Abstract
Single-dose intratracheal bleomycin has been instrumental for understanding fibrotic lung remodeling, but fails to recapitulate several features of idiopathic pulmonary fibrosis (IPF). Since IPF is thought to result from recurrent alveolar injury, we aimed to develop a repetitive bleomycin model that results in lung fibrosis with key characteristics of human disease, including alveolar epithelial cell (AEC) hyperplasia. Wild-type and cell fate reporter mice expressing β-galactosidase in cells of lung epithelial lineage were given intratracheal bleomycin after intubation, and lungs were harvested 2 wk after a single or eighth biweekly dose. Lungs were evaluated for fibrosis and collagen content. Bronchoalveolar lavage (BAL) was performed for cell counts. TUNEL staining and immunohistochemistry were performed for pro-surfactant protein C (pro-SP-C), Clara cell 10 (CC-10), β-galactosidase, S100A4, and α-smooth muscle actin. Lungs from repetitive bleomycin mice had marked fibrosis with prominent AEC hyperplasia, similar to usual interstitial pneumonia (UIP). Compared with single dosing, repetitive bleomycin mice had greater fibrosis by scoring, morphometry, and collagen content; increased TUNEL+ AECs; and reduced inflammatory cells in BAL. Sixty-four percent of pro-SP-C+ cells in areas of fibrosis expressed CC-10 in the repetitive model, suggesting expansion of a bronchoalveolar stem cell-like population. In reporter mice, 50% of S100A4+ lung fibroblasts were derived from epithelial mesenchymal transition compared with 33% in the single-dose model. With repetitive bleomycin, fibrotic remodeling persisted 10 wk after the eighth dose. Repetitive intratracheal bleomycin results in marked lung fibrosis with prominent AEC hyperplasia, features reminiscent of UIP.
Keywords: epithelial-mesenchymal transition, bronchoalveolar stem cell, lung
idiopathic pulmonary fibrosis (IPF) is a progressive lung disease characterized by progressive dyspnea, bilateral interstitial infiltrates, and restrictive physiology on pulmonary function testing (8). The estimated United States prevalence is 20 per 100,000 males and 13 per 100,000 females, and epidemiologic analysis implies that the incidence is on the rise (1). Diagnosis is often made by characteristic clinical and radiographic findings; however, often times a lung biopsy is required, which reveals usual interstitial pneumonia (UIP). Unfortunately, this devastating disease is often fatal within 3–5 years from diagnosis, and there is no effective treatment outside of lung transplantation.
The etiology of IPF is unknown, but a current hypothesis is that recurrent injury produces damage to the alveolar epithelial cells (AECs), inducing an aberrant wound healing response that is influenced by inflammatory cells, cytokine signaling, and genetic factors culminating in tissue fibrosis (8, 25). The patchy and heterogeneous pathology noted in UIP correlates with this sequential lung injury hypothesis.
Current efforts to understand disease in vivo rely heavily on murine models. The most common method to induce pulmonary fibrosis in the mouse is with the intratracheal administration of a single dose of bleomycin. While this method has provided insight into processes involved in lung fibrosis, there are clear limitations (22). One limitation is that the temporal and spatial scheme of disease development is different than human disease. Human disease most likely develops over time with repeated insults to the alveolar epithelium creating a cycle of injury and incomplete repair, ultimately resulting in progressive disease in the case of IPF. In contrast, in just 6 wk after single-dose bleomycin, the murine lung has repaired significantly, leaving minimal to no evidence of fibrosis in some investigations (6).
The microscopic pathology is also quite dissimilar. In UIP, features include heterogeneous distribution of injury juxtaposed between areas of relatively normal lung parenchyma. The areas of injury reveal increased extracellular matrix deposition with areas of fibroblastic foci surrounded by hyperplastic alveolar epithelium (14). Furthermore, neutrophilic inflammation is not a prominent feature in IPF. However, in murine bleomycin-induced lung fibrosis, there is often significant neutrophilic inflammation present, and the characteristic hyperplastic type II AECs are notably rare or absent (22).
Considering that IPF pathogenesis may include a component of recurrent lung injury, our goal was to develop a model of repetitive lung injury utilizing bleomycin that may be more reflective of some of the processes that occur in the development of human forms of fibrosing lung disease. With these studies, we have developed a repetitive lung injury model that can be easily and reproducibly performed that results in marked lung fibrosis with prominent type II AEC hyperplasia.
MATERIALS AND METHODS
Wild-type and transgenic mice.
All wild-type and transgenic mice used were in the C57BL/6J background, ranged in weight from 20–30 g, and were greater than or equal to 8 wk of age at the beginning of study. Transgenic mice expressing Cre recombinase under control of the 3.7-kb human surfactant protein C promoter (SPC.Cre) were obtained from Dr. Brigid Hogan (Duke Univ., Durham, NC). R26Rosa.Stop.LacZ reporter mice were obtained from Jackson Laboratories (Bar Harbor, ME). With these mice, the R26Rosa endogenous promoter drives expression of a construct that consists of a loxP flanked STOP cassette upstream of lacZ (whose gene product is β-gal), and a polyadenylation sequence (30). SPC.Cre mice were mated to R26Rosa.Stop.LacZ reporter mice, resulting in R26Rosa.Stop.LacZ.SPC.Cre mice that serve as a lung epithelium cell fate reporter system as described previously (34). Mice were housed in the central animal care facility at Vanderbilt University Medical Center (Nashville, TN) and were given food and water ad libitum. The experimental protocol was reviewed and approved by the Institutional Animal Care and Utilization Committee at Vanderbilt University.
Bleomycin model.
