Abstract
TNFα, a proinflammatory cytokine known to be involved in the pathogenesis of allergic asthma, has been shown to induce hyperalgesia in somatic tissue via a sensitizing effect on dorsal root ganglion neurons expressing transient receptor potential vanilloid type 1 receptor (TRPV1). Because TRPV1-expressing pulmonary sensory neurons play an important role in regulating airway function, this study was carried out to determine whether TNFα alters the sensitivity of these neurons to chemical activators. Responses of isolated nodose and jugular ganglion neurons innervating the rat lungs were determined by measuring the transient increase in intracellular Ca2+ concentration ([Ca2+]i). Our results showed the following. 1) A pretreatment with TNFα (50 ng/ml) for ∼24 h increased significantly the peak Δ[Ca2+]i evoked by capsaicin (Cap) in these neurons. A pretreatment with the same concentration of TNFα for a longer duration (∼48 h) did not further increase the response, but pretreatment for a shorter duration (1 h) or with a lower concentration (25 ng/ml, 24 h) failed to enhance the Cap sensitivity. 2) The same TNFα pretreatment also induced similar but less pronounced and less uniform increases in the responses to acid (pH 6.5–5.5), 2-aminoethoxydiphenyl borate (2-APB), a common activator of TRPV1, V2, and V3 channels, and allyl isothiocyanate (AITC), a selective activator of TRPA1 channel. 3) In sharp contrast, the responses to ATP, ACh, and KCl were not affected by TNFα. 4) The TNFα-induced hypersensitivity to Cap was not prevented by pretreatment with indomethacin (30 μM). 5) The immunoreactivity to both TNF receptor types 1 and 2 were detected in rat vagal pulmonary sensory neurons. In conclusion, prolonged treatment with TNFα induces a pronounced potentiating effect on the responses of isolated pulmonary sensory neurons to TRPV1 activators. This action of TNFα may contribute in part to the airway hyperresponsiveness induced by this cytokine.
Keywords: intracellular calcium, pulmonary afferent, transient receptor potential vanilloid type 1, airway inflammation, asthma
an important role of TNFα in the pathogenesis of allergic asthma has been extensively documented (6, 9, 28, 47). TNFα can be released from a variety of cell types in the airways, particularly macrophages, monocytes, and mast cells. TNFα, either newly synthesized or preformed and stored constitutively within the mast cell granules, is coreleased with other potent mediators such as histamine and tryptase via the IgE-dependent mechanism (9, 21, 47). TNFα was detected in bronchoalveolar lavage fluid, sputum, exhaled breath condensate, and serum of asthmatic patients during asthmatic attack or following antigen inhalation challenge (12, 33, 39). Furthermore, it has been shown that TNFα is released from sensitized human and mouse lung tissue in response to antigen challenge (21, 33, 47). TNFα is known to exert multiple potent effects on a number of effector cells in the airways; for example, it is a chemoattractant for neutrophils and eosinophils, upregulates the leukocyte-endothelial cell adhesive molecule E-selectin, enhances the production of TH2 cytokines, and increases microvascular permeability (8, 34, 48). Indeed, inhalation of TNFα can induce airway hyperresponsiveness associated with neutrophil infiltration and airway inflammation in healthy humans (48). However, its effect on airway sensory nerves remains to be determined.
Previous investigators have reported a sensitizing effect of TNFα on nociceptive neurons in dorsal root ganglia (DRG) and its important role in the development of lingering inflammatory pain in somatic tissues (15, 42, 45). Furthermore, this effect seems to involve specifically an upregulation of the sensitivity and/or expression of transient receptor potential vanilloid type 1 (TRPV1) induced by the TNFα pretreatment in DRG neurons; TRPV1 is a polymodal transducer and expressed predominantly in nonmyelinated (C-fiber) afferents (11, 30). Bronchopulmonary C-fibers represent >75% of the vagal afferents innervating the lungs and airways and are the counterpart of the DRG nociceptive endings in the lung structures. It is well-documented that vagal bronchopulmonary C-fiber afferents play an important role in regulating cardiopulmonary function, particularly in airway inflammatory diseases such as asthma (37, 38). However, whether the sensitivity of these vagal afferents is altered by TNFα is yet unknown.
In light of TNFα as an important contributing factor in the pathogenesis of allergic asthma and its potential role in the neural-immune interaction during airway inflammatory reaction, this study was carried out to investigate whether TNFα alters the sensitivity to TRPV1 and non-TRPV1 activators in pulmonary sensory neurons. Because intracellular Ca2+ is an important signal transduction molecule and plays a critical role in the regulation of neuronal membrane excitability (22) and neurotransmitter release (44), the responses of isolated nodose and jugular ganglion neurons innervating the rat lungs were determined by measuring the transient increase in intracellular Ca2+ concentration ([Ca2+]i) in this study.
MATERIALS AND METHODS
The procedures described below were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals [DHEW Publication No. (NIH) 85–23, Revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205] and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
Retrograde labeling of vagal pulmonary sensory neurons.
Sensory neurons innervating the lungs and airways were identified by retrograde labeling from the lungs by using the fluorescent tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Sigma, St. Louis, MO), a lipophilic fluorescent dye. Young Sprague-Dawley rats (∼200 g) were anesthetized with continuous inhalation of isoflurane administered via a nose cone connected to a vaporizing machine (AM Bickford, New York City, NY). A small (∼0.5 cm) midline incision was made on the ventral neck skin to expose the trachea. With the animal lying in the supine position and head tilted upward at ∼45°, DiI (0.2 mg/ml for cell culture and 1 mg/ml for immunohistochemistry; 0.05 ml in volume) was instilled into the trachea and lung via a needle (30 gauge), and the incision was then closed. DiI was initially sonicated, dissolved in ethanol, and diluted in saline (1% ethanol vol/vol). Our (25, 41) previous studies have shown that after >7 days, the soma of pulmonary sensory neurons located in the vagal sensory ganglia were labeled with DiI.
