Intact and T5X rats. Left ventricular NGF content was significantly higher in T5X rats (∼5.4-fold).
Determination of Left Ventricular Sympathetic Innervation Density
Tyrosine hydroxylase immunohistochemistry was used to determine sympathetic innervation density on the remaining LV free wall of Intact and T5X rats, because tyrosine hydroxylase is the rate-limiting enzyme in norepinephrine synthesis and is routinely used to determine cardiac sympathetic innervation density (20, 47). Innervation density was expressed as the percent area of tyrosine hydroxylase-positive fibers. Specifically, the area of the tyrosine hydroxylase-positive fibers was divided by the area of the section. Four sections, at least 150 microns apart, from each animal were analyzed, and the data were averaged to obtain one data point per animal. Left ventricular sympathetic nerve fiber density (Fig. 4A) and the number of LV sympathetic nerve fibers (Fig. 4B) were increased in T5X rats. These results were consistent with Fig. 4C showing photomicrographs of tyrosine hydroxylase immunoreactive sympathetic fibers in one Intact (left) and one T5X rat (right). Left ventricular sympathetic nerve fiber density and number were significantly higher in the T5X group compared with the Intact group.
Determination of Cardiac Sympathetic Preganglionic Dendritic Intersections: Structural Analysis
Morphological features of cardiac SPNs in the six Intact and seven T5X spinal cords were analyzed using the Sholl analysis of dendritic branching, which assumes that dendritic arborization is an indirect measure of available postsynaptic space. A series of concentric rings calibrated at 10-μm intervals were superimposed on each neuron and centered on the cell body. Intersections between dendrites and each concentric ring were then counted. To identify SPNs, CTB was injected into the stellate ganglia to retrogradely label SPNs projecting mainly to the heart. This is an important procedure because it is well documented that the majority of cardiac sympathetic axons and nerve terminals originate in the cell bodies of the bilateral stellate ganglia. Specifically, bilateral stellate ganglionectomy in the rat reduces cardiac norepinephrine content by 89–100% (64, 90), suggesting that > 90% of cardiac sympathetic innervation arises from the stellate ganglia. In this context, we mainly examined cardiac projecting SPNs. Specifically, Fig. 3 presents the Sholl analysis of the mean number of intersections per CTB-labeled cardiac SPN between each ring in a series of concentric rings. The Sholl analysis was evaluated by the use of a two-way repeated-measures ANOVA applied to the dendritic intersections found at each concentric ring. Specifically, a two-way repeated-measures ANOVA [group (Intact or T5X) × branching order] was applied to the numbers of dendrites according to their order of branching (Fig. 3). Significance was set at P < 0.05. The two-way ANOVA revealed a significant group effect, significant branching order effect, and significant group by branching order interaction. Post hoc Tukey analysis documented a significant difference between the two groups for every point on Fig. 3A. These results suggest that SPN arborization was increased in T5X rats.
Determination of SPN Soma Area, Dendritic Length, and Total Number of Dendritic Intersections
SPN soma area (Fig. 6A), maximum dendritic length (Fig. 6B), and the total dendritic field (Fig. 6C) were increased in T5X rats. The increased SPN soma size and maximum dendritic length are consistent with the increase in the total number of dendrites (Fig. 6C), since processes within the cell body, such as synthesis of proteins, are responsible for the maintenance of overall cell structure.
Determination of the Size and Number of Cardiac Projecting Postganglionic Neurons in the Stellate Ganglia
Nissl staining with cresyl violet was used to determine the size and number of cardiac projecting postganglionic neurons in the entire stellate ganglia. The cardiac sympathetic postganglionic cell bodies, on every 10th section, were counted and measured with the aid of a ×20 objective and MicroBrightField Neurolucida software interfaced with a BH-2 Olympus microscope (50, 51). Only neurons with distinct, prominent nucleoli were counted. There were no changes in stellate neuron number or size (Fig. 7A and B) between Intact and T5X rats.
