Abstract
Intracellular cAMP is compartmentalized to near membrane domains in endothelium, where it strengthens endothelial cell barrier function. Phosphodiesterase 4D4 (PDE4D4) interacts with the spectrin membrane skeleton and prevents cAMP from accessing microtubules. Expression of a dominant-negative PDE4D4 peptide enables cAMP to access microtubules, where it results in phosphorylation of the nonneuronal microtubule-associated protein tau at serine 214. Presently, we sought to determine whether PKA is responsible for tau-Ser214 phosphorylation and furthermore whether PKA phosphorylation of tau-Ser214 is sufficient to reorganize microtubules and induce endothelial cell gaps. In cells expressing the dominant-negative PDE4D4 peptide, forskolin activated transmembrane adenylyl cyclases, increased cAMP, and induced tau-Ser214 phosphorylation that was accompanied by microtubule reorganization. PKA catalytic and regulatory I subunits, but not the regulatory II subunit, coassociated with reorganized microtubules. To determine the functional consequence of tau-Ser214 phosphorylation, wild-type human tau40 and tau40 engineered to possess an alanine point mutation (S214A) were stably expressed in endothelium. In cells expressing the dominant-negative PDE4D4 peptide and tau-S214A, PKA-dependent phosphorylation of both the endogenous and heterologously expressed tau were abolished. Expression of tau-S214A prevented forskolin from depolymerizing microtubules, inducing intercellular gaps, and increasing macromolecular permeability. These findings therefore identify nonneuronal tau as a critical cAMP-responsive microtubule-associated protein that controls microtubule architecture and endothelial cell barrier function.
Keywords: adenylyl cyclase, cytoskeleton, phosphodiesterase
endothelial cells form a restrictive barrier that limits fluid, solute, and macromolecule access to the interstitium. The microtubule network plays a key role in establishing endothelial cell shape and hence barrier integrity (17, 41). Whereas stabilizing microtubules enhances endothelial barrier function, disrupting microtubules induces interendothelial cell gap formation that increases permeability (33, 47).
Tau and other microtubule-associated proteins bind to and stabilize microtubules (3, 45). Although tau is a relatively small protein, it shares a conserved 18-amino acid microtubule-binding repeat in common with larger microtubule-associated proteins (3, 44). Tau isoforms vary in their number of repeat domains (20), with the longest tau40 isoform having 4 microtubule-binding repeats at residues 256–273, 287–304, 318–335, and 350–367.
Tau40 possesses multiple consensus phosphorylation sites (3, 25); 20% of the tau40 amino acid sequence comprises serine, threonine, and tyrosine residues that can potentially be phosphorylated (25). At least two types of kinase phosphorylation sites exist in tau proteins: proline-direct kinase sites, which possess a threonine-proline (TP)-serine-proline (SP) sequence, and nonproline-directed sites. Most of the TP-SP sites are located in either the NH2- or COOH-terminal regions, outside of the microtubule-binding repeat domains. Kinases like glycogen synthase kinase-3β, mitogen-activated protein kinase, and cyclin-dependent kinase phosphorylate TP-SP sites. Nonproline-directed sites are located within (e.g., Ser262) or adjacent to (e.g., Ser214) the microtubule-binding repeats. Serines 262 and 356, for example, are found within a microtubule affinity-regulating kinase (MARK)-specific motif, KXGS. Multiple putative PKA-specific motifs, R(R)XSX, have been identified in tau40, including serines 129, 214, 352, and 409.
Phosphorylation controls tau binding to microtubules (3, 25). Increased tau phosphorylation reduces its microtubule-binding affinity necessary to adjust neurite outgrowth, axonal transport, and cytoskeleton dynamics (9, 25, 32) and increases the amount of depolymerized total or phosphorylated (phospho-) tau in the cytosol (25, 40). Tau overphosphorylation causes oligomerization and aggregation, ultimately leading to generation of the so-called paired helical filaments that are characteristic of pretangles in the neurons of Alzheimer's patients (19, 25). Tau-serines 214 and 409 are both PKA-phosphorylated in paired helical filaments (24, 25, 51), incriminating PKA phosphorylation of these sites in control of microtubule organization.
Recently, a role for tau in control of the microtubule architecture that adjusts endothelial cell integrity has been identified (6), although at present it is not clear how PKA-dependent tau phosphorylation influences microtubule architecture and barrier function. PKA activation attenuates nocodazole-induced microtubule disassembly in intact cells (8). This protective action of PKA likely reflects compartmentation of the cAMP signal (12, 35, 37), as near membrane cAMP signals strengthen the cortical actin rim and reduce endothelial cell permeability. In pulmonary microvascular endothelial cells (PMVECs), phosphodiesterase 4D4 (PDE4D4) limits cAMP access to nonneuronal tau (13). Inhibiting PDE4D4 enables cAMP to access the cytosol, where it promotes tau-Ser214 phosphorylation. Our present studies sought to determine whether PKA is responsible for tau-Ser214 phosphorylation and furthermore whether PKA phosphorylation of tau-Ser214 is sufficient to depolymerize microtubules, induce endothelial cell gaps, and increase permeability.
MATERIALS AND METHODS
Ethical approval.
All animal use studies were approved by the University of South Alabama Institutional Animal Care and Use Committee.
Cell cultures.
Rat PMVECs were isolated and cultured in DMEM supplemented with 10% FBS and penicillin/streptomycin using previously described methods (13, 34). Cells infected with retrovirus to deliver genes of interest, i.e., PDE4D41–166-green fluorescent protein (GFP), human tau (hTau)-Ser214, hTau-S214A alanine mutation, and vector [GFP or hemagglutinin (HA) tag] controls, were maintained in culture under the same conditions.
Microtubule-enriched cytosolic and membrane extractions.