Bleomycin was prepared by mixing sterile bleomycin sulfate powder (Teva Parenteral Medicines, Irvine, CA) with sterile normal saline. Bleomycin was injected intratracheally via an intubation procedure at a dose of 0.04 units in a total volume of 100 μl of sterile saline. For this procedure, mice were anesthetized with isoflurane by inhalation and then suspended by their front teeth on a wire attached to an angled fiberglass stand. The tongue was lifted with the gentle use of forceps, and then the palate was lifted with the use of a small scoop, similar to a Miller blade on a laryngoscope, allowing an unobstructed view of the trachea. A 26 French angiocatheter was inserted into the trachea, and 100 μl of bleomycin solution was administered. The mice were observed following intubation to ensure they recovered from anesthesia completely. At designated time points after bleomycin administration, mice were euthanized by exposure to carbon dioxide, lungs were harvested for histological preparations, and frozen tissue or bronchoalveolar lavage was performed as detailed below and as previously described (19, 20, 34).
Histology and microscopy.
For tissue harvesting, the lungs were perfused with normal saline from right to left ventricle of the heart. For wild-type mice, the right hilum was identified, tied off, and surgically removed with the separate lobes flash-frozen immediately in liquid nitrogen and stored at −70°C. The trachea was then isolated, and, using a blunt tip needle and syringe, the remaining left lung was inflated with 10% neutral buffered formalin by a 25-cm pressure column. The trachea was then tied off, and the lung was removed for fixation overnight in formalin followed by embedding in paraffin. Five-micrometer sections were cut for hematoxylin and eosin and trichrome blue stains as well as for immunohistochemistry studies. For cell fate mapping, frozen sections were processed as previously described (34). Briefly, lungs were perfused with normal saline and then inflated with 4% paraformaldehyde by a 25-cm pressure column. The trachea was then tied off, and the lungs were kept in 4% paraformaldehyde for 2 h at 4°C and then transferred into a 20% sucrose solution for 24 h. At this time, the lungs were flash-frozen in liquid nitrogen and transferred to a −70°C freezer until processed on a cryostat for frozen tissue sectioning. Light and fluorescent microscopy was performed using an Olympus IX81 Inverted Research Microscope configured with an Olympus IX2 Biological Disk Scanning Unit (Tokyo, Japan).
Lung lavage and cell counts.
Bronchoalveolar lavage (BAL) was performed as detailed previously (19). After euthanasia, three 800-μl lavages of sterile saline were performed using a 20 g blunt tipped needle inserted into the trachea. Samples were centrifuged at 400 g for 10 min, and the supernatant was discarded. Cell counts were performed by manual counting under light microscopy using a hemocytometer. Approximately 30,000 cells from each specimen were loaded onto slides using a Cytospin 2 (Shandon Southern Products, England). These slide preparations were then stained using a modified Wright stain and reviewed under light microscopy for differential white blood cell counts.
Human lung biopsies.
This investigation was approved by the Vanderbilt University Institutional Review Board, and informed consent was obtained. Lung samples were obtained from surgical lung biopsies performed for evaluation of interstitial lung disease. Diagnosis of IPF was made in accordance with American Thoracic Society/European Respiratory Society Consensus Statements (1, 2).
Immunohistochemistry and TUNEL.
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue sections using techniques as previously described (19, 20, 34). Primary antibodies were used followed by a standard immunoperoxidase/avidin-biotin complex protocol using an appropriate Vectastain ABC kit (Vector Laboratories, Burlingame, CA). The following primary antibodies were used: S100A4 biotinylated rabbit polyclonal antibody (obtained from Dr. Eric Neilson, Vanderbilt Univ., Nashville, TN); pro-surfactant protein C (pro-SP-C) rabbit polyclonal antibody (Millipore, Billerica, MA); and Clara cell protein 10 (CC-10) goat polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). TUNEL assays were performed using a commercially available kit in accordance with the manufacturer's instructions (In Situ Cell Death Detection Kit; Roche Molecular Biochemicals, Indianapolis, IN). Counterstains for immunohistochemistry preparations were performed with hematoxylin.
Immunofluorescence staining.
Frozen lung tissue blocks were cut into 5 μM sections and fixed in 0.2% paraformaldehyde in PIPES buffer [0.1 M PIPES (Sigma, St. Louis, MO), 2 mM MgCl2, 5 mM EGTA]. After blocking with 3% BSA, sections were incubated with primary antibodies against S100A4 (non-biotinylated antibody rabbit polyclonal antibody; obtained from Dr. Eric Neilson as above) and β-galactosidase (chicken polyclonal antibody; Abcam, Cambridge, MA), at 4°C overnight. After the sections were washed with PBS, they were stained with fluorescent secondary antibodies (Jackson Immunoresearch, West Grove, PA). For α-smooth muscle actin (α-SMA) staining, sections were incubated with Cy3-conjugated antibody (Sigma). In a separate evaluation, lung sections were stained with the same pro-SP-C and CC-10 antibodies as above followed by appropriate fluorescent secondary antibodies (Jackson Immunoresearch). Nuclear staining was done with DAPI using Vectashield mounting medium (Vector Laboratories).
LacZ staining.
Xgal substrate staining was performed as previously described (34). Frozen lung tissue sections were fixed in LacZ fixative solution containing 0.2% glutaraldehyde, 5 mM ethyleneglycoltetraacetic acid (pH 7.3), and 100 mM MgCl2 in 0.1 M NaPO4 (pH 7.3) for 15 min at 4°C, washed three times in PBS, and then incubated at 37°C in 1 mg/ml Xgal (Sigma), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, 0.2% Nonidet P-40, and 0.1% sodium deoxycholate in PBS for 16 h. Sections were counterstained with eosin and mounted with parmount (Sigma).
Semiquantitative scoring.
Quantification of lung fibrosis on histological specimens was performed by an investigator blinded to the group using a semiquantitative score as previously described (19). Briefly, slides were evaluated on 10 sequential, nonoverlapping fields (magnification ×300) of lung parenchyma from each specimen. Lung fibrosis was evaluated on trichrome-stained lung sections using a 0–4 point scale, with a score of 0, normal lung architecture; 1, increased thickness of some (≤50%) of interalveolar septa; 2, thickening of >50% of interalveolar septa without formation of fibrotic lesions; 3, thickening of the interalveolar septa with formation of isolated fibrotic lesions; and 4, formation of multiple fibrotic lesions with total or subtotal distortion of parenchymal architecture. The mean score for the 10 fields represented the score for each individual specimen.