DISCUSSION
In this study, we assessed the potential mechanisms by which spinal cord transection at T5 alters the autonomic nervous system, in particular, the augmentation of tonic sympathetic outflow. We hypothesize that T5X would be associated with increased LV NGF content, increased LV sympathetic innervation density, and increased cardiac SPN arborization. Sham-operated Intact rats were compared with paraplegic (9 wk post-T5X) rats. Following T5X, there was a significant increase in LV NGF content (Fig. 5), LV sympathetic innervation density (Fig. 4), and cardiac SPN arborization (Figs. 3 and 6). In contrast, T5X did not alter soma number or size of stellate ganglia neurons (Fig. 7). These structural neuroplastic changes in cardiac innervation may contribute to the cardiovascular dysfunction associated with SCI.
In addition to cardiovascular dysfunction, several additional complications associated with SCI (e.g., chronic pain, spasticity, and autonomic dysreflexia) are also mediated, in part, by NGF-induced structural neuroplastic changes within the spinal cord and peripheral tissues. Specifically, SCI produces changes in small-diameter peptidergic afferent fibers, increases the density of lumbosacral propriospinal projections, induces sprouting of the corticospinal tract, causes a reorganization of the neural pathways regulating bladder and urethral sphincter function, and alters vascular responsiveness (3, 5, 16, 22, 24, 34, 36, 46, 61, 62, 77, 89).
Importantly, trophic factors, such as NGF, mediate many of these structural neuroplastic changes. For example, it is well known that increased levels of NGF in the bladder, spinal cord, and dorsal root ganglia are associated with bladder hyperreflexia after SCI. Furthermore, immunoneutralization of NGF in the spinal cord suppressed NGF levels and also suppressed bladder hyperreflexia (77). Similarly, central sprouting of small-diameter primary afferent fibers in the dorsal horn of the spinal cord occurs concurrently with the development of autonomic dysreflexia. Furthermore, blocking intraspinal NGF prevented small-diameter afferent sprouting and decreased (by 43%) the hypertension induced by colon stimulation (36, 38, 55).
Our recent work (13, 14, 48, 49, 70, 71) and the present study extend the concept of structural neuroplastic changes, associated with NGF content, mediating complications associated with SCI. Furthermore, these results are consistent with recent work documenting that individuals and animals with paraplegia have elevated heart rates (23), increased blood pressure variability (71), episodic bouts of life-threatening hypertension as part of a condition termed autonomic dysreflexia (12, 13), and elevated sympathetic activity above the level of the lesion (14, 31, 48, 70). Paraplegia also alters cardiac electrophysiology and the abundance of Ca2+ regulatory proteins in a manner that increases the susceptibility to electrically-induced as well as ischemia and reperfusion-induced ventricular arrhythmias (14, 48, 49, 70, 71). These findings are important because patients with high-level spinal cord injuries prioritize the recovery of autonomic functions, such as cardiovascular and sexual function and bowel and bladder control, above the ability to walk (1). As stated profoundly by Christopher Reeve, “Spinal cord injury is a ferocious assault on the body that leaves havoc in its wake. Paralysis is certainly part of its legacy, but there are other equally devastating consequences including autonomic dysfunction: compromised cardiovascular, bowel, bladder, and sexual function. Treatments and cures for these losses would greatly improve the quality of life for all of us living with SCI” [September 30, 2004; (69)].
T5X Increased LV NGF Content
T5X increased LV NGF content (Fig. 5). These results confirm a recent report (48). NGF is expressed in the heart and other sympathetic targets (33). The production of NGF in target organs determines the density of innervation by the sympathetic nervous system (32). For example, overexpression of NGF within the heart of transgenic mice causes hyperinnervation (21). Accordingly, individuals and animals with paraplegia have elevated heart rates (23). Elevated heart rates are an important consideration because several long-term cohort studies have found resting heart rate to be a risk factor for mortality from coronary heart disease, cardiovascular diseases, cancer, or all causes (19, 86).