Preconfluent cells were treated with forskolin, washed with PBS, and collected in cold TME-PP buffer [20 mM Tris·HCl, 5 mM MgAC2, 1 mM EDTA, pH 7.4, supplemented with protease inhibitors and protein phosphatase inhibitors 10 μM Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK), 2 μM leupeptin, 2 μM pepstatin A, 10 μM benzamidine, 2,000 units of aprotinin/ml, 1 mM PMSF, 10 mM NaF, and 1 mM Na3VO4]. For microtubule enrichment, cells suspended in TME-PP buffer were kept on ice for 30 min before homogenization to facilitate polymerized microtubule release to the cytosol. After centrifugation at 20,000 g for 20 min at 4°C, the supernatant (microtubule-enriched cytosolic fraction) was collected from the homogenate. The particulate pellet (membrane fraction) was resuspended in Triton lysis buffer (20 mM Tris·HCl, pH 7.5, with 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1% Triton X-100; Cell Signaling Technology) and extracted at 4°C for 15 min. The extracts were centrifuged as described above, and the supernatant was used as the membrane fraction. The cytosolic and membrane extractions were used directly for immunoprecipitation or prepared for Western blot using the methanol-chloroform precipitation approach, as previously described (52).
Immunocytochemistry.
Cells were seeded onto 12-mm coverslips and grown to preconfluence. For most experiments, cells were directly fixed in cold methanol and kept at −20°C for 5 min. After washing with PBS, cells were permeabilized with 0.1% Triton X-100 in PBS for 15 min at room temperature and blocked in 3% BSA for 1 h. Antibodies for β-tubulin (Covance, Berkeley, CA), pan-tau, phospho-tau at serines 214, 262, or 356 (Biosource, Camarillo, CA), and PKA catalytic and regulatory I and II subunits (Santa Cruz Biotechnology, Santa Cruz, CA) were added to the cells for 1 h. Following three rinses with PBS, cells were incubated with conjugated fluorescent secondary antibodies (Molecular Probes) for 1 h and mounted (DakoCytomation, Carpinteria, CA). For microtubule costaining with filamentous (F)-actin, cells were fixed in 1% paraffin supplemented with 30% methanol and kept at room temperature for 20 min. Rhodamine-conjugated phalloidin (Chemicon International, Temecula, CA) and β-tubulin were applied to cells with secondary antibodies. Fluorescent images were taken using either epifluorescence or confocal (Leica TCS SP2; Leica Microsystems Heidelberg) microscopes.
Immunoprecipitation and Western blot analysis.
Cell extractions in lysis buffer were incubated with antibodies, including selective antibodies for polymerized (SMI62) and depolymerized (SMI61) microtubules (Covance), and EZview Red Protein A Affinity Gel beads (Sigma) at 4°C for 12 h, as previously described (13). The gels were washed with the same incubation buffer and used for Western blot assays. The protein samples and immunoprecipitated proteins from the affinity gel were dissolved in SDS buffer for loading onto 7% precast Novex gels (Invitrogen, Carlsbad, CA). The standard Western blot and alkaline phosphatase-conjugated secondary antibodies were used to visualize proteins after color developing using NBT and BCIP as the substrates (52).
Mutation of hTau40 and generation of retroviral constructs.
Full-length hTau40 cDNA kindly provided by Dr. Lester I. Binder (North Western University) was used to generate a retroviral construct pMA2533 that encoded a protein with an NH2-terminal HA tag. Briefly, the coding sequence of hTau40 was amplified from Tau(Bamf) gcgggatccatggctgagccccgccagga and Tau(Notr) gcgcggccgctcacaaaccctgcttggccag. The 1,345-bp product was cloned into the EcoR I site of the pMA1373 plasmid (unpublished observations). The 5′ and 3′ nucleotide sequences of the resulting plasmid, pMA2449, were verified up to the Sac II site at the 5′ end and up to the Hind III site at the 3′ end by double-stranded DNA sequencing (Louisiana State University School of Veterinary Medicine). Then, the unsequenced internal Sac II-Hind III fragment of pMA2449 was replaced with the wild-type fragment from the original plasmid, thus generating pMA2516. The apurinic/apyrimidinic endonuclease (APE1)/redox factor-1 (Ref-1) gene in pMA2464 [a retroviral vector encoding puromycin resistance and cytomegalovirus (CMV)-HA-APE1 construct; unpublished observations] was replaced with the wild-type hTau40 gene (Ser214) from pMA2516, resulting in pMA2533. Mutations of S214A were introduced into the wild-type Tau40 gene by overlap extension PCR using the following primers: TauFint, ggtaaaacgaagatcgccac; TauRint, gactggactctgtccttgaag; TauS214Af, tcccgcaccccggcccttccaacccca; and TauS214Ar, tggggttggaagggccggggtgcggga. The PCR products were cloned into the pBluescript II SK (+) plasmid, mutations were verified by sequencing, and mutated fragments were used to replace the corresponding wild-type region in pMA2449, generating pMA2466 for the S214A mutation. These plasmids were used to replace the APE1 gene in pMA2464, thus generating retroviral vectors pMA2181 and pMA2182, respectively. Retroviral supernatants were produced in the Phoenix Ampho packaging cell line [American Type Culture Collection (ATCC)] using standard protocols. The final retroviral constructs were used to generate retroviral supernatants using the Phoenix Ampho packaging cell line (a kind gift of Dr. Gary Nolan, Stanford University). Retroviral supernatants were incubated with PMVECs and 5-μl polybrene for 24 h. The stably transfected cells were selected to homogeneity in 20 μg/ml puromycin for 72 h. hTau40 expression efficiency was confirmed by immunoblotting using HA and tau (pan)-specific antibodies in whole cell extractions. The established hTau40-expressing PMVECs were then cotransfected with retroviral constructs encoding PDE4D41–166-GFP or GFP control and sorted for high enhanced GFP (EGFP) expression by flow cytometry. The final coexpression efficiency was confirmed by Western blotting using GFP antibodies.