Neutrophils were counted on 10 non-overlapping high-power fields on lung tissue sections. The average of the 10 fields represented the score for each individual specimen.
For evaluation of TUNEL staining, the percentage of cells with TUNEL-positive nuclei on 10 sequential, non-overlapping high-power fields from each specimen was recorded. The mean percentage of TUNEL-positive cells on 10 sequential fields represented the score for each individual specimen. For quantitation of immunofluorescent positive cells in tissue preparations, green fluorescent, red fluorescent, and dual fluorescent cells were counted on 10 non-overlapping high-power fields at ×600 magnification. The average number as well as mean percentage represented the score for each individual specimen.
Lung morphometry measurements were performed using techniques as previously described (28). The percentage of lung parenchyma containing fibrotic patches was measured by determining the area of fibrotic patches divided by the area of each lung section using calibrated image analysis using Image-Pro Express (Media Cybernetics, Silver Spring, MD). For each sample, the percentage of lung parenchyma affected by fibrosis was determined on 10 non-overlapping sections at ×40 magnification. The mean percentage represented the score for each individual specimen.
Lung collagen content determination.
Frozen right lower lobe lung tissue samples were hydrolyzed in 6 N HCl, and hydroxyproline content was quantitated using high pressure liquid chromatography (HPLC) as previously described (10, 19). Lung collagen content was calculated from these results as hydroxyproline accounts for ∼13.3% of collagen by weight.
Statistics.
Statistical analyses were performed using GraphPad InStat (GraphPad Software, San Diego, CA). Differences among groups were assessed using one-way ANOVA or Kruskal-Wallis rank analysis of variance. Differences between pairs were assessed using a Student's t-test or a Mann-Whitney test. Results are presented as means ± SE. P values < 0.05 were considered significant.
RESULTS
Repetitive bleomycin injury results in prominence of AEC lining areas of lung fibrosis.
To create a model of repetitive injury, we utilized direct laryngeal intubation. To help guide a dosing strategy, we analyzed TUNEL assays from the lungs of wild-type mice harvested 1–4 wk post a single dose of 0.04 units of bleomycin. The greatest degree of apoptotic and necrotic cells is at 1 wk post-bleomycin with a steep decline by week 2 (Supplemental Fig. 1; Supplemental Material for this article is available online at the Journal website.). This suggests that the second week post-bleomycin injury may represent a time frame at which the alveolar epithelium is attempting repair and therefore is sensitive to a new insult. For this reason, we chose a biweekly dosing strategy for the chronic injury model. Specifically, mice were given 0.04 units of bleomycin in 100 μl of saline every other week for eight doses, and then lungs were harvested at 2 wk after the last bleomycin dose. In initial model development, repetitive doses of saline were done as well with no difference compared with untreated mice. For these studies, a mortality rate of 8.3% was observed in the single-dose bleomycin group vs. 33.3% in the repetitive-dose group. Most of the mortality in the repetitive group was in the first 6 wk (or 3 doses). Two weeks after a single dose of bleomycin, the mice on average lost 1.4 g of weight from baseline. For those mice that remained in the repetitive dosing arm, weights were stabilized by the fourth dose of bleomycin, with the average weight 0.7 g above baseline at 8 wk and 0.8 g above baseline at 16 wk (harvest). For these studies, to best control the comparison between single- and repetitive-dose models, mice were harvested from both dosing strategies at 2 wk after bleomycin, allowing for evaluation of cell injury, inflammation, lung fibroblasts, and fibrosis.
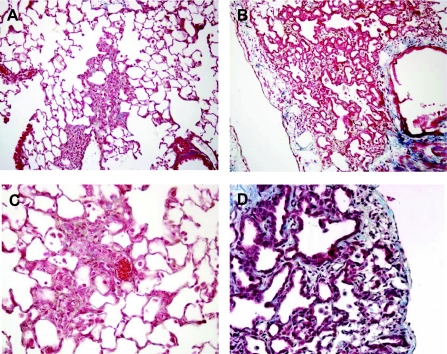
Evaluation of lung histology from mice receiving repetitive dosing revealed extensive fibrosis and extracellular matrix deposition based on trichrome blue collagen staining. Interestingly, lung histology from the repetitive injury model revealed a prominence of hyperplastic AECs lining the areas of fibrosis (Fig. 1). Such prominent cuboidal-shaped AECs are rarely encountered in the single-dose bleomycin model, even with higher doses, but are a central finding in human IPF lung biopsy sections (31, 37). Furthermore, the histological pattern of lung fibrosis was different in the repetitive dosing model with areas of fibrosis that appeared to follow the pattern of alveolar structure consistent with marked septal thickening rather than forming large single areas of collagen deposition as seen in the single-dose model (Fig. 1). The appearance of the lung suggested temporal heterogeneity with areas of fibrotic scar, active remodeling with presence of hyperplastic AECs, and normal lung in the same tissue sections (Supplemental Fig. 2). Areas consistent with a fibroblast focus, although rare, were occasionally encountered in the repetitive-dose model (Supplemental Fig. 3), but were not found in the single-dose model.
Fig. 1.
Trichrome blue-stained lung sections post-bleomycin. A and C: patchy areas of fibrosis were noted on trichrome-stained lung sections from mice 2 wk after a single dose of 0.04 units of bleomycin. B and D: in contrast, fibrosis was more prominent in trichrome-stained lung sections from mice 2 wk after the 8th biweekly repetitive dose of 0.04 units of bleomycin. Furthermore, alveolar epithelial cell (AEC) hyperplasia was prominent with the repetitive model. Magnification in A and B, ×40; magnification in C and D, × 400.
Repetitive bleomycin dosing results in greater lung fibrosis, less neutrophilic inflammation, greater cell death, and more prominent EMT compared with the single-dose model.
