Abstract
Cellular senescence is a response to nonlethal stress that results in persistent cytostasis with a distinct morphological and biochemical phenotype. The senescence phenotype, detected in tumors through the expression of mRNA and protein markers, can be generated in cancer cells lacking functional p53 and retinoblastoma protein. Current research suggests that therapy-induced senescence (TIS) represents a novel functional target that may improve cancer therapy. TIS can be induced in immortal and transformed cancer cells by selected anticancer compounds or radiation, and accumulating data indicate that TIS may produce reduced toxicity-related side effects and increased tumor-specific immune activity. This review examines the current status of TIS-regulated mechanisms, agents, and senescence biomarkers with the goal of encouraging further development of this approach to cancer therapy. Remaining hurdles include the lack of efficient senescence-inducing agents and incomplete biological data on tumor response. The identification of additional compounds and other targeted approaches to senescence induction will further the development of TIS in the clinical treatment of cancer.
Cancer therapy has traditionally relied on cytotoxic treatment strategies on the assumption that complete cellular destruction of tumors optimizes the potential for patient survival. This view has limited the treatment options that oncologists have at their disposal to toxic compounds and high dose radiation. These approaches may produce complete cell death within a solid tumor and can cause severe side effects in patients. Such cancers often develop resistance to treatment and recur or progress to advanced primary and metastatic tumors. An alternative strategy is the induction of cytostasis, which permanently disables the proliferative capacity of cells without inducing cancer cell death. Initial clinical studies utilizing cytostatic treatments have yielded promising preliminary results (1–3), suggesting that these treatments may be as effective as cytotoxic therapies in preventing continued tumor growth. This approach to treatment could provide equivalent or prolonged survival with fewer and less severe side effects related to cytotoxicity and may provide a more realistic goal for the chronic management of some cancers.
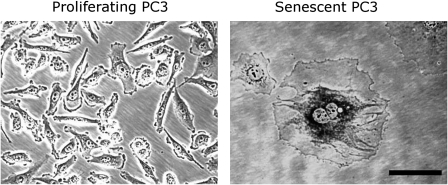
A promising approach to induction of cytostasis in tumor cells is therapy-induced senescence (TIS) (4). Replicative senescence was first observed in vitro in primary cells after extensive culture and replicative exhaustion linked to telomere shortening (5,6). More recently, DNA damage, increased oncogenic signaling, and oxidative stress have been found to result in induced or accelerated senescence (7). Senescent cells remain viable and metabolically active but are permanently growth arrested (7). In contrast to cells undergoing apoptosis or mitotic catastrophe in response to conventional cytotoxic drugs, senescent cells may persist indefinitely. Growth arrest is achieved and maintained in either the G1 or G2/M stage of the cell cycle, in part, by the increased expression of specific cyclin-dependent kinase inhibitors (CDKIs), including p16Ink4a (CDKN2A), p21Waf1 (CDKN1A, CIP1), and p27Kip1 (CDKN1B) (8). Transformed neoplastic cells that lack senescence-associated tumor suppressors present in nontransformed cells, including genes such as p53 and retinoblastoma protein (Rb,RB1), retain the capacity to become senescent when exposed to certain stresses, including those generated by select anticancer agents and ionizing radiation (9–11). Cultured in vitro, senescent cells develop a distinct and recognizable flattened and enlarged morphology with a prominent nucleus and increased cytoplasmic granularity. Most notably, these cells can be visualized with a staining technique that is a widely accepted and used marker, senescence-associated β-galactosidase (SA-β-gal) activity (12), which stains the perinuclear compartment blue (Figure 1).
Figure 1.
Proliferating and drug-induced senescent PC3 prostate cancer cells. Senescent cancer cells exhibit the characteristic morphology and increased SA-β-gal activity seen in replicative senescent cells. PC3 cells were cultured in drug-free medium or in medium containing 250 nM quinone diaziquone for 3 days followed by 2 days in drug-free medium (13), fixing, and staining (12). Cells were visualized under ×200 magnification using phase contrast microscopy. Scale bar = 100 μm; SA-β-gal = senescence-associated β-galactosidase.
It has been shown that senescence-associated mechanisms prevent cells from proliferating indefinitely in vitro and that immortalization circumvents this tumor suppressor mechanism (7). Oncogene-induced senescence has been identified in nonmalignant human tissues as one mechanism of senescence tumor suppression (14). Benign melanocytic nevi (ie, skin moles) result from the increased activity of the mutant oncogene v-raf murine sarcoma viral oncogene homolog B1 (BRAF). After increased proliferation and growth, melanocytes arrest, increase the expression of p16Ink4a, and stain positive for SA-β-gal (14). Increased expression of CDKI proteins and increased SA-β-gal staining are also observed in lung adenomas, but not in adenocarcinomas, suggesting that senescence suppresses malignant transformation (15). Senescence has also been identified in cases of more extensive benign prostatic hyperplasia and premalignant prostatic intraepithelial neoplasia (16,17). These data suggest that senescence may be an endogenous barrier to malignant transformation and that senescence in tumors may indicate a more benign or favorable outcome.
It is of clinical interest that transformed cancer cells can be induced to a similar senescent state in vitro and in vivo with specific conventional anticancer treatments. TIS has been identified in tumors after radiation or genotoxic chemotherapy (10,18,19), although it occurs only in a subset of the treated cells. As a therapeutic goal, TIS may provide an effective means to induce a persistent growth inhibitory response in both early- and late-stage cancers while limiting toxicity. Experimental evidence indicates that there are benefits to the induction of senescence in immunocompetent patients (20,21). Moreover, evidence suggests that TIS may function as a “back-up” response to therapy in cancer cells in which apoptotic pathways are disabled (20).
In this review, we discuss current information on treatments that induce senescence in cancer cells and the molecular pathways that regulate these events. Markers and other characteristics by which cellular senescence may be identified in tissue samples, and their potential prognostic uses, are addressed.
The Genetics of Senescence Induction
The antagonistic relationship between senescence and oncogenic transformation is central to tumor suppression induced by both endogenous processes and as part of a treatment strategy. In the 1980s, early genetic studies of senescence were performed by fusing mortal cells, which retain the capacity to become replicatively senescent, and immortalized transformed cells that do not (22–25). This fusion resulted in cells with limited replicative capacity, suggesting that immortalization occurs by “turning off” or bypassing senescence-inducing genes and pathways and that active processes are replaced or re-engaged to induce senescence in the hybrids. In a series of elegant experiments, Pereira-Smith and Smith (23) found that fusion of specific cancer cell lines results in mortal hybrids and that these cells could be placed into one of the four genetic complementation groups (24). Importantly, these experiments suggest that four genetic pathways regulate senescence and that only one deficient process needs replacement to reactivate senescence in cancer cells (24).
Although the identity of these molecular pathways has yet to be completely defined, several chromosomes and distinct genes have been found to induce senescence when reintroduced into cancer cell lines that have necessarily bypassed or inactivated senescence (Table 1). Several of these genes, including p53 and Rb, have known growth inhibitory functions (4,5,42). Cells with functional Rb and p53 appear more sensitive to stress and oncogene activities that stimulate senescence (7,43). However, it is noteworthy from a therapeutic standpoint that cancer cells lacking functional Rb, p53, and other tumor suppressors retain the capacity for TIS. In SAOS-2 osteosarcoma and DU145 prostate cancer cells, both lacking Rb and p53, doxorubicin induced senescence in more than 50% of cells in vitro (9,11). A comparison of TIS in cancer cells containing functional p53 or a homozygous knockout demonstrated similar robust responses, indicating limited p53 dependence in drug-induced senescence (44,45). These p53/pRb-independent pathways involve stress- and damage response signaling mechanisms that sense damage and directly affect transcription without the direct involvement of these classic tumor suppressor genes.
Table 1.