Permeability assay.
PMVECs were trypsinized and suspended in DMEM containing 10% serum. Cells were seeded onto the top chamber of a Transwell (0.2 ml, 2 × 104 cells) plate with 0.5 ml of medium in the bottom chamber settled in 24-well plates. Cells were grown to 100% confluence and permeability to macromolecules assessed as previously described (26). Briefly, dextran-Texas Red (Invitrogen) was added to the top well in serum-free DMEM (mol mass 40,000 Da with final concentrations at 0.5 mg/ml). Additional agents were added in the top chamber in 20-μl aliquots 15 min after dextran. Dextran transfer from the upper to lower Transwell chamber was measured at 30-, 60-, 90-, 150-, and 210-min time points. DMEM containing dextran-Texas Red (0.2 ml) was extracted from the bottom well and placed in 96-well plates to make fluorescence measurements, with excitation and emission wavelengths set at 590 and 615 nm, respectively.
Statistical analysis.
Results were analyzed using two-way ANOVA with post hoc test, where appropriate, using GraphPad Prism 3.1 software. All data represent means ± SE. P < 0.05 was considered statistically significant for the comparisons.
RESULTS
Inhibition of membrane PDE4D4 reveals forskolin-induced tau-Ser214 phosphorylation and increased endothelial cell permeability.
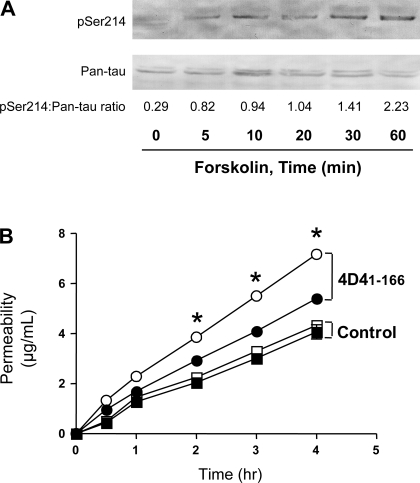
We (13) recently demonstrated that a catalytically inactive PDE4D4 peptide, PDE4D41–166, inhibits endogenous membrane PDE4D4 activity. In cells expressing PDE4D41–166, forskolin stimulation of transmembrane adenylyl cyclases resulted in tau-Ser214 phosphorylation and microtubule reorganization. Presently, we confirmed this observation, as forskolin increased tau-Ser214 phosphorylation in PDE4D41–166-expressing PMVECs over a 60-min time course. Western blots using a phospho-tau-Ser214 antibody (Fig. 1A) revealed that forskolin induced tau-Ser214 phosphorylation within 10 min, which continued to increase throughout the time course.
Fig. 1.
Forskolin increases tau-serine 214 phosphorylation and promotes endothelial permeability in pulmonary microvascular endothelial cells (PMVECs) expressing the catalytically inactive phosphodiesterase 4D4 (PDE4D4) peptide. A: Western blot illustrates the time course for increased phosphorylated tau-Ser214 (pSer214) in the microtubule-enriched cytosolic fractionation from PDE4D41–166-expressing cells, with the total tau level shown using a pan-tau antibody. B: forskolin increases permeability in PDE4D41–166-expressing PMVECs (○; 4D41–166) but not in vector control cells (□), measured using fluorescent dextran-Texas Red (mol mass 40,000 Da) Transwell analysis (n = 3). PDE4D41–166-expressing control experiments are shown using ●, and vector controls are shown using ■. *P < 0.05.
Forskolin induced gaps between adjacent endothelial cells in PDE4D41–166-expressing cells, a result that was not observed in wild-type or vector control cells (13). To examine whether these gaps formed a paracellular pathway for macromolecular permeability, dextran permeability was measured over a 4-h time course. As seen in Fig. 1B, forskolin increased dextran permeability across a confluent PMVEC monolayer in PDE4D41–166-expressing cells, but not in vector control cells, confirming that formation of interendothelial cell gaps results in increased macromolecular permeability.
cAMP-activated PKA colocalizes with disrupted microtubules.
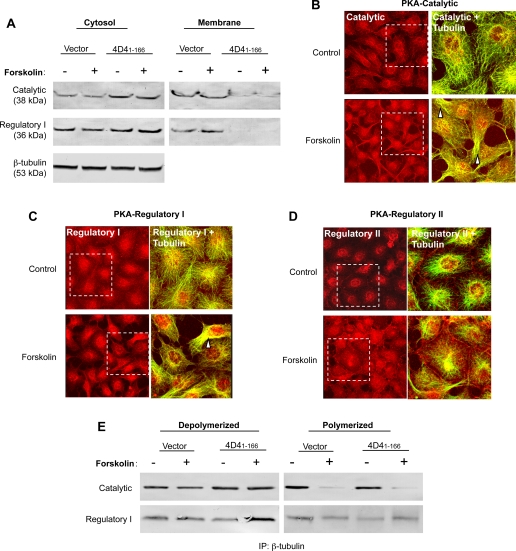
Since PDE4D41–166-expressing cells possessed greater basal and forskolin-stimulated PKA activity than did control cells (13), we next determined whether PDE4D41–166 expression changes the distribution of catalytic and regulatory PKA subunits. In support of this idea, Western blots of the catalytic PKA subunit revealed that the majority of this subunit was found in the cytosolic fraction of PDE4D41–166-expressing cells, whereas the majority of the catalytic subunit was resolved in the membrane fraction of control cells (Fig. 2A). Summary data from three separate experiments demonstrate that the cytosol-to-membrane ratio was 0.5 ± 0.1 in control PMVEC and 4.2 ± 0.4 in PDE4D41–166-expressing PMVECs (P < 0.05). Similarly, the regulatory I PKA subunit was prominently localized to the cytosol in PDE4D41–166-expressing cells, whereas it was found in both membrane and cytosolic fractions of control cells. Forskolin treatment did not impact on subunit distribution in either cell type. Consistent with our earlier report (13), these findings suggest that PDE4D41–166 expression allows higher cAMP concentrations to access the cytosol and activate PKA or to promote PKA translocation to the cytosol.
