Abstract
Lung development requires coordinated signaling between airway and vascular growth, but the link between these processes remains unclear. Mammalian target of rapamycin complex-1 (mTORC1) can amplify hypoxia-inducible factor-1α (HIF-1α) vasculogenic activity through an NH2-terminal mTOR binding (TOS) motif. We hypothesized that this mechanism coordinates vasculogenesis with the fibroblast growth factor (FGF)-10/FGF-receptor2b/Spry2 regulator of airway branching. First, we tested if the HIF-1α TOS motif participated in epithelial-mesenchymal vascular signaling. mTORC1 activation by insulin significantly amplified HIF-1α activity at fetal Po2 (23 mmHg) in human bronchial epithelium (16HBE14o-) and induced vascular traits (Flk1, sprouting) in cocultured human embryonic lung mesenchyme (HEL-12469). This enhanced activation of HIF-1α by mTORC1 was abolished on expression of a HIF-1α (F99A) TOS-mutant and also suppressed vascular differentiation of HEL-12469 cocultures. Next, we determined if vasculogenesis in fetal lung involved regulation of mTORC1 by the FGF-10/FGFR2b/Spry2 pathway. Fetal airway epithelium displayed distinct mTORC1 activity in situ, and its hyperactivation by TSC1−/− knockout induced widespread VEGF expression and disaggregation of Tie2-positive vascular bundles. FGF-10-coated beads grafted into fetal lung explants from Tie2-LacZ transgenic mice induced localized vascular differentiation in the peripheral mesenchyme. In rat fetal distal lung epithelial (FDLE) cells cultured at fetal Po2, FGF-10 induced mTORC1 and amplified HIF-1α activity and VEGF secretion without induction of ERK1/2. This was accompanied by the formation of a complex between Spry2, the cCBL ubiquitin ligase, and the mTOR repressor, TSC2, which abolished GTPase activity directed against Rheb, the G protein inducer of mTORC1. Thus, mTORC1 links HIF-1α-driven vasculogenesis with the FGF-10/FGFR2b/Spry2 airway branching periodicity regulator.
Keywords: lung development, epithelium, mesenchyme, hypoxia, rheb, tuberous sclerosis complex
hypoxia is an important physiological context for fetal development, but its role in organ morphogenesis is not well understood. In the embryonic lung, low Po2 favors airway branching and bud formation (18, 19, 54), yet a critical morphogenic role for hypoxia is unlikely, as branched airway growth occurs at higher oxygen tensions (7, 37, 46, 49, 54). Vascular branching, by contrast, is almost completely dependent on the low Po2 of the fetal lung and is abolished by experimental increases in oxygen tension (1, 22, 54). Despite these different sensitivities to oxygen, airway and vascular systems develop in tandem to form an interwoven tubular network with a shared semifractal pattern of branching to the level of the respiratory acinus (57). Thus, although hypoxia is necessary for proper lung development, its effects are secondary to the signaling network that links airway and vascular growth together.
Airway branching morphogenesis begins by evagination of the laryngo-tracheal groove into the surrounding mesenchyme tissue. After dividing into two distal buds, a series of sequential branching events occurs to form the conductive airways of the respiratory tree. Outgrowth of the airways is induced by fibroblast growth factor-10 (FGF-10), which is transiently expressed in the mesenchyme tissue surrounding each prospective bud. This is believed to activate Ras/Raf/MEK1 signaling to the extracellular-signal regulated kinases 1 and 2 (ERK1/2) through its receptor, FGFR2b, expressed in the airway epithelium (7, 52, 53). Negative feedback of this pathway is mediated by Sprouty2 (Spry2), whose activity is induced as an additional, but time-delayed, response to FGFR2b/ERK1/2 activation (37, 53). Thus, the temporal, spatial, and concentration-dependent activation of FGFR2b by FGF-10 and inhibition by Spry2 control the length of each branch and the location of bifurcations. This mechanism acts as an airway branching periodicity regulator or “clock” that defines the semifractal branching pattern of the conducting airways in the lung (40, 55).
The mechanism that links vasculogenesis to this process remains unknown. The differentiation of mesenchyme progenitor cells into vascular tissue is initiated by the secretion of vascular endothelial growth factor (VEGF) from the airway epithelium. VEGF expression is regulated by hypoxia-inducible factors (HIFs), a family of transcription factors whose hypoxia-activated α-subunit is required for transcriptional activity. Three HIF-α isoforms are expressed in the fetal lung (21, 47). HIF-1α occurs in the branching epithelium, and its homozygous knockout in mice causes severe vascular defects and death at embryonic day (E) 10.5 (29). HIF-2α occurs in the epithelium and the interstitium. Its knockout does not affect the growth of the conducting vasculature but produces vascular defects during alveolar septation (10). HIF-3α is a truncated isoform that lacks the COOH-terminal transactivation domain present in HIF-1α and 2α and so may act as a repressor of these transcription factors (6). Although it is present in the developing lung, its function remains unknown.
Of these, HIF-1α plays the major role in early pulmonary vasculogenesis and so essentially defines the growth of the conducting vasculature (21, 29, 47). HIF-1α activity is regulated by O2-dependent prolyl-hydroylase domain proteins (PHD1, 2, 3) and the asparagine hydroxylase factor inhibiting HIF (FIH-1) (reviewed in Ref. 28). In normoxia, these enzymes silence HIF signaling by catalyzing the hydroxylation of proline residues 402 and 564, and so allow the proteasomal degradation of HIF-1α by the von Hippel-Lindau E3 ubiquitin ligase. In addition, FIH-1 catalyzes the hydroxylation of asparagine 803 and blocks the interaction of the HIF-1α transactivation domain with the p300 transcriptional coactivator. In hypoxia, degradation of PHD proteins by another E3 ubiquitin ligase, SIAH (seven in absentia homolog), allows rapid stabilization, nuclear translocation, and transactivation of HIF-1α leading to the expression of hypoxia-regulated proteins (45).
PHD proteins critically regulate HIF-1–3α stability during lung development, as their inhibition augments HIF target gene expression and promotes blood vessel growth and formation of the blood gas barrier (2, 3, 22). This effect is so profound that vascular signaling and growth can be sustained in explants cultured in extreme hyperoxia [95% O2, (20)]. These studies fail to explain, however, the temporal and spatial regulation of HIF activity that would be necessary to form a branched vascular and airway tubule network. One possibility is that oxygen gradients radiating from the fetal pulmonary vasculature may result in subtle differences in PHD1–3 activity that coordinate HIF transcription between the two systems. In the fetal lung, however, any such gradient would be shallow [∼15 mmHg (20 μM)]: difference between the Po2 of the umbilical vein (∼30 mmHg) and the Po2 of the amniotic fluid (∼15 mmHg; Ref. 31) and ∼10-fold below the Km O2 of the PHD enzymes (230–250 μM; Ref. 26). A second, more plausible, scenario suggests that morphogens that are transiently expressed in the growing tip of the airway bud locally regulate HIF-1α and vascular signaling. Thus, the low Po2 of the fetal lung maintains a baseline level of HIF-1α stability and activity that is regionally amplified by growth-regulating signals. Such events might be considered to confer contextual regulation over HIF proteins, directing their activity subsequent to PHD and FIH induction.
We have discovered that the mammalian target of rapamycin (mTOR), a nutrient-sensitive regulator of growth and differentiation, amplifies PHD/FIH-induced HIF-1α activity by interacting with a conserved mTOR signaling (TOS) motif within the HIF-1α PAS-A domain (33). This interaction, which requires the mTOR complex 1 (mTORC1) adaptor protein, raptor, promotes the binding of the p300 transcription initiation complex to the HIF-1α COOH-terminal transactivation domain (CTAD) and substantially amplifies HIF-1α-evoked expression of vascular genes such as VEGF. HIF2α lacks this TOS motif, and HIF-3α lacks the CTAD, thus the positive effect of this interaction on hypoxic gene expression is specific to HIF-1α. This complements a growing number of studies that show HIF-1α transactivation to be similarly regulated by other kinases (9, 20, 41, 48). Here, we aimed to determine if mTORC1 could, in principle, regulate HIF-1α and vasculogenic signaling in a cell line culture model of the lung epithelial-mesenchymal interface. We then investigated if this interaction influenced vasculogenesis in the fetal lung in vivo and whether it was subject to regulation by airway branching morphogens in primary cultures of fetal distal lung epithelium (FDLE). Our results demonstrate that FGF-10 induces mTORC1 activity via Spry2 in fetal airway epithelium and that this amplifies HIF-1α-evoked vascular signaling beyond the level induced by exposure to fetal Po2 alone. We postulate a model whereby mTORC1 coordinates HIF-1α vascular signaling with the FGF-10/FGFR2b/Spry2 airway branching periodicity clock.
MATERIALS AND METHODS
Chemicals and reagents.
Recombinant human FGF-10 was from R&D Systems (Abingdon, UK). Rapamycin, LY-294002, and U0126 were obtained from Merck Biosciences (Nottingham, UK). Antibodies against Flk1 (A-3), -Flt-1, and VEGF (A-20) were from Santa Cruz Biotechnology; Tie2 (ab24859) and Spry2 (ab50317) were from Abcam (Cambridge, UK); and S6K1, S6K1 phospho-T389, ERK1/2, ERK1/2 phospho-(T202/Y204, T183/Y185), TSC1, and TSC2 were from Cell Signaling Technology (Danvers, MA). Antibodies against epitope tags and all other reagents (unless stated) were purchased from Sigma-Aldrich (Dorset, UK).
Plasmids and molecular biology.
NH2-terminal flag-tagged pRK7-Rheb, pRK7-HA-HIF-1α, and pRK7-HA-HIF-1α(F99A) and the HIF-1α luciferase reporter gene, pHRE3-TK-GL2, were generated as described (33). pRL incorporating the minimal thymidine kinase (TK) promoter was from Promega. Kinase dead (D2357E) mTOR was kindly provided by Dr. Stuart Schreiber (Harvard Univ.), and pXJ40-FLAG-human(h)Spry2, pXJ40-FLAG-Spry2 (Y55F), and pXJ40-HA-Rheb were kindly provided by P. Yusoff and G. Guy (Proteos, Singapore).