Genes regulating senescence induction in cancer cells*
| Mechanism and gene | Function | p53 Dependent† | p53 Independent† | Reference |
| Mitotic and stress signaling | ||||
| Raf1 | Mitogenic/stress signaling | + | (26) | |
| MAP2K6/p38 | Mitogenic/stress signaling | + | (27) | |
| Aurora kinase A | Mitogenic signaling | + | (28) | |
| PTEN | Oncogenic stress signaling | + | (29) | |
| SKP2 | Mitogenic/stress signaling | (30) | ||
| Major tumor suppressors | ||||
| p53 | Transcription factor | + | (31) | |
| p63 | Transcription factor | + | (32) | |
| p73 | Transcription factor | + | (32) | |
| Rb | Transcription regulator | + | (33) | |
| CDKIs | ||||
| p21Waf1/Cip1 | Kinase inhibitor | + | (34) | |
| p16Ink4a | Kinase inhibitor | + | (35) | |
| p57Kip2 | Kinase inhibitor | (36) | ||
| p15Ink4b | Kinase inhibitor | (37) | ||
| Mitochondrial integrity and function | ||||
| OPA1 | Mitochondrial membrane structure | + | (38) | |
| Proinflammatory signaling | ||||
| IL-6/CXCR2 | Cytokine/receptor | + | (39) | |
| IGFBP-rP1 | Cytokine/IGF signaling, modulators | (40) | ||
| IGFBP7 | + | + | (41) |
CXCR2 = chemokine (C-X-C motif) receptor 2; IL-6 = interleukin 6; IGFBP-rP1= insulinlike growth factor–binding protein-related protein 1; IGFBP7 = insulinlike growth factor–binding protein 7; MAP2K6 = mitogen-activated protein kinase kinase 6; OPA1= optic atrophy 1; p38 = mitogen-activated protein kinase 14; p21Waf1/Cip1 = CDKN1A/cyclin-dependent kinase inhibitor 1A; p16Ink4a = CDKN2A/cyclin-dependent kinase inhibitor 2a; p57Kip2 = cyclin-dependent kinase inhibitor 1C; p15Ink4b = cyclin-dependent kinase inhibitor 2B; PTEN = phosphatase and tensin homolog; Raf1 = raf-1 murine leukemia viral oncogene homolog 1; Rb = retinoblastoma protein; Skp2 = S-phase kinase-associated protein 2.
Senescence-inducing activity shown to be dependent or independent of intact p53 function, denoted by (+). Blank means undetermined.
Several genes, not necessarily related to p53 and Rb, are active during senescence and induce senescence when overexpressed in cancer cell lines. These genes include the CDKIs p16Ink4a, p21Waf1/Cip1, and p27Kip1 (5,8). We have demonstrated in prostate epithelial and urothelial cells that p21Waf1/Cip1 appears more important in the cell cycle arrest associated with early senescence, whereas p16Ink4a is more important to maintaining this phenotype (46). The p53-related proteins p63 and p73 also regulate senescence induction by mechanisms similar to those of p53 (4,47–49). Tumor cell senescence has been induced by insulinlike growth factor–binding protein-related protein 1 (IGFBP-rP1), a member of the insulinlike growth factor (IGF) protein family (40). Other IGF members are overexpressed during senescence (eg, IGFBP3, IGFBP5, and IGFBP7), suggesting an important role in regulating both proliferation and senescence (41,50,51). Suppressing apoptosis by expression of the pro-survival gene B-cell CLL/lymphoma 2 (BCL2) during chemotherapy has also been found to induce senescence, dependent on p27Kip1 expression (20,52). These studies suggest that an intricate balance exists among proliferation, apoptosis, and senescence, which could be exploited therapeutically.
Timing and Mechanisms of Senescence Induction in Cancer Cells
Known drugs and other therapies that induce senescence typically take several days for the full TIS phenotype to develop, in contrast to the rapid activation of apoptotic processes that commit a cell to apoptotic destruction within 24 hours. Senescence, both in vitro and in vivo, does not fully develop its characteristic expression of SA-β-gal and morphology for 3–7 days after exposure to an agent (7,9,11,19). However, knockout of p53 appears only to delay senescence rather than abrogate the TIS response in cancer cells (44,45). Senescence requires that cells sense damage and respond with active changes in gene expression, which mediate the development of the characteristic senescent phenotype (53).
The cellular decision between apoptosis and senescence is dependent, in part, on the magnitude of stress to which cancer cells are exposed. Lower levels of damage may trigger senescence-associated antiproliferative responses without activating the cascades of caspase activity that commit the cell to apoptosis (9). In prostate cancer cell lines, 250 nM dosing of doxorubicin generates apoptosis, whereas lower 25 nM doses induce TIS (11,13). Although similar stresses can induce both apoptosis and senescence, these pathways diverge, and the regulation of these processes is distinct. Studies show that senescence results when apoptosis is blocked by the overexpression of BCL2 or the inhibition of caspases (20,52,54). These data imply that the point at which these pathways diverge occurs upstream from caspase activation. Identification and characterization of this regulatory nodal point is an area of active research interest.
The senescence response of cancer cells to select drugs with specific identified targets has resulted in many of these pathways being implicated in TIS. Many senescence-inducing drugs generate DNA damage to produce single- and double-strand breaks, highlighting the close relationship between senescence and genomic stress. In oncogene-induced senescence and replicative senescence, DNA damage response pathways play a central role functioning through ataxia telangiectasia–mutated (ATM), ataxia telangiectasia and Rad3-related (ATR), and checkpoint homologs 1 and 2 (Chk1 and Chk2) activity and the CDKN2A/p16Ink4a/p19ARF tumor suppressor (55,56). Aspects of this DNA checkpoint response appear to play a role in TIS. Recently, senescence was induced in cancer cells, regardless of p53 status, by targeting the mitosis-regulating Aurora kinase A using small molecule inhibitors (28). Other agents induce senescence through mechanisms that alter DNA structure and function. These agents include the DNA methyltransferase inhibitor 5-azacytidine, which inhibits DNA methylation, and Sirtinol, a histone DNA acetylase inhibitor that alters normal chromatin structure. DU145 and LNCaP cancer cells cultured continuously in 400 nM 5-azacytidine become senescent within 7 days, whereas MCF7 breast and H1299 lung cancer cells become senescent within 10 days after a 24-hour exposure to 100 nM Sirtinol (11,57).
Stress signaling through the p38 (mitogen-activated protein kinase 14, MAPK14), c-JUN N-terminal kinase (MAPK8), and extracellular signal-regulated kinase (MAPK1) pathways occurs in oncogene-induced senescence and replicative senescence, although this has not been widely investigated in TIS (42,55,58). Recently, senescence-inducing compounds that target proteins not closely associated with TIS have been identified (59,60). The diterpene esters TPA, PEP005, and PEP008 have been shown to induce senescence through the activation of protein kinase C isoforms and MAPKs (59,60). Senescence may occur either as a direct result of increased protein kinase C signaling activity or because of genomic stress that results from pro-mitotic signaling, but these issues have not been thoroughly investigated.
Increased intracellular reactive oxygen species are closely associated with senescence induction in both normal and immortal cells by causing DNA, lipid, and protein damage (61). The Zn2+ ionophore pyrithione generates oxidative stress inducing growth arrest and senescence (13,62). Recently, TIS was also induced in cancer cells in mouse models and human xenografts exposed to the phosphatase and tensin homolog (PTEN) inhibitor VO-OHpic, but only in PTEN hemizygous cells (29). This suggests the possibility that some cancers may be conditionally susceptible to TIS by compounds affecting particular stress-signaling responses. In addition, this induction of TIS occurred independently of DNA damage and DNA damage response signaling (29). TIS was also achieved in cancers by targeting S phase kinase-associated protein 2 (Skp2) expression dependent on the presence of “oncogenic stress” that results from PTEN or p19ARF mutation, independently of p53 and DNA damage response signaling (30). With recent evidence demonstrating that senescence-inducing DNA damage response signaling may be uncoupled from DNA damage (63), TIS may be achieved either by inducing direct damage or by targeting events that regulate the damage response.