Fig. 2.
Type I PKA is resolved in cytosolic microtubule fractions of PDE4D41–166-expressing PMVECs. A: immunoblotting revealed an increase in the PKA catalytic subunit in the cytosolic fractions of PDE4D41–166-expressing cells compared with vector control cells. This increase in the PKA catalytic subunit observed in the cytosolic fraction was paralleled by a decrease in the membrane fraction of PDE4D41–166-expressing cells. Similarly, the type I regulatory subunit of PKA was enriched in the cytosolic fractions and absent in the membrane fractions of PDE4D41–166-expressing cells compared with controls. Forskolin (1 μM for 30 min) treatment did not further change the amounts of catalytic or regulatory proteins in either the cytosol or membrane fractions. PDE4D41–166 expression did not change the β-tubulin levels in the cytosol of PMVECs. Immunofluorescence shows the PKA catalytic (B, red) and type I regulatory (C, red) subunits colocalize with microtubules (B and C, green) in basal and forskolin-stimulated PDE4D41–166-expressing PMVECs. Following forskolin treatment, PKA catalytic and regulatory subunits were enriched in reorganized microtubules. The type II regulatory PKA subunit (red) was primarily localized to the perinuclear region (D) and did not intensely interact with microtubules (green) under basal conditions or after forskolin stimulation in PDE4D41–166-expressing PMVECs. For B–D, the white boxes denote the area enlarged on the right of each image set. Colocalization is indicated by yellow in the merged images (arrowheads). E: catalytic and type I regulatory PKA subunits were principally found in the depolymerized pool of microtubules. β-Tubulin was immunoprecipitated (IP), and catalytic and type I regulatory subunits were immunoblotted from either depolymerized or polymerized microtubules. Forskolin activation did not increase the coimmunoprecipitation of PKA subunits with β-tubulin.
Colocalization of PKA with reorganized microtubules was tested in PDE4D41–166-expressing cells under basal conditions and following forskolin-activation. Catalytic (Fig. 2B) and regulatory I (Fig. 2C) PKA subunits were localized in the cytosol with microtubules and were particularly prominent in reorganized microtubules following forskolin stimulation. The observed distribution for the regulatory II PKA subunit was different than that seen for the regulatory I subunit. The regulatory II subunit was predominantly localized at the nuclear and perinuclear region and did not appear to colocalize with microtubules following forskolin activation (Fig. 2D). In control PMVECs, the catalytic, regulatory I, and regulatory II PKA subunit distribution was similar to unstimulated PDE4D41–166-expressing cells; forskolin stimulation did not impact the distribution of any of these PKA subunits in control cells (data not shown).
Since catalytic and regulatory I PKA subunits colocalized with reorganized microtubules in PDE4D41–166-expressing cells, we examined whether these PKA subunits coimmunoprecipitate with depolymerized microtubules. The catalytic PKA subunit was resolved in both depolymerized and polymerized β-tubulin pools (Fig. 2E). A moderate increase of the catalytic subunit was seen in depolymerized microtubules in PDE4D41–166-expressing cells compared with controls. Forskolin activation did not change the abundance of the catalytic PKA subunits that coimmunoprecipitated with depolymerized β-tubulin but decreased their association with polymerized microtubules in both PDE4D41–166-expressing and control cells. The regulatory I PKA subunit was abundant in the depolymerized microtubule pool. Forskolin did not significantly increase the amount of regulatory I PKA subunit that was resolved in the depolymerized microtubule pool in either PDE4D41–166-expressing or control cells.
Mutating tau-Ser214 to its nonphosphorylatable form, S214A.
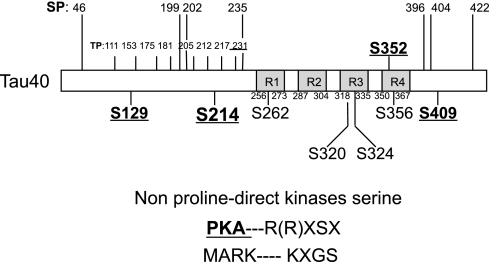
To verify whether tau-Ser214 was PKA-phosphorylated in PDE4D41–166-expressing cells, a detailed tau protein phosphorylation site map was analyzed using National Center for Biotechnology Information (NCBI) GenBank, and putative serine, threonine, and tyrosine phosphorylation sites were matched to the various kinase-specific motifs. Results confirmed that hTau40 possesses putative PKA phosphorylation sites, including serines 129, 214, 352, and 409, each with an R(R)XSX motif. Ser262 and Ser356 represent other nonproline-direct kinase phosphorylation targets, e.g., MARK KXGS motifs (Fig. 3). These findings suggest that PKA is a kinase that contributes to forskolin-induced phosphorylation of tau-Ser214 in PDE4D41–166-expressing PMVECs.
Fig. 3.