Isolation of nodose and jugular ganglion neurons.
Seven to ten days after receiving DiI instillation, rats were decapitated after being anesthetized by halothane inhalation. The head was immediately immersed in ice-cold DMEM/F-12 solution followed by quick extraction of nodose and jugular ganglia under a dissecting microscope. Each ganglion was then desheathed, cut into ∼8 pieces, placed in a 0.04% type IV collagenase and 0.02% dispase II mixture, and incubated for 80 min in 5% CO2 in air at 37°C. After digestion, the ganglion suspension was centrifuged at 150 g for 5 min followed by resuspension in a modified DMEM/F-12 solution (DMEM/F-12 supplemented with 10% vol/vol heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μM MEM nonessential amino acids). Nerve growth factors were not used in the culture to avoid their sensitizing effect on these neurons (38). Cells were then dissociated by gentle trituration with a small-bore, fire-polished Pasteur pipette. Myelin debris was separated and discarded after centrifugation of the dispersed cell suspension (500 g, 8 min) through a layer of 15% bovine serum albumin. The pellets were resuspended in the modified DMEM/F-12 solution, plated onto poly-l-lysine-coated glass coverslips, and then incubated overnight (5% CO2 in air at 37°C). Cells isolated from nodose and jugular ganglia were cultured and studied separately in each experiment.
Ca2+-imaging recording.
For all experiments, cells were washed or maintained in an extracellular solution (ECS): 5.4 mM KCl, 136 mM NaCl, 1 mM MgCl2, 1.8 mM CaCl2, 0.33 mM NaH2PO4, 10 mM glucose, 10 mM HEPES, pH adjusted to 7.4 with NaOH and osmolarity to 300 mosM. Ca2+ transients were measured in these cells with a Zeiss digital fluorescence microscope unit equipped with a variable filter wheel (Sutter Instruments, Novato, CA) and a digital CCD camera (Princeton Instruments, Trenton, NJ), as previously described (24). In brief, nodose and jugular neurons were incubated with 5 μM Ca2+ indicator fura 2-AM (Molecular Probes, Eugene, OR) for 30 min at 37°C in tissue culture medium and then rinsed (3×) with ECS and allowed to deesterify for at least 30 min before use. Dual images (340- and 380-nm excitation, 510-nm emission) were collected, and pseudocolor ratiometric images were monitored during the experiments by using the software Axon Imaging Workbench (Axon Instruments, Union City, CA). The imaging system was standardized with a two-point calibration using a Ca2+-free standard (−) and a Ca2+-saturated standard (+). Both standards contained 11 μM fura 2 [44 μl of 10 mM fura 2 Penta K+ salt (Molecular Probes), 8 ml of 20 mM HEPES-Na (pH 7.4), 32 ml H2O] and were prepared as follows: (− standard) 18 ml of fura 2, 1.98 ml of 10 mM EGTA-Na (pH 7.6); (+ standard) 18 ml of fura 2, 1.98 ml of 10 mM CaCl2. The parameters used for the two-point calibration include the dissociation constant of fura 2 (Kd: 285), the ratio values for the (−) and (+) concentration standards (Rmin and Rmax), and the denominator wavelength intensities for the (−) and (+) concentration standards (Denmin and Denmax). The concentration of Ca2+ was calculated according to the following equation described by Grynkiewicz et al. (23): [Ca2+]i = Kd (R − Rmin)/(Rmax − R) (Denmin/Denmax). Typical Rmin and Rmax values were 0.225 and 1.45, respectively.
Following the incubation period with fura 2-AM, the coverslip containing cells was mounted into a chamber placed on the stage of the microscope. Each cell, viewed under light field and selected on the basis of its regular, symmetrical shape and no attachment to other cells, was closely outlined within a circular or elliptic region of interest for the measurement of the [Ca2+]i. The equivalent diameter of the cell was calculated based on the measured area (A) of the region of interest: d = 2 × (A/π)1/2. Cells were continuously perfused with the ECS at a constant flow rate of 2 ml/min; a complete change of the bath solution occurred in ∼6 s. Pharmacological agents were perfused through the chamber by a gravity-fed valve-control system (VC-6 Valve Controller; Warner Instruments, Hamden, CT).
Immunohistochemistry.
Rats (150–250 g) were anesthetized with isoflurane inhalation, euthanized, and transcardially perfused with 0.01 M PBS (pH 7.4) followed by freshly prepared 0.01 M phosphate buffer (pH 7.4) containing 4% paraformaldehyde. The nodose and jugular ganglia were dissected and fixed in 4% paraformaldehyde for 4 h. The ganglia were then incubated in 20% sucrose in PBS overnight at 4°C, embedded in optimal cutting temperature compound, and cryosectioned at 12 μm. Sections were incubated in 5% normal goat serum in 0.01 M PBS for 1 h at room temperature and incubated overnight with anti-rat TNF receptor 1 (TNFR1; p55 receptor) antibody (1:500; rabbit polyclonal; Abcam, Cambridge, MA) or anti-rat TNF receptor 2 (TNFR2; p75 receptor) antibody (1:500; rabbit polyclonal; Sigma) that diluted in PBS containing 5% normal goat serum and 0.1% Triton X-100. Sections were then washed (3 × 10-min) with PBS and incubated with FITC-labeled goat anti-rabbit secondary IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature. After several washings with PBS, sections were mounted with coverslips and visualized with a Nikon Eclipse TE2000-U fluorescent microscope. In control experiments, primary antibodies were omitted to confirm the specificity of receptor-like immunoreactivity.
Protocols.