Agents Capable of Inducing Senescence in Cancer
Observations that some tumor cells can be forced into senescence by agents used in the management of human cancers are of clinical interest (Table 2). These findings indicate that many cancer cells possess intact, but silenced, signaling pathways that can be manipulated to stimulate senescence. This ability of tumor cells to undergo senescence in response to stress and damage has been noted with both radiation and chemotherapeutic drugs (4,10). When equitoxic levels of different agents were applied to HT1080 fibrosarcoma cells in vitro, the strongest induction of the senescent phenotype (>50% senescent based on SA β-gal and cell cycle analysis) was seen with the DNA-interactive agents, doxorubicin and cisplatin (9). Lesser responses were observed with ionizing radiation and etoposide and the lowest were observed with the microtubule-targeting drugs docetaxel and vincristine, which typically induce mitotic catastrophe. Furthermore, drugs such as doxorubicin induced senescence in numerous cancer cell lines, including those lacking p53 and p21Waf1/Cip1 (9,44). We have shown that prostate cancer cell lines express different CDKIs at senescence when compared with primary prostate epithelial cells, suggesting that compensatory mechanisms are involved in cancer cell senescence (46). Whereas many drugs are inefficient in inducing senescence, in which case senescence occurs in only a subset of treated cells, these results suggest that damage responses that signal senescence remain competent in cancers lacking major tumor suppressors.
Table 2.
Drugs that induce senescence in cancer cell lines and tumors*
| Agent | p53 status† | Mechanism | In vitro‡ | In vivo§ | Reference |
| Aphidocolin | + | DNA polymerase inhibitor | + | (9) | |
| Bleomycin | + | DNA damage | + | (64) | |
| Camptothecin | +/− | DNA damage | + | (65) | |
| Carboplatin + docetaxel | +/− | DNA damage | + | Human lung tumors | (18) |
| Cisplatin | +/− | DNA damage | + | (9) | |
| Cyclophosphamide + doxorubicin + 5-Fluorouracil | DNA damage | Human breast tumors | (19) | ||
| Diaziquone/AZQ | +/− | DNA damage | + | Prostate xenograft tumors | (13) |
| Doxorubicin | +/− | DNA damage | + | (9) | |
| Epigallocatechin gallate | + | Telomerase inhibition | + | (66) | |
| Etoposide | +/− | DNA damage | + | (9) | |
| Gamma irradiation | +/− | DNA damage | + | + | (10) |
| Hydroxyurea | + | ROS | + | (67) | |
| K858 | +/− | KIF11 | + | Xenografts tumors | (68) |
| Lovastatin | − | HMG-CoA-reductase inhibitor | + | (69) | |
| Mitoxantrone | +/− | DNA damage | + | Human prostate tumors | (70) |
| MLN4924 | −/+ | Cul1 SCF subunit inhibitor | + | Prostate xenografts tumors | |
| MLN8054 | + | Aurora kinase A inhibitor | + | Colon xenograft tumors | (28) |
| Pyrithione | +/− | Zinc/calcium regulation, ROS | + | (13) | |
| Resveratrol | + | ROS | + | (71) | |
| Retinols | + | Differentiation | + | (9) | |
| TPA, PEP005, PEP008 | + | PKC activating | + | (59,60) | |
| VO-OHpic | + | PTEN | + | Prostate xenograft tumors | (29) |
Cul1= cullin 1; HMG-Co-A reductase = 3-hydroxy-3-methylglutaryl-CoA-reductase; KIF11 = kinesin family member 11; PKC = protein kinase C; PTEN = phosphatase and tensin homolog; ROS = reactive oxygen species; SCF = Skp1/Cul1/F-box protein complex.
p53 status of cells in which the drug induces senescence. (+) denotes active p53 in cancer cells in which the drug induces senescence, whereas (−) denotes senescence induction by the drug in cancer cells in which p53 is deleted or mutated.
Senescence-inducing activity of drug in cancer cells in vitro; (+) denotes induction of senescence in vitro, whereas an empty cell denotes that results were not determined.
Senescence-inducing activity of drug in vivo in patients or tumor models. An empty cell denotes that results are not determined; (+) denotes senescence induction in various in vivo tumor models.
A limiting factor in the identification of new compounds that induce senescence efficiently has been the lack of methods to rapidly screen compounds and small molecules. Until recently, the senescence-inducing activity of compounds was evaluated individually in a time-intensive and focused manner. In response to this lack of screening techniques for senescence, our laboratory developed a method for screening small molecule and other compound libraries for senescence activity using a robotic fluid handler and plate reader (13). This whole cell assay is based on identifying several characteristics of senescent cells, including the development of permanent growth arrest, characteristic senescent morphology, and positive SA-β-gal staining. This method was used to screen a 4160 compound library of known bioactive compounds and natural products at a 10-μM dose. Of the four most effective novel compounds identified, the quinone diaziquone (AZQ) had been previously found to induce tumor stasis in experimental solid tumor models in the 1980s but was not pursued further because of the lack of tumor regression (72,73). Postulated mechanisms for these drugs include DNA damage and oxidative stress, as well as novel approaches including post-translational modifications involved in proteolytic processing and kinase signaling. Further library screening should permit the identification of additional senescence-inducing agents as well as provide additional tools to understand the molecular basis for this response.
Notably, senescence has been identified in patient tumors removed after genotoxic treatments. Areas of increased SA-β-gal staining were observed in 41% of breast tumors after treatment with a regimen of cyclophosphamide, doxorubicin, and 5-fluorouracil (19). This staining was confined to tumor cells, with no detection in normal tissues. Senescence markers have also been observed in lung tumors after treatment with carboplatin and docetaxel (18). Despite the relative inefficiency of these regimens for inducing senescence, these studies suggest that senescence may be a more prevalent tumor response to current anticancer therapy than previously realized (18,19).
Cellular Senescence: Friend or Foe?
TIS in tumor cells induces several features that may be beneficial to the treatment of cancer. Importantly, senescence stimulates a persistent terminal growth arrest. Cells remain viable but are typically arrested at the G1 or G2/M phases of the cell cycle and fail to proceed even after mitogen stimulation (7), in part, because of the increased expression of one or more cyclin-dependent kinase inhibitors including p16Ink4a, p21Waf1/Cip1, and p27Kip1 (8). These cells may persist indefinitely in a stable state even in vivo. Senescent melanocytes have been identified in benign nevi that remain indolent for years (14). We have recently demonstrated the persistence of a subset of senescent prostate cancer cells at least 6 weeks after the establishment of xenografts with doxorubicin-induced senescent prostate cancer cells (74). In vivo, senescent cells may survive over prolonged periods but may become nonviable and undergo phagocytosis. The enrichment of lysosomal β-galactosidase activity with the development of the senescent phenotype suggests that some senescent cells may eventually undergo autophagy (75).
The presence of senescence in tumor cells can stimulate an immune response. In human melanoma cells co-expressing mutants of neuroblastoma RAS viral (v-ras) oncogene homolog (NRAS) and v-raf murine sarcoma viral oncogene homolog B1 (BRAF), senescence increased susceptibility to cell-mediated cytotoxicity in vitro by lymphokine-activated killer cells (76). This response was also observed in a mouse hepatocarcinoma model in which the conditional expression of functional p53 induced senescence resulting in tumor regression (21). This response may be instrumental in the prolonged survival found in a mouse lymphoma model in which senescence was induced by chemotherapy and BCL2 expression (20). However, this benefit has yet to be specifically investigated in TIS tumor models.