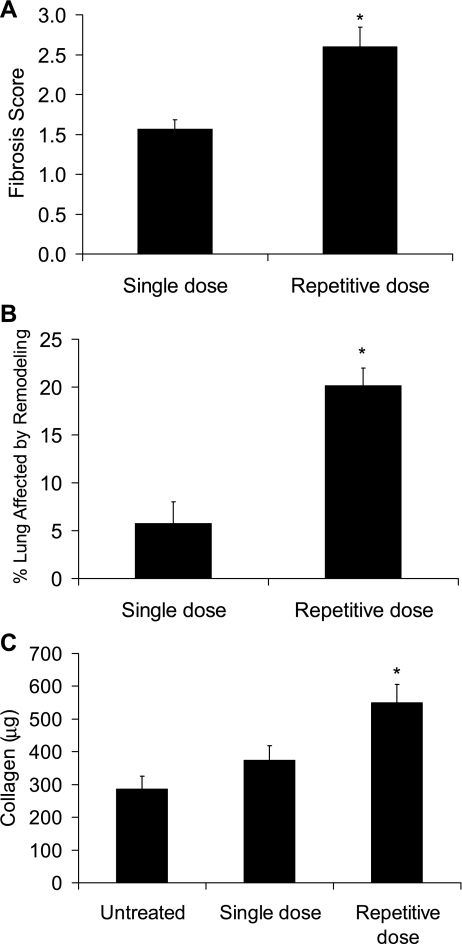
After noting these striking findings in our model development studies, we wanted to compare the degree of lung fibrosis in the single-dose model to the repetitive-dose model. Here, wild-type C57BL/6J mice were given 0.04 units of bleomycin by endotracheal intubation and then harvested 2 wk after a single dose (single-dose model) or 2 wk after the eighth biweekly dose (repetitive-dose model). Trichrome-stained lung sections were scored semiquantitatively for degree of fibrosis in a blinded fashion using a 0–4 scale that we have published previously (19). With this analysis, lung sections from the repetitive-dose model had greater fibrosis than the single-dose model (Fig. 2A). As the appearance of the fibrosis was strikingly different between the single- and repetitive-dose models, we sought to define the degree of architectural distortion further with the use of morphometry. Here, image capture techniques, as detailed in materials and methods, were used to determine the percentage of lung parenchyma for each specimen that contained tissue fibrosis. From these evaluations, it was found that a markedly greater percentage of lung had fibrotic involvement in the repetitive model (Fig. 2B). Finally, lung hydroxyproline content was greater in the biweekly repetitive bleomycin model as determined by HPLC (Fig. 2C). In a previous paper, we noted that lung architectural distortion peaks at 3 wk after single-dose bleomycin (20), so we also did studies comparing the repetitive-dose strategy results to a single-dose 3 wk time point and noted similar differences in fibrosis evaluations (Supplemental Fig. 4).
Fig. 2.
Lung fibrosis was greater in the repetitive bleomycin model compared with the single-dose model. A: lung fibrosis was scored on trichrome blue-stained lung sections, revealing a greater score with the repetitive model. N = 5 for each column. B: morphometry measurements of lung sections revealed a greater percentage of lung tissue affected by fibrosis in the repetitive model. N = 5 for each column. C: by HPLC assay, hydroxyproline content was greater in lungs from the repetitive model. N = 3 for untreated and 6 for single and repetitive. *P < 0.05 compared with single dose.
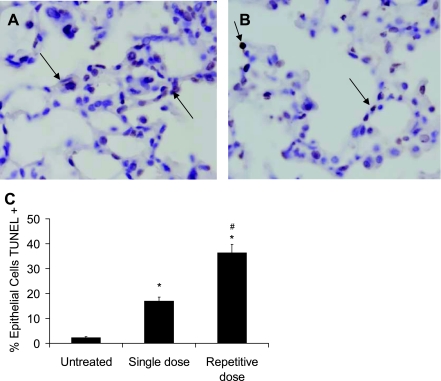
With the finding of greater fibrosis, studies were then performed to determine if greater AEC death was present as assessed by TUNEL staining. From these studies, the repetitive-dose group had a significant increase in the number of TUNEL-positive cells compared with the single-dose group, with 36% and 17% TUNEL-positive cells, respectively (P < 0.05) (Fig. 3).
Fig. 3.
TUNEL+ cells were greater in the repetitive bleomycin model compared with the single-dose model. Representative images from lung sections from 2 wk after a single dose of 0.04 units of bleomycin (A) and 2 wk after the 8th biweekly repetitive dose of 0.04 units of bleomycin (B). Magnification, ×800. Arrows point to TUNEL+ cells. C: when quantitated per high-power field, a greater percentage of cells were TUNEL+ in the repetitive bleomycin model. N = 5 per column. #P < 0.01 compared with single dose. *P < 0.01 compared with untreated.
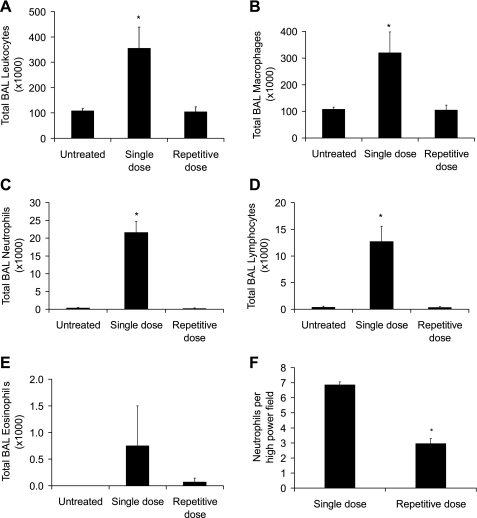
Based on histological evaluation, it appeared that lung tissue in the biweekly repetitive bleomycin model had less neutrophilic inflammatory infiltration, so we wanted to evaluate this more quantitatively with lavage studies. For this evaluation, lung lavage was performed in mice 2 wk after a single dose of bleomycin and 2 wk after the last dose in the eight-dose biweekly strategy. In the biweekly repetitive bleomycin model, BAL cell analysis revealed that the majority of the cells were macrophages with very few neutrophils or lymphocytes, compared with the single-dose model, which not only had greater numbers of macrophages, but also had more neutrophils and lymphocytes present (Fig. 4, A–E). In fact, the cell count and differential in the repetitive dosing strategy was similar to that observed in the untreated group. This likely represents a modification in the cellular influx over time. Initially, there is an acute neutrophilic infiltration in response to the acute injury that over weeks changes to a more monocytic cell population. After noting the increased BAL neutrophils in the single-dose group compared with the repetitive-dose group, neutrophils were counted in lung tissue sections and were observed to be lower in the repetitive bleomycin group, supporting the BAL findings (Fig. 4F).