T5X Increased LV Sympathetic Innervation Density
The increased LV NGF content was associated with an increased LV sympathetic innervation density (Fig. 4). The concept of plasticity is well established with respect to heart muscle; however, the importance of plasticity of cardiac innervation has only recently received attention (83). In this context, investigators have recently provided evidence implicating sympathetic nerve sprouting in ventricular arrhythmogenesis and sudden cardiac death (7, 10, 43). For example, hypercholesterolemia, a change in rate or activation sequence, or enhanced sympathetic nerve activity induces proarrhythmic nerve sprouting and sympathetic hyperinnervation. The hyperinnervation is associated with dispersion of repolarization, changes in calcium currents, and increased susceptibility to ventricular arrhythmias (41, 43, 91). Importantly, myocardial damage and cardiac dyssynchrony, related to calcium overload, begins as early as 15 min after SCI in humans and animals (78). Thus, cardiac dyssynchrony, associated with midthoracic SCI, may increase cardiac NGF content. The increased cardiac NGF content may increase cardiac sympathetic innervation density and increase the susceptibility to ventricular tachyarrhythmias induced by programmed electrical stimulation (70, 71), myocardial reperfusion (49), and myocardial ischemia (48).
T5X Altered the Morphology of Cardiac Projecting SPNs
Specifically, T5X increased soma size, maximum dendritic length, and dendritic number of mainly cardiac projecting SPNs rostral to the injury (Figs. 3 and 6). These results are in agreement with a recent report by Kalincik et al. (30) who documented that T4 spinal cord transection increased the soma size of SPNs rostral to the transection, but differ from the results of Krassioukov and Weaver (34, 35), where alterations of soma size were not seen rostrally at 7 or 30 days postinjury. The innovative feature of the present study is that stellate ganglia projecting SPNs were analyzed. In the previous studies (30, 34, 35), heterogenous populations of SPNs with unknown targets were analyzed. In sharp contrast, in the present study, a nearly homogenous population of SPNs projecting to the heart was studied. Specifically, we injected CTB into the stellate ganglia to retrogradely label mainly SPNs projecting to the heart. It is well documented that the majority of cardiac sympathetic axons and nerve terminals originate in the cell bodies of the bilateral stellate ganglia. Bilateral stellate ganglionectomy in the rat reduces cardiac norepinephrine content by 89–100% (64, 90) suggesting that > 90% of cardiac sympathetic innervation arises from the stellate ganglia. Therefore, this paper presents the first report examining morphometric features of SPNs mainly projecting to the heart following SCI.
Potential Mechanisms
Although the mechanisms mediating the increased LV NGF content and structural neuroplastic changes are unknown, it is important to note that increases in cardiac NGF content due to abnormal cardiac electrical conduction, disease, or injury can produce adverse sympathetic remodeling (7). Importantly, abnormal electrical conduction (71), unbalanced autonomic activity (48), and myocardial damage related to calcium overload begin as early as 15 min after SCI in humans and animals (78). This may be due, in part, to a baroreflex-mediated increased cardiac sympathetic efferent activity and decreased cardiac parasympathetic activity following acute hypotension due to loss of sympathetic vasoconstrictor drive below the site of the lesion. The baroreflex-induced unbalanced autonomic activity may cause myocardial electrical uncoupling, arrhythmias, myocardial damage, and thus stimulate NGF production, which further promotes cardiac sympathetic hyperinnervation. Thus the initial hypotension and baroreflex-mediated unbalanced autonomic activity may cause abnormal cardiac conduction and thus explain, in part, the increased cardiac NGF content and LV sympathetic hyperinnervation. However, it is unclear whether cardiac-produced NGF is transported to the spinal cord. In this context, Krenz and Weaver (37) documented increased NGF levels around the lesion site following T4X spinal cord transection. The mechanisms responsible for the production of NGF, as well as the site of origin, remain unclear. However, these data suggest that the demand for trophic factors may be activity dependent. Specifically, SPNs with intact descending innervation (and thus active) may require trophic factors. This is suggested because it is well known that neurotrophins act as modulators of activity-dependent plasticity (4). For example, the trophic effect of brain-derived neurotrophic factor on dendritic length and branching in retinal ganglion cells is dependent on their activity (11). In a similar manner, Kalincik et al. (30) documented differential regulation of SPNs above vs. below a T4 spinal cord transection. Finally, activity in locomotor circuits is well known to lead to structural plasticity, changes in electrophysiological properties, and altered function within the spinal cord (6, 88). Thus altered morphology of SPNs could be associated with their hyperactivity and altered cardiac electrophysiology in spinal cord-injured humans and animals (14).