The observation that lower concentrations of active drugs induce senescence suggests another potential benefit of TIS. We recently screened a series of concentrations of doxorubicin, 5-azacytidine, and docetaxel for their ability to generate senescence (11). At lower doses, the senescent phenotype was predominant, albeit inefficient, in inducing senescence in most prostate cancer cell lines. Higher doses that lead to elevated DNA damage and stress are associated with a more pronounced apoptotic response (11), suggesting that the induction of senescence in tumors may be achieved with lower drug doses and, especially if administered chronically, may potentially limit treatment-related toxic side effects.
Other features associated with senescence have generated concern for oncologists. One is the idea that senescence may be a reversible process, at least in fibroblasts, if proteins involved in its maintenance are lost. The overexpression of simian virus 40 large T-antigen protein or the forced inactivation/underexpression of p53 and p16Ink4a results in proliferation in senescent fibroblasts (43,77). However, these induced cells had a limited proliferative capacity, undergoing only a few cell divisions before becoming apoptotic. Notably, whether drug-induced senescence is a reversible process has not been addressed experimentally. However, it appears unlikely that the expression of proteins that block proliferation, notably the CDKIs, and the extensive changes in nuclear structure shown to maintain senescence could be reversed in senescent tumors.
Senescence in fibroblasts may result in the resistance of these cells to programmed cell death. Senescent fibroblasts resist the apoptotic effects of serum starvation (78) and hydrogen peroxide (79). However, senescent human umbilical vascular endothelial cells are more prone to apoptosis than fibroblasts, suggesting that this phenomenon is cell type specific (80). DeJesus et al. (81) found that proapoptotic signaling via ceramide and tumor necrosis factor-α is interrupted in senescent fibroblasts and may be a mechanism by which apoptosis is avoided in these cells. To date, the resistance of TIS cells to apoptosis has not been clearly addressed.
Studies in aging have suggested an association between age-related senescence and the promotion of carcinogenesis in surrounding tissues (82). Senescent fibroblasts secrete characteristic proinflammatory immune cytokines, including interleukin (IL)-6 and IL-8, which have the potential to promote bystander cell proliferation and may account for the development of some age-related cancers (70). Although secretion of these cytokines may mediate senescence-related effects of aging, the relevance of this phenomenon to treatment-induced senescence in cancers is unclear. Evidence suggests that secreted factors may prove to be a benefit in the context of TIS. Studies have shown that IGFBP7, IL-6, and IL-8 secreted by senescent cells stimulate autocrine signaling loops that are required to reinforce and maintain senescence induction (41,39). Additional autocrine factors regulating this process are likely to be identified, some of which may be involved in TIS. Secreted inflammatory factors also likely facilitate the cell-mediated tumor clearance observed after senescence induction in a mouse hepatocarcinoma model (21). We and other researchers have demonstrated that senescent gene expression patterns, although similar, vary markedly among fibroblasts, epithelial cells, and cancer cells (50,70,74,83), implying that each cell type may secrete specific factors with different effects on bystander cells.
Recently, we examined the effect of doxorubicin-induced senescent prostate cancer cells on the growth of surrounding proliferating cancer cells (74). In a series of experiments, we demonstrated that over time tumor growth was not affected by increasing numbers of senescent cells and that the proliferation rate of bystander cancer cells was not increased (84). Also, an antiproliferative effect was observed in vitro using MCF7 breast cancer cells induced to senescence by doxorubicin (84). Recent in vivo studies of TIS using drugs targeting PTEN, Aurora kinase A, and SKP2 activities in tumor models did not report complications resulting from bystander proliferation (28–30). Finally, one of the most notable findings suggesting that senescent cells have no impact on proliferation in situ is the observation that benign nevi containing senescent cells persist chronically for years, yet remain uniformly nonmalignant and stable (14). The effects of senescence on bystander cell proliferation remain an area of debate. It is possible that the senescent bystander effect in aging tissues requires exposure to surrounding tissues on a timescale of a lifetime to promote oncogenesis, whereas TIS of tumors would occur on a timescale of years and decades. Another distinct possibility is that sarcomas and other non–epithelial-derived cancers may produce a bystander response to TIS that is different from that observed with epithelial tumor models. Ultimately, the effect and consequences of senescence induction as a therapeutic strategy may vary with the cancer type as well as the drug used to induce senescence.
The Identification of Senescence In Vitro and In Vivo
The ability to identify markers associated with senescence is important for the use of this phenotype in clinical practice. Senescence has been routinely identified by staining for SA-β-gal activity (12), and this has been used as a marker for senescence in aging tissues (12) as well as in tumor tissues after chemotherapy (18,19). It has been suggested that this staining may also be induced by transforming growth factor-β signaling independent of senescence, generating concern regarding its specificity (85). SA-β-gal staining is dependent on increased lysosomal activity and requires fresh or frozen tissue for staining. Thus, this technique is incompatible with many immunohistological techniques routinely used in clinical pathology laboratories, especially with respect to archived tissues. The gene associated with SA-β-gal activity, the lysosomal galactosidase beta-1 (GLB1) is not required for senescence growth arrest and may be uncoupled from senescence in some cancer cell lines (86). The development of immunohistological methods to detect GLB1 protein expression and localization in paraffin-embedded tissue, although not improving the reliability of this marker, would nonetheless facilitate its use in archival samples.
Although specific senescent biomarkers have yet to be fully developed, senescent cells may be identified on the basis of multiple characteristics (Table 3). When cells enter senescence, they develop a distinctive morphology, becoming enlarged, flattened, and multinucleated (Figure 1). This morphology, however, is most easily identified in vitro and may not be apparent in tissues. Many senescent cells also develop extensive vacuoles in the cytoplasm associated with an increase in cellular complexity. This senescent morphology can be measured by flow cytometry as increased side scatter (4). However, the most important characteristic of senescence is the irreversible loss of cell proliferative capacity. Flow cytometric cell cycle profiling typically shows that the number of cells in S phase decreases and the number in G1 or G2/M increases. In addition, cells become multinucleated, identified by the occurrence of additional 2N and 4N peaks. Taken together, these simple techniques can be used to identify characteristics of senescence in cultured cells.
Table 3.
Cellular characteristics and molecular markers of senescence in wild-type and cancer cells*
| Marker | Marker type | Reference |
| Cellular phenotype | ||
| Morphology† | Visual | (4,6) |
| SA-β-gal activity | Enzyme/staining | (12) |
| Glb1 | RNA, IHC | (86) |
| SSC | Flow cytometry | (4) |
| Proliferation arrest | ||
| BrdU Incorporation | IHC, flow cytometry | (13) |
| DAPI/Hoechst 33342 | DNA stain | (13) |
| Decreased KI-67 | IHC | (15,21) |
| Apoptosis exclusion | ||
| Propidium iodide/annexin V staining | Flow cytometry | (13) |
| Cleaved PARP | IHC, western | (13,21) |
| Cleaved caspase 2/3/9 | IHC, western | (13,21) |
| TUNEL staining | IHC | (21) |
| CKIs | ||
| p16Ink4a | IHC | (5,8,14) |
| p21waf1/cip1 | IHC | (8,45,65) |
| p27kip1 | IHC | (7,8,17) |
| Heterochromatin foci | ||
| DAPI/Hoechst 33342 | DNA Stain | (87) |
| HIRA | IHC | (87) |
| H3K9-methyl3 | IHC | (87) |
| HP1-γ | IHC | (87) |
| Secretory proteins | ||
| IGF2 | RNA, IHC | (50,51) |
| IGFBP3, IGFBP5, IGFBP7 | RNA, IHC | (39,44,50,50) |
| IL-6, IL-8, CXCR2, and others | RNA, IHC | (39,70,88) |
| Miscellaneous | ||
| Versican§ | RNA | (11) |
| CXCL14§ | RNA | (11) |
| Mitochondrial fusion/hFis1/OPA1¶ | IHC | (38) |
| Dec1║ | IHC | (15) |
| DcR2║ | IHC | (15) |
BrdU = bromo-deoxy-uridine; CXCR2 = chemokine (C-X-C motif) receptor 2; CXCL14 = chemokine (C-X-C motif) ligand 14; DAPI = 4′,6-diamidino-2-phenylindole; DcR2 = tumor necrosis factor receptor superfamily, member 10d, decoy truncated death domain; Dec1 = basic helix–loop–helix family, member e40; Glb1 = lysosomal galactosidase 1; H3K9-methyl3 = trimethylated histone 3 at lysine 9; HIRA = HIR histone cell cycle regulation defective homolog A; HP1-γ = histone protein 1-gamma; hFIS1 = human fission 1 homolog; IGF2 = insulinlike growth factor 2; IGFBP = insulinlike growth factor–binding protein; IHC = immunohistochemistry; IL-6 = interleukin 6; IL-8 = interleukin 8; KI-67 = antigen identified by monoclonal antibody Ki-67; OPA1 = optic atrophy 1; PARP = poly-ADP ribosyl polymerase; SA-β-gal = senescence-associated β-galactosidase; SSC = side scatter; TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling.