Fig. 4.
For bronchoalveolar lavage (BAL) cell studies, total leukocyte (A), macrophage (B), neutrophil (C), and lymphocyte (D) counts in BAL were less in the repetitive model compared with the single-dose model. E: eosinophil counts were not different among the groups. F: neutrophil counts per high-power field were lower in the repetitive bleomycin group compared with the single-dose group. N = 5 per column. *P < 0.05 compared with other columns.
In a recent manuscript, we used a cell fate mapping model to determine the contribution of epithelial-mesenchymal transition (EMT)-derived fibroblasts in the single-dose bleomycin model (34). In this model, SPC.Cre mice are crossed to R26Rosa.Stop.LacZ reporter mice. The resulting double transgenic mice (SPC.Cre.R26Rosa.Stop.LacZ) have irreversible expression of β-gal in the lung epithelium because of the expression of SPC during lung development (26). X-gal staining of a lung section from a mouse that has undergone repetitive bleomycin is shown in Supplemental Fig. 5.
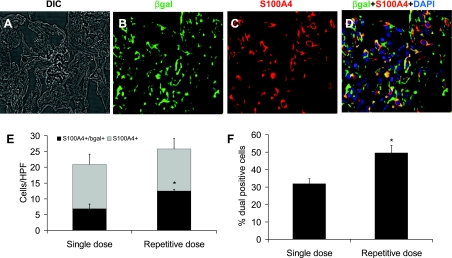
To evaluate the extent to which EMT contributes to the single and repetitive models, we performed confocal dual immunofluorescence staining on frozen lung sections for β-gal and the fibroblast marker S100A4, also referred to as fibroblast specific protein 1 (FSP1) (12, 20, 33). As we had noted in our previous paper (34), S100A4+ cells were prominent in areas of fibrosis in both the single-dose and repetitive-dose models, and some of these cells were dual S100A4+/β-gal+ (Fig. 5, A–D). To quantitate these cells, 10 high-power fields were evaluated for each specimen, and the number of S100A4+ and S100A4+/β-gal+ cells was determined in areas of fibrosis. Since we focused specifically in areas of fibrosis, it was not surprising that the number of S100A4+ cells per high-power field in areas of fibrosis was not significantly different between the two models. However, the repetitive model had a greater number of epithelial-derived S100A4+ cells per high-power field (Fig. 5E). Presented differently, lung sections from the repetitive-dose model revealed that approximately one-half of the S100A4+ fibroblasts were of epithelial lineage, compared with approximately one-third in the single-dose model (a number similar to what we had observed in our previous paper) (Fig. 5F) (34). In addition to evaluation of S100A4+ cells, we also performed similar studies for α-SMA. As with our previous paper (34), we noticed occasional dual α-SMA+/β-gal+ cells in areas of fibrosis (Supplemental Fig. 6), but at a level not easily quantitated.
Fig. 5.
Epithelial-mesenchymal transition (EMT)-derived cells are detected in the lung following bleomycin treatment. Confocal microscopy was performed to detect fluorescent immunostaining for β-gal (green), S100A4 (red), and DAPI (blue) in R26Rosa.Stop.LacZ.SPC.Cre reporter mice. A–D: representative images from lung sections from mice 2 wk after the 8th biweekly repetitive dose of 0.04 units of bleomycin. Dual fluorescent (yellow) cells indicate S100A4+ fibroblasts that are derived from lung epithelial lineage. DIC, differential interference contrast. Magnification, ×600. Comparing the single-dose bleomycin model to the repetitive-dose bleomycin model, quantitation of the number of S100A4+ cells per high-power field (HPF) in areas of fibrosis was not statistically different between the 2 groups (E). However, the number of S100A4+/β-gal+ cells per HPF was higher in the repetitive group. F: the percentage of S100A4+ cells that were also β-gal+ in the repetitive-dose group approached 50% compared with ∼33% in the single-dose group. N = 3 per column. *P < 0.05 compared with single dose.
A major issue with the single-dose bleomycin model is that the development of lung fibrosis is followed by resolution, and, in fact, by 6 wk, the lungs have remodeled remarkably back toward normal (19). To determine if the repetitive dosing strategy leads to a persistent fibrotic process, we performed an experiment in which four mice underwent eight biweekly repetitive doses of 0.04 units of bleomycin. These mice all survived and stabilized their weight after the third or fourth dose of bleomycin. Following the eighth dose of bleomycin, rather than harvest 2 wk later as was done above, we waited 10 wk after the last repetitive dose and then harvested the lungs. Lung tissue sections on these mice revealed marked tissue distortion, collagen deposition, and prominent type II AEC hyperplasia in a similar pattern to that observed in our earlier studies (Supplemental Fig. 7).
Epithelial cells lining areas of fibrosis in the repetitive model express the type II AEC marker pro-SP-C in a pattern similar to that seen in IPF.