Limitations
It is well known that sympathetic innervation of the heart is directed by chemoattractive and chemorepulsive factors (18, 27). Specifically, the chemoattractive factor, NGF, supports sympathetic neuron survival and promotes cardiac axon outgrowth (15, 18). In contrast, the chemorepulsive factor semaphorin 3a attenuates sympathetic axon extension in the heart (27). Importantly, a gradient of chemoattractive and chemorepulsive factors, and accordingly, heterogeneity of sympathetic innervation density, exists across the ventricle with more sympathetic fibers in the subepicardium than in the subendocardium (27, 68). This is an important consideration because heterogeneous sympathetic innervation density in the left ventricle increases the occurrence of ventricular arrhythmias (7, 8, 27, 47, 72). In the present study, there was no attempt to determine regional sympathetic innervation density, and this is a limitation to the study. However, this is the first report documenting increased LV sympathetic innervation density following midthoracic SCI. Furthermore, hyperinnervation is associated with dispersion of repolarization, changes in calcium currents, and increased susceptibility to ventricular arrhythmias (41, 43, 91).
As stated in the introduction, NGF is produced in the target tissue (heart) and is transported back to the ganglia, where NGF promotes an increase in sympathetic innervation density. Thus, NGF levels should be elevated in the ganglia following T5X. However, we did not determine NGF levels in the stellate ganglia following T5X. Having measurements of stellate ganglia NGF content would have provided important data supporting the conclusions of this study. In addition, what is less clear is whether cardiac NGF is transported back to the spinal cord. As stated above, Krenz and Weaver (37) demonstrated that SCI induces increases in NGF content within certain populations of microglia and astroglia. Furthermore, B and T cells responding to the injury were found to contain NGF. These results suggest that neuronal remodeling of SPN following SCI is not mediated by cardiac NGF, but possibly from NGF released from the site of the injury. This possibility merits further investigation.
We performed bilateral stellate ganglia injections in this study as well as in two recent studies (50, 51) to identify all SPNs with the potential to promote ventricular arrhythmias in animals with spinal cord injuries (48, 49). However, unilateral injections may have provided more specific regional information. Specifically, sympathetic control of heart rate is primarily exerted by the right stellate ganglion (67, 74), while the left stellate ganglion is primarily responsible for ventricular arrhythmic events (74–76). However, the response to ischemia is complex and involves the cardio-cardiac sympathetic reflex with the efferent limb mainly through the right stellate ganglion and the afferent limb mainly through the left stellate ganglion (52–54). Specifically, cardiac sympathetic afferents are excited by ischemia and elicit a cardio-cardiac sympathetic reflex (9, 53) that plays a major role in the genesis of ventricular arrhythmias (73). Since the response to ischemia involves afferent and efferent fibers from both ganglia, we designed the present study to examine both components.
Finally, > 90% of the sympathetic supply to the heart originates from the stellate ganglion (64, 65). However, the stellate ganglia is not a pure cardiac sympathetic ganglion, because a small minority of its neurons innervate other structures (2, 39, 87). Thus, some noncardiac projecting SPNs may have been analyzed. Nevertheless, the approach used in this study (bilateral stellate ganglia injections) was viewed as a significant advantage over nonspecific labeling of all SPNs with unknown targets, since the majority of SPNs labeled project to the heart.
Conclusion
T5X increased LV NGF content, LV sympathetic innervation density, and cardiac SPN arborization. These results provide mechanistic support of previous findings that paraplegia increases cardiac sympathetic tonus (48), alters cardiac electrophysiology (14), and increases the susceptibility to ventricular tachyarrhythmias induced by programmed electrical stimulation (70, 71) as well as myocardial reperfusion (49) and the clinically relevant ischemia-induced sustained ventricular tachycardia (48) in conscious animals.
Cardiovascular diseases are a growing concern for individuals with SCI. In fact, the prevalence of cardiovascular diseases in individuals with paraplegia is much higher than in the general population (82). Furthermore, the morbidity and mortality from cardiovascular disease exceeds that caused by renal and pulmonary complications, the primary cause of mortality in previous decades (56). Excessive sympathetic activity is responsible for, and/or contributes to, the morbidity and mortality associated with cardiovascular disease. Accordingly, the increased susceptibility to cardiovascular diseases may be mediated, in part, by NGF-induced cardiac sympathetic hyperinnervation. This hypothesis merits further investigation.