Culture of cell lines.
The diploid human embryonic lung mesenchymal fibroblast (HELMF) cell line, HEL-12469, was obtained from the European Collection of Cell Cultures. An immortalized human bronchial airway epithelial cell line [16HBE14o-, termed HBE here (12)] was obtained from Dr. D. Gruenert, California Pacific Medical Center, San Francisco, CA. Both were routinely cultured in DMEM supplemented with Ham's F-12 nutrient mix (DMEM/F-12) and 8.5% FCS from Invitrogen (Paisley, UK) in a humidified atmosphere containing 21% O2 and 5% CO2. For coculture experiments, HELMF were seeded into Costar six-well culture dishes at a density of 5 × 105 cells/ml−1 and allowed to establish for 4 days before each experiment. HBE, seeded separately at a density of 2.5 × 105 cells·ml−1 onto 2.4-cm diameter Costar Transwell permeable supports, were otherwise treated identically. Sixteen hours before the start of each experiment, the cells were placed into serum-free MEM (SFM), and then the HBE and HELMF cells were combined under the experimental conditions described in each figure by placing Transwell filters into the wells containing the HELMF cells.
Primary culture of rodent fetal lung cells and organ explants.
FDLE were isolated from gestation day 19 rat fetuses as described previously (5, 32). All procedures conformed with the Animals (Scientific Procedures) Act, 1986, UK. FDLE were seeded in DMEM/F-12 containing 8.5% FCS onto Transwell permeable supports at a density of 5 mg of packed cell wt·ml−1, whereas fetal mesenchymal lung fibroblasts (FMLF) obtained as a byproduct of the FDLE isolation procedure were seeded in the same media at a density of 1 × 106 cells/well in six-well plastic dishes. Twenty-four hours after isolation, FDLE and FMLF were placed into DMEM/F-12 supplemented with 2% charcoal and ion-exchange resin-stripped serum (stripped serum) and placed at fetal Po2 until use.
Lung explants from gestation day 13 or 14 Sprague-Dawley rat fetuses or gestation day 12 Tie2-LacZ mouse fetuses were cultured in DMEM/F-12 supplemented with 2% stripped serum on Whatman polycarbonate filter supports and cultured at fetal Po2. For bead experiments, heparin-coated acrylic beads (Sigma) were prepared as described in Supplemental Fig. S1 and grafted into an incision in the peripheral mesenchyme (Supplemental material for this article is available online at the Journal website). In all cases, digital images were captured at the start and end of each experiment using a Leica DC 300F digital camera mounted on an MZ FLIII binocular microscope under identical magnification settings (Leica Camera, Milton Keynes, UK).
All cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. The oxygen content of the atmosphere was maintained at the level stated in each figure using a MACS VA500 Microaerophilic Workstation (Don Whitley Scientific, Shipley, West Yorkshire, UK).
Transfection and cell lysis.
HBE, HELMF, and FDLE were transfected at 60–80% confluency using Lipofectamine 2000 (Invitrogen, Paisley, UK) according to the manufacturer's instructions. To measure transfection efficiency, HBE and HELMF cells were transfected with a CMV-driven GFP reporter gene, and the number of GFP fluorescing cells was expressed as a proportion of DRAQ-5-stained nuclei. Transfection efficiency was routinely >95% for both HELMF and HBE. pRL was used to assess even transfection of FDLE and was not found to vary significantly between experiments. To create lysates, cells or explants were washed twice in PBS and then harvested in kinase lysis buffer containing Complete Protease Inhibitor Cocktail tablets (Roche) as described previously (33).
Measurement of HIF-1α transcriptional activity.
HIF-1α transcriptional activity in HBE was measured by luciferase assay as described previously (33). For HBE and HELMF cells, medium was replaced after transfection with fresh SFM, and cells were allowed to acclimatize to the respective experimental Po2 for 16 h. Subsequent treatments with insulin or rapamycin were then performed for 6 h before lysis. Experiments involving FGF-10 and/or Spry2 overexpression varied from this format as described in the legend to the relevant figures. In all cases, luciferase activity was corrected for protein content and expressed as change relative to the control group stated in the figure legend. HIF-1α transcriptional activity in FDLE was measured using the same procedure in conjunction with pRL (transfection ratio of 8:1, pHRE3-TK-GL2:pRL) to control for potential variation in transfection efficiency between preparations. These results were adjusted for protein content, normalized against Renilla expression, and expressed as fold change relative to the stated control group.
Quantitative PCR.
Detection of pluripotency marker genes in HELMF (Oct4, Sox2, and NANOG) and FGFR2b and Spry2 in HBE was performed as described in Supplemental Figs. S2 and S4.
Immunoprecipitation and Western blotting.
Total protein content of cell lysates was determined using the BioRad protein assay. For immunoprecipitations (IP), 150 μg of total protein was precleared for 1 h at 4°C in nonconjugated protein G-sepharose. IP was then performed for a further 3 h using cCBL or FLAG antibodies conjugated to protein G-sepharose beads as appropriate. Beads were then washed twice with buffer A [50 mM HEPES (pH 7.4), 100 mM NaCl, 10 mM MgCl2, 1 mg·ml−1 BSA, 1 mM dithiothreitol, and 1% Triton] and buffer B [50 mM HEPES (pH 7.4), 100 mM NaCl, 10 mM MgCl2, and 0.1% Triton] with Complete Protease Inhibitor Cocktail (Roche) included in all buffers. IP complexes eluting with the beads were dissolved in Laemmli sample buffer and processed by Western blotting. Western blots were performed on either cell lysates or IP samples by SDS-PAGE and transferred to nitrocellulose as described before (33). Even transfer onto nitrocellulose was confirmed using Ponceau S and by reference to actin.
VEGF ELISA.
ELISA for human or rat VEGF-A was performed as described before (29) using the DuoSet ELISA system from R&D Systems.
Sprouting assay.
HELMF were induced to form spheroids by nonadherent culture on soft agar. Spheroids of 400- to 600-μm diameter were placed into growth factor-reduced Matrigel (BD Biosciences), and HBE-conditioned medium was laid above the collagen dome. Images of spheroids were collected at 24-h intervals, and the compound sprout length was calculated at 48 h. Spheroid images were digitally separated into quadrants of equal size, and sprout length was measured using Scion Image 4.0.2 software at five evenly spaced intervals per quadrant. Measurements were pooled from four spheroids for each treatment, and each series of treatments was derived from independent experiments.
Measurement of airway surface complexity and epithelial cell height.
Airway surface complexity [ASC; perimeter (mm)/√area (mm2)] was determined from digital images by tracing the dimensions of the airway perimeter as described (Ref. 46 and Refs. therein). Epithelial height was measured from images of hematoxylin and eosin-stained sections generated from paraformaldehyde-fixed and paraffin wax-embedded gestation day 14 explants after 48 h of culture. Ten measurements were taken at even intervals around each airway cross-section using an Openlab camera-calibrated micrometer. Frequency distribution of epithelial cell height is represented as box plots and are compared with equivalent measurements from gestation day 16 fetal rat lungs.
Immunohistochemistry.
Paraformaldehyde-fixed fetuses of wild-type (WT) and homozygous TSC1−/− mice (E12.5) were kindly provided by Prof. Jeremy Cheadle (Univ. of Cardiff, Wales, UK). A Histostain Plus broad spectrum DAB kit was used to visualize antibody-specific staining. Sections incubated in preimmune serum were treated as controls. Antigen retrieval and quenching of endogenous peroxide activity were performed according to the manufacturer's instructions. Images were obtained using a Zeiss Axiovert microscope and Improvision 5 software.
Rheb-GAP assay.
The ratio of GTP-to-GDP-bound Rheb was determined in FDLE as described by Tee et al. (51). Briefly, FDLE transfected with FLAG-Rheb were incubated at fetal or ambient Po2 for 6 h in medium containing FGF-10 at the indicated concentrations and 125 kBq/cm−2 [32P]O4 in DMEM containing 2% charcoal-stripped medium. After lysis, FLAG-Rheb was immunoprecipitated, and the proportion of eluted GTP or GDP was determined by TLC on PEI cellulose using 0.5 MKH2PO4 (pH 3.3) as the mobile phase. The migration position of radiolabeled spots (Rf; ratio of spot migration:mobile phase migration) was visualized using a phosphorimager and were 0.65 (GTP) and 0.81 (GDP), matching equivalent values calculated from Tee et al. (51).
Statistics.
All values are expressed as means ± SE unless otherwise stated in the figure legend. The value of n for HBE/HELMF studies was determined as the number of experimental repeats on cells of different passage. For FDLEs and explants, n was determined as the number of pregnant dams used to generate independent preparations unless otherwise stated in the figure legend. In all cases, Student's t-test was used to assess significance between paired single treatments, and one-way ANOVA with post hoc significance assessed with the Tukey or Dunn test was used for multiple comparisons. A P value < 0.05 was considered to be statistically significant.
RESULTS
Part 1: Role of the HIF-1α TOS motif in vascular signaling.