Six study series were carried out to answer the following specific questions. Study 1: to determine the effect of a pretreatment with TNFα on the responses of Ca2+ transient to increasing concentrations of capsaicin (Cap) in vagal pulmonary sensory neurons; two matching dishes of pulmonary nodose or jugular neurons cultured from the same ganglion of the same rat were incubated with vehicle (as control) and 50 ng/ml TNFα in culture medium for ∼24 h, respectively, and their responses to three concentrations of Cap (30, 100, and 300 nM; 30-s application and 10-min recovery) were then determined. Study 2: to establish the effective concentration range of TNFα, pulmonary sensory neurons cultured from the same ganglion were divided into four dishes, incubated with four different concentrations of TNFα (0 as control, 25, 50, and 100 ng/ml), and their responses to Cap were then determined ∼24 h later. Study 3: to determine the duration of TNFα pretreatment required to produce the observed effect; pulmonary sensory neurons cultured from the same ganglion were divided into six dishes and incubated with vehicle (control) and TNFα (50 ng/ml) each for three different durations (1, ∼24, and ∼48 h), and their responses to Cap were compared. The 1-h incubation with TNFα was administered just before the test after the cells had been dissociated and cultured for ∼24 h. Study 4: to evaluate whether the potentiating effect of TNFα was specific on the TRPV1 channel, the same protocols as described in Study 1 were applied to determine the responses to the following known chemical activators of pulmonary sensory neurons: 1) 2-aminoethoxydiphenyl borate (2-APB; 30, 100, and 300 μM); 2) acid solution (pH 6.5, 6.0, and 5.5); 3) allyl isothiocyanate (AITC; 30, 100, and 300 μM); 4) ATP (0.3, 1.0, and 3.0 μM); 5) ACh (10, 30, and 100 μM); and 6) KCl (5, 15, and 45 mM). Study 5: to determine whether the effect of TNFα was caused by synthesis and release of cyclooxygenase metabolites of arachidonic acid (42), two matching dishes of pulmonary sensory neurons were incubated with TNFα (50 ng/ml) alone and a combination of TNFα (50 ng/ml) and indomethacin (30 μM; a cyclooxygenase inhibitor), respectively; in the latter preparation, indomethacin was administered 1 h before the combined application. Approximately 24 h later, their responses to Cap were determined and compared. Study 6: to determine whether either or both types of TNF receptors, TNFR1 (p55) and TNFR2 (p75), are expressed in vagal pulmonary sensory neurons, immunohistochemical studies were performed in the nodose and jugular ganglia from the rats that had received intratracheal instillation of DiI 7–10 days earlier.
Chemicals.
All chemicals were purchased from Sigma except TNFα (ProSpec-Tany, Rehovot, Israel), 2-APB (Tocris, Ellisville, MO), and dispase II (Roche, Indianapolis, IN). A stock solution of TNFα (100 μg/ml) was diluted in DMEM/F-12 culture medium to the desired concentration daily. A stock solution of Cap (1 mM) was prepared in 1% Tween 80, 1% ethanol, and 98% saline and that of 2-APB (100 mM) in dimethyl sulfoxide; solutions of these chemical agents at desired concentrations were then prepared daily by dilution with ECS.
Statistical analysis.
Data were analyzed with a one- or two-way repeated-measure ANOVA unless mentioned otherwise (e.g., the χ2-test was used to determine size-related changes in the cell responses in Study 1). In the two-way ANOVA, one factor was the treatment effect of TNFα, and the other factor was the effect of chemical activators. When the ANOVA showed a significant interaction, pairwise comparisons were made with a post hoc analysis (Fisher protected least significant differences test). A P value <0.05 was considered significant. Data are reported as means ± SE.
RESULTS
The cells examined in each study series were selected based on two criteria. 1) Only the neurons innervating the lung structure (exhibiting DiI fluorescence) were selected. 2) A high concentration of KCl (100 mM, 15-s perfusion) was applied at the end of each experimental run to test for cell viability. Cells that did not respond to the KCl challenge were considered nonexcitable, and data were not included for analysis.
Study 1.
Cap evoked a rapid and transient increase in the [Ca2+]i in a concentration-dependent manner in pulmonary sensory neurons (Fig. 1). This Cap-evoked Ca2+ transient was markedly enhanced after a pretreatment with TNFα (50 ng/ml) for ∼24 h (Fig. 2A) in both nodose and jugular neurons, and the difference in response between the control and treated groups increased at higher concentrations of Cap (e.g., 100 and 300 nM; Fig. 2A). In sharp contrast, the response to the challenge of KCl (100 mM) was not different between control and TNFα-treated groups (P > 0.05; Fig. 2A). The pretreatment with TNFα did not significantly alter the baseline [Ca2+]i, which were 128.5 ± 12.5 and 120.1 ± 13.8 nM (P > 0.5) in control and TNFα-treated groups, respectively, in nodose neurons and 150.7 ± 14.7 and 139.1 ± 11.8 nM (P > 0.5) in control and TNFα-treated groups, respectively, in jugular neurons.
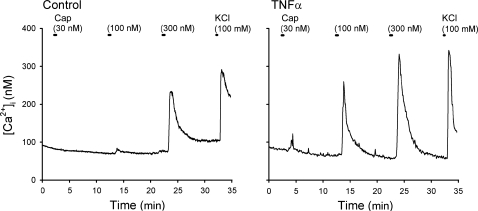
Fig. 1.
Representative experimental records illustrating the change in intracellular Ca2+ concentration ([Ca2+]i) evoked by increasing concentrations of capsaicin (Cap) in vagal sensory neurons labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI). Left: a control neuron (diameter 33.3 μm) incubated with vehicle (culture medium: modified DMEM/F-12 solution) for ∼24 h. Right: a neuron (diameter 24.8 μm) incubated with TNFα (50 ng/ml in culture medium) for ∼24 h. Both neurons were isolated from nodose ganglia of the same rat (185 g). Cap was applied for 30 s each, and KCl solution (100 mM, 15 s) was applied to test cell vitality at the end of both experiments.