In vitro only.
Marker senescence demonstrated in prostate and prostate cancer only.
Demonstrated in HeLa cells.
Marker may be specific to only nontransformed tissue or fibroblast cells.
The analysis of senescence in tissue samples can be more challenging because many of the in vitro techniques are difficult to perform in patient samples, especially those that are paraffin embedded. Several classes of markers that can assist in the identification of senescent cells in tissues have been identified (Table 3). Senescence-associated heterochromatic foci (SAHF) are condensed regions of heterochromatin that accumulate during senescence (87,89). These composite foci contain methylated and deacetylated histones and other associated proteins. SAHF have been used to identify senescence in vitro in fibroblasts and other non-immortalized cells. Widely tested markers in this category include methylation of histone 3 at lysines 9 and 27 and phosphorylation of H2A histone family, member X (γ-H2AX), all of which colocalize in SAHF (90). In cancer, SAHF staining using homolog protein 1 gamma has been used to identify senescence in MCF7 cells (91). In cancers in which chromatin maintenance is dysregulated, the occurrence and composition of these foci may vary. The utility of these markers to identify senescence in patient tissues is as yet unexplored.
A more promising class of markers include the CDKIs whose increased expression mediates senescence cell cycle arrest (Table 1). Amplified expression of the CDKIs p16Ink4a and related Ink4 proteins, p57Kip2, p21Waf1/Cip1, and notably p27Kip1, has been observed in senescent cells and tissues (7,8). However, many CDKIs are inactivated during senescence bypass, making them less reliable markers. In cancer, the downregulation of p27Kip1 and expression of its regulator ubiquitin ligase SKP2 has been identified in prostate and other cancers (92,93), as well as in precancerous lesions (17). In an AKT1 (v-akt murine thymoma viral oncogene homolog 1)-driven murine prostate cancer model, p27Kip1 is a key checkpoint for senescence (17). The CDKN1B/p27Kip1 gene itself is infrequently mutated or deleted in many cancers, suggesting that its induction may represent a more promising marker of senescence.
Senescence is also characterized by a large protein secretory response. This phenotype in fibroblasts and some cancer cells includes proteins involved in IGF signaling (including IGF2 and IGFBPs 3, 5, 6, and 7) (41,44,50,51), immuno-inflammatory cytokines (eg, IL-6, IL-8, and related proteins) (39,70,88) and chemokine (C-X-C motif) ligand 14 (BRAK/CXCL14), whose function remains largely undefined (11). IL-8, IGFBP7, and the IL-6 receptor chemokine (C-X-C motif) receptor 2 (CXCR2) have been shown by immunohistochemistry to be expressed in lesions undergoing tumor-suppressive oncogene-induced senescence (39,41,88). The induction of these secreted factors in senescence may potentially serve as serum-based markers for the identification of patients undergoing senescence responses.
We used microarrays to screen a series of genes with increased expression during epithelial senescence for their role as markers of senescence in cancer (11). In a series of cancer lines using a number of senescence-inducing drugs, transcripts of versican, filamin A–interacting protein 1–like (FILIP1L), and chromosome 5 open reading frame 13/P311 RNA were found to represent specific markers of senescence that are not induced during apoptosis. Changes in mitochondrial architecture may also be used. Mitochondria in proliferating fibroblasts are distinct and small, whereas in senescent cells, mitochondria fuse into elongated and integrated networks (38). The expression and localization of the integral mitochondrial proteins human fission protein 1 (hFIS1) and optic atrophy 1 (OPA1) regulate these changes and the development of senescence (38). Finally, the proteins Dec1 (BHLHB2) and DcR1 (TNFRSF10D) have been associated with senescence in noncancer tissues (15). Although these proteins may be detected by immunohistochemistry, the utility of these potential markers in identifying senescence in fixed patient tumors has yet to be investigated.
Recently, quantitative modeling was used to assess validity of senescence markers in nontransformed cells as they become replicatively senescent (90). Decreased proliferation of senescent cells was associated with measured increases in SA-β-gal activity and the combined detection of phosphorylated H2A histone family, member X (γ-H2AX, H2AFX) and decreased expression of the proliferation marker protein KI-67 (antigen identified by monoclonal antibody Ki-67, MKI67). This pattern of staining predicted the extent of senescence more closely than detection of other senescence-associated markers, individually or in combination. Dual-detection of KI-67 and extensive γ-H2AX phosphorylation was also associated with SA-β-gal staining in mouse intestinal crypts. Although these detection methods have not been used with other cell types or other mechanisms to induce senescence, they provide a computational framework for developing and validating senescence biomarkers and for predicting the frequency of markers in senescent tissue.
In summary, the identification of multiple markers in tissues currently provides more reliable evidence for senescence than that provided by a single marker. The most widely used marker of senescence is SA-β-gal, which provides strong evidence for identifying senescence when used in vitro with changes in morphology, increased side scatter, and accumulation in phases G0/G1 and G2/M. Other useful in vitro markers include the CDKIs p21Waf1, p16Ink4a and p27Kip1; versican and FILIP1L; and the increased expression of secreted cytokines. In vivo, SA-β-gal staining in conjunction with CDKI protein induction and other markers of decreased proliferation provide evidence for the presence of senescence.
Clinical Potential for Senescence-Based Tumor Suppression
Therapeutic senescence is a potential mechanism to induce cytostasis in cancer. The goal of this strategy is to inhibit tumor growth rather than to cause regression or ablation. Given various in vitro studies (9,11) and the effects of retinoic acid (9) and AZQ (our unpublished data) on xenograft tumors, senescence can be achieved by the chronic administration of low doses of senescence-inducing drugs. Effective dosing to achieve senescence will vary with the drug but may involve lower doses than those that generate apoptosis (13). Notably, tumor models with inactivated apoptotic signaling pathways respond to senescence-inducing drugs, which have been demonstrated to lead to improved survival after chemotherapy in a mouse Eμ-myc lymphoma model (20).
As with many anticancer approaches, TIS can generate a heterogeneous response in tumors in vivo. Only a subset of cells responds with senescence to current chemotherapeutics in human cancer (18,19). Recent data suggest that the expression of cytokines and secreted factors by senescent cancer cells may have an inhibitory effect on the growth of surrounding bystander cells (39,41,84,88), resulting in inhibitory or growth-neutral tumor effects (21,74,84). Alternatively, this heterogeneous response may have a growth-promoting effect in some situations (7,82). With the further identification of precise pathways that regulate senescence and additional specific senescence-inducing agents, we foresee a wider exploitation of this approach in cancer treatment.
Several scenarios can be anticipated for the utilization of senescence in clinical cancer therapy. One is its use in clinically advanced tumors. These cancers frequently contain cells that have bypassed senescence-associated barriers during oncogenic progression (5). However, data from our laboratory and others indicate that even in these advanced cancers, senescence can be induced through use of specific drugs (9,11). Tumor tissues may be monitored for changes in size, senescence activity (SA β-gal, p27Kip1), and other markers of proliferation such as KI-67. Given the robust secretory activity of senescent cells, serum markers of senescence (eg, BRAK/CXCL14, IL-8, CSF, and IGFBPs) might be used to measure response.