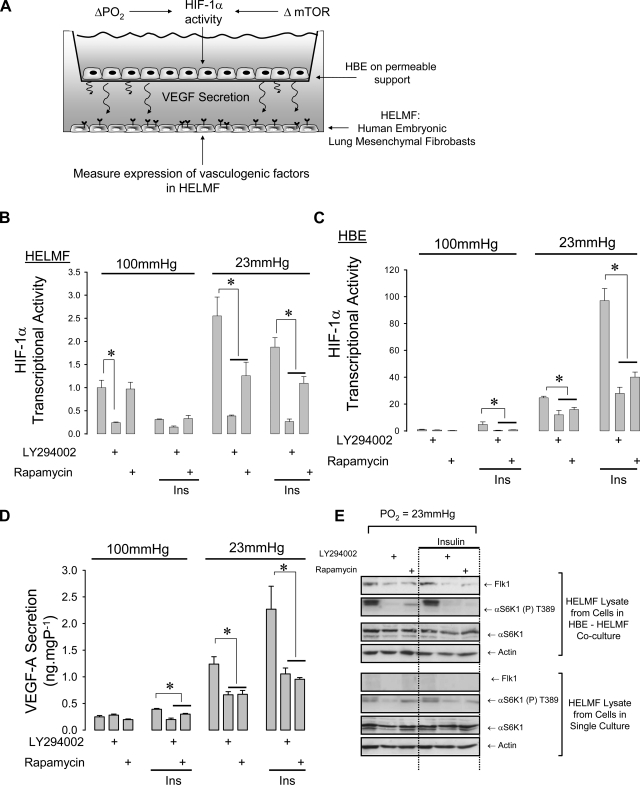
The initial goal of our study was to establish a culture model that could be used to explore the requirement for the HIF-1α TOS motif in vascular signaling. For ease of culture, transgene expression, and relevance to the airway environment, we selected HBE cells as a well-characterized model of the bronchial epithelium (12) and HELMF cells as a fetal lung-derived mesenchymal cell line with progenitor cell characteristics (constitutive expression of the pluripotency markers, Oct4, Sox2, and NANOG, under basal conditions. See Supplemental Fig. S2). A bicameral chamber was used to determine if HBE grown on a permeable support in the upper half of the chamber displayed vasculogenic activity (HIF-1α activity, VEGF release) when maintained at the Po2 of the fetal lung (23 mmHg). HELMF cells were maintained beneath the permeable support and probed for the appearance of vascular markers (Flk-1 expression, sprouting) (Fig. 1A). HIF-1α activity was determined by transfecting each culture with a HIF-driven reporter gene, and mTOR-dependent modulation of this response was determined by treating cells for 6 h with 20 nM insulin in combination with the selective mTOR inhibitor, rapamycin. LY-294002 (50 μM) was used to block the activity of PI3-kinase, which lies upstream of the mTORC1 complex. Concentration of insulin and LY-294002 were based on the effective doses shown to respectively induce and inhibit a PI3K-regulated Na+ current in H441 human bronchial epithelial cells (27). Choice of 100 nM rapamycin is based on the recommended concentration for these compounds to achieve selective and complete inhibition of mTORC1 (4). Culture of HELMF at fetal Po2 induced HIF-1α activity 2.5-fold above that at alveolar Po2. Insulin had no observable effect; however, rapamycin and LY-294002 each produced a significant blockade of this endogenous activity (Fig. 1B). The scope for HIF-1α activation at fetal Po2 (25-fold) was substantially greater in HBE compared with HELMF and was inhibited by ∼50% by either rapamycin or LY-294002. Insulin augmented this activity by a further fourfold. Rapamycin and LY-294002 suppressed this induced activity to the level observed without insulin treatment (Fig. 1C). VEGF-A secretion was significantly induced by culture at fetal Po2 and was augmented further by insulin. Rapamycin and LY-294002 lowered the insulin-evoked VEGF-A secretion to the spontaneous level induced by culture at fetal Po2 alone (Fig. 1D).
Fig. 1.
mTOR amplifies hypoxia-inducible factor-1α (HIF-1α) activity and vasculogenic signaling in HBE-HELMF cocultures. A: the bicameral culture method for examining epithelial-mesenchymal trophic signaling. Human bronchial epithelial cells (HBE) were cultured on permeable supports suspended above a monolayer of HEL-12469 human embryonic lung fibroblast cells (HELMF) in serum-free MEM (SFM). Single culture was performed by culturing HBE and HELMF in SFM separately. B: HIF-1α-driven luciferase reporter gene expression in HELMF at alveolar (100 mmHg) or fetal (23 mmHg) Po2. Values are expressed as fold change over untreated cells at alveolar Po2, n = 4, *P < 0.05. C: HIF-1α activity in HBE; n = 4, *P < 0.05. D: VEGF secretion in HBE is elevated at fetal Po2 and is inhibited by rapamycin and LY-294002. VEGF production was undetectable from HELMF cells (data not shown); *P < 0.05, n = 4. E: Flk-1 expression at fetal Po2 in HELMF cocultured with HBE (top) or alone (HELMF single culture, bottom). Representative of 3 independent experiments.
The VEGF-A receptor, Flk1, promotes angioblast differentiation and is a critical signaling component of epithelial-endothelial cross talk, which is necessary for proper branching morphogenesis (14). We therefore examined whether HELMF maintained in coculture with HBE displayed Flk-1 expression in response to culture at fetal Po2 or insulin (Fig. 1E). Both conditions were found to induce Flk-1 protein expression in HELMF, and this corresponded with increased αS6K1 (T389) phosphorylation, a specific substrate of mTORC1. Rapamycin and LY-294002 abolished this activation and led to a reduction in Flk-1 expression. HELMF cells subjected to the same treatment but in the absence of HBE did not express Flk-1 protein.
An intact HIF-1α TOS motif is required for vasculogenic trophic signaling between HBE and HELMF.
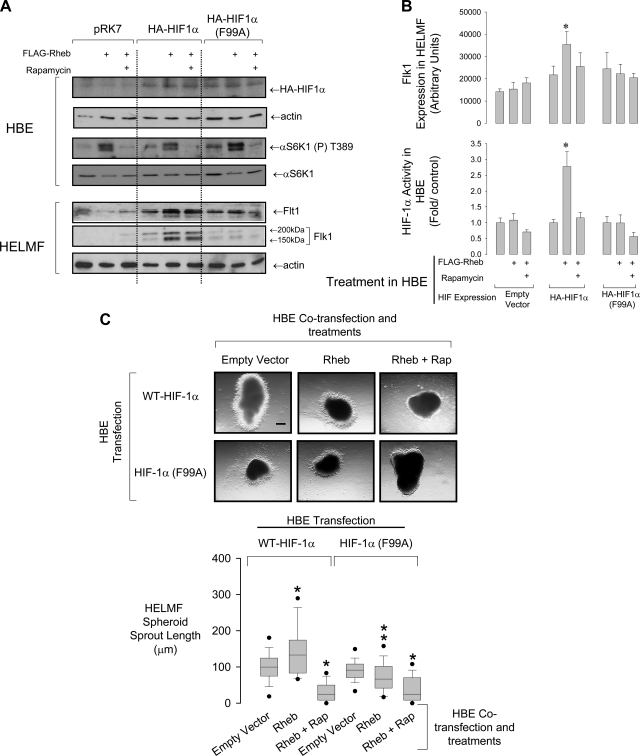
mTORC1 can amplify HIF-1α activity by interacting with a mTOR signaling (TOS) motif within the HIF-1α PAS-A domain. This effect is independent of the prolyl hydroxylase regulation of HIF-1α but promotes the binding of the p300 translation initiation complex to its COOH-terminal transactivation domain (33). We therefore determined if mutation of the first phenylalanine residue of the TOS motif (F99A) interfered with vasculogenic signaling from HBE to HELMF. Overexpression of the upstream activating G protein of mTOR, Rheb, together with rapamycin treatment were used to further specify observed effects to the mTOR pathway. Flk-1 expression and mTORC1 activity (αS6K1-T389 phosphorylation) was induced in HELMF exposed to conditioned medium from the HBE cells coexpressing WT HA-HIF-1α and Rheb (Fig. 2, A and B). Although this Flk-1 expression was insensitive to rapamycin, HELMF treated with conditioned medium from HBE expressing the HIF-1α TOS mutant failed to express Flk-1 despite mTORC1 activation by Rheb. Reporter gene assays conducted in parallel showed that Rheb augmented HIF-1α activity in cells overexpressing WT but not TOS-mutant HA-HIF-1α.
Fig. 2.
The HIF-1α F99A TOS mutation suppresses trophic signaling between HBE and HELMF. A, top blots: conditional phenotype of HBE cells used to generate conditioned medium for HELMF culture. Rapamycin (100 nM) was used to suppress mTOR signaling. Bottom blots: expression of Flt1 and Flk1 [nascent (150 kDa) and glycosylated (200 kDa) forms shown] in HELMF following 18-h culture in HBE-conditioned medium. Blots are representative of 4 experiments. B: top histogram shows the actin-corrected change in Flk1 protein abundance in HELMF cultured in HBE-conditioned SFM. *P < 0.05, n = 5. Bottom histogram shows the activity of HIF-1α in the HBE used to generate the conditioned medium. Activity is expressed as fold change over empty vector (pRK7) control. *P < 0.05 relative to control group; n = 5. C: sprouting from HELMF spheroids in Matrigel following 48-h culture in medium obtained from the HBE in A. Scale bar: 182 μm. Box plots at bottom show the data distribution of sprout length. (From bottom: 5th percentile, smallest nonoutlier, lower quartile, median, top quartile, 95th percentile. Bars indicate the data range.) *P < 0.05 relative to wild-type transfected control group; **P < 0.001 relative to wild-type rheb-transfected group; n = 4. One-way ANOVA on ranks with Dunn's post hoc test.
Sprouting assays were used to measure the potentiation of vascular outgrowth from HELMF spheroids induced by conditioned medium from HBE treated as in Fig. 2A. Sprout branch length was significantly elevated from spheroids exposed to medium from HBE transfected with both WT-HIF-1α and Rheb (Fig. 2C). Exposure to medium from HBE expressing the HIF-1α TOS mutant suppressed sprouting, and, in both cases, was almost completely abolished by rapamycin.
Part 2: mTOR-dependent regulation of vascular signaling in the fetal lung.
Experiments in Part 1 establish that HBE/HELMF cells can be used to explore vascular signaling in vitro and establish the principle that mTORC1 positively modulates HIF-1α-mediated vasculogenesis through the NH2-terminal TOS motif. We next explored the role of mTORC1 in fetal lung development and asked if it could act as a critical point of coordination between vascular signaling with the FGF-10/FGFR2b/Spry2 airway branching periodicity regulator.
mTORC-1 is active in airway epithelium and participates in airway morphogenesis.
The pattern of mTORC1 activity in gestation day 19 fetal rat lungs in situ was determined from the distribution of αS6K1-T389 phosphorylation. Nonimmune serum was used as a control. Intense staining was apparent in the nascent airway endodermal epithelium and in the mesothelium lining the lung periphery (Fig. 3). αS6K1-T389 phosphorylation was not uniform around the endodermal tubes appearing more intense within the airway tip region.