Serines (S) 129, 214, 352, and 409 on tau represent putative PKA phosphorylation targets. Serine and threonine phosphorylation sites for nonproline-directed kinases have been identified in hTau40. Nonproline-directed kinase consensus sites are normally localized inside or between the microtubule-binding repeat domains, R1–R4. Among them, putative PKA consensus sites, with specific R(R)XSX motifs, have been found at serines 129, 214, 352, and 409 via gene alignment analysis. Other kinase phosphorylation sites, e.g., the specific KXGS motif for microtubule affinity-regulating kinase (MARK), have been identified at serines 262 and 356. Multiple phosphorylation sites for proline-directed kinase sites (TP, SP) are indicated at the NH2 and COOH termini of tau, outside of the R1–R4 domains.
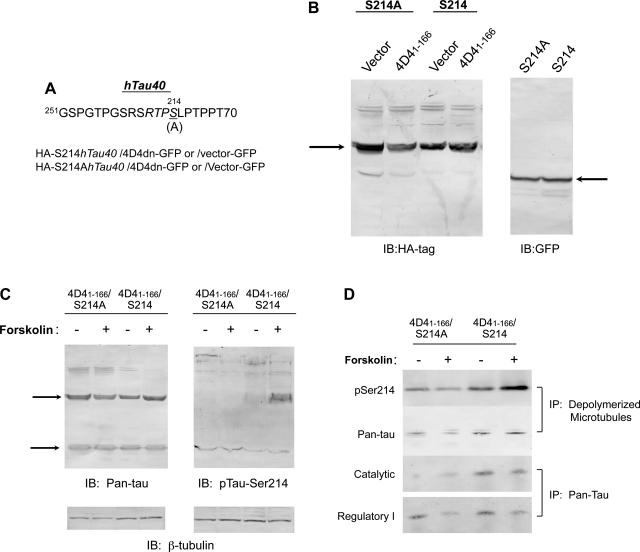
We next sought to determine whether PKA phosphorylation of tau-Ser214 is essential for forskolin-induced barrier disruption in PDE4D41–166-expressing PMVECs. To mask PKA-mediated phosphorylation of endogenous tau at Ser214, a point mutation in the hTau40 cDNA clone was inserted, which encoded for a S214A mutation (Fig. 4A). Retroviral constructs were generated containing either wild-type S214hTau40 or mutated S214AhTau40, each possessing HA tags, and these constructs were stably cotransfected in PMVECs with vector-GFP or PDE4D41–166-GFP. Western blots using an HA-tagged antibody indicated that vector control and PDE4D41–166-expressing cells both possessed a similar abundance of the S214hTau40 and S214AhTau40 proteins. Using GFP immunoblotting, identical PDE4D41–166-GFP expression was observed in S214hTau40 and S214AhTau40-coexpressing cells (Fig. 4B). The corresponding major hTau40 band was confirmed in PDE4D41–166-expressing cells cotransfected with either Ser214 or S214A mutation constructs, using the pan-tau antibody (Fig. 4C). Endogenous rat tau was equally abundant in Ser214- and S214A-expressing cells, indicating heterologously expressed hTau40 did not change endogenous tau protein expression in PMVECs. Forskolin treatment did not change the S214hTau40 or S214AhTau abundance, and it did not change expression of the endogenous rat tau protein. Forskolin induced the phosphorylation of S214hTau40 in PDE4D41–166-expressing cells. The S214A mutation abolished forskolin-induced tau-Ser214 phosphorylation in PDE4D41–166-expressing cells, and there was no increase in phosphorylation of the endogenous tau (Fig. 4C).
Fig. 4.
Mutation of tau-Ser214 inhibits PKA-mediated phosphorylation. A: human (h) tau40 cDNA at Ser214 (S214) was mutated to alanine (S214A). Both hemagglutinin (HA)-tagged S214hTau and mutated S214AhTau40 in retroviral constructs were transfected into PMVECs with the coexpression of PDE4D41–166-green fluorescent protein (GFP) or vector-GFP. B: the expression of S214hTau and mutated S214AhTau40 were detected using an HA-tagged antibody. S214hTau and S214AhTau expression was identical in both vector control and PDE4D41–166-expressing cells (arrow). Identical PDE4D41–166-GFP expression in S214AhTau- and S214AhTau40-expressing cells was confirmed using a GFP antibody (arrow). C: using a pan-tau antibody, S214hTau and mutated S214AhTau40 were resolved, as was endogenous rat tau (arrows). Forskolin treatment (1 μM for 30 min) induced phosphorylation of the major band in cells expressing S214hTau40 and PDE4D41–166. Forskolin did not induce phosphorylation of the 62- or 32-kDa band in cells expressing S214AhTau40 and PDE4D41–166. D: in cells expressing S214AhTau and PDE4D41–166, low amounts of phosphorylated tau-Ser214 (pSer214) coimmunoprecipitated with depolymerized microtubules, and this effect was not potentiated by forskolin pretreatment (1 μM for 30 min). In cells expressing S214hTau40 and PDE4D41–166, pSer214 coimmunoprecipitated with depolymerized microtubules at high levels, and this effect was potentiated by forskolin activation. The S214A also reduced catalytic PKA subunit interaction with tau under basal conditions, assessed using a pan-tau antibody for immunoprecipitation. No effect of type I regulatory PKA subunit binding to tau was observed. IB, immunoblot.
Since heterologously expressed tau was prominently phosphorylated in S214hTau40-expressing cells, we determined whether S214hTau40 interacts with depolymerized microtubules, as does the endogenously expressed rat tau following its phosphorylation (13). β-Tubulin was immunoprecipitated from the depolymerized microtubule pool and immunoblotted for phospho-tau-Ser214. Increased phospho-tau-Ser214 was seen in forskolin-treated cells coexpressing PDE4D41–166 and S214hTau40 immunoprecipitated with depolymerized β-tubulin. In contrast, forskolin did not enhance tau-Ser214 phosphorylation in S214A-expressing cells (Fig. 4D). A greater total tau protein abundance was coimmunoprecipitated with β-tubulin in the depolymerized microtubule fraction from cells expressing both PDE4D41–166 and S214hTau40 compared with cells coexpressing PDE4D41–166 and S214AhTau40.