Perspective and Significance
It is important to note that worldwide efforts are in progress for the regenerative repair of injured spinal cords (25, 42). However, it is unknown whether regenerating or sprouting axons will form functionally appropriate connections and avoid forming aberrant synapses (63). Results from this study and many others (36, 55, 77) document that several complications associated with SCI are mediated, in part, by structural neuroplastic changes within the spinal cord and peripheral tissues. The potential complications of inappropriate autonomic rewiring are a serious concern and merit further investigation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-74122.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 21: 1371–1383, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Arbab MA, Wiklund L, Delgado T, Svendgaard NA. Stellate ganglion innervation of the vertebro-basilar arterial system demonstrated in the rat with anterograde and retrograde WGA-HRP tracing. Brain Res 445: 175–180, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Arnold JM, Feng QP, Delaney GA, Teasell RW. Autonomic dysreflexia in tetraplegic patients: evidence for α-adrenoceptor hyper-responsiveness. Clin Auton Res 5: 267–270, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14: 2919–2937, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Brock JA, Yeoh M, McLachlan EM. Enhanced neurally evoked responses and inhibition of norepinephrine reuptake in rat mesenteric arteries after spinal transection. Am J Physiol Heart Circ Physiol 290: H398–H405, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cai LL, Courtine G, Fong AJ, Burdick JW, Roy RR, Edgerton VR. Plasticity of functional connectivity in the adult spinal cord. Philos Trans R Soc Lond B Biol Sci 361: 1635–1646, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res 86: 816–21, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101: 1960–1969, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Casati R, Lombardi F, Malliani A. Afferent sympathetic unmyelinated fibres with left ventricular endings in cats. J Physiol 292: 135–148, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res 50: 409–416, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Cory S. BDNF modulates, but does not mediate, activity-dependent branching and remodeling of optic axon arbors in vivo. J Neurosci 19: 9996–10003, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins HL, DiCarlo SE. Acute exercise reduces the response to colon distension in T5 spinal rats. Am J Physiol Heart Circ Physiol 282: H1566–H1570, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Collins HL, DiCarlo SE. TENS attenuates response to colon distension in paraplegic and quadriplegic rats. Am J Physiol Heart Circ Physiol 283: H1734–H1739, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Collins HL, Rodenbaugh DW, DiCarlo SE. Paraplegia alters cardiac electrophysiology and increases the susceptibility to ventricular arrhythmias. In: Progress in Brain Research, edited by Weaver L, Polosa C. New York: Elsevier Science, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76: 1001–1011, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Fouad K, Pedersen V, Schwab ME, Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol 11: 1766–1770, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 43: 408–416, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci 24: 743–751, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenland P, Daviglus ML, Dyer AR, Liu K, Huang CF, Goldberger JJ, Stamler J. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. Am J Epidemiol 149: 853–862, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Habecker BA, Bilimoria P, Linick C, Gritman K, Lorentz CU, Woodward W, Birren SJ. Regulation of cardiac innervation and function via the p75 neurotrophin receptor. Auton Neurosci 140: 40–48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassankhani A, Steinhelper ME, Soonpaa MH, Katz EB, Taylor DA, Andrade-Rozental A, Factor SM, Steinberg JJ, Field LJ, Federoff HJ. Overexpression of NGF within the heart of transgenic mice causes hyperinnervation, cardiac enlargement, and hyperplasia of ectopic cells. Dev Biol 169: 309–21, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Hill CE, Beattie MS, Bresnahan JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol 171: 153–169, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation 65: 457–464, 1982 [DOI] [PubMed] [Google Scholar]