Fig. 3.
mTORC1 activity in the pseudoglandular rat lung in vivo. Gestation day 19 fetal rat lungs were fixed and stained with a phospho-antibody against the mTORC1 substrate, p70S6K1-T389. A: low-power (×40) image of fetal lung in situ. p70S6K-T389 phosphorylation is apparent in the nascent airway epithelium (brown stain). B: control section treated with nonimmune IgG at same magnification. C: mid-power (×200) image showing airway (a), mesenchyme (m), and endothelial tube (et) structures. Note apparent polarization of mTORC1 activity around the tube (white arrows). D: high-power (×400) image showing mTOR activity extending laterally from the epithelium indicating activity within a possible branching node. Localized activity in cells of the mesenchyme is evident (black arrows). E: high mTOR activity in the mesothelial lung lining (mth), a site of FGF9 expression (56). White arrow indicates regional activity in an epithelial lateral branch node. F and G: ×200 and ×400 images of sections treated with nonimmune IgG.
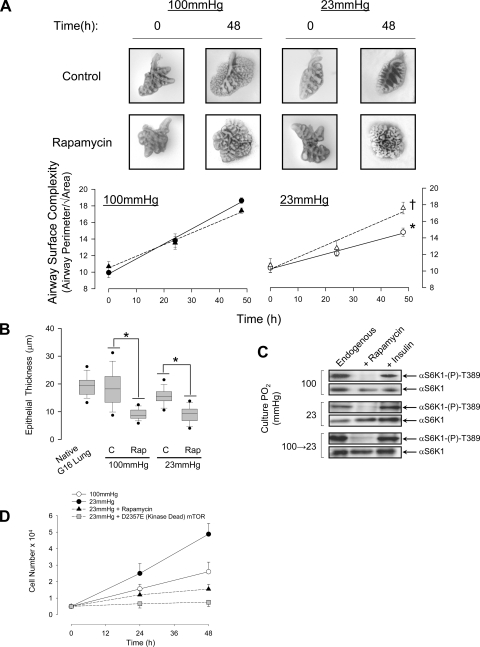
These results suggest that endogenous factors induce mTORC1 in the endodermal epithelium of the developing airway and that mTORC1 may participate in lung morphogenesis. This was confirmed in isolated pseudoglandular stage (gestation day 14) rat lung explants where inhibition of endogenous mTORC1 activity with rapamycin (0.1 μM) significantly augmented airway epithelial surface folding complexity (ASC, a measure of branching tendency) and promoted epithelial flattening (an indicator of epithelial cell maturation) at fetal Po2 (Fig. 4, A–C). The rise in ASC appeared to be accompanied by a loss of mesenchyme mass; therefore, we examined the effect of Po2 and mTOR activity on rat fetal mesenchymal lung fibroblast (FMLF) proliferation. Growth rate was greater at fetal compared with alveolar Po2 and was inhibited by either rapamycin or transfection with a kinase dead (D2357E) mutant of mTOR (Fig. 4D) suggesting an additional role for both hypoxia and mTOR in regulating mesenchyme cell density.
Fig. 4.
Endogenous mTOR activity is critical for morphogenesis of fetal rat lung explants. A: influence of culture Po2 and rapamycin on airway surface complexity [ASC; perimeter/√airway area (46)] in lung explants from gestation day (G) 14 fetal rats. Circles, Control; triangles, 0.1 μM rapamycin. *P < 0.05 23 mmHg control vs. 100 mmHg control, n = 15; †P < 0.05 rapamycin vs. control at 23 mmHg, n = 15. B: box plots showing data distribution of epithelial thickness in G14 explants maintained for 48 h in the presence or absence of rapamycin (0.1 μM) at the indicated Po2. Measurements from G16 lung in situ are included for comparison (from bottom: 5th percentile, smallest nonoutlier, lower quartile, median, top quartile, 95th percentile. Bars indicate the data range). *P < 0.05, n = 4. One-way ANOVA on ranks with Dunn's post hoc test. C: endogenous activity of mTOR [αS6K-(P)-T389] in explants cultured in SFM for 48 h at fetal or alveolar Po2. Insulin (20 nM) was used as a positive control, and rapamycin (0.1 μM) was used to block mTOR activity. Representative of 4 replicates. D: growth rate of fetal rat lung fibroblasts determined over 48 h at fetal or alveolar Po2 in the presence of rapamycin or following overexpression of a kinase dead (D2357E) mutant of mTOR. *P < 0.05 relative to 23 mmHg treatment, n = 6.
FGF-10 induces discrete expression of Tie2 in fetal mouse lung explants.
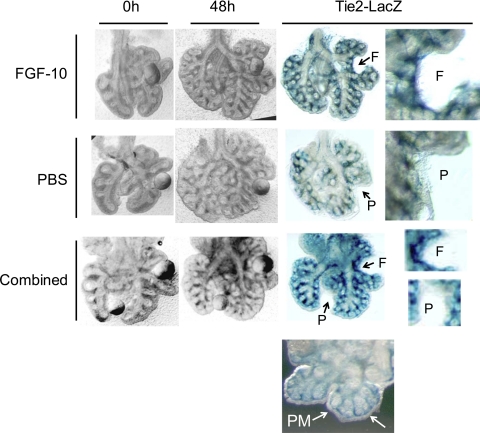
These experiments demonstrate that mTORC1 participates in lung morphogenesis, and our experiments in HBE/HELMF predict that growth factor activation of mTORC1 will induce vasculogenic signaling in the fetal lung. We therefore explored whether FGF-10, a central inducer of airway outgrowth, induced vascular differentiation in murine fetal lung organ explants. Beads soaked in FGF-10 (Supplemental Fig. S1) were grafted into the peripheral mesenchyme of gestation day 12 fetal lung explants from mice bearing a Tie2-driven LacZ reporter gene. Explants were cultured at fetal Po2 for 48 h and then stained to reveal the pattern of Tie2 gene activation. This expression was absent from the peripheral mesenchyme surrounding the distal airway tips but was evident around the proximal airway and in the nexus of airway branches. FGF-10-coated beads grafted into the peripheral mesenchyme induced Tie2 gene activation around the bead, which was not observed with PBS-soaked control beads (Fig. 5). Note that the distortion of airway and peripheral mesenchyme tissue around the bead position indicates intimate contact between bead and tissue. This establishes the principle that a localized FGF-10 signal can promote vascular differentiation of mesenchyme tissue at fetal Po2.
Fig. 5.
Vascular differentiation is induced by FGF-10 in E12 fetal murine lung explants. Beads coated in FGF-10 or PBS (control) were grafted onto E12 murine fetal lung explants from Tie2-LacZ transgenic mice. Explants were maintained at fetal Po2 for 48 h, fixed, and then stained for LacZ reporter expression. Grayscale images on left show bead location and morphogenesis over 48 h; color images on right show subsequent expression of Tie2-LacZ reporter gene with bead excised. Bead position is indicated as: F (FGF-10), P (PBS). Combined treatment indicates PBS and FGF-10-coated beads grafted onto the same explant. Note lack of Tie2-LacZ expression in the peripheral mesenchyme (PM) shown in bottom image. Findings shown in these images were replicated across explants isolated from 3 independent litters.
Homozygous knockout of TSC1−/− causes aberrant vascular growth in the fetal lung in situ.
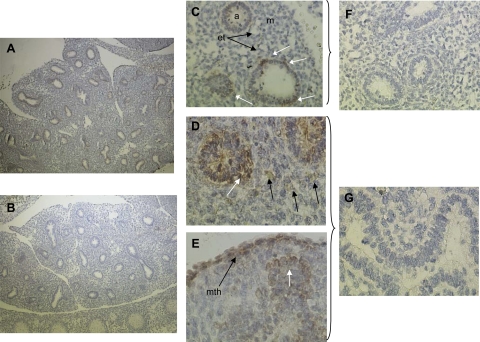
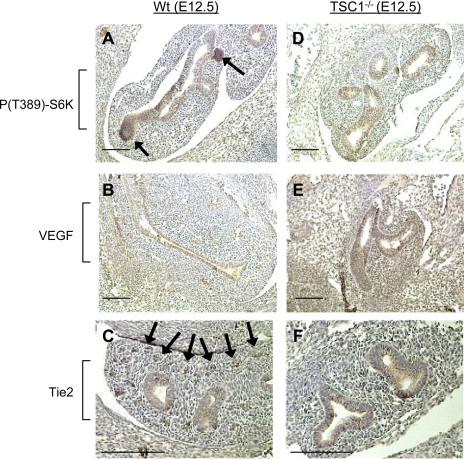
TSC1−/− produces a hyperactive mTOR phenotype that is fatal in mice from gestation day 13.5 (58). To determine if mTOR hyperactivity produces a hypervascular lung, we performed immunohistochemistry on fixed sections of gestation day 12.5 from WT and TSC1−/− mouse fetuses. As with pseudoglandular stage rat lung, mTORC1 phosphorylation of αS6K1-T389 in WT fetal lungs was distinct in airway epithelium and was particularly pronounced at the growing tips of airway buds. VEGF staining was confined to airway epithelium, and Tie2 angiopoietin receptor expression was confined to discrete regions of mesenchymal tissue corresponding to nascent vascular bundles (Fig. 6, A–C). TSC1−/− mouse lungs also displayed apparently normal airway growth; however, mTORC1 phosphorylation of αS6K1-T389 was homogenously distributed in the airway epithelium. Similarly, VEGF expression was widely distributed throughout the lung and was accompanied by disaggregation of Tie2-positive vascular bundles (Fig. 6, D–F).
Fig. 6.
Knockout of the upstream repressor of mTOR, TSC1, alters vasculogenic signaling in the murine lung. Wild-type (A–C) and TSC1−/− (D–F) embryonic day (E) 12.5 murine fetuses were sectioned and stained for mTOR activity [S6K1 (T389) phosphorylation], VEGF, and Tie2 expression. Arrows in A indicate raised mTOR activity at sites of airway epithelial outgrowth in wild-type animals. Arrows in C indicate Tie2 receptor expression corresponding to nascent vascular bundles. Images are representative of 4 fetuses from independent litters. Bar = 200 μm.