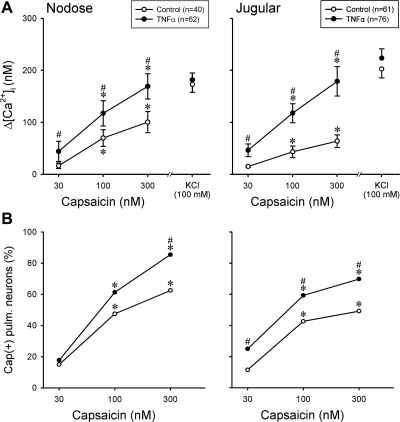
Fig. 2.
Effect of TNFα pretreatment on the response to Cap in pulmonary (pulm.) sensory neurons. A: changes in [Ca2+]i evoked by Cap challenges in DiI-labeled nodose and jugular ganglion neurons. B: percentage of the Cap-sensitive [Cap(+)] neurons in all the DiI-labeled neurons that were activated by KCl; a neuron was considered Cap(+) if its Cap-evoked Ca2+ transient exceeded 20% of its peak Ca2+ transient generated by 100 mM KCl. The total number of neurons presented in B was the same as that in A. In both A and B, control (○) and treated (●) neurons were tested after incubation with vehicle and TNFα (50 ng/ml), respectively, in culture medium for ∼24 h. Data in A were analyzed by 2-way ANOVA, and data in B by χ2-test and McNemar test (see text for details). *P < 0.05, significantly different from the response to 30 nM Cap. #P < 0.05, significantly different from the corresponding data in control neurons.
These results were analyzed to determine whether the TNFα-induced increase in response to Cap was related to an increased percentage of Cap-sensitive [Cap(+)] neurons in the treated group. A cell was considered Cap(+) if Cap evoked a change in [Ca2+]i exceeding 20% of its maximal response to KCl (100 mM). Indeed, a significantly higher percentage of Cap(+) neurons was found in the TNFα-treated group (Fig. 2B); for example, Cap (300 nM) activated 53 of the 62 nodose neurons (85.5%) in the TNFα-treated group but only 25 of 40 (62.5%) neurons in the control group. The data analysis and comparisons between control and TNFα groups were made using χ2-test, and the within-group comparisons (between different Cap concentrations) were made using McNemar test for correlated proportions (52).
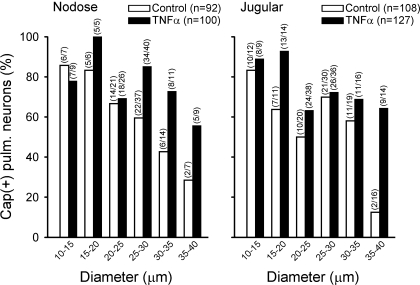
This difference was then further analyzed to determine whether the TNFα-induced increase in the percentage of Cap(+) neurons was size dependent. Similar to that found in our (13) previous study, ∼80% of the cultured pulmonary neurons were in the diameter range of 20–35 μm. However, to reach a sufficient number of cells for a more accurate analysis, the numbers of small (<20 μm) and large (>35 μm) cells higher than their normal distribution were selected and tested in this study (Fig. 3). Figure 3 shows a comparison of the size distributions of the Cap(+) nodose and jugular pulmonary neurons between control and TNFα-treated groups, when cells were grouped based on their size (in 5-μm increments of cell diameter). It appears that the TNFα pretreatment induced an increase in the percentage of Cap(+) neurons in cells of all sizes in general, but the increase was more consistent in the medium- and large-size nodose neurons (diameter >25 μm) and in the large-size jugular neurons (>35 μm) (Fig. 3).
Fig. 3.
Effect of TNFα pretreatment on the size distributions of Cap-sensitive pulmonary sensory neurons. Cells were grouped based on their size (in 5-μm increments of cell diameter), and Cap(+) neurons were depicted as the percentage of all the DiI-labeled nodose and jugular ganglion neurons that were activated by KCl in each size category. A neuron was considered Cap(+) if its Cap (300 nM)-evoked Ca2+ transient exceeded 20% of its peak Ca2+ transient generated by KCl (100 mM). Closed and open bars represent treated neurons (pretreated with 50 ng/ml TNFα for ∼24 h) and control neurons (pretreated with vehicle for ∼24 h), respectively. To have a sufficient number of cells for a more accurate analysis, the numbers of small (<20 μm) and large (>35 μm) cells higher than their normal distribution were selected and tested in this study (see text for details).
Study 2.
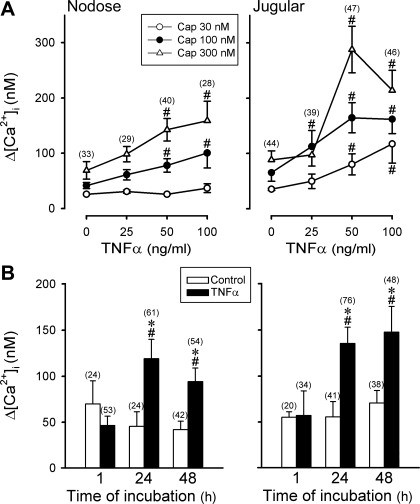
When pulmonary sensory neurons were incubated with three different concentrations of TNFα, the response to Cap was markedly elevated in the neurons pretreated with higher concentrations of TNFα (50 and 100 ng/ml) but was not significantly changed with lower concentration of TNFα (25 ng/ml) (Fig. 4A). There was no major difference in the potentiating effect of TNFα between nodose and jugular neurons (Fig. 4A).
Fig. 4.