An alternate use for senescence-inducing compounds would be the treatment of premalignant or early cancers. Research has suggested that cells in the premalignant prostatic lesion, prostatic intraepithelial neoplasia, are frequently senescent, with more than 50% staining positive in four of seven specimens (16,17). These findings may represent senescence as tumor suppression. Retinoids are a class of drugs used in chemoprevention and have demonstrated senescence-inducing activity in several cancers (4). Senescence has also been shown to be triggered in osteosarcoma, colon adenocarcinoma, and skin cancer cell lines by grape seed–derived resveratrol, raising its potential as a chemopreventive agent against these diseases (71,94,95). Further studies are required to determine whether other chemoprevention agents induce senescence. Whether premalignant and early cancer cells are more sensitive to pro-senescent drugs than surrounding normal tissue is unclear.
Senescence markers and characteristics could also be used to assess tumor prognosis. Given that senescent cells display persistent growth arrest, the presence of senescence in a cancer may indicate slower overall tumor growth or decreased metastatic potential. This relationship is illustrated by the identification of senescence markers in nevi, lung adenomas, and other nonmalignant growths, including prostatic intraepithelial neoplasia (14–17). Other markers associated with senescence, including p27Kip1 and p16Ink4a, are selectively expressed in cancers, and their presence is associated with improved prognosis and lower probability of relapse after treatment (96). A similar finding has been demonstrated for IGFBP3 and maspin in prostate and other cancers (11,44,97). Our group and others have noted that a subpopulation within proliferating tumor cell lines (eg, LNCaP, DU145, PC3, MCF7, and HCT116) appear to senesce spontaneously (9,11,18), which may also occur in patient tumors in vivo. Sporadic SA-β-gal staining has been observed in 30% of lung and 20% of breast tumors in untreated control patients, whereas no staining was observed in normal tissues (18,19), suggesting that some malignancies can form without bypassing pro-senescent pressure and may have intact senescence machinery, especially in early cancers. These intriguing observations suggest that senescence may provide prognostic data, but further investigation is required.
Conclusions
In the war on cancer, the focus has been on achieving complete cure through tumor eradication. However, tumor cells are typically heterogeneous and adapt rapidly to toxic chemicals and varying environments. Increasing information supports an approach that incorporates the induction of senescence in cancer therapy. Other data suggest that the presence of cancer cells sensitive to therapy may suppress the growth of resistant clones (98). Furthermore, the obliteration of these sensitive cells using cytotoxic chemotherapy may result in the rapid unchecked proliferation of resistant clones. Approaches designed to maintain a stable tumor volume may actually lead to improved survival (98).
The therapeutic induction of senescence is a potential means to treat cancer through induction of a persistent cytostatic state in tumors. In many cell types, senescence is an endogenous mechanism to limit the growth of nonmalignant neoplasias. Accumulating data indicate that cancer cells that have bypassed many major tumor suppressor blocks remain sensitive to induced senescence, suggesting that TIS may be widely applicable. Other advantages of senescence include cytostasis, low toxicity-related side effects, and immune stimulation. The finding of endogenous senescence in some premalignant lesions and cancers may have favorable implications with regard to tumor biology and prognosis. Some hurdles still remain, including development of robust senescence-inducing agents, identification of more reliable markers, and continued investigations of the biological implications of senescence in tumors.
Cancer therapy to date has focused on complete eradication at the expense of treatment-related complications. TIS may lead to chronic tumors that allow patients to maintain quality and quantity of life. Given the expected increase in cancer with the aging population, we need to consider senescence as part of our armamentarium to treat cancer patients as effectively as possible while maintaining the quality of their lives.
Funding
This work was supported by the National Institutes of Health (R01CA97131) and the University of Wisconsin George M. O’Brien Urology Research Center (1P50DK065303), the John Livesey Endowment, and the Department of Defense Prostate Cancer Research Program (DAMD17-02-1-0163 to D.F.J. and J.A.D.). J.A.E. is supported through a Ruth L. Kirchstein National Research Service Award (T32 CA009681-14).
Footnotes
The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors claim no professional or financial conflicts of interest.
References
- 1.Desai AA, Stadler WM. Novel kinase inhibitors in renal cell carcinoma: progressive development of static agents. Curr Urol Rep. 2006;7(1):16–22. doi: 10.1007/s11934-006-0033-x. [DOI] [PubMed] [Google Scholar]
- 2.Martin L, Schilder RJ. Novel non-cytotoxic therapy in ovarian cancer: current status and future prospects. J Natl Compr Canc Netw. 2006;4(9):955–966. doi: 10.6004/jnccn.2006.0079. [DOI] [PubMed] [Google Scholar]
- 3.Winquist E, Waldron T, Berry S, Ernst DS, Hotte S, Lukka H. Non-hormonal systemic therapy in men with hormone-refractory prostate cancer and metastases: a systematic review from the Cancer Care Ontario Program in Evidence-based Care’s Genitourinary Cancer Disease Site Group. BMC Cancer. 2006;6:112. doi: 10.1186/1471-2407-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63(11):2705–2715. [PubMed] [Google Scholar]
- 5.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 7.Campisi J. d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 8.Bringold F, Serrano M. Tumor suppressors and oncogenes in cellular senescence. Exp Gerontol. 2000;35(3):317–329. doi: 10.1016/s0531-5565(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 9.Chang BD, Broude EV, Dokmanovic M, et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999;59(15):3761–3767. [PubMed] [Google Scholar]
- 10.Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76(8):947–957. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Schwarze SR, Fu VX, Desotelle JA, Kenowski ML, Jarrard DF. The identification of senescence-specific genes during the induction of senescence in prostate cancer cells. Neoplasia. 2005;7(9):816–823. doi: 10.1593/neo.05250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewald JA, Peters N, Desotelle JA, Hoffmann FM, Jarrard DF. A high-throughput method to identify novel senescence-inducing compounds. J Biomol Screen. 2009;14(7):853–858. doi: 10.1177/1087057109340314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaloglou C, Vredeveld LC, Soengas MS, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436(7051):720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 15.Collado M, Gil J, Efeyan A, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436(7051):642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, Shendrik I, Peacocke M, et al. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000;56(1):160–166. doi: 10.1016/s0090-4295(00)00538-0. [DOI] [PubMed] [Google Scholar]
- 17.Majumder PK, Grisanzio C, O’Connell F, et al. A prostatic intraepithelial neoplasia-dependent p27 Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression. Cancer Cell. 2008;14(2):146–155. doi: 10.1016/j.ccr.2008.06.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberson RS, Kussick SJ, Vallieres E, Chen SY, Wu DY. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res. 2005;65(7):2795–2803. doi: 10.1158/0008-5472.CAN-04-1270. [DOI] [PubMed] [Google Scholar]
- 19.te Poele RH Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62(6):1876–1883. [PubMed] [Google Scholar]