FGF-10 regulates HIF-1α activity via mTORC1 in FDLE.
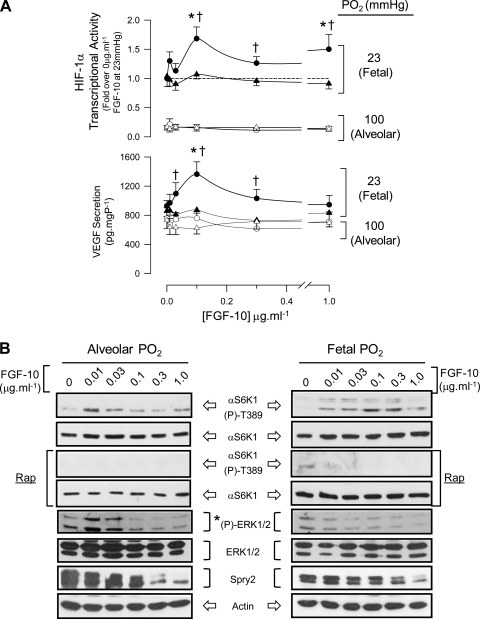
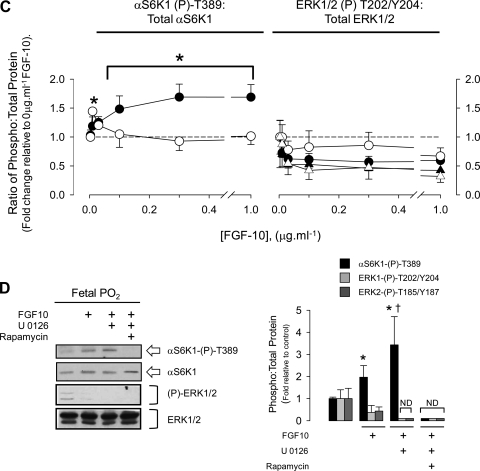
Having established that endogenous factors sustain mTORC1 activity in the fetal lung and that localized release of FGF-10 promotes vascular differentiation in mesenchyme, we next tested the hypothesis that FGF-10 regulates mTORC1-dependent HIF-1α and vascular signaling using primary cultures of FDLE at either fetal or alveolar Po2. By culturing FDLE on permeable supports, we aimed to maintain apical/basolateral polarization of these cells as shown before (5, 32, 42). FGF-10 produced a dose-dependent, rapamycin-sensitive activation of HIF-1α and VEGF secretion at fetal Po2, which was absent at alveolar Po2 (Fig. 7A). mTORC1 activity was dose dependently induced by FGF-10 at fetal Po2 but was not sustained at FGF-10 concentrations ≥0.01 μg·ml−1 at alveolar Po2 (Fig. 7, B and C). As FGF-10 is reported to induce ERK1/2 signaling in adult murine airway epithelia (53) and can, in principle, activate mTORC1 by repressing TSC1/2, we measured ERK1/2 phosphorylation in parallel with mTORC1. Basal ERK1/2 phosphorylation was low in FDLE, and enhanced blot images showed it to be further suppressed by FGF-10 (Fig. 7, B and C). ERK1/2 activity was not required for mTORC1 activation by FGF-10 at fetal Po2 as S6K1-T389 phosphorylation was sustained in the presence of the MEK1 inhibitor, U0126 (Fig. 7D). Notably, the low endogenous activity of ERK1/2 in FDLE was echoed in perinatal rat lung, which showed little measurable ERK1/2 activity in gestation days 19 and 21 homogenates but a significant activation during the early postnatal period (Supplemental Fig. S3).
Fig. 7.
FGF-10 positively modulates HIF-1α activity via mTORC1 in polarized, primary FDLE cells. A: top graph shows HIF-1α activity measured from FDLE transfected with pHRE3-TK-GL2 and pRL (8:1) and cultured at fetal (23 mmHg; closed symbols) or alveolar (100 mmHg; open symbols) Po2 for 6 h in the presence of increasing concentrations of FGF-10. Rapamycin (0.1 μM; triangles) was added to a parallel set of cells to determine mTORC1-sensitive HIF-1α activity. Values are normalized to 0 μg·ml−1 FGF-10 treatment at fetal Po2. Bottom graph shows the level of VEGF secreted into the medium beneath the permeable support from the same cells. *P < 0.05 relative to control at fetal Po2; †P < 0.05 relative to paired rapamycin treatment, n = 5. B: representative blots showing mTORC1 [αS6K-(p)-T389 phosphorylation] and ERK1/2 activation (T202/Y204 and T185/Y187 phosphorylation) and Spry2 protein abundance measured in samples from A. *Contrast is enhanced separately from total ERK blot to show decline in ERK1/2 phosphorylation with FGF-10. C: densitometry of pooled experimental blots represented in B. Open/closed symbols are as described in A; triangles represent ERK2 values. *P < 0.05 relative to control, n = 4. D: the MEK1 inhibitor U0126 (10 μM) does not prevent mTORC1 activation (S6K1-T389 phosphorylation) by 0.1 μg/ml−1 FGF-10 in FDLE at fetal Po2. Rapamycin (0.1 μM) was added to show inhibition of mTORC1. Histogram (right) shows pooled densitometry data for mTORC1 activity and ERK1/2 phosphorylation. *P < 0.05 relative to control; †not significantly different from FGF-10 treatment. ND, not detectable; n = 5.
Parallel studies in HBE cells revealed a similar induction of HIF-1α activity by FGF-10, which was significantly inhibited by rapamycin but declined at the highest concentrations of FGF-10 (Supplemental Fig. S4). In contrast to our findings in FDLE, the induction of mTORC1 by FGF-10 was accompanied by a transient activation of ERK1/2 and was significantly repressed by U0126 (Supplemental Fig. S5).
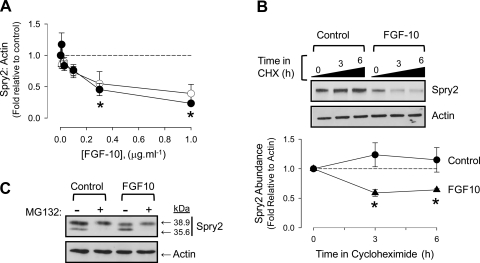
FGF-10 destabilizes Spry2 and promotes interaction between cCBL and TSC2 in FDLE.
Current models of lung development suggest that FGF-10-FGFR2b signaling is antagonized by Spry2 and that this mechanism regulates airway branching periodicity. Given the lack of apparent control of mTORC1 by ERK1/2 in FDLE, we focused on the role of Spry2 as a potential regulator of the mTORC1-HIF-1α interaction. Spry2 was resolved in FDLE as a double band with masses of 38.9 (full length) and 35.6 kDa (truncated) using an NH2-terminal epitope antibody (Abcam, ab50317). FGF-10 induced a dose-dependent degradation of full-length Spry2 at both alveolar and fetal Po2 that correlated with the rise in mTORC1 activation, HIF-1α reporter gene expression, and VEGF production (Figs. 7, A and B, and 8A). The cleavage of full-length Spry2 occurred by proteolysis as blockade of de novo synthesis using cycloheximide led to its clearance within 3 h of addition of 0.1 μg/ml−1 FGF-10. This cleavage was proteasomal as addition of MG132 abolished the truncated form of Spry2 and stabilized the full-length form in the presence of FGF-10 (Fig. 8, B and C).
Fig. 8.
FGF-10 destabilizes Spry2 in FDLE. A: abundance of Spry2 declines with increasing FGF-10 concentration at fetal (closed circles) and alveolar (open circles) Po2. Densitometry performed on full-length Spry2 in ratio to actin. *P < 0.05 relative to control, n = 4. B: Spry2 becomes unstable in the presence of FGF-10. FDLE were treated with 100 μM cycloheximide (CHX) and 0.1 μg·ml−1 FGF-10 as indicated at fetal Po2. Representative blot is accompanied by graph showing full-length Spry2 abundance measured in ratio to actin. Circles, control; triangles, 0.1 μg·ml−1 FGF-10. *P < 0.05, n = 4 relative to time 0. Error bars may be within symbols. C: Spry2 cleavage is proteasomal. FDLE were treated with 10 μM MG132 and 0.1 μg·ml−1 FGF-10 at fetal Po2 for 3 h as indicated. Proteins were separated by 10% SDS-PAGE to resolve both full-length and cleaved Spry2 product. Blot is representative of n = 3.
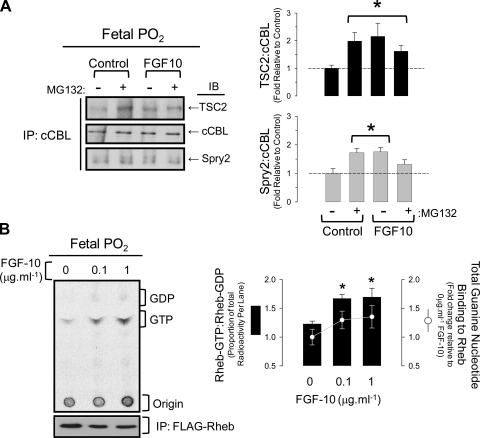
To explain the association between Spry2 clearance and mTORC1 activation, we investigated whether repression of mTORC1 by TSC1/2 was dependent on cCBL, an E3 ubiquitin ligase that binds to the phosphorylated Y55 residue of Spry2 (17). TSC2 consistently immunoprecipitated with cCBL, and this association increased significantly on exposure to 0.1 μg/ml−1 FGF-10 and the proteasomal inhibitor, MG132. Surprisingly, Spry2 interaction with cCBL also increased with FGF-10 treatment, suggesting that TSC2 preferentially associates with the intact Spry2-cCBL complex (Fig. 9A). When in complex with TSC1, TSC2 inhibits mTORC1 assembly by acting as a GTPase activating protein (GAP) towards Rheb; therefore, we determined if the interaction between TSC2 and cCBL/Spry2 corresponded with a decrease in Rheb-GAP activity and a subsequent rise in GTP-bound Rheb (Fig. 9B). FGF-10 tended to increase guanine nucleotide binding to FLAG-Rheb expressed in FDLE at fetal Po2; however, the proportion of radioactivity eluting with GTP was significantly greater at 0.1 and 1 μg·ml−1 FGF-10 compared with controls and is consistent with decreased TSC1/2 GAP activity.