S214AhTau40 inhibits forskolin-induced PMVEC gap formation and -increased macromolecular permeability.
PMVEC shape was examined in cells coexpressing PDE4D41–166 and S214hTau40 and in cells coexpressing PDE4D41–166 and S214AhTau40. Rhodamine-labeled phalloidin was used to visualize F-actin. Cells coexpressing PDE4D41–166 and either S214hTau or S214AhTau maintained a regular F-actin architecture (Fig. 5A). However, in response to forskolin treatment in PDE4D41–166 and S214hTau40-coexpressing cells, obvious intercellular gaps formed. In contrast, forskolin did not induce intercellular gaps in S214A-expressing cells. To examine the extent to which forskolin breached PMVEC barrier function in PDE4D41–166 and S214hTau40-coexpressing cells, macromolecular permeability was measured over a 5-h time course (Fig. 5B). Forskolin increased dextran permeability in PDE4D41–166 and S214hTau40-coexpressing cells. By comparison, cells expressing the S214AhTau mutation displayed reduced basal dextran permeability and an attenuated permeability response to forskolin.
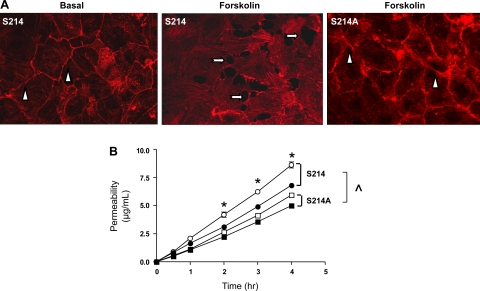
Fig. 5.
S214A mutation blocks the forskolin-induced endothelial cell barrier disruption and -increased permeability. A: phalloidin was used to resolve the cortical actin rim in cells expressing PDE4D41–166 and either S214hTau40 or S214AhTau40. Under basal conditions, a prominent cortical actin rim was denoted (arrowheads). Forskolin (1 μM for 30 min) diminished the cortical actin rim and induced interendothelial cell gaps in S214hTau40-expressing cells (arrows). However, following forskolin stimulation, the cortical actin rim remained intact, and interendothelial cell gaps did not form, in S214AhTau40-expressing cells. B: permeability was measured using fluorescent dextran-Texas Red (mol mass 40,000 Da) Transwell analysis (n = 3). Cells expressing PDE4D41–166 and either S214hTau40 or S214AhTau40 were grown to confluence on Transwell inserts, and permeability was measured over a 5-h time course. Basal permeability measurements (closed symbols) were higher in S214hTau40-expressing cells (S214) than in S214AhTau40-expressing cells (S214A) (^P < 0.05; n = 3). Forskolin (1 μM; open symbols) increased permeability in S214hTau40-expressing cells but was without effect in S214AhTau40-expressing cells (*P < 0.05; n = 3).
DISCUSSION
Ligand-receptor binding triggers a complex series of intracellular responses that enable cells to rapidly adjust to the demands of their extracellular environment. Efficiency of this intracellular response is aided by the nanomolecular organization of signaling modules, which place receptor complexes in immediate proximity to G proteins, signal-generating second messenger systems, and their effectors, altogether promoting the preferential activation of physiologically appropriate targets (5, 50). In endothelial cells, receptors coupled to adenylyl cyclase generate a membrane-delimited cAMP signal that activates PKA (30, 42), which phosphorylates and/or activates filamin (21–23, 48) and Rac1, which stabilize the cortical actin rim (39, 49). In addition, membrane cAMP activates exchange protein activated by cAMP (1, 4, 18, 27, 31), which inserts adherens junction proteins into the plasma membrane necessary to strengthen cell-cell contacts (7, 14, 29). In contrast to the mammalian transmembrane adenylyl cyclases, bacteria have evolved mechanisms to insert soluble adenylyl cyclases into the host cell cytosol. Soluble adenylyl cyclases generate a cAMP signal that reorganizes microtubules, generates intercellular gaps, and increases endothelial cell permeability (34, 37, 38). These diverse physiological outcomes bring into question how the cAMP signal emanating from mammalian transmembrane adenylyl cyclases is directed to barrier-enhancing, rather than barrier-disrupting, effector targets. We (13) recently demonstrated that PDE4D4 is a part of the endogenous adenylyl cyclase-cAMP signaling module and is necessary to direct the cAMP signal to its barrier-enhancing targets. Indeed, expression of a PDE4D4 dominant-negative peptide, PDE4D41–166, mislocalizes the cAMP signal, resulting in microtubule reorganization and intercellular gap formation, reminiscent of the actions of bacterial soluble adenylyl cyclases. A major unanswered question is how the mislocalized cAMP signal initiates microtubule reorganization that is responsible for gap formation. In the present studies, we demonstrate that inhibition of membrane PDE4D4 allows the cAMP signal to activate cytosolic PKA, resulting in tau-Ser214 phosphorylation sufficient to reorganize microtubules.
Four lines of evidence implicate PKA as the critical kinase responsible for tau-Ser214 phosphorylation in PDE4D41–166-expressing cells. First, PMVECs expressing PDE4D41–166 display increased basal and forskolin-activated cytosolic PKA activity (13). Whereas wild-type cells possess a cytosol-to-membrane PKA activity ratio of 0.83, in PDE4D41–166-expressing cells, this ratio is increased to 1.27. Increased cytosolic PKA activity is due to enzyme activation and/or translocation, not increased enzyme expression. Second, catalytic and regulatory I PKA subunits, but not the regulatory II PKA subunit, colocalized with reorganized microtubules. These reorganized microtubules possess a population of depolymerized microtubules that interact with phospho-tau-Ser214. Third, Ser214 resides within a consensus PKA phosphorylation site in tau40, and a PKA antagonist inhibited forskolin-induced tau-Ser214 phosphorylation (13). Moreover, other putative phosphorylation sites, such as Ser262 and Ser356, were not responsive to PKA phosphorylation. Fourth, site-directed mutagenesis to replace Ser214 with a nonphosphorylatable residue prevented forskolin-activated tau phosphorylation.