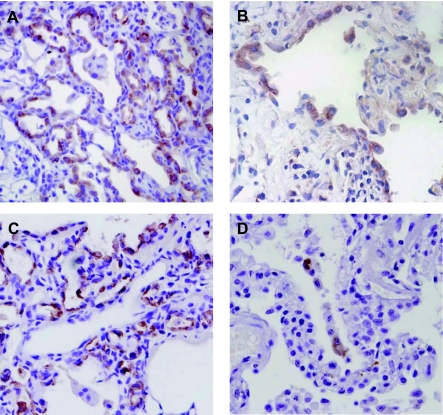
In the section above, some key findings were noted in the repetitive model that are also noted in IPF. First, neutrophilic inflammation is not prominent in the later time points in the repetitive model. Second, an active fibrotic process persists well after the last dose of bleomycin, suggesting an ongoing aberrant repair process. Finally, the repetitive model results in marked AEC hyperplasia, a pattern also noted in UIP biopsy specimens. Therefore, we wanted to analyze these hyperplastic AECs further. In the repetitive bleomycin model, immunohistochemistry for pro-SP-C confirmed that hyperplastic cells lining areas of fibrosis and tissue distortion were type II AECs (Fig. 6A). This pattern is reminiscent of human UIP, where the AECs lining areas of fibrosis are also positive for pro-SP-C expression (Fig. 6B) (18).
Fig. 6.
Hyperplastic AECs in the repetitive model express both pro-surfactant protein C (pro-SP-C) and Clara cell 10 (CC-10). A: immunohistochemistry (IHC) on lung sections from the repetitive model revealed that the hyperplastic AECs lining the areas of lung fibrosis were immunostained positive for pro-SP-C, a pattern similar to the hyperplastic epithelial cells noted in a UIP lung biopsy (B). C: by IHC, many of the hyperplastic AECs also immunostained positive for CC-10. D: in contrast, only rare hyperplastic AECs in UIP lung biopsies were positive for CC-10 by IHC. Magnification, ×600.
Recent studies have suggested that the lung has a progenitor cell population known as bronchoalveolar stem cells (BASCs) that characteristically express both pro-SP-C and CC-10 (15). To determine if a BASC-like population was expanded in the lungs of mice exposed to repetitive bleomycin, we first performed immunohistochemistry on lung sections for expression of CC-10. There were distinct cells that stained CC-10 positive within the hyperplastic cell population in the repetitive bleomycin model (Fig. 6C). In contrast, very few hyperplastic cells from IPF lung tissue were CC-10+ (Fig. 6D).
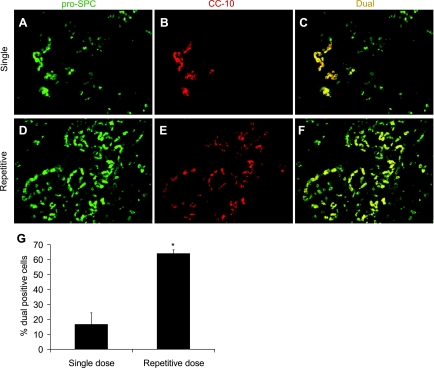
For better analysis of the BASC-like cell population, dual immunofluorescence imaging for pro-SP-C and CC-10 was performed on lung tissue sections from both the single-dose and repetitive-dose models. These cells were not detected in untreated mice (data not shown) but were present in occasional patches in the single-dose model (Fig. 7, A–C). However, in the repetitive group, a high percentage of the hyperplastic AECs lining areas of fibrosis were dual pro-SP-C+/CC-10+ (Fig. 7, D–F), as demonstrated by semiquantitative scoring of the number of dual-positive cells (Fig. 7G).
Fig. 7.
Characterization of bronchoalveolar stem cell (BASC)-like cells in areas of fibrosis. Confocal microscopy was performed to detect fluorescent immunostaining for pro-SP-C (green) and CC-10 (red). A–C: in lungs from mice 2 wk after a single dose of 0.04 units of bleomycin, occasional patches of dual fluorescent (yellow) cells that were pro-SP-C+/CC-10+ were noted. D–F: in contrast, many of the hyperplastic AECs lining areas of fibrosis in the repetitive-dose bleomycin model were dual pro-SP-C+/CC-10+ by immunofluorescence. Magnification in A–F, ×600. G: quantitation of the percentage of pro-SP-C+ cells that also expressed CC-10 revealed a greater percentage of dual-positive cells in lungs from the repetitive model. N = 5 per column. *P < 0.02 compared with single dose.
Ten human IPF lung biopsy samples were analyzed to determine if this same BASC-like population could be identified. Among the hyperplastic cells lining areas of fibrosis, however, only rare dual pro-SP-C+/CC-10+ were identified (Supplemental Fig. 8).
DISCUSSION
With these studies, we have developed a murine lung fibrosis model that we think will be useful in future studies to help further define some of the critical aspects of processes noted in human forms of pulmonary fibrosis. In this model, repetitive lung injury with bleomycin produces lung fibrosis, which has a pattern different from that seen with the single-dose model. Specifically, lung fibrosis is induced with this repetitive bleomycin model, which has less inflammation at later doses, and provides substantial lung architectural distortion, marked collagen deposition, enhanced EMT, and does not resolve spontaneously even when the stimulus is removed. This model recapitulates some of the features of UIP with prominent type II AEC hyperplasia and temporal heterogeneity. In addition, this model results in a population of BASC-like cells that has yet to be described to this prominence in the alveolar units in a fibrosis model.
Multiple approaches have been utilized by investigators to model human forms of pulmonary fibrosis in mice, including exposure to bleomycin, FITC, silica, or irradiation. Transgenic mice of viral vectors expressing specific genes of interest have also been used recently to induce lung fibrosis (22). Exposure of mice to silica or irradiation are favorable approaches to consider for investigators who are modeling pulmonary silicosis or radiation pneumonitis and fibrosis. Utilization of either transgenic mice or adenoviral vectors allows investigators to express specific genes of interest, including ones that when overexpressed result in lung fibrosis, as illustrated by studies of transforming growth factor-β (3, 4, 24). However, with these studies, the level of gene expression may be significantly higher than that seen in disease situations. Intratracheal FITC has recently emerged as a model for lung fibrosis (5, 29). The FITC model has some distinct strengths including ease of administration and a fibrotic response that persists for at least several months. The ability to visualize the area of the lung where FITC is deposited may be beneficial in many studies, but can also be perceived as problematic if one has studies in which fluorescent antibodies are required or green fluorescent protein reporter mice are used.