- 24.Hou S, Duale H, Cameron AA, Abshire SM, Lyttle TS, Rabchevsky AG. Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J Comp Neurol 509: 382–399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang H, Chen L, Wang H, Xi H, Gou C, Zhang J, Zhang F, Liu Y. Safety of fetal olfactory ensheathing cell transplantation in patients with chronic spinal cord injury. A 38-month follow-up with MRI. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 20: 439–443, 2006 [PubMed] [Google Scholar]
- 26.Ieda M, Fukuda K, Hisaka Y, Kimura K, Kawaguchi H, Fujita J, Shimoda K, Takeshita E, Okano H, Kurihara Y, Kurihara H, Ishida J, Fukamizu A, Federoff HJ, Ogawa S. Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression. J Clin Invest 113: 876–84, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ieda M, Kanazawa H, Kimura K, Hattori F, Ieda Y, Taniguchi M, Lee JK, Matsumura K, Tomita Y, Miyoshi S, Shimoda K, Makino S, Sano M, Kodama I, Ogawa S, Fukuda K. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat Med 13: 604–612, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Inskip J, Plunet W, Ramer L, Ramsey JB, Yung A, Kozlowski P, Ramer M, Krassioukov A. Cardiometabolic risk factors in experimental spinal cord injury. J Neurotrauma 27: 275–285, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Jones R. Split nucleoli as a source of error in nerve cell counts. Stain Technol 19: 91–95, 1937 [Google Scholar]
- 30.Kalincik T, Choi EA, Feron F, Bianco J, Sutharsan R, Hayward I, Mackay-Sim A, Carrive P, Waite PM. Olfactory ensheathing cells reduce duration of autonomic dysreflexia in rats with high spinal cord injury. Auton Neurosci 154: 20–29, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Karlsson AK, Friberg P, Lonnroth P, Sullivan L, Elam M. Regional sympathetic function in high spinal cord injury during mental stress and autonomic dysreflexia. Brain 121: 1711–1719, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Korsching S, Thoenen H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: correlation with density of sympathetic innervation. Proc Natl Acad Sci USA 80: 3513–3516, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korsching S, Thoenen H. Developmental changes of nerve growth factor levels in sympathetic ganglia and their target organs. Dev Biol 126: 40–46, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Krassioukov AV, Weaver LC. Reflex and morphological changes in spinal preganglionic neurons after cord injury in rats. Clin Exp Hypertens 17: 361–373, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Krassioukov AV, Weaver LC. Morphological changes in sympathetic preganglionic neurons after spinal cord injury in rats. Neuroscience 70: 211–225, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Krenz NR, Meakin SO, Krassioukov AV, Weaver LC. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J Neurosci 19: 7405–7414, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krenz NR, Weaver LC. Nerve growth factor in glia and inflammatory cells of the injured rat spinal cord. J Neurochem 74: 730–739, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience 85: 443–458, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49: 715–737, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Levi-Montalcini R. The nerve growth factor: its role in growth, differentiation and function of the sympathetic adrenergic neuron. Prog Brain Res 45: 235–258, 1976 [DOI] [PubMed] [Google Scholar]
- 41.Libbus I, Rosenbaum DS. Remodeling of cardiac repolarization: mechanisms and implications of memory. Card Electrophysiol Rev 6: 302–10, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med 29: 191–203, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu YB, Wu CC, Lu LS, Su MJ, Lin CW, Lin SF, Chen LS, Fishbein MC, Chen PS, Lee YT. Sympathetic nerve sprouting, electrical remodeling, and increased vulnerability to ventricular fibrillation in hypercholesterolemic rabbits. Circ Res 92: 1145–1152, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Llewellyn-Smith IJ, DiCarlo SE, Collins HL, Keast JR. Enkephalin-immunoreactive interneurons extensively innervate sympathetic preganglionic neurons regulating the pelvic viscera. J Comp Neurol 488: 278–89, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Llewellyn-Smith IJ, Martin CL, Arnolda LF, Minson JB. Retrogradely transported CTB-saporin kills sympathetic preganglionic neurons. Neuroreport 10: 307–312, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Llewellyn-Smith IJ, Weaver LC. Changes in synaptic inputs to sympathetic preganglionic neurons after spinal