Influences of TNFα concentration and incubation time on the changes in Cap sensitivity in pulmonary sensory neurons. A: effect of increasing the TNFα concentration on Cap sensitivity in DiI-labeled nodose and jugular ganglion neurons. Changes in [Ca2+]i evoked by Cap challenge were measured in neurons after incubation with TNFα for ∼24 h. The number in each parenthesis represents the number of cells pretreated with a given concentration of TNFα. B: effect of TNFα (50 ng/ml) on the response of Δ[Ca2+]i evoked by Cap (100 nM) was altered by increasing incubation time in pulmonary sensory neurons. Different groups of neurons were used in the studies in A and B. #P < 0.05, significantly different from the response in corresponding control neurons (pretreated with vehicle, 0 ng/ml TNFα, for ∼24 h). *P < 0.05, significantly different from the response after TNFα incubation for 1 h. Data are means ± SE; when SE bars overlap between 2 adjacent data points, only 1 side is shown.
Study 3.
When pulmonary sensory neurons were incubated with TNFα (50 ng/ml) for three different durations, the response to Cap (100 nM) was significantly elevated after ∼24 and ∼48 h compared with control (incubated with vehicle) but not different after TNFα pretreatment for only 1 h just before the test (Fig. 4B). This time-dependent phenomenon of the potentiating effect of TNFα pretreatment was found in both nodose and jugular neurons (Fig. 4B) and was also observed in the response to challenges with 300 nM Cap (data not shown).
Study 4.
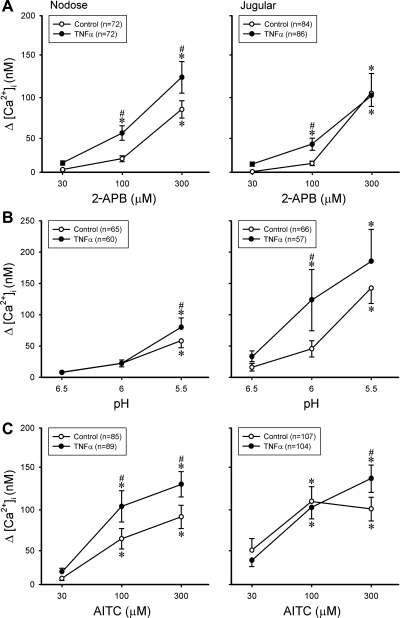
2-APB is a common activator of TRPV1, V2, and V3 channels (26); acid (low pH) solution is known to activate both TRPV1 and acid-sensing ion channels (ASICs) (11, 25, 50); AITC is a selective activator of TRP ankyrin type 1 receptor (TRPA1) (5, 19). Pretreatment with TNFα (50 ng/ml, ∼24 h) also increased the responses to 2-APB, low pH, and AITC in pulmonary nodose and jugular neurons, but the potentiating effects were not as pronounced and uniform as that seen in the response to Cap (Fig. 5). For example, TNFα pretreatment failed to increase the responses to pH 6.0 in nodose neurons and pH 5.5 in jugular neurons (Fig. 5).
Fig. 5.
A–C: changes in [Ca2+]i evoked by 2-aminoethoxydiphenyl borate (2-APB), low pH, and allyl isothiocyanate (AITC) in DiI-labeled nodose and jugular ganglion neurons. ○, Control neurons (incubated with vehicle for ∼24 h); ●, neurons incubated with TNFα (50 ng/ml) for ∼24 h. *P < 0.05, significantly different from response to 30 μM 2-APB, pH 6.5, or 30 μM AITC. #P < 0.05, significantly different from the corresponding data in control neurons.
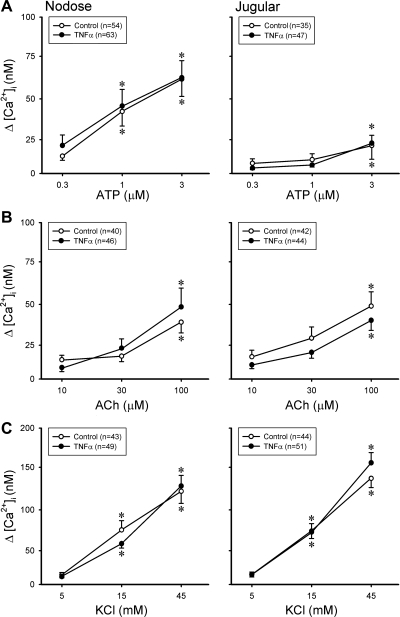
However, in sharp contrast, the same TNFα pretreatment did not generate any potentiation on the response to ATP, ACh, or KCl in either pulmonary nodose or jugular neurons (Fig. 6). ATP is an activator of purinoceptors P2X2 and P2X3 (10, 35); ACh is an agonist of nicotinic ACh receptors in pulmonary sensory neurons (27); KCl can cause membrane depolarization and evoke Ca2+ transient via voltage-gated Ca2+ channels. None of them is known to activate either TRPV1 or TRPA1 channels.
Fig. 6.
A–C: changes in [Ca2+]i evoked by ATP, ACh, and KCl in DiI-labeled nodose and jugular ganglion neurons. ○, Control neurons (incubated with vehicle for ∼24 h); ●, neurons incubated with TNFα (50 ng/ml) for ∼24 h. *P < 0.05, significantly different from the response to 0.3 μM ATP, 10 μM ACh, or 5 mM KCl. Data are means ± SE; when SE bars overlap between 2 adjacent data points, only 1 side is shown.
Study 5.