Bleomycin is used extensively to model lung fibrosis in mice and can be given by multiple routes: intratracheal, intranasal, subcutaneous, intraperitoneal, or intravenous. The subcutaneous, intraperitoneal, or intravenous routes deliver the drug systemically and provide clinically relevant models for human forms of bleomycin lung toxicity. However, the single-dose intratracheal bleomycin model has long been used as the most common method to induce experimental lung fibrosis because of its ease of administration and ability to induce lung fibrosis in a relatively short time frame. It has been unquestionably beneficial in improving our understanding of many facets in the pathogenesis of pulmonary fibrosis. The single-dose bleomycin model has helped to elucidate myriad cytokines and chemokines important to fibrosis development, including helping to establish the role of TGF-β as a cornerstone of the fibrotic response (7, 38). Furthermore, the single-dose model has helped to define the role of fibroblasts and myofibroblasts in pulmonary fibrosis, from defining their behavior to understanding their origins (9, 17, 23, 27, 34). An improved understanding of the alveolar epithelium's roles in pulmonary fibrosis has also been obtained with the single-dose bleomycin model, from studies implicating AECs as producers of profibrotic cytokines to studies demonstrating the importance of AEC apoptosis in lung fibrosis (21, 35, 36).
While it is clear that the single-dose bleomycin model has been and will continue to be useful, there are some limitations that must be addressed with this model (22). We believe that the biweekly repetitive bleomycin model offers some improvements over the single-dose bleomycin model and other existing models. Notably, it draws on the strengths of the single-dose bleomycin model, which are to easily and reproducibly induce experimental lung fibrosis, but turns an acute injury model into a chronic lung disease model. Furthermore, it has some features that compare more favorably to the pathology of UIP.
With single-dose intratracheal bleomycin, the initial phase of bleomycin-induced injury is primarily a proinflammatory acute lung injury model with an influx of neutrophils in addition to a mononuclear infiltrate (22). In IPF, an inflammatory component is not as prominent. With the repetitive bleomycin model, neutrophilic inflammation is undoubtedly present early in the process, but is attenuated as the model timeline progresses.
All of the models in current use lack many of the histological correlations with human UIP. One of the major hallmarks in UIP is the presence of hyperplastic type II AECs lining areas of fibrosis. This is notably absent or minimal in the single-dose bleomycin model and other models. In contrast, one of the most important aspects of the repetitive bleomycin model is that hyperplastic AECs are a prominent and reproducible feature. Little is actually known about the hyperplastic epithelial cells in IPF; in fact, it is not known if they are beneficial as a population for AEC renewal or perhaps harmful as producers of profibrotic signals. Without their prominence in a model, these questions cannot be answered. The repetitive bleomycin model provides us with a model that allows us to investigate this potentially crucial cell type.
The timing of disease development is certainly an issue when comparing clinical disease vs. animal models. Human fibrotic lung diseases such as IPF likely develop following a series of repeated injuries culminating in progressive disease. Indeed, the temporal heterogeneity of lung fibrosis with areas of normal lung juxtaposed to areas of dense fibrosis as a hallmark of UIP suggests the possibility of a chronic or sequential injury. Thus, it is difficult to envision a model of a single episode of injury adequately reflecting events that occur in such human lung diseases. In contrast to the single-dose bleomycin model and other fibrosis models, the recurrent nature of lung injury with the repetitive bleomycin model was selected to better mimic the hypothesized recurrent injury that occurs in IPF. In fact, histological lung sections from the repetitive model suggest temporal heterogeneity with areas of normal lung, dense scar, and active fibrosis present in different parts of the lung. One of the hallmark findings in the pathology of UIP is the presence of fibroblastic foci. With the repetitive-dose bleomycin model, we have observed an occasional, although rare, fibroblastic focus in lung tissue sections, illustrating an important difference between the lung pathology in the repetitive bleomycin model and UIP. Furthermore, the architectural distortion seen with the repetitive bleomycin model raises questions on whether these findings could be a prequel to the appearance of honeycombing noted in UIP. Future studies will be directed toward determining whether this honeycombing pattern progresses and fibroblast foci prevalence increases with longer experimental courses, but these are currently beyond the scope of this investigation.
In the single-dose bleomycin model, there is spontaneous resolution of the fibrosis without any intervention beginning after day 21 (6). In fact, this has often been criticized as the main limitation of this model (22), especially when using it as a model for IPF/UIP. In a previous paper, Chung et al. (6) investigated three weekly doses of intratracheal bleomycin, but noted that lung fibrosis had resolved by 6 wk post-bleomycin as well. Furthermore, prominent AEC hyperplasia was not noted either. In contrast, fibrosis appears to persist in our eight biweekly dose-repetitive bleomycin model. In fact, there are active areas of remodeling with areas of fibrosis adjacent to hyperplastic epithelium in lung sections 10 wk after the last repetitive dose of bleomycin. This pattern of persistent active remodeling may be a better model for the persistent disease process that occurs in IPF.
Recent evaluations have delineated a progenitor cell population in the lung that appears to reside near the bronchoalveolar junction and may be involved in repair of the lung epithelium following injury (15, 32). From these studies, these BASCs express both pro-SP-C and CC-10. We think that one of the more intriguing findings in this study is that a significant component of the hyperplastic epithelial cells lining areas of fibrosis in the repetitive model is dual pro-SP-C+/CC-10+, suggesting that these cells may represent an expansion of BASC-like cells. At this point, we speculate that the expansion of this cell population may represent an attempt at lung repair in the setting of repetitive injury. However, the degree to which these cells are reparative in nature or whether they actually may propagate the fibrotic process is not known. These questions provide important avenues of investigation for future studies.