cord injury. J Comp Neurol 435: 226–40, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Lorentz CU, Alston EN, Belcik JT, Lindner JR, Giraud GD, Habecker BA. Heterogeneous ventricular sympathetic innervation, altered β-adrenergic receptor expression, and rhythm instability in mice lacking p75 neurotrophin receptor. Am J Physiol Heart Circ Physiol 298: H1652–H1660, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lujan HL, Chen Y, DiCarlo SE. Paraplegia increased cardiac NGF content, sympathetic tonus and the susceptibility to ischemia-induced ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 296: H1364–H1372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lujan HL, DiCarlo SE. T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am J Physiol Heart Circ Physiol 293: H3333–H3339, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Lujan HL, Palani G, Chen Y, Peduzzi-Nelson J, DiCarlo SE. Targeted ablation of cardiac sympathetic neurons reduces resting, reflex and exercise-induced sympathetic activation in conscious rats. Am J Physiol Heart Circ Physiol 296: H1305–H1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lujan HL, Palani G, Zhang L, DiCarlo SE. Targeted ablation of cardiac sympathetic neurons reduces the susceptibility to ischemia-induced sustained ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 298: H1330–H1339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malliani A, Parks M, Tuckett RP, Brown AM. Reflex increases in heart rate elicited by stimulation of afferent cardiac sympathetic nerve fibers in the cat. Circ Res 32: 9–14, 1973 [PubMed] [Google Scholar]
- 53.Malliani A, Recordati G, Schwartz PJ. Nervous activity of afferent cardiac sympathetic fibres with atrial and ventricular endings. J Physiol 229: 457–469, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malliani A, Schwartz PJ, Zanchetti A. A sympathetic reflex elicited by experimental coronary occlusion. Am J Physiol 217: 703–709, 1969 [DOI] [PubMed] [Google Scholar]
- 55.Marsh DR, Wong ST, Meakin SO, MacDonald JI, Hamilton EF, Weaver LC. Neutralizing intraspinal nerve growth factor with a trkA-IgG fusion protein blocks the development of autonomic dysreflexia in a clip-compression model of spinal cord injury. J Neurotrauma 19: 1531–1541, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil 86: 142–152, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil 86: 142–152, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Nelson AJ, Iwamoto GA. Reversibility of exercise-induced dendritic attenuation in brain cardiorespiratory and locomotor areas following exercise detraining. J Appl Physiol 101: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Nelson AJ, Juraska JM, Musch TI, Iwamoto GA. Neuroplastic adaptations to exercise: neuronal remodeling in cardiorespiratory and locomotor areas. J Appl Physiol 99: 2312–22, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Nelson AJ, Juraska JM, Ragan BG, Iwamoto GA. Effects of exercise training on dendritic morphology in the cardiorespiratory and locomotor centers of the mature rat brain. J Appl Physiol 108: 1582–1590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol 184: 373–380, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Pan B, Kim EJ, Schramm LP. Increased close appositions between corticospinal tract axons and spinal sympathetic neurons after spinal cord injury in rats. J Neurotrauma 22: 1399–410, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Pan B, Zahner MR, Kulikowicz E, Schramm LP. Effects of corticospinal tract stimulation on renal sympathetic nerve activity in rats with intact and chronically lesioned spinal cords. Am J Physiol Regul Integr Comp Physiol 293: R178–R184, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Pardini BJ, Lund DD, Schmid PG. Organization of the sympathetic postganglionic innervation of the rat heart. J Auton Nerv Syst 28: 193–201, 1989 [DOI] [PubMed] [Google Scholar]
- 65.Pardini BJ, Lund DD, Schmid PG. Innervation patterns of the middle cervical–stellate ganglion complex in the rat. Neurosci Lett 117: 300–306, 1990 [DOI] [PubMed] [Google Scholar]
- 66.Parrish DC, Gritman K, Van Winkle DM, Woodward WR, Bader M, Habecker BA. Postinfarct sympathetic hyperactivity differentially stimulates expression of tyrosine hydroxylase and norepinephrine transporter. Am J Physiol Heart Circ Physiol 294: H99–H106, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Randall WC, Rohse WG. The augmentor action of the sympathetic cardiac nerves. Circ Res 4: 470–475, 1956 [DOI] [PubMed] [Google Scholar]