Fig. 9.
Spry2 associates with cCBL and TSC2. A: immunoprecipitation (IP) of cCBL from FDLE at fetal Po2 treated with FGF-10 (0.1 μg·ml−1) or MG132 (10 μM) as indicated. Immunoblots (IB) were performed to detect TSC2, cCBL, and Spry2. Histograms on right show ratio of TSC2 or Spry2 to cCBL. *P < 0.05 relative to control, n = 5. B: effect of FGF-10 on TSC1/2 GAP activity towards the Rheb guanine nucleotide binding protein in FDLE at fetal or ambient Po2. TLC phosphorimage shows resolution of 32P-GTP and -GDP immunoprecipitating with FLAG-Rheb in cells treated with FGF-10. Immunoblot beneath shows even recovery of FLAG-Rheb by IP. Histogram shows ratio of GTP:GDP as a percentage of total radioactivity eluting with Rheb. The superimposed line graph shows fold change in total [32P]-labeled guanine nucleotide phosphates eluting with Rheb, *P < 0.05 relative to control at fetal Po2, n = 4.
FGF-10 and Sprouty2 regulate HIF-1α activity via mTORC1 in HBE.
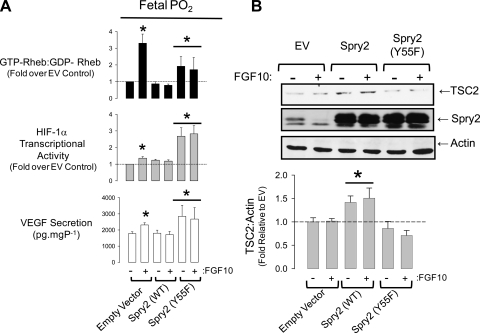
The preceding results are perplexing because they suggest that Spry2 cleavage is necessary for mTORC1 activation but that FGF-10 promotes a stable interaction between Spry2, cCBL, and TSC2. To discriminate between these outcomes, we reasoned that overexpression of WT Spry2 would quench the cleavage of Spry2 by FGF-10 and thus abolish mTORC1 and its induction of HIF-1α and VEGF secretion. Conversely, overexpression of a mutant form of Spry2, which cannot interact with cCBL [Spry2(Y55F); Ref. 17], would promote cCBL-mediated clearance of TSC2 and thus Rheb-GTP binding, HIF-1α activity, and VEGF expression. FDLE transfected with empty vector (EV) alone displayed Spry2 clearance in response to 0.1 μg·ml−1 FGF-10, which corresponded with increased Rheb-GTP binding, HIF-1α activation, and VEGF production (Fig. 10). Overexpression of WT-Spry2 sustained Spry2 abundance in the presence of FGF-10, prevented Rheb-GTP binding, and did not augment HIF-1α activity or VEGF secretion. Cells transfected with the Spry2(Y55F) mutant, however, showed marked induction of Rheb-GTP binding, HIF-1α activation, and VEGF secretion. Densitometry revealed that although TSC2 abundance changed little with FGF-10 treatment in the EV-transfected cells, overexpression of WT-Spry2 significantly induced TSC2 stability, whereas Spry2(Y55F) promoted TSC2 clearance. These data provide independent confirmation that Spry2 regulates mTORC1 at the level of the TSC1/2 complex and that this regulation is mediated through the Y55 binding site for cCBL. Moreover, our data illustrate that this effect is sufficient to significantly alter vasculogenic signaling through HIF-1α and VEGF.
Fig. 10.
Overexpression of Spry2 in FDLE abolishes FGF-10-evoked Rheb-GTP binding and is relieved by mutation of the Spry2 (Y55) cCBL docking site. A: TSC1/2 GAP activity (ratio of GTP:GDP bound Rheb), HIF-1α activity, and VEGF secretion in FDLE transfected with empty vector, wild-type, or Y55F Spry2. For GAP assays, the transfection mix included FLAG-Rheb (ratio of 1:4 Rheb to test vector), whereas for HIF-1α activity assays and VEGF secretion, the transfection mix included pHRE3-TK-GL2 (1:4). All experiments were performed at fetal Po2. *P < 0.05 relative to EV control; n = 4 (Rheb GAP assay), n = 6 (HIF-1α luciferase and VEGF secretion). B: representative blot showing TSC2, Spry2, and actin expression levels. Note that the Spry2 blot shows bands for the endogenous full-length and cleaved forms of Spry2 in EV lanes, which are superimposed by overexpressed Spry2 in other lanes. Histogram shows TSC2 abundance corrected for actin. *P < 0.05 relative to respective EV and Spry2 (Y55F) treatment, n = 6 independent transfections.
DISCUSSION
mTOR complex 1 is a central regulator of protein synthesis and cell growth and so acts as a hub where nutrient, metabolic, hormonal, and cytokine signals are integrated towards a defined pattern of growth. In mice, mTOR expression arises at E8.5 in the mid-thoracic region, coinciding with the initiation of lung development. Its inhibition by mutation or rapamycin treatment is lethal by E12.5 and is linked to forebrain defects, loss of somites, and an inability to turn around the embryonic axis (25). Ontogenic studies of mTOR function in rat fetuses show its activity is high during the early stages of development, declining towards term, but rebounds during the early neonatal period and corresponds with proportionate changes in translation efficiency during gestation and birth (42, 43).
Our study aimed to determine how the activity of HIF-1α, a critical regulator of vasculogenesis, is coordinated with the signals that control airway morphogenesis. We show that in both immortalized adult and primary fetal lung epithelia, exposure to fetal Po2 induces the activity of HIF-1α and VEGF. This is markedly amplified by growth-inducing factors such as insulin and FGF-10 and is sensitive to mTORC1 inhibition by rapamycin. In HBE cells, we demonstrate that mTORC1-dependent regulation of HIF-1α via the NH2-terminal TOS motif is sufficient to induce the appearance of vascular traits in cocultured mesenchyme cells (Figs. 1 and 2). In FDLE primary cultures, we show that stimulating this interaction using FGF-10, a critical initiator of airway budding, induces VEGF secretion and promotes localized differentiation of mesenchyme into vascular tissue in fetal lung explants. Although our study did not demonstrate that endogenous FGF-10 induces mTORC1 in fetal airway epithelium in situ, we do show mTORC1 activity is present in developing airway and that its hyperactivation strongly induces VEGF expression in fetal lung. Taken as a whole, these data establish that mTORC1 is a potent positive modulator of epithelial/mesenchymal vascular signaling that has the potential to coordinate HIF-1α activity with FGF-10, a growth factor that controls airway branch outgrowth.
These data imply that mTORC1 activity is insensitive to the low Po2 of the fetal environment. In tumor cell lines, hypoxic induction of HIF-1α regulated genes such as Redd1, Redd2 (8, 11), and Bnip3 (35) repress mTORC1 activity resulting in a suppression of tumor growth. Of these genes, Redd1 is expressed in fetal distal lung epithelial cells; however, it does not appear to mediate hypoxic repression of mTOR in these cells (42). We used immunohistochemistry to determine the distribution pattern of αS6K1-T389 phosphorylation as a readout of mTORC1 activity in pseudoglandular stage fetal rat and mice lungs in vivo. Our data show that mTORC1 activity was evident in airway endodermal growth buds and in the mesothelium lining the periphery of the lung, a site of FGF-9 expression (56) (Figs. 3 and 5). Experiments in isolated fetal rat lung explants, cultured at the Po2 of the fetal lung, demonstrate that this activity was necessary for balanced airway growth because exposure to rapamcyin produced significant morphological changes including accelerated airway branching (raised ASC in Fig. 4), epithelial flattening, and mesenchymal thinning. Overall, these data show mTOR signaling participates in tissue-specific growth events despite the relative hypoxia of the fetal environment.
This raises the question of whether mTOR is involved in controlling cell pluripotency/differentiation in the developing lung. In cancer, mTOR hyperactivation sustains cell proliferation and represses differentiation by positive induction of the Notch signaling pathway (36). Elsewhere, the role of mTOR in cell differentiation appears to be quite specific to cell type. For example, rapamycin promotes terminal differentiation of vascular smooth muscle cells (39) but maintains pluripotency of bone marrow-derived mesenchymal stem cells (24). Rapamycin also abolishes transforming growth factor-β-evoked increases in cell size and migration/invasion during epithelial-to-mesenchymal transition in murine mammary epithelial cells (30). In the developing lung, mTOR-directed translation is active during early and mid-stages of development and declines in late gestation, but peaks again on postnatal day 1 (42, 43). This late-gestation decline in translation efficiency is accompanied by a switch to mTOR-independent transcript expression involving genes linked to the development of immunity, antioxidant defense, and the acquisition of a terminally differentiated epithelial phenotype [e.g., SCGB3A1, (44)] and may be necessary to prepare the lung for air breathing and exposure to airborne pathogens.
mTOR is recognized as an important inducer of vascular growth by modulating angiogenic signaling as well as endothelial cell proliferation and differentiation (reviewed in Ref. 34). To determine if mTOR signaling was necessary for vasculogenesis in the fetal lung in vivo, we examined how targeted knockout of the upstream repressor of mTOR, TSC1, altered the distribution and expression of VEGF and the angiopoietin receptor, Tie2. Mice lacking the TSC1 allele display constitutively active mTOR activity, develop exencephaly and abnormal vacuolarization of myocardial cells in utero, and fail to develop beyond E13 (58). We found that airway growth proceeds up to at least E12.5 in TSC1−/− fetal lungs and that gross airway morphology (epithelial thickness, airway patency, mesenchyme thickness) differs little from age-matched WT lungs (Fig. 6). However, mTOR activity appeared to be uniform in the airway epithelium of TSC1−/− lungs and did not show the distinct localization at growth tips observed in WT lungs. TSC1−/− lungs also showed excessive staining for VEGF and an absence of discretely organized Tie2-positive vascular bundles around the airways. VEGF is a HIF-regulated target gene, and its raised expression in TSC1 knockout mouse lungs is consistent with our observations in Figs. 1, 2, and 7 that mTOR activation amplifies HIF-1α transcriptional activity and VEGF expression. The reason for the decline in coordinated vasculogenesis, which follows, may arise from dysfunctional regulation of Tie2 signaling caused by hyperexpression of VEGF. Tie2 and its ligands, angiopoietin 1 and 2 (Ang1 and 2), control the proliferation and maturation of nascent blood vessels. Proteolytic cleavage of Tie2 results in the shedding of a truncated soluble form of this receptor, sTie2, which is capable of binding Ang1 and 2 and so competitively inhibits the uncleaved membrane-bound Tie2 receptor. VEGF accelerates the cleavage and shedding of sTie2 and so its elevated expression decreases Tie-2-dependent blood vessel wall assembly but promotes the proliferation of vascular endothelial cells (16).