In contrast to the mouse studies, dual pro-SP-C+/CC-10+ cells were rarely encountered in lung sections from patients with advanced IPF. The reasons for this discrepancy are not entirely clear, but suggest differences in disease processes as well as differences in lung regenerative capacity. First, this repetitive model does not completely recapitulate the pathological findings of UIP. Although a more chronic injury pattern than with the single-dose model, this repetitive dosing strategy still falls significantly short of the time period involved with IPF pathogenesis. During a prolonged process, it is possible that a stem or progenitor cell population may become more depleted, which could fuel the process of fibrosis. Second, the mouse lung has a robust regenerative capacity (11), more than that of the human lung, and thus inherently may have a more easily recruited stem or progenitor cell population. Nevertheless, this cell population in the repetitive model provides an opportunity to gain improved understanding of the normal and aberrant processes involved in regeneration of the alveolar epithelium following injury.
A relatively new and exciting aspect of fibrotic disease is the study of cellular plasticity and the ability of epithelial cells to change phenotype and contribute to the fibroblast population, a process termed epithelial-mesenchymal transition (13). Recent studies from Kim et al. (16, 17) and our lab (34) have used cell fate mapping models to demonstrate that EMT contributes to the lung fibroblast population in experimentally induced lung fibrosis. In our previous paper (34), we observed that approximately one-third of the S100A4+ lung fibroblasts at 2 wk post-single-dose bleomycin were of epithelial lineage, a number similar to our single-dose results in this study. Here, in the repetitive model, we found that almost one-half of the S100A4+ fibroblasts were derived via EMT. We suspect that the fact that the alveolar epithelium is undergoing repetitive injury leads to a greater propensity to undergo EMT in this model.
From this study, we think that the repetitive bleomcyin injury model offers new benefits in the study of pulmonary fibrosis, including some important findings that are more reflective of some of the histological features seen in UIP. However, we do recognize that even this model falls short of completely recapitulating the pathological findings of UIP, as is the case with all other experimental lung fibrosis models. While the time investment is longer with this model, there are certainly major improvements that may benefit future applications and investigations, potentially helping to provide important insights into crucial components of lung fibrotic remodeling.
GRANTS
Grant funding for this manuscript was provided from National Institutes of Health (NIH) and from non-profit organizations. Funding sources are as follows: NIH Grants HL-85317, HL-85406, HL-86825, HL-87738, and NCRR UL1 RR-024975; American Thoracic Society/Coalition for Pulmonary Fibrosis Research Grants; American Lung Association Dalsemer Research Grant; and IPFNet Cowlin Fellowship Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank the Mouse Pathology and Immunostaining Core Facility at Vanderbilt University Medical Center for assistance with some of the lung tissue slide preparations.
REFERENCES
- 1.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society and the European Respiratory Society. Am J Respir Crit Care Med 161: 646–664, 2000 [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 165: 277–304, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-beta and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol 175: 5390–5395, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bonniaud P, Margetts PJ, Kolb M, Haberberger T, Kelly M, Robertson J, Gauldie J. Adenoviral gene transfer of connective tissue growth factor in the lung induces transient fibrosis. Am J Respir Crit Care Med 168: 770–778, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Christensen PJ, Goodman RE, Pastoriza L, Moore B, Toews GB. Induction of lung fibrosis in the mouse by intratracheal instillation of fluorescein isothiocyanate is not T-cell-dependent. Am J Pathol 155: 1773–1779, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung MP, Monick MM, Hamzeh NY, Butler NS, Powers LS, Hunninghake GW. Role of repeated lung injury and genetic background in bleomycin-induced fibrosis. Am J Respir Cell Mol Biol 29: 375–380, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Goodwin A, Jenkins G. Role of integrin-mediated TGFbeta activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans 37: 849–854, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 345: 517–525, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 113: 243–252, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoff CR, Perkins DR, Davidson JM. Elastin gene expression is upregulated during pulmonary fibrosis. Connect Tissue Res 40: 145–153, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Hsia CC. Signals and mechanisms of compensatory lung growth. J Appl Physiol 97: 1992–1998, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzenstein AL, Mukhopadhyay S, Myers JL. Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases. Hum Pathol 39: 1275–1294, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 103: 13180–13185, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, Chapman HA. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 119: 213–224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 294: L1119–L1126, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, Li B, Donnelly EF, Holburn GE, Lewis KG, Collins RD, Hull WM, Glasser SW, Whitsett JA, Blackwell TS. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol 167: 1267–1277, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawson WE, Polosukhin VV, Zoia O, Stathopoulos GT, Han W, Plieth D, Loyd JE, Neilson EG, Blackwell TS. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med 171: 899–907, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Li X, Shu R, Filippatos G, Uhal BD. Apoptosis in lung injury and remodeling. J Appl Physiol 97: 1535–1542, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L152–L160, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 35: 175–181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, Miyazono K, Iwamoto I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest 104: 5–11, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardo A, Selman M. Molecular mechanisms of pulmonary fibrosis. Front Biosci 7: d1743–d1761, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 99: 10482–10487, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 114: 438–446, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polosukhin VV, Stathopoulos GT, Lawson WE, Blackwell TS. Variability of interalveolar septal remodeling after bleomycin treatment in mice. Ultrastruct Pathol 29: 53–64, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Roberts SN, Howie SE, Wallace WA, Brown DM, Lamb D, Ramage EA, Donaldson K. A novel model for human interstitial lung disease: hapten-driven lung fibrosis in rodents. J Pathol 176: 309–318, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Strieter RM. Pathogenesis and natural history of usual interstitial pneumonia: the whole story or the last chapter of a long novel. Chest 128: 526S–532S, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 5: 328–333, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, Lawson WE. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 180: 657–665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Uhal BD. Apoptosis in lung fibrosis and repair. Chest 122: 293S–298S, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc 3: 322–329, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr, Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 282: L585–L593, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.