- 68.Randall WC, Szentivanyi M, Pace JB, Wechsler JS, Kaye MP. Patterns of sympathetic nerve projections onto the canine heart. Circ Res 22: 315–323, 1968. [DOI] [PubMed] [Google Scholar]
- 69.Reeve C. Dedication. In: Progress in Brain Research, Autonomic Dysfunction after Spinal Cord Injury, edited by Weaver L, Polosa C. Amsterdam: Elsevier Science, 2005, p. ix [Google Scholar]
- 70.Rodenbaugh DW, Collins HL, DiCarlo SE. Increased susceptibility to ventricular arrhythmias in hypertensive paraplegic rats. Clin Exp Hypertens 25: 349–58, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Rodenbaugh DW, Collins HL, Nowacek DG, DiCarlo SE. Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am J Physiol Heart Circ Physiol 285: H2605–H2613, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest 115: 2305–2315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartz PJ, Foreman RD, Stone HL, Brown AM. Effect of dorsal root section on the arrhythmias associated with coronary occlusion. Am J Physiol 231: 923–928, 1976 [DOI] [PubMed] [Google Scholar]
- 74.Schwartz PJ, Stone HL. Effects of unilateral stellectomy upon cardiac performance during exercise in dogs. Circ Res 44: 637–645, 1979 [DOI] [PubMed] [Google Scholar]
- 75.Schwartz PJ, Stone HL. Left stellectomy in the prevention of ventricular fibrillation caused by acute myocardial ischemia in conscious dogs with anterior myocardial infarction. Circulation 62: 1256–1265, 1980 [DOI] [PubMed] [Google Scholar]
- 76.Schwartz PJ, Stone HL, Brown AM. Effects of unilateral stellate ganglion blockade on the arrhythmias associated with coronary occlusion. Am Heart J 92: 589–599, 1976 [DOI] [PubMed] [Google Scholar]
- 77.Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol 168: 2269–2274, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Sharov VG, Galakhin KA. [Myocardial changes after spinal cord injuries in humans and experimental animals]. Arkh Patol 46: 17–20, 1984. [PubMed] [Google Scholar]
- 79.Sholl D. The quantification of neuronal connectivity. In: Organization of the Cerebral Cortex, edited by Sholl DA. New York: Wiley, 1956 [Google Scholar]
- 80.Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res 455: 187–191, 1988 [DOI] [PubMed] [Google Scholar]
- 81.Strauss DJ, DeVivo MJ, Paculdo DR, Shavelle RM. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil 87: 1079–1085, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Thomas T, Haase N, Rosamond W, Howard V, Rumsfeld J, Manolio T, Zheng Z, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DJ, Hong Y, Members of the Statistics Committee, and Stroke Statistics Subcommittee. Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics-2006 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation 113: 85–151, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Verrier RL, Kwaku KF. Frayed nerves in myocardial infarction: the importance of rewiring. Circ Res 95: 5–6, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Von Euler M, Akesson E, Samuelsson EB, Seiger A, Sundstrom E. Motor performance score: a new algorithm for accurate behavioral testing of spinal cord injury in rats. Exp Neurol 137: 242–254, 1996 [DOI] [PubMed] [Google Scholar]
- 85.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 143: 387–93, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Wannamethee G, Shaper AG, Macfarlane PW. Heart rate, physical activity, and mortality from cancer and other noncardiovascular diseases. Am J Epidemiol 137: 735–748, 1993 [DOI] [PubMed] [Google Scholar]
- 87.Widenfalk B, Elfvin LG, Wiberg M. Origin of sympathetic and sensory innervation of the elbow joint in the rat: a retrograde axonal tracing study with wheat germ agglutinin conjugated horseradish peroxidase. J Comp Neurol 271: 313–318, 1988 [DOI] [PubMed] [Google Scholar]
- 88.Wolpaw JR. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol (Oxf) 189: 155–169, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Yeoh M, McLachlan EM, Brock JA. Chronic decentralization potentiates neurovascular transmission in the isolated rat tail artery, mimicking the effects of spinal transection. J Physiol 561: 583–596, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshimoto M, Wehrwein EA, Novotny M, Swain GM, Kreulen DL, Osborn JW. Effect of stellate ganglionectomy on basal cardiovascular function and responses to beta1-adrenoceptor blockade in the rat. Am J Physiol Heart Circ Physiol 295: H2447–H2454, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 95: 76–83, 2004 [DOI] [PubMed] [Google Scholar]