Regulatory PKA subunits are responsible for anchoring the kinase to subcellular loci through A-kinase anchoring proteins (AKAP) (2, 11, 15, 16, 28, 36, 46). The mechanism accounting for PKA localization with tau40 is not presently known. However, there is precedence for PKA interaction with microtubule-associated proteins. Indeed, microtubule-associated protein-2 was among the first AKAPs identified. Microtubule-associated protein-2 and other AKAPs possess a conserved hydrophobic surface along one side of an amphipathic helix that tethers to both regulatory I and II subunits. Recently, Carlson and colleagues (10) identified an 18-residue consensus sequence in the AKAP amphipathic helix (LKQYANQLASQVIKEATE) that more selectively interacts with regulatory I than II subunits. Our sequence analysis did not resolve this conserved AKAP helix domain in tau40 (data not shown). Nonetheless, nonneuronal tau coimmunoprecipitated with the regulatory I and catalytic subunits of PKA, suggesting that tau40 functions as an AKAP or, alternatively, that it interacts with other proteins promoting such an interaction.
PKA phosphorylation of tau-Ser214 caused microtubule reorganization, loss of cell-cell tethering, and increased macromolecular permeability. The idea that tau binding to microtubules stabilizes them in a way that promotes endothelial cell barrier integrity has been advanced previously. Tar and colleagues (43) demonstrated that protein phosphatase 2A dephosphorylates HSP27 and tau and that this phosphatase action strengthened the endothelial cell barrier. In these studies, however, a role for PKA phosphorylation of tau-Ser214 was not investigated. Our prior work identified that expression of the PDE4D41–166 peptide-enabled stimulation of transmembrane adenylyl cyclases to phosphorylate tau-Ser214, yet it was unclear whether this phosphorylation event is causally related to interendothelial cell gap formation (13). Herein, by performing site-directed mutagenesis to replace tau-S214A, we demonstrate that the PKA phosphorylation of this single residue is essential for a cAMP signal to reorganize microtubules and increase macromolecular permeability.
In summary, the activation of transmembrane adenylyl cyclases generates a cAMP pool with restricted diffusion properties. PDE4D4 prevents cAMP from accessing cytoskeletal targets that disrupt the endothelial cell barrier. Inhibiting this action of PDE4D4 enables the cAMP signal to activate PKA, which phosphorylates tau-Ser214, disassembles microtubules into elongated bundles, and disrupts cell-cell adhesion. Loss of cell adhesion is sufficient to increase macromolecular permeability. These findings illustrate molecular mechanisms responsible for cAMP compartmentation in endothelium and reveal a previously unrecognized mechanism of endothelial cell barrier regulation. In addition, they highlight putative ways in which pathogenic bacteria may utilize soluble adenylyl cyclases to reorganize microtubules, disrupt the endothelial cell barrier, and cause tissue edema.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-60024 and HL-66299 to T. Stevens and an American Heart Association Award to B. Zhu.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Anna Buford and Lynn Ayers for their assistance in cell culture.
REFERENCES
- 1.Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol 294: H1188– H1196, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Angelo R, Rubin CS. Molecular characterization of an anchor protein (AKAPCE) that binds the RI subunit (RCE) of type I protein kinase A from Caenorhabditis elegans. J Biol Chem 273: 14633– 14643, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev 84: 361– 384, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Baumer Y, Drenckhahn D, Waschke J. cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem Cell Biol 129: 765– 778, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Beene DL, Scott JD. A-kinase anchoring proteins take shape. Curr Opin Cell Biol 19: 192– 198, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 18: 1879– 1890, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Birukova AA, Burdette D, Moldobaeva N, Xing J, Fu P, Birukov KG. Rac GTPase is a hub for protein kinase A and Epac signaling in endothelial barrier protection by cAMP. Microvasc Res 79: 128– 138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birukova AA, Liu F, Garcia JG, Verin AD. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 287: L86– L93, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Bunker JM, Wilson L, Jordan MA, Feinstein SC. Modulation of microtubule dynamics by tau in living cells: implications for development and neurodegeneration. Mol Biol Cell 15: 2720– 2728, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson CR, Lygren B, Berge T, Hoshi N, Wong W, Tasken K, Scott JD. Delineation of type I protein kinase A-selective signaling events using an RI anchoring disruptor. J Biol Chem 281: 21535– 21545, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Lin RY, Rubin CS. Organelle-specific targeting of protein kinase AII (PKAII). Molecular and in situ characterization of murine A kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J Biol Chem 272: 15247– 15257, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Cioffi DL, Moore TM, Schaack J, Creighton JR, Cooper DM, Stevens T. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol 157: 1267– 1278, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creighton J, Zhu B, Alexeyev M, Stevens T. Spectrin-anchored phosphodiesterase 4D4 restricts cAMP from disrupting microtubules and inducing endothelial cell gap formation. J Cell Sci 121: 110– 119, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950– 1955, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Davare MA, Dong F, Rubin CS, Hell JW. The A-kinase anchor protein MAP2B and cAMP-dependent protein kinase are associated with class C l-type calcium channels in neurons. J Biol Chem 274: 30280– 30287, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Diviani D, Scott JD. AKAP signaling complexes at the cytoskeleton. J Cell Sci 114: 1431– 1437, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487– 1500, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136– 146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3: 519– 526, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Goode BL, Chau M, Denis PE, Feinstein SC. Structural and