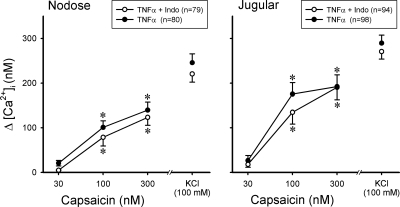
Prior and continuous treatment of pulmonary sensory neurons with indomethacin (30 μM) did not prevent the elevated response to Cap induced by TNFα (Fig. 7). For example, Δ[Ca2+]i evoked by 100 and 300 nM Cap were 175.5 ± 25.7 and 192.2 ± 22.8 nM, respectively, in jugular neurons pretreated with TNFα alone (n = 98), which were not different from 134.5 ± 26.4 nM (P > 0.05) and 190.6 ± 28.1 nM (P > 0.05), respectively, evoked by the same Cap challenges in those pretreated with a combination of indomethacin and TNFα (n = 94). Similarly, indomethacin failed to produce any difference in the responses of nodose neurons to Cap after the TNFα pretreatment (Fig. 7).
Fig. 7.
Lack of influence of indomethacin pretreatment on the sensitizing effect of TNFα on pulmonary sensory neurons. Changes in [Ca2+]i evoked by Cap challenges were measured in DiI-labeled nodose and jugular ganglion neurons. ○, Neurons were first treated with indomethacin (Indo; 30 μM) for 1 h and then incubated with a combination of Indo (30 μM) and TNFα (50 ng/ml) for ∼24 h; ●, neurons were incubated with TNFα (50 ng/ml) alone for ∼24 h. *P < 0.05, significantly different from the response to 30 nM Cap.
Study 6.
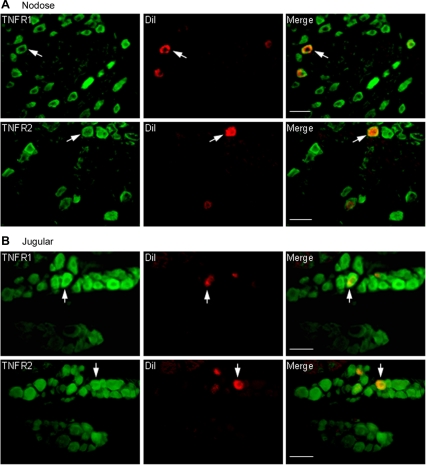
The immunoreactivity for both TNFR1 and TNFR2 were detected in nodose and jugular ganglion neurons, including those innervating the lung structures as identified by DiI labeling (Fig. 8). Although we did not correlate the expression of TNFR1 and TNFR2 with the cell size, the receptor-like immunoreactivity for both types of TNF receptors appeared uniformly distributed among different size of neurons. There was no immunoreactivity in the absence of the primary antibodies.
Fig. 8.
Expression of TNF receptors in rat vagal pulmonary sensory neurons. The sections of nodose (A) and jugular (B) ganglia were subject to immunohistochemistry for the TNF receptors TNFR1 (p55) and TNFR2 (p75). Left: TNF receptor immunoreactivity in green. Middle: DiI labeling in red. Right: the merged image. Arrowheads depict colocalization of TNF receptor staining and DiI labeling in the same neurons. Scale bar, 50 μm.
DISCUSSION
Results in this study showed that isolated pulmonary nodose and jugular sensory neurons incubated with TNFα for ∼24 h or longer developed an elevated response of Ca2+ transient to a number of chemical activators of these neurons, particularly those activating the TRPV1 channel. In contrast, the same TNFα pretreatment did not affect the responses of these neurons to some other chemical activators such as ATP, ACh, and KCl that are not known to activate TRPV1 channels. The mechanism underlying this sensitizing effect of TNFα is not yet fully understood, but several factors that are possibly involved should be considered.
Shortening the TNFα pretreatment to 1 h failed to induce the same potentiated responses to TRPV1 activation in these neurons. Although we cannot completely rule out the possibility that a posttranslational modulation of these channels is also involved, the relative long duration required for the potentiating effect of TNFα to take place seems to favor the probability of a transcriptional upregulation of the expression of TRP channels in this study. This postulation is further supported by a recent report that exposure of DRG neurons to TNFα for 48 h induced a pronounced increase in the proportion of these neurons expressing TRPV1-like immunoreactivity (29). In addition, their observation of the increased TRPV1 expression occurring mostly in the large-diameter neurons, which normally have either no or very low level of TRPV1 expression, is also in general agreement with our results. In the present study, the TNFα-induced increase in the percentage of Cap-sensitive neurons was greater in the medium- and large-size nodose pulmonary neurons (diameter >25 μm) and in the large-size jugular pulmonary neurons (>35 μm), although the increase was also found in small-size neurons (e.g., jugular, 15–20 μm). These large-size cells were presumably myelinated neurons, but we did not verify them in this study. Several cellular signaling pathways are possibly involved in the TNFα-induced increase in TRPV1 expression, but the primary mechanisms are not yet fully understood.
After the membrane TNFα is cleaved, they form free TNFα and highly active homotrimers. The biological effects of these molecules are mediated by activation of two distinct types of TNF receptors on the cell surface, TNFR1 and TNFR2 (4); the former is believed to be responsible for most of the proinflammatory effects induced by TNFα in the pathogenesis of asthma (6, 28). Coincidentally, it has been reported that the TNFα-induced upregulation of TRPV1 expression in DRG neurons was most probably mediated through the activation of TNFR1 because the potentiating effect was absent in the DRG neurons isolated from mice lacking TNFR1 (29). Activation of these receptors is known to induce activation of several signaling pathways such as the NF-κB pathway and mitogen-activated protein kinase pathways, including ERK (3, 4, 18). Activated ERK are translocated to the nucleus and can alter the gene expression, which has been implicated in the TNFα-induced overexpression of TRPV1 in DRG nociceptive neurons (29, 49). Whether these signaling pathways are also involved in the potentiating effect of TNFα on pulmonary sensory neurons in this study remains to be determined. Nonetheless, the expression of both TNFR1 and TNFR2 receptors in the pulmonary sensory neurons (Fig. 8) seems to support this possibility.