- 20.Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109(3):335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 21.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunn CL, Tarrant GM. Limited lifespan in somatic cell hybrids and cybrids. Exp Cell Res. 1980;127(2):385–396. doi: 10.1016/0014-4827(80)90443-7. [DOI] [PubMed] [Google Scholar]
- 23.Pereira-Smith OM, Smith JR. Expression of SV40 T antigen in finite life-span hybrids of normal and SV40-transformed fibroblasts. Somatic Cell Genet. 1981;7(4):411–421. doi: 10.1007/BF01542986. [DOI] [PubMed] [Google Scholar]
- 24.Smith JR, Ning Y, Pereira-Smith OM. Why are transformed cells immortal? Is the process reversible? Am J Clin Nutr. 1992;55(6 suppl):1215S–1221S. doi: 10.1093/ajcn/55.6.1215S. [DOI] [PubMed] [Google Scholar]
- 25.Muggleton-Harris AL, DeSimone DW. Replicative potentials of various fusion products between WI-38 and SV40 transformed WI-38 cells and their components. Somatic Cell Genet. 1980;6(6):689–698. doi: 10.1007/BF01538968. [DOI] [PubMed] [Google Scholar]
- 26.Ravi RK, McMahon M, Yangang Z, et al. Raf-1-induced cell cycle arrest in LNCaP human prostate cancer cells. J Cell Biochem. 1999;72(4):458–469. doi: 10.1002/(sici)1097-4644(19990315)72:4<458::aid-jcb2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 27.Haq R, Brenton JD, Takahashi M, et al. Constitutive p38HOG mitogen-activated protein kinase activation induces permanent cell cycle arrest and senescence. Cancer Res. 2002;62(17):5076–5082. [PubMed] [Google Scholar]
- 28.Huck JJ, Zhang M, McDonald A, et al. MLN8054, an Inhibitor of Aurora A kinase, induces senescence in human tumor cells both in vitro and in vivo. Mol Cancer Res. 2010;8(3):373–384. doi: 10.1158/1541-7786.MCR-09-0300. [DOI] [PubMed] [Google Scholar]
- 29.Alimonti A, Nardella C, Chen Z, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 2010;120(3):681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin HK, Chen Z, Wang G, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464(7287):374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugrue MM, Shin DY, Lee SW, Aaronson SA. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc Natl Acad Sci U S A. 1997;94(18):9648–9653. doi: 10.1073/pnas.94.18.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung MS, Yun J, Chae HD, et al. p53 and its homologues, p63 and p73, induce a replicative senescence through inactivation of NF-Y transcription factor. Oncogene. 2001;20(41):5818–5825. doi: 10.1038/sj.onc.1204748. [DOI] [PubMed] [Google Scholar]
- 33.Xu HJ, Zhou Y, Ji W, et al. Reexpression of the retinoblastoma protein in tumor cells induces senescence and telomerase inhibition. Oncogene. 1997;15(21):2589–2596. doi: 10.1038/sj.onc.1201446. [DOI] [PubMed] [Google Scholar]
- 34.Fang L, Igarashi M, Leung J, Sugrue MM, Lee SW, Aaronson SA. p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene. 1999;18(18):2789–2797. doi: 10.1038/sj.onc.1202615. [DOI] [PubMed] [Google Scholar]
- 35.Dai CY, Enders GH. p16 INK4a can initiate an autonomous senescence program. Oncogene. 2000;19(13):1613–1622. doi: 10.1038/sj.onc.1203438. [DOI] [PubMed] [Google Scholar]
- 36.Tsugu A, Sakai K, Dirks PB, et al. Expression of p57(KIP2) potently blocks the growth of human astrocytomas and induces cell senescence. Am J Pathol. 2000;157(3):919–932. doi: 10.1016/S0002-9440(10)64605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuxe J, Akusjarvi G, Goike HM, Roos G, Collins VP, Pettersson RF. Adenovirus-mediated overexpression of p15INK4B inhibits human glioma cell growth, induces replicative senescence, and inhibits telomerase activity similarly to p16INK4A. Cell Growth Differ. 2000;11(7):373–384. [PubMed] [Google Scholar]
- 38.Lee S, Jeong SY, Lim WC, et al. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007;282(31):22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- 39.Kuilman T, Michaloglou C, Vredeveld LC, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 40.Sprenger CC, Vail ME, Evans K, Simurdak J, Plymate SR. Over-expression of insulin-like growth factor binding protein-related protein-1(IGFBP-rP1/mac25) in the M12 prostate cancer cell line alters tumor growth by a delay in G1 and cyclin A associated apoptosis. Oncogene. 2002;21(1):140–147. doi: 10.1038/sj.onc.1205021. [DOI] [PubMed] [Google Scholar]
- 41.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132(3):363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37(5):961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Beausejour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22(16):4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang BD, Swift ME, Shen M, Fang J, Broude EV, Roninson IB. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc Natl Acad Sci U S A. 2002;99(1):389–394. doi: 10.1073/pnas.012602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang BD, Xuan Y, Broude EV, et al. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 1999;18(34):4808–4818. doi: 10.1038/sj.onc.1203078. [DOI] [PubMed] [Google Scholar]
- 46.Schwarze SR, Shi Y, Fu VX, Watson PA, Jarrard DF. Role of cyclin-dependent kinase inhibitors in the growth arrest at senescence in human prostate epithelial and uroepithelial cells. Oncogene. 2001;20(57):8184–8192. doi: 10.1038/sj.onc.1205049. [DOI] [PubMed] [Google Scholar]
- 47.Guo X, Mills AA. p63, cellular senescence and tumor development. Cell Cycle. 2007;6(3):305–311. doi: 10.4161/cc.6.3.3794. [DOI] [PubMed] [Google Scholar]
- 48.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24(17):2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 49.Keyes WM, Mills AA. p63: a new link between senescence and aging. Cell Cycle. 2006;5(3):260–265. doi: 10.4161/cc.5.3.2415. [DOI] [PubMed] [Google Scholar]
- 50.Schwarze SR, DePrimo SE, Grabert LM, Fu VX, Brooks JD, Jarrard DF. Novel pathways associated with bypassing cellular senescence in human prostate epithelial cells. J Biol Chem. 2002;277(17):14877–14883. doi: 10.1074/jbc.M200373200. [DOI] [PubMed] [Google Scholar]
- 51.Untergasser G, Koch HB, Menssen A, Hermeking H. Characterization of epithelial senescence by serial analysis of gene expression: identification of genes potentially involved in prostate cancer. Cancer Res. 2002;62(21):6255–6262. [PubMed] [Google Scholar]
- 52.Crescenzi E, Palumbo G, Brady HJ. Bcl-2 activates a programme of premature senescence in human carcinoma cells. Biochem J. 2003;375(pt 2):263–274. doi: 10.1042/BJ20030868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsson O, Scheele C, Liang Z, Moll J, Karlsson C, Wahlestedt C. Kinetics of senescence-associated changes of gene expression in an epithelial, temperature-sensitive SV40 large T antigen model. Cancer Res. 2004;64(2):482–489. doi: 10.1158/0008-5472.can-03-1872. [DOI] [PubMed] [Google Scholar]
- 54.Rebbaa A, Zheng X, Chou PM, Mirkin BL. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene. 2003;22(18):2805–2811. doi: 10.1038/sj.onc.1206366. [DOI] [PubMed] [Google Scholar]
- 55.d’Adda di Fagagna F Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8(7):512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 56.Mallette FA, Gaumont-Leclerc MF, Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 2007;21(1):43–48. doi: 10.1101/gad.1487307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ota H, Tokunaga E, Chang K, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25(2):176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 58.Ishikawa F. Cellular senescence as a stress response. Cornea. 2006;25(10) suppl 1:S3–S6. doi: 10.1097/01.ico.0000247206.47897.92. [DOI] [PubMed] [Google Scholar]
- 59.Cozzi SJ, Parsons PG, Ogbourne SM, Pedley J, Boyle GM. Induction of senescence in diterpene ester-treated melanoma cells via protein kinase C-dependent hyperactivation of the mitogen-activated protein kinase pathway. Cancer Res. 2006;66(20):10083–10091. doi: 10.1158/0008-5472.CAN-06-0348. [DOI] [PubMed] [Google Scholar]