The final part of this study determined how mTORC1-dependent regulation of HIF-1α and VEGF secretion is linked to the FGF-10/FGFR2b/Spry2 pathway, a central regulator of airway outgrowth and branching. FGF-10-coated beads produced a localized increase in Tie2-promoted reporter gene expression in fetal murine lung explants. More detailed examination of the link between mTORC1 and FGF-10 in FDLE (Figs. 7–10) and HBE (Supplemental Figs. S4 and S5) showed a clear induction of HIF-1α activity and VEGF secretion with increasing FGF-10 dosage, which was suppressed to the basal hypoxic level by rapamycin. Given that FGF-10 has been reported to induce ERK1/2 activation and proliferation of airway epithelium (53), we anticipated that mTORC1 activation would arise by ERK1/2-dependent phosphorylation and subsequent inactivation of the TSC1/2 complex. We were therefore surprised to discover that FGF-10 actually suppressed ERK1/2 activity in FDLE cultured at fetal Po2 and that signaling through the mTORC1 pathway was unaltered by inhibition of the upstream kinase to ERK1/2, MEK1. Induction of ERK1/2 by FGF-10 was originally reported in an immortalized adult murine lung epithelial cell line (MLE15) that was cultured, unpolarized, at ambient Po2 (53). We repeated these experiments using unpolarized, immortalized adult lung-derived HBE cells and observed transient induction of ERK1/2 by FGF-10, which was not sustained with time or increased FGF-10 dosage (Supplemental Figs. S4 and S5). Although the magnitude of ERK1/2 signaling was greater at alveolar compared with fetal Po2, mTORC1 induction by FGF-10 was, nevertheless, ERK1/2 dependent, leading us to suggest that ERK1/2 signaling induces, but does not sustain, mTORC1 activity in HBE. These differences in FGF-10 signaling responses between FDLE and MLE15/HBE probably reflect variation in tyrosine kinase receptor expression linked to development, culture, and oxygen exposure; however, our finding that overall ERK1/2 activity is low in FDLE as well as in the late-term fetal lung, but is augmented by oxygen in HBE and increases in postnatal lung with the onset of breathing (Supplementary Data Figs. S3–S5), suggests that low oxygen tensions could have a role to play in constraining ERK1/2 signaling during fetal development. Numerous studies in embryonic stem cells have established that low oxygen tensions conserve pluripotency, and it is now shown that temporal activation of ERK1/2 induces differentiation to a defined phenotype (50). It is possible, then, that the constrained ERK1/2 activity we observed in fetal tissue may be critical to conserve populations of progenitor cells in the fetal lung.
Our studies thus highlight mTORC1, and not ERK1/2, as an important downstream target of FGF-10 in FDLE. Given that Spry2 antagonism of the FGF-10 receptor, FGFR2b, is believed to be an important regulator of airway branching periodicity, it follows that mTORC1 activity and its regulation of HIF-1α should be closely governed by Spry2. We found the activation of mTORC1 by FGF-10 was accompanied by three key events: 1) proteasomal cleavage of Spry2 to a truncated form, 2) formation of a stable complex between Spry2, cCBL, and TSC2, and 3) decreased TSC1/2 GAP activity towards Rheb as indicated by increased Rheb-GTP binding. Our Spry2 overexpression experiments demonstrated that enforced maintenance of Spry2 levels during FGF-10 treatment sustained TSC2 expression levels and suppressed Rheb-GTP binding, whereas overexpression of the Spry2 Y55F mutant, which lacks a functional cCBL binding domain (17), caused TSC2 clearance, Rheb-GTP binding, and induced HIF-1α activity and VEGF secretion. Together, these results suggest that Spry2 is a potent regulator of mTORC1 activation and that this regulation depends on an interaction between cCBL and TSC2.
Spry2 cleavage in hand with the formation of an apparently stable Spry2-cCBL-TSC2 complex accords with several proposed physiological roles for Spry2 (reviewed in Ref. 23). One scenario suggests that Spry2 sequesters cCBL activity. Cleavage of Spry2 by another ubiquitin ligase (SIAH2), or cCBL itself, targets downstream elements of RTK signaling pathways for proteasomal clearance. Two variations on this theme suggest that Spry2 performs either a targeting or adaptor-protein function that is necessary for cCBL to interact with its substrate proteins. Our finding that Spry2 was consistently cleaved by FGF-10 treatment and that relief of the Spry2-cCBL interaction by overexpression of the Spry2-Y55F mutant led to TSC2 clearance and Rheb-GTP binding suggests that a cCBL sequestering role for Spry2 is possible in unstimulated cells. However, immunoprecipitations performed against endogenous cCBL showed that FGF-10 treatment does not lead to the complete cleavage of Spry2 with some remaining associated with cCBL when in complex with TSC2, thus a targeting or adaptor-protein role is also possible. Spry2 binds protein phosphatases (SHP2; PP2A) that can mediate its own dephosphorylation as well as that of other phosphoproteins. As TSC2 is a substrate of both Shp2 and PP2A (38, 59) and Spry2 has been shown to associate with Shp2 in fetal airway epithelium (52), it is conceivable that the decline in Spry2 abundance with FGF-10 disrupts phosphatase activity directed towards TSC2 thereby promoting Rheb-GTP binding and mTORC1 activation (38, 59). Finally, we acknowledge that Spry2 can regulate HIF-1α independently of RTKs by targeting prolyl hydroxylases 1 and 3 for degradation via the Siah2 ubiquitin ligase (45); however, this mechanism was not investigated further here.
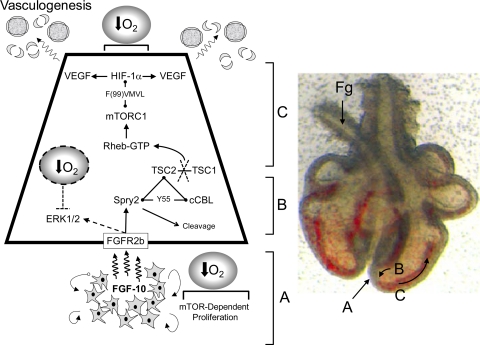
We have incorporated these results into a model that links vascular signaling with the FGF-10/FGFR2b/Spry2 signaling pathway in the developing fetal lung (Fig. 11). Our model differs from the classic view of branched airway morphogenesis in that we exclude a significant role for ERK1/2 and highlight, for the first time, the central importance of mTORC1 as a coordinator of airway and vascular morphogenesis. This model also highlights the importance of the low oxygen tension of the fetal environment for priming key developmental events, particularly, establishing a basal activation of HIF-1α and the maintenance of a mesenchymal cell population. By implication, therapies that target the HIF signaling system to treat developmental vascular diseases such as bronchopulmonary dysplasia need to take into account the role of the mTOR signaling pathway as a major determinant of temporal and spatial vascular signaling.
Fig. 11.
Model of integrated airway and vasculogenesis in the developing lung. Model is represented next to a late embryonic stage (E12) rat lung showing formation of airway branches extending into the mesenchyme tissue surrounded by blood-filled vascular structures proximal to the airway tip. Foregut (Fg) is shown displaced to left of lung. Zone A: FGF-10 expressed in the mesenchyme ahead of nascent airway buds induces Spry2 cleavage by an unknown ubiquitin ligase (possibly SIAH2 or cCBL) in the progenitor epithelial cells of the airway tip. This cleavage event, although necessary for mTORC1 activation, is accompanied by the formation of a complex between Spry2, cCBL, and TSC2 (Figs. 7 and 8), which dislocates the TSC1/2 complex through the proteolytic cleavage of TSC2 (Fig. 10). This disrupts TSC1/2 GTPase activating protein (GAP) activity allowing GTP interaction with Rheb and activation of mTORC1. Zones B and C: trophic signaling, involving VEGF release by airway epithelium into the mesenchyme and subsequent vasculogenesis, proceeds proximal to the airway tip by binding of mTORC1 to the HIF-1α TOS motif and subsequent interaction with the p300 transcriptional initiation complex. This interaction locally amplifies the transcriptional activity of HIF-1α causing increased release of VEGF and the appearance of vascular structures behind the growing tip of the airway. Our model acknowledges that there are 2 oxygen-dependent points of control that are critical for proper lung morphogenesis. These are: 1) the proliferation of mesenchyme tissue that requires low-oxygen tension and mTOR activity for sustained growth, and 2) hypoxic priming of HIF-1α transcriptional activity, which can be positively modulated by locally expressed growth factors. The ERK1/2 activation by FGF-10 reported by Tefft et al. (53) and observed by us in HBE (Supplementary Figs. S4 and S5) is represented by a dotted line. Our study shows that the magnitude of this response is also oxygen sensitive as it is suppressed at fetal compared with alveolar Po2, leading us to speculate a secondary role for oxygen in regulating ERK1/2 signaling in the fetal lung. This phenomenon could be linked to the conservation of progenitor cell populations during lung morphogenesis (50).
GRANTS
This work was supported by grants to S. C. Land from The Wellcome Trust (088032/z/08/z), TENOVUS (Scotland), and the Anonymous Trust. A. R. Tee is supported by the Association of International Cancer Research through a Career Development Fellowship (06-914/915).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Claire Cockburn for assistance with qPCR determinations of pluripotency markers in HELMF, Prof. Jerry Cheadle and Dr. Cleo Bonett (Cardiff Univ.) for provision of fixed TSC1−/− fetuses, and Drs. Graeme Guy and Permeen Yusoff for provision of Spry2 vectors and related advice.