In our pilot experiments, we have observed that intratracheal administration of TNFα (5–10 μg/kg) induced an increased vagal pulmonary C-fiber sensitivity to right atrial bolus injection of Cap (0.5 μg/kg) in anesthetized mice and rats (data not shown). However, the diverse actions of TNFα on a number of cell types in the airways made it impossible to selectively evaluate its sensitizing effect on pulmonary sensory nerves in a whole animal preparation. For example, TNFα is known as a chemoattractant and can cause leukocyte infiltration and degranulation of mast cells and neutrophils in the airways (47, 48). Inflammatory autacoids released from these cells may in turn generate a secondary stimulatory effect on these afferent endings (14, 38). To avoid these potential indirect actions of TNFα, this study was therefore carried out in isolated pulmonary sensory neurons. However, even in the cultured neuron preparation, we cannot dismiss the possibility that TNFα may interact with microglial cells (e.g., satellite cells) present in the culture and trigger the release of chemical mediators and other cytokines that can sensitize these neurons. Previous investigators have reported that TNFα can induce an increased production of cyclooxygenase metabolites of arachidonic acid, particularly PGE2, in cultured DRG cells (17). PGE2 is known to enhance the sensitivity of pulmonary sensory neurons via the cyclic AMP and protein kinase A signaling pathway (24, 36). However, the results of this study suggest that the cyclooxygenase metabolites do not play a major role in the TNFα-induced hypersensitivity of these neurons because indomethacin pretreatment failed to attenuate the TNFα-induced potentiating effect on the response to Cap in these neurons.
A distinct potentiating effect of TNFα was found in the response of these neurons to Cap (Fig. 2), a selective activator of TRPV1, and a similar but less uniform and less pronounced effect on the response to AITC (Fig. 5C), a selective TRPA1 activator. TRPV1 and TRPA1 are members of the vanilloid and ankyrin subfamilies, respectively, in the TRP ion channel family; both are nonselective cation channels with a high permeability to Ca2+ (43). The responses of these neurons to acid (low pH) and 2-APB were also potentiated after TNFα treatment, but the increased responses were not as consistent as that to Cap (Fig. 5). In addition to TRPV1, acid in the range of pH applied in this study can also activate several amiloride-sensitive ASICs, and their relative contributions to the response to acid vary substantially between individual pulmonary sensory neurons and also depend on the level of acidity (25). On the other hand, blocking TRPV1 by capsazepine only abolished approximately half of the inward current evoked by 2-APB in isolated pulmonary sensory neurons; the remaining response was caused by activation of TRPV2 and TRPV3 (26). Therefore, the varying degree of TNFα-induced potentiation between the responses to Cap, low pH, and 2-APB observed in this study may be related to the relative role of TRPV1 in the responses of individual neurons to these chemical activators.
TRPV1 is widely expressed in somatic and visceral nociceptive neurons and is a polymodal transducer responsible for detecting chemical, thermal, and mechanical nociceptive stimuli. In healthy lungs, TRPV1 is selectively expressed in small-diameter, slow-conducting nonmyelinated (C-) fibers that represent a majority (>75%) of vagal afferents innervating the lung and airways (30, 31), and TRPA1 is found to be coexpressed with TRPV1 in a subset of these vagal C-fiber afferent nerves (5, 40). Both TRPV1 and TRPA1 are recognized as the primary transducers located in the bronchopulmonary C-fiber sensory terminals that can be activated by a wide range of endogenous inflammatory mediators and activators (e.g., protons, hyperthermia, bradykinin, reactive oxygen radicals, etc.) and inhaled chemical irritants (e.g., acrolein, toluene diisocyanate, etc.) (7, 25, 41, 46). Immunohistochemical studies have shown that TRPV1 channels are also colocalized with certain sensory neuropeptides, such as tachykinins and CGRP, in the sensory terminals (51). These TRPV1- and CGRP-expressing C-fiber endings innervate the entire respiratory tract, from upper airways to lung parenchyma (51). They are found in the mucosa as well as in the deeper regions of the airway structure (e.g., near airway smooth muscles). In the conducting airways, they exhibit extensive axonal arborization extending into the space between epithelial cells or forming network-like plexus underneath the airway epithelial basement membrane (1, 2, 51). The sensitivity of these C-fiber afferents to certain endogenously released autacoids and a wide range of inhaled chemical irritants is well-documented (14, 37, 38). Activation of these sensory nerves is known to elicit centrally mediated reflex responses including reflex bronchoconstriction and mucus hypersecretion via the cholinergic pathway, accompanied by the sensation of airway irritation and urge to cough (14, 36, 37). Activation of TRPV1 also triggers Ca2+ influx and release of tachykinins and CGRP from the sensory terminals. These sensory neuropeptides can act on a number of effector cells in the respiratory tract (e.g., airway smooth muscles, cholinergic ganglia, mucous glands, immune cells) and elicit the local “axon reflexes” such as protein extravasation, inflammatory cell chemotaxis, etc. (16, 38). Therefore, it is believed that these C-fiber sensory nerves play an important part in the manifestation of various symptoms of airway hypersensitivity in patients with airway inflammatory diseases such as asthma, bronchitis, and viral infection (7, 20, 32, 38). Taken together, this study suggests that the sensitizing effect of TNFα on the pulmonary sensory neurons may at least in part contribute to the pathogenesis of airway hyperresponsiveness induced by this cytokine.
GRANTS
This study was supported in part by grants from the National Heart, Lung, and Blood Institute (HL-58686 and HL-96914).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Michelle Lim for technical assistance. We also thank Drs. Brian E. Cairns and Xu-Dong Dong (University of British Columbia) for their valuable advice regarding the primary antibodies of TNF receptors.