- 60.Mason SA, Cozzi SJ, Pierce CJ, Pavey SJ, Parsons PG, Boyle GM. The induction of senescence-like growth arrest by protein kinase C-activating diterpene esters in solid tumor cells. Invest New Drugs. 2010;28(5):575–586. doi: 10.1007/s10637-009-9292-y. [DOI] [PubMed] [Google Scholar]
- 61.Lu T, Finkel T. Free radicals and senescence. Exp Cell Res. 2008;314(9):1918–1922. doi: 10.1016/j.yexcr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magda D, Lecane P, Wang Z, et al. Synthesis and anticancer properties of water-soluble zinc ionophores. Cancer Res. 2008;68(13):5318–5325. doi: 10.1158/0008-5472.CAN-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV. Pseudo-DNA damage response in senescent cells. Cell Cycle. 2009;8(24):4112–4118. doi: 10.4161/cc.8.24.10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Linge A, Weinhold K, Blasche R, Kasper M, Barth K. Downregulation of caveolin-1 affects bleomycin-induced growth arrest and cellular senescence in A549 cells. Int J Biochem Cell Biol. 2007;39(10):1964–1974. doi: 10.1016/j.biocel.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 65.Han Z, Wei W, Dunaway S, et al. Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J Biol Chem. 2002;277(19):17154–17160. doi: 10.1074/jbc.M112401200. [DOI] [PubMed] [Google Scholar]
- 66.Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008;103(2):509–519. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Narath R, Ambros IM, Kowalska A, Bozsaky E, Boukamp P, Ambros PF. Induction of senescence in MYCN amplified neuroblastoma cell lines by hydroxyurea. Genes Chromosomes Cancer. 2007;46(2):130–142. doi: 10.1002/gcc.20393. [DOI] [PubMed] [Google Scholar]
- 68.Nakai R, Iida S, Takahashi T, et al. K858, a novel inhibitor of mitotic kinesin Eg5 and antitumor agent, induces cell death in cancer cells. Cancer Res. 2009;69(9):3901–3909. doi: 10.1158/0008-5472.CAN-08-4373. [DOI] [PubMed] [Google Scholar]
- 69.Lee J, Lee I, Park C, Kang WK. Lovastatin-induced RhoA modulation and its effect on senescence in prostate cancer cells. Biochem Biophys Res Commun. 2006;339(3):748–754. doi: 10.1016/j.bbrc.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 70.Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heiss EH, Schilder YD, Dirsch VM. Chronic treatment with resveratrol induces redox stress- and ataxia telangiectasia-mutated (ATM)-dependent senescence in p53-positive cancer cells. J Biol Chem. 2007;282(37):26759–26766. doi: 10.1074/jbc.M703229200. [DOI] [PubMed] [Google Scholar]
- 72.Bender JF, Grillo-Lopez AJ, Posada JG., Jr. Diaziquone (AZQ) Invest New Drugs. 1983;1(1):71–84. doi: 10.1007/BF00180194. [DOI] [PubMed] [Google Scholar]
- 73.Bukowski RM, Fleming TR, Macdonald JS, Oishi N, Taylor SA, Baker LH. Evaluation of combination chemotherapy and phase II agents in pancreatic adenocarcinoma. A Southwest Oncology Group study. Cancer. 1993;71(2):322–325. doi: 10.1002/1097-0142(19930115)71:2<322::aid-cncr2820710209>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 74.Ewald JA, Desotelle JA, Almassi N, Jarrard DF. Drug-induced senescence bystander proliferation in prostate cancer cells in vitro and in vivo. Br J Cancer. 2008;98(7):1244–1249. doi: 10.1038/sj.bjc.6604288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerland LM, Peyrol S, Lallemand C, Branche R, Magaud JP, Ffrench M. Association of increased autophagic inclusions labeled for beta-galactosidase with fibroblastic aging. Exp Gerontol. 2003;38(8):887–895. doi: 10.1016/s0531-5565(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 76.Petti C, Molla A, Vegetti C, Ferrone S, Anichini A, Sensi M. Coexpression of NRASQ61R and BRAFV600E in human melanoma cells activates senescence and increases susceptibility to cell-mediated cytotoxicity. Cancer Res. 2006;66(13):6503–6511. doi: 10.1158/0008-5472.CAN-05-4671. [DOI] [PubMed] [Google Scholar]
- 77.Dirac AM, Bernards R. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J Biol Chem. 2003;278(14):11731–11734. doi: 10.1074/jbc.C300023200. [DOI] [PubMed] [Google Scholar]
- 78.Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 1995;55(11):2284–2292. [PubMed] [Google Scholar]
- 79.Sasaki M, Kumazaki T, Takano H, Nishiyama M, Mitsui Y. Senescent cells are resistant to death despite low Bcl-2 level. Mech Ageing Dev. 2001;122(15):1695–1706. doi: 10.1016/s0047-6374(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 80.Hampel B, Malisan F, Niederegger H, Testi R, Jansen-Durr P. Differential regulation of apoptotic cell death in senescent human cells. Exp Gerontol. 2004;39(11–12):1713–1721. doi: 10.1016/j.exger.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 81.DeJesus V, Rios I, Davis C, et al. Induction of apoptosis in human replicative senescent fibroblasts. Exp Cell Res. 2002;274(1):92–99. doi: 10.1006/excr.2001.5425. [DOI] [PubMed] [Google Scholar]
- 82.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98(21):12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H, Pan KH, Cohen SN. Senescence-specific gene expression fingerprints reveal cell-type-dependent physical clustering of up-regulated chromosomal loci. Proc Natl Acad Sci U S A. 2003;100(6):3251–3256. doi: 10.1073/pnas.2627983100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di X, Bright AT, Bellott R, et al. A chemotherapy-associated senescence bystander effect in breast cancer cells. Cancer Biol Ther. 2008;7(6):864–872. doi: 10.4161/cbt.7.6.5861. [DOI] [PubMed] [Google Scholar]
- 85.Untergasser G, Gander R, Rumpold H, Heinrich E, Plas E, Berger P. TGF-beta cytokines increase senescence-associated beta-galactosidase activity in human prostate basal cells by supporting differentiation processes, but not cellular senescence. Exp Gerontol. 2003;38(10):1179–1188. doi: 10.1016/j.exger.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 86.Lee BY, Han JA, Im JS, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5(2):187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 87.Zhang R, Adams PD. Heterochromatin and its relationship to cell senescence and cancer therapy. Cell Cycle. 2007;6(7):784–789. doi: 10.4161/cc.6.7.4079. [DOI] [PubMed] [Google Scholar]
- 88.Acosta JC, O’Loghlen A, Banito A, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133(6):1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 89.Zhang R, Chen W, Adams PD. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27(6):2343–2358. doi: 10.1128/MCB.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawless C, Wang C, Jurk D, Merz A, Zglinicki TV, Passos JF. Quantitative assessment of markers for cell senescence. Exp Gerontol. 2010 doi: 10.1016/j.exger.2010.01.018. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 91.Rastogi S, Joshi B, Dasgupta P, Morris M, Wright K, Chellappan S. Prohibitin facilitates cellular senescence by recruiting specific corepressors to inhibit E2F target genes. Mol Cell Biol. 2006;26(11):4161–4171. doi: 10.1128/MCB.02142-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8(4):253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 93.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8(6):438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19(9):1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 95.Rusin M, Zajkowicz A, Butkiewicz D. Resveratrol induces senescence-like growth inhibition of U-2 OS cells associated with the instability of telomeric DNA and upregulation of BRCA1. Mech Ageing Dev. 2009;130(8):528–537. doi: 10.1016/j.mad.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 96.Halvorsen OJ. Molecular and prognostic markers in prostate cancer. A study of cell-cycle regulators, angiogenesis and candidate markers. APMIS Suppl. 2008;(123):5–62. [PubMed] [Google Scholar]
- 97.Bensalah K, Lotan Y, Karam JA, Shariat SF. New circulating biomarkers for prostate cancer. Prostate Cancer Prostatic Dis. 2008;11(2):112–120. doi: 10.1038/sj.pcan.4501026. [DOI] [PubMed] [Google Scholar]
- 98.Gatenby RA. A change of strategy in the war on cancer. Nature. 2009;459(7246):508–509. doi: 10.1038/459508a. [DOI] [PubMed] [Google Scholar]