functional differences between 3-repeat and 4-repeat tau isoforms. Implications for normal tau function and the onset of neurodegenetative disease. J Biol Chem 275: 38182– 38189, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Hastie LE, Patton WF, Hechtman HB, Shepro D. H2O2-induced filamin redistribution in endothelial cells is modulated by the cyclic AMP-dependent protein kinase pathway. J Cell Physiol 172: 373– 381, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Jay D, Garcia EJ, de la Luz Ibarra M. In situ determination of a PKA phosphorylation site in the C-terminal region of filamin. Mol Cell Biochem 260: 49– 53, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Jay D, Garcia EJ, Lara JE, Medina MA, de la Luz Ibarra M. Determination of a cAMP-dependent protein kinase phosphorylation site in the C-terminal region of human endothelial actin-binding protein. Arch Biochem Biophys 377: 80– 84, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Jicha GA, Weaver C, Lane E, Vianna C, Kress Y, Rockwood J, Davies P. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer's disease. J Neurosci 19: 7486– 7494, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci 117: 5721– 5729, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kelly JJ, Moore TM, Babal P, Diwan AH, Stevens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol Lung Cell Mol Physiol 274: L810– L819, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett 579: 4966– 4972, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lester LB, Coghlan VM, Nauert B, Scott JD. Cloning and characterization of a novel A-kinase anchoring protein. AKAP 220, association with testicular peroxisomes. J Biol Chem 271: 9460– 9465, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Lorenowicz MJ, Fernandez-Borja M, Kooistra MR, Bos JL, Hordijk PL. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur J Cell Biol 87: 779– 792, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Moore TM, Chetham PM, Kelly JJ, Stevens T. Signal transduction and regulation of lung endothelial cell permeability. Interaction between calcium and cAMP. Am J Physiol Lung Cell Mol Physiol 275: L203– L222, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Netherton SJ, Sutton JA, Wilson LS, Carter RL, Maurice DH. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ Res 101: 768– 776, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Panda D, Samuel JC, Massie M, Feinstein SC, Wilson L. Differential regulation of microtubule dynamics by three- and four-repeat tau: implications for the onset of neurodegenerative disease. Proc Natl Acad Sci USA 100: 9548– 9553, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol 28: 574– 581, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Prasain N, Alexeyev M, Balczon R, Stevens T. Soluble adenylyl cyclase-dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol 297: L73– L83, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol 116: 147– 161, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvador LM, Flynn MP, Avila J, Reierstad S, Maizels ET, Alam H, Park Y, Scott JD, Carr DW, Hunzicker-Dunn M. Neuronal microtubule-associated protein 2D is a dual A-kinase anchoring protein expressed in rat ovarian granulosa cells. J Biol Chem 279: 27621– 27632, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sayner SL, Alexeyev M, Dessauer CW, Stevens T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res 98: 675– 681, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res 95: 196– 203, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Schlegel N, Waschke J. VASP is involved in cAMP-mediated Rac 1 activation in microvascular endothelial cells. Am J Physiol Cell Physiol 296: C453– C462, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38: 3549– 3558, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Shasby DM, Shasby SS, Sullivan JM, Peach MJ. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res 51: 657– 661, 1982 [DOI] [PubMed] [Google Scholar]
- 42.Stelzner TJ, Weil JV, O'Brien RF. Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J Cell Physiol 139: 157– 166, 1989 [DOI] [PubMed] [Google Scholar]
- 43.Tar K, Birukova AA, Csortos C, Bako E, Garcia JG, Verin AD. Phosphatase 2A is involved in endothelial cell microtubule remodeling and barrier regulation. J Cell Biochem 92: 534– 546, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Tokuraku K, Matsushima K, Matui T, Nakagawa H, Katsuki M, Majima R, Kotani S. The number of repeat sequences in microtubule-associated protein 4 affects the microtubule surface properties. J Biol Chem 278: 29609– 29618, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Trinczek B, Brajenovic M, Ebneth A, Drewes G. MARK4 is a novel microtubule-associated proteins/microtubule affinity-regulating kinase that binds to the cellular microtubule network and to centrosomes. J Biol Chem 279: 5915– 5923, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Verde I, Pahlke G, Salanova M, Zhang G, Wang S, Coletti D, Onuffer J, Jin SL, Conti M. Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem 276: 11189– 11198, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Verin AD, Birukova A, Wang P, Liu F, Becker P, Birukov K, Garcia JG. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol 281: L565– L574, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Wang Q, Patton WF, Chiang ET, Hechtman HB, Shepro D. Filamin translocation is an early endothelial cell inflammatory response to bradykinin: regulation by calcium, protein kinases, and protein phosphatases. J Cell Biochem 62: 383– 396, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Waschke J, Drenckhahn D, Adamson RH, Barth H, Curry FE. cAMP protects endothelial barrier functions by preventing Rac-1 inhibition. Am J Physiol Heart Circ Physiol 287: H2427– H2433, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965– 1010, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Zheng-Fischhofer Q, Biernat J, Mandelkow EM, Illenberger S, Godemann R, Mandelkow E. Sequential phosphorylation of Tau by glycogen synthase kinase-3beta and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur J Biochem 252: 542– 552, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Zhu B, Kelly J, Vemavarapu L, Thompson WJ, Strada SJ. Activation and induction of cyclic AMP phosphodiesterase (PDE4) in rat pulmonary microvascular endothelial cells. Biochem Pharmacol 68: 479– 491, 2004 [DOI] [PubMed] [Google Scholar]