REFERENCES
- 1.Adriaensen D, Timmermans JP, Brouns I, Berthoud HR, Neuhuber WL, Scheuermann DW. Pulmonary intraepithelial vagal nodose afferent nerve terminals are confined to neuroepithelial bodies: an anterograde tracing and confocal microscopy study in adult rats. Cell Tissue Res 293: 395– 405, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Baluk P, Nadel JA, McDonald DM. Substance P-immunoreactive sensory axons in the rat respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol 319: 586– 598, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Barbin G, Roisin MP, Zalc B. Tumor necrosis factor alpha activates the phosphorylation of ERK, SAPK/JNK, and P38 kinase in primary cultures of neurons. Neurochem Res 26: 107– 112, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11: 372– 377, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102: 12248– 12252, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med 354: 697– 708, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23: 360– 370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol 10: 471– 480, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol 121: 5–10; quiz 11–12, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci 22: 182– 188, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816– 824, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Cembrzynska-Nowak M, Szklarz E, Inglot AD, Teodorczyk-Injeyan JA. Elevated release of tumor necrosis factor-alpha and interferon-gamma by bronchoalveolar leukocytes from patients with bronchial asthma. Am Rev Respir Dis 147: 291– 295, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Chung E, Gu Q, Kwong K, Arden W, Lee LY. Comparison of capsaicin-evoked calcium transients between rat nodose and jugular ganglion neurons. Auton Neurosci 97: 83– 88, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Coleridge JC, Coleridge HM. Afferent vagal C fiber innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1– 110, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol 107: 660– 664, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Swert KO, Joos GF. Extending the understanding of sensory neuropeptides. Eur J Pharmacol 533: 171– 181, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fehrenbacher JC, Burkey TH, Nicol GD, Vasko MR. Tumor necrosis factor alpha and interleukin-1beta stimulate the expression of cyclooxygenase II but do not alter prostaglandin E2 receptor mRNA levels in cultured dorsal root ganglia cells. Pain 113: 113– 122, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem 70: 1876– 1886, 1998 [DOI] [PubMed] [Google Scholar]
- 19.García-Añoveros J, Nagata K. TRPA1. Handb Exp Pharmacol 179: 347– 362, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol 533: 207– 214, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Gosset P, Tsicopoulos A, Wallaert B, Joseph M, Capron A, Tonnel AB. Tumor necrosis factor alpha and interleukin-6 production by human mononuclear phagocytes from allergic asthmatics after IgE-dependent stimulation. Am Rev Respir Dis 146: 768– 774, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Gover TD, Moreira TH, Weinreich D. Role of calcium in regulating primary sensory neuronal excitability. Handb Exp Pharmacol 194: 563– 587, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440– 3450, 1985 [PubMed] [Google Scholar]
- 24.Gu Q, Kwong K, Lee LY. Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: role of cAMP/PKA signaling pathway. J Neurol 89: 1985– 1993, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 291: L58– L65, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Q, Lin RL, Hu HZ, Zhu MX, Lee LY. 2-Aminoethoxydiphenyl borate stimulates pulmonary C neurons via the activation of TRPV channels. Am J Physiol Lung Cell Mol Physiol 288: L932– L941, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu Q, Ni D, Lee LY. Expression of neuronal nicotinic acetylcholine receptors in rat vagal pulmonary sensory neurons. Respir Physiol Neurobiol 161: 87– 91, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heffler E, Berry M, Pavord ID. Tumor necrosis factor-alpha: a promising therapeutic target for asthma? BioDrugs 21: 345– 349, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Hensellek S, Brell P, Schaible HG, Brauer R, Segond von Banchet G. The cytokine TNFalpha increases the proportion of DRG neurons expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci 36: 381– 391, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127: 113– 124, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst 5: 165– 176, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta 1772: 915– 927, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Keatings VM, O'Connor BJ, Wright LG, Huston DP, Corrigan CJ, Barnes PJ. Late response to allergen is associated with increased concentrations of tumor necrosis factor-alpha and IL-5 in induced sputum. J Allergy Clin Immunol 99: 693– 698, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Kips JC, Tavernier J, Pauwels RA. Tumor necrosis factor causes bronchial hyperresponsiveness in rats. Am Rev Respir Dis 145: 332– 336, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol 295: L858– L865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee LY. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir Physiol Neurobiol 167: 26– 35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47– 65, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Lee LY, Undem BJ. Brochopulmonary vagal sensory nerves. In: Advances in Vagal Afferent Neurobiology, edited by Undem BJ, Weinreich D. Boca Raton, FL: CRC (Chemical Rubber Company Press), 2005, chapt. 11, p. 279–313 [Google Scholar]
- 39.Matsunaga K, Yanagisawa S, Ichikawa T, Ueshima K, Akamatsu K, Hirano T, Nakanishi M, Yamagata T, Minakata Y, Ichinose M. Airway cytokine expression measured by means of protein array in exhaled breath condensate: correlation with physiologic properties in asthmatic patients. J Allergy Clin Immunol 118: 84– 90, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586: 1595– 1604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, Lee LY. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol 291: R541– R550, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci 17: 975– 982, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165– 217, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol 15: 266– 274, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 81: 255– 262, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respir Cell Mol Biol 40: 756– 762, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas PS. Tumour necrosis factor-alpha: the role of this multifunctional cytokine in asthma. Immunol Cell Biol 79: 132– 140, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med 152: 76– 80, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Utreras E, Futatsugi A, Rudrabhatla P, Keller J, Iadarola MJ, Pant HC, Kulkarni AB. Tumor necrosis factor-alpha regulates cyclin-dependent kinase 5 activity during pain signaling through transcriptional activation of p35. J Biol Chem 284: 2275– 2284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173– 177, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV. Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 141: 1533– 1543, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Zar JH. Biostatistical Analysis (3rd ed.). Englewood Cliffs, NJ: Prentice-Hall, 1996 [Google Scholar]