REFERENCES
- 1.Acarregui MJ, Penisten ST, Goss KL, Ramirez K, Snyder JM. Vascular endothelial growth factor gene expression in human fetal lung in vitro. Am J Respir Cell Mol Biol 20: 14–23, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Asikainen TM, Chang LY, Coalson JJ, Schneider BK, Waleh NS, Ikegami M, Shannon JM, Winter VT, Grubb P, Clyman RI, Yoder BA, Crapo JD, White CW. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J 20: 1698–1700, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho WB, Flippin LA, Gunzler V, White CW. Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung. Proc Natl Acad Sci USA 102: 10212–10217, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines DL, Ramminger SJ, Collett A, Haddad JJ, Best OG, Land SC, Olver RE, Wilson SM. Oxygen-evoked Na+ transport in rat fetal distal lung epithelial cells. J Physiol 532: 105–113, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardos JI, Ashcroft M. Negative and positive regulation of HIF-1: a complex network. Biochim Biophys Acta 1755: 107–120, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bellusci Grindley SJ, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124: 4867–4878, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev 18: 2893–2904, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho H, Ahn DR, Park H, Yang EG. Modulation of p300 binding by posttranslational modifications of the C-terminal activation domain of hypoxia-inducible factor-1α. FEBS Lett 581: 1542–1548, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8: 702–710, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem 280: 9769–9772, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol 10: 38–47, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Davies DM, Johnson SR, Tattersfield AE, Kingswood JC, Cox JA, McCartney DL, Doyle T, Elmslie F, Saggar A, de Vries PJ, Sampson JR. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med 10: 200–203, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, Warburton D. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol 290: 177–188, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: signalling inputs, substrates and feedback mechanisms. Cell Signal 21: 827–835, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Findley CM, Cudmore MJ, Ahmed A, Kontos CD. VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler Thromb Vasc Biol 27: 2619–2626, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Fong CW, Leong HF, Wong ES, Lim J, Yusoff P, Guy GR. Tyrosine phosphorylation of Sprouty2 enhances its interaction with c-Cbl and is crucial for its function. J Biol Chem 278: 33456–33464, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Gebb SA, Fox K, Vaughn J, McKean D, Jones PL. Fetal oxygen tension promotes tenascin-C-dependent lung branching morphogenesis. Dev Dyn 234: 1–10, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Gebb SA, Jones PL. Hypoxia and lung branching morphogenesis. Adv Exp Med Biol 543: 117–125, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Gradin K, Takasaki C, Fujii-Kuriyama Y, Sogawa K. The transcriptional activation function of the HIF-like factor requires phosphorylation at a conserved threonine. J Biol Chem 277: 23508–23514, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Groenman F, Rutter M, Caniggia I, Tibboel D, Post M. Hypoxia-inducible factors in the first trimester human lung. J Histochem Cytochem 55: 355–363, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Groenman FA, Rutter M, Wang J, Caniggia I, Tibboel D, Post M. Effect of chemical stabilizers of hypoxia-inducible factors on early lung development. Am J Physiol Lung Cell Mol Physiol 293: L557–L567, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Guy GR, Jackson RA, Yusoff P, Chow SY. Sprouty proteins: modified modulators, matchmakers or missing links? J Endocrinol 203: 191–202, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Hegner B, Weber M, Dragun D, Schulze-Lohoff E. Differential regulation of smooth muscle markers in human bone marrow-derived mesenchymal stem cells. J Hypertens 23: 1191–1202, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Hentges KE, Sirry B, Gingeras AC, Sarbassov D, Sonenberg N, Sabatini D, Peterson AS. FRAP/mTOR is required for proliferation and patterning during embryonic development in the mouse. Proc Natl Acad Sci USA 98: 13796–13801, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsilä M, Koivunen P, Günzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem 278: 30772–30780, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Inglis SK, Gallacher M, Brown SG, McTavish N, Getty J, Husband EM, Murray JT, Wilson SM. SGK1 activity in Na+ absorbing airway epithelial cells monitored by assaying NDRG1-Thr346/356/366 phosphorylation. Pflügers Arch 457: 1287–1301, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Kenneth NS, Rocha S. Regulation of gene expression by hypoxia. Biochem J 414: 19–29, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol 209: 254–267, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol 178: 437–451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Land S. Oxygen-sensing patghways and the development of mammalian gas exchange. Redox Report 8: 326–340, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Land SC, Collett A. Detection of Cl− flux in the apical microenvironment of cultured foetal distal lung epithelial cells. J Exp Biol 204: 785–795, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Land SC, Tee AR. Hypoxia-inducible factor 1α is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem 282: 20534–20543, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Li DF, Huang MC. All roads lead to mTOR: integrating inflammation and tumor angiogenesis. Cell Cycle 6: 3011–3014, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Wang Y, Kim E, Beemiller P, Wang CY, Swanson J, You M, Guan KL. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J Biol Chem 282: 35803–35813, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Ma J, Meng Y, Kwiatkowski DJ, Chen X, Peng H, Sun Q, Zha X, Wang F, Wang Y, Jing Y, Zhang S, Chen R, Wang L, Wu E, Cai G, Malinowska-Kolodziej I, Liao Q, Liu Y, Zhao Y, Sun Q, Xu K, Dai J, Han J, Wu L, Zhao RC, Shen H, Zhang H. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J Clin Invest 120: 103–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, Bellusci S. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech Dev 102: 81–94, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Marin TM, Clemente CF, Santos AM, Picardi PK, Pascoal VD, Lopes-Cendes I, Saad MJ, Franchini KG. Shp2 negatively regulates growth in cardiomyocytes by controlling focal adhesion kinase/Src and mTOR pathways. Circ Res 103: 813–824, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, Brown DJ, Chiu HY, Shyu M, Drapeau BL, Wagner RJ, Powell RJ. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem 282: 36112–36120, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature 453: 745–750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mylonis I, Chachami G, Samiotaki M, Panayotou G, Paraskeva E, Kalousi A, Georgatsou E, Bonanou S, Simos G. Identification of MAPK phosphorylation sites and their role in the localization and activity of hypoxia-inducible factor-1α. J Biol Chem 281: 33095–33106, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Otulakowski G, Duan W, Gandhi S, O'Brodovich H. Steroid and oxygen effects on eIF4F complex, mTOR, and ENaC translation in fetal lung epithelia. Am J Respir Cell Mol Biol 37: 457–466, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Otulakowski G, Duan W, O'Brodovich H. Global and gene-specific translational regulation in rat lung development. Am J Respir Cell Mol Biol 40: 555–567, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Porter D, Lahti-Domenici J, Torres-Arzayus M, Chin L, Polyak K. Expression of high in normal-1 (HIN-1) and uteroglobin related protein-1 (UGRP-1) in adult and developing tissues. Mech Dev 114: 201–204, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Qi J, Nakayama K, Gaitonde S, Goydos JS, Krajewski S, Eroshkin A, Bar-Sagi D, Bowtell D, Ronai Z. The ubiquitin ligase Siah2 regulates tumorigenesis and metastasis by HIF-dependent and -independent pathways. Proc Natl Acad Sci USA 105: 16713–16718, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rae C, Cherry JI, Land FM, Land SC. Endotoxin-induced nitric oxide production rescues airway growth and maturation in atrophic fetal rat lung explants. Biochem Biophys Res Commun 349: 416–425, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Rajatapiti P, van der Horst IW, de Rooij JD, Tran MG, Maxwell PH, Tibboel D, Rottier R, de Krijger RR. Expression of hypoxia-inducible factors in normal human lung development. Pediatr Dev Pathol 11: 193–199, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Sang N, Stiehl DP, Bohensky J, Leshchinsky I, Srinivas V, Caro J. MAPK signalling upregulates the activity of hypoxia-inducible factors by its effects on p300. J Biol Chem 278: 14013–14019, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shinkai T, Shinkai M, Pirker ME, Montedonico S, Puri P. The role of oxygen tension in the regulation of embryonic lung development. J Pediatr Surg 40: 32–35, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Stavridis MP, Collins BJ, Storey KG. Retinoic acid orchestrates fibroblast growth factor signalling to drive embryonic stem cell differentiation. Development 137: 881–890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tee AR, Blenis J, Proud CG. Analysis of mTOR signaling by the small G-proteins, Rheb and RhebL1. FEBS Lett 579: 4763–4768, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Tefft D, De Langhe SP, Del Moral PM, Sala F, Shi W, Bellusci S, Warburton D. A novel function for the protein tyrosine phosphatase Shp2 during lung branching morphogenesis. Dev Biol 282: 422–431, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Tefft D, Lee M, Smith S, Crowe DL, Bellusci S, Warburton D. mSprouty2 inhibits FGF10-activated MAP kinase by differentially binding to upstream target proteins. Am J Physiol Lung Cell Mol Physiol 283: L700–L706, 2002 [DOI] [PubMed] [Google Scholar]
- 54.van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol 288: L167–L178, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Warburton D. Developmental biology: order in the lung. Nature 453: 733–735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol 258: 169–184, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Weibel ER. Fractal geometry: a design principle for living organisms. Am J Physiol Lung Cell Mol Physiol 261: L361–L369, 1991 [DOI] [PubMed] [Google Scholar]
- 58.Wilson C, Idziaszczyk S, Parry L, Guy C, Griffiths DF, Lazda E, Bayne RA, Smith AJ, Sampson JR, Cheadle JP. A mouse model of tuberous sclerosis 1 showing background specific early postnatal mortality and metastatic renal cell carcinoma. Hum Mol Genet 14: 1839–1850, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Xia H, Nho R, Kleidon J, Kahm J, Henke CA. Polymerized collagen inhibits fibroblast proliferation via a mechanism involving the formation of a beta1 integrin-protein phosphatase 2A-tuberous sclerosis complex 2 complex that suppresses S6K1 activity. J Biol Chem 283: 20350–20360, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.